Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes
Abstract
1. Introduction
2. Materials and Methods
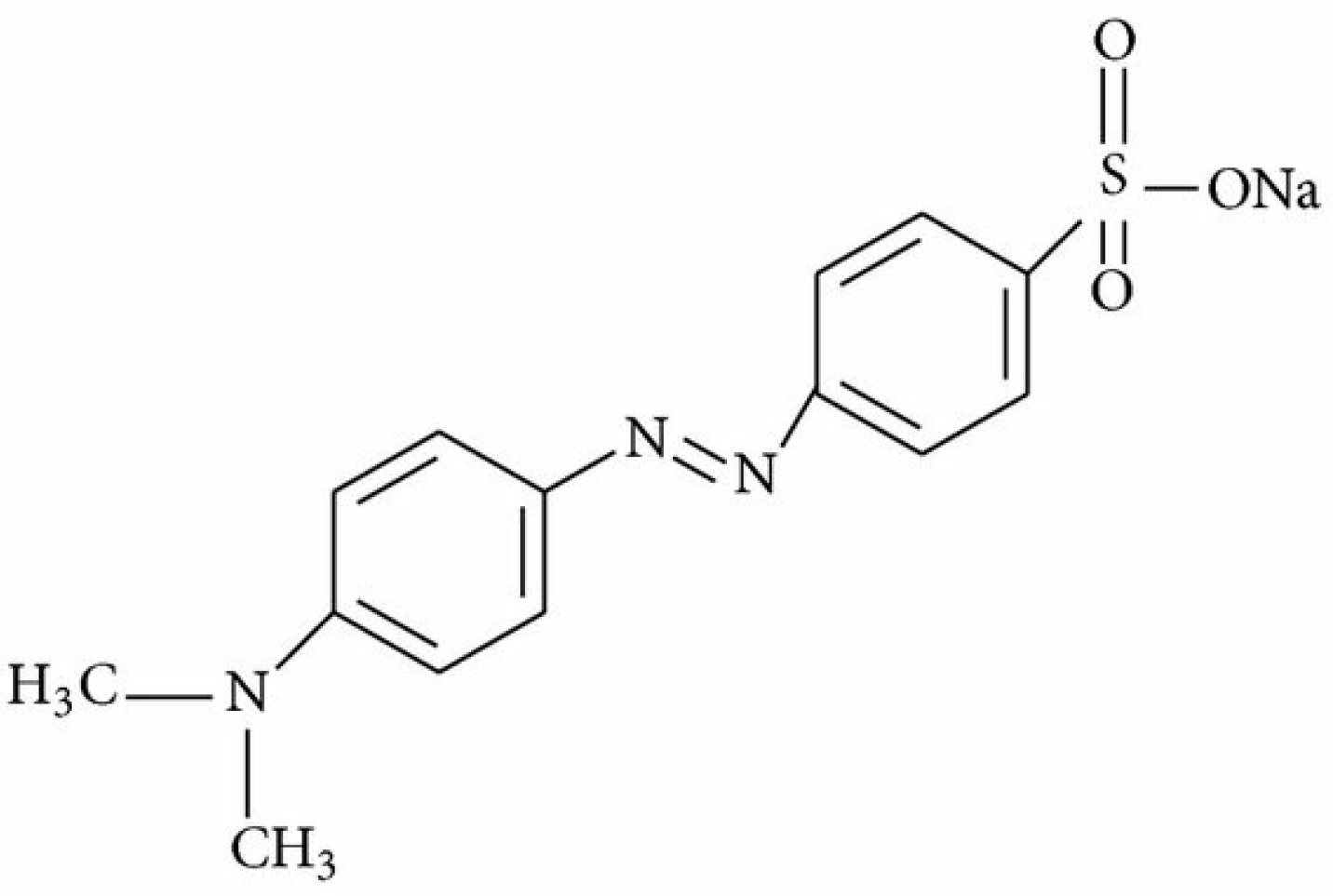
2.1. Chemicals
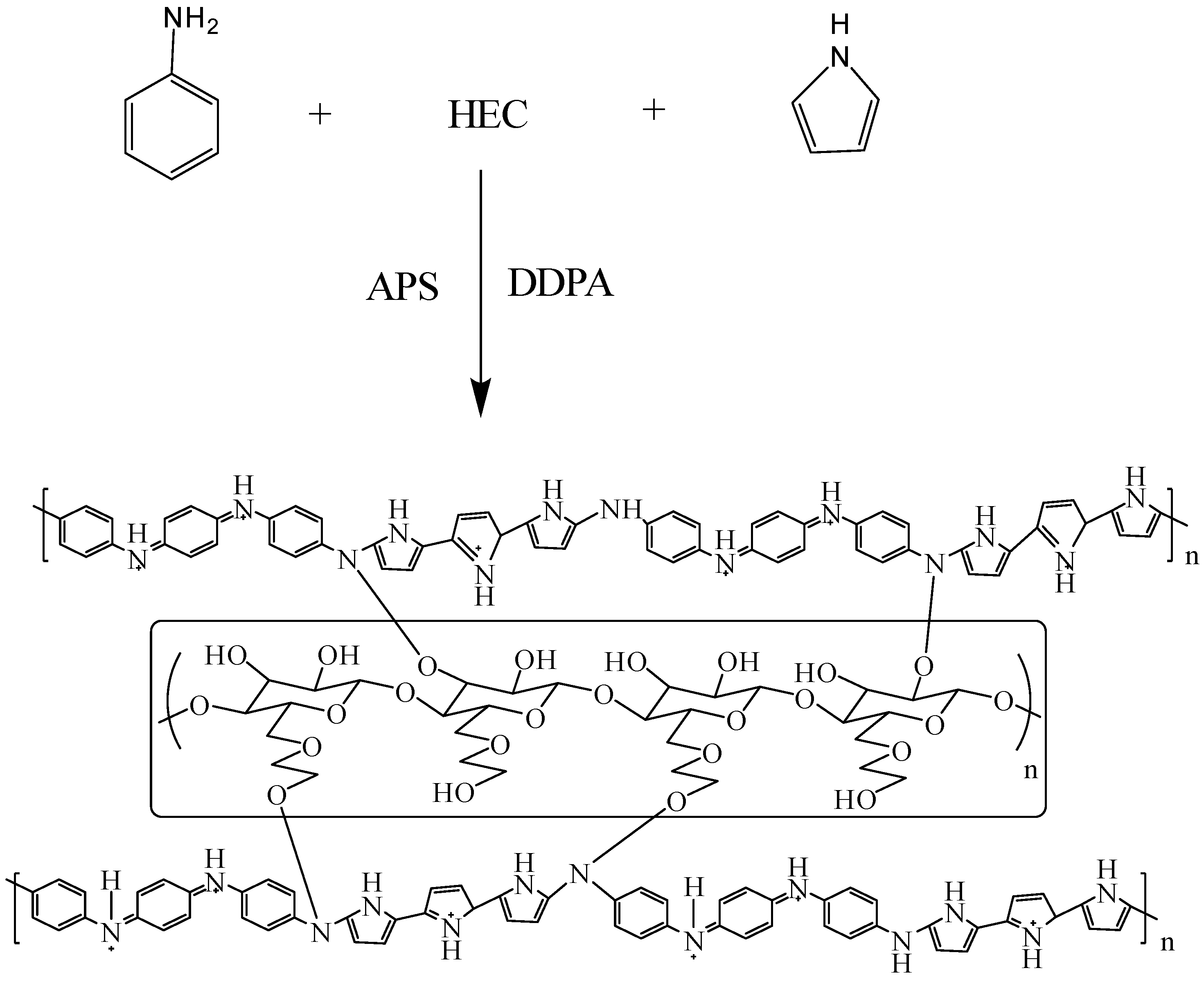
2.2. Preparation of HEC/PANI-PPy
2.3. Characterization of HEC/PANI-PPy
3. Results and Discussion
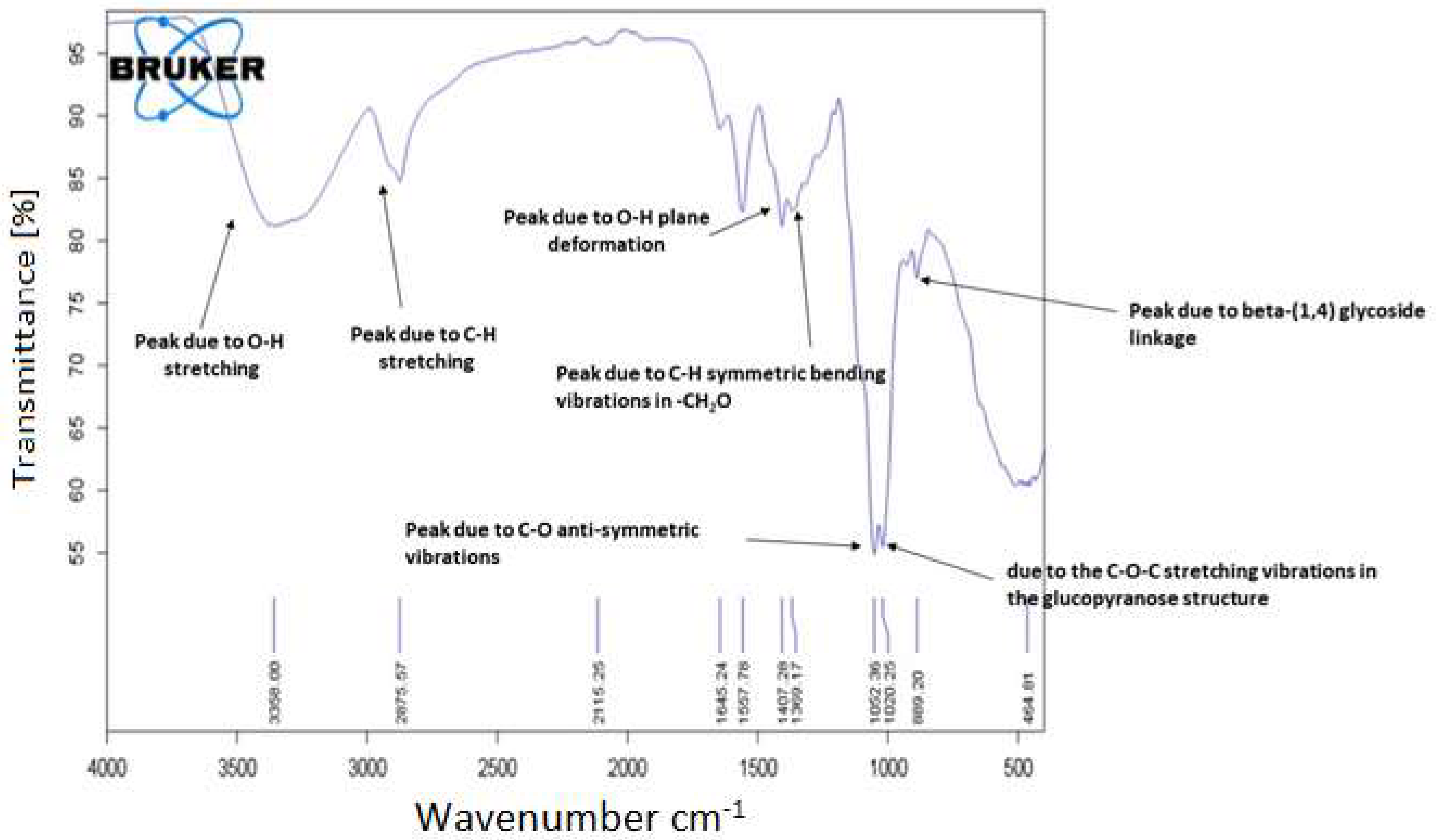
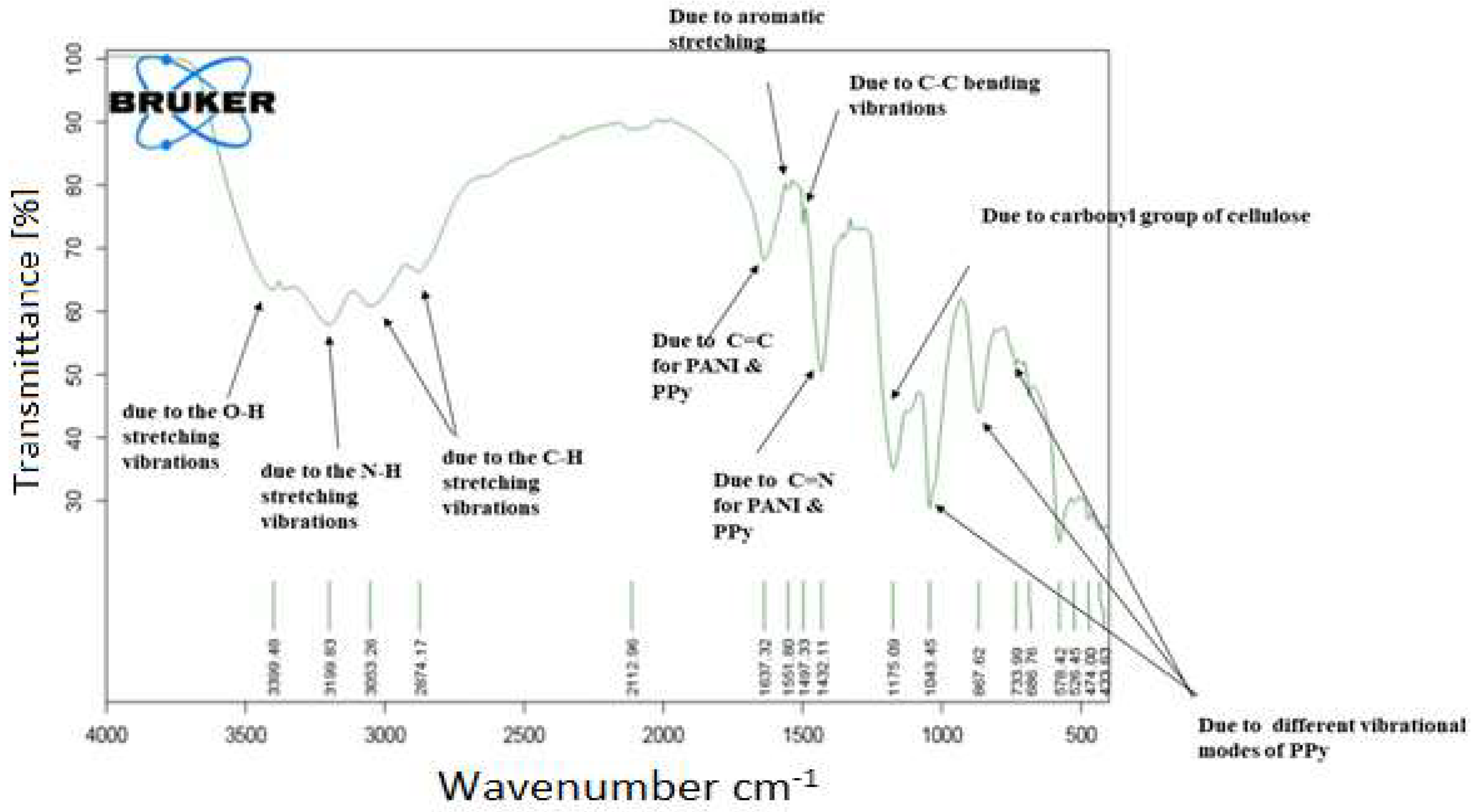
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
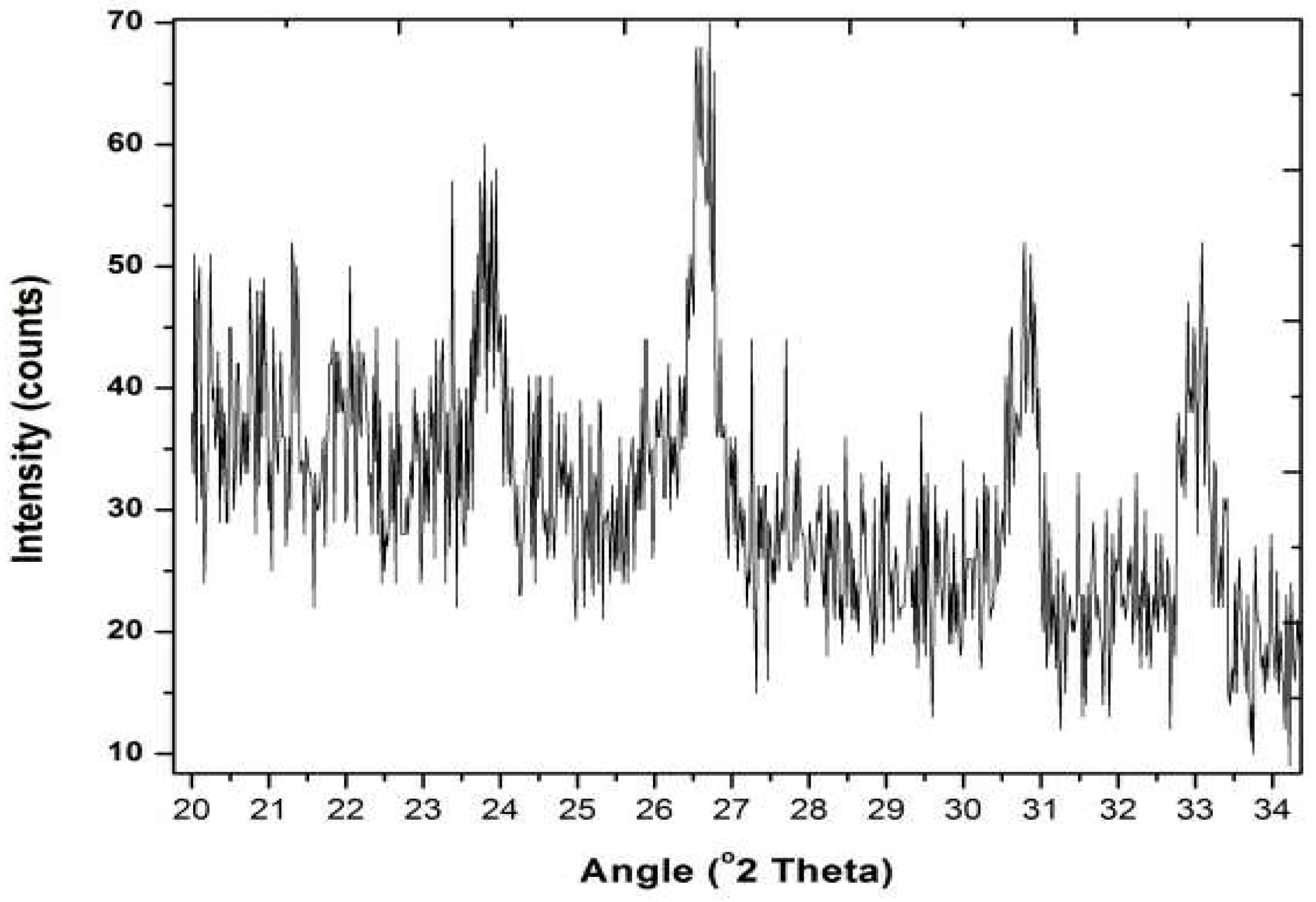
3.2. X-rays Diffraction (XRD) Analysis
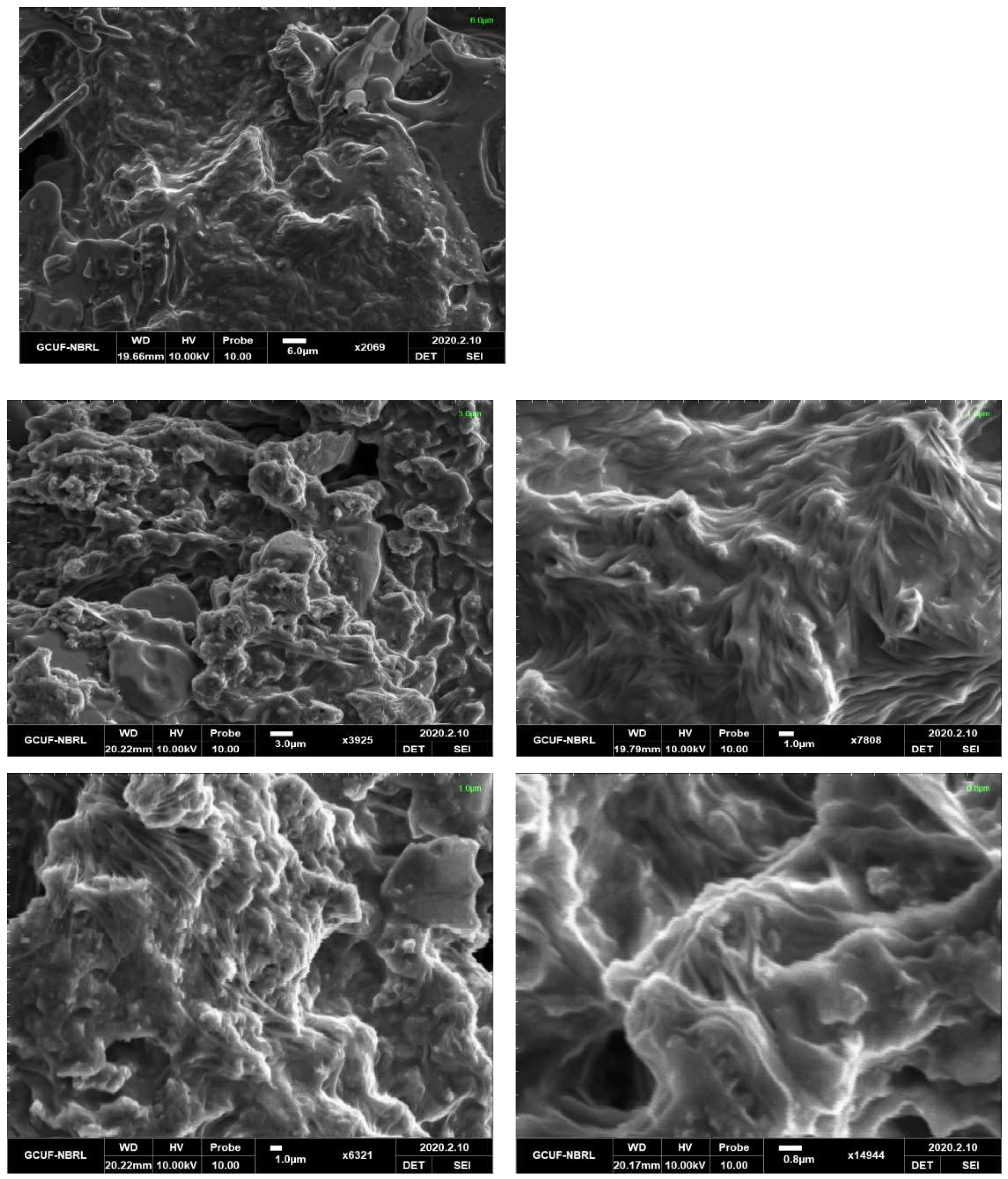
3.3. Morphological Study by Scanning Electron Microscopy (SEM)
3.4. Brunauer-Emmett-Teller (BET)
3.5. Adsorption of Dyes
4. Dyes Adsorption and Effect of Different Parameters
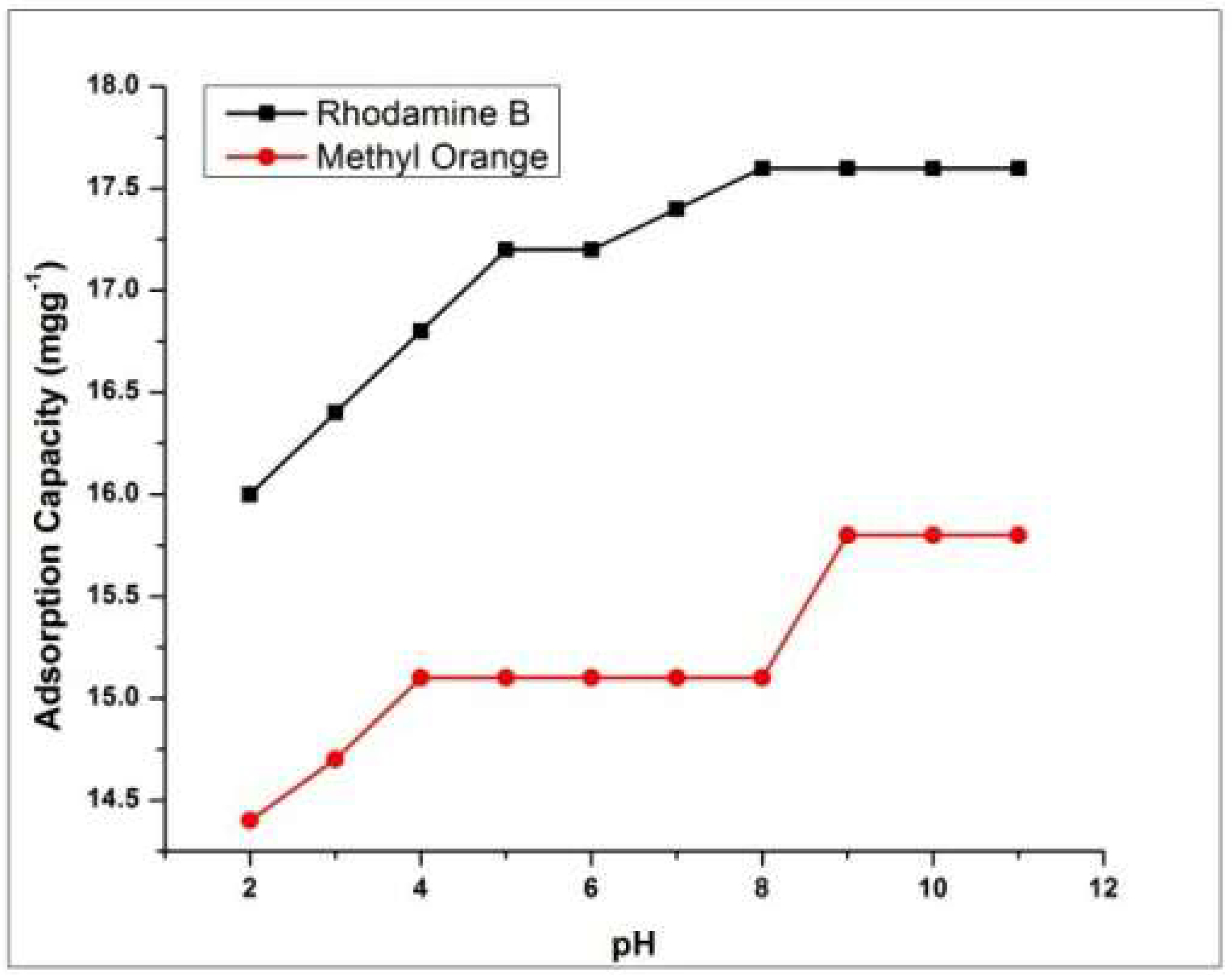
4.1. Effect of pH
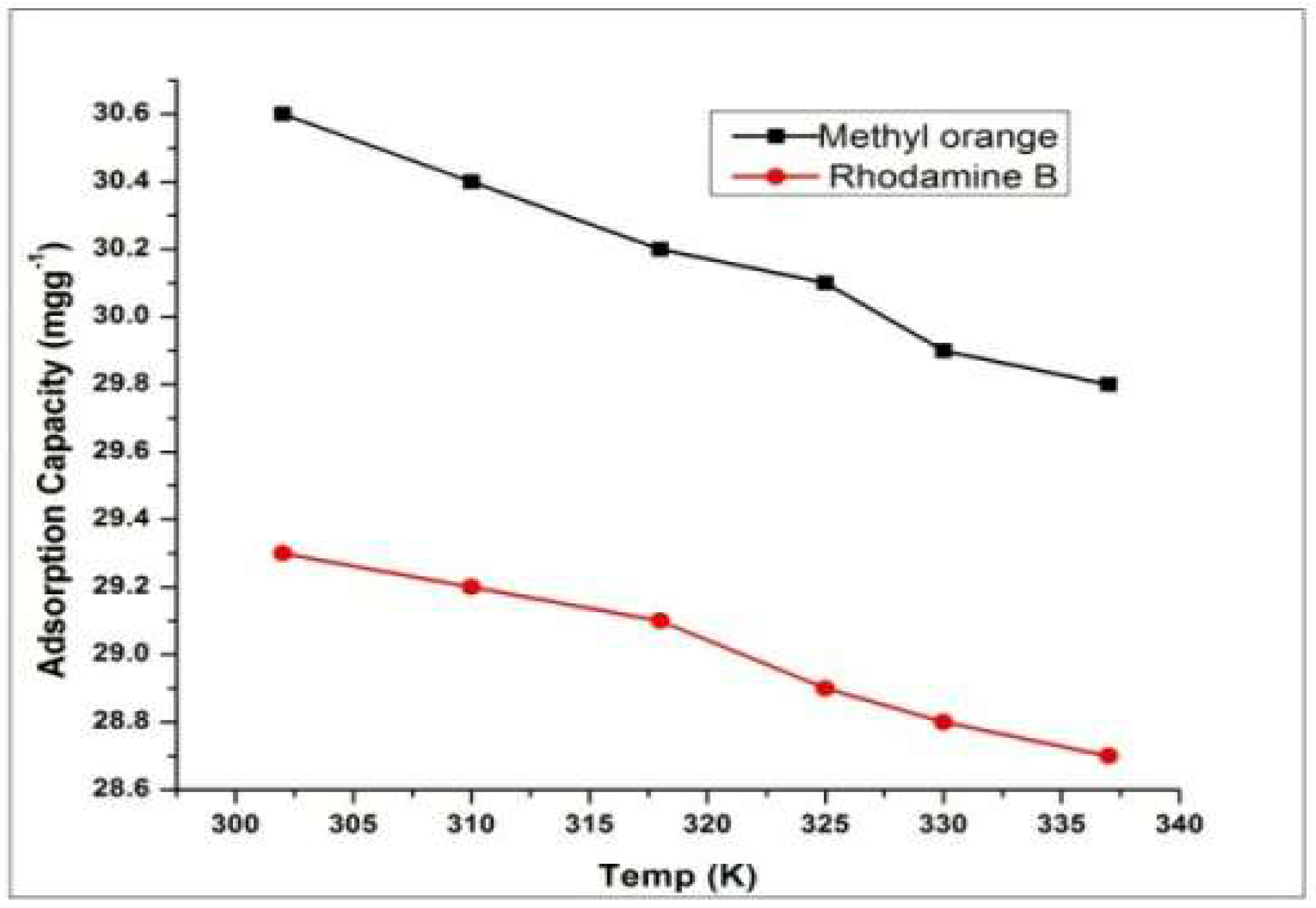
4.2. Effect of Temperature
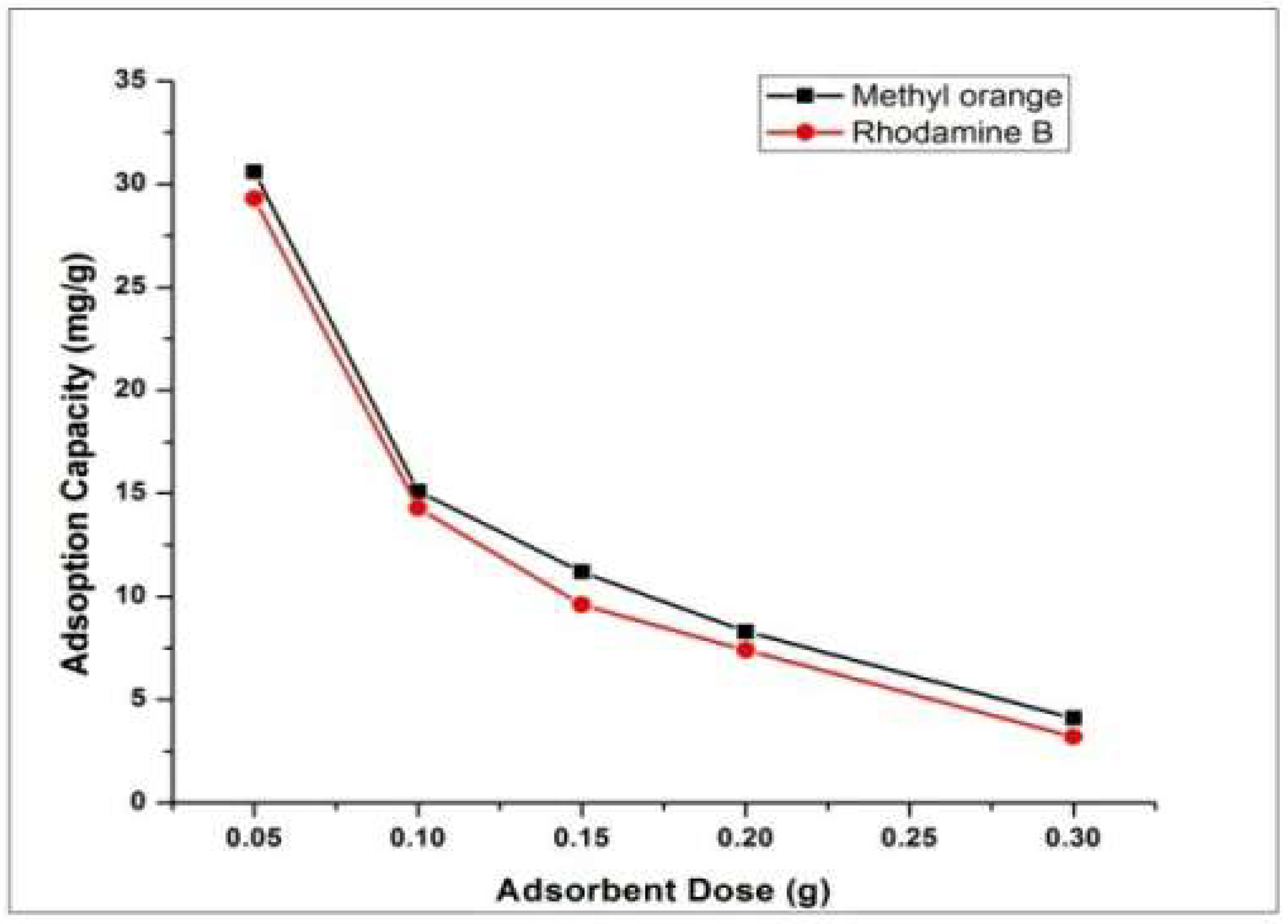
4.3. Effect of Adsorbent Dose
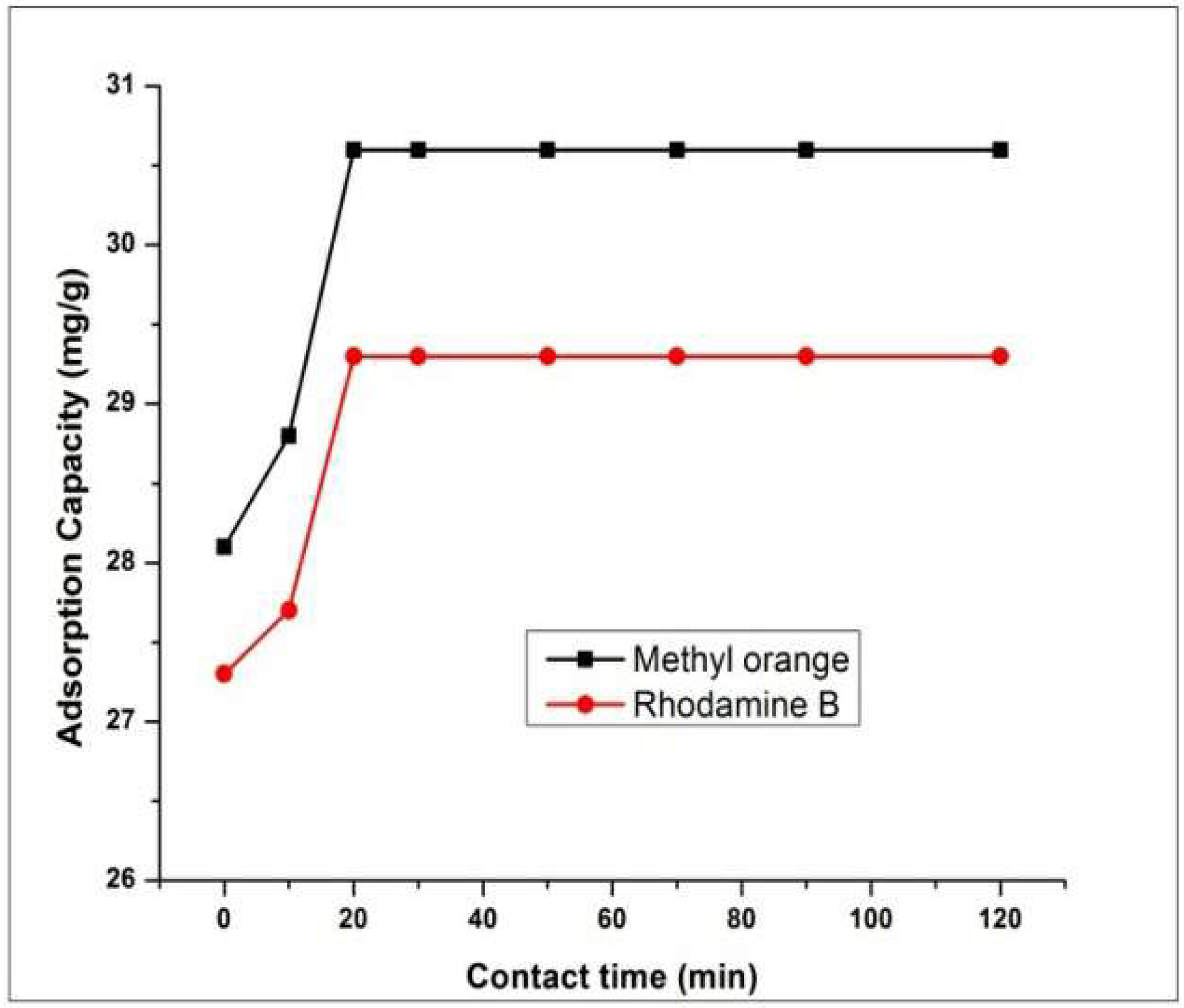
4.4. Effect of Contact Time
4.5. Effect of Initial Dye Concentration
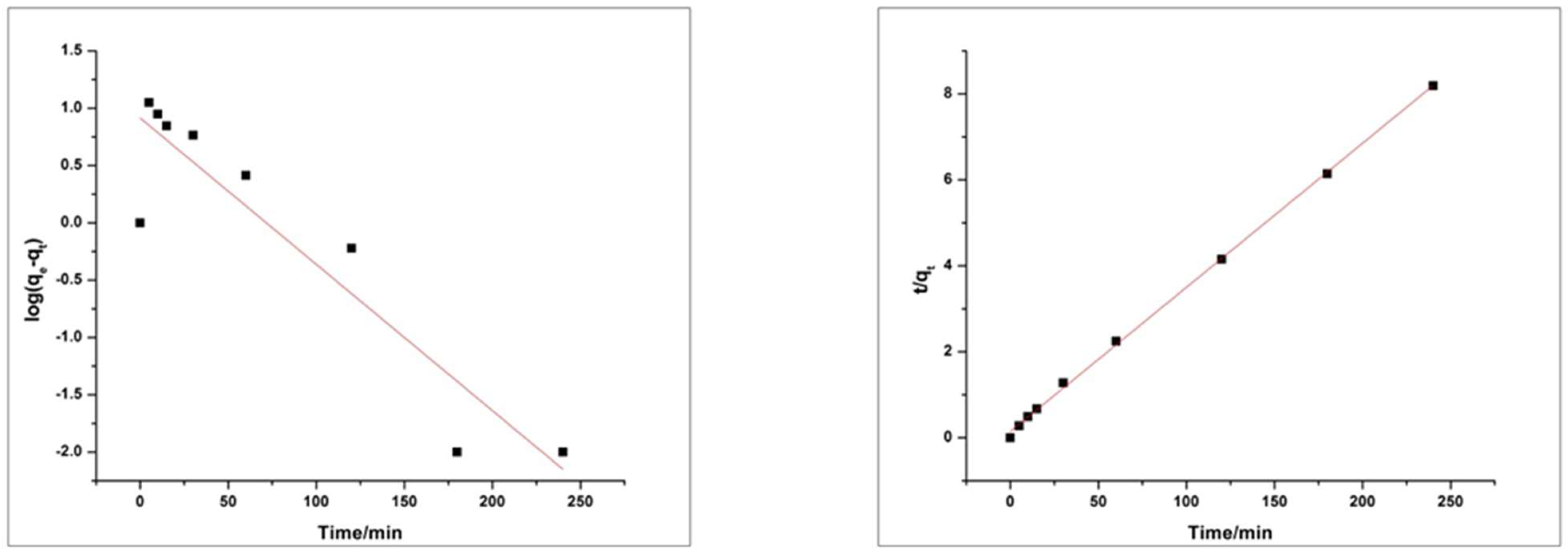
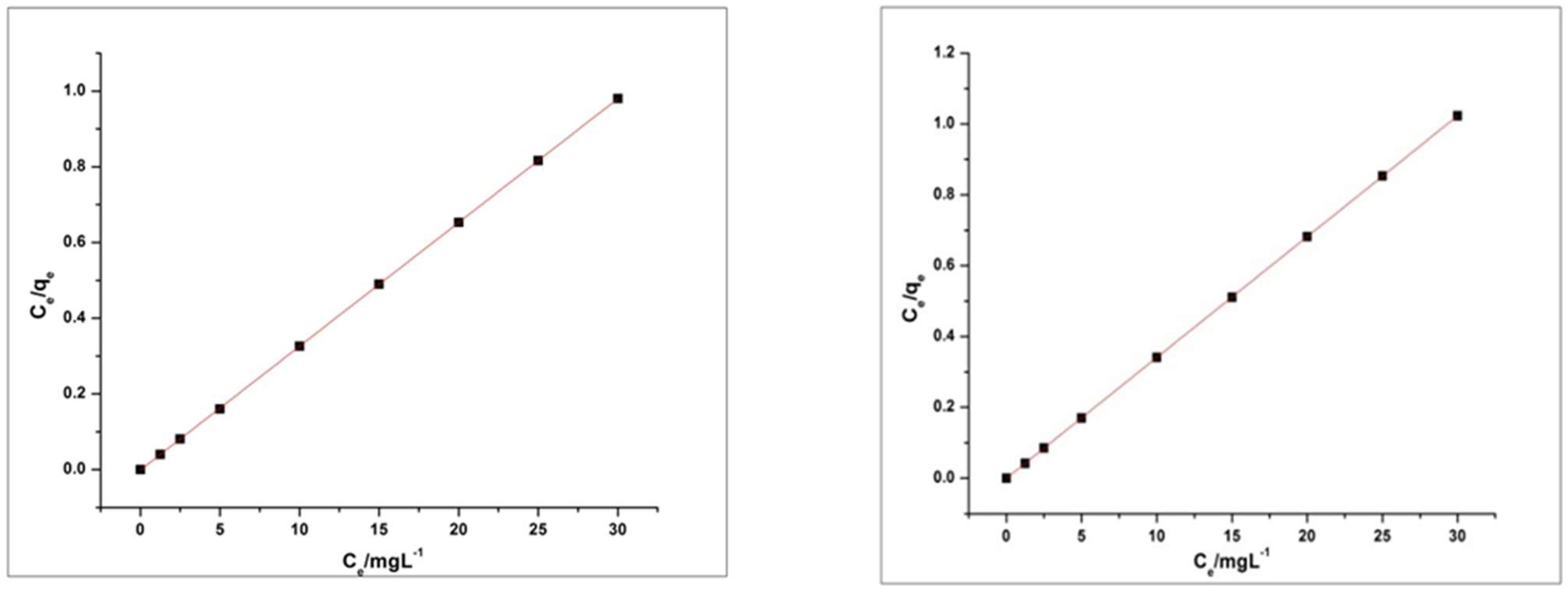
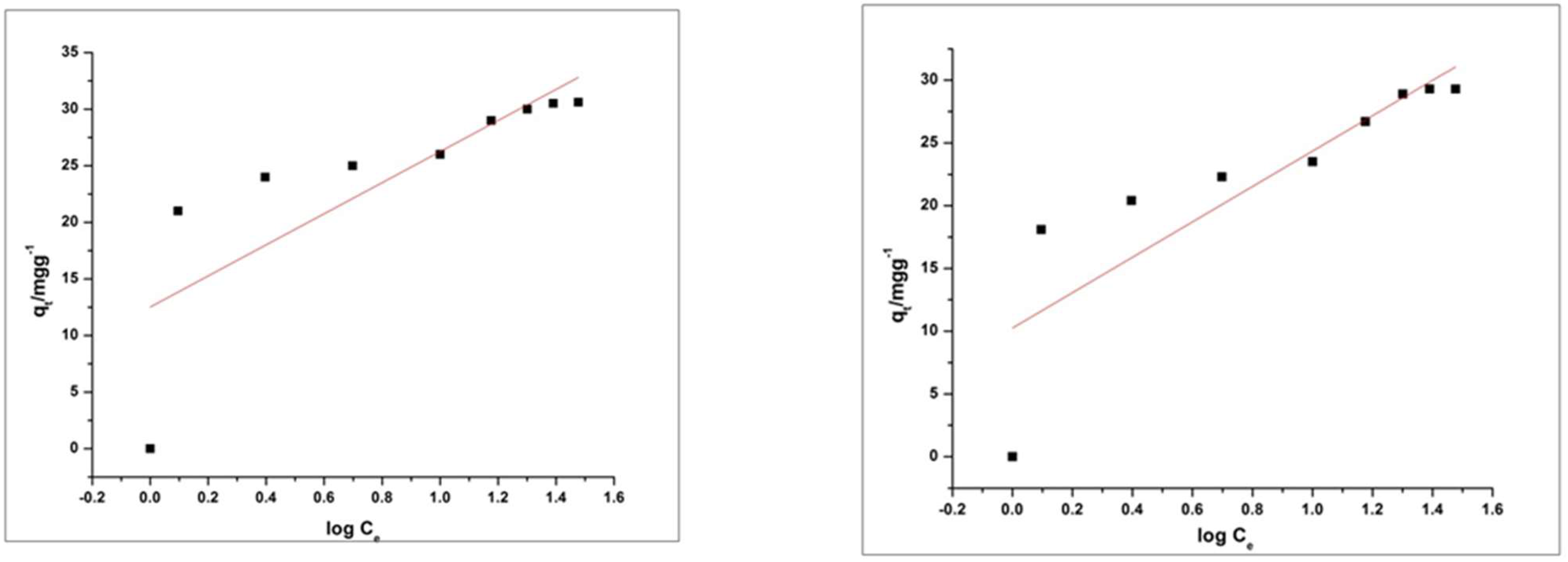
4.6. Adsorption Kinetics and Isotherms
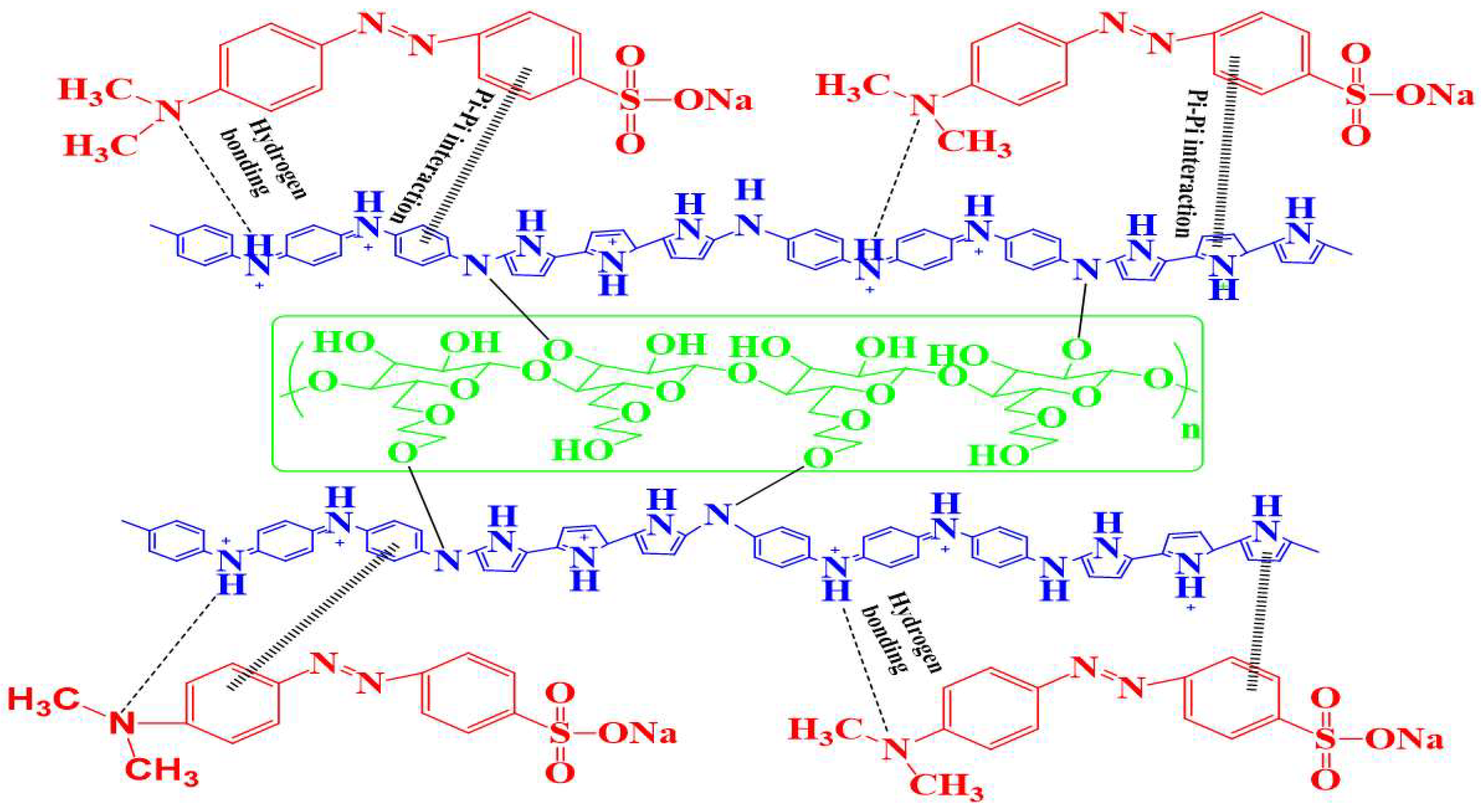
4.7. Adsorption Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Mandal, S.; Pramanick, A.; Chakraborty, S.; Dey, P.P. Phase determination of ZrB2-B4C ceramic composite material using XRD and rietveld refinement analysis. Mater. Today Proc. 2020, 33, 5664–5666. [Google Scholar] [CrossRef]
- Ben Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Abubakar, A.S.; Mahmud, H.N.M.E.; Kutty, S.R.M.; Jagaba, A.H.; Abdullahi SS, a.; Zango, Z.U. Application of Agricultural Wastes for Cationic Dyes Removal from Wastewater Textile Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 239–274. [Google Scholar]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Mirghaffari, N.; Salman, A.D.; Juzsakova, T.; Abdullah, T.A. Removal of organic pollutants from produced water by batch adsorption treatment. Clean Technol. Environ. Policy 2022, 24, 713–720. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Ghoniem, M.; Nguyen-Tri, P.; Baaloudj, O.; Guesmi, A.; AlGethami, F.K.; Amer, M.S.; Assadi, A. Superior removal of dyes by mesoporous MgO/g-C3N4 fabricated through ultrasound method: Adsorption mechanism and process modeling. Environ. Res. 2022, 205, 112543. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process Eng. 2022, 46, 102641. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, S.; Pal, B. Superior adsorptive removal of brilliant green and phenol red dyes mixture by CaO nanoparticles extracted from egg shells. J. Nanostructure Chem. 2022, 12, 207–221. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically modified sugarcane bagasse-based biocomposites for efficient removal of acid red 1 dye: Kinetics, isotherms, thermodynamics, and desorption studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
- Ferkous, H.; Rouibah, K.; Hammoudi, N.-E.; Alam, M.; Djilani, C.; Delimi, A.; Laraba, O.; Yadav, K.K.; Ahn, H.-J.; Jeon, B.-H.; et al. The Removal of a Textile Dye from an Aqueous Solution Using a Biocomposite Adsorbent. Polymers 2022, 14, 2396. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.; Kumar, P.S.; Karishma, S. Review on biopolymers and composites—Evolving material as adsorbents in removal of environmental pollutants. Environ. Res. 2021, 212, 113114. [Google Scholar] [CrossRef]
- Peramune, D.; Manatunga, D.C.; Dassanayake, R.S.; Premalal, V.; Liyanage, R.N.; Gunathilake, C.; Abidi, N. Recent advances in biopolymer-based advanced oxidation processes for dye removal applications: A review. Environ. Res. 2022, 215, 114242. [Google Scholar] [CrossRef]
- Gao, Z.-Z.; Qi, N.; Chen, W.-J.; Zhao, H. Construction of hydroxyethyl cellulose/silica/graphitic carbon nitride solid foam for adsorption and photocatalytic degradation of dyes. Arab. J. Chem. 2022, 15, 104105. [Google Scholar] [CrossRef]
- Gelaw, T.B.; Sarojini, B.K.; Kodoth, A.K. Chitosan/Hydroxyethyl Cellulose Gel Immobilized Polyaniline/CuO/ZnO Adsorptive-Photocatalytic Hybrid Nanocomposite for Congo Red Removal. J. Polym. Environ. 2022, 30, 4086–4101. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, Y.-Q.; Zhu, H.-Y.; Yao, J.; Xiao, L. Removal of methyl orange from aqueous solutions by magnetic maghemite/chitosan nanocomposite films: Adsorption kinetics and equilibrium. J. Appl. Polym. Sci. 2012, 125, E540–E549. [Google Scholar] [CrossRef]
- Wegermann, C.A.; da Rocha, J.C.; Drechsel, S.M.; Nunes, F.S. Semi-empirical ZINDO/S description of the electronic structure and the spectral features of methyl orange and its products of oxidation. A study of relationship between molecular geometry and spectroscopic properties. Dye. Pigment. 2013, 99, 839–849. [Google Scholar] [CrossRef]
- Soylu, M. The effect of thickness of organic layer on electronic properties of Al/Rhodamine B/p-Si structure. Mater. Sci. Semicond. Process. 2011, 14, 212–218. [Google Scholar] [CrossRef]
- Youssef, A.; Hasanin, M.; El-Aziz, M.A.; Turky, G. Conducting chitosan/hydroxylethyl cellulose/polyaniline bionanocomposites hydrogel based on graphene oxide doped with Ag-NPs. Int. J. Biol. Macromol. 2020, 167, 1435–1444. [Google Scholar] [CrossRef]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr. Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Barkany, S.; El Idrissi, A.; Zannagui, C.; Fatima Ezahra, T.; Maaroufi, A.; Abou-Salama, M.; Hassan, A. Homogeneous Grafting of New Amido Groups onto Hydroxyethyl Cellulose Acetate Microfibrils: Solubility Study. Mor. J. Chem. 2016, 4, 743–758. [Google Scholar]
- El Idrissi, A.; El Barkany, S.; Amhamdi, H.; Maaroufi, A.-K. Physicochemical characterization of celluloses extracted from Esparto “Stipa tenacissima” of Eastern Morocco. J. Appl. Polym. Sci. 2013, 128, 537–548. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rathika, R.; Byung-Taek, O.; Vishnukumar, B.; Shanthi, K.; Kamala-Kannan, S.; Janaki, V. Synthesis, characterization and application of polypyrrole-cellulose nanocomposite for efficient Ni(II) removal from aqueous solution: Box-Behnken design optimization. e-Polymers 2018, 18, 287–295. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, X.; Dong, L.; Yuan, Z.; Wang, W.; Wu, S.; Chen, S.; Zheng, G.; Zhang, W.; Zhang, D.; et al. Effective nondestructive evaluations on UHMWPE/Recycled-PA6 blends using FTIR imaging and dynamic mechanical analysis. Polym. Test. 2017, 59, 371–376. [Google Scholar] [CrossRef]
- Alam, M.; Ansari, A.A.; Shaik, M.R.; Alandis, N.M. Optical and electrical conducting properties of Polyaniline/Tin oxide nanocomposite. Arab. J. Chem. 2013, 6, 341–345. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Huang, D.; Wang, A. Kapok fiber oriented-polyaniline nanofibers for efficient Cr(VI) removal. Chem. Eng. J. 2012, 191, 154–161. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ma, G.; Wang, Z.; Liu, K.; Liu, H. Ethylene glycol reduced graphene oxide/polypyrrole composite for supercapacitor. Electrochim. Acta 2013, 88, 519–525. [Google Scholar] [CrossRef]
- Mehra, S.; Singh, M.; Chadha, P. Adverse impact of textile dyes on the aquatic environment as well as on human beings. Toxicol. Int. 2021, 28, 165–176. [Google Scholar]
- Abdi, M.M.; Tahir, P.; Liyana, R.; Javahershenas, R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules 2018, 23, 2470. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, F.; Chen, J.; Li, F.; Weng, W. Metal-organic framework MIL-125(Ti) for efficient adsorptive removal of Rhodamine B from aqueous solution. Appl. Organomet. Chem. 2015, 29, 12–19. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, K.; Luo, X. Small sample image recognition using improved Convolutional Neural Network. J. Vis. Commun. Image Represent. 2018, 55, 640–647. [Google Scholar] [CrossRef]
- Xu, T.; Hou, X.; Liu, S.; Liu, B. One-step synthesis of magnetic and porous Ni@MOF-74(Ni) composite. Microporous Mesoporous Mater. 2018, 259, 178–183. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, L.; Long, Y.; Shi, X. Trichloroacetic acid-modulated synthesis of polyoxometalate@UiO-66 for selective adsorption of cationic dyes. J. Colloid Interface Sci. 2018, 516, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.N.; Zia, K.M.; Zhu, L.; Haroonur, R.; Ahmad, M.N.; Zuber, M.; Tang, H. Adsorption of methyl orange using self-assembled porous microspheres of poly(o-chloroaniline). Korean J. Chem. Eng. 2014, 31, 2192–2197. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Zayed, A.M.; Wahed, M.S.A.; Mohamed, E.A.; Sillanpää, M. Insights on the role of organic matters of some Egyptian clays in methyl orange adsorption: Isotherm and kinetic studies. Appl. Clay Sci. 2018, 166, 49–60. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, J.; Dong, L.; Chai, J.; Zhao, N.; Ullah, S.; Wang, H.; Zhang, D.; Imtiaz, S.; Shan, G.; et al. Self-assembly of 2D-metal–organic framework/graphene oxide membranes as highly efficient adsorbents for the removal of Cs+ from aqueous solutions. RSC Adv. 2018, 8, 40813–40822. [Google Scholar] [CrossRef]
- Hajlaoui, O.; Khiari, R.; Ajili, L.; Batis, N.; Bergaoui, L. Design and Characterization of Type I Cellulose-Polyaniline Composites from Various Cellulose Sources: A Comparative Study. Chem. Afr. 2020, 3, 783–792. [Google Scholar] [CrossRef]
- Duan, Y.-T.; Sangani, C.B.; Ameta, R.K. Thermal, SEM, AFM, BET and biological analysis of newly synthesized Fe2+/Fe3+ based MOIFs. J. Mol. Liq. 2019, 295, 111709. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, K.; Li, X.; Huang, L.; Liang, J.; Zheng, G.; Shan, G. Nickel-metal-organic framework nanobelt based composite membranes for efficient Sr2+ removal from aqueous solution. Environ. Sci. Ecotechnology 2020, 3, 100035. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z.; Coomes, A.; Haghseresht, F.; Lu, G. The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. J. Colloid Interface Sci. 2005, 284, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ali, I. Removal of lead and chromium from wastewater using bagasse fly ash—A sugar industry waste. J. Colloid Interface Sci. 2004, 271, 321–328. [Google Scholar] [CrossRef]
- Khattri, S.; Singh, M. Use of Sagaun sawdust as an adsorbent for the removal of crystal violet dye from simulated wastewater. Environ. Prog. Sustain. Energy 2012, 31, 435–442. [Google Scholar] [CrossRef]
- Yusra, S.; Haq, N.B.; Safa, Y.; Bhatti, H.N. Adsorptive removal of direct dyes by low cost rice husk: Effect of treatments and modifications. Afr. J. Biotechnol. 2011, 10, 3128–3142. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2015, 119, 132–140. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, M.; Liu, Y.; Zhang, L.; Han, R. Study of congo red adsorption onto chitosan coated magnetic iron oxide in batch mode. Desalination Water Treat. 2012, 37, 46–54. [Google Scholar] [CrossRef]










| Adsorbent | Dye | Adsorption Capacities (mg/g) | References |
|---|---|---|---|
| MIL-125 (Ti) | RhB | 59.92 | [33] |
| CZIF-867 | RhB | 119.9 | [34] |
| Ni@MOF-74(Ni) | RhB | 209.2 | [35] |
| POM@UiO-66 | RhB | 225.7 | [36] |
| POC porous microspheres | MO | 35.21 | [37] |
| Banana peel | MO | 21.0 | [38] |
| COMRC | MO | 34.48 | [39] |
| Nano-composite film Maghemite/chitosan | MO | 29.41 | [18] |
| HEC/PANI-PPy | MO | 30.6 | This work |
| HEC/PANI-PPy | RhB | 29.3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajaber, M.A.; Anjum, M.N.; Ibrahim, M.; Farooq, T.; Ahmad, M.N.; Abideen, Z.u. Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes. Molecules 2022, 27, 8238. https://doi.org/10.3390/molecules27238238
Bajaber MA, Anjum MN, Ibrahim M, Farooq T, Ahmad MN, Abideen Zu. Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes. Molecules. 2022; 27(23):8238. https://doi.org/10.3390/molecules27238238
Chicago/Turabian StyleBajaber, Majed A., Muhammad Naveed Anjum, Muhammad Ibrahim, Tahir Farooq, Mirza Nadeem Ahmad, and Zain ul Abideen. 2022. "Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes" Molecules 27, no. 23: 8238. https://doi.org/10.3390/molecules27238238
APA StyleBajaber, M. A., Anjum, M. N., Ibrahim, M., Farooq, T., Ahmad, M. N., & Abideen, Z. u. (2022). Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes. Molecules, 27(23), 8238. https://doi.org/10.3390/molecules27238238








