Long-Range Proton Transfer in 7-Hydroxy-Quinoline-Based Azomethine Dyes: A Hidden Reason for the Low Efficiency
Abstract
1. Introduction
2. Results and Discussion
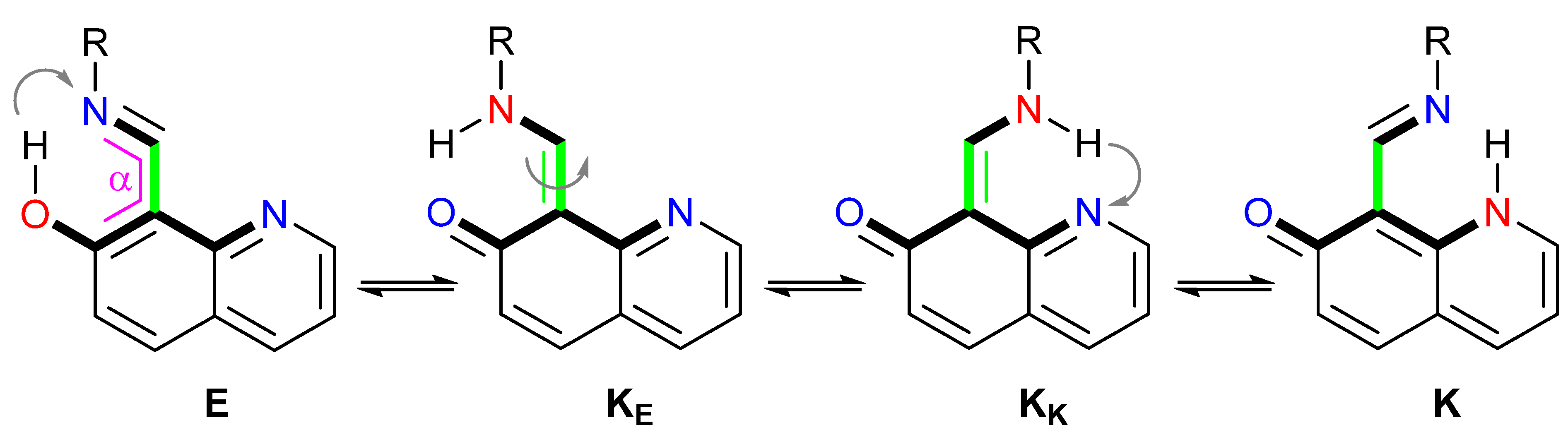
2.1. Ground State Tautomerization and Isomerization
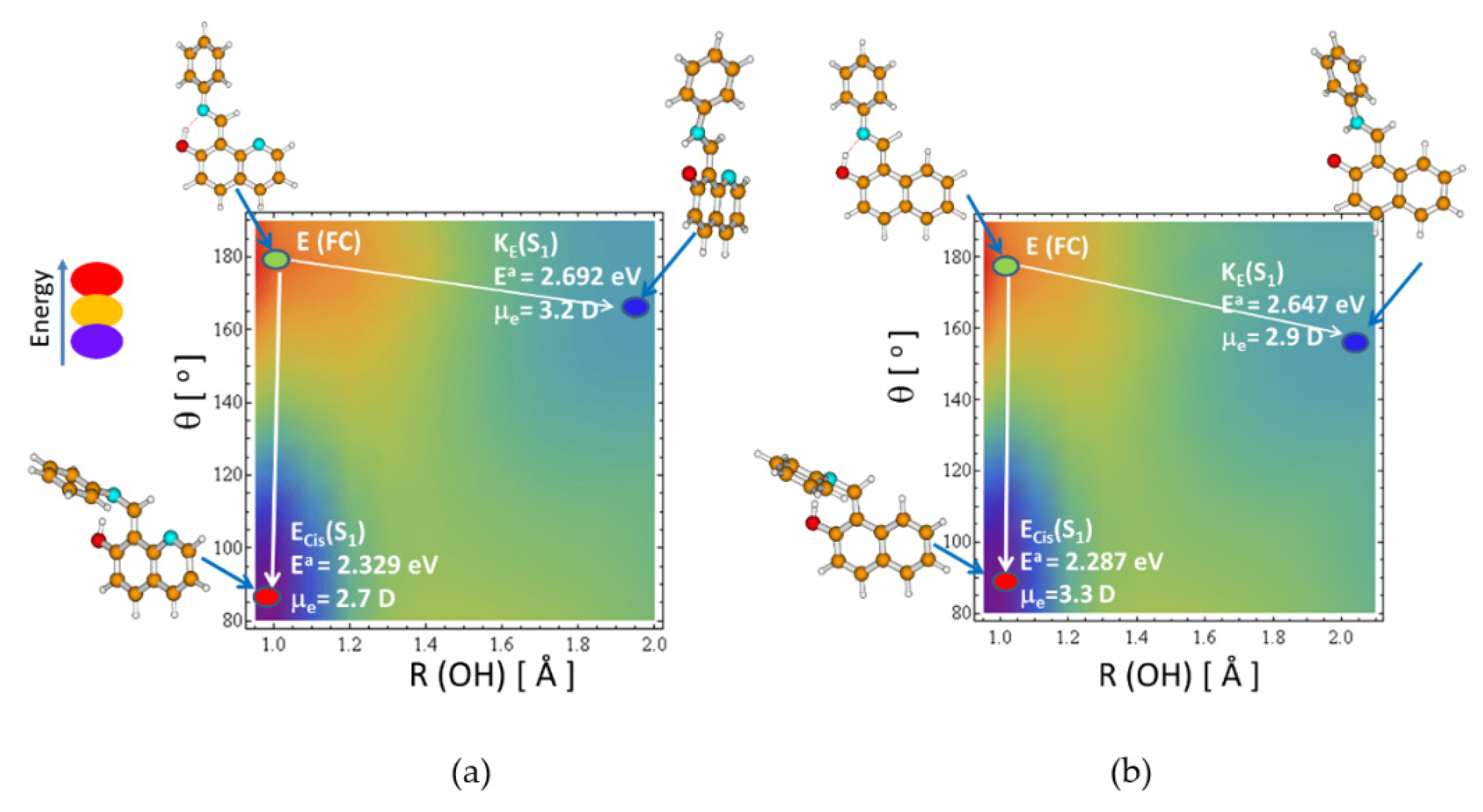
2.2. ESIPT vs. Trans–cis Isomerization in S1
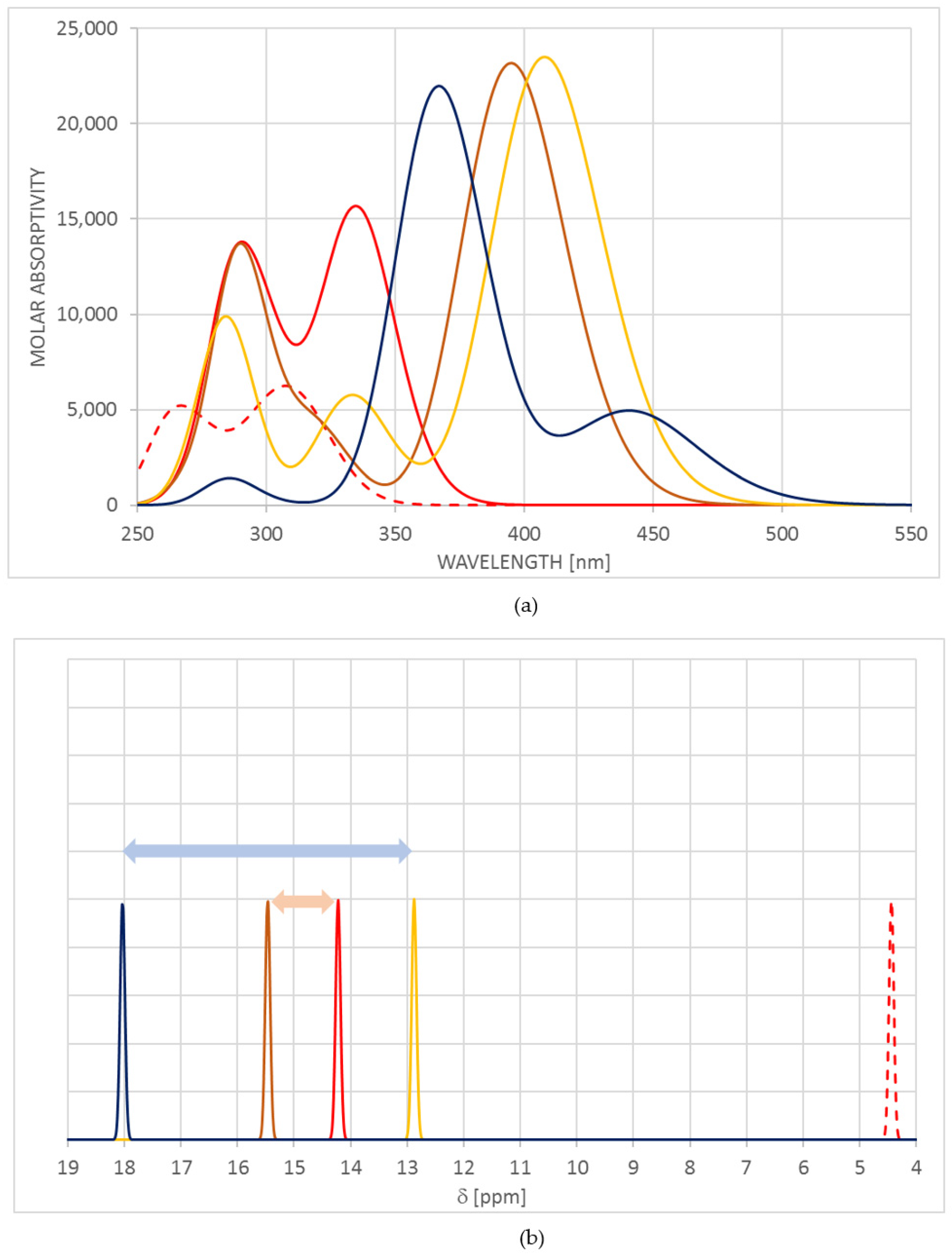
2.3. Long-Range PT and Trans–cis Isomerization in Terms of Spectral Changes to Be Experimentally Detected
3. Theoretical Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jalink, C.J.; van Ingen, W.M.; Huizer, A.H.; Varma, C.A.G.O. Prospects for Using Photoinduced Intramolecular Proton Transfer to Study the Dynamics of Conformational Changes in Flexible Molecular Chains. J. Chem. Soc. Faraday Trans. 1991, 87, 1103. [Google Scholar] [CrossRef]
- Jalink, C.J.; Huizer, A.H.; Varma, C.A.G.O. Rate-Limiting Action of a Proton Crane in Long-Range Intramolecular Proton Transfer. J. Chem. Soc. Faraday Trans. 1992, 88, 2655. [Google Scholar] [CrossRef]
- de Bekker, E.J.A.; Geerlings, J.D.; Varma, C.A.G.O. Mechanism of a Photoinduced Solvent-Assisted Transfer of a Proton to a Specified Remote Target. J. Phys. Chem. A 2000, 104, 5916–5927. [Google Scholar] [CrossRef]
- de Bekker, E.J.A.; Pugzlys, A.; Varma, C.A.G.O. Elementary Processes in Photoinduced Proton Transfers in 2-Hydroxy-1-(N-Morpholinomethyl)Naphthalene and 7-Hydroxy-8-(N-Morpholinomethyl)Quinoline in Liquid Solutions. J. Phys. Chem. A 2001, 105, 399–409. [Google Scholar] [CrossRef]
- Sobolewski, A.L. Reversible Molecular Switch Driven by Excited-State Hydrogen Transfer. Phys. Chem. Chem. Phys. 2008, 10, 1243. [Google Scholar] [CrossRef]
- van der Loop, T.H.; Ruesink, F.; Amirjalayer, S.; Sanders, H.J.; Buma, W.J.; Woutersen, S. Unraveling the Mechanism of a Reversible Photoactivated Molecular Proton Crane. J. Phys. Chem. B 2014, 118, 12965–12971. [Google Scholar] [CrossRef]
- Ewing, G.W.; Steck, E.A. Absorption Spectra of Heterocyclic Compounds. I. Quinolinols and Isoquinolinols 1. J. Am. Chem. Soc. 1946, 68, 2181–2187. [Google Scholar] [CrossRef]
- Mason, S.F. The Tautomerism of N-Heteroaromatic Hydroxy-Compounds. Part I. Infrared Spectra. J. Chem. Soc. Resumed 1957, 980, 4874–4880. [Google Scholar] [CrossRef]
- Mason, S.F. The Tautomerism of N-Heteroaromatic Hydroxy-Compounds. Part II. Ultraviolet Spectra. J. Chem. Soc. Resumed 1957, 1002, 5010–5017. [Google Scholar] [CrossRef]
- Mason, S.F.; Philp, J.; Smith, B.E. Prototropic Equilibria of Electronically Excited Molecules. Part II. 3-, 6-, and 7-Hydroxyquinoline. J. Chem. Soc. Inorg. Phys. Theor. 1968, 3051–3056. [Google Scholar] [CrossRef]
- Lee, S.-I.; Jang, D.-J. Proton Transfers of Aqueous 7-Hydroxyquinoline in the First Excited Singlet, Lowest Triplet, and Ground States. J. Phys. Chem. 1995, 99, 7537–7541. [Google Scholar] [CrossRef]
- García-Ochoa, I.; Bisht, P.B.; Sánchez, F.; Martinez-Atáz, E.; Santos, L.; Tripathi, H.B.; Douhal, A. Experimental and Theoretical Studies of the Proton-Hopping Reaction of 7-Hydroxyquinoline in Viscous Hydroxylic Media. J. Phys. Chem. A 1998, 102, 8871–8880. [Google Scholar] [CrossRef]
- Chou, P.-T.; Wei, C.-Y.; Chris Wang, C.-R.; Hung, F.-T.; Chang, C.-P. Proton-Transfer Tautomerism of 7-Hydroxyquinolines Mediated by Hydrogen-Bonded Complexes. J. Phys. Chem. A 1999, 103, 1939–1949. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Lee, Y.-S.; Yoo, B.K.; Jang, D.-J. Excited-State Triple Proton Transfer of 7-Hydroxyquinoline along a Hydrogen-Bonded Alcohol Chain: Vibrationally Assisted Proton Tunneling. Angew. Chem. Int. Ed. 2006, 45, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lee, Y.-S.; Kwon, O.-H.; Jang, D.-J. Proton Transport of Water in Acid–Base Reactions of 7-Hydroxyquinoline. Chem. Commun. 2009, 8, 926. [Google Scholar] [CrossRef]
- Park, S.-Y.; Jang, D.-J. Excited-State Hydrogen Relay along a Blended-Alcohol Chain as a Model System of a Proton Wire: Deuterium Effect on the Reaction Dynamics. Phys. Chem. Chem. Phys. 2012, 14, 8885. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Lang, B.; Rosspeintner, A.; Vauthey, E. Ultrafast Elementary Photochemical Processes of Organic Molecules in Liquid Solution. Chem. Rev. 2017, 117, 10826–10939. [Google Scholar] [CrossRef]
- Vetokhina, V.; Nowacki, J.; Pietrzak, M.; Rode, M.F.; Sobolewski, A.L.; Waluk, J.; Herbich, J. 7-Hydroxyquinoline-8-Carbaldehydes. 1. Ground- and Excited-State Long-Range Prototropic Tautomerization. J. Phys. Chem. A 2013, 117, 9127–9146. [Google Scholar] [CrossRef]
- Rode, M.F.; Sobolewski, A.L. Effect of Chemical Substituents on the Energetical Landscape of a Molecular Photoswitch: An Ab Initio Study. J. Phys. Chem. A 2010, 114, 11879–11889. [Google Scholar] [CrossRef]
- Csehi, A.; Illés, L.; Halász, G.J.; Vibók, Á. The Effect of Chemical Substituents on the Functionality of a Molecular Switch System: A Theoretical Study of Several Quinoline Compounds. Phys. Chem. Chem. Phys. 2013, 15, 18048. [Google Scholar] [CrossRef]
- Csehi, A.; Halász, G.J.; Vibók, Á. Molecular Switch Properties of 7-Hydroxyquinoline Compounds. Int. J. Quantum Chem. 2014, 114, 1135–1145. [Google Scholar] [CrossRef]
- Lapinski, L.; Nowak, M.J.; Nowacki, J.; Rode, M.F.; Sobolewski, A.L. A Bistable Molecular Switch Driven by Photoinduced Hydrogen-Atom Transfer. ChemPhysChem 2009, 10, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Petek, A.; Hori, Y.; Georgiev, A.; Hirashima, S.; Matsushima, Y.; Yordanov, D.; Miura, T.; Antonov, L. Acylhydrazone Subunits as a Proton Cargo Delivery System in 7-Hydroxyquinoline. Chem.-Eur. J. 2021, 27, 11559–11566. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, A.; Yordanov, D.; Ivanova, N.; Deneva, V.; Vassilev, N.; Kamounah, F.S.; Pittelkow, M.; Crochet, A.; Fromm, K.M.; Antonov, L. 7-OH Quinoline Schiff Bases: Are They the Long Awaited Tautomeric Bistable Switches? Dyes Pigment. 2021, 195, 109739. [Google Scholar] [CrossRef]
- Nakashima, K.; Georgiev, A.; Yordanov, D.; Matsushima, Y.; Hirashima, S.; Miura, T.; Antonov, L. Solvent-Triggered Long-Range Proton Transport in 7-Hydroxyquinoline Using a Sulfonamide Transporter Group. J. Org. Chem. 2022, 87, 6794–6806. [Google Scholar] [CrossRef]
- Jankowska, J.; Rode, M.F.; Sadlej, J.; Sobolewski, A.L. Excited-State Intramolecular Proton Transfer: Photoswitching in Salicylidene Methylamine Derivatives. ChemPhysChem 2014, 15, 1643–1652. [Google Scholar] [CrossRef]
- Spörkel, L.; Jankowska, J.; Thiel, W. Photoswitching of Salicylidene Methylamine: A Theoretical Photodynamics Study. J. Phys. Chem. B 2015, 119, 2702–2710. [Google Scholar] [CrossRef]
- Antonov, L. Tautomerism in Azo and Azomethyne Dyes: When and If Theory Meets Experiment. Molecules 2019, 24, 2252. [Google Scholar] [CrossRef]
- Lochbrunner, S.; Schriever, C.; Riedle, E. Direct Observation of Nuclear Motion during Ultrafast Intramolecular Proton Transfer. In Hydrogen-Transfer Reactions; Hynes, J.T., Klinman, J.P., Limbach, H.-H., Schowen, R.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 349–375. ISBN 978-3-527-30777-7. [Google Scholar]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent DensityFunctional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Kleinpeter, E. NMR Spectroscopic Study of Tautomerism in Solution and in the Solid State. In Tautomerism: Methods and Theories; Antonov, L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 103–143. ISBN 978-3-527-65882-4. [Google Scholar]
- Nedeltcheva, D.; Antonov, L.; Lycka, A.; Damyanova, B.; Popov, S. Chemometric Models for Quantitative Analysis of Tautomeric Schiff Bases and Azo Dyes. Curr. Org. Chem. 2009, 13, 217–240. [Google Scholar] [CrossRef]
- Joshi, H.; Kamounah, F.S.; Gooijer, C.; van der Zwan, G.; Antonov, L. Excited State Intramolecular Proton Transfer in Some Tautomeric Azo Dyes and Schiff Bases Containing an Intramolecular Hydrogen Bond. J. Photochem. Photobiol. Chem. 2002, 152, 183–191. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Christiansen, O.; Koch, H.; Jørgensen, P. The second-order approximate coupled cluster singles and doubles model CC2. Chem. Phys. Lett. 1995, 243, 409–418. [Google Scholar] [CrossRef]
- Hättig, C.; Weigend, F. CC2 excitation energy calculations on large molecules using the resolution of the identity approximation. J. Chem. Phys. 2000, 113, 5154–5161. [Google Scholar] [CrossRef]
- Schirmer, J. Beyond the Random-Phase Approximation: A New Approximation Scheme for the Polarization Propagator. Phys. Rev. A At. Mol. Opt. Phys. 1982, 26, 2395–2416. [Google Scholar] [CrossRef]
- Trofimov, A.B.; Schirmer, J. An Efficient Polarization Propagator Approach to Valence Electron Excitation Spectra. J. Phys. B At. Mol. Opt. Phys. 1995, 28, 2299–2324. [Google Scholar] [CrossRef]
- Trofimov, A.B.; Stelter, G.; Schirmer, J. A Consistent Third-Order Propagator Method for Electronic Excitation. J. Chem. Phys. 1999, 111, 9982–9999. [Google Scholar] [CrossRef]
- Hättig, C. Structure Optimizations for Excited States with Correlated Second-Order Methods: CC2 and ADC(2). Adv. Quantum Chem. 2005, 50, 37–60. [Google Scholar]
- Dreuw, A.; Wormit, M. The Algebraic Diagrammatic Construction Scheme for the Polarization Propagator for the Calculation of Excited States. WIREs Comput. Mol. Sci. 2015, 5, 82–95. [Google Scholar] [CrossRef]
- Jacquemin, D.; Duchemin, I.; Blase, X. 0–0 Energies Using Hybrid Schemes: Benchmarks of TD-DFT, CIS(D), ADC(2), CC2, and BSE/GW Formalisms for 80 Real-Life Compounds. J. Chem. Theory Comput. 2015, 11, 5340–5359. [Google Scholar] [CrossRef] [PubMed]
- Loos, P.-F.; Lipparini, F.; Boggio-Pasqua, M.; Scemama, A.; Jacquemin, D. A Mountaineering Strategy to Excited States: Highly Accurate Energies and Benchmarks for Medium Sized Molecules. J. Chem. Theory Comput. 2020, 16, 1711–1741. [Google Scholar] [CrossRef] [PubMed]
- Turbomole V7.1 2016. A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, Turbomole GmbH, Since 2007. Available online: https://Www.Turbomole.Org (accessed on 19 November 2022).
- Antonov, L. Drawbacks of the Present Standards for Processing Absorption Spectra Recorded Linearly as a Function of Wavelength. TrAC Trends Anal. Chem. 1997, 16, 536–543. [Google Scholar] [CrossRef]
- Antonov, L.; Stoyanov, S. Analysis of the Overlapping Bands in UV-Vis Absorption Spectroscopy. Appl. Spectrosc. 1993, 47, 1030–1035. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]



| Tautomer | ΔEVE | f | μe | |
|---|---|---|---|---|
1E | S0 | 0.00 | μg = 3.2 | |
| S0→S1(ππ*) | 3.70 | 0.344 | 0.7 | |
| S0→S2(ππ*) | 4.11 | 0.129 | 2.7 | |
| S0→S3(ππ*) | 4.32 | 0.235 | 2.1 | |
| S0→S4(nπ*) | 4.40 | 0.004 | 1.2 | |
| S0→S5(ππ*) | 4.58 | 0.020 | 1.8 | |
| S0→S6(nπ*) | 4.75 | 0.000 | 1.9 | |
1KE | S0 | 0.203 a | μg = 2.5 | |
| S0→S1(ππ*) | 3.14 | 0.515 | 2.4 | |
| S0→S2(nπ*) | 3.40 | 0.000 | 1.7 | |
| S0→S3(ππ*) | 3.89 | 0.090 | 5.3 | |
| S0→S4(ππ*) | 4.28 | 0.298 | 4.7 | |
| S0→S5(nπ*) | 4.47 | 0.000 | 7.6 | |
| S0→S6(ππ*) | 4.61 | 0.022 | 2.9 | |
1KK | S0 | 0.274 a | μg = 4.7 | |
| S0→S1(ππ*) | 3.04 | 0.521 | 5.6 | |
| S0→S2(nπ*) | 3.15 | 0.001 | 0.9 | |
| S0→S3(ππ*) | 3.72 | 0.128 | 5.1 | |
| S0→S4(ππ*) | 4.35 | 0.207 | 6.1 | |
| S0→S5(ππ*) | 4.56 | 0.029 | 5.3 | |
| S0→S6(nπ*) | 4.57 | 0.000 | 8.2 | |
1K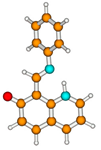 | S0 | 0.535 a | μg = 7.3 | |
| S0→S1(ππ*) | 2.81 | 0.109 | 5.4 | |
| S0→S2(nπ*) | 3.09 | 0.001 | 1.9 | |
| S0→S3(ππ*) | 3.38 | 0.488 | 6.1 | |
| S0→S4(nπ*) | 3.84 | 0.001 | 3.8 | |
| S0→S5(ππ*) | 4.33 | 0.028 | 7.0 | |
| S0→S6(nπ*) | 4.43 | 0.004 | 5.6 | |
1Ecis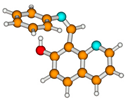 | S0 | 0.531 a | μg = 3.9 | |
| S0→S1(ππ*) | 3.93 | 0.086 | 2.8 | |
| S0→S2(ππ*) | 4.13 | 0.081 | 3.4 | |
| S0→S3(ππ*) | 4.42 | 0.038 | 2.2 | |
| S0→S4(nπ*) | 4.55 | 0.024 | 3.2 | |
| S0→S5(ππ*) | 4.66 | 0.052 | 4.1 | |
| S0→S6(nπ*) | 4.80 | 0.048 | 4.0 | |
| Tautomers | ΔEVE | f | μe | |
|---|---|---|---|---|
3E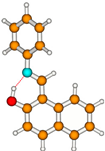 | S0 | 0.00 | μg = 2.4 | |
| S0→S1(ππ*) | 3.56 | 0.373 | 3.0 | |
| S0→S2(ππ*) | 4.13 | 0.214 | 2.8 | |
| S0→S3(nπ*) | 4.36 | 0.003 | 3.0 | |
| S0→S4(nπ*) | 4.46 | 0.001 | 0.9 | |
| S0→S5(nπ*) | 4.74 | 0.001 | 2.6 | |
| S0→S6(ππ*) | 4.95 | 0.098 | 0.3 | |
3KE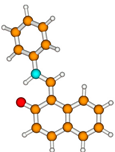 | S0 | 0.240 a | μg = 3.0 | |
| S0→S1(ππ*) | 3.05 | 0.487 | 3.5 | |
| S0→S2(nπ*) | 3.37 | 0.001 | 1.6 | |
| S0→S3(ππ*) | 3.94 | 0.027 | 4.2 | |
| S0→S4(ππ*) | 4.15 | 0.253 | 3.3 | |
| S0→S5(nπ*) | 4.56 | 0.018 | 3.5 | |
| S0→S6(nπ*) | 4.66 | 0.001 | 7.1 | |
3KK | S0 | 0.611 a | μg = 4.6 | |
| S0→S1(ππ*) | 3.07 | 0.269 | 4.6 | |
| S0→S2(nπ*) | 3.21 | 0.185 | 3.4 | |
| S0→S3(ππ*) | 3.98 | 0.005 | 3.7 | |
| S0→S4(ππ*) | 4.29 | 0.319 | 4.9 | |
| S0→S5(ππ*) | 4.53 | 0.017 | 4.6 | |
| S0→S6(nπ*) | 4.58 | 0.025 | 4.4 | |
3Ecis | S0 | 0.450 a | μg = 2.0 | |
| S0→S1(ππ*) | 3.82 | 0.107 | 2.8 | |
| S0→S2(ππ*) | 4.16 | 0.092 | 3.4 | |
| S0→S3(ππ*) | 4.39 | 0.033 | 4.2 | |
| S0→S4(nπ*) | 4.62 | 0.021 | 2.6 | |
| S0→S5(ππ*) | 4.74 | 0.001 | 1.5 | |
| S0→S6(nπ*) | 5.14 | 0.175 | 5.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rode, M.F.; Nedeltcheva-Antonova, D.; Antonov, L. Long-Range Proton Transfer in 7-Hydroxy-Quinoline-Based Azomethine Dyes: A Hidden Reason for the Low Efficiency. Molecules 2022, 27, 8225. https://doi.org/10.3390/molecules27238225
Rode MF, Nedeltcheva-Antonova D, Antonov L. Long-Range Proton Transfer in 7-Hydroxy-Quinoline-Based Azomethine Dyes: A Hidden Reason for the Low Efficiency. Molecules. 2022; 27(23):8225. https://doi.org/10.3390/molecules27238225
Chicago/Turabian StyleRode, Michał F., Daniela Nedeltcheva-Antonova, and Liudmil Antonov. 2022. "Long-Range Proton Transfer in 7-Hydroxy-Quinoline-Based Azomethine Dyes: A Hidden Reason for the Low Efficiency" Molecules 27, no. 23: 8225. https://doi.org/10.3390/molecules27238225
APA StyleRode, M. F., Nedeltcheva-Antonova, D., & Antonov, L. (2022). Long-Range Proton Transfer in 7-Hydroxy-Quinoline-Based Azomethine Dyes: A Hidden Reason for the Low Efficiency. Molecules, 27(23), 8225. https://doi.org/10.3390/molecules27238225







