Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Step
2.2. Optimization of Derivatization Step
2.3. Method Validation
2.4. Real Samples Application
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Standard Solutions
3.3. Apparatus and Chromatographic Conditions
3.4. Sample Preparation
3.5. Method Validation
3.6. Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Karovičová, J.; Kohajdová, Z. Biogenic amines in food. Chem. Pap. 2005, 59, 70–79. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Berthold, A.; Nowosielska, D. Biogenic amines in food. Med. Weter. 2008, 64, 745–748. [Google Scholar]
- Mayer, H.K.; Fiechter, G.; Fischer, E. A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. J. Chromatogr. B 2010, 1217, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J.; Little, C.L.; Grant, K.A.; Mithani, V. Scombrotoxic fish poisoning. J. Public Health 2005, 28, 61–62. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Bodmer, S.; Imark, C.; Kneubühl, M. Biogenic amines in food: Histamine and food processing. Inflamm. Res. 1999, 48, 296–300. [Google Scholar] [CrossRef]
- Hungerford, J. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic amines in cheese and other fermented foods: A review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Paparella, A. An overview of histamine and other biogenic amines in fish and fish products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Cieślik, I.; Migdał, W. Aminy biogenne w żywności. Bromat. Chem. Toksykol. 2011, 4, 1087–1096. [Google Scholar]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 11–14. [Google Scholar]
- Botello-Morte, L.; Moniente, M.; Gil-Ramírez, Y.; Virto, R.; García-Gonzalo, D.; Pagán, R. Identification by means of molecular tools of the microbiota responsible for the formation of histamine accumulated in commercial cheeses in Spain. Food Control 2022, 133, 108595. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Innocente, N.; Biasutti, M.; Padovese, M.; Moret, S. Determination of biogenic amines in cheese using HPLC technique and direct derivatization of acid extract. Food Chem. 2007, 101, 1285–1289. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.X.; Cui, W.Q.; Zhang, J.W.; Wu, D.Q.; Yu, X.R.; Luo, Y.B.; Jiang, X.Y.; Zhu, F.P.; Hussain, D.; et al. A simultaneous extraction/derivatization strategy coupled with liquid chromatography–tandem mass spectrometry for the determination of free catecholamines in biological fluids. J. Chromatogr. A 2021, 1654, 462474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhang, J.X.; Gao, M.; Cui, W.Q.; Xu, L.; Zhu, X.; Li, J.J.; Huang, Z.E.; Hussain, D.; Zhang, J.Y.; et al. Stable isotope labelling-flow injection analysis-mass spectrometry for rapid and high throughput quantitative analysis of 5-hydroxymethylfurfural in drinks. Food Control 2021, 130, 108386. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.H.; Cui, W.Q.; Zhang, J.X.; Zang, J.W.; Wu, D.Q.; Wang, Z.Y.; Yu, X.R.; Luo, Y.B.; Hussain, D.; et al. High throughput and very specific screening of anabolic-androgenic steroid adulterants in healthy foods based on stable isotope labelling and flow injection analysis-tandem mass spectrometry with simultaneous monitoring proton adduct ions and chloride adduct ions. J. Chromatogr. A 2022, 1667, 462891. [Google Scholar] [CrossRef] [PubMed]
- Tırıs, G.; Yanıkoğlu, R.S.; Ceylan, B.; Egeli, D.; Kepekci Tekkeli, E.; Önal, A. A review of the currently developed analytical methods for the determination of biogenic amines in food products. Food Chem. 2022, 398, 133919. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Kun Qian, K.; Shi, T.; Wang, F.; Li, J.; Cao, Y.; Hu, Q. Monitoring the contents of biogenic amines in sufu by HPLC with SPE and pre-column derivatization. Food Control 2011, 22, 1203–1208. [Google Scholar] [CrossRef]
- Duflos, P.; Dervin, C.; Malle, P.; Bouquelet, S. Relevance of matrix effect in determination of biogenic amines in plaice (Pleuronectes platessa) and whiting (Merlangus merlangus). J. AOAC Int. 1999, 82, 1097–1101. [Google Scholar] [CrossRef]
- Zdolec, N.; Bogdanović, T.; Severin, K.; Dobranić, V.; Kazazić, S.; Grbavac, J.; Pleadin, J.; Petričević, S.; Kiš, M. Biogenic amine content in retailed cheese varieties produced with commercial bacterial or mold cultures. Processes 2022, 10, 10. [Google Scholar] [CrossRef]
- Smĕlá, D.; Pechová, P.; Komprda, T.; Klejdus, B.; Kubáň, V. Liquid chromatographic determination of biogenic amines in a meat product during fermentation and long-term storage. Czech J. Food Sci. 2003, 21, 167–175. [Google Scholar] [CrossRef]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef]
- Bogdanović, T.; Petričević, S.; Brkljača, M.; Listeš, I.; Pleadin, J. Biogenic amines in selected foods of animal origin obtained from the Croatian retail market. Food Addit. Contam. Part A 2020, 37, 815–830. [Google Scholar] [CrossRef]
- Eerola, S.; Sagues, A.X.R.; Lilleberg, L.; Aalto, H. Biogenic amines in dry sausages during shelf-life storage. Eur. Food Res. Technol. 1997, 205, 351–355. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
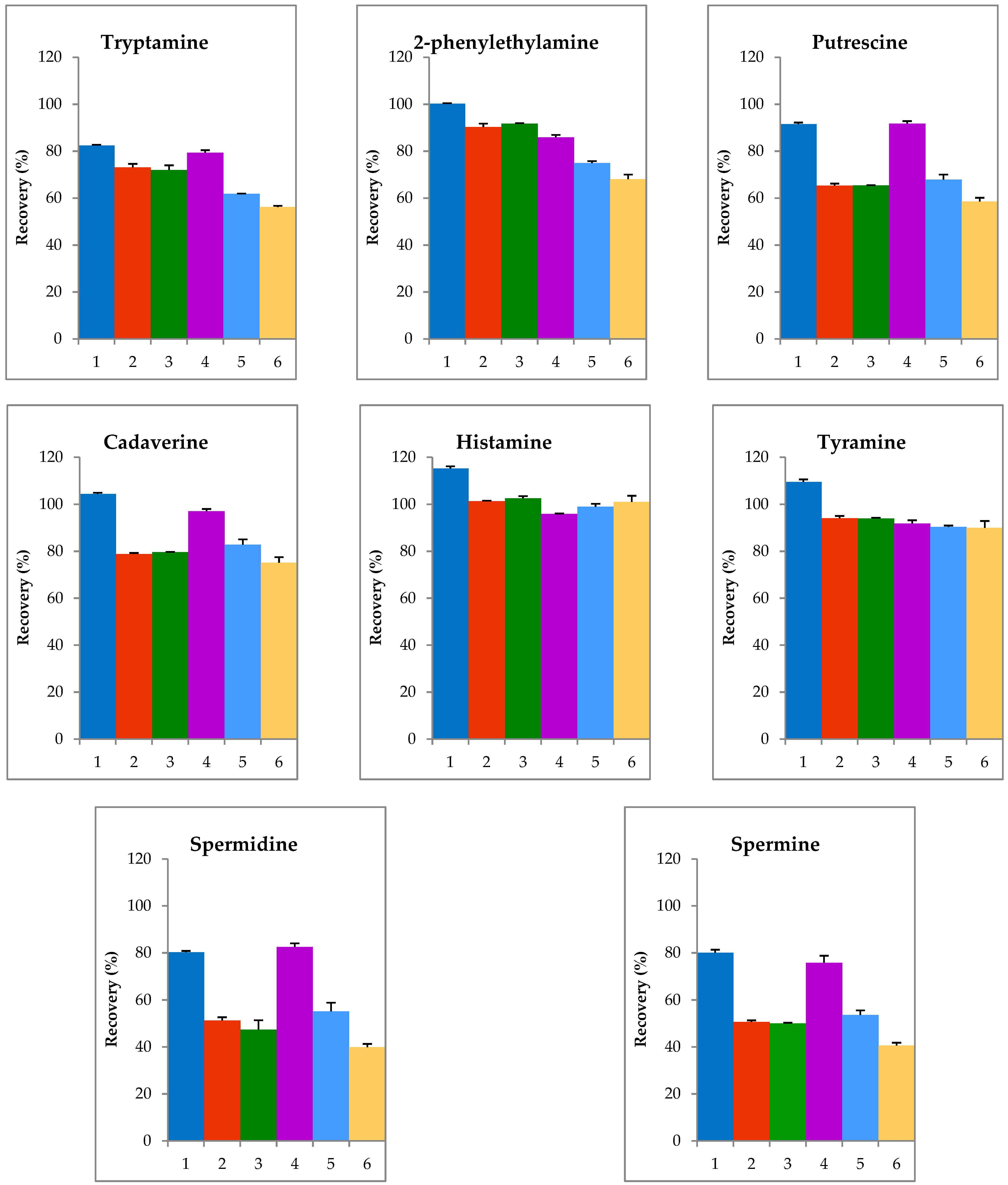
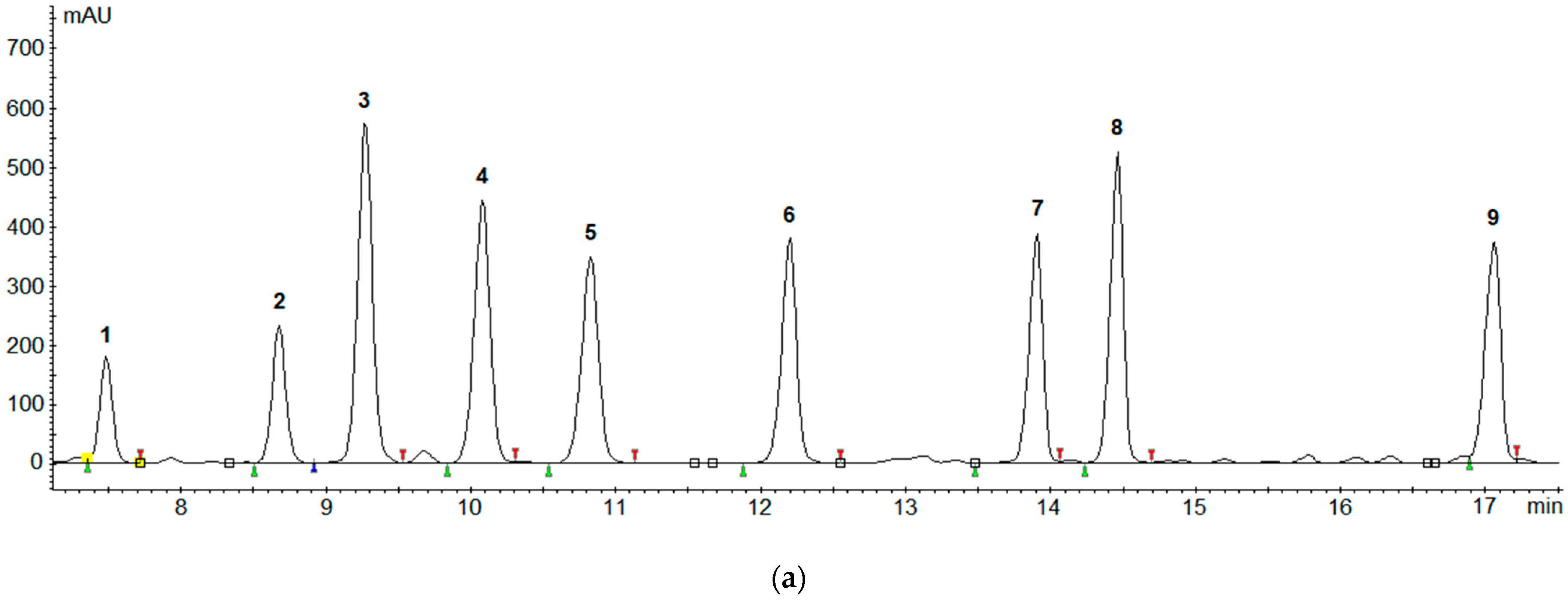
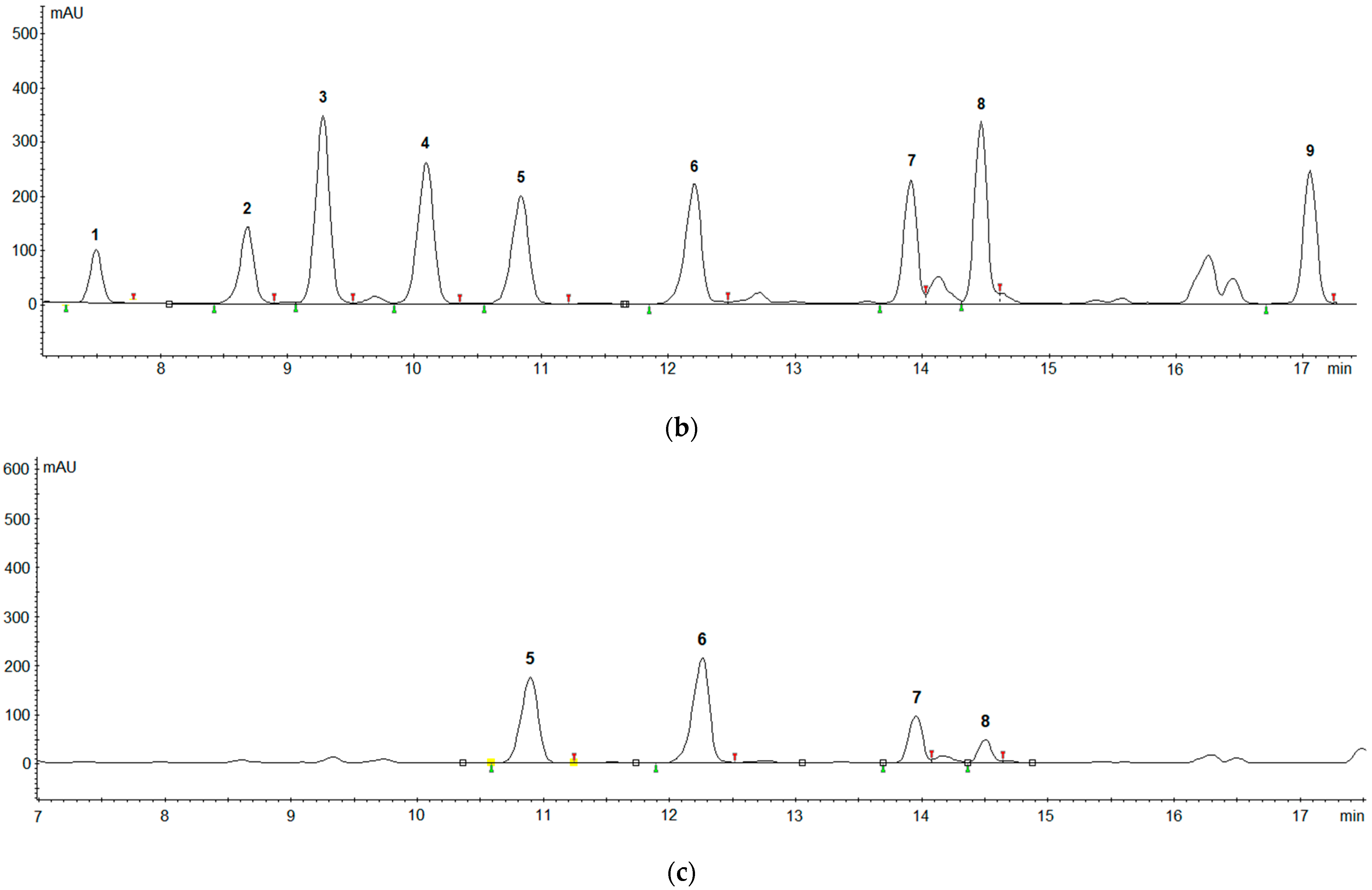
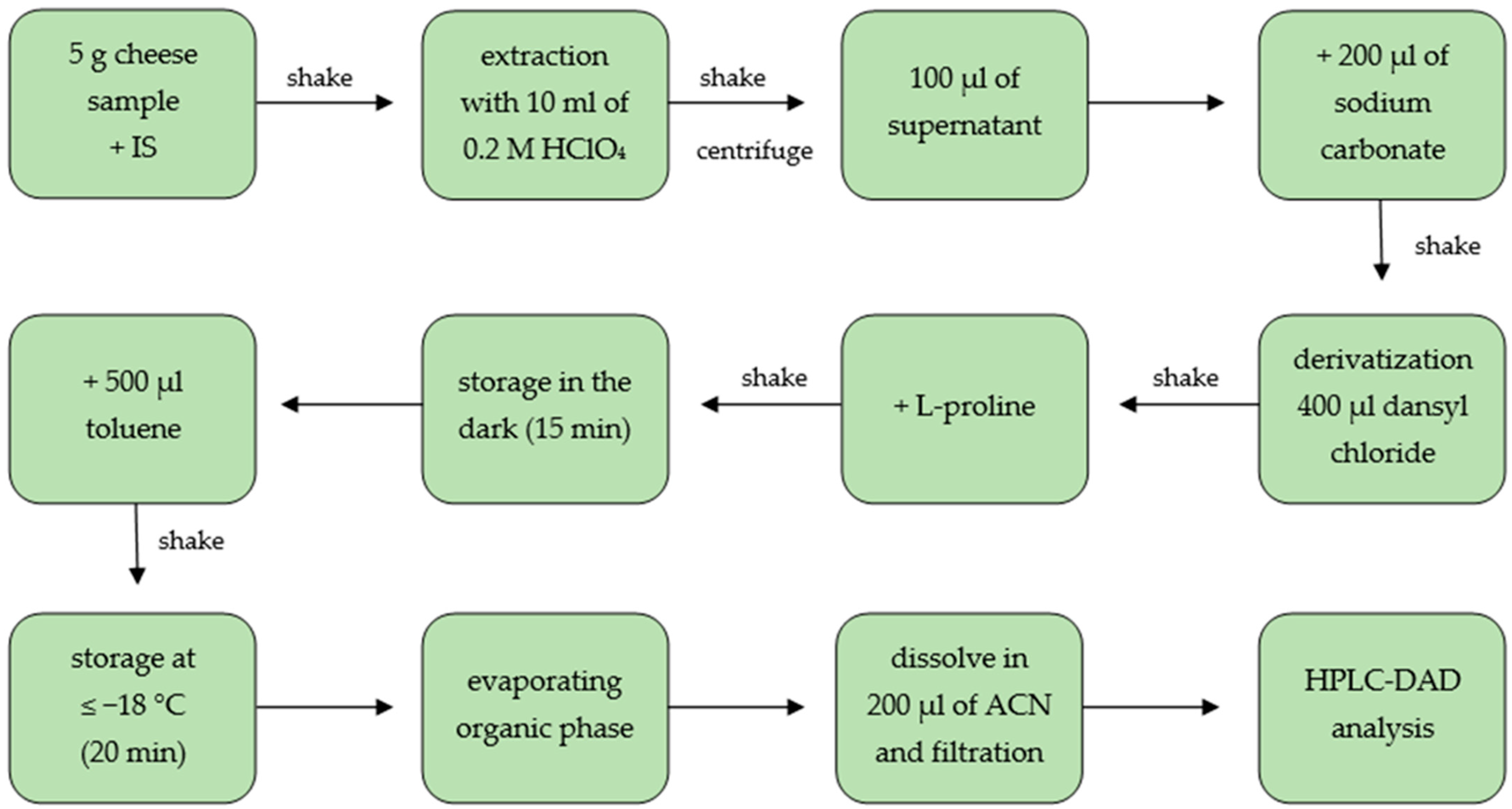
| Biogenic Amines | Recovery (%) | |
|---|---|---|
| Single Extraction | Double Extraction | |
| Tryptamine | 94.6 | 97.9 |
| 2-phenylethylamine | 106.3 | 106.6 |
| Putrescine | 83.8 | 79.0 |
| Cadaverine | 97.6 | 91.9 |
| Histamine | 109.4 | 103.1 |
| Tyramine | 99.8 | 98.4 |
| Spermidine | 75.6 | 68.9 |
| Spermine | 75.5 | 66.7 |
| Biogenic Amine | Derivatization Conditions | |||
|---|---|---|---|---|
| 25 °C for 15 min | 40 °C for 45 min | 60 °C for 5 min | 60 °C for 15 min | |
| Tryptamine | 20.14 | 19.41 | 19.02 | 19.80 |
| 2-phenylethylamine | 26.52 | 26.28 | 26.36 | 27.07 |
| Putrescine | 66.80 | 68.77 | 68.44 | 71.25 |
| Cadaverine | 58.74 | 58.89 | 58.45 | 60.73 |
| Histamine | 47.94 | 48.13 | 47.29 | 51.05 |
| Tyramine | 44.47 | 43.60 | 43.35 | 45.12 |
| Spermidine | 52.78 | 55.94 | 54.20 | 57.71 |
| Spermine | 42.19 | 47.23 | 44.89 | 48.51 |
| Biogenic Amines | LOD (mg/kg) | LOQ (mg/kg) | Regression Equation, Y | Linearity | R2 |
|---|---|---|---|---|---|
| Tryptamine | 1.55 | 5.18 | 0.0182x + 0.0045 | 5.18-200 | 0.9998 |
| 2-phenylethylamine | 1.59 | 5.31 | 0.0229x + 0.0056 | 5.31-200 | 0.9998 |
| Putrescine | 1.72 | 5.73 | 0.0591x + 0.0348 | 5.73-200 | 0.9997 |
| Cadaverine | 1.77 | 5.90 | 0.0513x + 0.0160 | 5.90-200 | 0.9997 |
| Histamine | 1.53 | 5.10 | 0.0425x + 0.0008 | 5.10-200 | 0.9998 |
| Tyramine | 1.60 | 5.33 | 0.0383x + 0.0092 | 5.33-200 | 0.9998 |
| Spermidine | 1.88 | 6.28 | 0.0497x + 0.0248 | 6.28-200 | 0.9997 |
| Spermine | 1.54 | 5.13 | 0.0432x + 0.0011 | 5.13-200 | 0.9998 |
| Biogenic Amine | Intra-Day Precision (% RSD) (n = 6) | Inter-Day Precision (% RSD) (n = 18) | Recovery (% R) (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L1 | L2 | L3 | L1 | L2 | L3 | |
| Tryptamine | 2.5 | 2.3 | 2.2 | 11.8 | 7.2 | 10.5 | 80.7 | 77.3 | 78.1 |
| 2-phenylethylamine | 2.9 | 2.0 | 2.4 | 8.0 | 6.7 | 7.0 | 98.0 | 91.8 | 93.3 |
| Putrescine | 2.6 | 3.0 | 1.1 | 10.5 | 4.3 | 5.9 | 96.1 | 89.0 | 91.3 |
| Cadaverine | 1.8 | 2.1 | 1.5 | 8.5 | 4.1 | 5.7 | 100.2 | 93.6 | 95.1 |
| Histamine | 2.1 | 1.4 | 2.0 | 12.4 | 8.1 | 7.7 | 94.2 | 93.0 | 91.0 |
| Tyramine | 1.7 | 0.7 | 2.2 | 9.3 | 12.2 | 13.1 | 85.2 | 82.9 | 80.8 |
| Spermidine | 2.6 | 2.3 | 0.7 | 9.7 | 8.9 | 6.4 | 81.0 | 80.3 | 80.7 |
| Spermine | 1.7 | 2.4 | 1.0 | 15.6 | 12.6 | 13.5 | 75.8 | 76.1 | 74.5 |
| Samples | Biogenic Amines Concentrations (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TRYP | PHE | PUT | CAD | HIS | TYR | SPD | SPM | Total | |
| Mould-ripened soft cheeses (n = 15) | |||||||||
| Brie | - | - | - | - | - | - | - | - | - |
| Brie | 9.84 | - | - | - | - | - | 9.82 | - | 19.7 |
| Camembert | - | - | - | - | - | - | 7.45 | - | 7.45 |
| Camembert | 6.46 | - | - | - | - | - | - | - | 6.46 |
| Camembert | - | - | - | - | - | - | 6.68 | - | 6.68 |
| Camembert | - | - | 22.9 | 162 | - | - | - | - | 185 |
| Caprice des dieux | - | - | - | - | - | - | 9.86 | - | 9.86 |
| Chruśniak z kozieradką | - | - | 40.1 | 10.1 | 13.1 | 102 | - | - | 165 |
| Limburger | 14.1 | - | - | - | 6.48 | 19.0 | - | - | 39.6 |
| Limburger | - | - | - | - | - | - | - | - | - |
| Saint Albray | - | - | - | - | - | - | - | - | - |
| Savaron | - | - | - | 81.3 | - | - | - | - | 81.3 |
| Snack a la francaise | - | - | - | - | - | - | - | - | - |
| Soft cheese | - | - | 20.6 | - | - | - | - | - | 20.6 |
| Soft cheese with chives | - | - | 31.9 | 208 | - | 692 | - | - | 932 |
| Blue-veined chesses (n = 10) | |||||||||
| Blu-fou | - | - | - | - | - | - | 7.97 | - | 7.97 |
| Bleu onctueux | 7.99 | 6.91 | 6.57 | 61.0 | - | - | 15.1 | - | 97.6 |
| Blu rival | - | - | - | - | - | - | 13.4 | - | 13.4 |
| Dorblu | - | - | - | - | - | - | - | - | - |
| Gorgonzola | - | - | - | - | 94.2 | - | 7.05 | - | 101 |
| Gorgonzola | - | 7.39 | - | - | 127 | - | - | - | 134 |
| Gorgonzola | - | - | - | - | 78.4 | 36.6 | 11.2 | - | 126 |
| Roquefort | - | 15.1 | 7.46 | 59.7 | - | 631 | - | - | 713 |
| Saint Agur | - | - | 7.97 | - | - | - | 10.3 | - | 18.3 |
| Srebrzysty | - | - | - | - | - | - | 14.5 | - | 14.5 |
| Hard cheeses (n = 10) | |||||||||
| Corregio | - | - | - | - | - | 7.28 | - | - | 7.28 |
| Emmental | - | 42.5 | 15.4 | 14.4 | 15.8 | 474 | - | - | 562 |
| Grana Padano | - | - | - | - | 84.7 | - | - | - | 84.7 |
| Grana Padano | - | - | - | - | 11.5 | - | - | - | 11.5 |
| Goat caciotta type | - | - | 24.9 | 10.5 | - | 53.0 | - | - | 88.4 |
| Goat caciotta type with pistachios | - | - | - | - | - | 34.3 | - | - | 34.3 |
| Le Gruyere | - | - | - | 55.8 | - | - | - | - | 55.8 |
| Parmigiano Reggiano | - | - | - | - | 5.81 | - | - | - | 5.81 |
| Raclette | - | 27.3 | 170 | 148 | - | 287 | - | - | 632 |
| Santtum | - | - | - | - | - | - | 7.31 | - | 7.31 |
| Samples | Biogenic Amines Concentrations (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TRYP | PHE | PUT | CAD | HIS | TYR | SPD | SPM | Total | |
| Mould-ripened soft cheeses (n = 15) | |||||||||
| Minimum | 6.46 | - | 20.6 | 10.1 | 6.48 | 19.0 | 6.68 | - | 6.46 |
| Mean | 10.3 | - | 28.9 | 115 | 9.79 | 271 | 8.45 | - | 73.7 |
| Median | 10.3 | - | 27.4 | 122 | 9.79 | 102 | 8.64 | - | 16.6 |
| 90 Percentile | 13.3 | - | 37.6 | 194 | 12.4 | 574 | 9.85 | - | 167 |
| 95 Percentile | 13.7 | - | 38.9 | 201 | 12.8 | 633 | 9.85 | - | 232 |
| Maximum | 14.1 | - | 40.1 | 208 | 13.1 | 692 | 9.86 | - | 692 |
| Blue-veined chesses (n = 10) | |||||||||
| Minimum | 7.99 | 6.91 | 6.57 | 59.7 | 78.4 | 36.6 | 7.05 | - | 6.57 |
| Mean | - | 9.80 | 7.33 | 60.4 | 99.9 | 334 | 11.4 | - | 58.4 |
| Median | - | 7.39 | 7.46 | 60.4 | 94.2 | 334 | 11.2 | - | 13.4 |
| 90 Percentile | - | 13.6 | 7.87 | 60.9 | 120 | 572 | 14.7 | - | 94.2 |
| 95 Percentile | - | 14.3 | 7.92 | 60.9 | 124 | 601 | 14.9 | - | 127 |
| Maximum | - | 15.1 | 7.97 | 61.0 | 127 | 631 | 15.1 | - | 631 |
| Hard cheeses (n = 10) | |||||||||
| Minimum | - | 27.3 | 15.4 | 10.5 | 5.81 | 7.28 | 7.31 | - | 5.81 |
| Mean | - | 34.9 | 70.1 | 57.2 | 29.5 | 171 | - | - | 78.4 |
| Median | - | 34.9 | 24.9 | 35.1 | 13.7 | 53.0 | - | - | 27.3 |
| 90 Percentile | - | 41.0 | 141 | 120 | 64.0 | 399 | - | - | 193 |
| 95 Percentile | - | 41.7 | 156 | 134 | 74.4 | 437 | - | - | 305 |
| Maximum | - | 42.5 | 170 | 148 | 84.7 | 474 | - | - | 474 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawul-Gruba, M.; Kiljanek, T.; Madejska, A.; Osek, J. Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses. Molecules 2022, 27, 8194. https://doi.org/10.3390/molecules27238194
Pawul-Gruba M, Kiljanek T, Madejska A, Osek J. Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses. Molecules. 2022; 27(23):8194. https://doi.org/10.3390/molecules27238194
Chicago/Turabian StylePawul-Gruba, Marzena, Tomasz Kiljanek, Anna Madejska, and Jacek Osek. 2022. "Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses" Molecules 27, no. 23: 8194. https://doi.org/10.3390/molecules27238194
APA StylePawul-Gruba, M., Kiljanek, T., Madejska, A., & Osek, J. (2022). Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses. Molecules, 27(23), 8194. https://doi.org/10.3390/molecules27238194






