Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study
Abstract
1. Introduction
2. Results
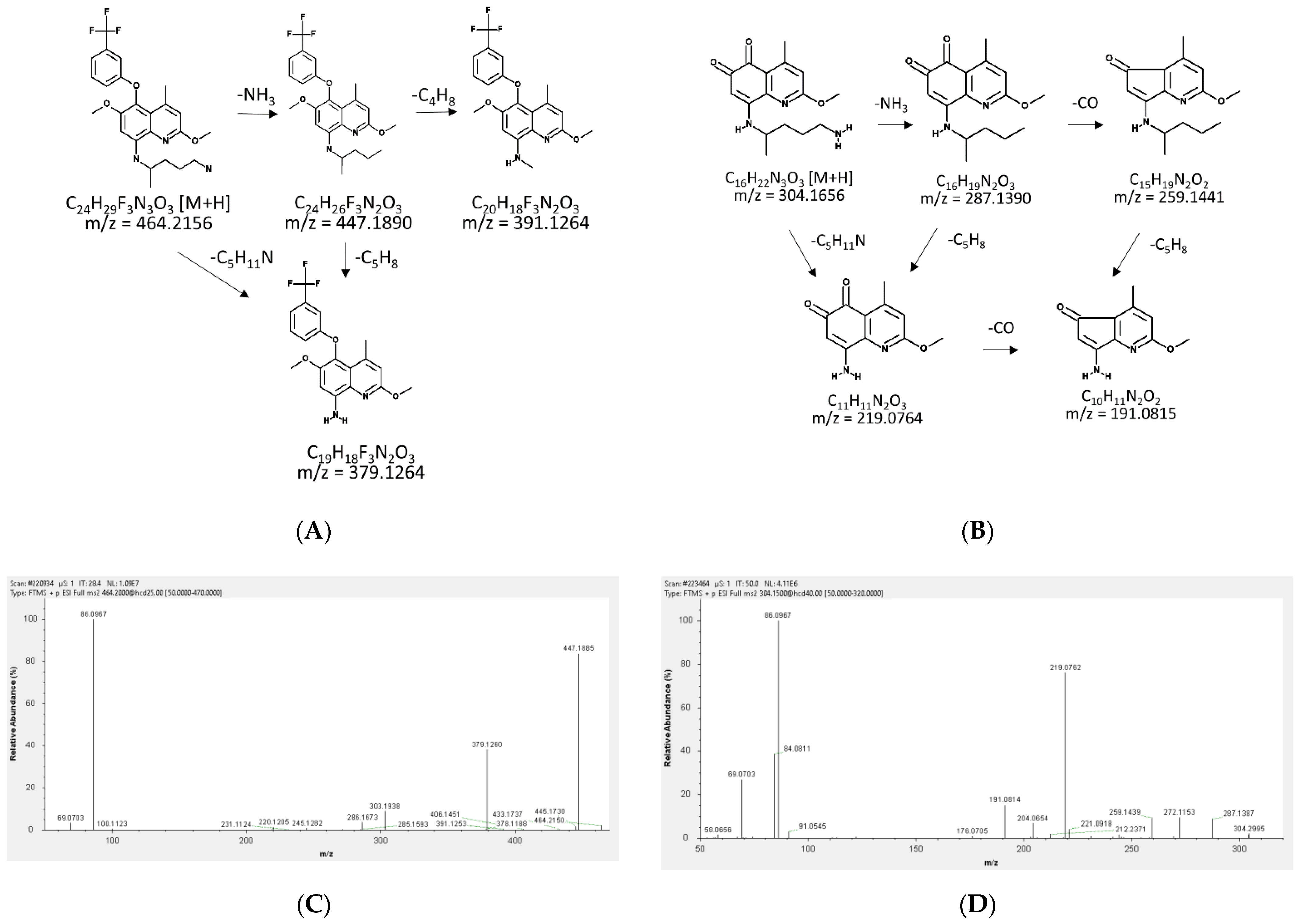
2.1. Fragmentation Patterns and MS/MS Spectra of TQ and 5,6-OQTQ
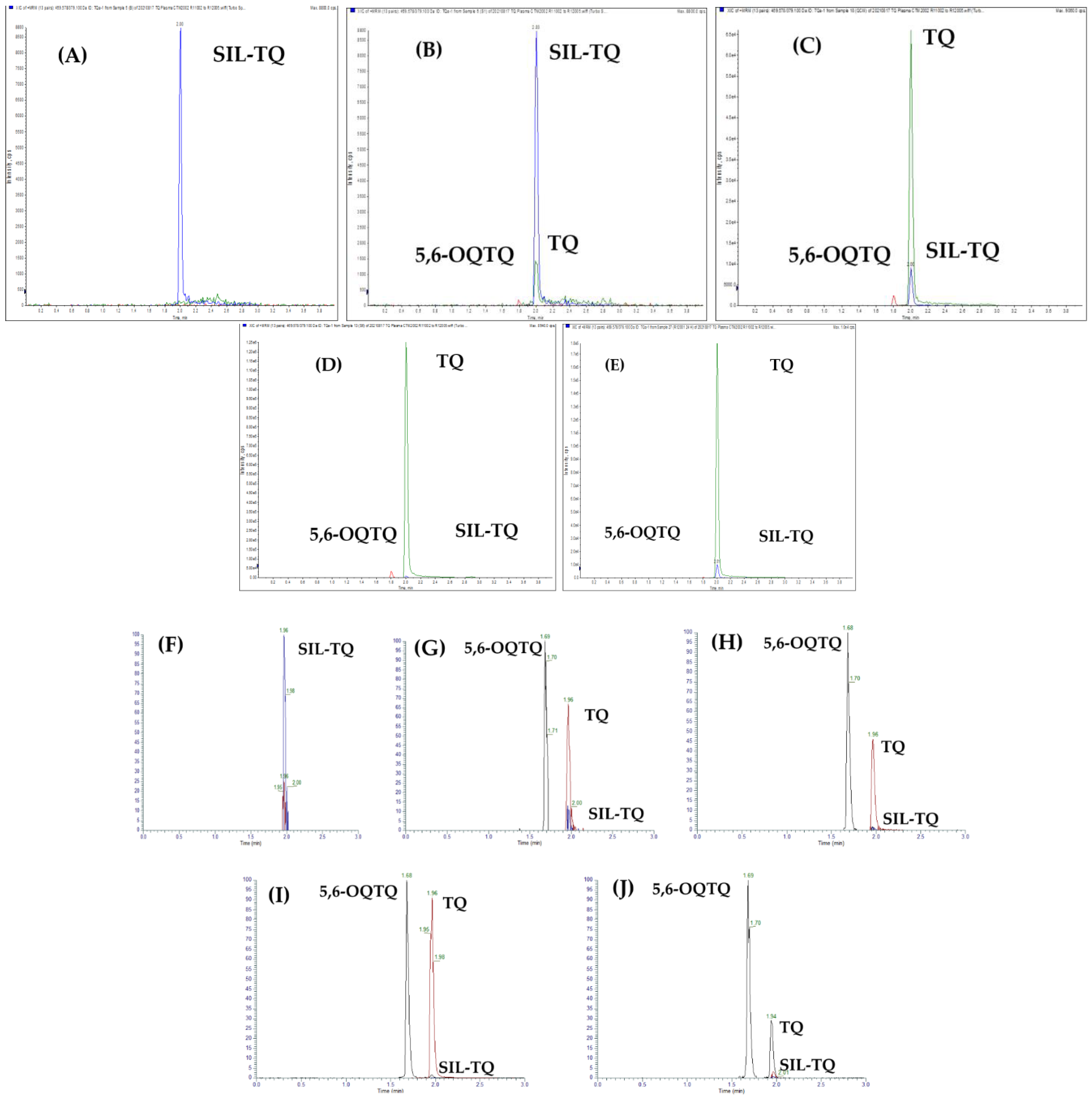
2.2. Detection and Quantification of 5,6-Tafenoquine, Stable Isotope Label-Tafenoquine and Tafenoquine
2.3. Method Validation
2.3.1. Selectivity and Specificity
2.3.2. Linearity and Sensitivity
2.3.3. Matrix Effect and Recovery
2.3.4. Accuracy and Precision
2.3.5. Stability
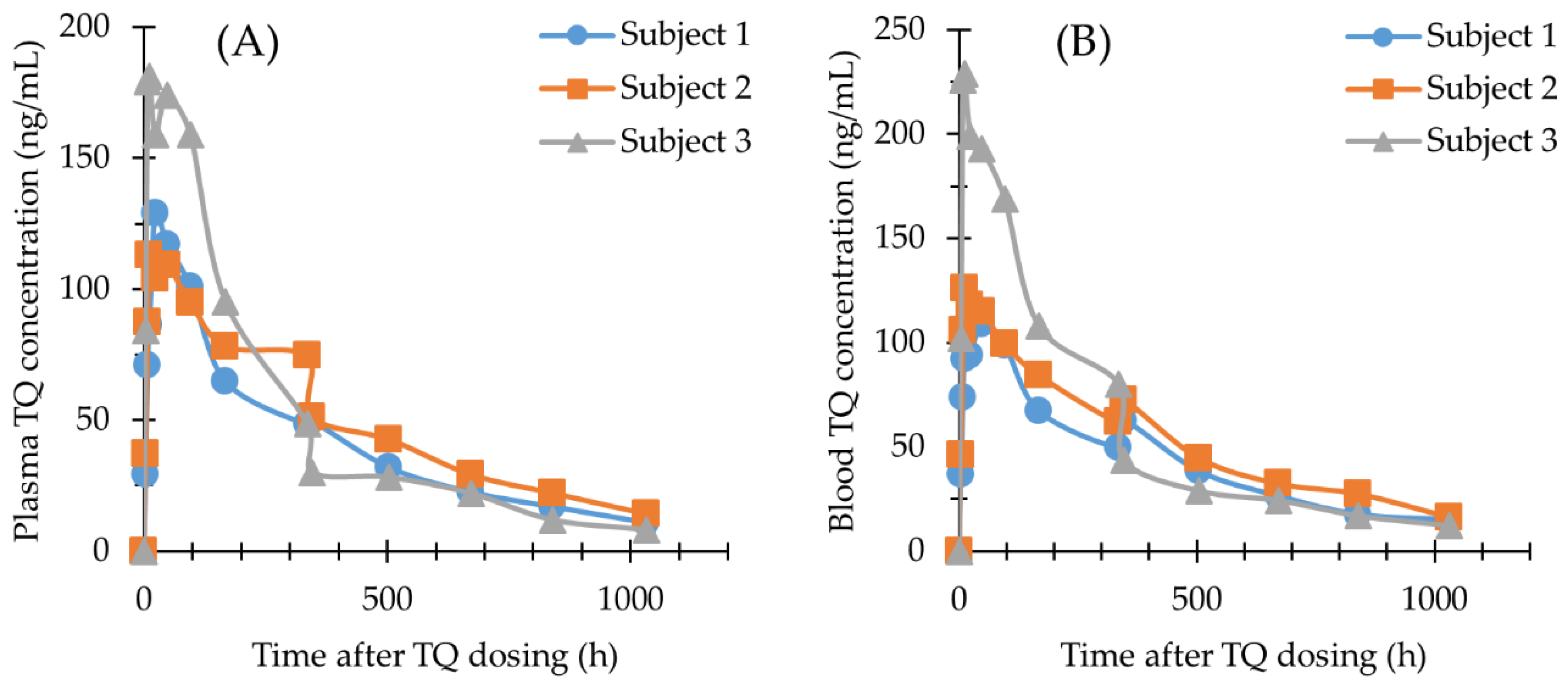
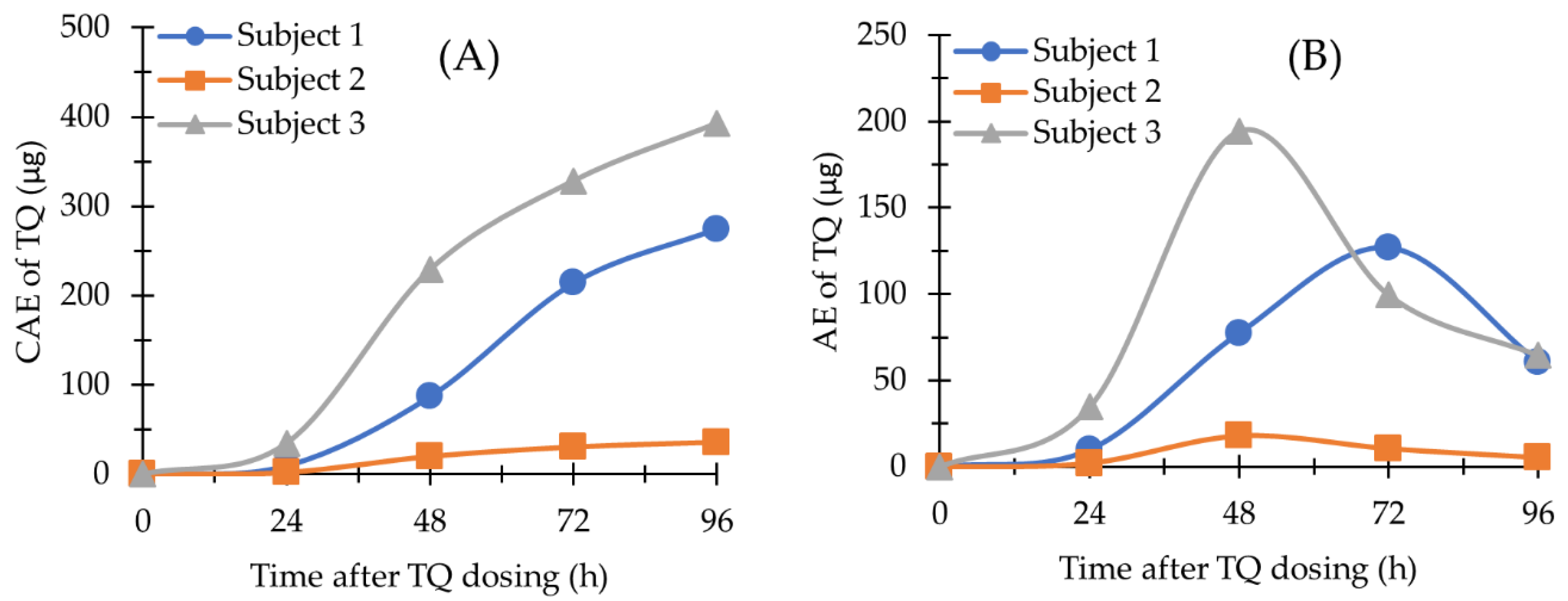
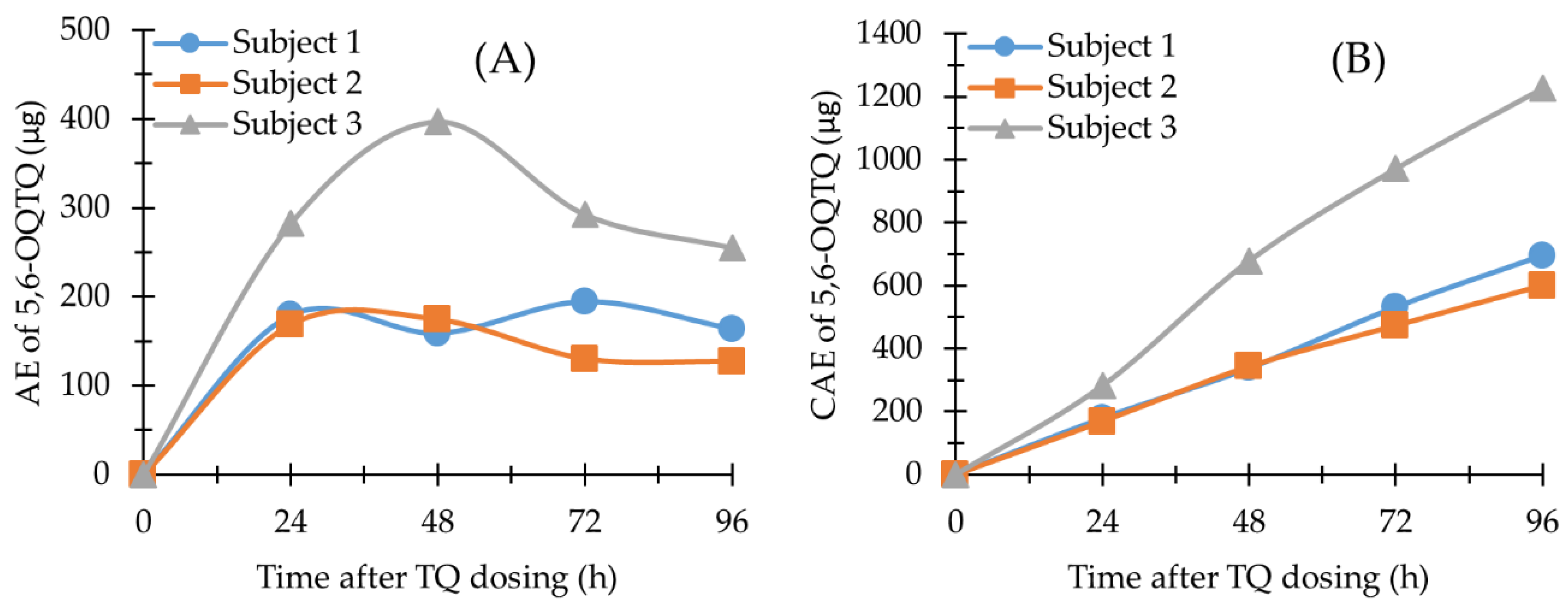
2.4. Application of the Methods
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation and Chromatographic Conditions
4.2.1. Analysis of TQ and 5,6-OQTQ in Blood and Plasma
4.2.2. Analysis of TQ and 5,6-OQTQ in Urine
4.3. Standard Stock Solutions Preparation
4.4. Method Validation
4.5. Application of the Method
4.5.1. Subjects and Sample Collection
4.5.2. Sample Preparation
4.5.3. Pharmacokinetic Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alving, A.S.; Arnold, J.; Robinson, D.H. Mass therapy of subclinical vivax malaria with primaquine. J. Am. Med. Assoc. 1952, 149, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K.; Rieckmann, K.H. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003, 19, 115–120. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Nanayakkara, N.P.D.; Wang, Y.H.; Chaurasiya, N.D.; Herath, H.M.B.; McChesney, J.D.; Avula, B.; Khan, I.; Tekwani, B.L.; Walker, L.A. Formation primaquine-5,6-orthoquinone, the putative active and toxic metabolite of primaquine via direct oxidation in human erythrocytes. Malar. J. 2019, 18, 30. [Google Scholar] [CrossRef]
- Thakkar, N.; Green, J.A.; Koh, G.; Duparc, S.; Tenero, D.; Goyal, N. Population Pharmacokinetics of Tafenoquine, a Novel Antimalarial. Antimicrob. Agents Chemother. 2018, 62, e00711-18. [Google Scholar] [CrossRef] [PubMed]
- Alving, A.S.; Carson, P.E.; Flanagan, C.L.; Ickes, C.E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 1956, 124, 484–485. [Google Scholar] [PubMed]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Constantino, L.; Paixao, P.; Moreira, R.; Portela, M.J.; Do Rosario, V.E.; Iley, J. Metabolism of primaquine by liver homogenate fractions. Evidence for monoamine oxidase and cytochrome P450 involvement in the oxidative deamination of primaquine to carboxyprimaquine. Exp. Toxicol. Pathol. 1999, 51, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Camarda, G.; Jirawatcharadech, P.; Priestley, R.S.; Saif, A.; March, S.; Wong, M.H.L.; Leung, S.; Miller, A.B.; Baker, D.A.; Alano, P.; et al. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019, 10, 3226. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Tekwani, B.L.; Nanayakkara, N.P.; Avula, B.; Herath, H.M.; Wang, Y.H.; Adelli, V.R.; Elsohly, M.A.; Khan, S.I.; Khan, I.A.; et al. Enantioselective metabolism of primaquine by human CYP2D6. Malar. J. 2014, 13, 507. [Google Scholar] [CrossRef]
- Vuong, C.; Xie, L.H.; Potter, B.M.; Zhang, J.; Zhang, P.; Duan, D.; Nolan, C.K.; Sciotti, R.J.; Zottig, V.E.; Nanayakkara, N.P.; et al. Differential cytochrome P450 2D metabolism alters tafenoquine pharmacokinetics. Antimicrob. Agents Chemother. 2015, 59, 3864–3869. [Google Scholar] [CrossRef] [PubMed]
- Pookmanee, W.; Thongthip, S.; Tankanitlert, J.; Mungthin, M.; Sukasem, C.; Wittayalertpanya, S. Simplified and Rapid Determination of Primaquine and 5,6-Orthoquinone Primaquine by UHPLC-MS/MS: Its Application to a Pharmacokinetic Study. Molecules 2021, 26, 4357. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.; Webster, R.; Marquart, L.; Rahman, A.; Amante, F.; Mitchell, H.; Gower, J.; Peters, J.; Heunis, J.; Jennings, H.; et al. A 2-part study to evaluate the asexual blood stage antimalarial activity, and the transmission blocking activity, of a single oral dose of tafenoquine in healthy subjects experimentally infected with plasmodium falciparum. Am. J. Soc. Trop. Med. Hyg. 2021, 105, 274. [Google Scholar]
- European Medicines Agency. Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 29 September 2022).
- Department of Health and Human Services. US Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 29 September 2022).
- Brueckner, R.P.; Coster, T.; Wesche, D.L.; Shmuklarsky, M.; Schuster, B.G. Prophylaxis of Plasmodium falciparum infection in a human challenge model with WR 238605, a new 8-aminoquinoline antimalarial. Antimicrob. Agents Chemother. 1998, 42, 1293–1294. [Google Scholar] [CrossRef]
- Karle, J.M.; Olmeda, R. Rapid and sensitive quantitative analysis of the new antimalarial N4-[2,6-dimethoxy-4-methyl-5-[(3-trifluoromethyl)phenoxy]-8- quinolinyl]-1,4-pentanediamine in plasma by liquid chromatography and electrochemical detection. J. Chromatogr. 1988, 424, 347–356. [Google Scholar] [CrossRef]
- Kocisko, D.A.; Walsh, D.S.; Eamsila, C.; Edstein, M.D. Measurement of tafenoquine (WR 238605) in human plasma and venous and capillary blood by high-pressure liquid chromatography. Ther. Drug Monit. 2000, 22, 184–189. [Google Scholar] [CrossRef]
- Araujo-Leon, J.A.; Ortiz-Andrade, R.; Vera-Sanchez, R.A.; Oney-Montalvo, J.E.; Coral-Martinez, T.I.; Cantillo-Ciau, Z. Development and Optimization of a High Sensitivity LC-MS/MS Method for the Determination of Hesperidin and Naringenin in Rat Plasma: Pharmacokinetic Approach. Molecules 2020, 25, 4241. [Google Scholar] [CrossRef]
- Spooner, N.; Anderson, K.D.; Siple, J.; Wickremsinhe, E.R.; Xu, Y.; Lee, M. Microsampling: Considerations for its use in pharmaceutical drug discovery and development. Bioanalysis 2019, 11, 1015–1038. [Google Scholar] [CrossRef]
- Bachhav, S.S.; Taylor, M.; Martin, A.; Green, J.A.; Duparc, S.; Rolfe, K.; Sharma, H.; Tan, L.K.; Goyal, N. A Pharmacometrics Approach to Assess the Feasibility of Capillary Microsampling to Replace Venous Sampling in Clinical Studies: Tafenoquine Case Study. Br. J. Clin. Pharmacol. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Avula, B.; Khan, S.I.; Tekwani, B.L.; Nanayakkara, N.P.; McChesney, J.D.; Walker, L.A.; Khan, I.A. Analysis of primaquine and its metabolite carboxyprimaquine in biological samples: Enantiomeric separation, method validation and quantification. Biomed. Chromatogr. 2011, 25, 1010–1017. [Google Scholar] [CrossRef]
- Bonato, P.S. Recent advances in the determination of enantiomeric drugs and their metabolites in biological fluids by capillary electrophoresis-mediated microanalysis. Electrophoresis 2003, 24, 4078–4094. [Google Scholar] [CrossRef] [PubMed]
- Spring, M.D.; Lon, C.; Sok, S.; Sea, D.; Wojnarski, M.; Chann, S.; Kuntawunginn, W.; Kheang Heng, T.; Nou, S.; Arsanok, M.; et al. Prevalence of CYP2D6 Genotypes and Predicted Phenotypes in a Cohort of Cambodians at High Risk for Infections with Plasmodium vivax. Am. J. Trop. Med. Hyg. 2020, 103, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.; Hartter, S.; Fuchs, K.; Sieghart, W.; Hiemke, C. CYP2D6 genotype and phenotyping by determination of dextromethorphan and metabolites in serum of healthy controls and of patients under psychotropic medication. Pharmacogenetics 1997, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K.; Louisa, M.; Noviyanti, R.; Ekawati, L.; Elyazar, I.; Subekti, D.; Chand, K.; Gayatri, A.; Instiaty, I.; Soebianto, S.; et al. Association of Impaired Cytochrome P450 2D6 Activity Genotype and Phenotype with Therapeutic Efficacy of Primaquine Treatment for Latent Plasmodium vivax Malaria. JAMA Netw. Open 2018, 1, e181449. [Google Scholar] [CrossRef] [PubMed]
- St Jean, P.L.; Xue, Z.; Carter, N.; Koh, G.C.; Duparc, S.; Taylor, M.; Beaumont, C.; Llanos-Cuentas, A.; Rueangweerayut, R.; Krudsood, S.; et al. Tafenoquine treatment of Plasmodium vivax malaria: Suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the Phase 2b DETECTIVE trial. Malar. J. 2016, 15, 97. [Google Scholar] [CrossRef] [PubMed]
| Blood | Plasma | Urine | ||||
|---|---|---|---|---|---|---|
| Analytes | Matrix Effect (%) (Mean ± SD/CV) | Recovery (%) (Mean ± SD/CV) | Matrix Effect (%) (Mean ± SD/CV) | Recovery (%) (Mean ± SD/CV) | Matrix Effect (%) (Mean ± SD/CV) | Recovery (%) (Mean ± SD/CV) |
| TQ | ||||||
| LQC | 97 ± 14/14.2 | 187 ± 6/11.9 | 99 ± 3/2.6 | 69 ± 5/7.7 | 87 ± 14/16.0 | 84 ± 10/11.3 |
| MQC | - | 106 ± 6/5.7 | - | 80 ± 6/7.2 | - | 85 ± 8.7/10.3 |
| HQC | 99 ± 3/3.5 | 116 ± 4/3.1 | 98 ± 1/1.0 | 92 ± 4/3.9 | 94 ± 3.5/3.7 | 96 ± 3.5/3.7 |
| 5,6-OQTQ | ||||||
| LQC | 64 ± 9/13.5 * | 61 ± 16/25.8 ** | 98 ± 4/3.9 | 83 ± 7/7.8 | 86 ± 13/15.5 | 98 ± 10/10.2 |
| MQC | 83 ± 8/10.1 | - | 110 ± 4/4.1 | - | 91 ± 10/10.5 | |
| HQC | 89 ± 5/5.5 | 82 ± 3.2/3.9 | 101 ± 7/7.0 | 94 ± 3/3.2 | 104 ± 19/17.9 | 101 ± 3/2.9 |
| Human Plasma | Human Blood | Human Urine | ||||
|---|---|---|---|---|---|---|
| Analytes | Intra-Day (n = 6) Accuracy/Precision (% Mean ± SD %, CV) | Inter-Day (n = 14) Accuracy/Precision (% Mean ± SD %, CV) | Intra-Day (n = 6) Accuracy/Precision (% Mean ± SD %, CV) | Inter-Day (n = 14) Accuracy/Precision (% Mean ± SD %, CV) | Intra-Day (n = 5) Accuracy/Precision (% mean ± SD %, CV) | Inter-Day (n = 14) Accuracy/Precision (% mean ± SD %, CV) |
| TQ | ||||||
| LLOQ | 100.5 ± 0.55/0.54 | 102.3 ± 8.35/8.16 | 99.82 ± 0.33/0.33 | 98.40 ± 9.69/9.85 | 103.00 ± 4.95/4.80 | 99.74 ± 8.15/8.17 |
| LQC | 98.90 ± 5.76/5.91 | 109.19 ± 6.34/5.85 | 100.43 ± 9.07/8.94 | 102.16 ± 8.61/8.43 | 107.35 ± 6.51/6.06 | 103.57 ± 5.88/5.68 |
| MQC | 98.35 ± 5.44/5.53 | 101.11 ± 2.93/2.89 | 102.62 ± 5.68/5.44 | 97.54 ± 4.78/4.81 | 104.71 ± 5.51/5.26 | 103.34 ± 6.98/6.76 |
| HQC | 103.35 ± 6.16/5.80 | 105.60 ± 4.65/4.42 | 94.42 ± 3.19/3.41 | 94.09 ± 3.98/4.22 | 104.74 ± 8.24/7.86 | 107.61 ± 9.15/8.50 |
| 5,6-OQTQ | ||||||
| LLOQ | 101.42 ± 4.27/4.21 | 98.68 ± 6.96/7.05 | - | - | 104.58 ± 6.01/5.74 | 99.81 ± 6.36/6.38 |
| LQC | 92.93 ± 6.04/6.58 | 107.87 ± 8.71/8.06 | - | - | 103.39 ± 8.00/7.74 | 100.64 ± 7.83/7.78 |
| MQC | 103.05 ± 6.94/6.57 | 105.14 ± 6.32/6.12 | - | - | 104.05 ± 5.78/5.55 | 103.43 ± 12.17/11.77 |
| HQC | 101.35 ± 5.04/4.97 | 104.51 ± 6.35/6.08 | - | - | 99.44 ± 2.82/2.83 | 102.58 ± 14.79/14.42 |
| Plasma TQ (ng/mL) | Venous Blood TQ (ng/mL) | Finger Prick Capillary Blood TQ (ng/mL) | Venous/Capillary Blood Mean (±SD) (ng/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Subject 1 | Subject 2 | Subject 3 | Subject 1 | Subject 2 | Subject 3 | Subject 1 | Subject 2 | Subject 3 | Subject 1 | Subject 2 | Subject 3 |
| 0 | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | N/D | N/D | N/D | N/D | N/D | N/D |
| 4 | 29.2 | 37.2 | 84.2 | 36.6 | 46.0 | 102 | N/D | N/D | N/D | N/D | N/D | N/D |
| 8 | 70.9 | 87.4 | 179 | 73.5 | 106 | 226 | N/D | N/D | N/D | N/D | N/D | N/D |
| 12 | 86.2 | 113 | 181 | 92.0 | 126 | 229 | 108 | 117 | 179 | 100 ± 11.3 | 122 ± 6.4 | 204 ± 35 |
| 24 | 129 | 104 | 159 | 93.9 | 118 | 199 | 112 | 124 | 199 | 103 ± 12.8 | 121 ± 4.2 | 199 ± 0.0 |
| 48 | 117 | 109 | 174 | 109 | 115 | 193 | N/D | N/D | N/D | N/D | N/D | N/D |
| 96 | 101 | 94.9 | 159 | 98.6 | 99.5 | 169 | 92.6 | 97.8 | 155 | 96 ± 4.2 | 99 ± 1.2 | 162 ± 9.9 |
| 168 | 64.7 | 78.1 | 95.2 | 67.0 | 84.3 | 108 | 72.8 | 64.7 | 75.0 | 70 ± 4.1 | 75 ± 13.9 | 92 ± 23.3 |
| 336 | 48.2 | 74.6 | 48.6 | 49.7 | 61.9 | 80.1 | N/D | N/D | N/D | N/D | N/D | N/D |
| 346 | 49.7 | 51.1 | 30.0 | 62.6 | 72.5 | 43.7 | N/D | N/D | N/D | N/D | N/D | N/D |
| 504 | 31.9 | 42.6 | 28.2 | 38.3 | 44.6 | 28.9 | N/D | N/D | N/D | N/D | N/D | N/D |
| 672 | 22.5 | 29.2 | 22.1 | 26.4 | 32.3 | 24.3 | N/D | N/D | N/D | N/D | N/D | N/D |
| 840 | 16.9 | 22.1 | 11.9 | 17.6 | 27.4 | 16.9 | N/D | N/D | N/D | N/D | N/D | N/D |
| 1032 | 10.9 | 14.2 | 8.2 | 15.0 | 16.1 | 12.2 | N/D | N/D | N/D | N/D | N/D | N/D |
| Subject | BW (kg) | Sex | BMI | Cmax (ng/mL) | Tmax (h) | AUC (ng/mL∗h) | AUC0-inf (ng/mL∗h) | t1/2 (h) | Recrudescence (day) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 95.0 | Male | 29.7 | 129 | 24 | 42,981 | 48,191 | 303.3 | 14 |
| 2 | 87.4 | Male | 28.8 | 113 | 12 | 50,387 | 57,902 | 300.8 | 9 |
| 3 | 60.6 | Female | 25.7 | 181 | 12 | 50,675 | 54,902 | 357.2 | 23 |
| Curtain gas | 40 |
| CAD gas | Medium |
| IS (V) | 5500 |
| Temp (°C) | 600 |
| GS1 | 50 |
| GS2 | 50 |
| IHE | On |
| Analyte | Q1 (Da) | Q3 (Da) | Time (msec) | DP (v) | EP (v) | CE (v) | CXP (v) |
|---|---|---|---|---|---|---|---|
| TQ | 464.124 | 447.000 | 150 | 76 | 10 | 25 | 12 |
| TQm | 304.150 | 219.100 | 150 | 66 | 10 | 27 | 20 |
| TQa | 469.252 | 378.900 | 150 | 46 | 10 | 31 | 28 |
| Mass | Formula | Species | Polarity | CE (V) | Identifier |
|---|---|---|---|---|---|
| 464.21555 | C24H28F3N3O3 | + H | Positive | 25 | TQ |
| 469.25468 | C24H33F3N3O3 | + H | Positive | 30 | SIL-TQ |
| 304.16557 | C16H21N3O3 | + H | Positive | 25 | 5,6-OQTQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birrell, G.W.; Van Breda, K.; Barber, B.; Webster, R.; McCarthy, J.S.; Shanks, G.D.; Edstein, M.D. Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study. Molecules 2022, 27, 8186. https://doi.org/10.3390/molecules27238186
Birrell GW, Van Breda K, Barber B, Webster R, McCarthy JS, Shanks GD, Edstein MD. Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study. Molecules. 2022; 27(23):8186. https://doi.org/10.3390/molecules27238186
Chicago/Turabian StyleBirrell, Geoffrey W., Karin Van Breda, Bridget Barber, Rebecca Webster, James S. McCarthy, G. Dennis Shanks, and Michael D. Edstein. 2022. "Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study" Molecules 27, no. 23: 8186. https://doi.org/10.3390/molecules27238186
APA StyleBirrell, G. W., Van Breda, K., Barber, B., Webster, R., McCarthy, J. S., Shanks, G. D., & Edstein, M. D. (2022). Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study. Molecules, 27(23), 8186. https://doi.org/10.3390/molecules27238186






