Abstract
Bismuth tellurides is one of the most promising thermoelectric (TE) material candidates in low-temperature application circumstances, but the n-type thermoelectric property is relatively low compared to the p-type counterpart and still needs to be improved. Herein, we incorporated different copper selenides (CuSe, Cu3Se2 and Cu2−xSe) into a Bi2Te3 matrix to create the alloy by grinding and successive sintering to enable higher thermoelectric performance. The results demonstrated that all alloys achieved n-type TE characteristics and Bi2Te3-CuSe exhibited the best Seebeck coefficient and power factor among them. Along with the low thermal conductivity, the maximum dimensionless TE figure of merit (ZT) value of 1.64 at 573 K was delivered for Bi2Te3-CuSe alloy, which is among the best reported results in the n-type Bi2Te3-based TE materials to the best of our knowledge. The improved TE properties should be related to the co-doping process of Se and Cu. Our investigation shows a new method to enhance the performance of n-type TE materials by appropriate co-doping or alloying.
1. Introduction
As both the practical need and the worldwide market for wearable and implantable electronics are increasing rapidly [1,2], flexible thermoelectric (FTE) materials, which are fundamental in the manufacturing of such devices, have been receiving particular attention in recent years. To maximize the electro-thermal conversion efficiency and reachability [3], the FTE materials should exhibit satisfactory thermoelectric properties at a relatively low temperature range [4,5]. Among all kinds of TE materials, bismuth telluride-based materials are considered some the most attractive and promising FTE materials for both p-type and n-type TE applications to date [6,7,8,9]. The advantages of bismuth telluride-based materials include its highest ZT value and stable performance around room temperature, ease of preparation and moderate mechanical properties [5,10]. Until now, the ZT values of p-type bismuth telluride-based materials are usually reported higher than those of the n-type counterpart [11,12,13,14,15,16,17,18,19,20]. For instance, the highest ZT value of p-type bismuth telluride-based materials has reached about 1.86 [13], whilst the highest value is merely 1.47 for the n-type counterpart and most of other reported high ZT values are around 1.2 [18,19,20]. For the sake of maximizing the energy conversion efficiency of a practical TE device, the ZT value of the p-type and n-type materials should be equally high and close to each other [21]. Thus, the TE performance of n-type bismuth telluride-based materials still needs to be further improved.
In recent years, nano-structuring and alloying have been shown to be the simple and effective strategies to prompt TE properties [7,22]. For instance, Lou et al. discovered that Na-doping can influence the Fermi level properly, and thereby optimizing the electrical and thermal transport properties of Bi2Te3 [23]. Therefore, the peak ZT value of the doped sample attained a 70% enhancement compared to that of the pristine sample. Zhu et al. also boosted the ZT value of n-type Bi2Te3 to 1.35 by W and Cl co-doping [24]. They revealed that co-doping can optimize the carrier concentration and enlarge van der Waals gap for better scattering of phonons. These reports demonstrated that proper alloying or co-doping of Bi2Te3 can boost TE performance by band tuning or promoting the boundary scattering of phonons. However, it is still unclear how to wisely choose the suitable doping/alloying components to enhance the properties of Bi2Te3, and there is still a relatively large amount of room for a more favorable n-type TE performance by trail-and-error.
Similar to bismuth tellurides, copper selenides are another candidate of promising TE materials. It was revealed that the β-phase of Cu2−xSe exhibits the ideal phonon-glass electron-crystal model, and is also relatively economical and eco-friendly [25,26,27,28]. More importantly, the very high ZT value, up to 2.3~2.7, of Cu2Se-based materials was demonstrated as a characteristic of the p-type TE, due to the optimal carrier concentration and intensive phonon scattering [29,30]. Some researchers have revealed that alloying with Se or some metallic cations can easily affect the conduction band structure, which is effective to tune the carrier concentration and transport property [23,31,32,33,34]. When copper selenides is incorporated into the Bi2Te3 matrix, it might very probably realize a better n-type TE performance.
In this work, we synthesized Bi2Te3-based alloys with copper selenides (CuSe, Cu3Se2 and Cu2−xSe) and studied the effects of adding copper selenides. Our results revealed that three alloys showed a negative Seebeck coefficient characteristic, and the Bi2Te3-CuSe alloy exhibited the highest absolute value, over 200 μV/K around 433 K, among them. Moreover, the electrical conductivity of Bi2Te3-CuSe is higher than the reference Bi2Te3. The thermal conductivity remained relatively low and displayed a decreasing trend with the temperature rising from 303 to 573 K. Therefore, the synergistic effect of three parameters resulted in the highest ZT values, up to 1.64 at 573 K for the Bi2Te3-CuSe alloy, which is remarkable for the n-type TE materials. Our results demonstrated a novel method for enhancing the ZT value of efficient n-type TE materials.
2. Experimental Section
Fabrication of bismuth telluride-copper selenide alloys: Bi2Te3 powders (Macklin, 99.9%) were purchased commercially. The copper selenides with different stoichiometric compositions (CuSe, Cu3Se2 and Cu2−xSe) were prepared by wet chemical methods, which can be seen in our previous article [25]. Bi2Te3 and copper selenides powders were mixed in an agate mortar, and then fully ground for about 20 min. The molar ratio of Bi2Te3 and different copper selenides were all set as 1:0.62. Additionally, samples with different molar ratios of Bi2Te3 versus CuSe (1:1~1:0.2) were also prepared to investigate the best alloyed proportion. The evenly mixed powders were transferred into a cylindrical mold and pressed under 30 MPa pressure which lasted for 10 min. After that, the samples turned into hard disks with diameters of about 1.3 cm and were successively sintered in a tube furnace at 573 K for 2 h under flowing Ar stream.
Characterization of samples: The crystal structures of the samples were measured by X-ray diffractometry (XRD, Ultima IV, Rigaku Corporation, Tokyo, Japan). Further structural and phase identification were examined by transmission electron microscopy and high-resolution transmission electron microscopy (TEM and HRTEM, Talos F200x, FEI, Waltham, MA, USA), and elemental mapping were conducted under TEM characterization.
Seebeck coefficient and electrical conductivity of the samples were both tested by Seebeck coefficient measurement (LSR-3, Linseis, Selb, Germany) at the range between 303 to 573 K, which was conducted in He atmosphere. Thermal conductivity was directly measured by thermal conductivity analyzer (TPS 2500S, Hot Disk, Göteborg, Sweden) at the temperature range of 333 to 573 K.
3. Results and Discussion
The crystal structures of Bi2Te3-copper selenides alloys with/without calcination were characterized using XRD method, where the commercial Bi2Te3 was used as reference. As shown in Figure 1a, one can notice that the untreated Bi2Te3 displays a typical XRD pattern of mineral tellurobismuthite Bi2Te3 (JCPSD Card No. 15-0863), and the blended composite without calcination shows superposed patterns of Bi2Te3 and the corresponding copper selenides. For example, the diffraction peaks at 31.1° and 46.0° of the Bi2Te3-CuSe composite belong to the klockmannite CuSe (JCPDS Card No. 34-0171), and the peaks at 25.0°, 28.7°, etc., of the Bi2Te3-Cu3Se2 composite belong to the umangite Cu3Se2 (JCPDS Card No. 47-1745), and the peak at 26.7° of the Bi2Te3-Cu2−xSe composite belongs to the berzelianite Cu2−xSe (JCPDS Card No. 06-0680). However, the XRD patterns of the alloys with calcination treatment turned out to be very different, as displayed in Figure 1b. Several new peaks appear in the patterns and they are hard to classify. Such peaks possibly reflect the existence of non-stoichiometric compounds composed of Bi, Te, Cu and Se, which should have an impact on their TE properties.
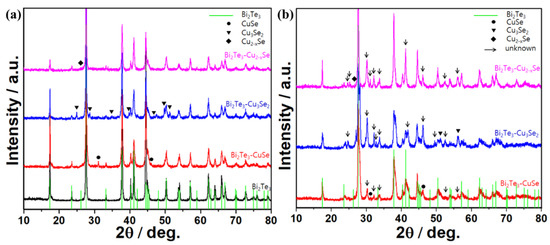
Figure 1.
(a) XRD patterns of pristine Bi2Te3 and the composites without calcination. (b) XRD patterns of Bi2Te3-based alloys with calcination.
The sintered alloys were crushed into powders and then examined with TEM, HR-TEM and elemental mapping characterizations, and the relevant images are shown in Figure 2. All the alloys are irregular fragments with bulks with sizes of hundreds of nanometers, and no specific characteristics can be recognized in TEM images. The interplanar space distances of the alloys estimated from HRTEM images are 0.28 nm, 0.33 nm and 0.29 nm for Bi2Te3-CuSe, Bi2Te3-Cu3Se2 and Bi2Te3-Cu2−xSe2, respectively. Such values are close to the lattice spacing (0.32 nm) of (015) of Bi2Te3, but do not match well with any other typical crystalline data of Bi2Te3 or copper selenides. This may result from the co-doping of Se and Cu which changes the crystalline structure of Bi2Te3. In the elemental mapping images shown in Figure 2g–i, it can be observed that four elements of Bi, Te, Cu and Se are all evenly distributed throughout the alloy bulk, indicating that copper selenides fused well with the Bi2Te3 substrate. Such result can also be seen in the SEM and EDS mapping images which are shown in the Supplementary Materials (Figures S1 and S2).
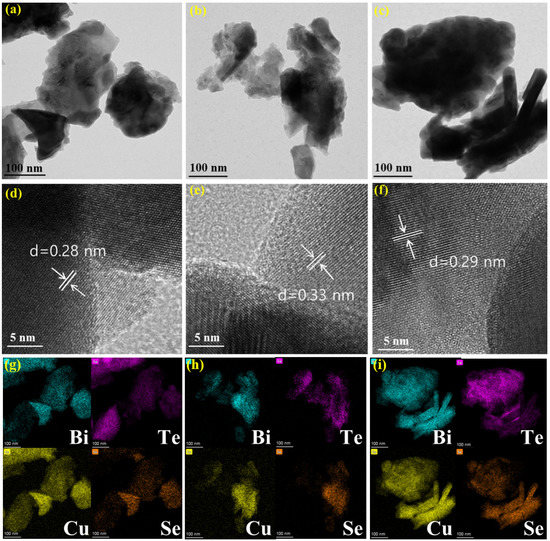
Figure 2.
TEM (a–c), HRTEM (d–f) and corresponding elemental mapping (g–i) images of the Bi2Te3-CuSe, Bi2Te3-Cu3Se2 and Bi2Te3-Cu2−xSe alloys, respectively. Bi, Te, Cu and Se elements are marked by blue, magenta, yellow and orange in (g–i), respectively.
We further comparatively studied the effect of different copper selenide on TE properties of the sintered alloys, which are shown in Figure 3. Figure 3a shows the dependence of their Seebeck coefficients (S) on temperature. It can be seen that commercial Bi2Te3 exhibits the p-type TE characteristic between 303~453 K, but the Seebeck coefficient turns negative at the temperature range of 453~573 K, which is mainly as a result of the sensitivity to particle size, surface defects and manufacturing processes [35,36]. In the cases of the synthesized Bi2Te3-copper selenide alloys, all of them maintain the n-type characteristic in the entire testing temperature range, and Bi2Te3-Cu3Se2 and Bi2Te3-Cu2−xSe alloys have relatively lower absolute S values around 15~80 μV·K−1. Nevertheless, the absolute S value of the Bi2Te3-CuSe alloy first rises at 313~433 K and then slightly decreases over 433 K, resulting in an oblate peak value of 201.8 μV K−1 at about 433 K. The very high S value of Bi2Te3-CuSe is 2~12 times larger than that of other alloys at the same temperature. The absolute S value of Bi2Te3-CuSe is comparable to the best results reported in other n-type Bi2Te3-based TE materials [18,19,20,21,23], which is about 170~240 μV/K at the temperature range of room temperature to 575 K. Some recent reports disclosed that both the doped Se and metal atoms can tune the Fermi level and the band structure of Bi2Te3, which can greatly impact the TE performance [6,23,34]. The measured carrier concentration and calculated effective mass results also indicate the excellent S value of Bi2Te3-CuSe, which is discussed in the Supplementary Materials (Table S1). Thus, it is reasonable that the higher Seebeck coefficient of Bi2Te3-CuSe alloy is speculated to be related to the appropriate doping of Se and Cu into the Bi2Te3 crystallites to form an alloy structure (as shown in Figure 1 and Figure 2).
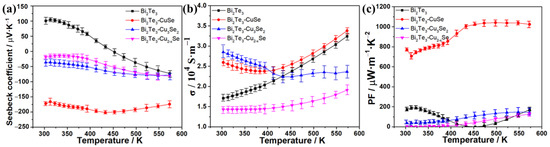
Figure 3.
Temperature dependence of Seebeck coefficient (a), electrical conductivity (b) and power factor (c) of pristine Bi2Te3 and Bi2Te3-copper selenide-based alloys.
The curves of electrical conductivity (σ)-temperature are shown in Figure 3b. Both Bi2Te3-CuSe and Bi2Te3-Cu3Se2 display comparably high electrical conductivity in the range of 303~413 K, but the latter decreases to be lower than Bi2Te3 when the temperature increases over 413 K. Conversely, the Bi2Te3-CuSe alloy maintains the continuously increasing trend after crossing the lowest point at 393 K, which is similar to that of reference Bi2Te3, and the corresponding σ value remains at the highest levels in the 413~573 K. Bi2Te3-Cu2−xSe shows only a sluggish increase with the rise of temperature, and the σ value is less than 2.0 × 104 S m−1.
Furthermore, the power factors (PF) of these alloys and the reference Bi2Te3 were calculated from the equation of PF = S2σ, and the PF–temperature curves are shown in Figure 3c. As expected, the Bi2Te3-CuSe alloy exhibits the strongest power factors because of the excellent Seebeck coefficient and electrical conductivity. In particular, the highest PF value reached over 1000 μW·m−1·K−2 in the temperature range of 433~573 K, which is over six times as large as the other alloys and the pristine Bi2Te3. The PF in the 300~433 K range is slightly lower than the plateau values, mainly resulting from the slightly decreased electrical conductivity. We also fine-tuned the CuSe component in the corresponding alloy materials to further improve the TE properties. Figure 4a shows the Seebeck coefficient results, and the 1:0.62 sample exhibits the highest absolute Seebeck coefficient values throughout the tested temperature range. Figure 4b shows the electrical conductivity result. Overall, when the amount of CuSe increases, the electrical conductivity increases, except that the 1:0.62 sample is slightly higher than the 1:0.5 sample. The calculated PF result is shown in Figure 4c. Thanks to the highest absolute Seebeck coefficient values and the relatively high electrical conductivity, the 1:0.62 sample also demonstrates the highest PF in most of the tested temperature ranges. Furthermore, the thermal conductivity (κ) at different temperatures was determined, and the resulting ZT values are estimated from the equation of ZT = S2σT/κ, which is shown in Figure 5. The Bi2Te3-CuSe and Bi2Te3-Cu3Se2 alloys exhibit similar thermal conductivity to the reference materials Bi2Te3 in the entire temperature range, while the value is shown to be largest for the Bi2Te3-Cu2−xSe alloy. However, all the three alloys exhibit lower lattice thermal conductivity and better phonon scattering than the pristine Bi2Te3, which can be seen in the Supplementary Materials (Figure S3). Therefore, the Bi2Te3-CuSe alloy possesses the highest ZT values in the tested temperature ranges. It is worth noting that, although the ZT value of 0.46 at 323 K is moderate compared to many other reported near-room-temperature n-type Bi2Te3-based TE materials, it can be almost linearly augmented to a remarkable level of 1.64 when the temperature rises to 573 K. In fact, the trend is different from the results reported in other studies in the literature [19,20,21,23,37]. This should be ascribed to the fact that the addition of a certain amount of Se may enlarge the bandgap and tune the carrier concentration, thus moving the peak ZT value to a higher temperature, as explained by Pan et al. [38].
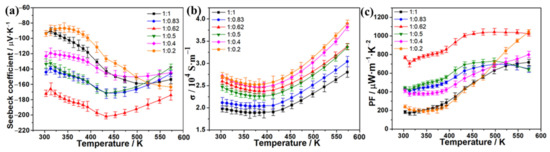
Figure 4.
Temperature dependence of Seebeck coefficient (a), electrical conductivity (b), and power factor (c) of the Bi2Te3-CuSe alloys with different proportions.
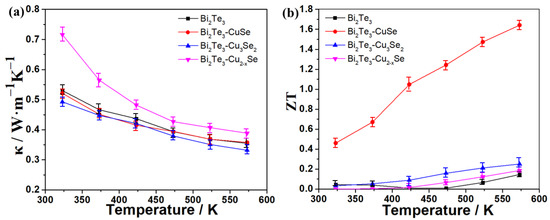
Figure 5.
Temperature dependence of thermal conductivity (a) and ZT value (b) of Bi2Te3 and the alloys.
4. Conclusions
In summary, the Bi2Te3-based alloys with various copper selenides were prepared by grinding and successive sintering for an efficient thermoelectric conversion. It was found that all of the synthesized alloys exhibited the n-type TE characteristic. Among them, Bi2Te3-CuSe attained the highest Seebeck coefficient throughout 303~573 K. Additionally, the increasing electrical conductivity and decreasing thermal conductivity led to its peak ZT value reaching 1.64 at 573 K, and such a result is remarkable in n-type Bi2Te3-based TE materials. The improved TE properties should be related to the alloying structure of Bi2Te3 and CuSe. Our results demonstrated an efficient n-type TE material with a high ZT value with future practical thermoelectric applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238183/s1, Figure S1: SEM and EDS mapping images of the crushed powders of the alloyed samples; Figure S2: Surface SEM and EDS mapping images of the bulks of the alloyed samples; Table S1: Hall carrier concentration (nH) and Hall carrier mobility (μH) of the samples measured at 303 K, and the calculated m* results; Figure S3: the calculated electronic thermal conductivity (кe) and the lattice thermal conductivity (кl) results of the samples.
Author Contributions
L.L.: Conceptualization, Methodology, Investigation, Data Curation and Writing—Original Draft. J.J.: Writing—Review and Editing and Visualization. C.S.: Resources. W.Z.: Validation, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (Grant No. 52073066) and the GDAS Project of Science and Technology Development (No. 2020GDASYL-20200102028, No. 2020GDASYL-20200103130).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Massetti, M.; Jiao, F.; Ferguson, A.J.; Zhao, D.; Wijeratne, K.; Wurger, A.; Blackburn, J.L.; Crispin, X.; Fabiano, S. Unconventional thermoelectric materials for energy harvesting and sensing applications. Chem. Rev. 2021, 121, 12465–12547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Shi, X.; Shi, X.; Chen, L.; Dargusch, M.S.; Zou, J.; Chen, Z. Flexible thermoelectric materials and generators: Challenges and innovations. Adv. Mater. 2019, 31, 1807916. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nie, X.; Sun, C.; Ke, S.; Xu, W.; Zhao, Y.; Zhu, W.; Zhao, W.; Zhang, Q. Realizing High-performance BiSbTe magnetic flexible films via acceleration movement and hopping migration of carriers. Adv. Funct. Mater. 2022, 32, 2111373. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, B.; Ouyang, H.; Fan, Y.; Wang, Z.L.; Li, Z. Emerging implantable energy harvesters and self-powered implantable medical electronics. ACS Nano 2020, 14, 6436–6448. [Google Scholar] [CrossRef]
- Li, C.; Jiang, F.; Liu, C.; Liu, P.; Xu, J. Present and future thermoelectric materials toward wearable energy harvesting. Appl. Mater. Today 2019, 15, 543–557. [Google Scholar] [CrossRef]
- Witting, I.T.; Ricci, F.; Chasapis, T.C.; Hautier, G.; Snyder, G.J. The thermoelectric properties of n-type bismuth telluride: Bismuth selenide alloys Bi2Te3−xSex. Research 2020, 2020, 4361703. [Google Scholar] [CrossRef]
- El-Makaty, F.M.; Ahmed, H.K.; Youssef, K.M. Review: The effect of different nanofiller materials on the thermoelectric behavior of bismuth telluride. Mater. Des. 2021, 209, 109974. [Google Scholar] [CrossRef]
- Cen, J.; Pallikara, I.; Skelton, J.M. Structural dynamics and thermal transport in bismuth chalcogenide alloys. Chem. Mater. 2021, 33, 8404–8417. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ghosh, T.K. Thermoelectric materials for textile applications. Molecules 2021, 26, 3154. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The thermoelectric properties of bismuth telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S.; Kim, Y.; Kim, H.; Park, T.; Kim, S.W. Cu nanoparticle-processed n-type Bi2Te2.7Se0.3 alloys for low-temperature thermoelectric power generation. J. Alloys Compd. 2021, 884, 161060. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; McCuskey, S.R.; Chen, L.; Bazan, G.C.; Liang, Z. Recent advances in n-type thermoelectric nanocomposites. Adv. Electron. Mater. 2019, 5, 1800943. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, L.; Ma, Z.; Song, P.; Zhang, M.; Ma, J.; Yang, F.; Wang, X. Review of current high-ZT thermoelectric materials. J. Mater. Sci. 2020, 55, 12642–12704. [Google Scholar] [CrossRef]
- Zhang, C.; de la Mata, M.; Li, Z.; Belarre, F.J.; Arbiol, J.; Khor, K.A.; Poletti, D.; Zhu, B.; Yan, Q.; Xiong, Q. Enhanced thermoelectric performance of solution-derived bismuth telluride based nanocomposites via liquid-phase sintering. Nano Energy 2016, 30, 630–638. [Google Scholar] [CrossRef]
- Shin, W.H.; Roh, J.W.; Ryu, B.; Chang, H.J.; Kim, H.S.; Lee, S.; Seo, W.S.; Ahn, K. Enhancing thermoelectric performances of bismuth antimony telluride via synergistic combination of multiscale structuring and band alignment by FeTe2 incorporation. ACS Appl. Mater. Interfaces 2018, 10, 3689–3698. [Google Scholar] [CrossRef]
- Yu, Y.; He, D.; Zhang, S.; Cojocaru-Mirédin, O.; Schwarz, T.; Stoffers, A.; Wang, X.; Zheng, S.; Zhu, B.; Scheu, C.; et al. Simultaneous optimization of electrical and thermal transport properties of Bi0.5Sb1.5Te3 thermoelectric alloy by twin boundary engineering. Nano Energy 2017, 37, 203–213. [Google Scholar] [CrossRef]
- Kato, K.; Hatasako, Y.; Uchino, M.; Nakata, Y.; Suzuki, Y.; Hayakawa, T.; Adachi, C.; Miyazaki, K. Flexible porous bismuth telluride thin films with enhanced figure of merit using micro-phase separation of block copolymer. Adv. Mater. Interfaces 2014, 1, 1300015. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Zhu, T.; Fu, C.; He, J.; Ying, P.; Zhao, X. Tuning multiscale microstructures to enhance thermoelectric performance of n-type bismuth-telluride-based solid solutions. Adv. Energy Mater. 2015, 5, 1500411. [Google Scholar] [CrossRef]
- Li, D.; Li, J.M.; Li, J.C.; Wang, Y.S.; Zhang, J.; Qin, X.Y.; Cao, Y.; Li, Y.S.; Tang, G.D. High thermoelectric performance of n-type Bi2Te2.7Se0.3 via nanostructure engineering. J. Mater. Chem. A 2018, 6, 9642–9649. [Google Scholar] [CrossRef]
- Cho, H.; Yun, J.; Back, S.Y.; Lee, J.; Kang, N.; Jang, Y.; Lim, J.; Son, J.; Park, J.; Kim, J.; et al. Superior thermoelectric cooling performance by suppressing bipolar diffusion effect and enhancing anisotropic texture in p-/n-type Bi2Te3 based compounds. J. Alloys Compd. 2021, 888, 161572. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Zou, J. Fundamental and progress of Bi2Te3-based thermoelectric materials. Chin. Phys. B 2018, 27, 048403. [Google Scholar] [CrossRef]
- Lou, L.; Yang, J.; Zhu, Y.; Liang, H.; Zhang, Y.; Feng, J.; He, J.; Ge, Z.; Zhao, L. Tunable Electrical Conductivity and Simultaneously Enhanced Thermoelectric and Mechanical Properties in n-type Bi2Te3. Adv. Sci. 2022, 9, 2203250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, W.; Cui, J.; He, J. Point defect engineering: Co-doping synergy realizing superior performance in n-type Bi2Te3 thermoelectric materials. Small 2021, 17, 2101328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Shi, C.; Zeng, W.; Liao, B.; Zhang, M.; Tao, X. Facile synthesis of copper selenides with different stoichiometric compositions and their thermoelectric performance at a low temperature range. RSC Adv. 2021, 11, 25955. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, L.; Chen, Z.; Zou, J. Promising and eco-friendly Cu2X-based thermoelectric materials: Progress and applications. Adv. Mater. 2020, 32, 1905703. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef]
- Olvera, A.A.; Moroza, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial indium solubility induces chemical stability and colossal thermoelectric figure of merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Li, J.; Bai, H.; Wang, S.; Li, Z.; Tang, H.; Tang, K.; Luo, T.; Yan, Y.; et al. Blocking ion migration stabilizes the high thermoelectric performance in Cu2Se composites. Adv. Mater. 2020, 32, 2003730. [Google Scholar] [CrossRef]
- Nazrul-Islam, S.M.K.; Rahman, M.R.; Ahmed, A.J.; Yun, F.F.; Cortie, D.L.; Wang, X.; Cortie, M.B. Beneficial effect of Na2CO3 additions on the thermoelectric performance of melt-route Cu2Se. Adv. Electro. Mater. 2022, 8, 2100802. [Google Scholar] [CrossRef]
- Zeier, W.G.; Zevalkink, A.; Gibbs, Z.M.; Hautier, G.; Kanatzidis, M.G.; Snyder, G.J. Thinking like a chemist: Intuition in thermoelectric materials. Angew. Chem. Int. Ed. 2016, 55, 6826–6841. [Google Scholar] [CrossRef]
- Adam, A.M.; Diab, A.K.; Ataalla, M.; Alotaibi, M.F.; Alharbi, A.N.; Elsehly, E.M. Optimized thermoelectric performance in thin (Bi2Se3)1−x(Bi2Te3)x alloyed films. J. Alloys Compd. 2022, 898, 162888. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, P.; Zhang, J.; Li, L.; Zhu, W.; Nie, X.; Sang, X.; Zhang, Q.; Zhao, W. Fabrication and excellent performances of bismuth telluride-based thermoelectric devices. ACS Appl. Mater. Interfaces 2022, 14, 12276–12283. [Google Scholar] [CrossRef]
- Ma, R.; Yang, D.; Tian, Z.; Song, H.; Zhang, Y. Effects of Bi2Te3 doping on the thermoelectric properties of Cu2Se alloys. Appl. Phys. A 2022, 128, 531. [Google Scholar] [CrossRef]
- Zhang, C.C.; Fan, X.A.; Hu, J.; Jiang, C.P.; Feng, B.; Xiang, Q.S.; Li, G.Q.; Li, Y.W. The effect of porosity and milling induced defects on the thermoelectric properties of p-Type Bi2Te3-based bulks. Adv. Eng. Mater. 2016, 18, 1777–1784. [Google Scholar] [CrossRef]
- Mølnås, H.; Russ, B.; Farrell, S.L.; Gordon, M.P.; Urban, J.J.; Sahu, A. N-Type doping of a solution processed p-type semiconductor using isoelectronic surface dopants for homojunction fabrication. Appl. Surf. Sci. 2022, 590, 153089. [Google Scholar] [CrossRef]
- Chen, C.; Wang, T.; Yu, Z.; Hutabalian, Y.; Vankayala, R.K.; Chen, C.; Hsieh, W.; Jeng, H.; Wei, D.; Chen, Y. Modulation doping enables ultrahigh power factor and thermoelectric ZT in n-Type Bi2Te2.7Se0.3. Adv. Sci. 2022, 9, 2201353. [Google Scholar] [CrossRef]
- Pan, Y.; Wei, T.R.; Wu, C.F.; Li, J.F. Electrical and thermal transport properties of spark plasma sintered n-type Bi2Te3-xSex alloys: The combined effect of point defect and Se content. J. Mater. Chem. C 2015, 3, 10583. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).