Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes
Abstract
1. Introduction
2. Results and Discussion
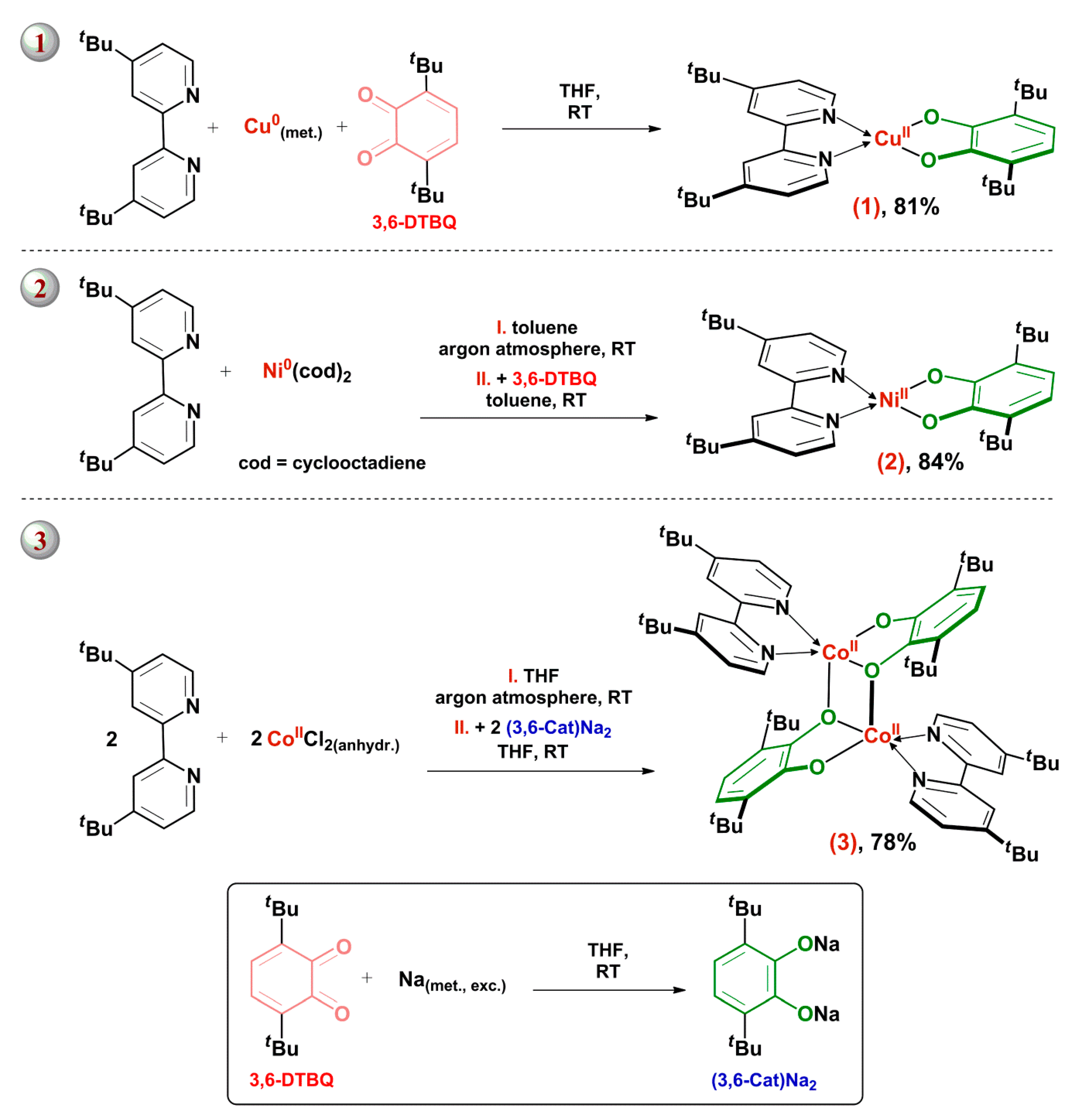
2.1. Syntheses of Complexes 1–3
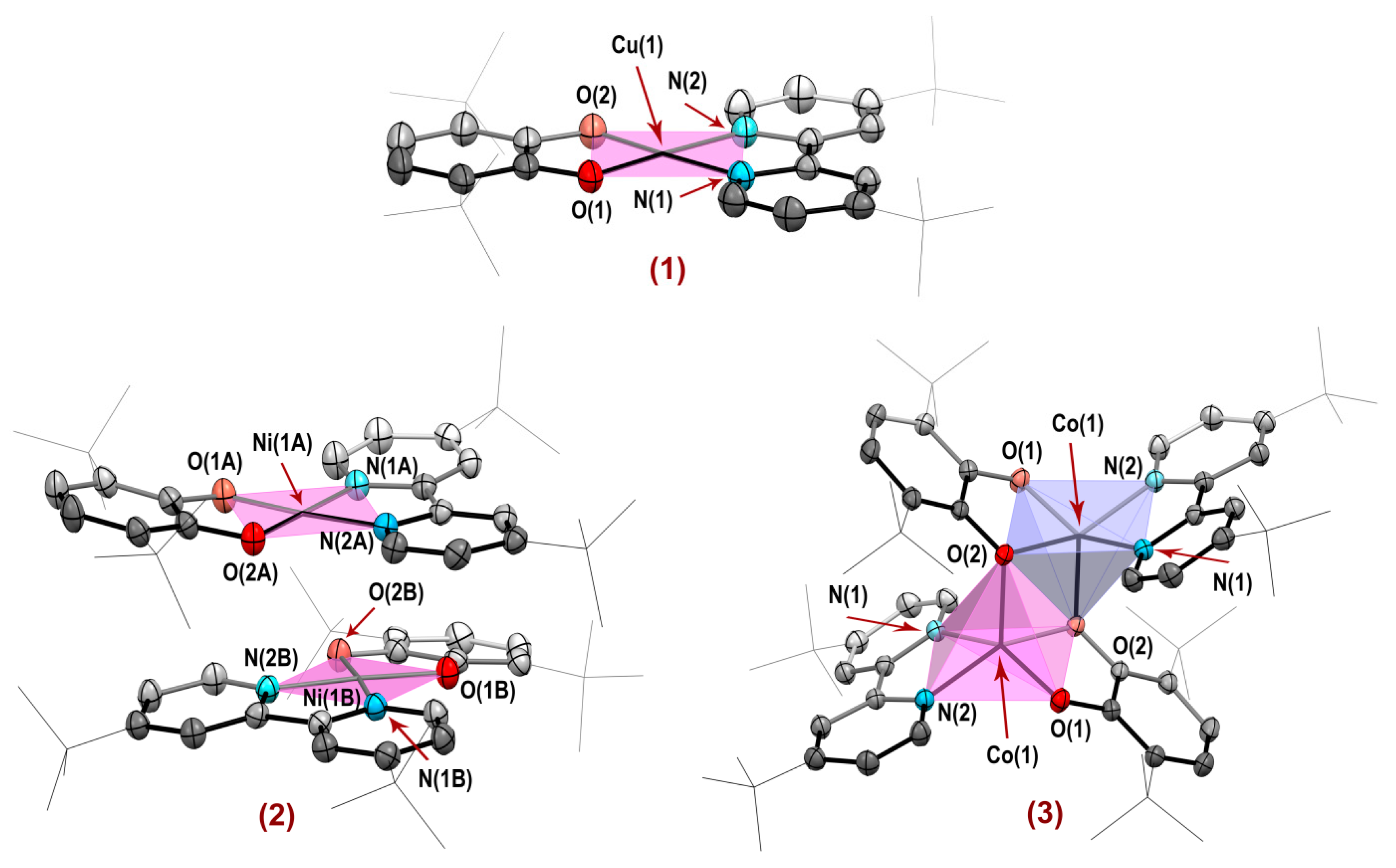
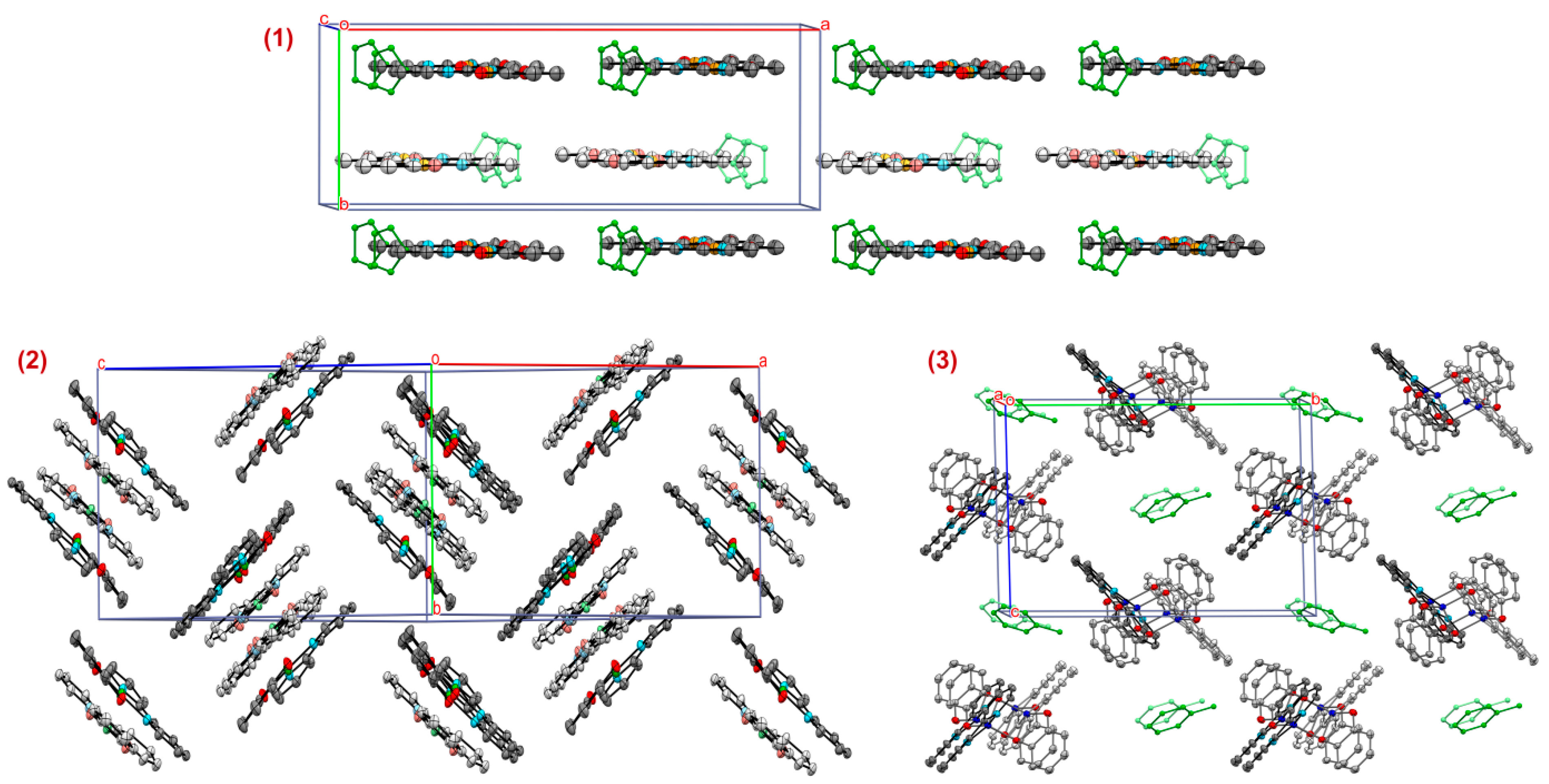
2.2. Molecular Structures of Complexes 1–3
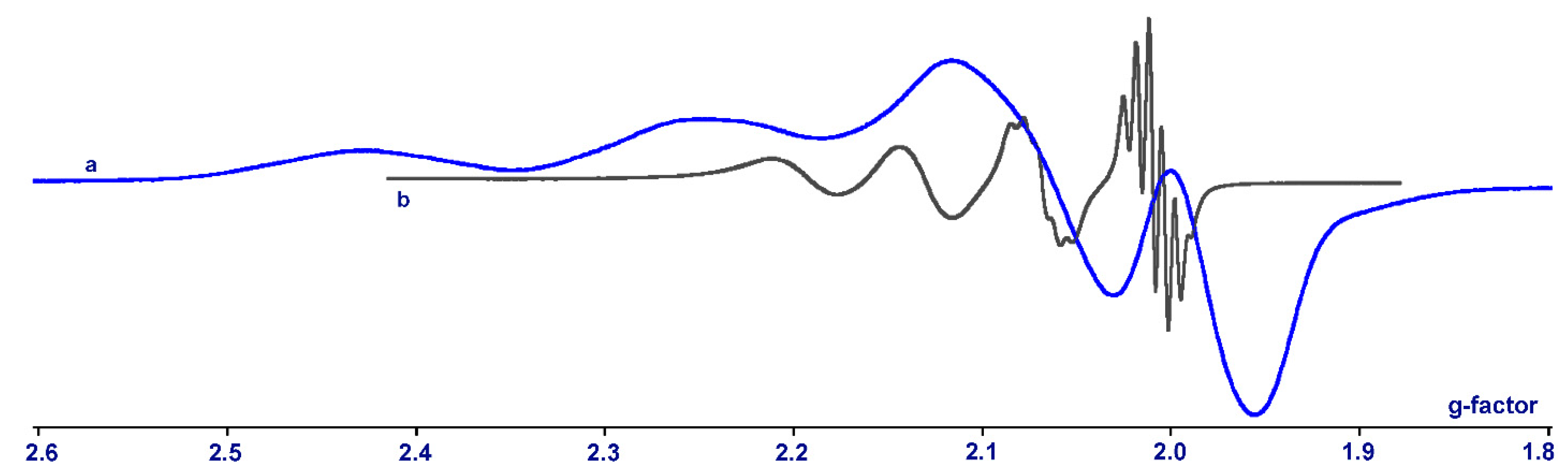
2.3. EPR Investigation for Complex 1
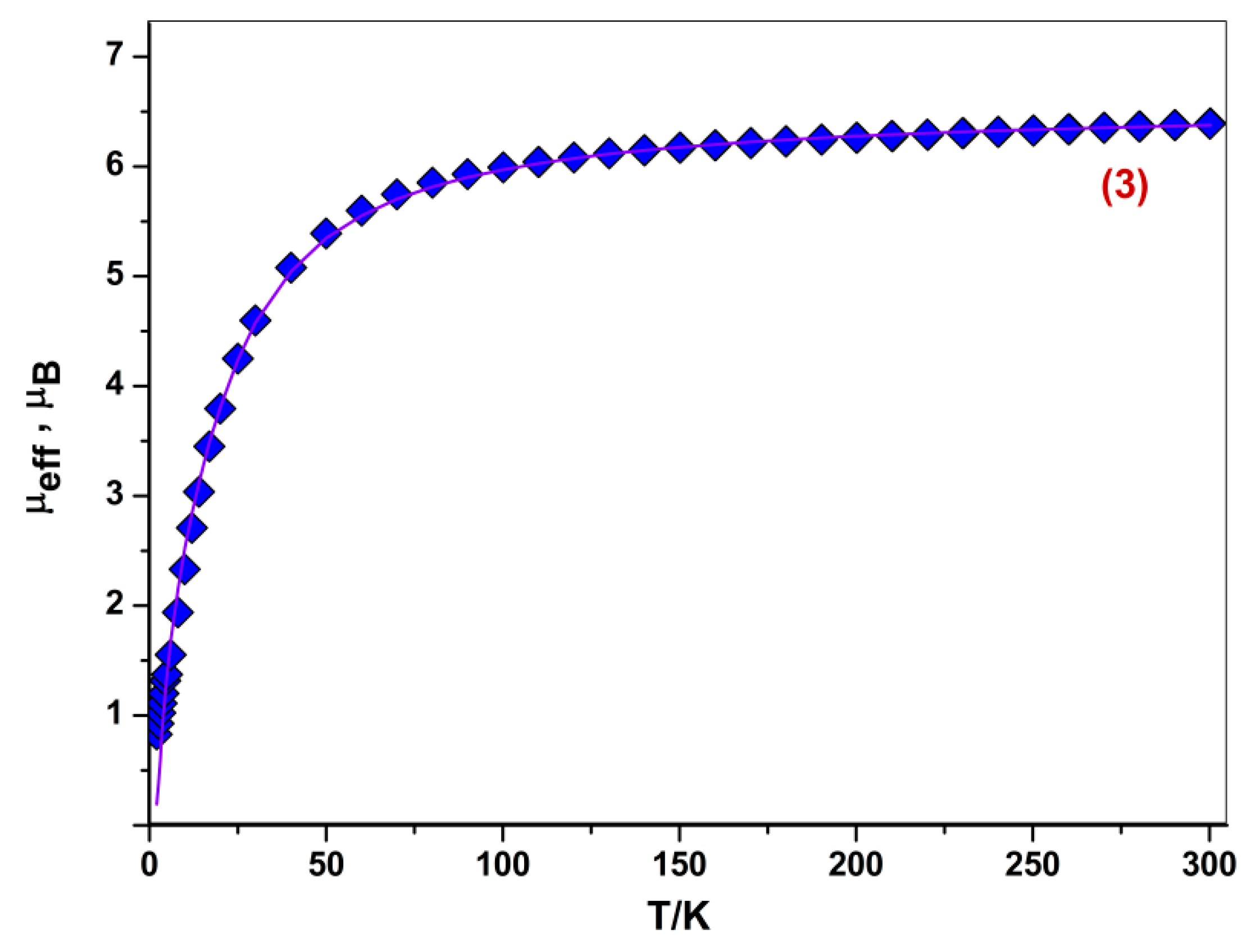
2.4. Magnetochemical Study for Complex 3
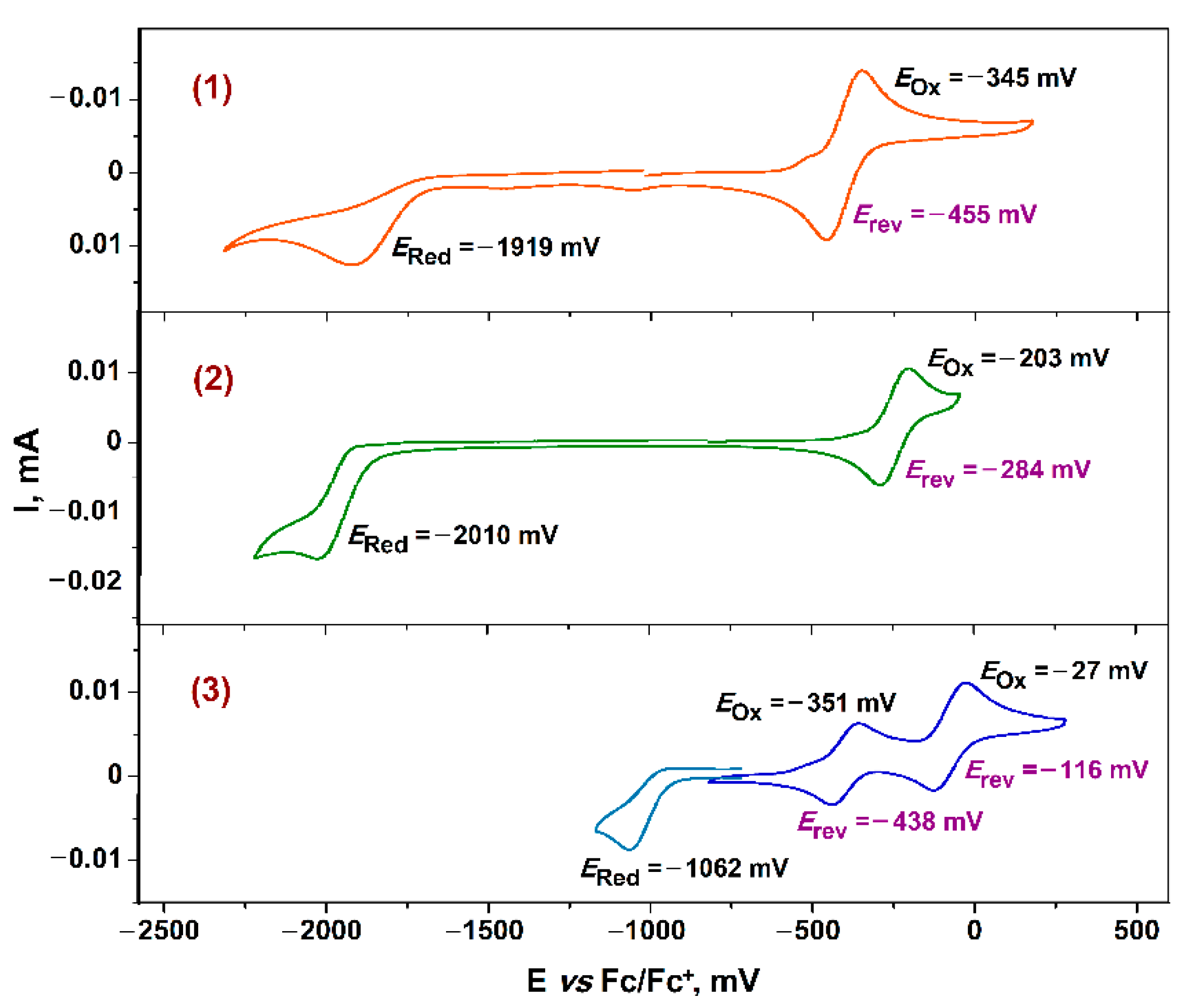
2.5. Electrochemical Study for Complexes 1–3
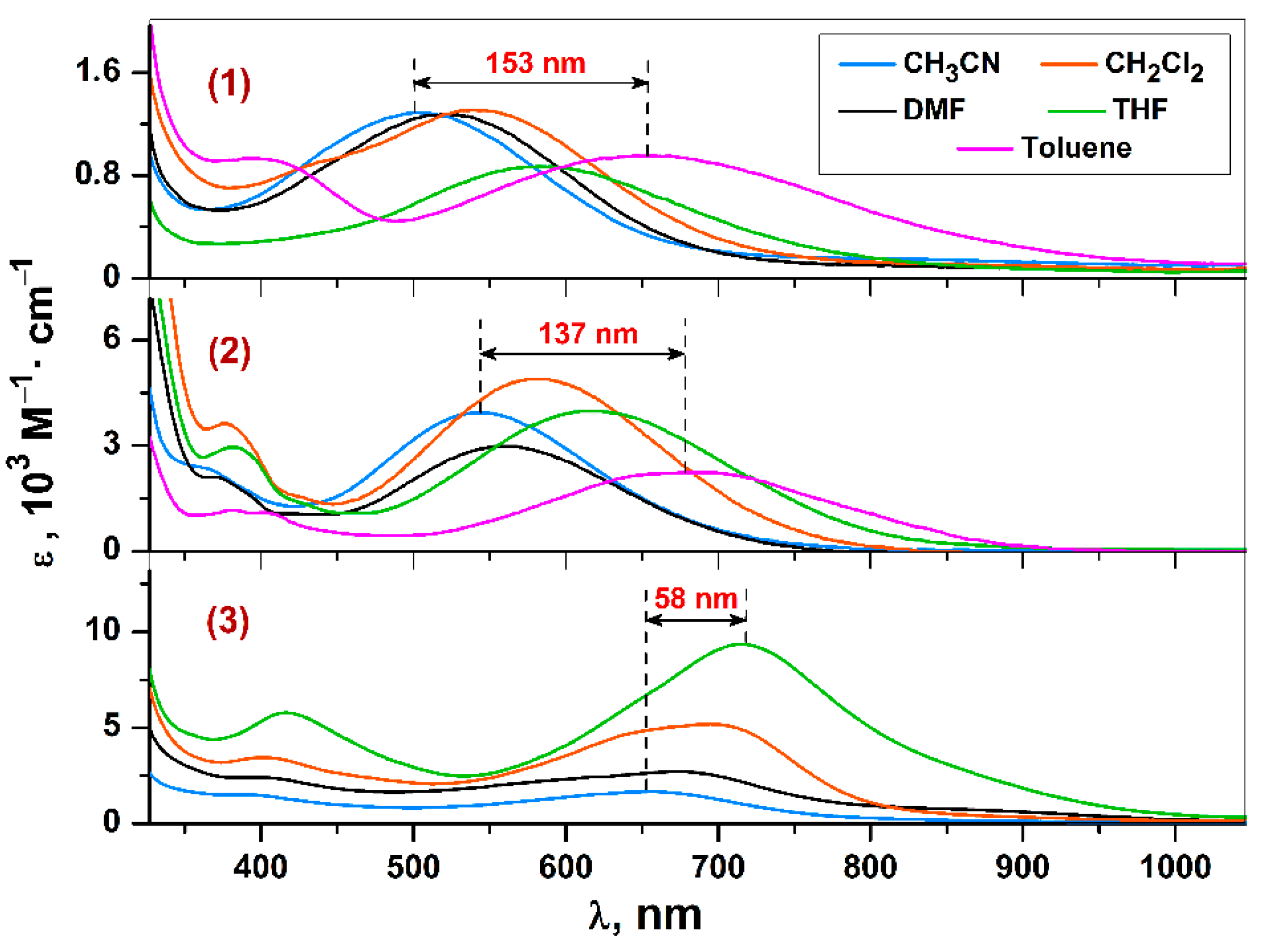
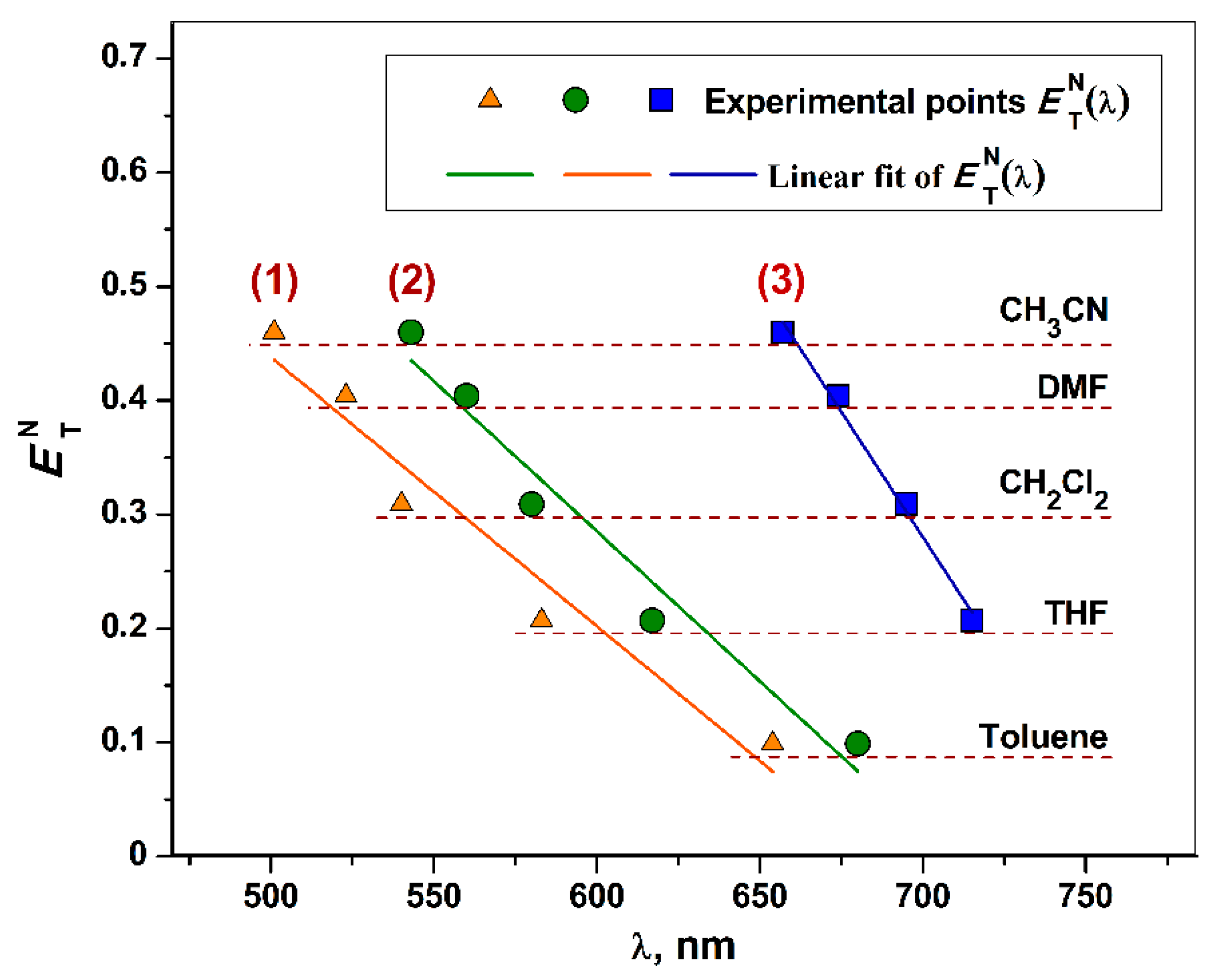
2.6. UV-vis-NIR Spectroscopy for Complexes 1–3
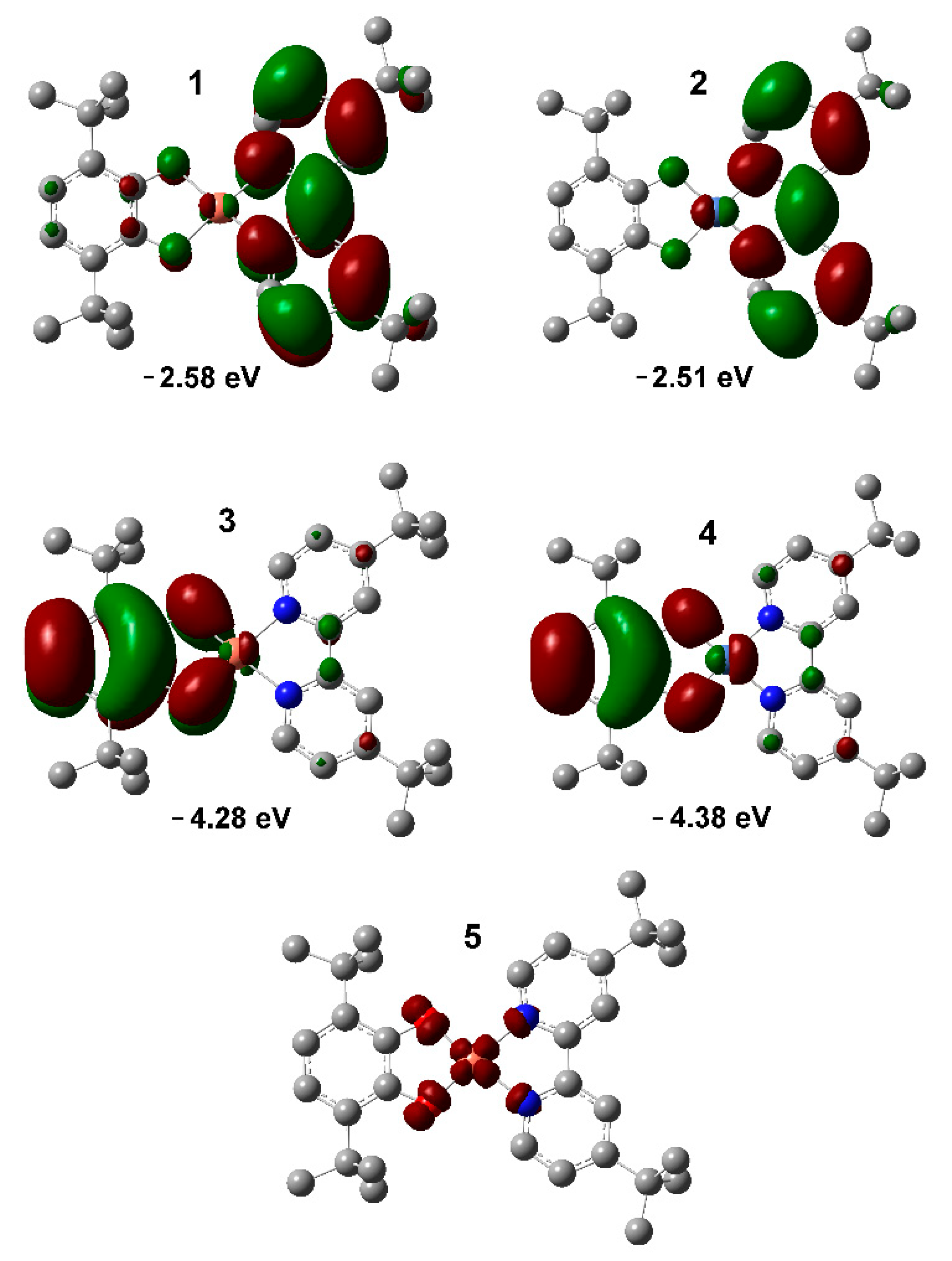
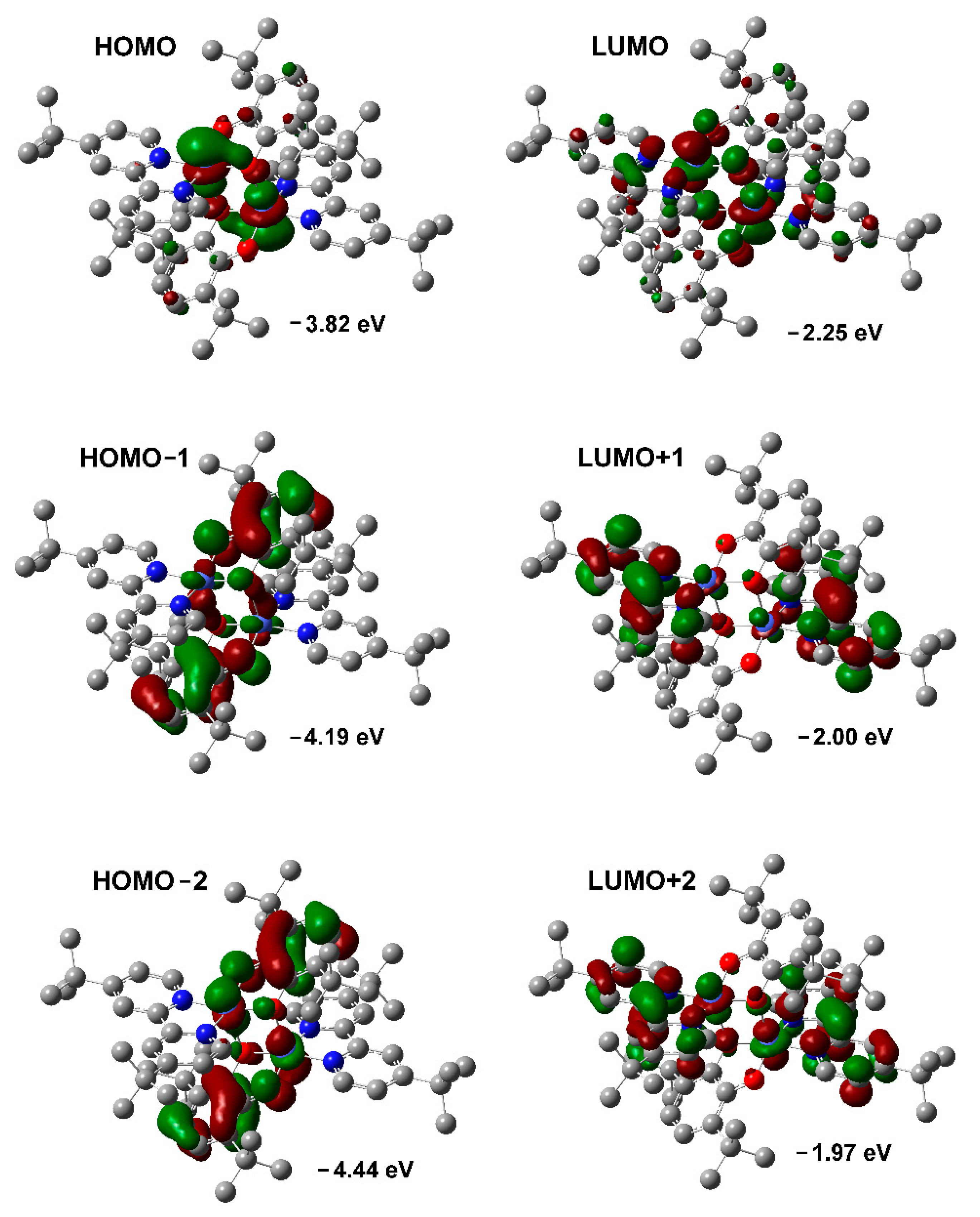
2.7. DFT Calculations
3. Materials and Methods
Syntheses and Characterization of Complexes 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bechtold, T.; Mussak, R. Handbook of Natural Colorants; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Müllen, K. The rylene colorant family—tailored nanoemitters for photonics research and applications. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, L.; Kanaparthi, R.K.; Velkannan, V. Molecular engineering of sensitizers for dye-sensitized solar cell applications. Chem. Rec. 2012, 12, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Besedina, M.; Karushev, M.; Vasil’ev, V.; Timonov, A. Photogalvanic and photovoltaic effects in systems based on metal complexes of Schiff bases. Russ. J. Phys. Chem. A 2016, 90, 1088–1094. [Google Scholar] [CrossRef]
- Sekar, N.; Gehlot, V.Y. Metal complex dyes for dye-sensitized solar cells: Recent developments. Resonance 2010, 15, 819–831. [Google Scholar] [CrossRef]
- Neuthe, K.; Popeney, C.; Bialecka, K.; Hinsch, A.; Sokolowski, A.; Veurmann, W.; Haag, R. Simple NIR complexes and their applicability in dye-sensitized solar cells. Polyhedron 2014, 81, 583–587. [Google Scholar] [CrossRef]
- Shubbak, M.H. Advances in solar photovoltaics: Technology review and patent trends. Renew. Sustain. Energy Rev. 2019, 115, 109383. [Google Scholar] [CrossRef]
- Tyagi, V.; Rahim, N.A.; Rahim, N.; Jeyraj, A.; Selvaraj, L. Progress in solar PV technology: Research and achievement. Renew. Sustain. Energy Rev. 2013, 20, 443–461. [Google Scholar] [CrossRef]
- Archer, S.; Weinstein, J.A. Charge-separated excited states in platinum(II) chromophores: Photophysics, formation, stabilization and utilization in solar energy conversion. Coord. Chem. Rev. 2012, 256, 2530–2561. [Google Scholar] [CrossRef]
- Tahara, K.; Kadowaki, T.; Kikuchi, J.-I.; Ozawa, Y.; Yoshimoto, S.; Abe, M. Synthesis and characterization of a new series of binuclear Pd(II) biscatecholato complexes: Non-innocent ligand-based approach to a wide range of variation in near-infrared absorptions of mixed-valence complexes. Bull. Chem. Soc. Jpn. 2018, 91, 1630–1639. [Google Scholar] [CrossRef]
- Baise, A.; Teucher, I.; Labes, M. Effect of charge-transfer acceptors on dynamic scattering in a nematic liquid crystal. Appl. Phys. Lett. 1972, 21, 142–143. [Google Scholar] [CrossRef]
- Krebs, P.; Sackmann, E.; Schwarz, J. Triplet ESR-spectra of charge-transfer complexes in liquid and single crystals. Chem. Phys. Lett. 1971, 8, 417–420. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Accorsi, G.; Virgili, D.; Bell, Z.R.; Lazarides, T.; Calogero, G.; Armaroli, N.; Ward, M.D. Syntheses and crystal structures of dinuclear complexes containing d-block and f-block luminophores. Sensitization of NIR luminescence from Yb(III), Nd(III), and Er(III) centers by energy transfer from Re(I)− and Pt(II)−bipyrimidine metal centers. Inorg. Chem. 2005, 44, 61–72. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Heinze, K.; Reinhardt, S. Platinum(II) complexes with non-innocent ligands: Solid-phase synthesis, redox chemistry and luminescence. Chem. Eur. J. 2008, 14, 9482–9486. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one-and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Chem. Soc. Rev. 1997, 26, 147–156. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Bange, K.; Gambke, T. Electrochromic materials for optical switching devices. Adv. Mater. 1990, 2, 10–16. [Google Scholar] [CrossRef]
- Monk, P.; Mortimer, R.; Rosseinsky, D. Electrochromism: Fundamentals and Applications, Angewandte Chemie-English Edition; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Granqvist, C.G. Handbook of inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Mortimer, R.J. Organic electrochromic materials. Electrochim. Acta 1999, 44, 2971–2981. [Google Scholar] [CrossRef]
- Granqvist, C. Electrochromism and smart window design. Solid State 1992, 53, 479–489. [Google Scholar] [CrossRef]
- Nejad, M.A.F.; Ranjbar, S.; Parolo, C.; Nguyen, E.P.; Álvarez-Diduk, R.; Hormozi-Nezhad, M.R.; Merkoçi, A. Electrochromism: An emerging and promising approach in (bio)sensing technology. Today 2021, 50, 476–498. [Google Scholar]
- Ward, M.D. Near-infrared electrochromic materials for optical attenuation based on transition-metal coordination complexes. J. Solid State Electrochem. 2005, 9, 778–787. [Google Scholar] [CrossRef]
- Sobottka, S.; Nößler, M.; Ostericher, A.L.; Hermann, G.; Subat, N.Z.; Beerhues, J.; Behr-van der Meer, M.; Suntrup, L.; Albold, U.; Hohloch, S. Tuning PtII-based donor–acceptor systems through ligand design: Effects on frontier orbitals, redox potentials, UV/Vis/NIR absorptions, electrochromism, and photocatalysis. Chemistry 2020, 26, 1314. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Pashanova, K.I.; Poddel′sky, A.I.; Piskunov, A.V. Complexes of “late” transition metals of the 3d row based on functionalized o-iminobenzoquinone type ligands: Interrelation of molecular and electronic structure, magnetic behaviour. Coord. Chem. Rev. 2022, 459, 214399. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Cherkasov, V.K.; Abakumov, G.A. Transition metal complexes with bulky 4,6-di-tert-butyl-N-aryl(alkyl)-o-iminobenzoquinonato ligands: Structure, EPR and magnetism. Coord. Chem. Rev. 2009, 253, 291–324. [Google Scholar] [CrossRef]
- Pashanova, K.I.; Bitkina, V.O.; Yakushev, I.A.; Arsenyev, M.V.; Piskunov, A.V. Square-planar heteroleptic complexes of α-diimine-NiII-catecholate type: Intramolecular ligand-to-ligand charge transfer. Molecules 2021, 26, 4622. [Google Scholar] [CrossRef] [PubMed]
- Atallah, H.; Taliaferro, C.M.; Wells, K.A.; Castellano, F.N. Photophysics and ultrafast processes in rhenium(I) diimine dicarbonyls. Dalton Trans. 2020, 49, 11565–11576. [Google Scholar] [CrossRef] [PubMed]
- García-Cañadas, J.; Meacham, A.P.; Peter, L.M.; Ward, M.D. A Near-infrared electrochromic window based on an Sb-doped SnO2 electrode modified with a Ru–dioxolene complex. Angew. Chem. 2003, 115, 3119–3122. [Google Scholar] [CrossRef]
- Tsai, M.-K.; Rochford, J.; Polyansky, D.E.; Wada, T.; Tanaka, K.; Fujita, E.; Muckerman, J.T. Characterization of redox states of Ru(OH2)(Q)(tpy)2+ (Q = 3,5-di-tert-butyl-1,2-benzoquinone, tpy = 2, 2′: 6′, 2″-terpyridine) and related species through experimental and theoretical studies. Inorg. Chem. 2009, 48, 4372–4383. [Google Scholar] [CrossRef] [PubMed]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper complexes with tetradentate ligands for enhanced charge transport in dye-sensitized solar cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. In Renewable Energy; Routledge: London, UK, 2018; pp. 208–213. [Google Scholar]
- Miao, Q.; Gao, J.; Wang, Z.; Yu, H.; Luo, Y.; Ma, T. Syntheses and characterization of several nickel bis(dithiolene) complexes with strong and broad near-IR absorption. Inorg. Chim. Acta 2011, 376, 619–627. [Google Scholar] [CrossRef]
- Linfoot, C.L.; Richardson, P.; McCall, K.L.; Durrant, J.R.; Morandeira, A.; Robertson, N. A nickel-complex sensitiser for dye-sensitised solar cells. Sol. Energy 2011, 85, 1195–1203. [Google Scholar] [CrossRef]
- Cameron, L.A.; Ziller, J.W.; Heyduk, A.F. Near-IR absorbing donor–acceptor ligand-to-ligand charge-transfer complexes of nickel(II). Chem. Sci. 2016, 7, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Kramer, W.W.; Cameron, L.A.; Zarkesh, R.A.; Ziller, J.W.; Heyduk, A.F. Donor–acceptor ligand-to-ligand charge-transfer coordination complexes of nickel(II). Inorg. Chem. 2014, 53, 8825–8837. [Google Scholar] [CrossRef] [PubMed]
- Bubnov, M.P.; Teplova, I.A.; Druzhkov, N.O.; Fukin, G.K.; Cherkasova, A.V.; Cherkasov, V.K. Catecholato complexes of cobalt and nickel with 1,4-disubstituted-1,4-diazabutadiens-1,3 and 1,2-bis(diphenylphosphino) ethane. J. Chem. Sci. 2015, 127, 527–535. [Google Scholar] [CrossRef]
- Brown, D.G.; Reinprecht, J.T.; Vogel, G.C. Synthesis and characterization of copper(II) catecholato complexes. Inorg. Nucl. Chem. Lett. 1976, 12, 399–404. [Google Scholar] [CrossRef]
- Brown, D.G.; Hughes, W.J. Copper(II) complexes containing 3-n-nonylcatechol: Preparation and ligand oxidation. Z. Für Nat. B 1979, 34, 1408–1412. [Google Scholar] [CrossRef]
- Brown, D.G.; Hughes, W.J.; Knerr, G. Electronic and X-ray photoelectron spectra of copper catecholate complexes. Inorg. Chim. Acta 1980, 46, 123–126. [Google Scholar] [CrossRef]
- Buchanan, R.M.; Wilson-Blumenberg, C.; Trapp, C.; Larsen, S.K.; Greene, D.L.; Pierpont, C.G. Counter ligand dependence of charge distribution in copper-quinone complexes. Structural and magnetic properties of (3, 5-di-tert-butylcatecholato)(bipyridine)copper(II). Inorg. Chem. 1986, 25, 3070–3076. [Google Scholar] [CrossRef]
- Poddel’sky, A.; Smolyaninov, I.; Skatova, A.; Lukoyanov, A.; Fukin, G.; Berberova, N.; Cherkasov, V.; Abakumov, G. Diradical bis-o-iminosemiquinonato zinc complex: Spectroscopy, magneto-and electrochemistry. Z. Für Anorg. Und Allg. Chem. 2008, 634, 1154–1160. [Google Scholar] [CrossRef]
- Spiro, T.G. Zinc Enzymes. Curr. Opin. Chem. Biol. 1983, 2, 222–234. [Google Scholar]
- Alsfasser, R.; Powell, A.K.; Vahrenkamp, H. Functional tetrahedral zinc complexes. Angew. Chem. Int. Ed. Engl. 1990, 29, 898–899. [Google Scholar] [CrossRef]
- Brock, S.L.; Kauzlarich, S.M. A2Zn3As2O2 (A = Ba, Sr): A rare example of square planar zinc. Inorg. Chem. 1994, 33, 2491–2492. [Google Scholar] [CrossRef]
- Rakshit, R.; Mukherjee, C. Secondary interactions versus intramolecular π–π interactions in CuII–diradical complexes. Eur. J. Inorg. Chem. 2016, 2016, 2731–2737. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Bubnov, M.P.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. New nitrosyl bis-o-iminobenzosemiquinonato complexes of M(ISQ)2(NO) type. Z. Für Anorg. Und Allg. Chem. 2008, 634, 1205–1209. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Cherkasov, V.K.; Fukin, G.K.; Bubnov, M.P.; Abakumova, L.G.; Abakumov, G.A. New four-and five-coordinated complexes of cobalt with sterically hindered o-iminobenzoquinone ligands: Synthesis and structure. Inorg. Chim. Acta 2004, 357, 3632–3640. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Pashanova, K.I.; Bogomyakov, A.S.; Smolyaninov, I.V.; Starikov, A.G.; Fukin, G.K. Cobalt complexes with hemilabile o-iminobenzoquinonate ligands: A novel example of redox-induced electron transfer. Dalton Trans. 2018, 47, 15049–15060. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.C.; Ghorai, S.; Mukherjee, C. Monoradical-containing four-coordinate Co(III) complexes: Homolytic S–S and Se–Se bond cleavage and catalytic isocyanate to urea conversion under sunlight. Chem. Commun. 2017, 53, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Piskunov, A.; Pashanova, K.; Ershova, I.; Bogomyakov, A.; Starikov, A.; Cherkasov, A. Synthesis of four-, five-, and six-coordinate cobalt(III) bis-o-iminobenzosemiquinone complexes. Russ. Chem. Bull. 2019, 68, 757–769. [Google Scholar] [CrossRef]
- Bill, E.; Bothe, E.; Chaudhuri, P.; Chlopek, K.; Herebian, D.; Kokatam, S.; Ray, K.; Weyhermüller, T.; Neese, F.; Wieghardt, K. Molecular and electronic structure of four-and five-coordinate cobalt complexes containing two o-phenylenediamine- or two o-aminophenol-type ligands at various oxidation levels: An experimental, density functional, and correlated ab initio study. Chem. Eur. J. 2005, 11, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Ershova, I.V.; Smolyaninov, I.V.; Bogomyakov, A.S.; Fedin, M.V.; Starikov, A.G.; Cherkasov, A.V.; Fukin, G.K.; Piskunov, A.V. Tetrahedral nickel(II) and cobalt(II) bis-o-iminobenzosemiquinonates. Dalton Trans. 2019, 48, 10723–10732. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Sazanovich, I.V.; Adams, H.; Bennett, R.D.; Davies, E.S.; Meijer, A.J.; Towrie, M.; Tikhomirov, S.A.; Bouganov, O.V.; Ward, M.D. Structure and ultrafast dynamics of the charge-transfer excited state and redox activity of the ground state of mono-and binuclear platinum(II) diimine catecholate and bis-catecholate complexes: A transient absorption, TRIR, DFT, and electrochemical study. Inorg. Chem. 2010, 49, 10041–10056. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.D.; Eisenberg, R. Tuning the excited-state properties of platinum(II) diimine dithiolate complexes. J. Am. Chem. Soc. 1996, 118, 1949–1960. [Google Scholar] [CrossRef]
- Scattergood, P.A.; Jesus, P.; Adams, H.; Delor, M.; Sazanovich, I.V.; Burrows, H.D.; Serpa, C.; Weinstein, J.A. Exploring excited states of Pt(II) diimine catecholates for photoinduced charge separation. Dalton Trans. 2015, 44, 11705–11716. [Google Scholar] [CrossRef] [PubMed]
- Shavaleev, N.M.; Davies, E.S.; Adams, H.; Best, J.; Weinstein, J.A. Platinum(II) diimine complexes with catecholate ligands bearing imide electron-acceptor groups: Synthesis, crystal structures, (spectro)electrochemical and EPR studies, and electronic structure. Inorg. Chem. 2008, 47, 1532–1547. [Google Scholar] [CrossRef]
- Weinstein, J.A.; Tierney, M.T.; Davies, E.S.; Base, K.; Robeiro, A.A.; Grinstaff, M.W. Probing the electronic structure of platinum(II) chromophores: Crystal structures, NMR structures, and photophysical properties of six new bis- and di-phenolate/thiolate Pt(II) diimine chromophores. Inorg. Chem. 2006, 45, 4544–4555. [Google Scholar] [CrossRef]
- Tichnell, C.R.; Daley, D.R.; Stein, B.W.; Shultz, D.A.; Kirk, M.L.; Danilov, E.O. Wave function control of charge-separated excited-state lifetimes. J. Am. Chem. Soc. 2019, 141, 3986–3992. [Google Scholar] [CrossRef]
- Yang, J.; Kersi, D.K.; Giles, L.J.; Stein, B.W.; Feng, C.; Tichnell, C.R.; Shultz, D.A.; Kirk, M.L. Ligand control of donor–acceptor excited-state lifetimes. Inorg. Chem. 2014, 53, 4791–4793. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Matsumoto, T.; Chang, H.C. Impact of group 10 metals on the solvent-induced disproportionation of o-semiquinonato complexes. Chem. Eur. J. 2019, 25, 8268–8278. [Google Scholar] [CrossRef] [PubMed]
- BaniKhaled, M.O.; Becker, J.D.; Koppang, M.; Sun, H. Perfluoroalkylation of square-planar transition metal complexes: A strategy to assemble them into solid state materials with a π–π stacked lamellar structure. Cryst. Growth Des. 2016, 16, 1869–1878. [Google Scholar] [CrossRef]
- Sun, X.; Chun, H.; Hildenbrand, K.; Bothe, E.; Weyhermüller, T.; Neese, F.; Wieghardt, K. o-Iminobenzosemiquinonato(1−) and o-amidophenolato(2−) complexes of palladium(II) and platinum(II): A combined experimental and density functional theoretical study. Inorg. Chem. 2002, 41, 4295–4303. [Google Scholar] [CrossRef]
- Deibel, N.; Schweinfurth, D.; Fiedler, J.; Záliš, S.; Sarkar, B. Isomeric separation in donor–acceptor systems of Pd(II) and Pt(II) and a combined structural, electrochemical and spectroelectrochemical study. Dalton Trans. 2011, 40, 9925–9934. [Google Scholar] [CrossRef]
- Tahara, K.; Ashihara, Y.; Higashino, T.; Ozawa, Y.; Kadoya, T.; Sugimoto, K.; Ueda, A.; Mori, H.; Abe, M. New π-extended catecholato complexes of Pt(II) and Pd(II) containing a benzothienobenzothiophene (BTBT) moiety: Synthesis, electrochemical behavior and charge transfer properties. Dalton Trans. 2019, 48, 7367–7377. [Google Scholar] [CrossRef]
- Ghosh, P.; Begum, A.; Herebian, D.; Bothe, E.; Hildenbrand, K.; Weyhermüller, T.; Wieghardt, K. Coordinated o-dithio-and o-iminothiobenzosemiquinonate(1−) π radicals in [MII(bpy)(L.)](PF6) complexes. Angew. Chem. Int. Ed. 2003, 42, 563–567. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Liu, B.; Su, J.-H.; Fedushkin, I.L.; Wu, B.; Yang, X.-J. Noninnocent ligands: Heteroleptic nickel complexes with α-diimine and 1,2-diketone derivatives. Dalton Trans. 2017, 46, 7857–7865. [Google Scholar] [CrossRef]
- Rauth, G.K.; Pal, S.; Das, D.; Sinha, C.; Slawin, A.M.; Woollins, J.D. Synthesis, spectral characterization and electrochemical studies of mixed-ligand complexes of platinum(II) with 2-(arylazo)pyridines and catechols. Single-crystal X-ray structure of dichloro{2-(phenylazo) pyridine}platinum(II). Polyhedron 2001, 20, 363–372. [Google Scholar] [CrossRef]
- Roy, R.; Chattopadhyay, P.; Sinha, C.; Chattopadhyay, S. Synthesis, spectral and electrochemical studies of arylazopyridine complexes of palladium(II) with dioxolenes. Polyhedron 1996, 15, 3361–3369. [Google Scholar] [CrossRef]
- Weinstein, J.; Zheligovskaya, N.; Mel’nikov, M. Spectroscopic (UV/VIS, resonance Raman) and spectroelectrochemical study of platinum(II) complexes with 2,2′-bipyridine and aromatic thiolate ligands. J. Chem. Soc. Dalton Trans. 1998, 15, 2459–2466. [Google Scholar] [CrossRef]
- Liu, W.; Heinze, K. Rhenium(I) and platinum(II) complexes with diimine ligands bearing acidic phenol substituents: Hydrogen-bonding, acid–base chemistry and optical properties. Dalton Trans. 2010, 39, 9554–9564. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.L.; Shultz, D.A.; Chen, J.; Hewitt, P.; Daley, D.; Paudel, S.; van Der Est, A. Metal ion control of photoinduced electron spin polarization in electronic ground states. J. Am. Chem. Soc. 2021, 143, 10519–10523. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.W.; Tichnell, C.R.; Chen, J.; Shultz, D.A.; Kirk, M.L. Excited state magnetic exchange interactions enable large spin polarization effects. J. Am. Chem. Soc. 2018, 140, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.; Ng, Y.; Kulkarni, S.; Muduli, S.; Xu, K.; Ganguly, R.; Lu, Y.; Hirao, H.; Soo, H. Development of bis(arylimino)acenaphthene (BIAN) copper complexes as visible light harvesters for potential photovoltaic applications. Inorg. Chem. Front. 2016, 3, 651–662. [Google Scholar] [CrossRef]
- Barnham, K.J.; Djuran, M.I.; Frey, U.; Mazid, M.A.; Sadler, P.J. [Pd(CBDCA-O,O′)(NH3)2]: The PdII analogue of a platinum anticancer drug (CBDCA= cyclobutane-1,1-dicarboxylate). J. Chem. Soc. Chem. Commun. 1994, 65–66. [Google Scholar] [CrossRef]
- Beagley, B.; Cruickshank, D.; McAuliffe, C.; Pritchard, R.; Zaki, A.; Beddoes, R.; Cernik, R.; Mills, O. The crystal and molecular structure of cis-diammine-1,1-cyclobutanedicarboxoplatinum(II) [cis-Pt(NH3)2CBDCA]. Dynamic puckering of the cyclobutane ring. J. Mol. Struct. 1985, 130, 97–102. [Google Scholar] [CrossRef]
- Neidle, S.; Ismail, I.M.; Sadler, P.J. The structure of the antitumor complex cis-(diammino)(1,1-cyclobutanedicarboxylato)-Pt(II): X ray and NMR studies. J. Inorg. Biochem. 1980, 13, 205–212. [Google Scholar] [CrossRef]
- Mansuri-Torshizi, H.; Ghadimy, S.; Akbarzadeh, N. Synthesis, characterization, DNA binding and cytotoxic studies of platinum(II) and palladium(II) complexes of the 2, 2′-bipyridine and an anion of 1,1-cyclobutanedicarboxylic acid. Chem. Pharm. Bull. 2001, 49, 1517–1520. [Google Scholar] [CrossRef]
- Jin, V.X.; Ranford, J.D. Complexes of platinum (II) or palladium (II) with 1, 10-phenanthroline and amino acids. Inorg. Chim. Acta 2000, 304, 38–44. [Google Scholar] [CrossRef]
- Nobuo, O.; Yu, M.; Mamiko, O. (Benzene-1,2-diolato-κ2O,O′)(di-2-pyridylamine-κ2N,N′)palladium(II) hemihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m2778–m2780. [Google Scholar] [CrossRef]
- Okabe, N.; Aziyama, T.; Odoko, M. (Benzene-1,2-diolato-κ2O,O′)(2,2′-bipyridine-κ2N,N′)palladium(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m1943–m1945. [Google Scholar] [CrossRef]
- Okabe, N.; Aziyama, T.; Odoko, M. (1,2-Benzenediolato-κ2O,O′)(2,2′-biquinoline-κ2N,N′)palladium(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m2154–m2156. [Google Scholar] [CrossRef]
- Okabe, N.; Hagihara, K.; Odoko, M.; Muranishi, Y. (2,2′-Bipyridine-κ2N,N′)(2,3-naphthalenediolato-κ2O,O′)palladium(II) and (2,2′-biquinoline-κ2N,N′)(2,3-naphthalenediolato-κ2O,O′)palladium(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, m150–m152. [Google Scholar] [CrossRef]
- Okabe, N.; Muranishi, Y.; Aziyama, T. (1,2-Benzenediolato-κ2O,O′)(1,10-phenanthroline-κ2N,N′)palladium(II) dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m936–m938. [Google Scholar] [CrossRef]
- Imer, A.G.; Syan, R.H.B.; Gülcan, M.; Ocak, Y.S.; Tombak, A. The novel pyridine based symmetrical Schiff base ligand and its transition metal complexes: Synthesis, spectral definitions and application in dye sensitized solar cells (DSSCs). J. Mater. Sci. Mater. Electron. 2018, 29, 898–905. [Google Scholar] [CrossRef]
- Islam, A.; Sugihara, H.; Hara, K.; Singh, L.P.; Katoh, R.; Yanagida, M.; Takahashi, Y.; Murata, S.; Arakawa, H. New platinum(II) polypyridyl photosensitizers for TiO2 solar cells. New J. Chem. 2000, 24, 343–345. [Google Scholar] [CrossRef]
- Gauthier, S.; Caro, B.; Robin-Le Guen, F.; Bhuvanesh, N.; Gladysz, J.A.; Wojcik, L.; Le Poul, N.; Planchat, A.; Pellegrin, Y.; Blart, E. Synthesis, photovoltaic performances and TD-DFT modeling of push–pull diacetylide platinum complexes in TiO2 based dye-sensitized solar cells. Dalton Trans. 2014, 43, 11233–11242. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, N.; Ho, C.-L.; Fu, Y.; Lau, W.-S.; Xie, Z.; Wang, L.; Wong, W.-Y. Synthesis, characterization, photophysical and photovoltaic properties of new donor–acceptor platinum(II) acetylide complexes. J. Organomet. Chem. 2016, 812, 2–12. [Google Scholar] [CrossRef]
- Brasse, M.; Campora, J.; Davies, M.; Teuma, E.; Palma, P.; Alvarez, E.; Sanz, E.; Reyes, M.L. Integrating catalyst and co-catalyst design in olefin polymerization catalysis: Transferable dianionic ligands for the activation of late transition metal polymerization catalysts. Adv. Synth. Catal. 2007, 349, 2111–2120. [Google Scholar] [CrossRef]
- Abakumov, G.; Cherkasov, V.; Lobanov, A.; Razuvaev, G. Oxidation of metallic copper and silver by orthoquinones in organic solvents. Bull. Acad. Sci. USSR Div. Chem. Sci. 1984, 33, 1478–1485. [Google Scholar] [CrossRef]
- Thompson, J.S.; Calabrese, J.C. Copper-catechol chemistry. Synthesis, spectroscopy, and structure of bis(3,5-di-tert-butyl-o-semiquinato)copper (II). J. Am. Chem. Soc. 1986, 108, 1903–1907. [Google Scholar] [CrossRef]
- Buchanan, R.M.; Fitzgerald, B.J.; Pierpont, C.G. Semiquinone radical anion coordination to divalent cobalt and nickel. Structural features of the bis (3,5-di-tert-butyl-1,2-semiquinone)cobalt(II) tetramer. Inorg. Chem. 1979, 18, 3439–3444. [Google Scholar] [CrossRef]
- Abakumov, G.; Cherkasov, V.; Piskunov, A.; Meshcheryakova, I.; Maleeva, A.; Poddel’skii, A.; Fukin, G. Zinc molecular complexes with sterically hindered o-quinone and o-iminoquinone. Dokl. Chem. 2009, 427, 168–171. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Lynch, M.W.; Buchanan, R.M.; Pierpont, C.G.; Hendrickson, D.N. Weak magnetic exchange interactions between paramagnetic metal ions and coordinated o-semiquinones in M(9, 10-phenanthrenesemiquinone)2(pyridine)2 [M = nickel(II) and cobalt(II)] and tetranuclear M4(o-semiquinone)8 complexes. Inorg. Chem. 1981, 20, 1038–1046. [Google Scholar] [CrossRef]
- Lange, C.W.; Conklin, B.J.; Pierpont, C.G. Radical superexchange in semiquinone complexes containing diamagnetic metal ions. 3,6-Di-tert-butyl-1,2-semiquinoate complexes of zinc(II), cobalt(III), gallium(III), and aluminum(III). Inorg. Chem. 1994, 33, 1276–1283. [Google Scholar] [CrossRef]
- Brown, S.N. Metrical oxidation states of 2-amidophenoxide and catecholate ligands: Structural signatures of metal–ligand π bonding in potentially noninnocent ligands. Inorg. Chem. 2012, 51, 1251–1260. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sarkar, B.; Hübner, R.; Pattacini, R.; Hartenbach, I. Combining two non-innocent ligands in isomeric complexes [Pt(pap)m Qn]0 (pap = phenylazopyridine, Q = 3,5-di-tert-butyl-benzoquinone. Dalton Trans. 2009, 4653–4655. [Google Scholar] [CrossRef] [PubMed]
- Deibel, N.; Schweinfurth, D.; Hohloch, S.; Fiedler, J.; Sarkar, B. Donor–acceptor systems of Pt(II) and redox-induced reactivity towards small molecules. Chem. Commun. 2012, 48, 2388–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mizubayashi, Y.; Odoko, M.; Okabe, N. (Di-2-pyridylamine-κ2N,N′)(naphthalene-2,3-diolato-κ2O,O′)palladium (II) monohydrate and (di-2-pyridylamine-κ2N,N′)(3-oxidonaphthalene-2-carboxylato-κ2O,O′)palladium(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005, 61, m67–m70. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, B.; Billing, D. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Nippe, M.; Khnayzer, R.S.; Panetier, J.A.; Zee, D.Z.; Olaiya, B.S.; Head-Gordon, M.; Chang, C.J.; Castellano, F.N.; Long, J.R. Catalytic proton reduction with transition metal complexes of the redox-active ligand bpy2PYMe. Chem. Sci. 2013, 4, 3934–3945. [Google Scholar] [CrossRef]
- De La Torre, P.; Derrick, J.S.; Snider, A.; Smith, P.T.; Loipersberger, M.; Head-Gordon, M.; Chang, C.J. Exchange coupling determines metal-dependent efficiency for iron-and cobalt-catalyzed photochemical CO2 reduction. ACS Catal. 2022, 12, 8484–8493. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Reichardt, C., Welton, T., Eds.; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Maleeva, A.V.; Ershova, I.V.; Trofimova, O.Y.; Arsenyeva, K.V.; Yakushev, I.A.; Piskunov, A.V. Near-IR absorbing donor–acceptor charge-transfer gallium complex, an example from non-transition metal chemistry. Mendeleev Commun. 2022, 32, 83–86. [Google Scholar] [CrossRef]
- Lado, A.; Piskunov, A.; Cherkasov, V.; Fukin, G.; Abakumov, G. Free radical fixation by tin(IV) diphenylcatecholate complexes. Russ. J. Coord. Chem. 2006, 32, 173–179. [Google Scholar] [CrossRef]
- Fukin, G.; Cherkasov, A.V.; Shurygina, M.P.; Druzhkov, N.O.; Kuropatov, V.A.; Chesnokov, S.A.; Abakumov, G.A. Geometrical and energetical aspects of structure of 3, 6-di-tert-butyl-o-benzoquinones. Struct. Chem. 2010, 21, 607–611. [Google Scholar]
- Gordon, A.; Ford, R. The Chemist’s Companion; Wiley & Sons: New York, NY, USA, 1972; p. 143. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, R.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, D.; Soncini, A.; Murray, S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d-and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System, Version 1.171.41.122a; Rigaku Corporation: Wroclaw, Poland, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3. [Google Scholar]
| Contact | 3∙Toluene | [CuII(3,5-Cat)(bipy)]2 [47] | [CuII(3,5-SQ)2]2 [97] | [CoII(3,5-SQ)2]4 [98] |
|---|---|---|---|---|
| dimer | dimer | dimer | tetramer | |
| M⋯M *, Å | 2.8847 (7) | 3.0742 (8) | 3.360 | 3.163/3.306/3.317 |
| M⋯O *, Å | 2.0222 (18) | 2.330 (2) | 2.416 (4) | 2.075/2.133/2.175 |
| Coordination polyhedron | ||||
| τ5 [102] ** | 0.087, square pyramid | 0.228, square pyramid | 0.420, square pyramid | - |
| - | - | - | - | octahedron |
| 1·THF | 2 (Molecule A)/(Molecule B) | 3∙Toluene | |||
|---|---|---|---|---|---|
| Bond | Å | Bond | Å | Bond | Å |
| Cu(1)-O(1) | 1.860 (2) | Ni(1)-O(1) | 1.8137 (14)/1.8110 (14) | Co(1)-O(1) | 1.9375 (18) |
| Cu(1)-O(2) | 1.857 (3) | Ni(1)-O(2) | 1.8096 (15)/1.8118 (15) | Co(1)-O(2)/Co(1)-O(2) * | 2.0552 (17)/2.0222 (18) |
| Cu(1)-N(1) | 1.975 (3) | Ni(1)-N(1) | 1.8755 (17)/1.8773 (18) | Co(1)-N(2) | 2.113 (2) |
| Cu(1)-N(2) | 1.975 (3) | Ni(1)-N(2) | 1.8782 (17)/1.8768 (17) | Co(1)-N(1) | 2.115 (2) |
| O(1)-C(1) | 1.348 (5) | O(1)-C(1) | 1.353 (3)/1.351 (3) | O(1)-C(1) | 1.335 (3) |
| O(2)-C(2) | 1.347 (4) | O(2)-C(2) | 1.357 (2)/1.356 (2) | O(2)-C(2) | 1.364 (3) |
| N(1)-C(13) | 1.335 (5) | N(1)-C(15) | 1.342 (3)/1.343 (3) | N(2)-C(24) | 1.340 (3) |
| N(1)-C(17) | 1.340 (4) | N(1)-C(19) | 1.349 (3)/1.352 (3) | N(2)-C(20) | 1.341 (3) |
| N(2)-C(18) | 1.346 (4) | N(2)-C(20) | 1.355 (3)/1.349 (3) | N(1)-C(19) | 1.344 (3) |
| N(2)-C(22) | 1.336 (5) | N(2)-C(24) | 1.339 (3)/1.339 (3) | N(1)-C(15) | 1.341 (3) |
| C(1)-C(2) | 1.408 (6) | C(1)-C(2) | 1.411 (3)/1.406 (3) | C(1)-C(2) | 1.434 (4) |
| C(2)-C(3) | 1.399 (6) | C(2)-C(3) | 1.393 (3)/1.391 (3) | C(2)-C(3) | 1.400 (4) |
| C(3)-C(4) | 1.388 (6) | C(3)-C(4) | 1.393 (3)/1.397 (3) | C(3)-C(4) | 1.401 (4) |
| C(4)-C(5) | 1.367 (7) | C(4)-C(5) | 1.378(3)/1.383 (3) | C(4)-C(5) | 1.367 (4) |
| C(5)-C(6) | 1.397 (6) | C(5)-C(6) | 1.394 (3)/1.393 (3) | C(5)-C(6) | 1.392 (4) |
| C(1)-C(6) | 1.401 (5) | C(1)-C(6) | 1.392 (3)/1.396 (3) | C(1)-C(6) | 1.407 (4) |
| Bond | CuII(3,5-Cat)(bipy) [47] | NiII(3,6-Cat)(bipy) [32] | CoII(3,6-Cat)(DAD) [43] |
|---|---|---|---|
| monomer | monomer | monomer | |
| M-O, Å | 1.901 (5), 1.870 (5) | 1.814 (3), 1.815 (3) | 1.8066 (13), 1.8104 (13) |
| M-N, Å | 1.993 (6), 1.999 (6) | 1.882 (4), 1.891 (3) | 1.8743 (16), 1.8711 (16) |
| Compound | E1/2Red, V | E1/2Ox, V | ΔEHOMO→LUMO, eV |
|---|---|---|---|
| 2 | −2.01 | −0.20 | 1.81 |
| NiII(3,6-Cat)(bipy) | −1.86 | −0.21 | 1.65 |
| Solvent | 1·THF | NiII(3,5-Cat) (bipytBu) [67] | 2 | NiII(3,6-Cat) (bipy) [32] | 3∙Toluene | NiII(3,6-CatGly) (bipy) [32] | |||
|---|---|---|---|---|---|---|---|---|---|
| λ (ε) | E | λ (ε) | λ (ε) | E | λ (ε) | λ (ε) | E | ||
| CH3CN | 501 (1.29) | 2.475 | - | 543 (3.94) | 2.284 | - | 657 (1.67) | 1.887 | - |
| DMF | 523 (1.28) | 2.371 | 554 (1.83) | 560 (2.98) | 2.214 | 580 (3.50) | 674 (2.74) | 1.840 | 618 (3.08) |
| CH2Cl2 | 540 (1.31) | 2.296 | 572 (2.09) | 580 (4.89) | 2.138 | 610 (4.35) | 695 (5.19) | 1.784 | 685 (4.04) |
| THF | 583 (0.87) | 2.127 | 618 (1.95) | 617 (3.98) | 2.010 | 640 (3.43) | 715 (9.36) | 1.734 | 720 (3.18) |
| Toluene | 654 (0.95) | 1.896 | 683 (1.70) | 680 (2.24) | 1.824 | 715 (3.57) | insoluble | - | 810 (3.38) |
| Parameters of solvatochromic shift | |||||||||
| Δλ(Tol–CH3CN) | 153 | - | 137 | - | - | - | |||
| Δλ(Tol–DMF) | 131 | 129 | 120 | 135 | - | 192 | |||
| Δλ(THF–CH3CN) | 82 | - | 74 | - | 58 | - | |||
| Δλ(THF–DMF) | 60 | 64 | 57 | 60 | 41 | 102 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashanova, K.I.; Ershova, I.V.; Trofimova, O.Y.; Rumyantsev, R.V.; Fukin, G.K.; Bogomyakov, A.S.; Arsenyev, M.V.; Piskunov, A.V. Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes. Molecules 2022, 27, 8175. https://doi.org/10.3390/molecules27238175
Pashanova KI, Ershova IV, Trofimova OY, Rumyantsev RV, Fukin GK, Bogomyakov AS, Arsenyev MV, Piskunov AV. Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes. Molecules. 2022; 27(23):8175. https://doi.org/10.3390/molecules27238175
Chicago/Turabian StylePashanova, Kira I., Irina V. Ershova, Olesya Yu. Trofimova, Roman V. Rumyantsev, Georgy K. Fukin, Artem S. Bogomyakov, Maxim V. Arsenyev, and Alexandr V. Piskunov. 2022. "Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes" Molecules 27, no. 23: 8175. https://doi.org/10.3390/molecules27238175
APA StylePashanova, K. I., Ershova, I. V., Trofimova, O. Y., Rumyantsev, R. V., Fukin, G. K., Bogomyakov, A. S., Arsenyev, M. V., & Piskunov, A. V. (2022). Charge Transfer Chromophores Derived from 3d-Row Transition Metal Complexes. Molecules, 27(23), 8175. https://doi.org/10.3390/molecules27238175







