Structure and Properties of Heterometallics Based on Lanthanides and Transition Metals with Methoxy-β-Diketonates
Abstract
1. Introduction
2. Results and Discussion
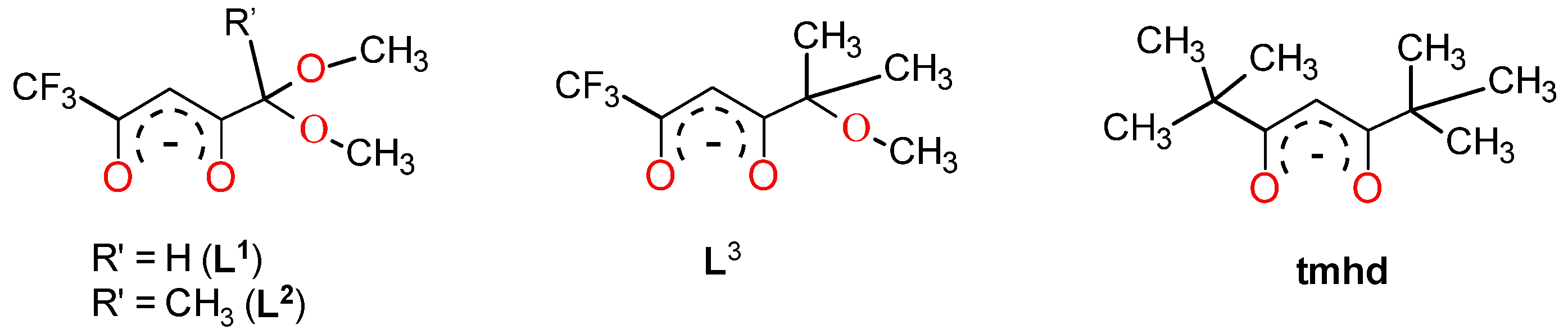
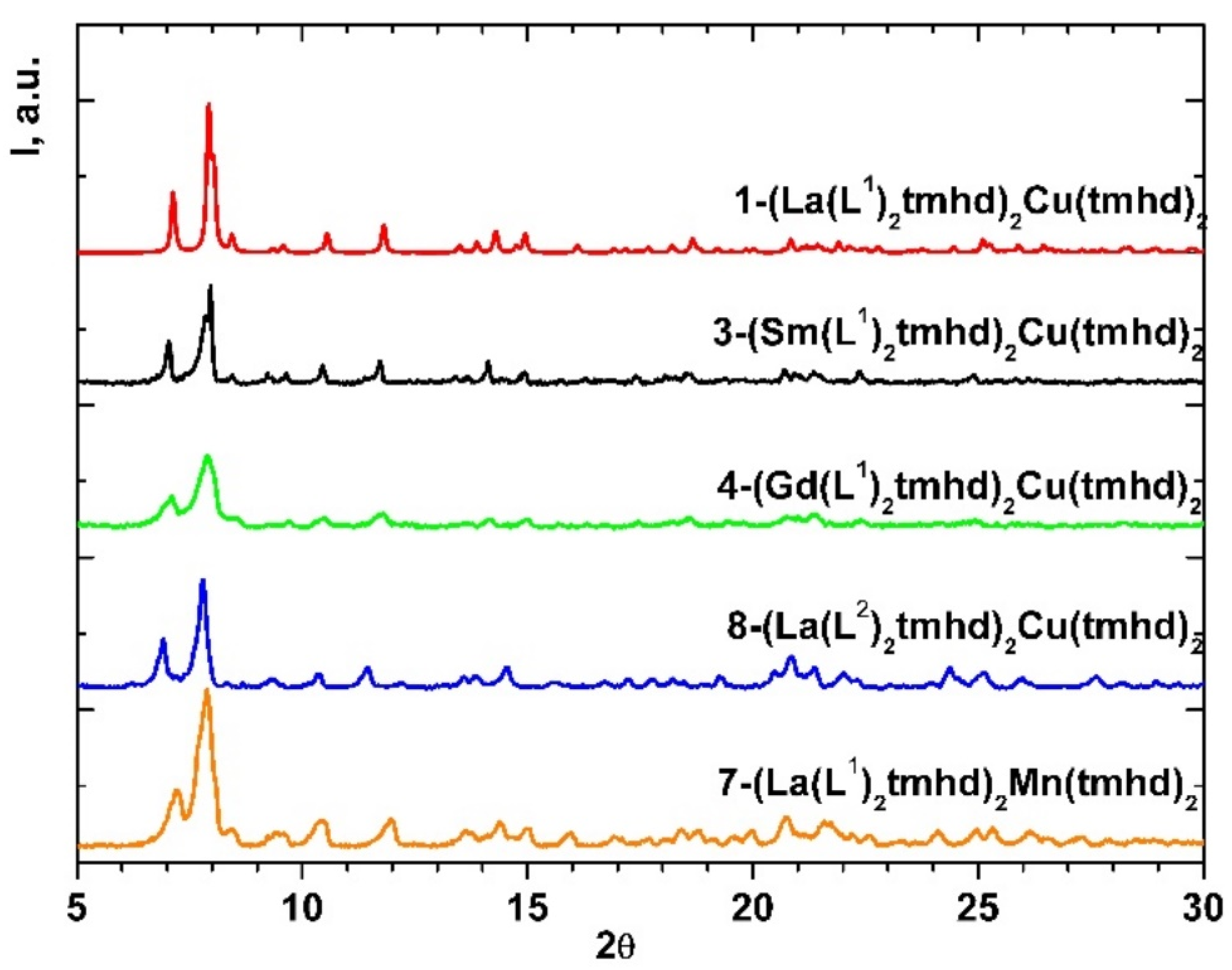
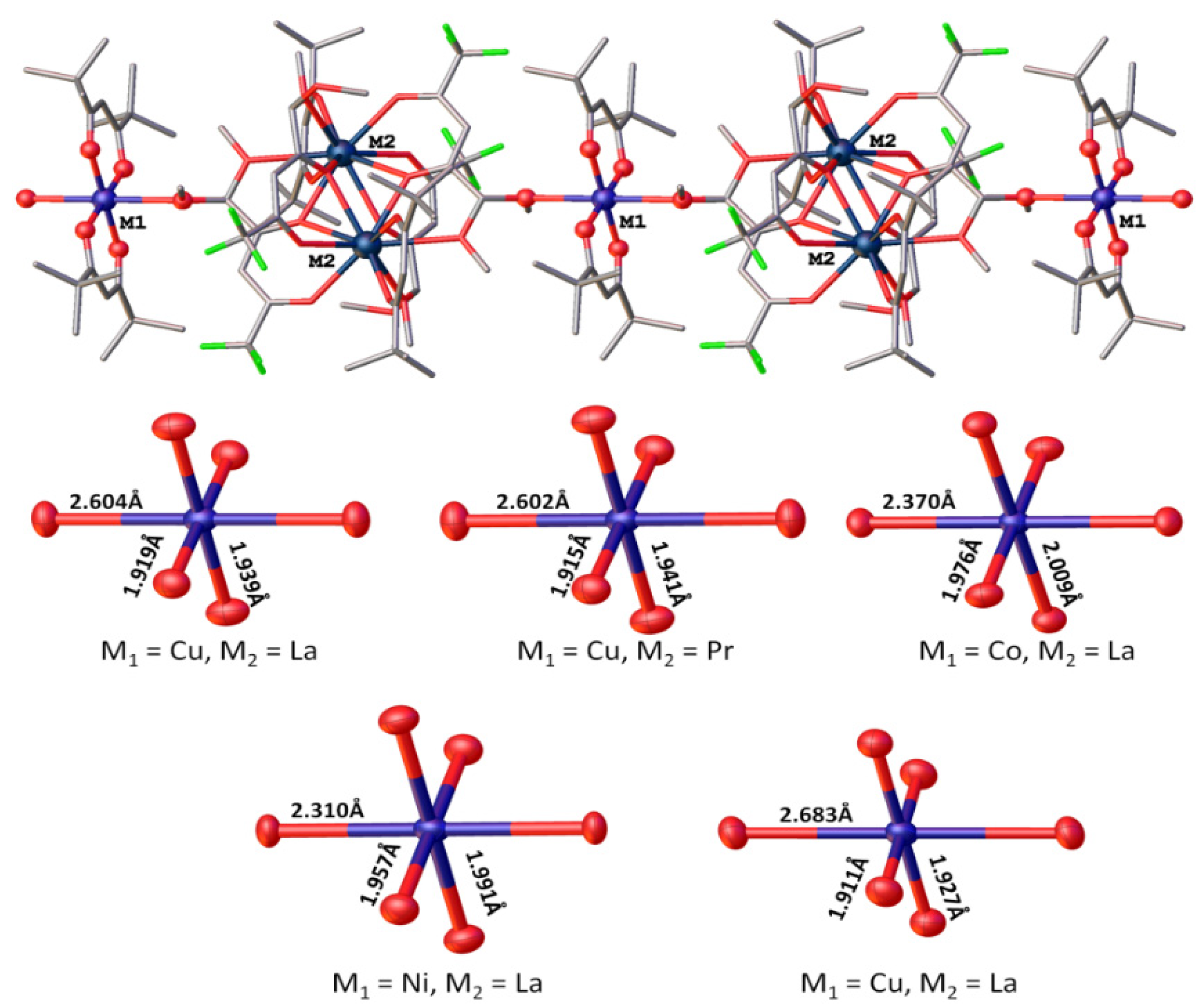
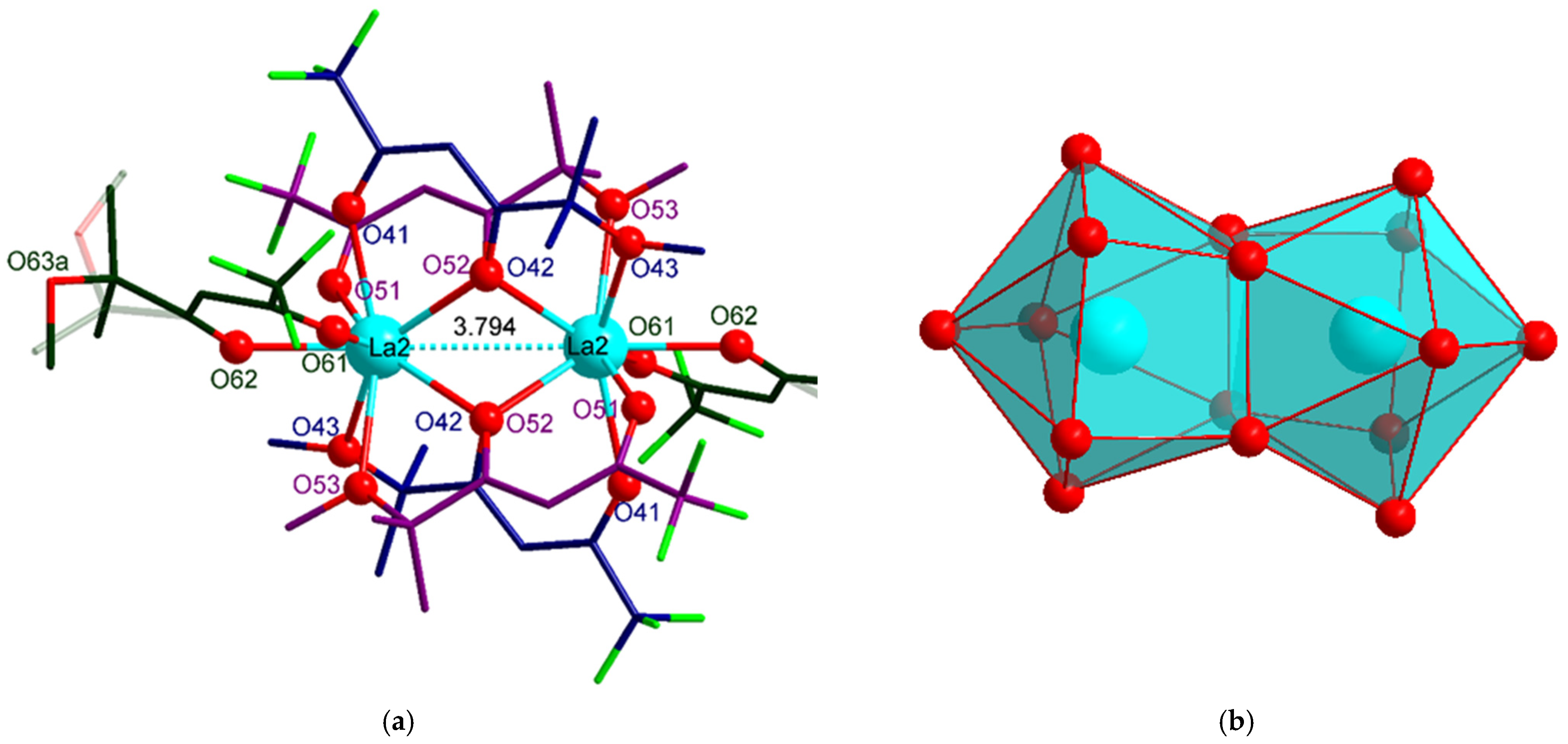
2.1. Synthesis and Structure
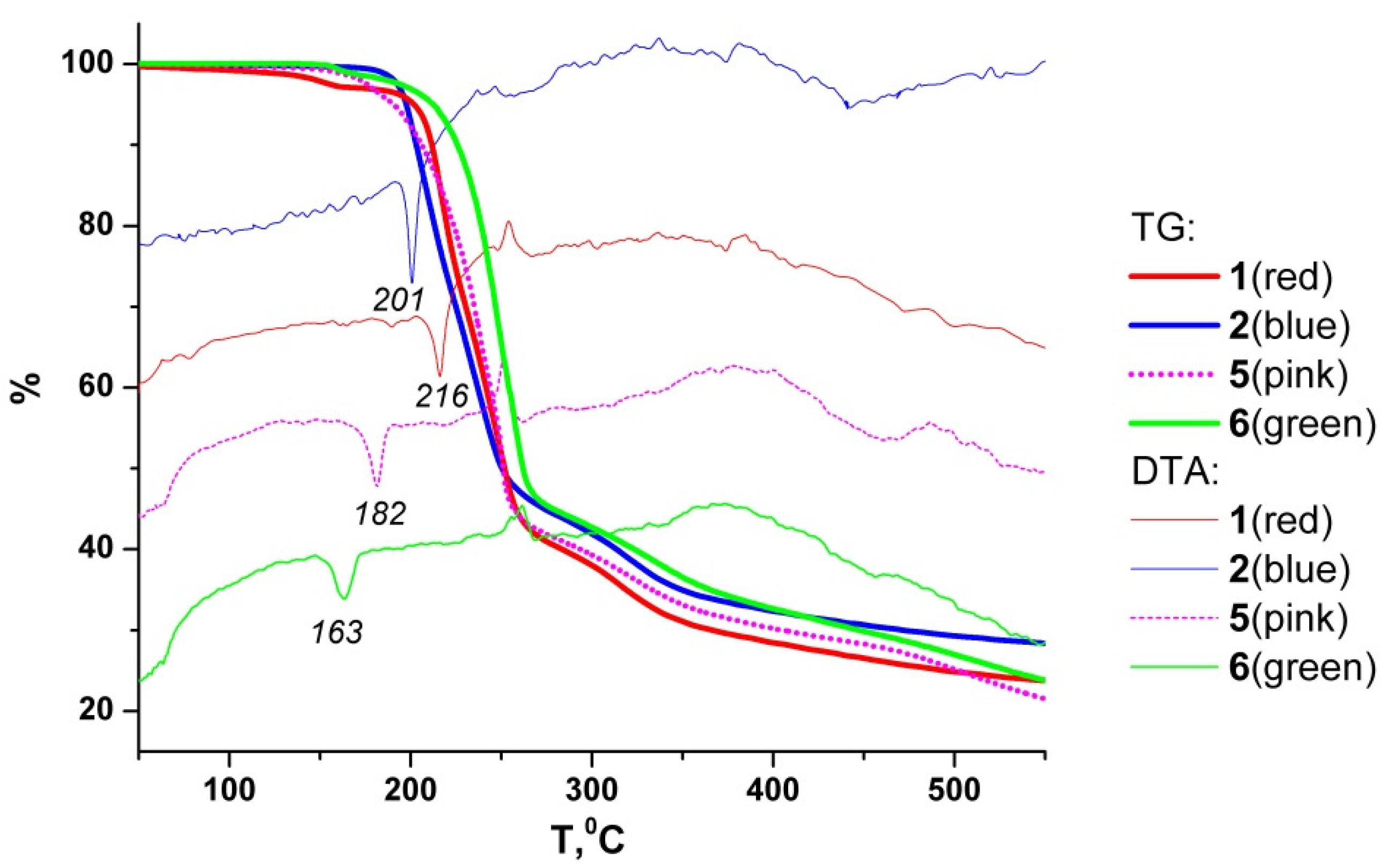
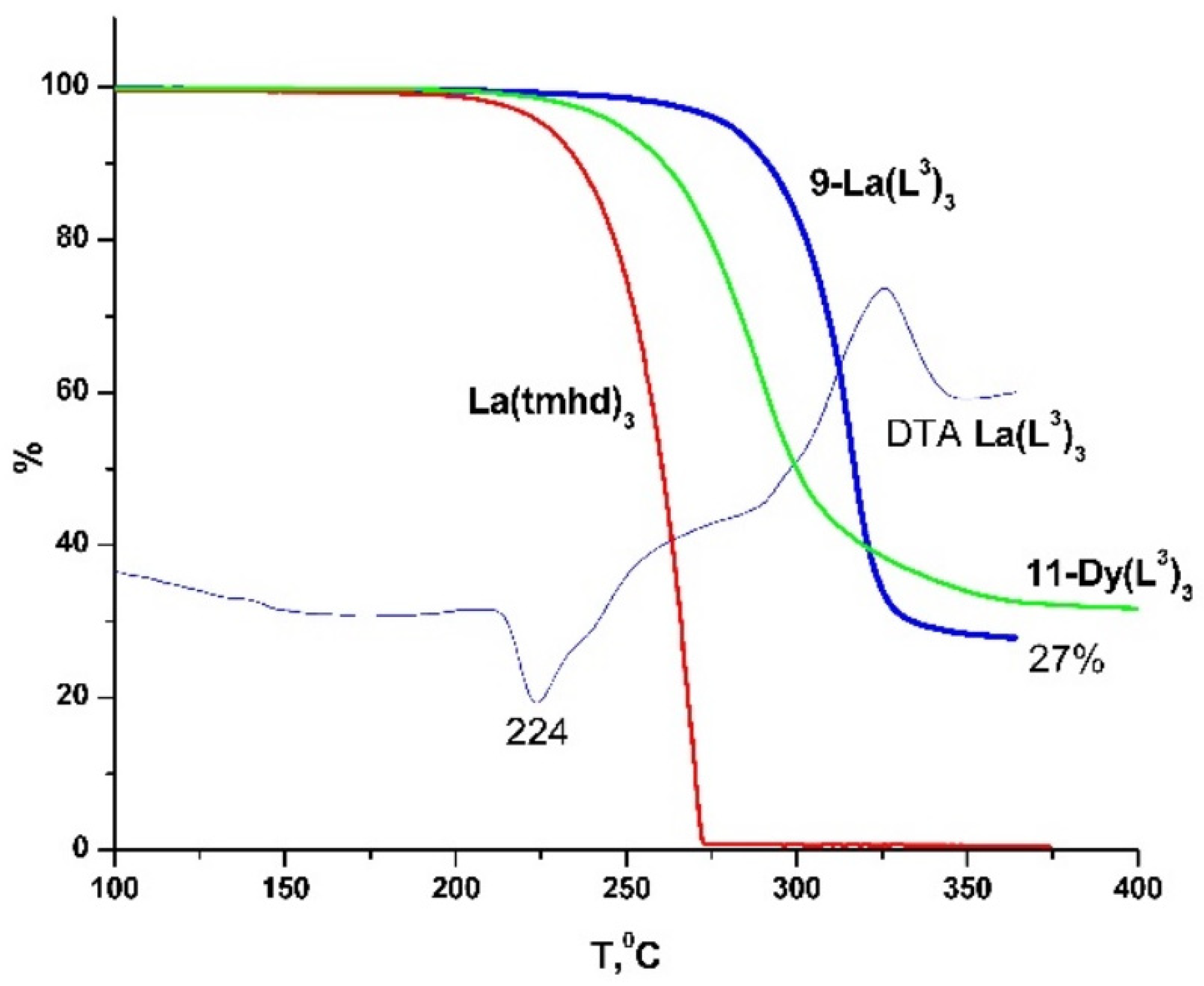
2.2. Thermal Properties
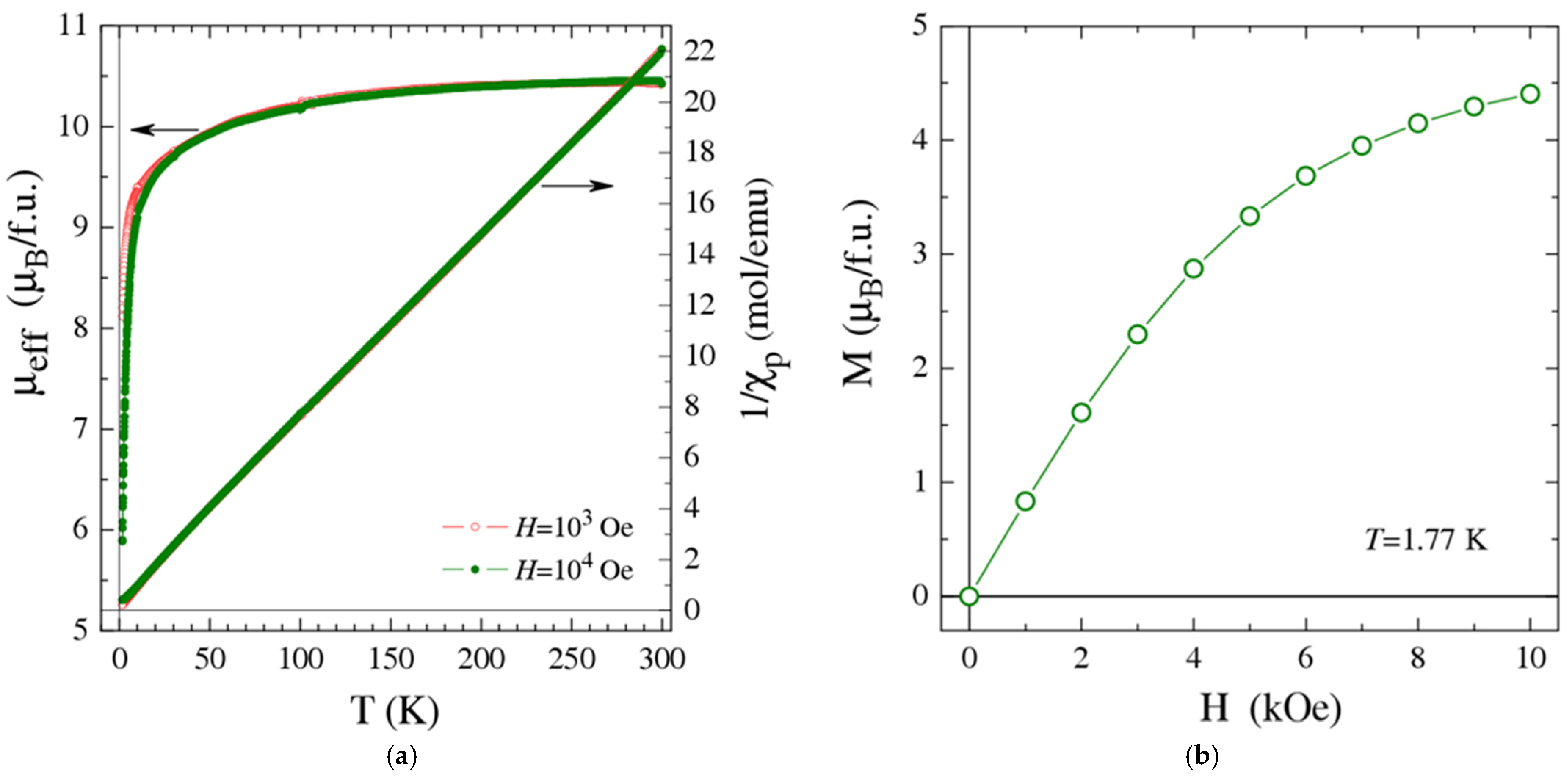
2.3. Magnetic Properties
3. Experimental
3.1. Synthesis
3.2. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Long, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J. Recent Advances in Luminescent Lanthanide Based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–70. [Google Scholar] [CrossRef]
- Jia, J.H.; Li, Q.W.; Chen, Y.C.; Liu, J.L.; Tong, M.L. Luminescent Single-molecule Magnets Based on Lanthanides: Design Strategies, Recent Advances and Magneto-luminescent Studies. Coord. Chem. Rev. 2019, 378, 365–381. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.Q.; Su, J.B.; Zhang, Y.Q.; Ben Zhu, D. Single-Molecule Magnet Behavior of 1D Coordination Polymers Based on DyZn2(salen)2 Units and Pyridin- N-Oxide-4-Carboxylate: Structural Divergence and Magnetic Regulation. Inorg. Chem. 2018, 57, 11077–11086. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Review of Purely 4f and Mixed-metal nd-4f Single-molecule Magnets Containing Only One Lanthanide Ion. Coord. Chem. Rev. 2014, 276, 1–33. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward Heterometallic Single-Molecule Magnets: Synthetic Strategy, Structures and Properties of 3d-4f Discrete Complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Skaribas, S.P.; Pomonis, P.J.; Sdoukos, A.T. Low-Temperature Synthesis of Perovskite Solids LaMO3 (M = Ni, Co, Mn) via Binuclear Complexes of Compartmental Ligand N,N′-bis(3-Carboxysalicylidene)ethylenediamine. J. Mater. Chem. 1991, 1, 781–784. [Google Scholar] [CrossRef]
- Hasegawa, E.; Aono, H.; Igoshi, T.; Sakamoto, M.; Traversa, E.; Sadaoka, Y. Preparation of YBa2Cu3O7. J. Alloys Compd. 1999, 287, 150–158. [Google Scholar] [CrossRef]
- Kuzmina, N.P.; Malkerova, I.P.; Alikhanyan, A.S.; Gleizes, A.N. The Use of 3d-Metal Complexes as Ligands to Prepare Volatile 4f-3d Heterobimetallic Complexes. J. Alloys Compd. 2004, 374, 315–319. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Metal–Organic Derivatives with Fluorinated Ligands as Precursors for Inorganic Nanomaterials. Chem. Rev. 2015, 115, 8379. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Urkasym Kyzy, S.; Rybalova, T.V.; Baidina, I.A.; Korolkov, I.V.; Chizhov, D.L.; Bazhin, D.N.; Kudyakova, Y.S. Isomerization as a Tool to Design Volatile Heterometallic Complexes with Methoxy-Substituted β-Diketonates. J. Coord. Chem. 2018, 71, 2194–2208. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Tkachev, S.V.; Baidina, I.A.; Korolkov, I.V.; Turgambaeva, A.E.; Igumenov, I.K. Volatile Pd-Pb and Cu-Pb Heterometallic Complexes: Structure, Properties, and Trans-to-cis Isomerization under Cocrystallization of Pd and Cu β-Diketonates with Pb Hexafluoroacetylacetonate. J. Coord. Chem. 2015, 68, 1890–1902. [Google Scholar] [CrossRef]
- Urkasym kyzy, S.; Krisyuk, V.V.; Turgambaeva, A.E.; Baidina, I.A.; Komarov, V.Y.; Korotaev, E.V.; Korolkov, I.V. Methoxy-Substituted Transition Metal β-Diketonates: Synthesis and Properties. J. Struct. Chem. 2019, 60, 1635–1647. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valov, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. A Rare Example of Discrete Lanthanide–Lithium Tetrakis-β-Diketonates: Synthesis, Structures, and Luminescence Properties. Rus. J. Coord. Chem. 2020, 46, 545–552. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. The Impact of the Alkali Metal Ion on the Crystal Structure and (Mechano)luminescence of Terbium(III) Tetrakis(β-diketonates). Eur. J. Inorg. Chem. 2020, 2020, 523–531. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Role of Alkyl Substituents in the Structure and Luminescence Properties of Discrete Terbium(III)-Lithium(I) β-Diketonates. J. Mol. Struct. 2021, 1226, 129331. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Functionalized Trifluoromethyl-Containing Lithium β-Diketonate in the Synthesis of Homo- and Heteronuclear Complexes of Rare-Earth Metals. Rus. J. Coord. Chem. 2021, 47, 280–295. [Google Scholar] [CrossRef]
- Yuikhan, L.; Mosyagina, S.A.; Stabnikov, P.A.; Alferova, N.I.; Korol‘kov, I.V.; Pervukhina, N.V.; Morozova, N.B. Structure of Lanthanum(III) tris-Dipivaloylmethanate. J. Struct. Chem. 2017, 58, 843–846. [Google Scholar] [CrossRef]
- Blundell, S. Magnetism in Condensed Matter; Oxford University Press: Oxford, UK, 2001; ISBN-10: 0198505914. [Google Scholar]
- Bernot, K.; Luzon, J.; Bogani, L.; Etienne, M.; Sangregorio, C.; Shanmugam, M.; Caneschi, A.; Sessoli, R.; Gatteschi, D. Magnetic Anisotropy of Dysprosium(III) in a Low-Symmetry Environment: A Theoretical and Experimental Investigation. J. Am. Chem. Soc. 2009, 131, 5573–5579. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.N.; Tang, J. Recent Advances in Dysprosium-Based Single Molecule Magnets: Structural Overview and Synthetic Strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Zvezdin, A.K.; Matveev, V.M.; Mukhin, A.A.; Popov, A.I. Rare Earth Ions in Magnetically Ordered Crystals; Nauka: Moscow, Russia, 1985. [Google Scholar]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Chizhov, D.L.; Belyaev, D.V.; Yachevskii, D.S.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Efficient and Scalable Synthesis of 3-(polyfluoroacyl)pyruvaldehydes Dimethyl Acetals: A Novel Functionalized Fluorinated Building-Block. J. Fluor. Chem. 2017, 199, 39–45. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Chizhov, D.L.; Röschenthaler, G.V.; Kudyakova, Y.S.; Burgart, Y.V.; Slepukhin, P.A.; Saloutin, V.I.; Charushin, V.N. A Concise Approach to CF3-Ccontaining Furan-3-ones, (Bis)pyrazoles from Novel Fluorinated Building Blocks Based on 2,3-Butanedione. Tetrahedron Lett. 2014, 55, 5714–5717. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Onoprienko, A.Y.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Effect of the Nature of a Fluorinated Substituent on the Synthesis of Functionalized 1,3-Diketones. Rus. Chem. Bull. Int. Ed. 2021, 70, 745–752. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]


| #-Ln | <Ln-Och> | <Ln-Ochb> | Ln-OCH3 * | Ln_Ln | TM | TM-Och | TM-OCH3 | <Och-Ln-Ochb> | Och-TM-Och |
|---|---|---|---|---|---|---|---|---|---|
| 1-La | 2.46(8) | 2.63(2) | 2.765(3) 2.759(3) | 3.854(3) | Cu | 1.915(3) 1.941(3) | 2.604(3) | 65.4(4) | 92.4(1) |
| 2-Pr | 2.42(8) | 2.59(1) | 2.752(3) 2.739(3) | 3.793(3) | Cu | 1.915(3) 1.941(3) | 2.602(3) | 66.2(4) | 92.5(1) |
| 5-La | 2.50(8) | 2.63(2) | 2.767(3) 2.747(3) | 3.834(3) | Co | 1.975(3) 2.009(3) | 2.370(3) | 65.3(4) | 70.3(1) |
| 6-La | 2.50(8) | 2.63(2) | 2.769(3) 2.753(3) | 3.838(3) | Ni | 1.956(3) 1.992(3) | 2.306(3) | 65.2(4) | 70.5(1) |
| 8-La | 2.47(8) | 2.65(2) | 2.774(3) 2.803(3) | 3.838(3) | Cu | 1.910(3) 1.928(3) | 2.685(3) | 65.3(4) | 92.4(1) |
| Bond Length, Å | Angle, ° | ||
|---|---|---|---|
| La2-O41 | 2.504(3) | O41-La2-O42 | 66.9(9) |
| La2-O42 | 2.613(3), 2.612 * | La2-O42-La2 | 93.1(9) |
| La2-O43 | 2.704(3) | O42-La2-O43 | 58.9(9) |
| La2-O51 | 2.505(3) | O51-La2-O52 | 65.7(9) |
| La2-O52 | 2.627(3), 2.622 * | La2-O52-La2 | 92.6(9) |
| La2-O53 | 2.702(3) | O52-La2-O53 | 56.1(9) |
| La2-O61 | 2.490(3) | O61-La2-O62 | 69.6(9) |
| La2-O62 | 2.454(3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisyuk, V.V.; Urkasym Kyzy, S.; Rybalova, T.V.; Korolkov, I.V.; Grebenkina, M.A.; Lavrov, A.N. Structure and Properties of Heterometallics Based on Lanthanides and Transition Metals with Methoxy-β-Diketonates. Molecules 2022, 27, 8400. https://doi.org/10.3390/molecules27238400
Krisyuk VV, Urkasym Kyzy S, Rybalova TV, Korolkov IV, Grebenkina MA, Lavrov AN. Structure and Properties of Heterometallics Based on Lanthanides and Transition Metals with Methoxy-β-Diketonates. Molecules. 2022; 27(23):8400. https://doi.org/10.3390/molecules27238400
Chicago/Turabian StyleKrisyuk, Vladislav V., Samara Urkasym Kyzy, Tatyana V. Rybalova, Ilya V. Korolkov, Mariya A. Grebenkina, and Alexander N. Lavrov. 2022. "Structure and Properties of Heterometallics Based on Lanthanides and Transition Metals with Methoxy-β-Diketonates" Molecules 27, no. 23: 8400. https://doi.org/10.3390/molecules27238400
APA StyleKrisyuk, V. V., Urkasym Kyzy, S., Rybalova, T. V., Korolkov, I. V., Grebenkina, M. A., & Lavrov, A. N. (2022). Structure and Properties of Heterometallics Based on Lanthanides and Transition Metals with Methoxy-β-Diketonates. Molecules, 27(23), 8400. https://doi.org/10.3390/molecules27238400








