Insights on Chemical Crosslinking Strategies for Proteins
Abstract
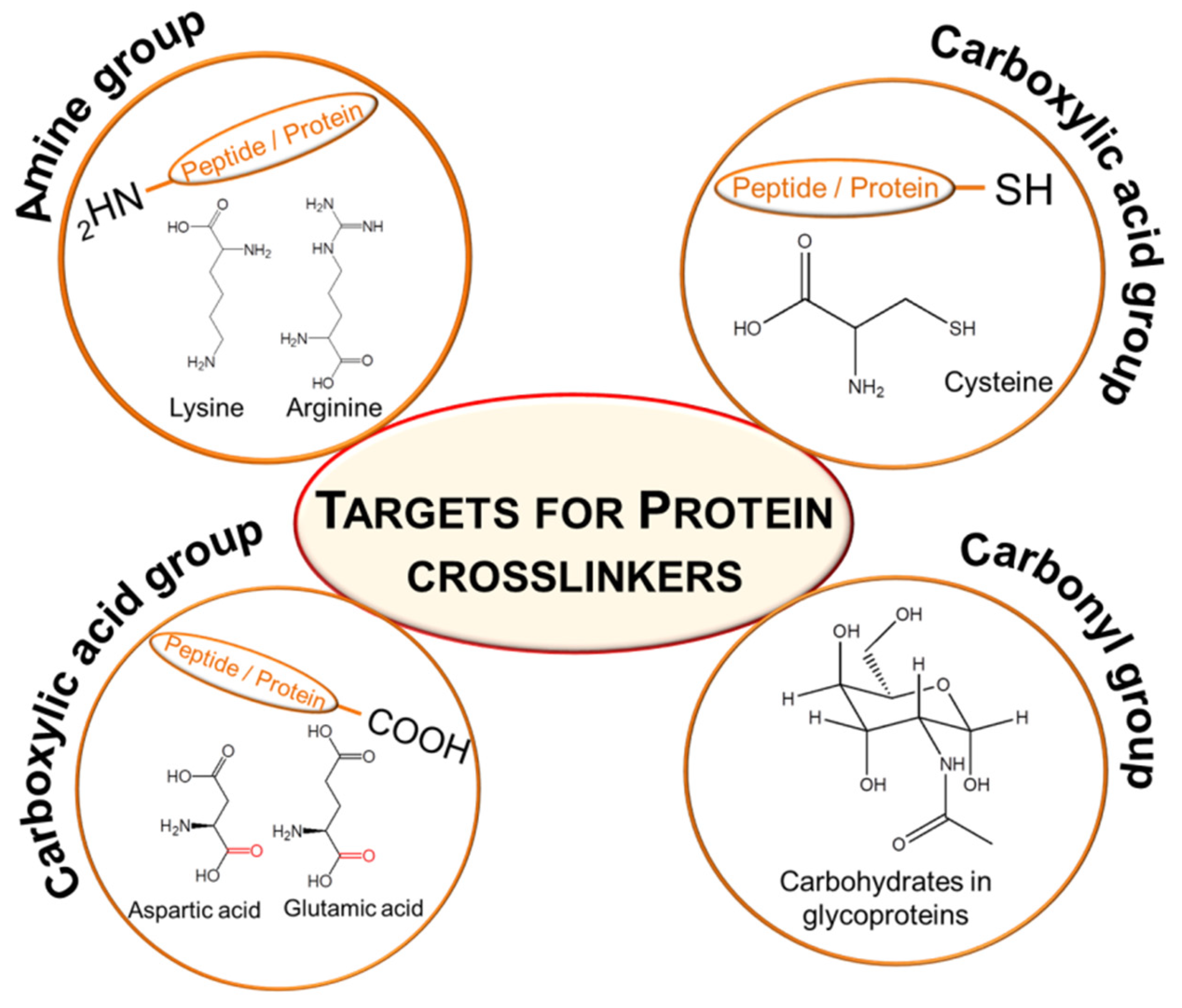
1. Introduction
2. Chemical Crosslinking of Proteins
2.1. Natural Crosslinkers
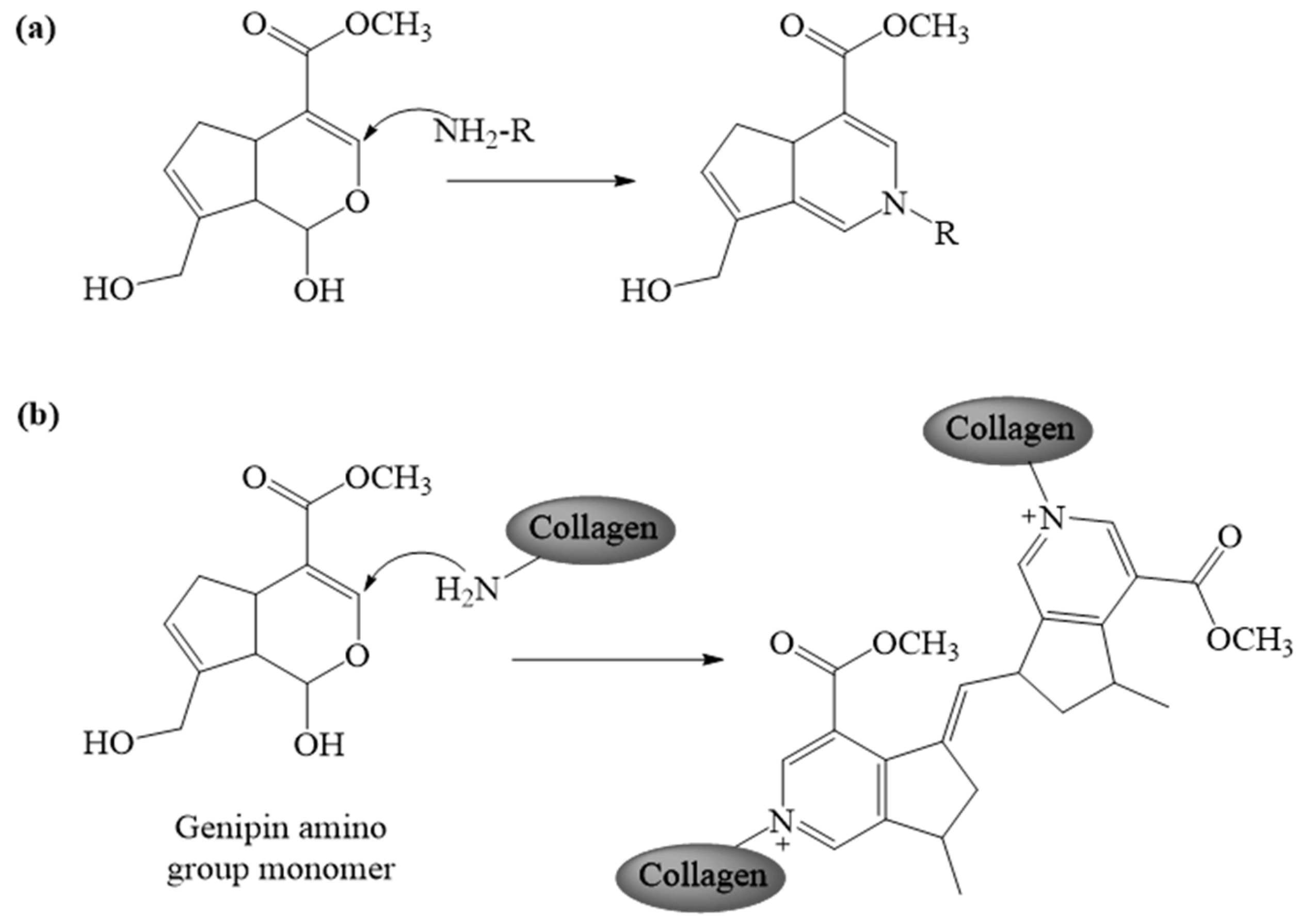
2.1.1. Genipin
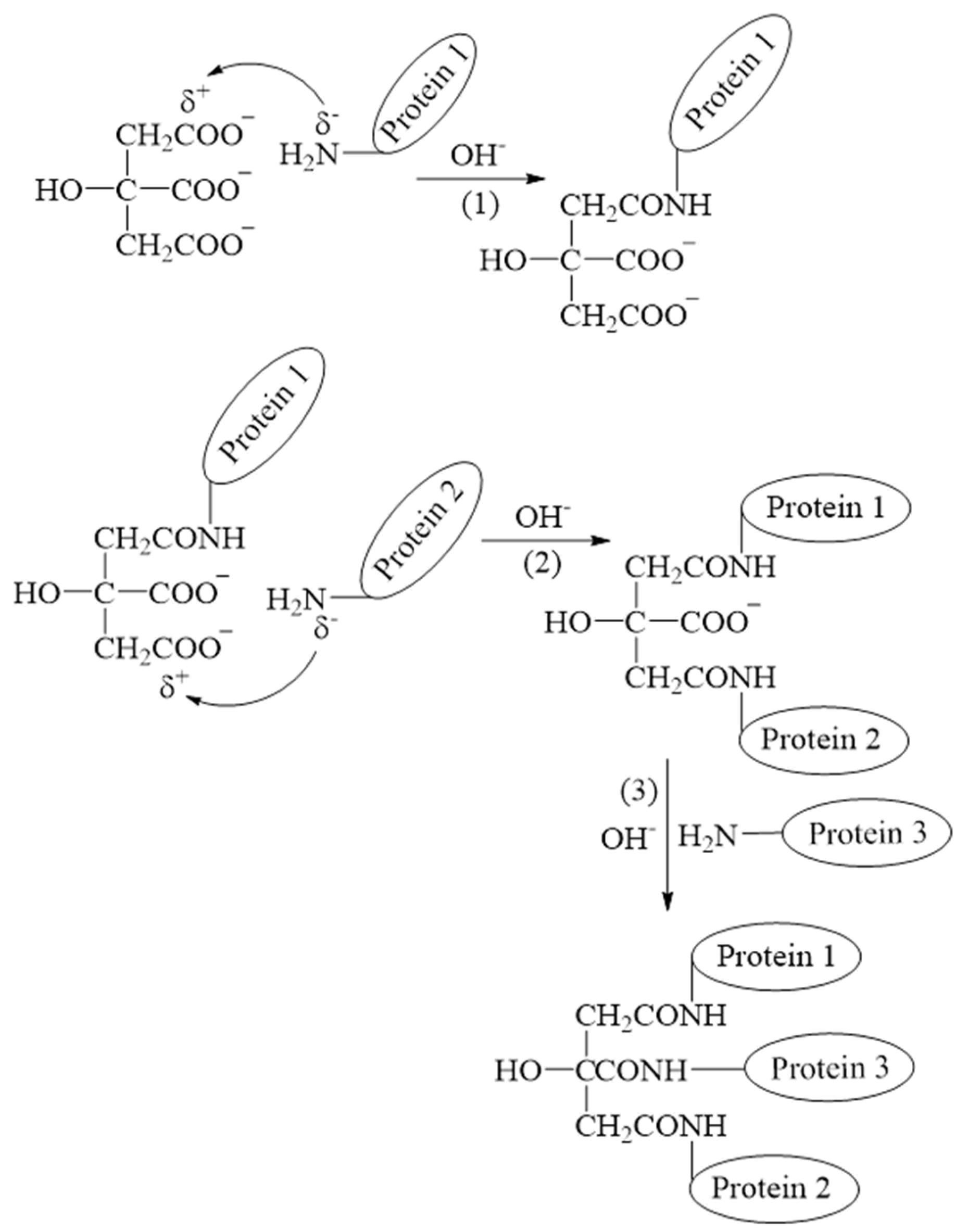
2.1.2. Citric Acid
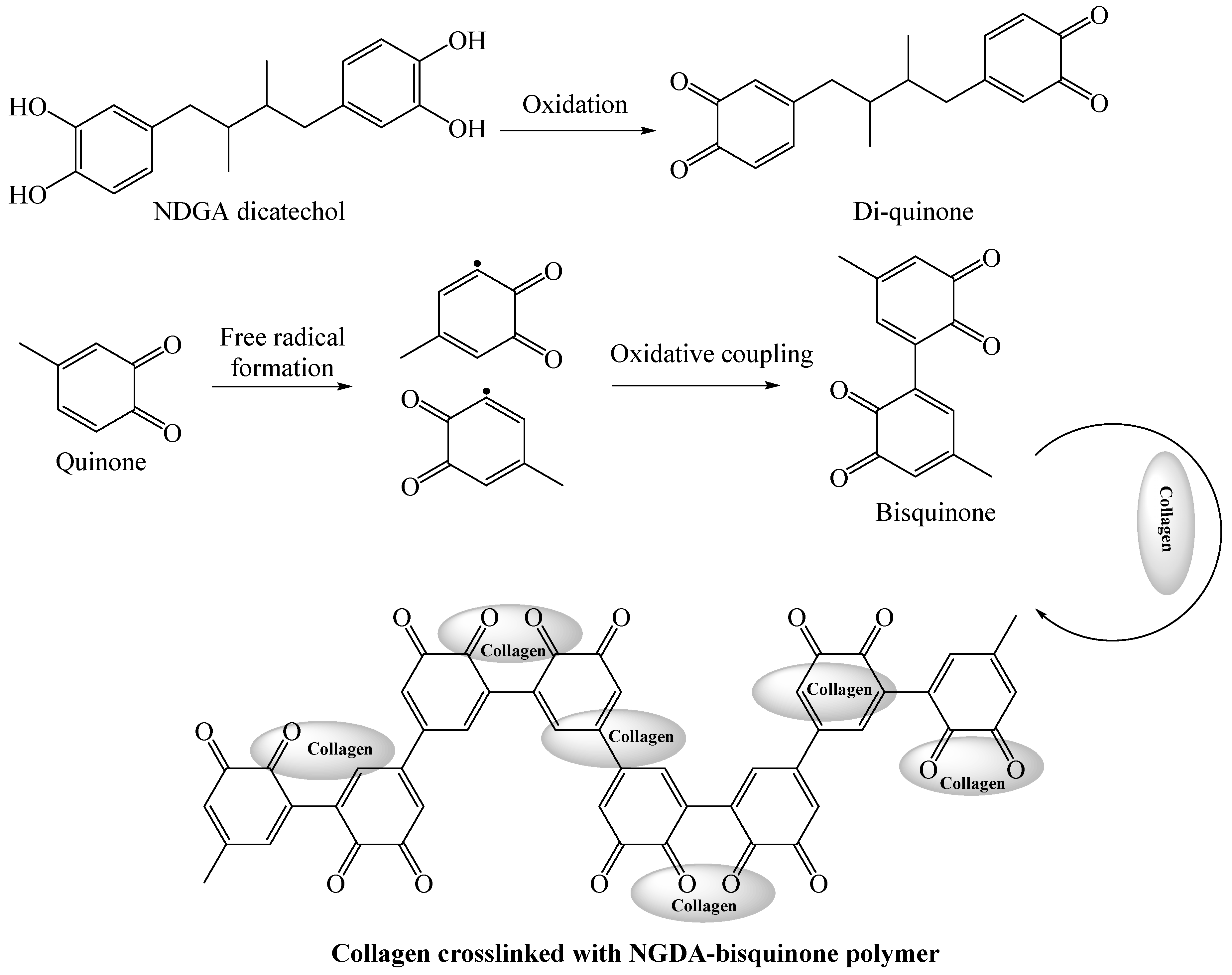
2.1.3. Nordihydroguaiaretic Acid
2.1.4. Procyanidins
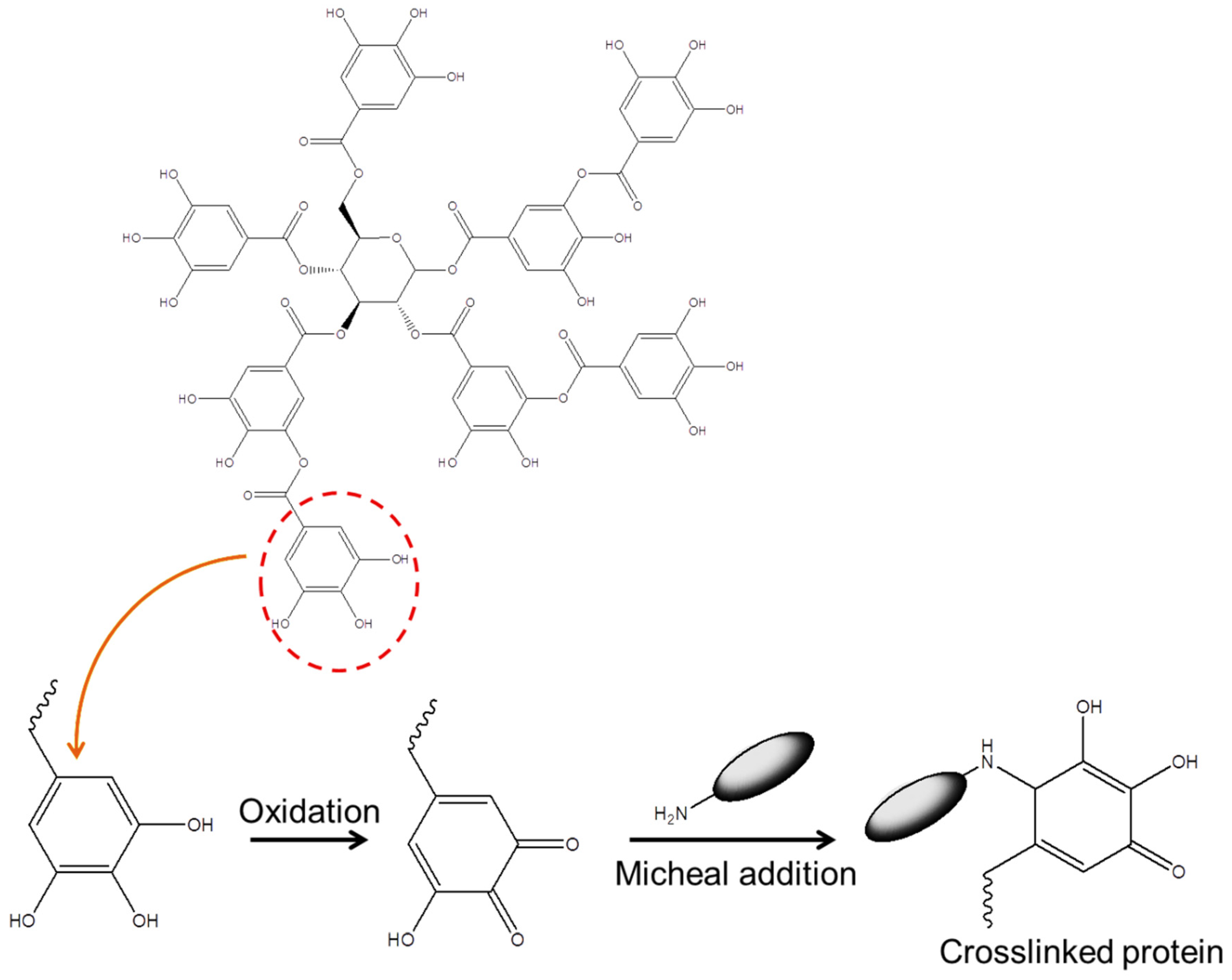
2.1.5. Tannic Acid
2.2. Synthetic Crosslinkers
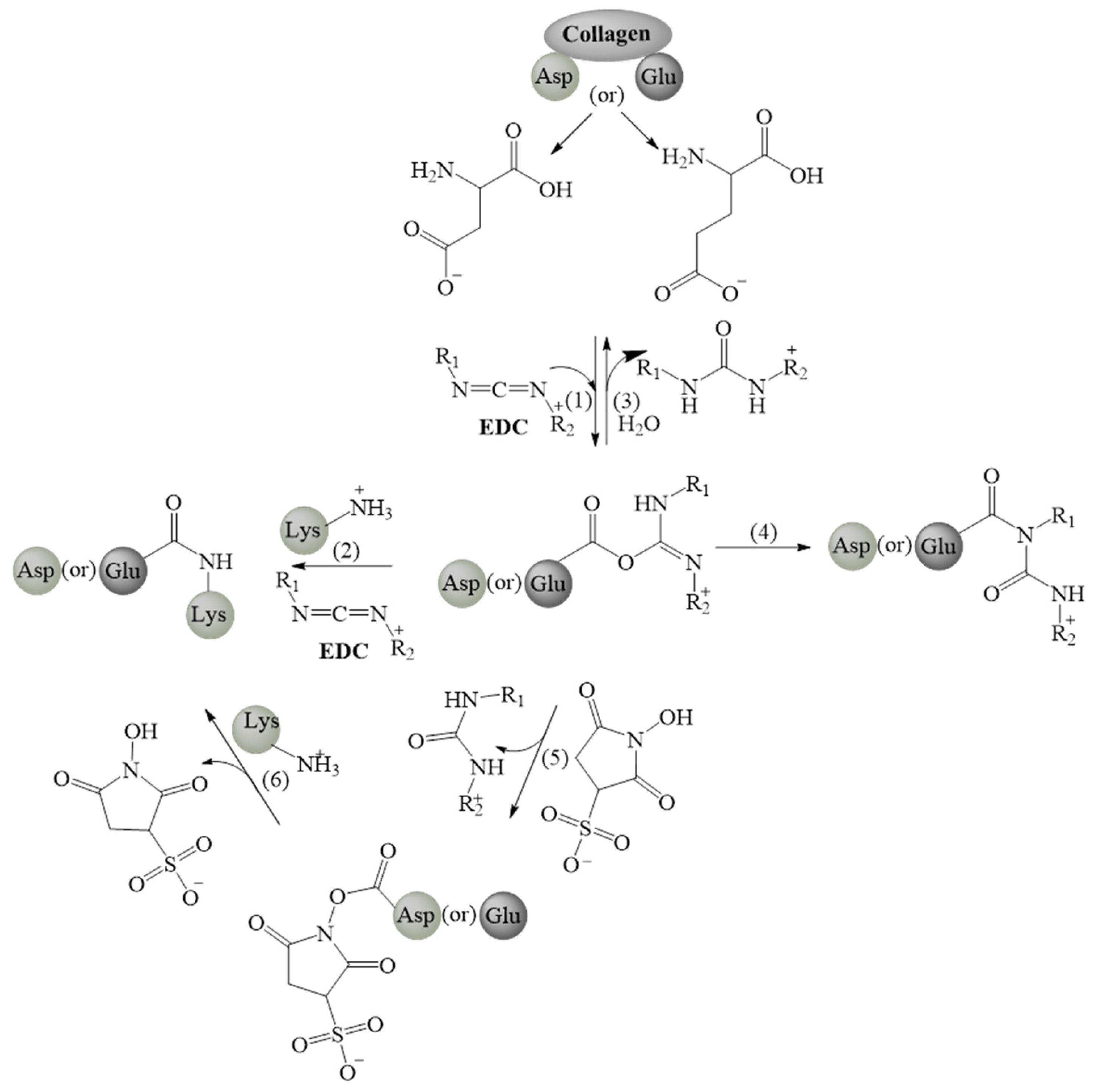
2.2.1. Carbodiimide Agents
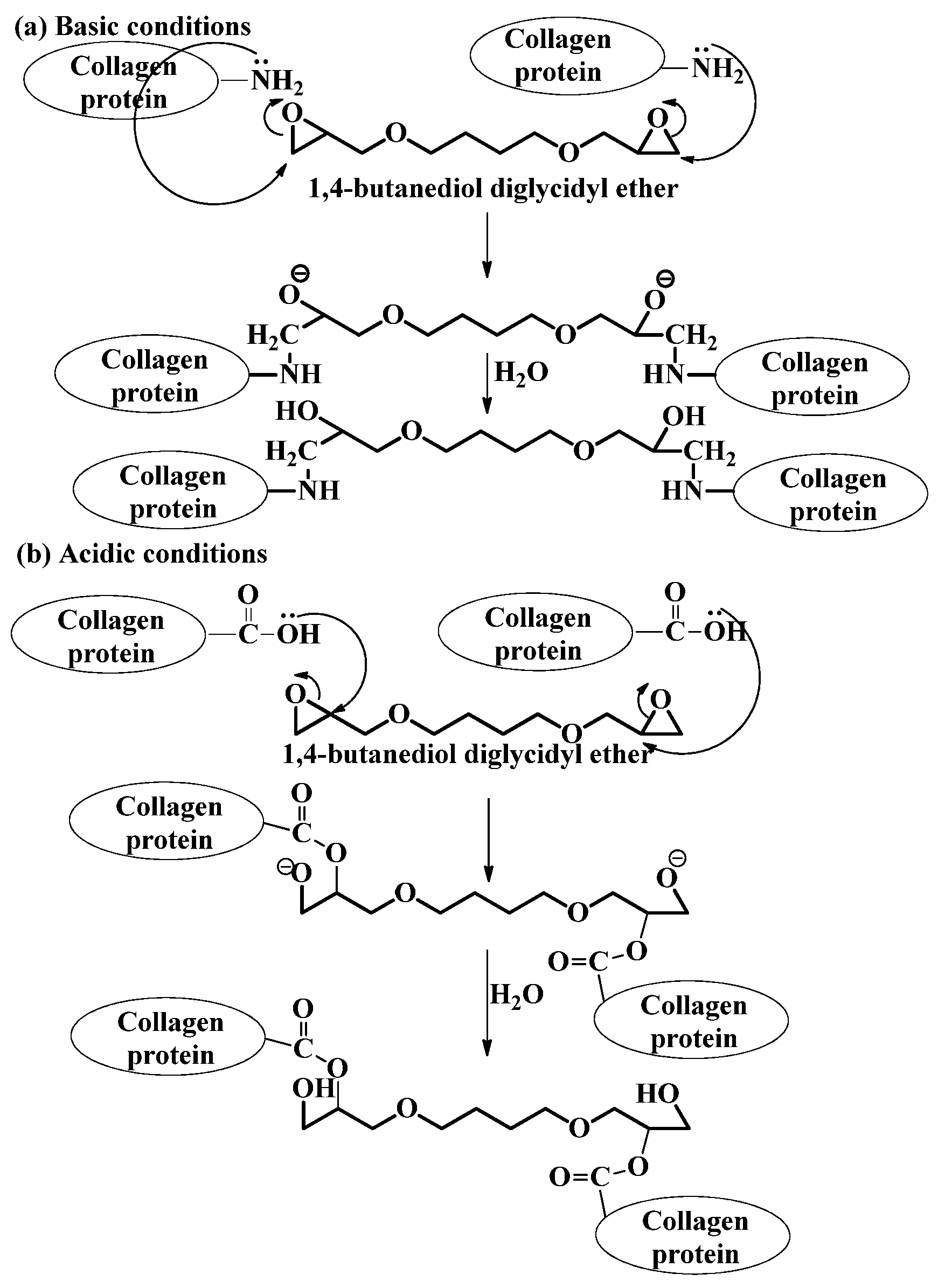
2.2.2. Epoxy Compounds
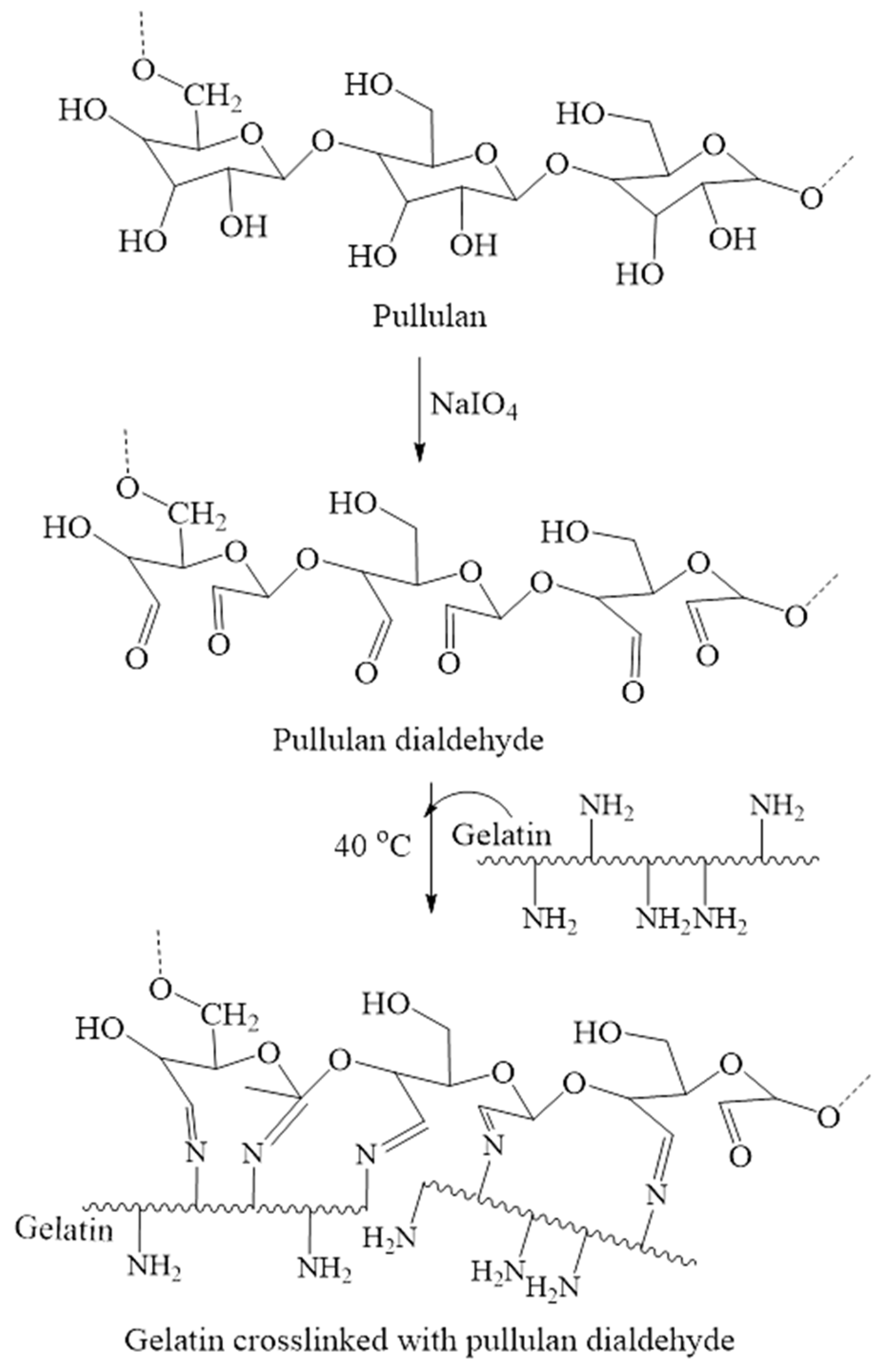
2.2.3. Polysaccharide Derivatives Containing Aldehyde Groups
2.2.4. N-hydroxysulfosuccinimide and Aryl Sulfonyl Fluoride
2.2.5. Quinone Methides
2.2.6. β-[tris(hydroxymethyl) phosphino] Propanoic Acid (THPP)
2.2.7. Protein Partners-SpyTag/SpyCatcher System
2.2.8. Miscellaneous Crosslinkers
3. Conclusions
4. Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuentes-Lemus, E.; Hägglund, P.; López-Alarcón, C.; Davies, M.J. Oxidative Crosslinking of Peptides and Proteins: Mechanisms of Formation, Detection, Characterization and Quantification. Molecules 2022, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Orqueda, M.E.; Gómez-Mascaraque, L.G.; Isla, M.I.; López-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.H.; Perwez, M.; Sardar, M. Protein crosslinking: Uses in chemistry, biology and biotechnology. Biocatal. Biotransformation 2020, 38, 178–201. [Google Scholar] [CrossRef]
- Wang, G.; Liu, N.; Guo, M. Use of whey protein as a natural polymer for tissue adhesive: Preliminary formulation and evaluation in vitro. Polymers 2018, 10, 843. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymer Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 95–118. [Google Scholar]
- Picchio, M.L.; Cuggino, J.C.; Nagel, G.; Wedepohl, S.; Minari, R.J.; Igarzabal, C.I.A.; Gugliotta, L.M.; Calderón, M. Crosslinked casein-based micelles as a dually responsive drug delivery system. Polym. Chem. 2018, 9, 3499–3510. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H.A.; Lee, M.; Shin, Y.; Song, J.J.; Kang, S.W.; Nam, D.H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef]
- Lenz, S.; Sinn, L.R.; O’Reilly, F.J.; Fischer, L.; Wegner, F.; Rappsilber, J. Reliable identification of protein-protein interactions by crosslinking mass spectrometry. Nat. Commun. 2021, 12, 3564. [Google Scholar] [CrossRef]
- Rai, K.; Sun, Y.; Shaliutina-Kolesova, A.; Nian, R.; Xian, M. Proteins: Natural polymers for tissue engineering. J. Biomater. Tissue Eng. 2018, 8, 295–308. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Brindha, J.; Balamurali, M.M.; Chanda, K. Evolutionary approaches in protein engineering towards biomaterial construction. RSC Adv. 2019, 9, 34720–34734. [Google Scholar]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-based drug-delivery materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Koh, L.-D.; Yeo, J.; Lee, Y.Y.; Ong, Q.; Han, M.; Tee, B.C. Advancing the frontiers of silk fibroin protein-based materials for futuristic electronics and clinical wound-healing (invited review). Mater. Sci. Eng. C 2018, 86, 151–172. [Google Scholar] [CrossRef]
- Humenik, M.; Winkler, A.; Scheibel, T. Patterning of protein-based materials. Biopolymers. 2021, 112, e23412. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Wang, L.; Yang, Z. Recombinant proteins as cross-linkers for hydrogelations. Chem. Soc. Rev. 2013, 42, 891–901. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Adams, D.J. Controlling peptidebased hydrogelation. Mater. Today 2012, 15, 500–507. [Google Scholar] [CrossRef]
- Li, H.; Kong, N.; Laver, B.; Liu, J. Hydrogels constructed from engineered proteins. Small 2016, 12, 973–987. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (elp-ha) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Tayri-Wilk, T.; Milhem, H.; Kalisman, N. Open Search Strategy for Inferring the Masses of Cross-Link Adducts on Proteins. Anal. Chem. 2020, 92, 15899–15907. [Google Scholar] [CrossRef] [PubMed]
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Isaschar-Ovdat, S.; Fishman, A. Crosslinking of food proteins mediated by oxidative enzymes—A review. Trends Food Sci. Technol. 2018, 72, 134–143. [Google Scholar] [CrossRef]
- Mishra, P.K.; Yoo, C.-M.; Hong, E.; Rhee, H.W. Photo-crosslinking: An Emerging Chemical Tool for Investigating Molecular Networks in Live Cells. ChemBioChem 2020, 21, 924. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Aslam, N.A.; Zheng, F.; Yang, B.; Cheng, R.; Wang, N.; Rozovsky, S.; Wang, P.G.; Wang, Q.; et al. Genetically encoding photocaged quinone methide to multitarget protein residues covalently in vivo. J. Am. Chem. Soc. 2019, 141, 9458–9462. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.a.J. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Granelli, J.; Lyubovitsky, J. Effects of zero-length and non-zero-length cross-linking reagents on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interface 2012, 4, 261–267. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C.; Chiu, C.T. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J. Biomed. Mater. Res. 1998, 42, 560–567. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.C.; Liang, H.C.; Sung, H.W. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 2002, 23, 2447–2457. [Google Scholar] [CrossRef]
- Levy, R.J.; Schoen, F.J.; Sherman, F.S.; Nichols, J.; Hawley, M.A.; Lund, S.A. Calcification of subcutaneously implanted type i collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am. J. Pathol. 1986, 122, 71–82. [Google Scholar] [PubMed]
- Liu, Z.; Zhou, Q.; Zhu, J.; Xiao, J.; Wan, P.; Zhou, C.; Huang, Z.; Qiang, N.; Zhang, W.; Wu, Z. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials 2012, 33, 7336–7346. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; May, R.D.; Bakirci, E.; Tekari, A.; Chan, S.C.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Genipin-enhanced fibrin hydrogel and novel silk for intervertebral disc repair in a loaded bovine organ culture model. J. Funct. Biomater. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Fessel, G.; Gerber, C.; Snedeker, J.G. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. J. Shoulder Elb. Surg. 2012, 21, 209–217. [Google Scholar] [CrossRef]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose-and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics–toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef]
- Martinez, A.W.; Caves, J.M.; Ravi, S.; Li, W.; Chaikof, E.L. Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers. Acta Biomater. 2014, 10, 26–33. [Google Scholar] [CrossRef]
- Hrabchak, C.; Rouleau, J.; Moss, I.; Woodhouse, K.; Akens, M.; Bellingham, C.; Keeley, F.; Dennis, M.; Yee, A. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 2010, 6, 2108–2115. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Liang, J.; Wang, H.; Zhu, D.; Leng, X.; Wang, C.; Kong, D.; Lv, F. A visible fluorescent nanovaccine based on functional genipin crosslinked ovalbumin protein nanoparticles. Nanomedicine 2018, 14, 1087–1098. [Google Scholar] [CrossRef]
- Lin, H.-C.; Wang, B.-J.; Weng, Y.-M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT 2019, 118, 108813. [Google Scholar] [CrossRef]
- Elliott, W.H.; Bonani, W.; Maniglio, D.; Motta, A.; Tan, W.; Migliaresi, C. Silk hydrogels of tunable structure and viscoelastic properties using different chronological orders of genipin and physical cross-linking. ACS Appl. Mater. Interfaces 2015, 7, 12099–12108. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Li, Y.; Yang, Y. Alkali-catalyzed low temperature wet crosslinking of plant proteins using carboxylic acids. Biotechnol. Prog. 2009, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, L.; Xu, L.; Yang, Y. Low-temperature crosslinking of proteins using non-toxic citric acid in neutral aqueous medium: Mechanism and kinetic study. Ind. Crops Prod. 2015, 74, 234–240. [Google Scholar] [CrossRef]
- Nataraj, D.; Sakkara, S.; Meenakshi, H.; Reddy, N. Properties and applications of citric acid crosslinked banana fibre-wheat gluten films. Ind. Crop Prod. 2018, 124, 265–272. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Emam-Djomeh, Z.; Momen, S.; Moosavi-Movahedi, A.A. Gelation of oil-in-water emulsions stabilized by heat-denatured and nanofibrillated whey proteins through ion bridging or citric acid-mediated cross-linking. Int. J. Biol. Macromol. 2018, 120, 2247–2258. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; de la Caba, K. The effect of cross-linking with citric acid on the properties of agar/fish gelatin films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.; Boiteux, J.; Silva, M.F. Green analytical chemistry metrics: Towards a sustainable phenolics extraction from medicinal plants. Microchem. J. 2018, 141, 438–443. [Google Scholar] [CrossRef]
- Fujimoto, N.; Kohta, R.; Kitamura, S. Estrogenic activity of an antioxidant, nordihydroguaiaretic acid (ndga). Life Sci. 2004, 74, 1417–1425. [Google Scholar] [CrossRef]
- Koob, T.J.; Hernandez, D.J. Mechanical and thermal properties of novel polymerized ndga–gelatin hydrogels. Biomaterials 2003, 24, 1285–1292. [Google Scholar] [CrossRef]
- Ju, Y.M.; Yu, B.; Koob, T.J.; Moussy, Y.; Moussy, F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J. Biomed. Mater. Res. Part A 2008, 87, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.-Q.; Xue, Z.-J.; Liao, S.-T.; Wu, Y.-B.; Liu, Y. The effect of ndga-modified etchant on the enzymatic degradation resistance and mechanical properties of collagen matrix. Chin. Chem. Lett. 2018, 29, 205–208. [Google Scholar] [CrossRef]
- Koob, T.J.; Pringle, D.; Hernandez, D. Methods of making high-strength NDGA polymerized collagen fibers and related collagen-prep methods, medical devices and constructs. U.S. Patent No. 9, 27 December 2007. [Google Scholar]
- Bagchi, D.; Sen, C.K.; Ray, S.D.; Das, D.K.; Bagchi, M.; Preuss, H.G.; Vinson, J.A. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. 2003, 523, 87–97. [Google Scholar] [CrossRef]
- Nomoto, H.; Iigo, M.; Hamada, H.; Kojima, S.; Tsuda, H. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr. Cancer 2004, 49, 81–88. [Google Scholar] [CrossRef]
- Strek, M.; Gorlach, S.; Podsedek, A.; Sosnowska, D.; Koziolkiewicz, M.; Hrabec, Z.; Hrabec, E. Procyanidin oligomers from japanese quince (chaenomeles japonica) fruit inhibit activity of mmp-2 and mmp-9 metalloproteinases. J. Agric. Food Chem. 2007, 55, 6447–6452. [Google Scholar] [CrossRef]
- Teissedre, P.L.; Frankel, E.N.; Waterhouse, A.L.; Peleg, H.; German, J.B. Inhibition ofin vitrohuman ldl oxidation by phenolic antioxidants from grapes and wines. J. Sci. Food Agric 1996, 70, 55–61. [Google Scholar] [CrossRef]
- Singh, R.P.; Tyagi, A.K.; Dhanalakshmi, S.; Agarwal, R.; Agarwal, C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int. J. Cancer 2004, 108, 733–740. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.F. Variability of the polyphenolic composition of cider apple (malus domestica) fruits and juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef]
- Zhai, W.; Chang, J.; Lü, X.; Wang, Z. Procyanidins-crosslinked heart valve matrix: Anticalcification effect. J. Biomed. Mater. Res. 2009, 90, 913–921. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, W.; Wu, C.; Ma, B.; Zhang, J.; Zhang, H.; Zhu, Z.; Chang, J. Procyanidins-crosslinked aortic elastin scaffolds with distinctive anti-calcification and biological properties. Acta Biomater. 2015, 16, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, B.; Chang, J. Preparation of decellularized vascular matrix by co-crosslinking of procyanidins and glutaraldehyde. Biomed. Mater. Eng. 2015, 26, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Rivero, S.; García, M.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer capsules of bovine serum albumin and tannic acid for controlled release by enzymatic degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef]
- Hedman, T.P.; Saito, H.; Vo, C.; Chuang, S.Y. Exogenous cross-linking increases the stability of spinal motion segments. Spine 2006, 31, E480–E485. [Google Scholar] [CrossRef]
- Popovich, J.M., Jr.; Yau, D.; Chuang, S.-Y.; Hedman, T.P. Exogenous collagen crosslinking of the intervertebral disc restores joint stability after lumbar posterior decompression surgery. Spine 2011, 36, 939–944. [Google Scholar] [CrossRef]
- Koob, T.J.; Willis, T.A.; Hernandez, D.J. Biocompatibility of ndga-polymerized collagen fibers. I. Evaluation of cytotoxicity with tendon fibroblasts in vitro. J. Biomed. Mater. Res. 2001, 56, 31–39. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Nie, X.; Gong, Y.; Wang, N.; Meng, X. Preparation and characterization of edible myofibrillar protein-based film incorporated with grape seed procyanidins and green tea polyphenol. LWT-Food Sci. Technol. 2015, 64, 1042–1046. [Google Scholar] [CrossRef]
- López-Alonso, J.P.; Diez-García, F.; Font, J.; Ribó, M.; Vilanova, M.; Scholtz, J.M.; González, C.; Vottariello, F.; Gotte, G.; Libonati, M. Carbodiimide edc induces cross-links that stabilize rnase a c-dimer against dissociation: Edc adducts can affect protein net charge, conformation, and activity. Bioconjug. Chem. 2009, 20, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Cammarata, C.R.; Park, H.J.; Rhodes, B.T.; Ofner, C.M., 3rd. Preparation, drug release, and cell growth inhibition of a gelatin: Doxorubicin conjugate. Pharm. Res. 2013, 30, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, C.R.; Hughes, M.E.; Ofner, C.M., 3rd. Carbodiimide induced cross-linking, ligand addition, and degradation in gelatin. Mol. Pharm. 2015, 12, 783–793. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Chou, S.F.; Luo, L.J.; Lai, J.Y.; Ma, D.H.K. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Mater. Sci. Eng. C 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; Van Wachem, P.; Van Luyn, M.; Hendriks, M.; Cahalan, P.; Feijen, J. Crosslinking and modification of dermal sheep collagen using 1, 4-butanediol diglycidyl ether. J. Biomed. Mater. Res. 1999, 46, 424–433. [Google Scholar] [CrossRef]
- Dulnik, J.; Sajkiewicz, P. Crosslinking of Gelatin in Bicomponent Electrospun Fibers. Materials 2021, 14, 3391–3403. [Google Scholar] [CrossRef]
- Martucci, J.F.; Espinosa, J.P.; Ruseckaite, R.A. Physicochemical properties of films based on bovine gelatin cross-linked with 1, 4-butanediol diglycidyl ether. Food Bioproc. Technol. 2015, 8, 1645–1656. [Google Scholar] [CrossRef]
- Dias, J.; Baptista-Silva, S.; de Oliveira, C.; Sousa, A.; Oliveira, A.L.; Bártolo, P.; Granja, P. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; Gomes da Silva, R.L.C.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. B 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Q.; Liu, J.; Pei, Y.; Tang, K. A unique high mechanical strength dialdehyde microfibrillated cellulose/gelatin composite hydrogel with a giant network structure. RSC Adv. 2016, 6, 71999–72007. [Google Scholar] [CrossRef]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin–peg composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, characterization and feasibility study of dialdehyde carboxymethyl cellulose as a novel crosslinking reagent. Carbohydr. Polym. 2016, 137, 632–641. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, W.; Liu, Y.; Li, W.; Fan, D.; Zhu, C.; Wang, Y. Hlc/pullulan and pullulan hydrogels: Their microstructure, engineering process and biocompatibility. Mater. Sci. Eng. C 2016, 58, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, H.; Schnier, P.D.; Liu, Y.; Liu, J.; Wang, N.; DeGrado, W.F.; Wang, L. Proximity-enhanced sufex chemical cross-linker for specific and multitargeting cross-linking mass spectrometry. Proc. Natl. Acad. Sci. USA 2018, 115, 11162–11167. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Tortosa, M. Para-quinone methide: A new player in asymmetric catalysis. ChemCatChem 2015, 7, 1524–1526. [Google Scholar] [CrossRef]
- Gnaim, S.; Shabat, D. Quinone-methide species, a gateway to functional molecular systems: From self-immolative dendrimers to long-wavelength fluorescent dyes. Acc. Chem. Res. 2014, 47, 2970–2984. [Google Scholar] [CrossRef]
- Bai, W.J.; David, J.G.; Feng, Z.G.; Weaver, M.G.; Wu, K.L.; Pettus, T.R. The domestication of ortho-quinone methides. Acc. Chem. Res. 2014, 47, 3655–3664. [Google Scholar] [CrossRef]
- Liu, J.; Cai, L.; Sun, W.; Cheng, R.; Wang, N.; Jin, L.; Rozovsky, S.; Seiple, I.; Wang, L. Photocaged quinone methide cross-linkers for light-controlled chemical cross-linking of protein-protein and protein-DNA complexes. Angew. Chem. Int. Ed. 2019, 58, 18839–18843. [Google Scholar] [CrossRef]
- Hu, W.; Yuan, Y.; Wang, C.H.; Tian, H.T.; Guo, A.D.; Nie, H.J.; Hu, H.; Tan, M.; Zhuo Tang, Z.; Chen, X.H. Genetically Encoded Residue-Selective Photo-Crosslinker to Capture Protein-Protein Interactions in Living Cells. Chem 2019, 5, 2955–2968. [Google Scholar] [CrossRef]
- Lim, D.W.; Nettles, D.L.; Setton, L.A.; Chilkoti, A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules 2007, 9, 222–230. [Google Scholar] [CrossRef]
- Chung, C.; Anderson, E.; Pera, R.R.; Pruitt, B.L.; Heilshorn, S.C. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3d cultures. Soft Matter 2012, 8, 10141–10148. [Google Scholar] [CrossRef]
- Chung, C.; Lampe, K.J.; Heilshorn, S.C. Tetrakis (hydroxymethyl) phosphonium chloride as a covalent cross-linking agent for cell encapsulation within protein-based hydrogels. Biomacromolecules 2012, 13, 3912–3916. [Google Scholar] [CrossRef] [PubMed]
- Vullo, W. Hydroxymethyl replacement reactions of tetrakis (hydroxymethyl) phosphonium chloride. Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 346–349. [Google Scholar] [CrossRef]
- Frank, A.W.; Daigle, D.J.; Vail, S.L. Chemistry of hydroxymethyl phosphorus compounds: Part iii. Phosphines, phosphine oxides, and phosphonium hydroxides. Text. Res. J. 1982, 52, 738–750. [Google Scholar] [CrossRef]
- Brindha, J.; Chanda, K.; Balamurali, M.M. Revisiting the insights and applications of protein engineered hydrogels. Mater. Sci. Eng. C 2019, 95, 312–327. [Google Scholar]
- Nettles, D.L.; Chilkoti, A.; Setton, L.A. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010, 62, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, W.-B.; Mahdavi, A.; Arnold, F.H.; Tirrell, D.A. Synthesis of bioactive protein hydrogels by genetically encoded spytag-spycatcher chemistry. Proc. Natl. Acad. Sci. USA 2014, 111, 11269–11274. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Sun, F.; Tirrell, D.A.; Arnold, F.H. Controlling macromolecular topology with genetically encoded spytag–spycatcher chemistry. J. Am. Chem. Soc. 2013, 135, 13988–13997. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yu, S.; Liu, J. Hierarchical Self-Assembly of Proteins Through Rationally Designed Supramolecular Interfaces. Front. Bioeng. Biotechnol. 2020, 8, 295. [Google Scholar] [CrossRef]
- Botyanszki, Z.; Tay, P.K.R.; Nguyen, P.Q.; Nussbaumer, M.G.; Joshi, N.S. Engineered catalytic biofilms: Site-specific enzyme immobilization onto e. Coli curli nanofibers. Biotechnol. Bioeng. 2015, 112, 2016–2024. [Google Scholar] [CrossRef]
- Bruins, J.J.; Albada, B.; van Delft, F. Ortho-quinones and analogues thereof: Highly reactive intermediates for fast and selective biofunctionalization. Chem. Eur. J. 2018, 24, 4749–4756. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, X.; Li, X.D. A bifunctional amino acid to study protein–protein interactions. RSC Adv. 2020, 10, 42076–42083. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]


| Natural Chemical Crosslinker | Reactive Moieties/Crosslinking Conditions | Limitations | Applications and References |
|---|---|---|---|
Genipin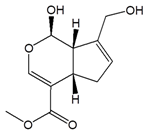 |
|
| |
|
| ||
Nordihydroguaiaretic acid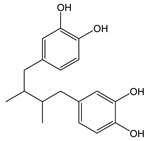 |
|
|
|
|
| ||
Tannic acid |
|
| |
| |||
Procyanidins |
|
|
|
|
| ||
Citric acid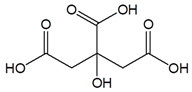 |
|
|
|
|
|
| Synthetic Crosslinkers | Reactive Moieties/Crosslinking Conditions | Limitations | Applications and References |
|---|---|---|---|
Carbodiimide agents- EDC-NHS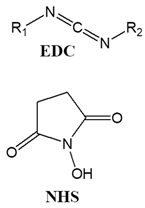 |
|
|
|
|
| ||
|
| ||
Epoxy compounds -1,4, butanediol diglycidyl ether (BDDGE) |
|
| |
|
| ||
Pullulan dialdehyde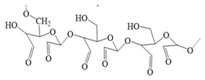 |
|
|
|
|
| ||
|
| ||
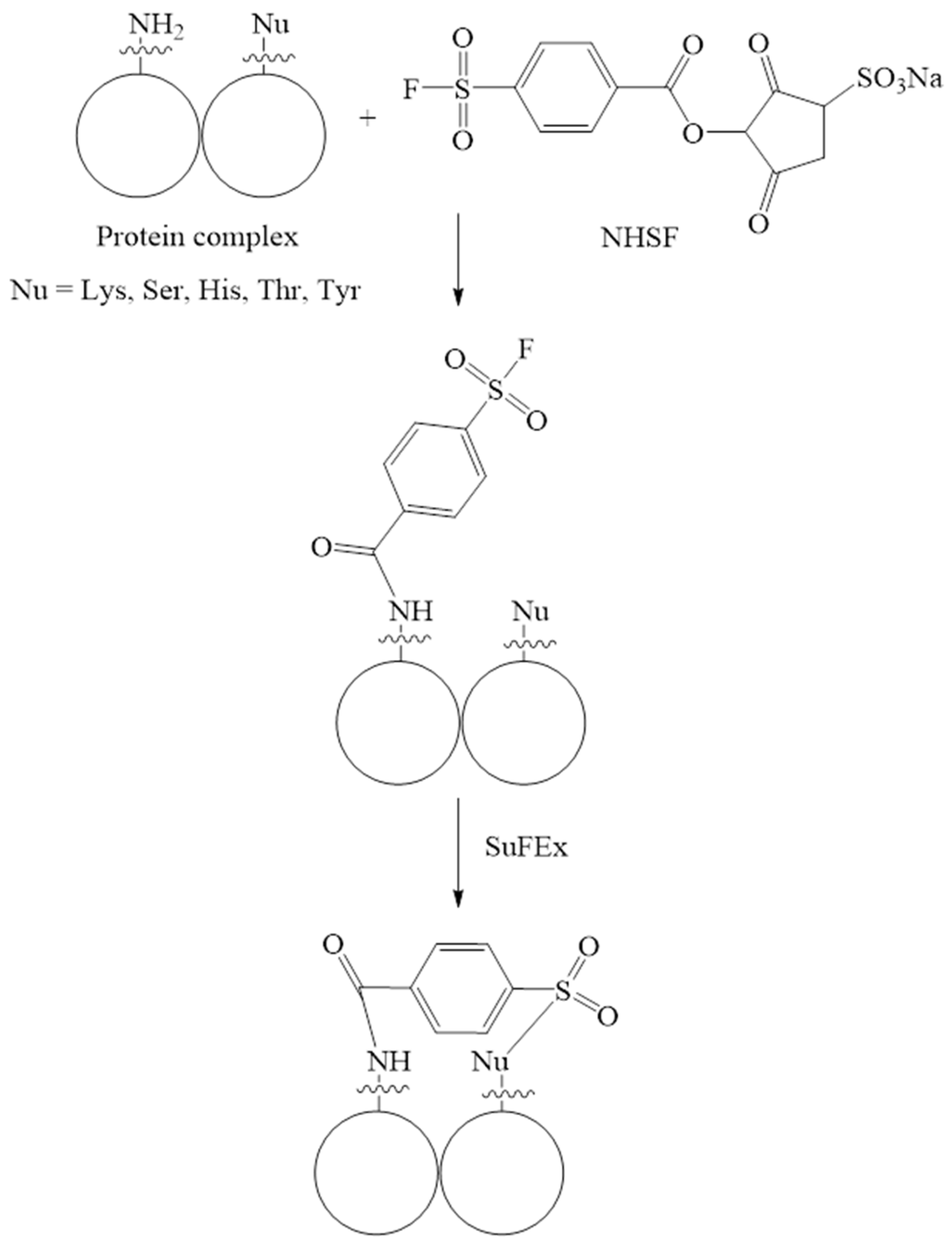
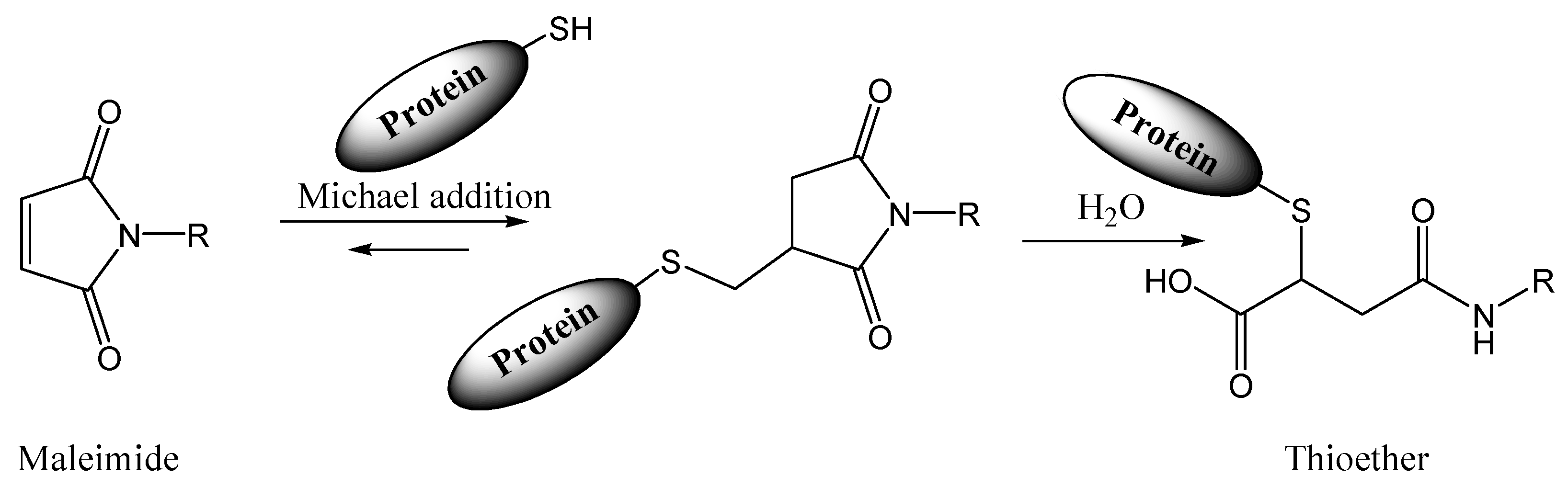
N-hydroxysulfosuccinimide and aryl sulfonyl fluoride (NHSF)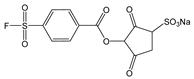 |
|
|
|
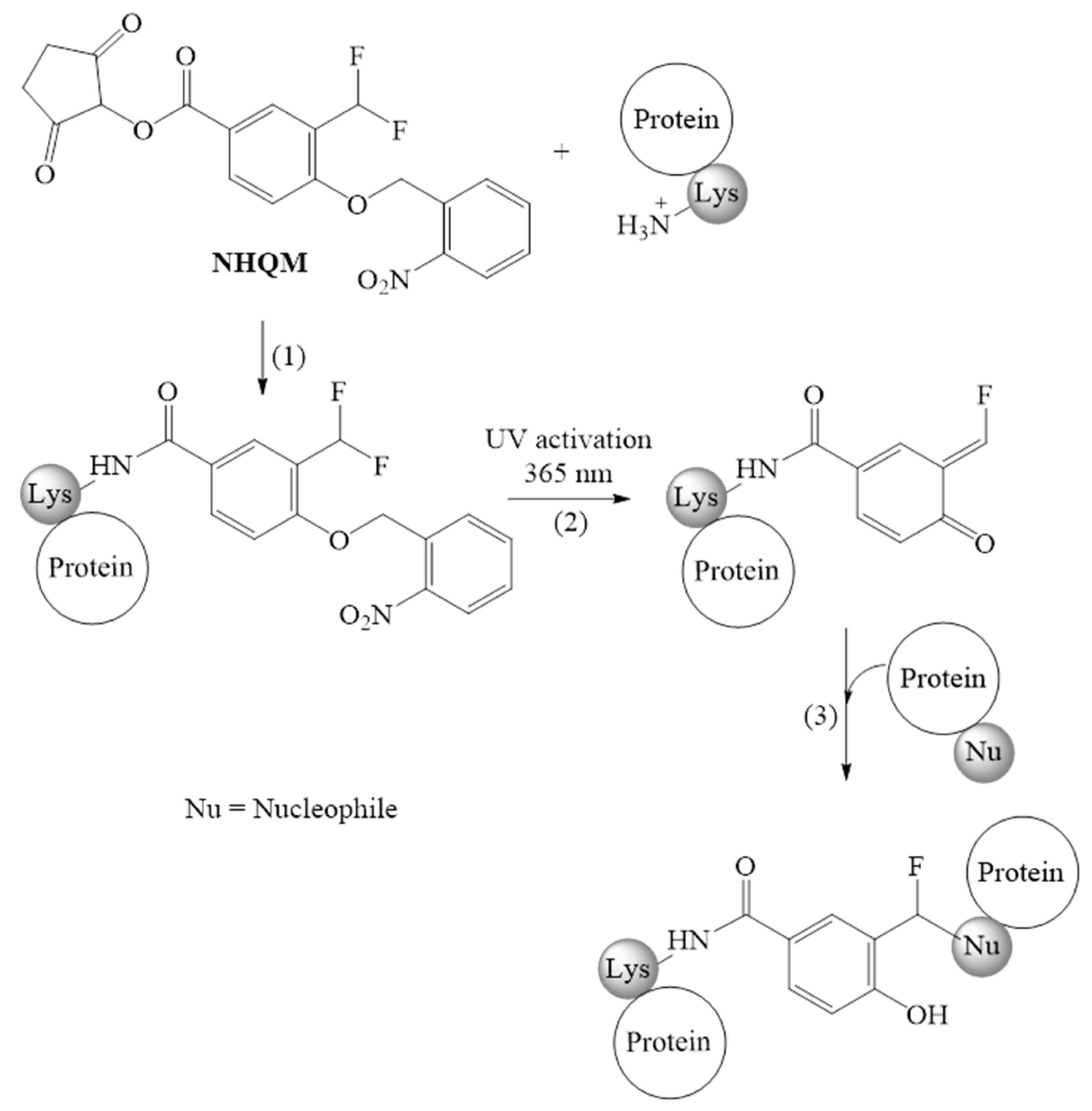
Quinone methides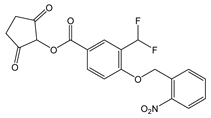 |
|
| |
|
| ||
|
| ||
|
| ||
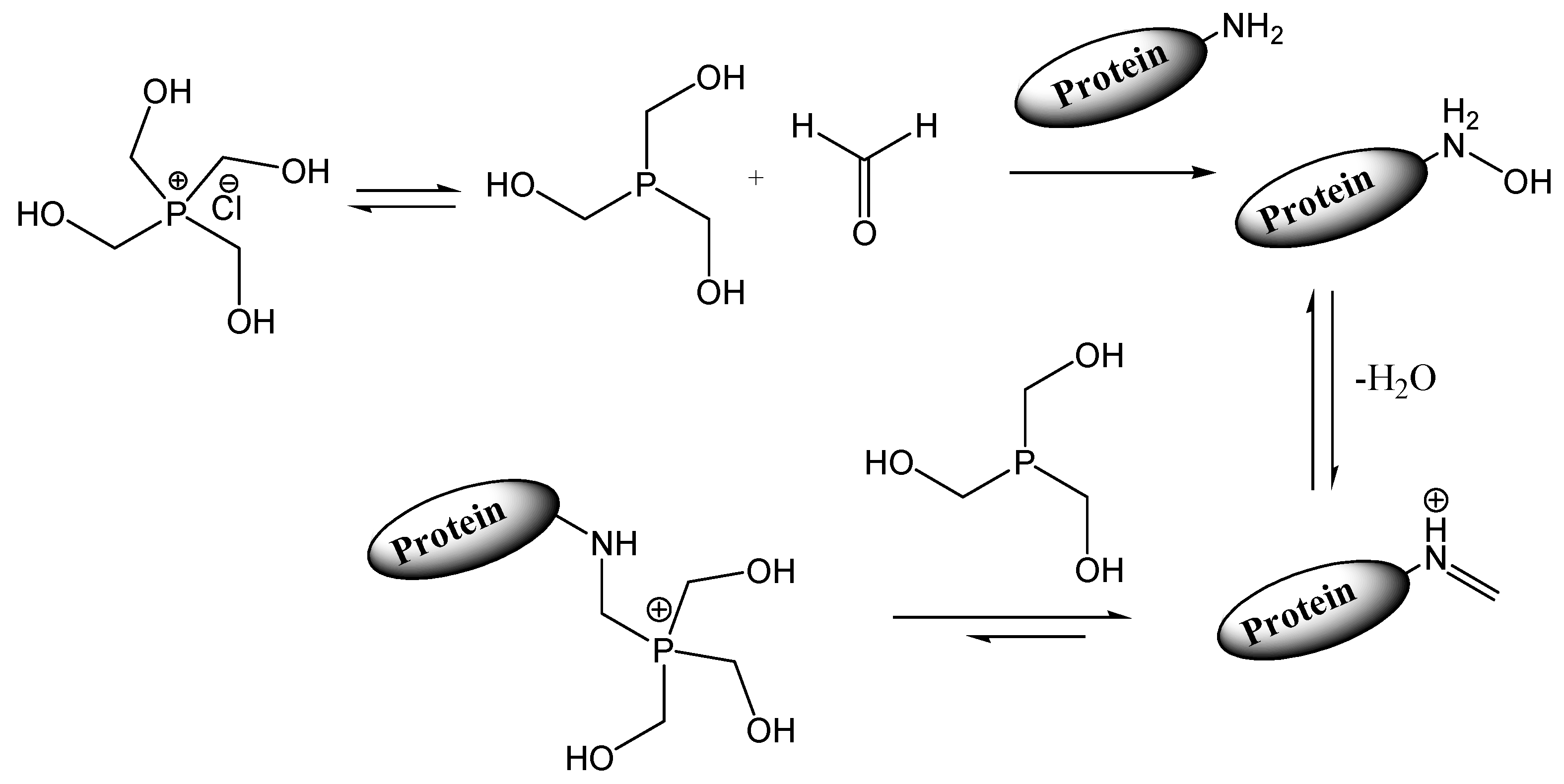
Tetrakis (hydroxymethyl) phosphonium chloride (THPC) |
|
| |
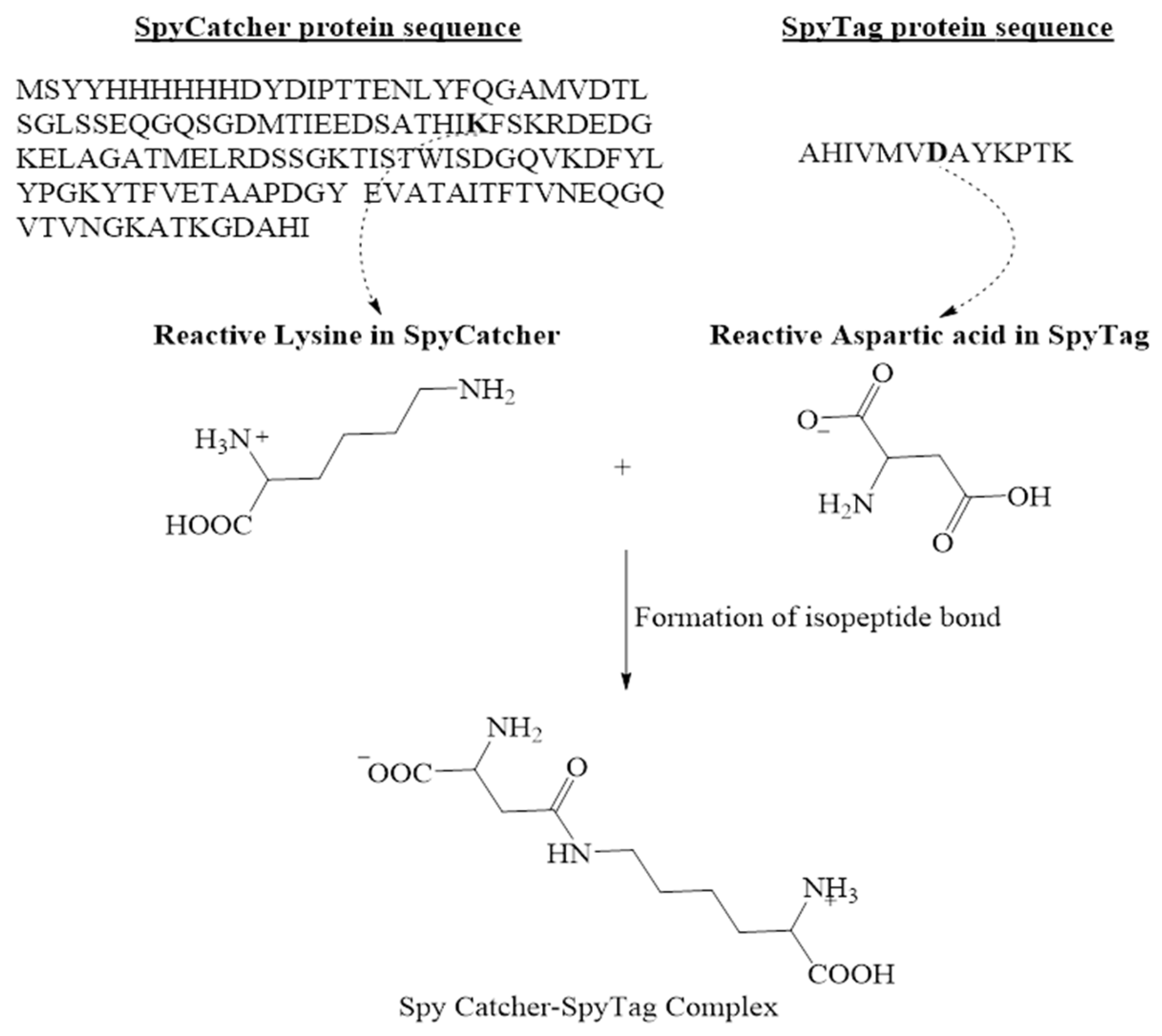
Protein partners-SpyTag/SpyCatcher system |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayachandran, B.; Parvin, T.N.; Alam, M.M.; Chanda, K.; MM, B. Insights on Chemical Crosslinking Strategies for Proteins. Molecules 2022, 27, 8124. https://doi.org/10.3390/molecules27238124
Jayachandran B, Parvin TN, Alam MM, Chanda K, MM B. Insights on Chemical Crosslinking Strategies for Proteins. Molecules. 2022; 27(23):8124. https://doi.org/10.3390/molecules27238124
Chicago/Turabian StyleJayachandran, Brindha, Thansila N Parvin, M Mujahid Alam, Kaushik Chanda, and Balamurali MM. 2022. "Insights on Chemical Crosslinking Strategies for Proteins" Molecules 27, no. 23: 8124. https://doi.org/10.3390/molecules27238124
APA StyleJayachandran, B., Parvin, T. N., Alam, M. M., Chanda, K., & MM, B. (2022). Insights on Chemical Crosslinking Strategies for Proteins. Molecules, 27(23), 8124. https://doi.org/10.3390/molecules27238124








