1. Introduction
An ever-increasing pressure on resources and environmental protection, especially in CO
2 reduction [
1], leads to “a systemic change in the use and recovery of resources in the economy” through a clear transition to a regenerative circular economy [
2,
3] by creating a closed-loop system that minimizes the use of resource inputs and the creation of wastes, pollution and overall carbon emissions [
3]. From this perspective, the reuse of opportunely treated municipal solid wastes (MSWs) represent a good starting point to fulfil the circular economy goals [
4]. To promote waste treatment options in line with the EU waste hierarchy based on prevention, preparing for reuse, recycling, recovery and disposal, the European Commission has adopted a Circular Economy Package [
5] that includes a ban on separately collected waste landfilling, a common EU target of 65% of recycled municipal waste and the reduction to maximum 10% of landfilled municipal waste by 2030.
The most attractive characteristic of the MSW is surely the possibility to extract the great amount of chemical energy stored in it. The calorific value which can be generated from municipal waste is around 8–12 MJ/kg [
6]. Up to date incineration is the most widespread approach for the energy recovery from MSWs [
7]. However, a number of post-treatments are necessary to eliminate the hazardous and toxic content from the ashes resulting from MSW incineration [
8]. In addition, flue gas emissions from the incineration of MSWs contain several pollutants as particulates, carbon oxides, nitrogen oxides, sulfur oxides, hydrochloric acid, heavy metals, dioxins (PCDD) and furans (PCDF) that require expensive cleaning systems. It has been reported that each ton of incinerated MSWs can produce 15–40 kg of hazardous wastes [
9]. The high variability and heterogeneity of MSW composition (e.g., seasonal, regional variability) [
10] heavily influences the emissions of pollutants generated during incineration as well as the thermochemical process performances, leading to a nonuniform thermal behavior and poor process outcome reproducibility [
11]. To overcome these difficulties and to respect the concepts of sustainability and the circular economy, based on material recycling and resource recovery, different MSW pretreatment approaches have been proposed. The aim of such pretreatments is to recover as many recyclable materials as possible, reduce the size of the feedstock and separate the combustible substances from noncombustible fraction and high-moisture materials (i.e., production of refuse-derived fuels, RDFs) [
12].
MSWs consist of three major fractions: a combustible fraction, a noncombustible fraction and moisture or volatile material. The combustible fraction is separated from the other two by specific sorting facilities [
12]. Typically, MSWs are processed to remove the recyclable fraction (e.g., metals), the inert fractions (e.g., glass) and separate the fine wet organic fraction (e.g., food and garden waste) containing high-moisture and high-ash material. There are two basic sorting methodologies which have been developed to produce MSWs-derived fuel [
13,
14]: the Mechanical Biological Treatment (MBT) and the Biological Drying Process. Since the RDF production concept ensures a certain degree of size reduction and the removal of organic and inert material, RDF is characterized, on average, by a higher heating value, lower ash content and a lower bulk density compared to untreated waste. As consequence, the composition [
15] and characteristics [
16] of RDF processed from MSWs showed narrower characteristics more suitable for thermochemical valorization purposes [
17] such as high calorific value (18–24 MJ/kg), low moisture content (3–6%) and high volatile (77–84%) and carbon (47–56%) content. It has to be mentioned that the use of RDF as a source of energy is encouraged as it is an integral part of waste management and it is regulated by EU regulations (EU Parliament Directive 2008/98/EC on waste) [
17].
Pyrolysis and gasification are emerging as promising thermochemical processes for MSW and RDF treatments. Compared to incineration, MSW pyrolysis and gasification are very attractive technologies as they increase energy recovery efficiency, reduce the dimension of the section of post-treatment for pollutants emissions control, prevent PCDD/PCDF formation (due to reducing conditions) and possibly generate value-added products as chemical platforms or fuels [
18]. Pyrolysis is a thermal degradation process to convert a carbon-rich matrix into solid, liquid and gas products in the absence of oxygen and at elevated temperature (400–700 °C) [
19]. Characteristics and yield of the pyrolysis products strongly depend on the choice of operating parameters such as process temperature, feedstock resident time, heating rate and volatiles residence time. Pressure is not expressly listed among the pyrolysis operating variables since it indirectly affects the volatiles residence time [
20,
21]. Depending on the operating conditions, the pyrolysis process can be classified as slow, conventional, fast or flash. In slow pyrolysis the heating rate is kept slow (approximately 0.1–1 °C/s) to obtain higher char yields at the expense of the other products. In conventional pyrolysis, moderate heating rates (up to 200–300 °C/min) are applied, leading to a more homogeneous distribution among the product yields. In fast pyrolysis, faster heating rates (between 10 and 200 °C/s) are applied to obtain high yields of liquid or gas products at the expense of a solid one. In flash pyrolysis, the heating rates are very high (>1000 °C/s) and the reaction times are of few to several seconds, allowing for the production of a high amount of liquid products [
20,
21].
Regarding the application of pyrolysis for waste treatment, many studies on the pyrolysis of MSWs and segregated MSWs are available [
22,
23,
24,
25], but it is necessary to underline that the pyrolysis process is more feasible for the treatment of homogeneous waste, which shows low variations in composition such as lignocellulosic biomasses. Indeed, the products obtained from the pyrolysis of MSW cannot be generalized because of the high variable thermal and chemical MSW compositions. Pyrolysis tests on MSWs and segregated MSWs have been mainly performed in fixed bed and tubular reactors, auger reactors, batch and semi batch reactors, fluidized-bed reactors and rotary kilns from lab scale up to pilot scale, mainly under slow and fast pyrolysis conditions. Very recently, microwave-assisted pyrolysis processes have also been proposed for MSW pyrolysis. In addition, in many cases catalytic pyrolysis processes are performed to improve the quality of gaseous and liquid products [
22,
23,
24,
25].
Gasification is a thermochemical process performed in presence of a sub-stoichiometric amount of oxygen with respect to that required for the complete combustion of a feedstock [
26]. The gasification process transforms an initial matrix into a gaseous energy carrier called a syngas, consisting mainly of H
2, CO, CH
4 and CO
2 [
27,
28]. However, this gas can be contaminated by inorganic and organic impurities such as solid particles, tars, sulfur and chlorine-containing compounds, etc. [
29]. More in detail, tar is a black-brown viscous liquid consisting of a mixture of high-molecular-weight hydrocarbons which can lead to machinery malfunctions and clogging [
30]. Different operating conditions are available for a gasification process: pressure can vary between 1 and 30 bar, the temperature can be set between 1000 and 1700 K, air, O
2 and steam can be used as gasifying agent and the gasifier can be a fixed bed, a fluidized bed, a rotary kiln, etc. [
28]. The quality of the gas is significantly affected by the gasification technology and the conditions applied. Typically, air-based gasifiers produce a syngas with a high nitrogen concentration and a lower heating value (4–7 MJ Nm
−3), while O
2/steam-based gasifiers produce a syngas with a high concentration of H
2 and CO and a LHV in the range of 10–20 MJ Nm
−3 [
28].
Gasification is a promising technology for the conversion of mixed solid waste into a valuable gas to be further used in power generation systems [
31,
32]. Batch and semibatch gasifiers [
33], fixed-bed gasifiers [
34], fluidized bed gasifiers [
35], entrained-flow gasifiers [
28], plasma-assisted gasifiers [
36] from the lab to pilot scale have been employed in the gasification of wastes, but a diffuse commercialization of such technologies still represent a challenge and only some of these plants (in Finland and Japan) are operating in the world. The high tar content in syngas is one of the most important disadvantages of the gasification process. The yield and characteristics of the tars depend on the feed composition and on the gasification technology used. Further reduction of tar can be achieved by physical or chemical ex situ methods such as scrubbing or secondary catalytic cracking [
37]. The interest towards pyrolysis or gasification as thermoconversion processes is not only related to the possibility to recover energy and fuels from RDFs, but also to recover added-value materials suitable for construction industry applications. Indeed, gasification and pyrolysis products (liquid and solids products) can be used as additives in the pavement industry, reducing the need of natural resources or petroleum derivatives [
4].
Despite the great interest in this field, the literature on the thermochemical treatment of a real RDF under pyrolysis and gasification conditions is still limited and a comprehensive characterization of the resulting products is rarely accomplished. In this paper, the results of pyrolysis and gasification tests on a real RDF in lab-scale reactors are reported. The performances obtained under the two different thermochemical processes (pyrolysis and gasification) are analyzed in terms of the yield, characteristics and quality of the products to highlight how the two thermochemical conversion processes can be used to accomplish waste-to-materials and waste-to-energy targets. In particular, pyrolysis tests have been performed in a fixed tubular reactor at three different temperatures (550, 650 and 750 °C) while the gasification test at 850 °C has been performed in a fluidized bed reactor to ensure extensive mixing between reagents and to keep a uniform process temperature with the aim of limiting the tar production.
The results herein disclose the aim to enrich the literature on the use of RDF as feedstock in thermochemical processes for the recovery of materials exploitable in the pavement industry. The circular-economy-based approach envisioned by this work is based on the exploitation of thermochemical processes to treat MSWs (or its fractions as Refuse-Derived Fuels, RDFs), whose residues (oil and char) can be used as additives in bitumen and asphalt preparation [
4]. In particular, the proposed approach is aimed to pursue different possible benefits: (i) replacement of petroleum-derived products (e.g., crude oil) with products from the transformation of urban solid wastes; (ii) improvement of the mechanical characteristics and the longevity of asphalts; (iii) rejuvenation of exhausted asphalts. These benefits are expected to greatly impact aged asphalts disposal in landfills, wastes treatment, CO
2 emission and production costs as a consequence of the increased asphalts duration.
3. Materials and Methods
3.1. Materials
The RDF (25 kg) was supplied by Calabra Maceri srl (Rende, CS, Italy) and stored in a dry place. The RDF presented a nonhomogeneous aspect (
Figure S2) and it was composed by large pieces of about 3 cm. Its average composition was: 84% plastics, 5.4% paper and cardboard, 3% multilayer packaging material, 2.5% wood; 0.7% organic fraction; 0.6% ceramics; 0.5% metals, 0.4% textile, 0.1% rubber; 0.1% glass, 0.1% leather, 2.6% others. The RDF-raw (residue-derived fuel as-received) was dried for 1 week (RDF-dry) at a controlled temperature (~30 °C) in order to reduce its moisture content (<10%) before its use in the pyrolysis and gasification tests, and grinded to reduce its size (RDF-milled), obtaining a more homogenous sample representative of the initial material to be used in characterization tests, in which a low amount of the sample is needed (elemental analysis, thermogravimetry).
In order to verify that the drying and the grinding treatments did not modify the feedstock main characteristics, the three different samples have been characterized using a macrobalance LECO 701 (as after reported), which allows to load the large pieces of about 3 cm of the starting material. It is possible to see from
Table S10 in the Supporting Information (SI) section that the values of the volatiles and the fixed carbon obtained on a dry basis or on a dry ash-free basis are very close to each other, confirming that the feedstock is not modified by the pretreatments. For this reason, only the label RDF was used to identify the feedstock.
3.2. Pyrolysis Tests
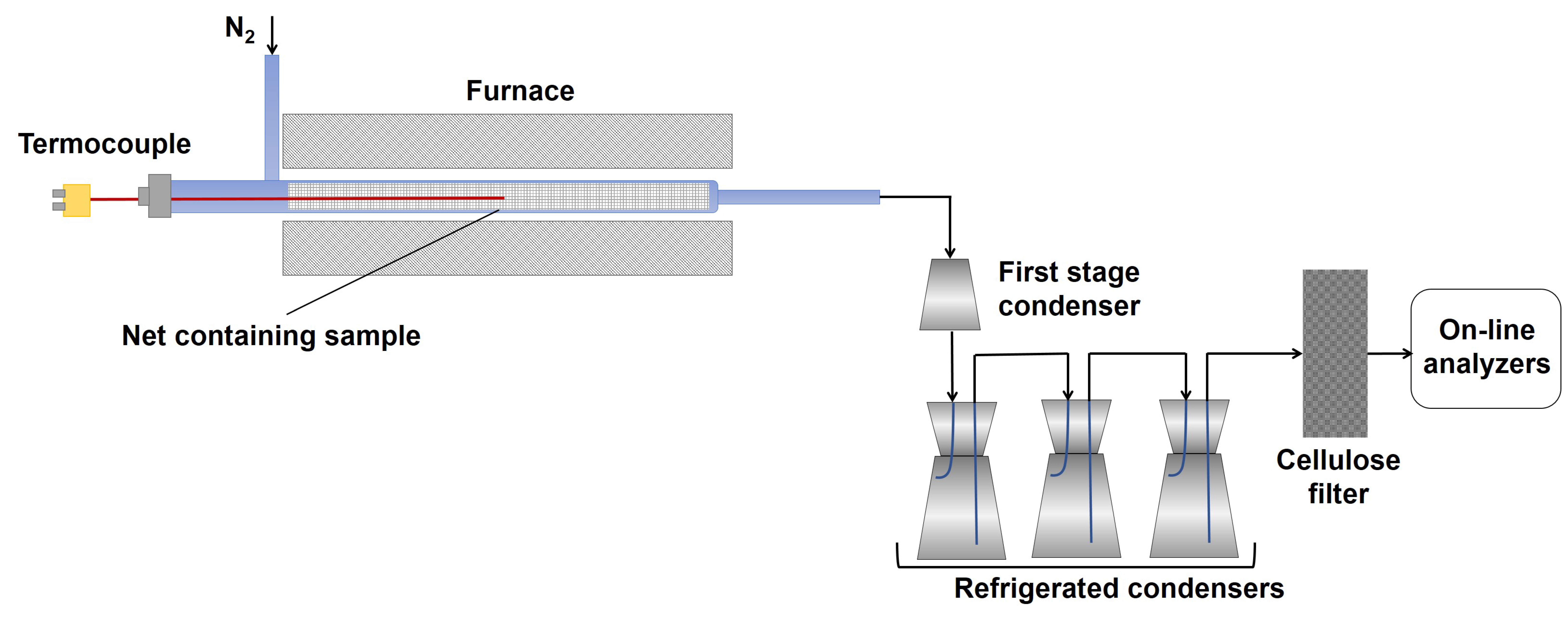
Three different pyrolysis tests have been performed at the three different final temperatures (550 °C, 650 °C, 750 °C), applying a heating rate (HR) of 30 °C/min under a nitrogen flux (60 NL/h). Two replicates of the same experimental run have been performed. The lab-scale plant used for pyrolysis testing is depicted in
Figure 12.
The pyrolysis lab-scale plant consists of a cylindric quartz reactor (internal diameter of 26 mm) located in a temperature-controlled furnace made up by a Kantal resistance wrapped by a high temperature (1000 °C) refractory ceramic mantel and of a condensing train made up by four Erlenmeyer flasks, one at room temperature (first condensing stage, named in the text as flask 1) and three flasks cooled at −12 °C (water/ice/NaCl cooling bath), referred in the text as flask 2.
For each test, about 3 g of the RDF was loaded into the reactor. The feedstock was preloaded in a basket of metal net (inox–stainless steel) before the introduction into the reactor to avoid the dragging of the lighter components of the feedstock and the clogging due to the possible melting of some feedstock components. The reactor was inserted into a furnace, and once the set point value was reached, the temperature was kept constant until no further gas production was detected.
The pyrolysis output consists of a solid residue (char), a mixture of condensable species (CS) and incondensable gases. The condensable species were collected in the four flasks, while the incondensable gases (CO
2, CH
4, CO, H
2) exiting the condensing train were continuously analyzed by online gas analyzers (ABB Magnos for O
2; ABB Uras 14 for CO and CO
2, ABB Caldos for H
2, Vario LUXX for CH
4) and also collected in sampling bags for offline analyses. The sampling bags were analyzed by a micro-gas chromatograph (Agilent 3000 A) equipped with 4 columns (OV-1, Alumina, PLOT-U, MS5A) for the determination of light hydrocarbons (C1–C6) in addition to H
2, CH
4, CO and CO
2. The characteristics and the overall analysis conditions are reported in the
SI section as Table S11, along with the detected species.
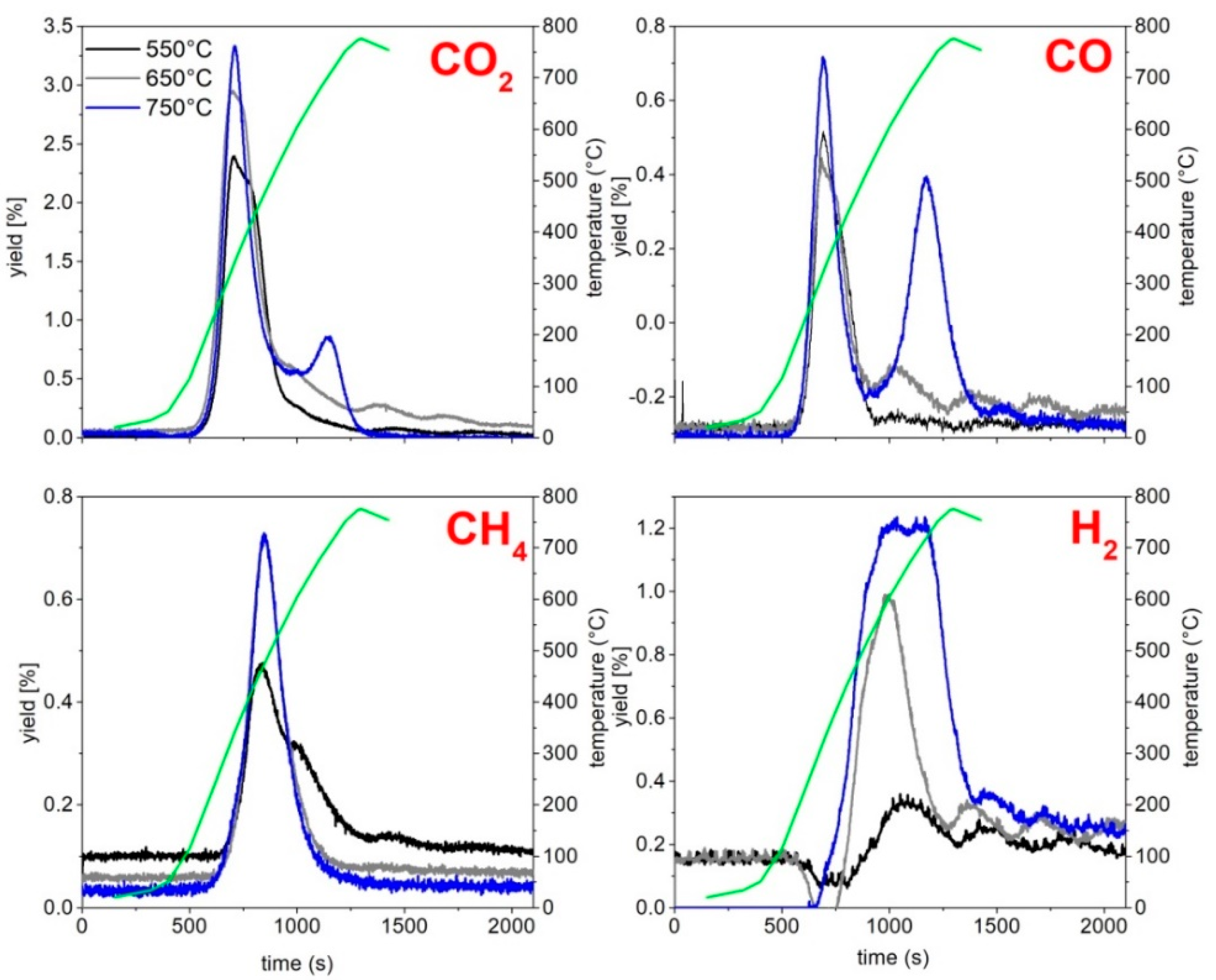
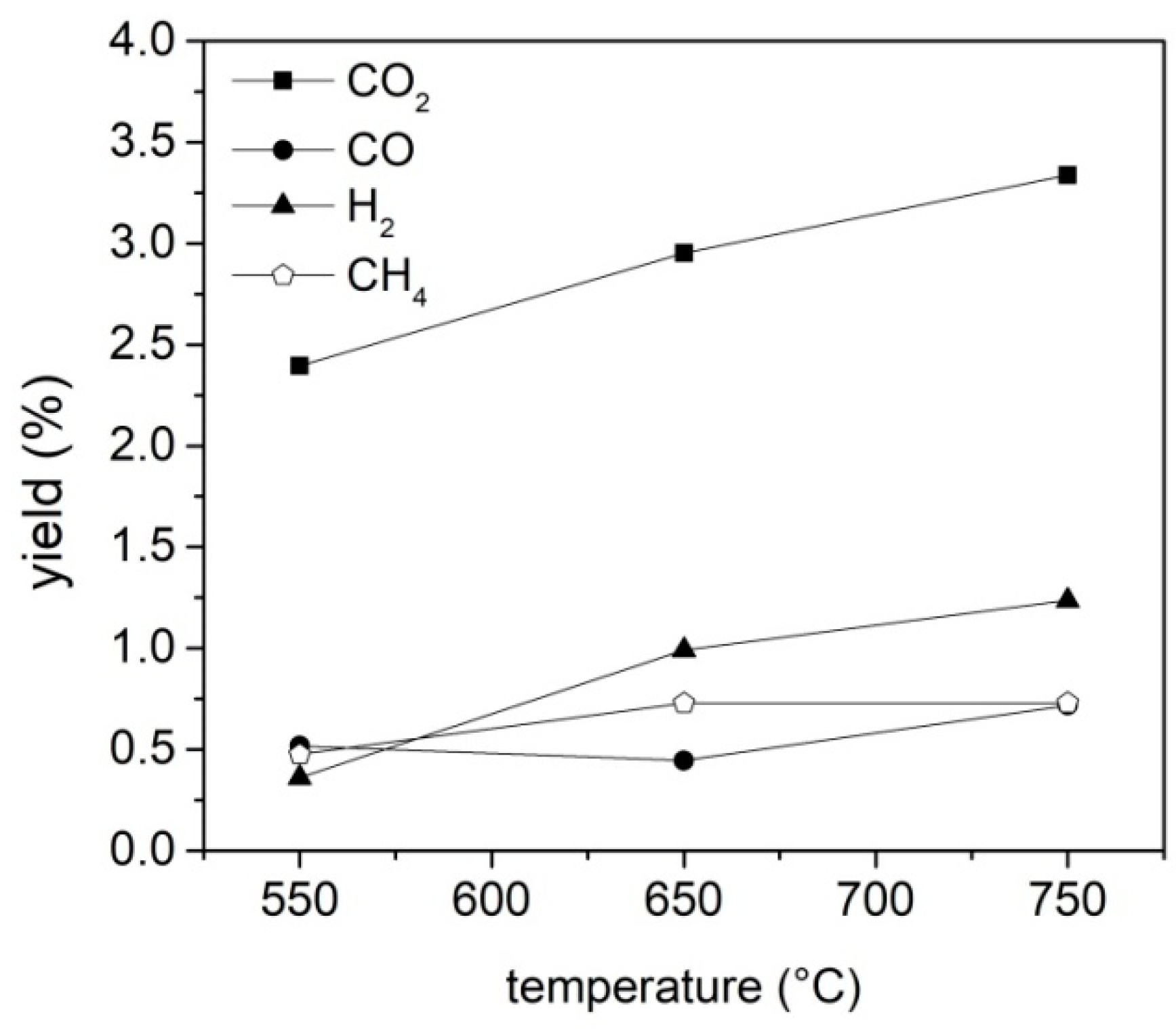
The amounts of CO
2, CH
4, CO and H
2 obtained during the tests have been evaluated by the integration of the gas profiles vs. time, while the amount of the other gaseous species detected in the bags (
Table S11) has been calculated by multiplying the mean concentration of each gas (obtained averaging the results of the analysis of each sampling bag) by the gas flow rate, assuming that the releasing time of each species was the same of that of methane estimated by online analysis.
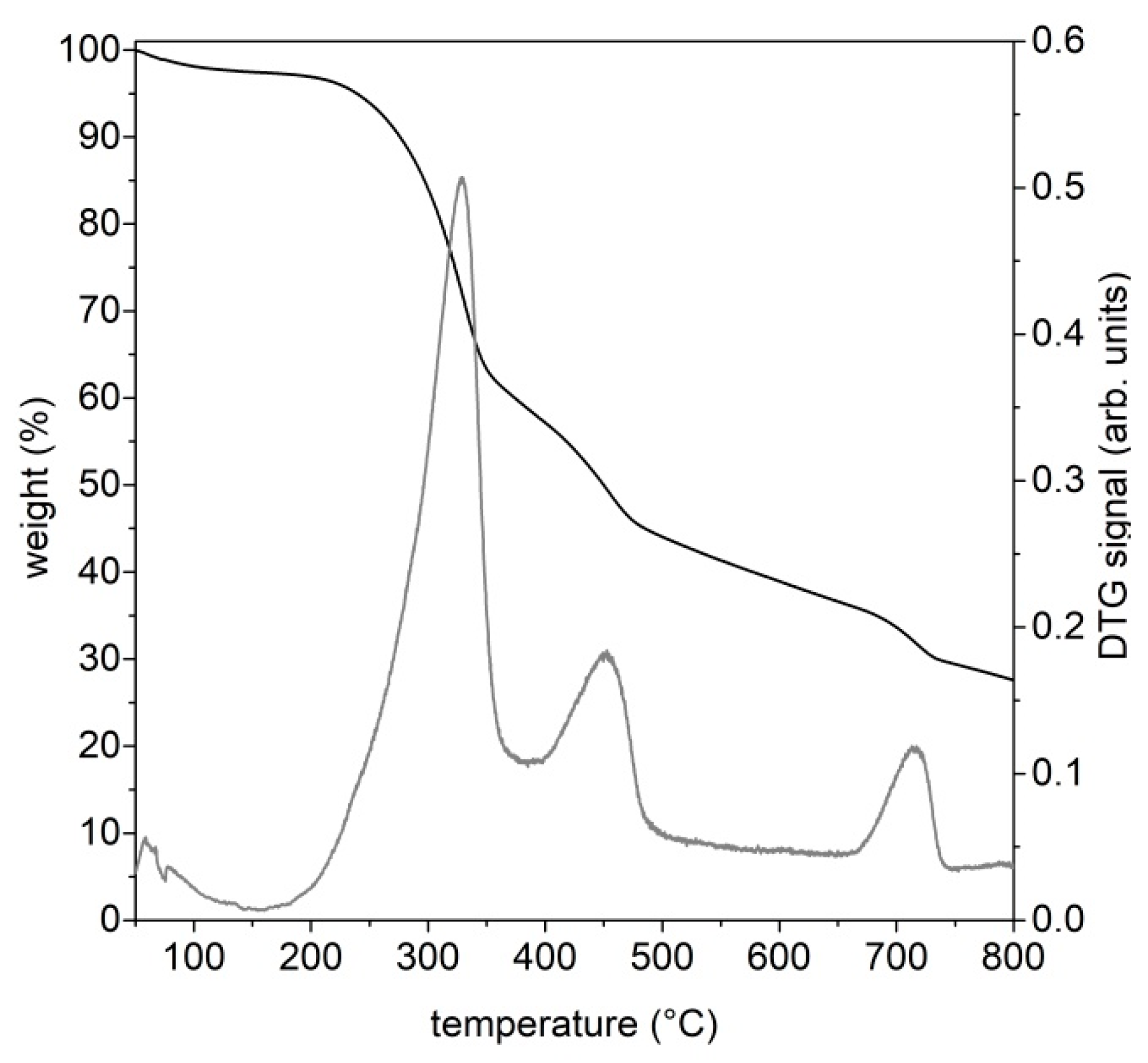
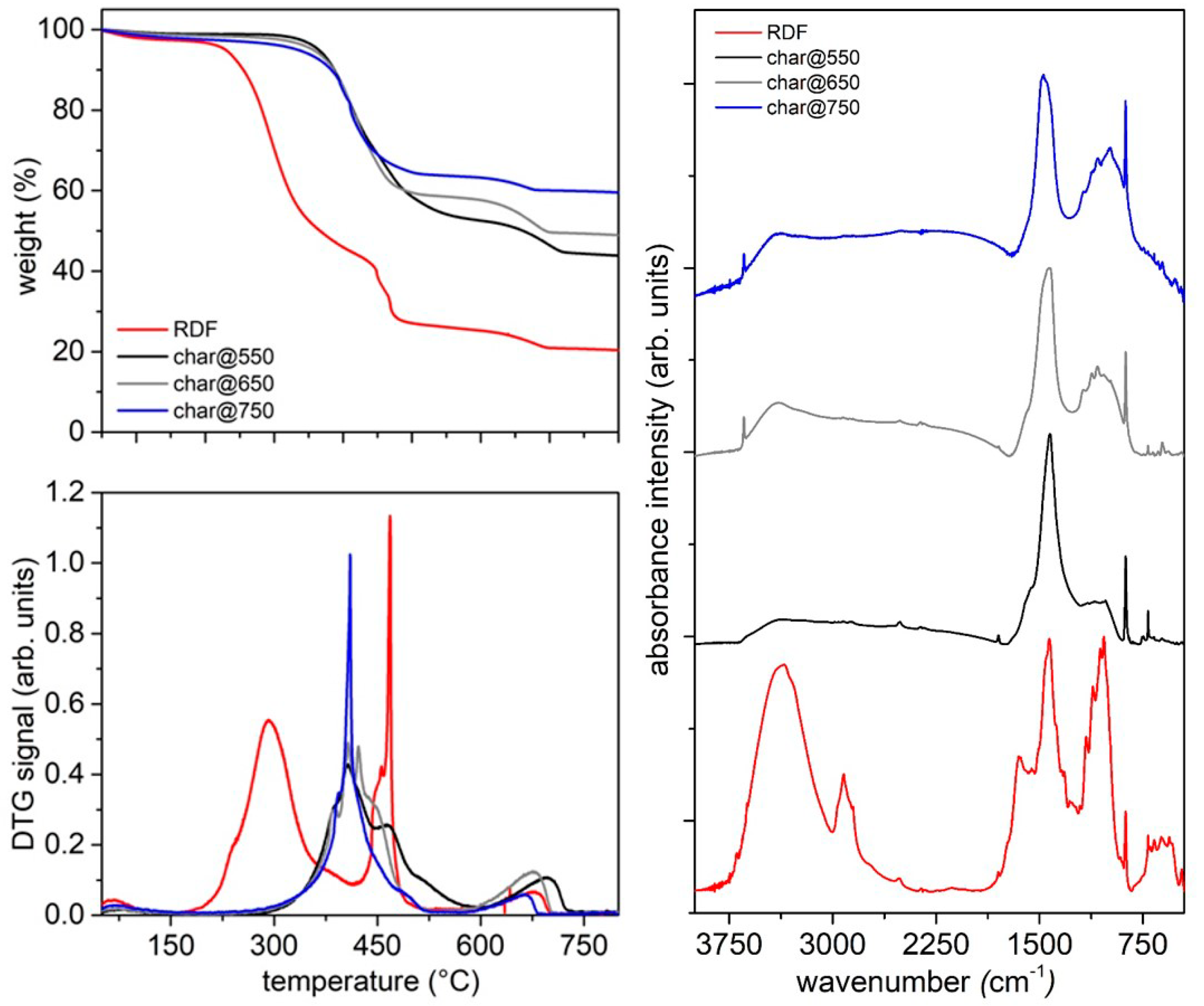
The amount of char recovered at the end of the pyrolysis test inside the cylindrical quartz reactor was determined by weighting each plant element (cylindrical quartz reactor, tubes, net and fittings) before and after each pyrolysis test. The solid (labeled in the text as char@T (T is the final pyrolysis temperature) was recovered and stored in a dry environment for further analysis.
The waxy residues stuck on the reactor wall and inside the connection tubes (
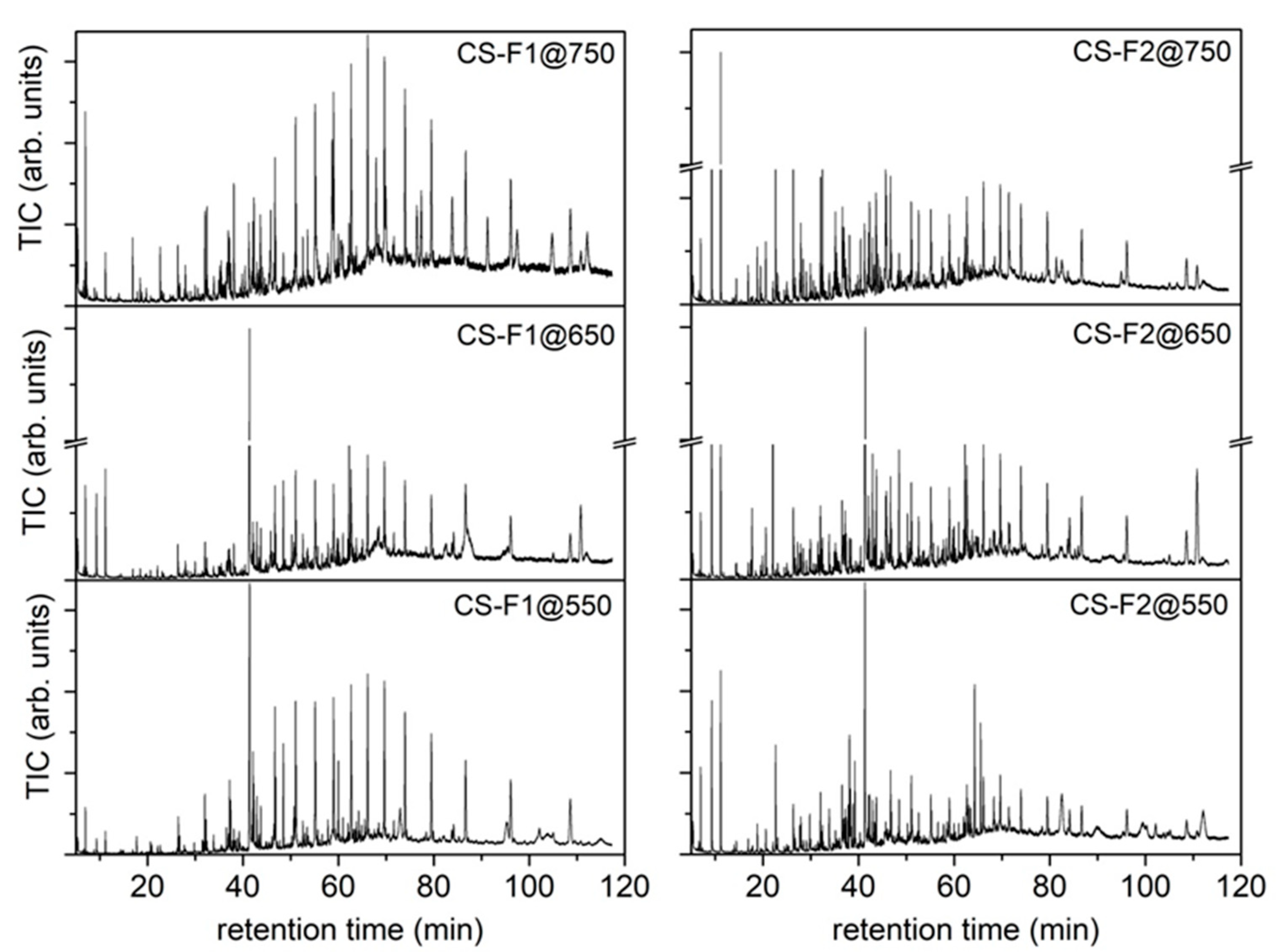
Figure S3 in SI section) were mechanically recovered and stored separately from the char. The waxy residue recovered from the wall of the reactor was labeled as wax-reactor@T, while the one recovered from the connection tubes and walls of the flasks was labeled as wax-Fx@T (T is the final pyrolysis temperature, and Fx is referred to flask 1 or flask 2). The total amount of condensable species was determined by weighting the flasks before and after the process. Dichloromethane (DCM) was used to recover the condensable species from the flasks. The condensable species trapped inside flask 1 were labeled as CS-F1@T, while those collected in the three cooled flaks (flask 2) were labeled as CS-F2@T.
3.3. Gasification Tests
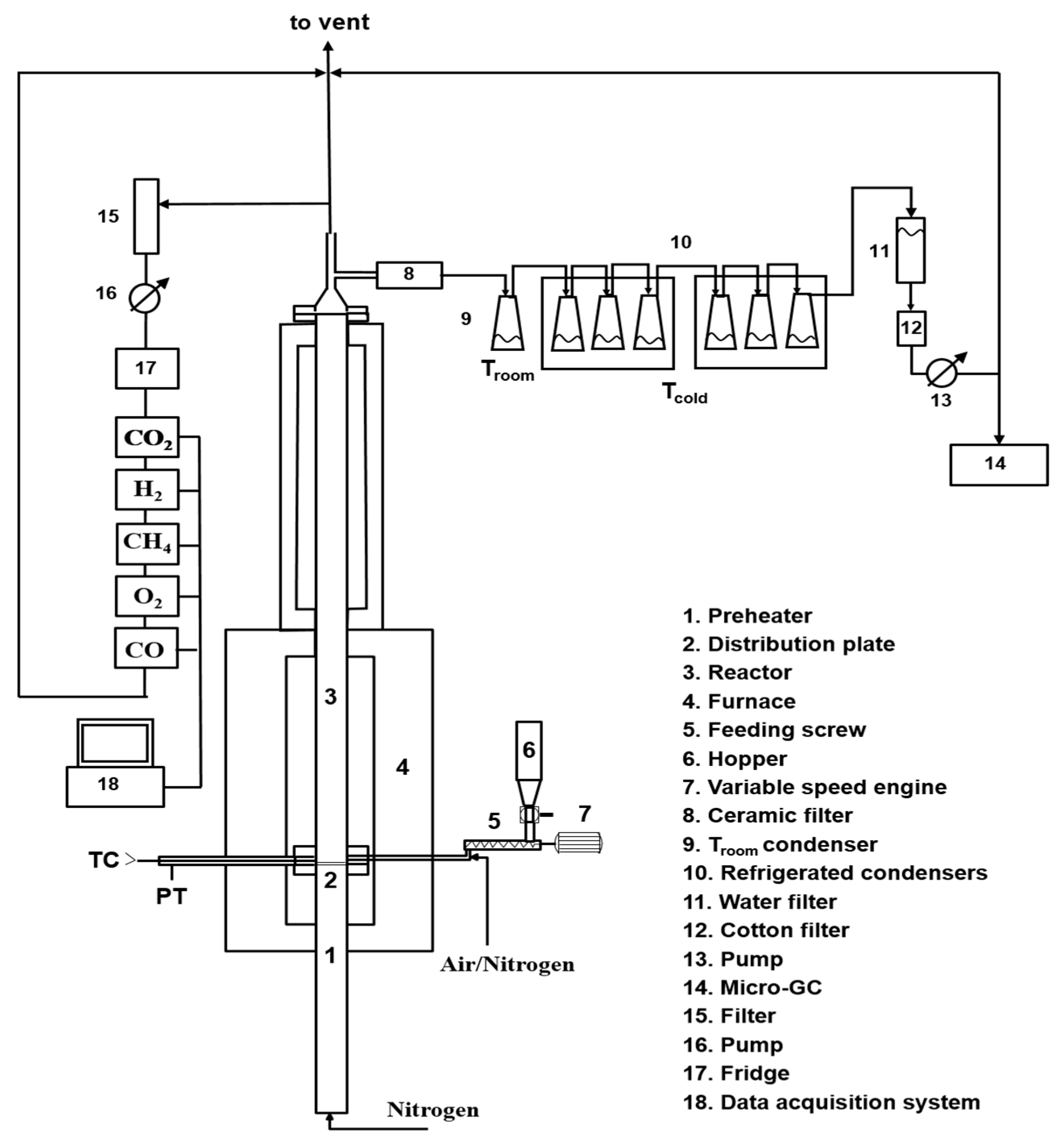
The lab-scale experimental setup used for the gasification test is schematically shown in
Figure 13.
The experimental rig consists of a fluidized reactor bed (cylindrical stainless-steel reactor with an internal diameter of 41 mm) inserted into a temperature-controlled furnace, and ancillary equipment for online monitoring and characterization of all system outputs.
The primary gas is fed from the bottom; it passes through a section acting as preheater (internal diameter of 41 mm and height of 600 mm) made in steel for high temperatures and filled with ceramic rings to favor the heat exchange between the gas and the surrounding heated surfaces and to make uniform the gaseous flow. The preheater section ends with a flange in which a seat has been made to house a series of metal meshes, which act both as a distributor of the fluidizing gas and as a support for the material making the bed. Above the preheater section, there is the fluidization column (internal diameter of 41 mm and height of 400 mm) made in steel for high temperatures. Both the preheater and the column are located in an oven made up by electric resistors placed inside two half-shells of low-density ceramic fiber able to generate a total power of 5 kW. A PID temperature controller ensures the control of the operating temperature by means of a type K thermocouple inserted into the bed just above the preheater section. A pressure transducer, located near the thermocouple ensures the detection of possible pressure drops of the bed. The thermal isolation of the system (ovens and column) is realized by using an insulating ceramic mantel covered by an external aluminum case. At the exit of the reactor, an aliquot of the gas stream was online analyzed by means of gas analyzers (ABB Magnos for O2; ABB Uras 14 for CO and CO2, ABB Caldos for H2, Vario LUXX for CH4), and another aliquot was sampled by means of a high-precision pump to quantify the tar amount (more details are reported in the following), and the remaining part was sent to the hood. Before entering the analyzers, the hot gases pass at first through a filter to remove the elutriated solids and then through a trap to remove the water present in the stream, preventing possible interferences during online analysis ascribable to the IR detector contamination.
To ensure a homogeneous and constant feedstock supply inside the gasification reactor, the RDF was pelletized by means of a pressing operation with a hydraulic press. Approximately 100 pellets of about 0.5 g (100 × 0.5 g = 50 g total) with a diameter around 1 cm and a thickness around 0.5–0.8 cm were prepared. RDF pellets were manually fed into the reactor from the top (one every 30 s), reproducing a semicontinuous feeding. In order to effectively calculate the fuel-feed rate, 3 sets of pellets were prepared (set1, set2, set3), each consisting of 32 pellets and for each set, the total weight (set1 = 17.2 g, set2 = 16.9 g, set3 = 16.9 g) and the time taken for its introduction into the reactor were considered. This procedure allows also to verify the reproducibility of the test (the average gas composition obtained analyzing the data related to each set of pellets shows a variation lower than 10%).
The experimental conditions adopted for the gasification test are shown in
Table 8.
As the fuel feeding started, the online monitoring of the process parameters and the sampling of the process outputs also started. During the test, the percentages of CO, CO
2, CH
4 and H
2 on a dry basis were achieved; recording the gas concentrations along the entire gasification test duration, the concentration profiles as a function of time were obtained. As reported before, to quantify the produced tars, a definite amount of the gas stream was sampled and sent to a train of condensers, which allowed the collection of condensable species. The condensation train consists in a series of seven flasks (250 mL): the first condensing flask (F1) was kept at room temperature, while the other six ( F2) were cooled at −12 °C (water/ice/NaCl cooling bath). The gases leaving the condensing train, deprived of the condensable components, similarly to what is reported for the pyrolysis tests, are sampled in bags and analyzed offline by means of a micro-gas chromatograph (Agilent 3000A micro-GC) to determine the concentration of light hydrocarbons (detection limit ppm). Further details on the instrument and the analysis conditions applied are available in
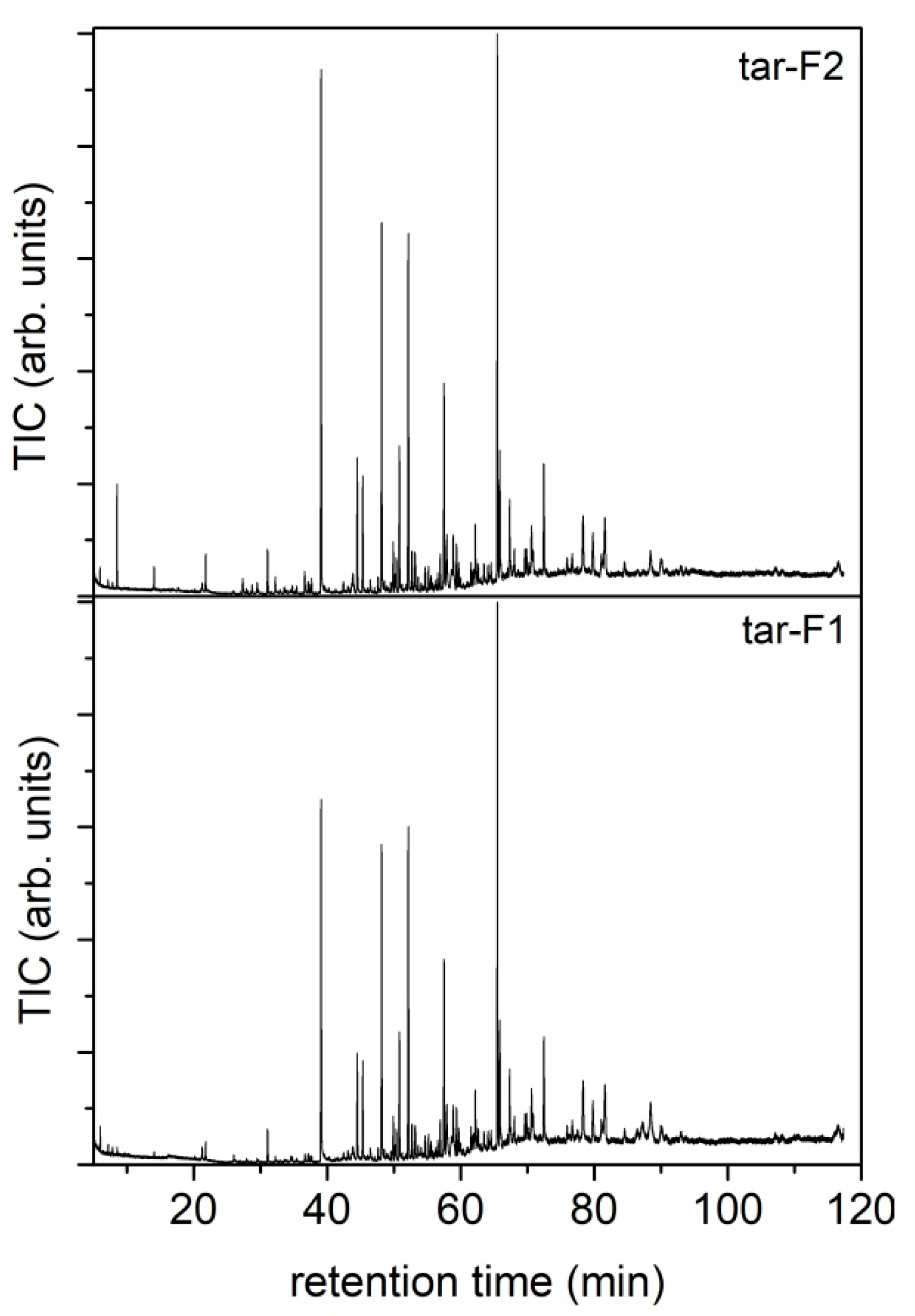
Table S11 as supporting materials. The total amount of condensable species was determined by weighting the flasks before and after the process. The condensed species were recovered from the flasks using acetone and analyzed. The condensed species from the flask at room temperature were collected separately (tar-F1) from those of the flasks maintained at −12 °C (tar-F2). The solid elutriated (named in the text as elutriated char) and collected in flask 1 was recovered and stored for further analysis.
At the end of the gasification test, the fuel supply was interrupted to start the “carbon-load” phase: to this aim, a stream of air was fed into the reactor to carry out the combustion of the char accumulated in the bed and the profiles of the produced gases acquired (mainly CO and CO2) over time. Through the integration of such profiles, the char content accumulated in the bed was estimated. After the carbon load phase, the reactor was cooled at room temperature.
3.4. Analytical Techniques
Proximate analysis to determine feedstock humidity, volatile, ashes and fixed carbon contents by means gravimetric evaluations (both direct and indirect) was performed on a LECO 701 thermobalance according to the standard ASTM D7582-15. Each measurement was repeated 4 times. C, H, N and S contents were determined by ultimate analysis following ASTM D3176-15 and ASTM D4239 standards. C, H and N contents were determined by using a LECO 628 analyzer after EDTA calibration (measurements were performed in triplicates). Sulfur content was determined by a LECO CS 144 analyzer calibrated with high-content (vanadyl sulphate pentahydrate) and low-content (low sulfur coal Leco 502-681) sulfur reagents (measurements were performed in duplicate).
Low-heating value (LHV) was determined in accordance with the ASTM D5865 by burning an amount of feedstock in pure oxygen in a PARR 6200 calorimeter (measurements were performed in duplicate).
The thermal behavior of the feedstock and of the pyrolysis and gasification products (both condensable species and char) was investigated through thermogravimetric analyses on a STA6000 Perkin-Elmer in inert (N2, 40 mL/min) or oxidizing (air, 40 mL/min) atmospheres. These conditions allowed for the evaluation of the thermal reactivity of each sample both in the absence and in the presence of oxygen. The thermal ramp was set from 30 °C to 800 °C, and depending on the analyzed material, the heating rate varied between 10 °C/min and 50 °C/min. An amount of 5–20 mg of material was loaded in an alumina crucible for each measurement. The alumina crucible was previously treated in furnace at 950 °C to guarantee an accurate solid residue determination.
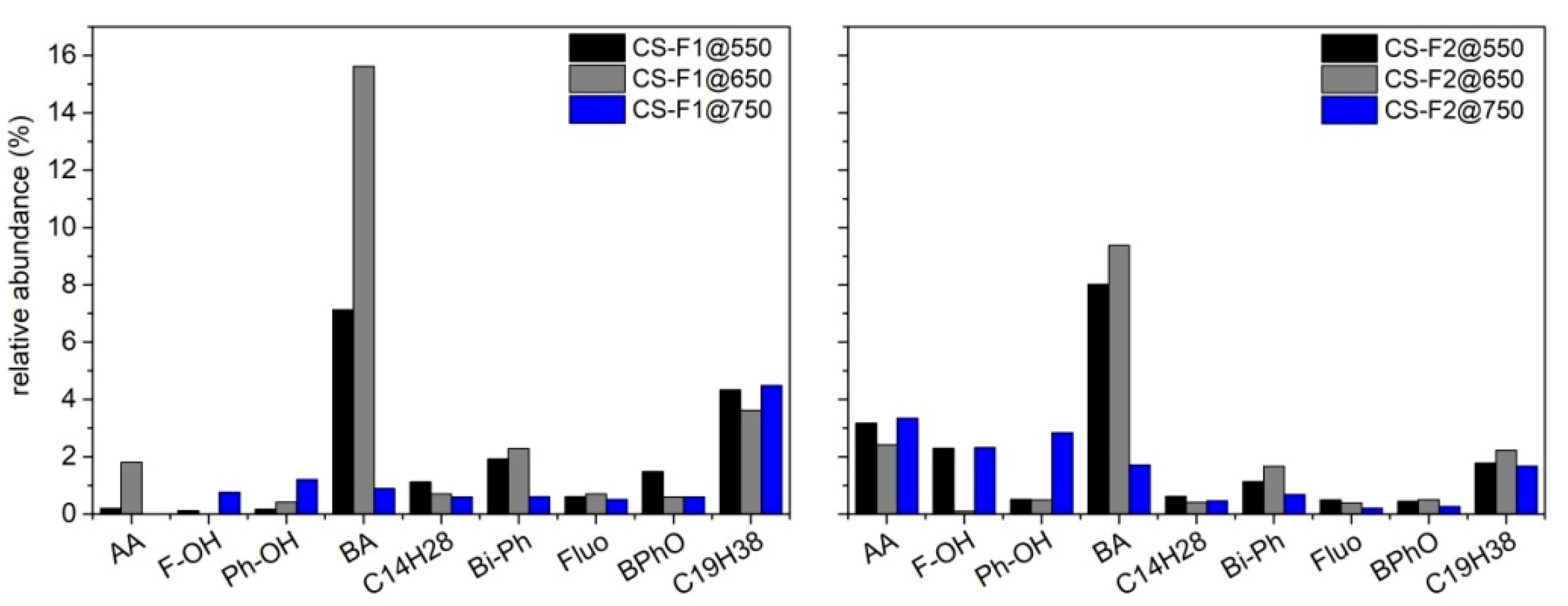
Qualitative (composition of each mixture) and quantitative (relative abundance of each species in a mixture) information about the composition of the condensed species was achieved by a gas chromatographic analysis implementing a mass spectrometer as detector (GC-MS system). Each liquid mixture was analyzed by GC-MS without any further derivatization. The samples dissolved in DCM or acetone were filtered on a PTFE filter (Millipore, 0.45 μm pore size and 47 mm membrane diameter) to remove water traces and undissolved particles before the analysis. An amount of 1 μL of each solution was analyzed by an Agilent GC-MS instrument (7890A/5972C) equipped with an Agilent DB-624 capillary column (30 m × 0.25 mm i.d., 1.40 μm film thickness) and using He as the carrier gas (1.0 mL/min). The mass spectrometer was operated in electron ionization mode and an m/z range from 30 to 400 was scanned. The oven temperature was programmed as follows: the starting oven T (45 °C) was held for 3 min, then it was raised to 235 °C at a heating rate of 3 °C/min and held for 50 min.
The water content of the condensed species was determined by Karl–Fisher titration (METTLER TOLEDO V20 instrument); for each pyrolysis liquid, the measurement was repeated thrice.
The content of major inorganic elements was determined by Inductively Coupled Plasma Mass Spectrometry (ICP/MS) using an Agilent 7500CE instrument in accordance with the US-EPA 3051 and US-EPA 3052 methods. To this aim, an amount of feedstock was suspended with distilled water and treated under microwave heating with concentrate HNO3 and H2O2 for 30 min. The digested sample, after filtration, was diluted with distilled water and analyzed with the ICP-MS system. The measurement was repeated thrice.
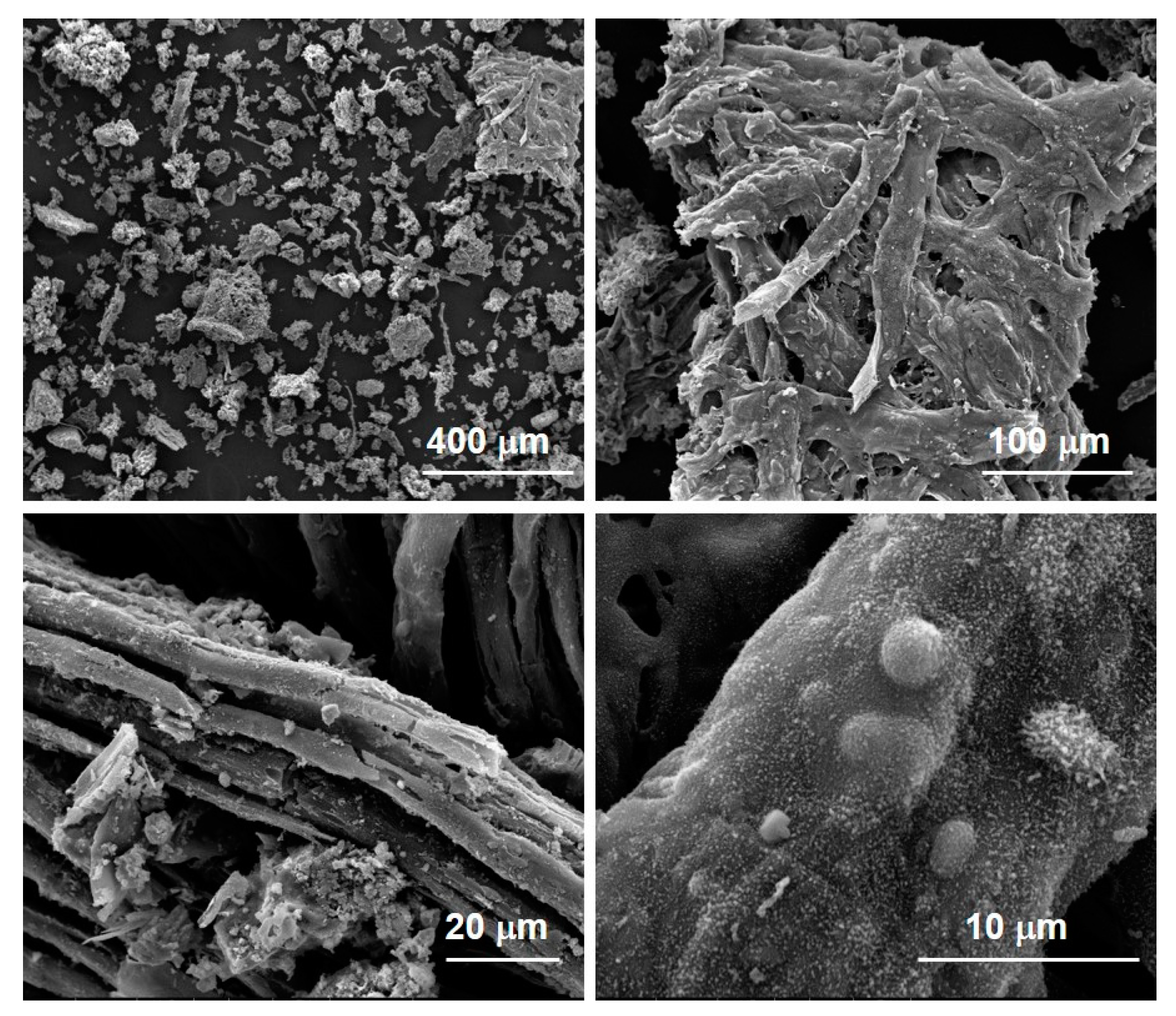
The morphology of the char samples was evaluated by scanning electron microscopy (SEM) imaging using a FEI Inspect microscope equipped with an EDS Oxford AZtecLiveLite probe and Xplore 30 detector for elemental analysis. The powdered samples were previously dried and sputter-coated with a thin layer of gold to avoid charging.
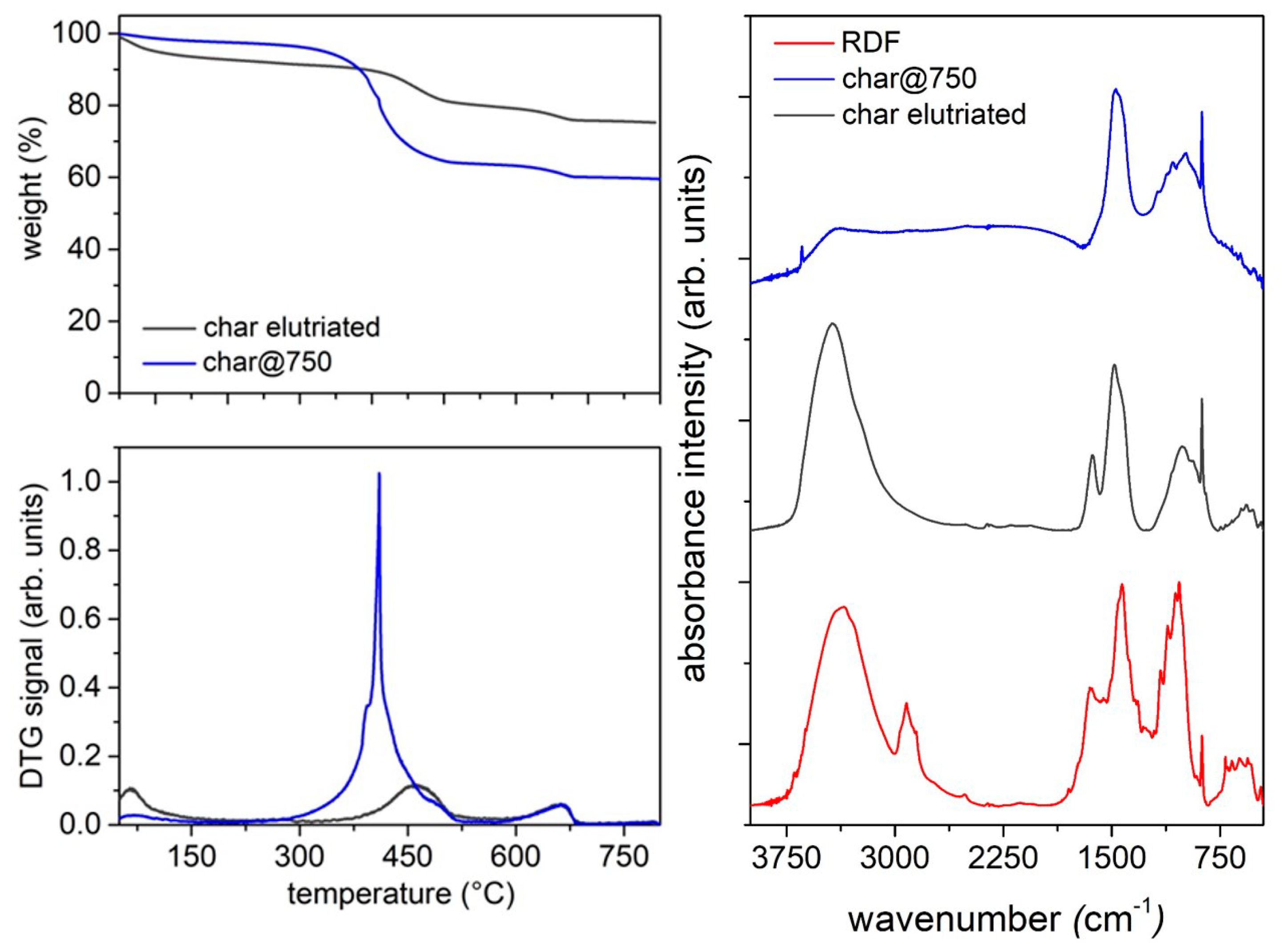
The surface chemistry of the char samples was investigated by infrared spectroscopy measuring FT-IR spectra in the 450–4000 cm−1 range on a Perkin–Elmer Frontier MIR spectrophotometer operated in transmittance mode. The spectra were acquired on KBr pellets (2 wt. %), collecting 8 scans and correcting the background noise.
4. Conclusions
Pyrolysis tests in a lab-scale tubular reactor up to 550, 650 and 750 °C and an air gasification test at 850 °C in a fluidized bed reactor have been performed and the results analyzed to highlight how the two processes can accomplish waste-to-materials and waste-to-energy targets.
Among the three products collected as the outputs of the pyrolysis process, the most abundant and also the most promising in terms of possible applications in the pavement industry is the fraction of condensable species, whose highest yield was achieved at 550 °C. The massive presence of waxes makes this fraction, when used as a whole and without fractionation, a potential candidate for the replacement of fossil-fuel-based additives in bitumen formulation and asphalt processing and rejuvenation. Different literature reports, indeed, have demonstrated that the hydrocarbons in bio-oil can enrich the poor fraction of the maltene phase in exhaust asphalts, promoting a rejuvenation process.
It is worth of noting also that the final pyrolysis temperature has a strong influence on the segregation of some critical species such as S and N in the char, opening the door to its reuse as adsorbent, catalyst, material for energy harvesting devices and additives for the pavement industry, depending on its composition. Char is mainly made up by carbonaceous particles and it is highly compatible with the organic nature of bitumen; as a consequence, the char addition is expected to reinforce the overall bitumen structure, increasing its mechanical properties and slowing down the molecular kinetics of its aging process. On the basis of these expectations, char is a low-cost carbon-based additive candidate for next-generation bitumen formulation.
The gasification process leads to the production of a syngas with a H2/CO ratio of 0.51. The result is in line with other literature reports, as well as those regarding pilot-scale systems, but to obtain a marketable product, a two-stage process implementing a catalytic or a reformer stage after the gasification one, also to reduce tar production, should be envisioned.
To sum up, with this work, the achievement of a possible integration of urban wastes and asphalt cycles is envisioned accordingly with the paradigm of the circular economy: thermochemical processes are used treat MSWs (or its fractions as Refuse-Derived Fuels, RDFs) under specific conditions, allowing to tune the characteristics of the resulting outputs (oil and char), making them feasible additives for bitumen and asphalt preparations. Since char can be used to prepare better performing and durable asphalts, and oil can be used to regenerate exhausted asphalts, avoiding their landfilling, the expected benefits of the proposed approach influence asphalts disposal, wastes treatment, CO2 emissions and overall production costs as a consequence of the increased asphalt duration.