Integrative Analysis of Proteomics and Metabolism Reveals the Potential Roles of Arachidonic Acid Metabolism in Hypoxia Response in Mouse Spleen
Abstract
1. Introduction
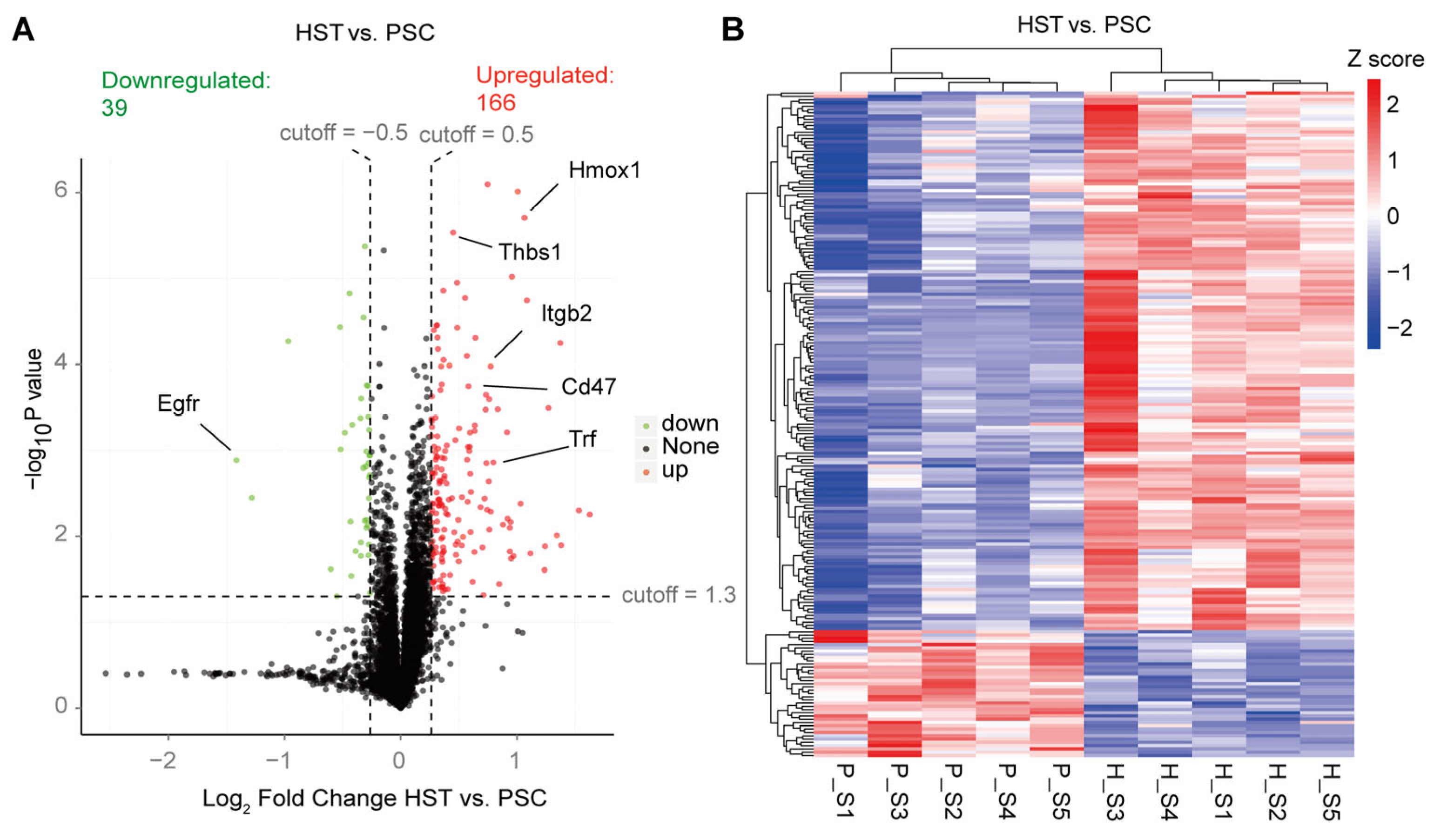
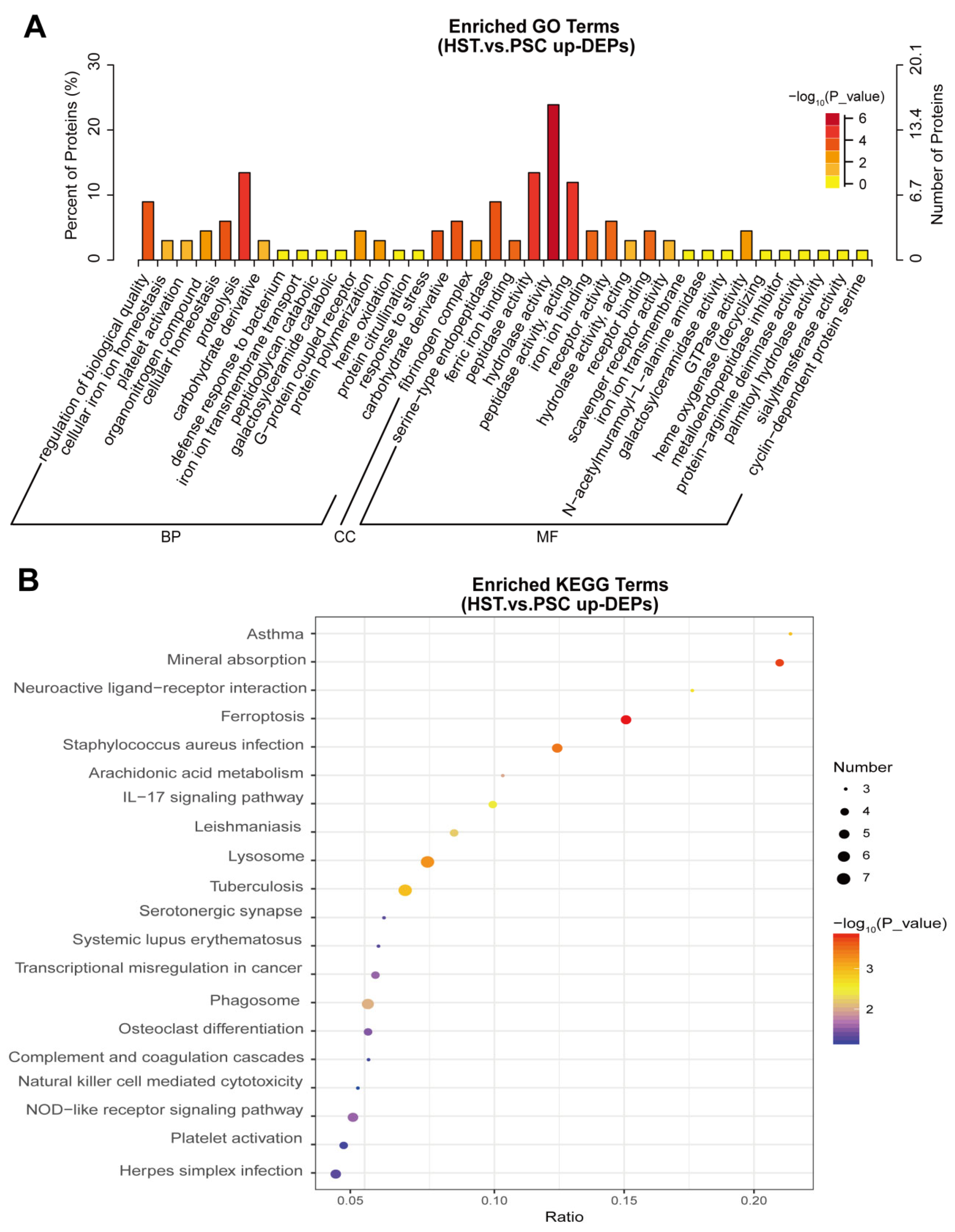
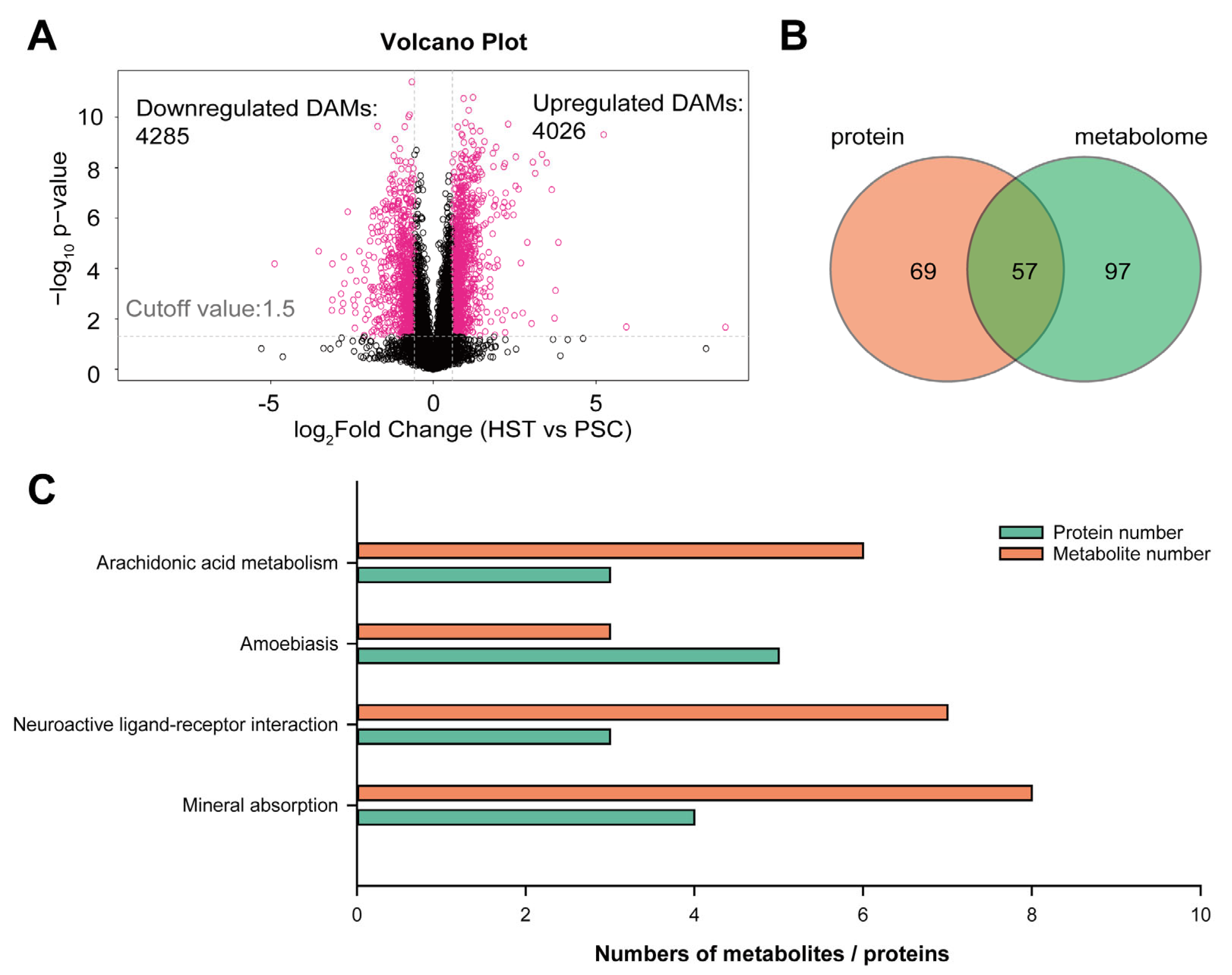
2. Results
3. Discussion
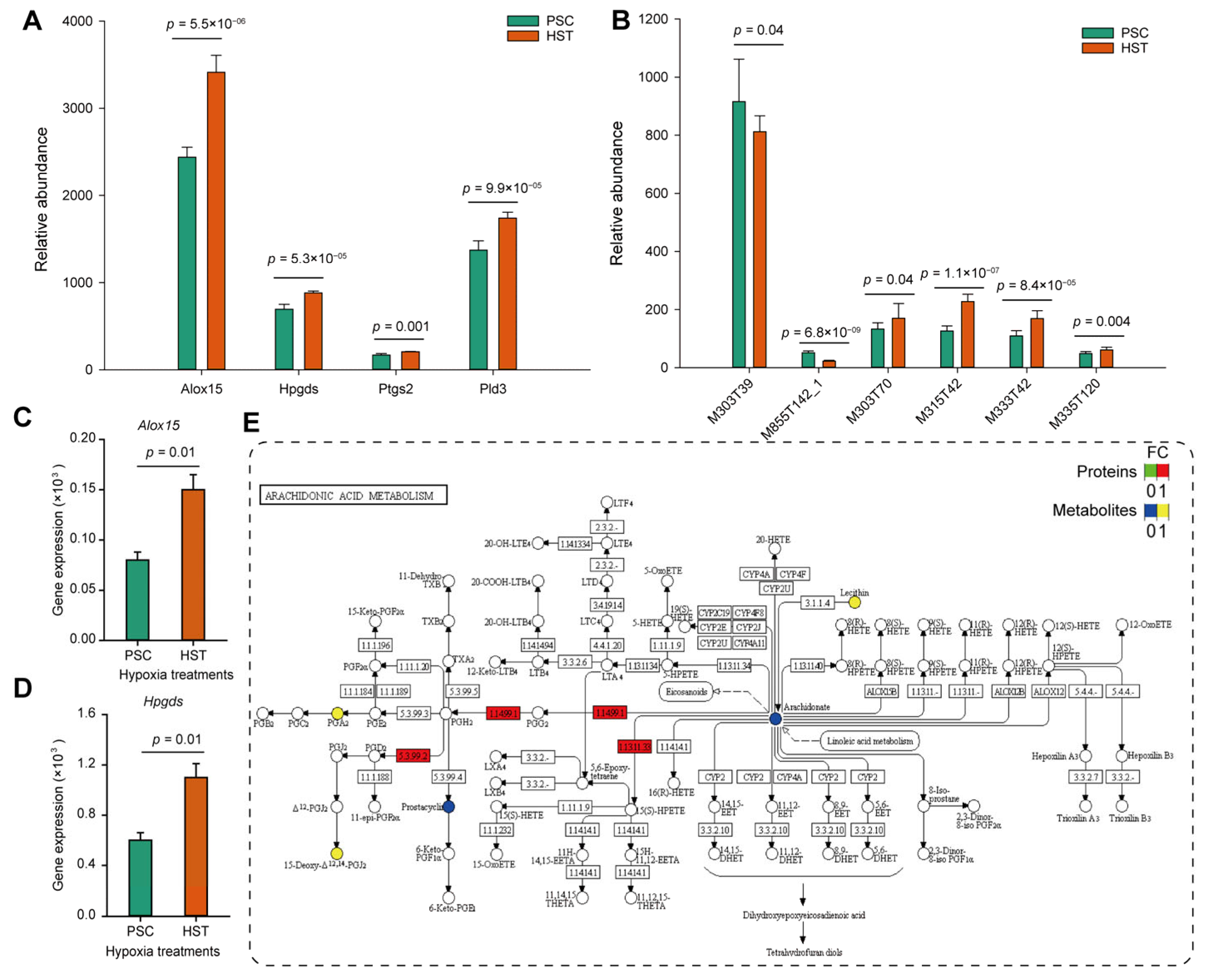
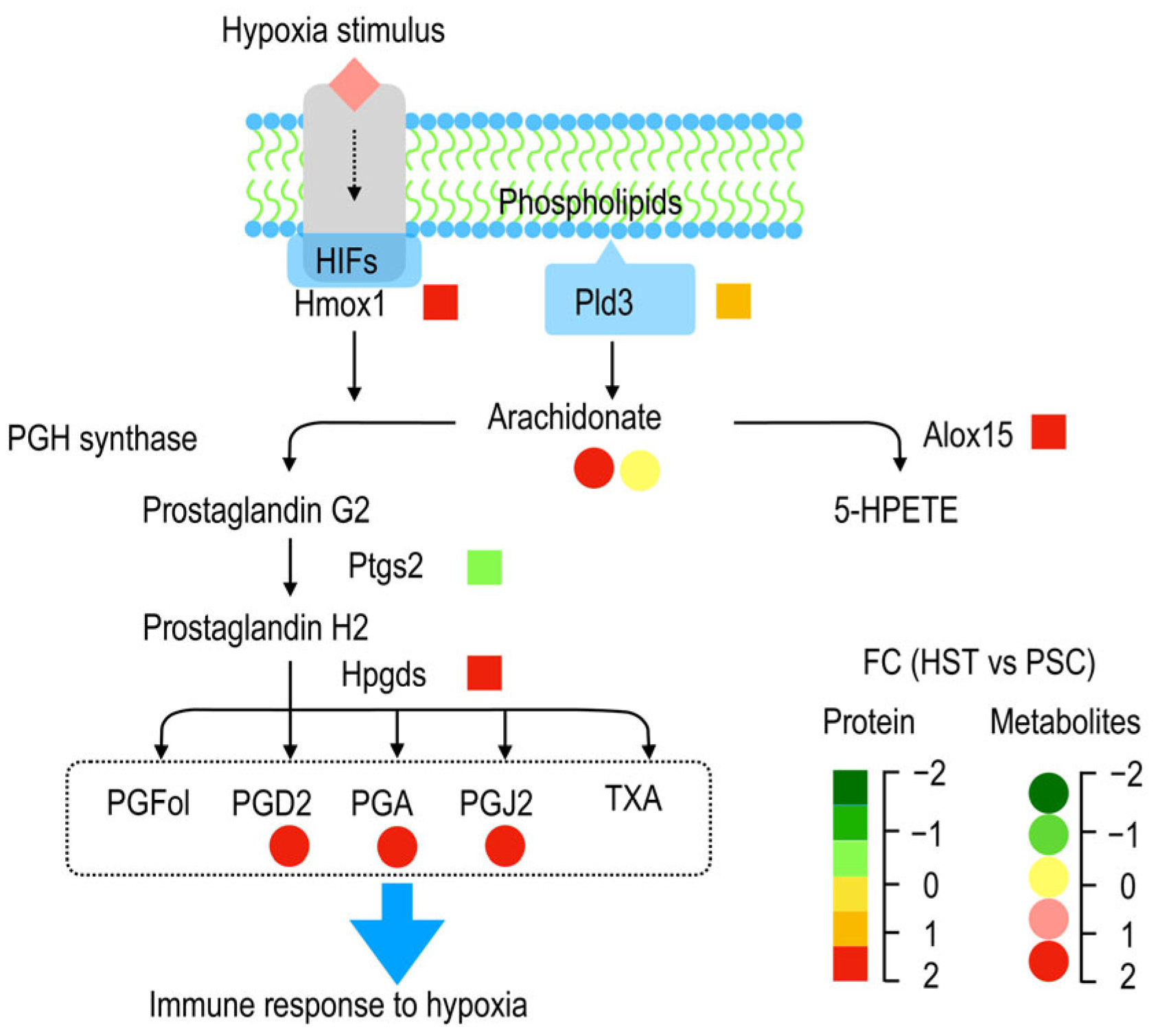
3.1. Arachidonic Acids Involved in T Cell Immune Response
3.2. Hypoxia Induced Accumulation of Arachidonic Acids Deteriotives
3.3. Energy Metabolism Promotes Immune Response by Hypoxia
4. Materials and Methods
4.1. Experimental Animals and Experiment Grouping
4.2. Proteomics Analysis
4.2.1. Protein Extraction
4.2.2. The Functional Analysis of Protein
4.2.3. Metabolic Dete rminations
4.2.4. qPCR Experiments
4.2.5. Western Blotting Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Joyce, K.E.; Lucas, S.J.E.; Imray, C.H.E.; Balanos, G.M.; Wright, A.D. Advances in the Available Non-Biological Pharmacotherapy Prevention and Treatment of Acute Mountain Sickness and High Altitude Cerebral and Pulmonary Oedema. Expert Opin. Pharmacother. 2018, 19, 1891–1902. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, Z.; Zhang, L.; Wang, X.; Dong, Y.; Wang, J.; Shao, L.; Tian, Y.; Wang, Z.; Zhao, T.; et al. Metabolic Risk Factors and Left Ventricular Diastolic Function in Middle-Aged Chinese Living in the Tibetan Plateau. J. Am. Hear. Assoc. 2019, 8, e010454. [Google Scholar] [CrossRef]
- Taylor, C.T.; Colgan, S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Damgaci, S.; Ibrahim-Hashim, A.; Enriquez-Navas, P.M.; Pilon-Thomas, S.; Guvenis, A.; Gillies, R.J. Hypoxia and Acidosis: Immune Suppressors and Therapeutic Targets. Immunology 2018, 154, 354–362. [Google Scholar] [CrossRef]
- Khanna, K.; Mishra, K.P.; Chanda, S.; Eslavath, M.R.; Ganju, L.; Kumar, B.; Singh, S.B. Effects of Acute Exposure to Hypobaric Hypoxia on Mucosal Barrier Injury and the Gastrointestinal Immune Axis in Rats. High Alt. Med. Biol. 2019, 20, 35–44. [Google Scholar] [CrossRef]
- Hartman-Ksycińska, A.; Kluz-Zawadzka, J.; Lewandowski, B. High Altitude Illness. Przegl. Epidemiol. 2016, 70, 490–499. [Google Scholar]
- Simancas-Racines, D.; Arevalo-Rodriguez, I.; Osorio, D.; Franco, J.V.; Xu, Y.; Hidalgo, R. Interventions for Treating Acute High Altitude Illness. Cochrane database Syst. Rev. 2018, 6, CD009567. [Google Scholar] [CrossRef]
- Lundeberg, J.; Feiner, J.R.; Schober, A.; Sall, J.W.; Eilers, H.; Bickler, P.E. Increased Cytokines at High Altitude: Lack of Effect of Ibuprofen on Acute Mountain Sickness, Physiological Variables, or Cytokine Levels. High Alt. Med. Biol. 2018, 19, 249–258. [Google Scholar] [CrossRef]
- Turolo, S.; Edefonti, A.; Mazzocchi, A.; Syren, M.L.; Morello, W.; Agostoni, C.; Montini, G. Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome. Int. J. Mol. Sci. 2021, 22, 5452. [Google Scholar] [CrossRef]
- Imaoka, S.; Funae, Y. [The Physiological Role of P450-Derived Arachidonic Acid Metabolites]. Nihon Yakurigaku Zasshi. 1998, 112, 23–31. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Sun, Q.-Y.; Zhou, H.-H.; Mao, X.-Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Balboa, M.A.; Balsinde, J. Lipid Droplets, Phospholipase A2, Arachidonic Acid, and Atherosclerosis. Biomed. 2021, 9, 1891. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yang, B.; Lai, Q.; Shi, J.-F.; Peng, J.-Y.; Zhang, Y.; Hu, K.-S.; Li, Y.-Q.; Peng, J.-W.; Yang, Z.-Z.; et al. Reprogramming of M6A Epitranscriptome Is Crucial for Shaping of Transcriptome and Proteome in Response to Hypoxia. RNA Biol. 2021, 18, 131–143. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, T.; Li, J.; Wang, H.; Li, X.; Xiong, J.; Xu, P.; Xue, M. Proteomic Analysis of the Mouse Brain after Repetitive Exposure to Hypoxia. Chem. Biol. Interact. 2015, 236, 57–66. [Google Scholar] [CrossRef]
- Gao, Y.; Ray, S.; Dai, S.; Ivanov, A.R.; Abu-Absi, N.R.; Lewis, A.M.; Huang, Z.; Xing, Z.; Borys, M.C.; Li, Z.J.; et al. Combined Metabolomics and Proteomics Reveals Hypoxia as a Cause of Lower Productivity on Scale-up to a 5000-Liter CHO Bioprocess. Biotechnol. J. 2016, 11, 1190–1200. [Google Scholar] [CrossRef]
- Martín-Bernabé, A.; Tarragó-Celada, J.; Cunin, V.; Michelland, S.; Cortés, R.; Poignant, J.; Boyault, C.; Rachidi, W.; Bourgoin-Voillard, S.; Cascante, M.; et al. Quantitative Proteomic Approach Reveals Altered Metabolic Pathways in Response to the Inhibition of Lysine Deacetylases in A549 Cells under Normoxia and Hypoxia. Int. J. Mol. Sci. 2021, 22, 3378. [Google Scholar] [CrossRef]
- Sun, X.; Tu, K.; Li, L.; Wu, B.; Wu, L.; Liu, Z.; Zhou, L.; Tian, J.; Yang, A. Integrated Transcriptome and Metabolome Analysis Reveals Molecular Responses of the Clams to Acute Hypoxia. Mar. Environ. Res. 2021, 168, 105317. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Schagatay, E.; Lunde, A.; Nilsson, S.; Palm, O.; Lodin-Sundström, A. Spleen Contraction Elevates Hemoglobin Concentration at High Altitude during Rest and Exercise. Eur. J. Appl. Physiol. 2020, 120, 2693–2704. [Google Scholar] [CrossRef]
- Ye, Z.; Shen, Y.; Jin, K.; Qiu, J.; Hu, B.; Jadhav, R.R.; Sheth, K.; Weyand, C.M.; Goronzy, J.J. Arachidonic Acid-Regulated Calcium Signaling in T Cells from Patients with Rheumatoid Arthritis Promotes Synovial Inflammation. Nat. Commun. 2021, 12, 907. [Google Scholar] [CrossRef]
- González-Cobos, J.C.; Zhang, X.; Zhang, W.; Ruhle, B.; Motiani, R.K.; Schindl, R.; Muik, M.; Spinelli, A.M.; Bisaillon, J.M.; Shinde, A.V.; et al. Store-Independent Orai1/3 Channels Activated by Intracrine Leukotriene C4: Role in Neointimal Hyperplasia. Circ. Res. 2013, 112, 1013–1025. [Google Scholar] [CrossRef]
- Carlsson, J.A.; Wold, A.E.; Sandberg, A.-S.; Östman, S.M. The Polyunsaturated Fatty Acids Arachidonic Acid and Docosahexaenoic Acid Induce Mouse Dendritic Cells Maturation but Reduce T-Cell Responses In Vitro. PLoS ONE 2015, 10, e0143741. [Google Scholar] [CrossRef]
- Oishi, Y.; Yoshida, K.; Scammell, T.E.; Urade, Y.; Lazarus, M.; Saper, C.B. The Roles of Prostaglandin E2 and D2 in Lipopolysaccharide-Mediated Changes in Sleep. Brain. Behav. Immun. 2015, 47, 172–177. [Google Scholar] [CrossRef]
- Poellinger, L.; Johnson, R.S. HIF-1 and Hypoxic Response: The Plot Thickens. Curr. Opin. Genet. Dev. 2004, 14, 81–85. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Seo, Y.; Jeon, H.; Hong, J.W.; Choi, J. Anti-Tumor Drug-Loaded Oxygen Nanobubbles for the Degradation of HIF-1α and the Upregulation of Reactive Oxygen Species in Tumor Cells. Cancers 2019, 11, 1464. [Google Scholar] [CrossRef]
- Li, J.; Essemine, J.; Shang, C.; Zhang, H.; Zhu, X.; Yu, J.; Chen, G.; Qu, M.; Sun, D. Combined Proteomics and Metabolism Analysis Unravels Prominent Roles of Antioxidant System in the Prevention of Alfalfa (Medicago Sativa, L.) against Salt Stress. Int. J. Mol. Sci. 2020, 21, 909. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange Provides Globally Coordinated Proteomics Data Submission and Dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Qu, M.; Chen, G.Y.; Bunce, J.A.; Zhu, X.; Sicher, R. Systematic Biology Analysis on Photosynthetic Carbon Metabolism of Maize Leaf Following Sudden Heat Shock under Elevated CO2. Sci. Rep. 2018, 8, 7849. [Google Scholar] [CrossRef]
- Qu, M.; Essemine, J.; Xu, J.; Ablat, G.; Perveen, S.; Wang, H.; Chen, K.; Zhao, Y.; Chen, G.Y.; Chu, C.C.; et al. Alterations in Stomatal Response to Fluctuating Light Increase Biomass and Yield of Rice Under Drought Conditions. Plant J. 2020, 104, 1334–1347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yong, S.; Xu, Y.; Hu, Y.; Li, J.; Long, Q.; Wang, X.; Gu, C.; Miao, Z. Integrative Analysis of Proteomics and Metabolism Reveals the Potential Roles of Arachidonic Acid Metabolism in Hypoxia Response in Mouse Spleen. Molecules 2022, 27, 8102. https://doi.org/10.3390/molecules27228102
Guo Y, Yong S, Xu Y, Hu Y, Li J, Long Q, Wang X, Gu C, Miao Z. Integrative Analysis of Proteomics and Metabolism Reveals the Potential Roles of Arachidonic Acid Metabolism in Hypoxia Response in Mouse Spleen. Molecules. 2022; 27(22):8102. https://doi.org/10.3390/molecules27228102
Chicago/Turabian StyleGuo, Yujing, Sheng Yong, Yuzhen Xu, Ying Hu, Jidong Li, Qifu Long, Xiaojun Wang, Cunlin Gu, and Zengqiang Miao. 2022. "Integrative Analysis of Proteomics and Metabolism Reveals the Potential Roles of Arachidonic Acid Metabolism in Hypoxia Response in Mouse Spleen" Molecules 27, no. 22: 8102. https://doi.org/10.3390/molecules27228102
APA StyleGuo, Y., Yong, S., Xu, Y., Hu, Y., Li, J., Long, Q., Wang, X., Gu, C., & Miao, Z. (2022). Integrative Analysis of Proteomics and Metabolism Reveals the Potential Roles of Arachidonic Acid Metabolism in Hypoxia Response in Mouse Spleen. Molecules, 27(22), 8102. https://doi.org/10.3390/molecules27228102





