Aggregation-Induced Intermolecular Charge Transfer Emission for Solution-Processable Bipolar Host Material via Adjusting the Length of Alkyl Chain
Abstract
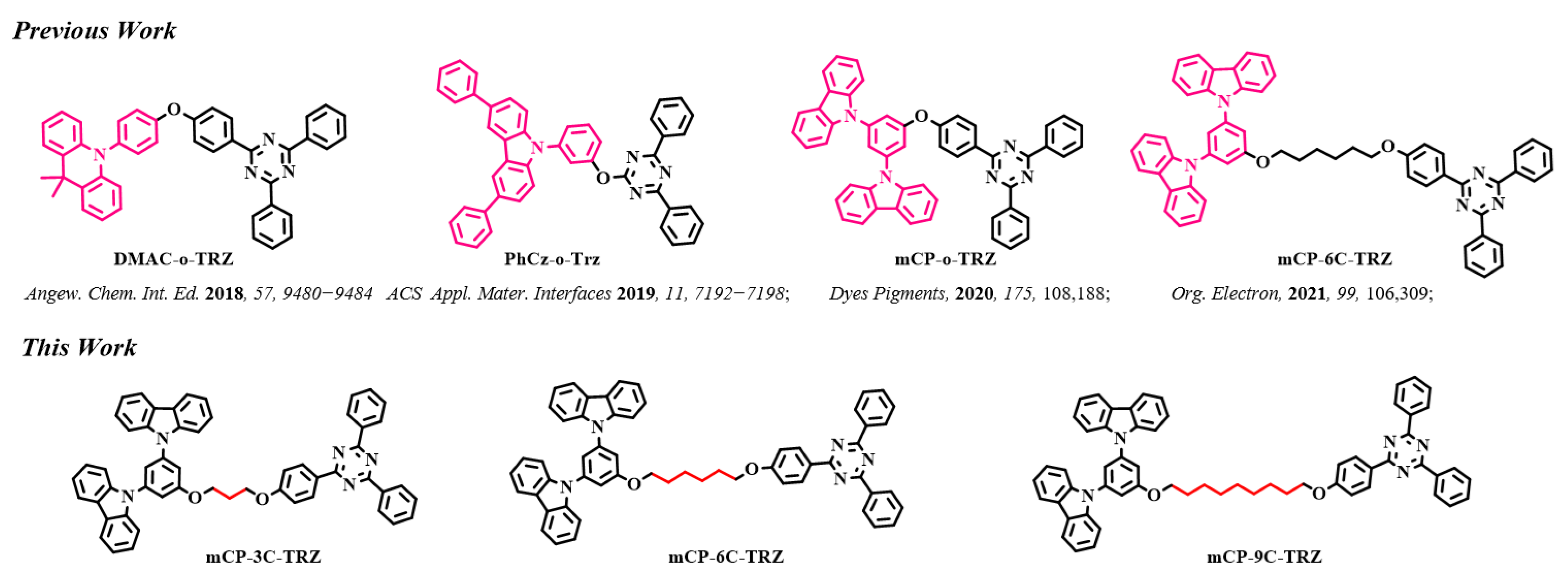
1. Introduction
2. Materials and Methods
2.1. Chemicals and Measurements
2.2. Synthesis
2.3. OLED Device Fabrication
3. Results and Discussions
3.1. Synthesis and Characterizations
3.2. Theoretical Calculations
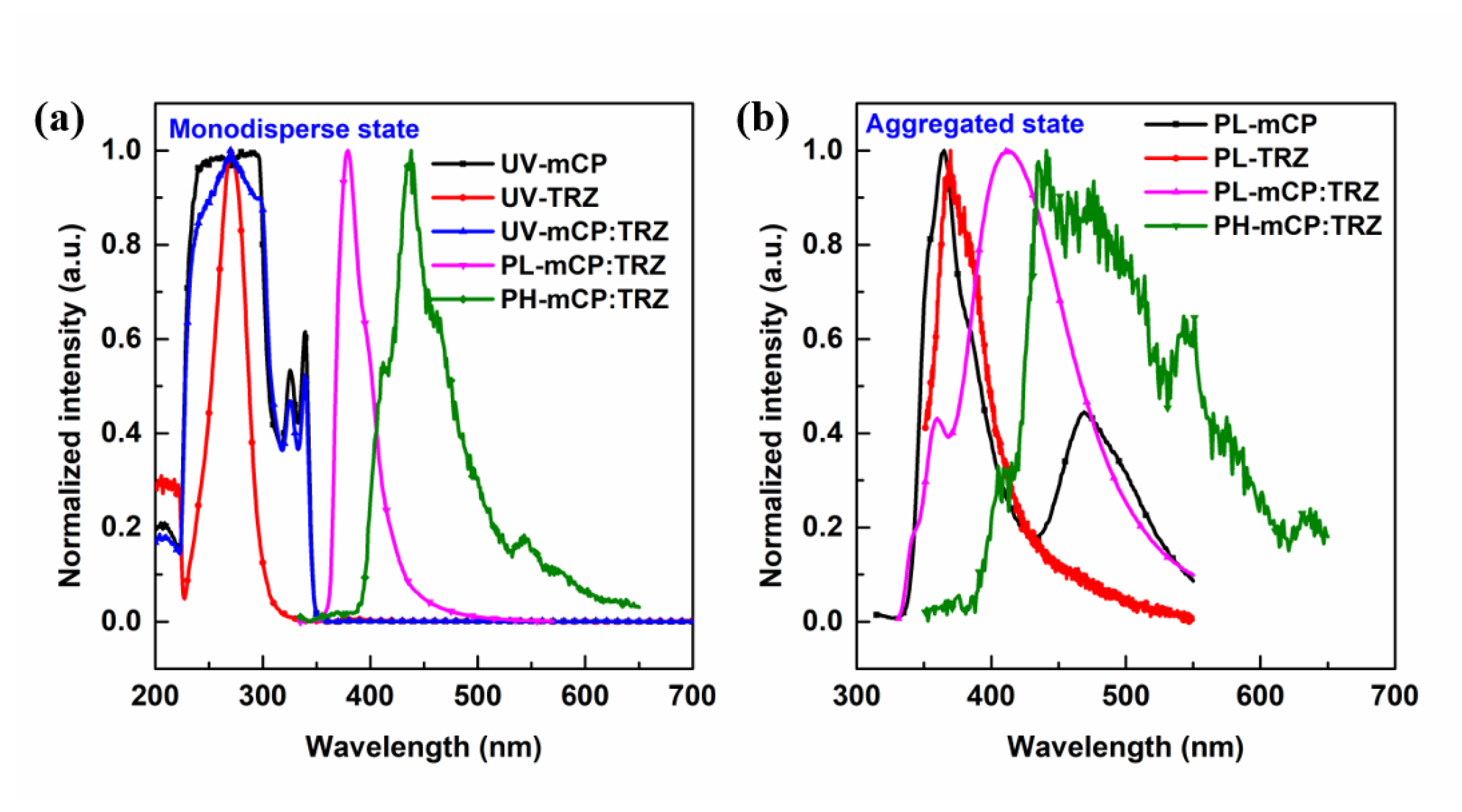
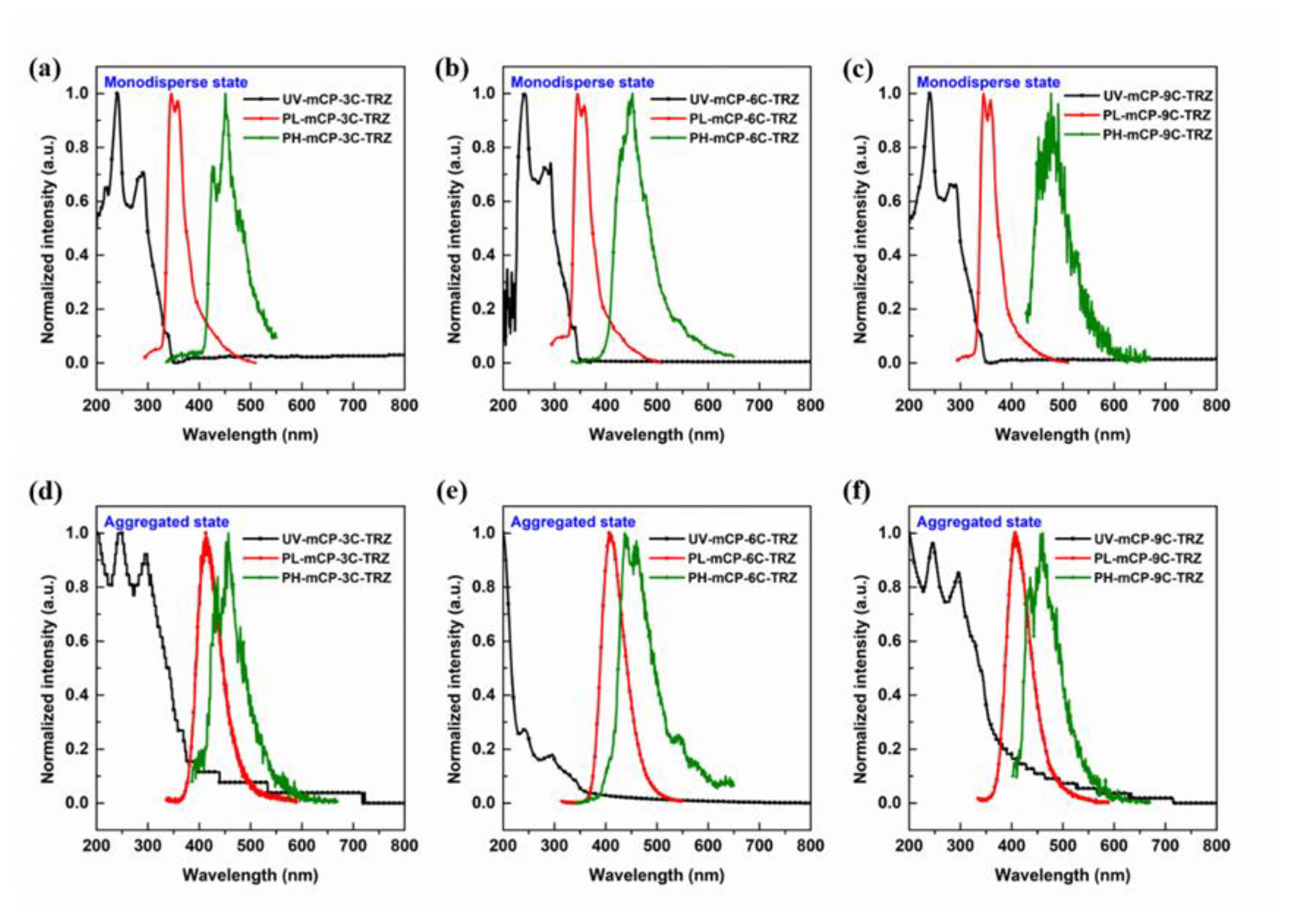
3.3. Photophysical Properties
3.4. Thermal Stability and Electrochemistry Properties
3.5. Device Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; VanSlykem, S. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Burroughes, J.; Bradley, D.; Brown, A.; Marks, R.; Mackay, K.; Friend, R.; Burns, P.; Holmes, A. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, T.; Duan, L. Emerging Self-Emissive Technologies for Flexible Displays. Adv. Mater. 2020, 32, 1902391. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Noh, I.; Seo, Y.; Han, J.; Park, Y.; Cho, E.; Choi, K. Parallel-Stacked Flexible Organic Light Emitting Diodes for Wearable Photodynamic Therapeutics and Color-Tunable Optoelectronics. ACS Nano 2020, 14, 15688–15699. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, J.; Yu, M.; Miao, J.; Xie, Z.; Qiu, Y.; Cao, X.; Yang, C. High-Performance Narrowband Pure-Red OLEDs with External Quantum Efficiencies up to 36.1% and Ultralow Efficiency Roll-Off. Adv. Mater. 2022, 34, 2201442. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Origin and Control of Orientation of Phosphorescent and TADF Dyes for High-Efficiency OLEDs. Adv. Mater. 2018, 30, 1705600. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, H.; Yang, D.; Ma, D.; Zhao, Z.; Tang, B.Z. Boosting external quantum efficiency to 38.6% of sky-blue delayed fluorescence molecules by optimizing horizontal dipole orientation. Sci. Adv. 2021, 7, eabj2504. [Google Scholar] [CrossRef]
- Peng, X.; Qiu, W.; Li, W.; Li, M.; Xie, W.; Li, W.; Lin, J.; Yang, J.; Li, X.; Su, S. Synergetic Horizontal Dipole Orientation Induction for Highly Efficient and Spectral Stable Thermally Activated Delayed Fluorescence White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2022, 32, 2203022. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, D.; Wang, P.; Huang, X.; Chen, H.; Zhang, Y.; Zhang, D.; Jiang, W.; Sun, Y.; Duan, L. Exceeding 30% External Quantum Efficiency in Non-doped OLEDs Utilizing Solution Processable TADF Emitters with High Horizontal Dipole Orientation via Anchoring Strategy. Angew. Chem. Int. Ed. 2022, 61, e202212861. [Google Scholar] [CrossRef]
- Burn, P.; Lo, S.; Samuel, D. The development of light-emitting dendrimers for displays. Adv. Mater. 2007, 19, 1675–1688. [Google Scholar] [CrossRef]
- Li, C.; Harrison, A.; Liu, Y.; Zhao, Z.; Zeng, C.; Dias, F.; Ren, Z.; Yan, S.; Bryce, M.R. Asymmetrical-Dendronized TADF Emitters for Efficient Non-doped Solution-Processed OLEDs by Eliminating Degenerate Excited States and Creating Solely Thermal Equilibrium Routes. Angew. Chem. Int. Ed. 2022, 61, e202115140. [Google Scholar]
- Sun, D.; Duda, E.; Fan, X.; Saxena, R.; Zhang, M.; Bagnich, S.; Zhang, X.; Köhler, A.; Zysman-Colman, E. Thermally Activated Delayed Fluorescent Dendrimers that Underpin High-efficiency Host-Free Solution-Processed Organic Light Emitting Diodes. Adv. Mater. 2022, 34, 2110344. [Google Scholar] [CrossRef]
- Ban, X.; Zhou, T.; Zhang, K.; Cao, Q.; Ge, F.; Zhang, D.; Zhu, P.; Liu, Z.; Li, Z.; Jiang, W. Developing homojunction exciplex for efficient multilayer solution-processed organic light emitting diodes. Chem. Eng. J. 2022, 441, 135898. [Google Scholar] [CrossRef]
- Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Solution-Processable Pure Green Thermally Activated Delayed Fluorescence Emitter Based on the Multiple Resonance Effect. Adv. Mater. 2020, 32, 2004072. [Google Scholar] [CrossRef]
- Oda, S.; Kawakami, B.; Yamasaki, Y.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Nakatsuka, S.; Hatakeyama, T. One-Shot Synthesis of Expanded Heterohelicene Exhibiting Narrowband Thermally Activated Delayed Fluorescence. J. Am. Chem. Soc. 2022, 144, 106–112. [Google Scholar] [CrossRef]
- Tan, H.; Yang, G.; Deng, Y.; Cao, C.; Tan, J.; Zhu, Z.; Chen, W.; Xiong, Y.; Jian, J.; Lee, C.-S.; et al. Deep-Blue OLEDs with Rec.2020 Blue Gamut Compliance and EQE over 22% Achieved by Conformation Engineering. Adv. Mater. 2022, 34, 220053. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, D.; Kong, F.; Zheng, Q.; Tang, X.; Zhu, Y.; Huang, C.; Feng, Z.; Fan, J.; Adachi, C.; et al. Steric Modulation of Spiro Structure for Highly Efficient Multiple Resonance Emitters. Angew. Chem. Int. Ed. 2022, 61, e202201886. [Google Scholar] [CrossRef]
- Cui, L.; Kim, J.; Nomura, H.; Nakanotani, H.; Adachi, C. Benzimidazobenzothiazole-Based Bipolar Hosts to Harvest Nearly All of the Excitons from Blue Delayed Fluorescence and Phosphorescent Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. 2016, 55, 6864–6868. [Google Scholar] [CrossRef]
- Zhang, D.; Song, X.; Cai, M.; Kaji, H.; Duan, L. Versatile Indolocarbazole-Isomer Derivatives as Highly Emissive Emitters and Ideal Hosts for Thermally Activated Delayed Fluorescent OLEDs with Alleviated Efficiency Roll-Off. Adv. Mater. 2018, 30, 1705406. [Google Scholar] [CrossRef]
- Sasabe, H.; Toyota, N.; Nakanishi, H.; Ishizaka, T.; Pu, Y.; Kido, J. 3,3′-Bicarbazole-Based Host Materials for High-Efficiency Blue Phosphorescent OLEDs with Extremely Low Driving Voltage. Adv. Mater. 2012, 24, 3212–3217. [Google Scholar] [CrossRef]
- Yook, K.; Lee, J. Small Molecule Host Materials for Solution Processed Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2014, 26, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhang, Y.; Quinton, C.; McIntosh, N.; Yang, S.; Rault-Berthelot, J.; Lucas, F.; Brouillac, C.; Jeannin, O.; Cornil, J.; et al. Pure Hydrocarbon Materials as Highly Efficient Host for White Phosphorescent Organic Light-Emitting Diodes: A New Molecular Design Approach. Angew. Chem. Int. Ed. 2022, 61, e202207. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, J.; Kim, H.; Lee, H.; Ha, J.; Woo, H.; Park, S.; Cho, M.; Choi, D. Novel V-Shaped Bipolar Host Materials for Solution-Processed Thermally Activated Delayed Fluorescence OLEDs. ACS Appl. Mater. Interfaces 2021, 13, 49076–49084. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ahn, H.; Kang, S.; Ko, S.; Song, D.; Um, H.; Kim, S.; Lee, Y.; Jeon, P.; Hwang, S.; et al. Exceptionally stable blue phosphorescent organic light-emitting diodes. Nat. Photonics 2022, 16, 212–218. [Google Scholar] [CrossRef]
- Braveenth, R.; Lee, H.; Park, J.; Yang, K.; Hwang, S.; Naveen, K.; Lampande, R.; Kwon, J. Achieving Narrow FWHM and High EQE Over 38% in Blue OLEDs Using Rigid Heteroatom Based Deep Blue TADF Sensitized Host. Adv. Funct. Mater. 2021, 31, 2105805. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Liu, Z.; Zhang, Y.; Wang, X.; Zhang, D.; Duan, L. A π-D and π-A Exciplex-Forming Host for High-Efficiency and Long-Lifetime Single-Emissive-Layer Fluorescent White Organic Light-Emitting Diodes. Adv. Mater. 2020, 32, 2004040. [Google Scholar] [CrossRef]
- Geng, Y.; D’Aleo, A.; Inada, K.; Cui, L.; Kim, J.; Nakanotani, H.; Adachi, C. Donor-σ-Acceptor motifs: Thermally activated delayed fluorescence emitters with dual upconversion. Angew. Chem. Int. Ed. 2017, 56, 16536–16540. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, K.; Li, X.; Dai, G.; Liu, W.; Ke, K.; Zhang, M.; Tao, S.; Zheng, C.; Ou, X.; et al. Intermolecular Charge-Transfer Transition Emitter Showing Thermally Activated Delayed Fluorescence for Efficient Non-Doped OLEDs. Angew. Chem. Int. Ed. 2018, 57, 9480–9484. [Google Scholar] [CrossRef]
- Zhang, D.; Suzuki, K.; Song, X.; Wada, Y.; Kubo, S.; Duan, L.; Kaji, H. Thermally Activated Delayed Fluorescent Materials Combining Intra- and Intermolecular Charge Transfers. ACS Appl. Mater. Interfaces 2019, 11, 7192–7198. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, D.; Tian, W.; Jiang, W.; Sun, Y. Rational molecular design of novel host material combing intra- and intermolecular charge transfers for efficient solution-processed organic light-emitting diodes. Dye. Pigment. 2020, 175, 108188. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, B.; Liu, D.; Ma, D.; Chen, H.; Tian, W.; Ban, X.; Jiang, W.; Sun, Y. Aggregation induced intermolecular charge transfer in simple nonconjugated donor-acceptor system. Org. Electron. 2021, 99, 106309. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.; Cheng, L.; Chen, H.; Qiu, C. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.; Tian, W.; Jiang, W.; Sun, Y.; Zhao, Z.; Tang, B.Z. Molecular core-shell structure design: Facilitating delayed fluorescence in aggregates toward highly efficient solution-processed OLEDs. Aggregate 2022, 3, e164. [Google Scholar] [CrossRef]
- Voskuhl, J.; Giese, M. Mesogens with aggregation-induced emission properties: Materials with a bright future. Aggregate 2022, 3, e124. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.; Wang, L.; McGonigal, P.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.; Lam, J.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef]
- Zhao, G.; He, Y.; Xu, Z.; Hou, J.; Zhang, M.; Min, J.; Chen, H.; Ye, M.; Hong, Z.; Yang, Y.; et al. Effect of Carbon Chain Length in the Substituent of PCBM-like Molecules on Their Photovoltaic Properties. Adv. Funct. Mater. 2010, 20, 1480–1487. [Google Scholar] [CrossRef]
- Liu, X.; He, B.; Garzón-Ruiz, A.; Navarro, A.; Chen, T.; Kolaczkowski, M.; Feng, S.; Zhang, L.; Anderson, C.; Chen, J.; et al. Unraveling the Main Chain and Side Chain Effects on Thin Film Morphology and Charge Transport in Quinoidal Conjugated Polymers. Adv. Funct. Mater. 2018, 28, 1801874. [Google Scholar] [CrossRef]
- Sheng, W.; Zheng, Y.; Wu, Q.; Chen, K.; Li, M.; Jiao, L.; Hao, E.; Wang, J.; Pei, J. Synthesis, characterization, and tunable semiconducting properties of aza-BODIPY derived polycyclic aromatic dyes. Sci. China Chem. 2020, 63, 1240–1245. [Google Scholar] [CrossRef]
- Wang, L.; Lu, N.; Huang, S.; Wang, M.; Chen, X.; Yang, H. Optically Active Nucleobase-Functionalized Polynorbornenes Mimicking Double-Helix DNA. CCS Chem. 2020, 2, 1787–1796. [Google Scholar] [CrossRef]
- Seguy, I.; Jolinat, P.; Destruel, P.; Farenc, J. Red organic light emitting device made from triphenylene hexaester and perylene tetraester. J. Appl. Phys. 2001, 89, 5442. [Google Scholar] [CrossRef]

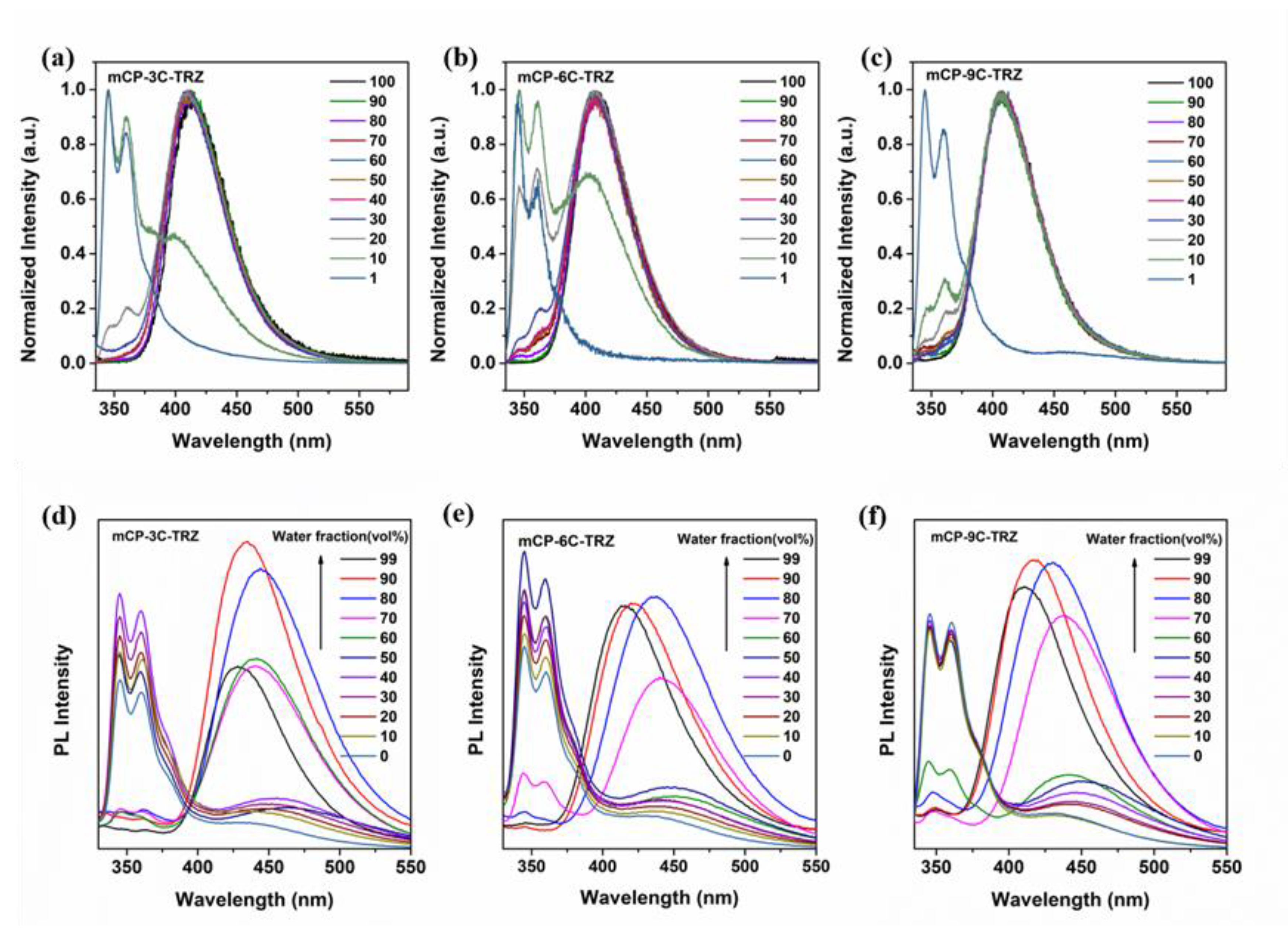
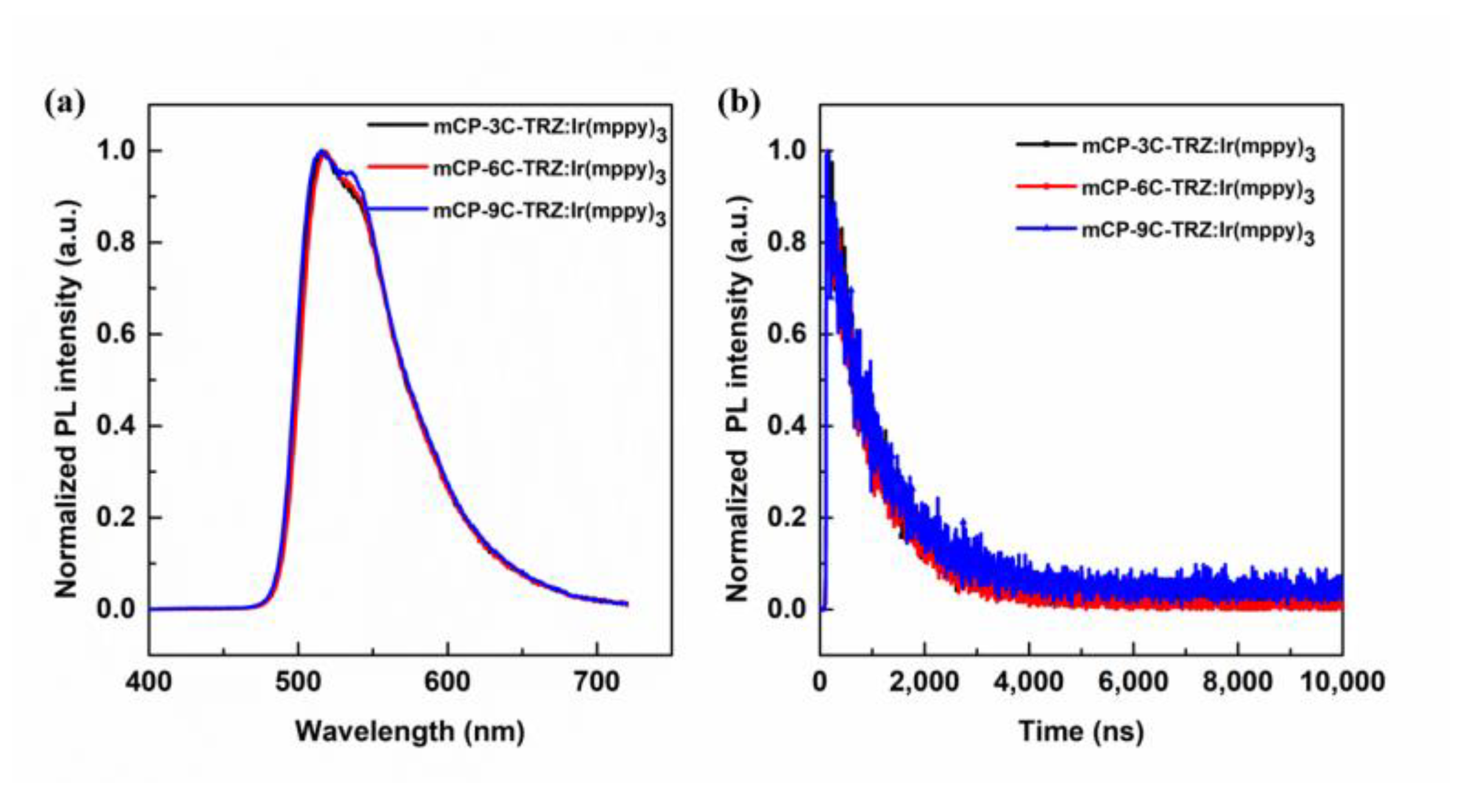
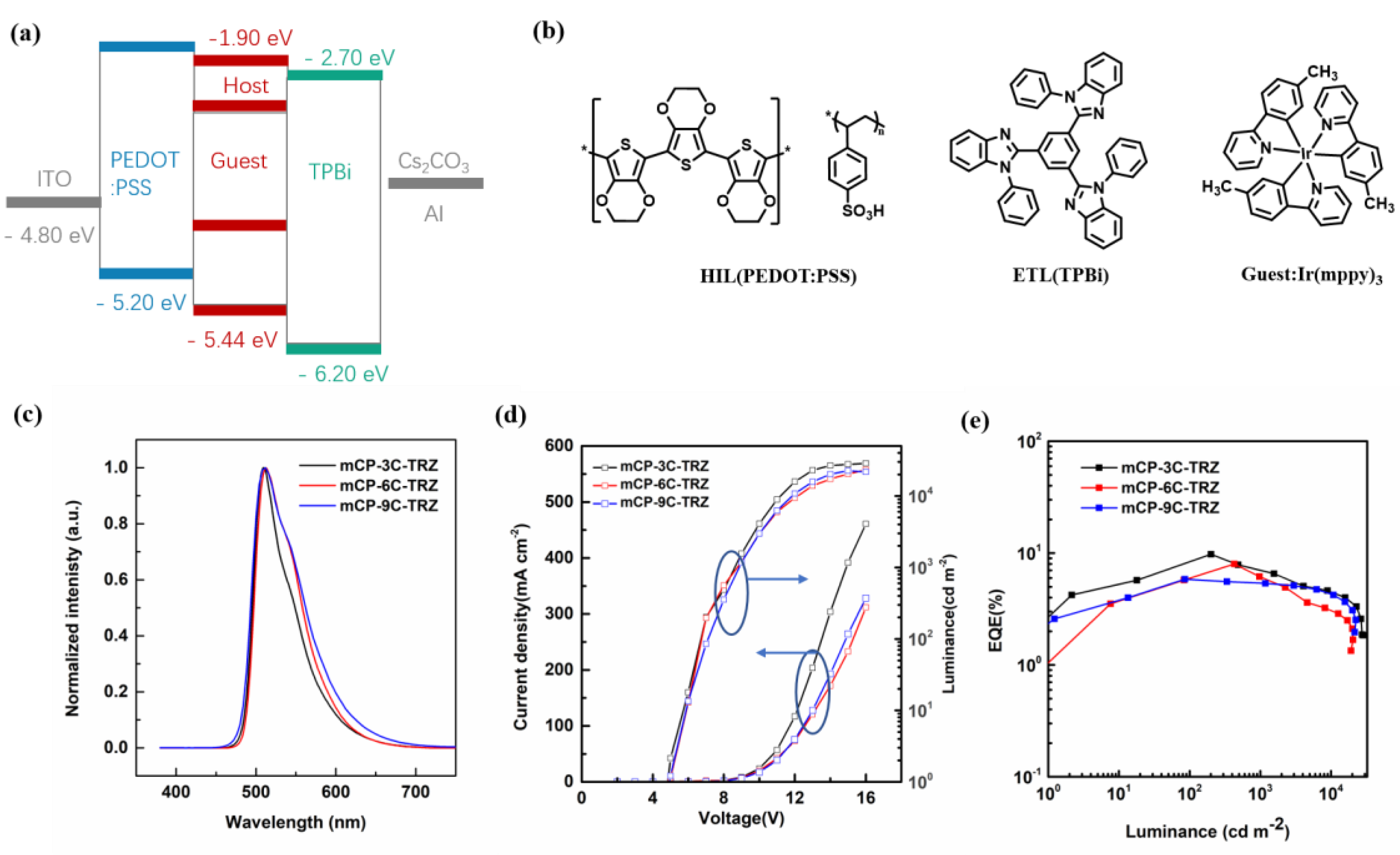
| Compound | Tg a (°C) | λabs † (nm) | λem b (nm) | λem c (nm) | Eg d (eV) | S1 e/T1 f (eV) | ΔEST g (eV) | HOMO h (eV) | LUMO i (eV) |
|---|---|---|---|---|---|---|---|---|---|
| mCP-3C-TRZ | 98 | 240, 292, 339 | 345, 360 | 413 | 3.65 | 3.28/ 3.02 | 0.26 | −5.44 | −1.90 |
| mCP-6C-TRZ | 92 | 240, 293 339 | 345, 359 | 413 | 3.65 | 3.29/ 3.05 | 0.24 | −5.45 | −1.91 |
| mCP-9C-TRZ | 73 | 240, 291, 339 | 344, 359 | 413 | 3.65 | 3.30/ 3.00 | 0.30 | −5.46 | −1.92 |
| Emitting Layer | EL a (nm) | Von b (V) | Lmax c (cd m−2) | CEmax d (cd A−1) | PEmax e (lm W−1) | EQEmax f (%) | CIE g (x, y) |
|---|---|---|---|---|---|---|---|
| mCP-3C-TRZ: Ir(mppy)3 | 512 | 4.5 | 28,589 | 29.2 | 13.1 | 9.8 | (0.29, 0.62) |
| mCP-6C-TRZ: Ir(mppy)3 | 512 | 4.8 | 20,190 | 20.9 | 9.4 | 7.0 | (0.29, 0.62) |
| mCP-9C-TRZ: Ir(mppy)3 | 512 | 4.9 | 22,600 | 17.3 | 7.8 | 5.8 | (0.29, 0.62) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Zhao, G.; Tian, W.; Sun, Y. Aggregation-Induced Intermolecular Charge Transfer Emission for Solution-Processable Bipolar Host Material via Adjusting the Length of Alkyl Chain. Molecules 2022, 27, 8099. https://doi.org/10.3390/molecules27228099
Jiang W, Zhao G, Tian W, Sun Y. Aggregation-Induced Intermolecular Charge Transfer Emission for Solution-Processable Bipolar Host Material via Adjusting the Length of Alkyl Chain. Molecules. 2022; 27(22):8099. https://doi.org/10.3390/molecules27228099
Chicago/Turabian StyleJiang, Wei, Guimin Zhao, Wenwen Tian, and Yueming Sun. 2022. "Aggregation-Induced Intermolecular Charge Transfer Emission for Solution-Processable Bipolar Host Material via Adjusting the Length of Alkyl Chain" Molecules 27, no. 22: 8099. https://doi.org/10.3390/molecules27228099
APA StyleJiang, W., Zhao, G., Tian, W., & Sun, Y. (2022). Aggregation-Induced Intermolecular Charge Transfer Emission for Solution-Processable Bipolar Host Material via Adjusting the Length of Alkyl Chain. Molecules, 27(22), 8099. https://doi.org/10.3390/molecules27228099