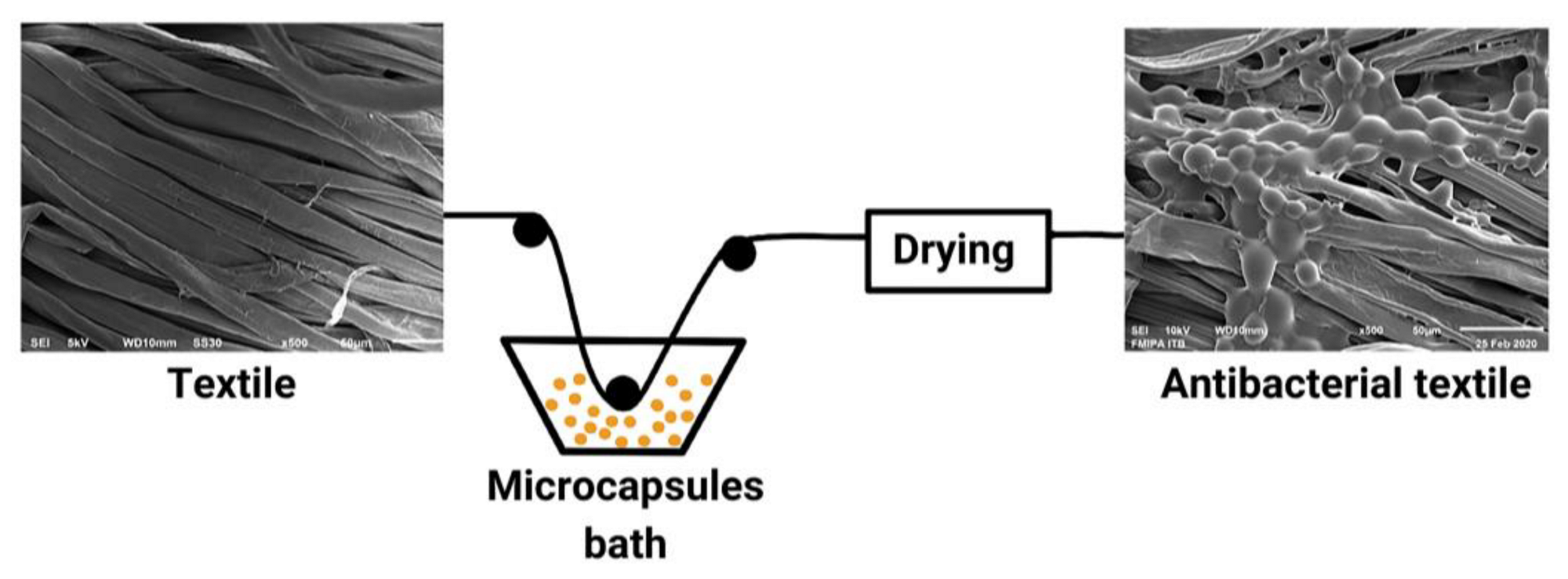
The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review
Abstract
1. Introduction
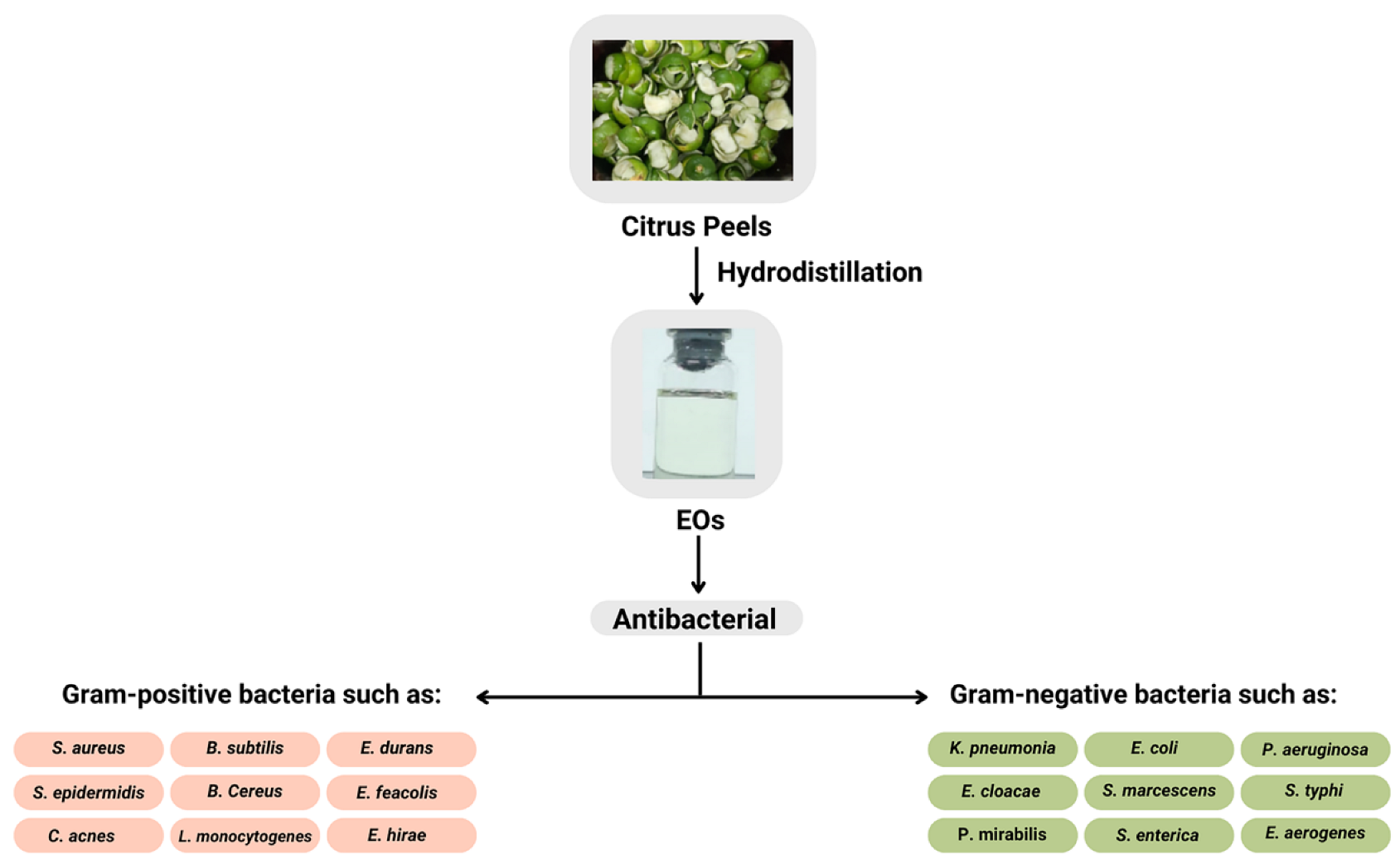
2. Potential of Citrus EOs as Antimicrobial Agents
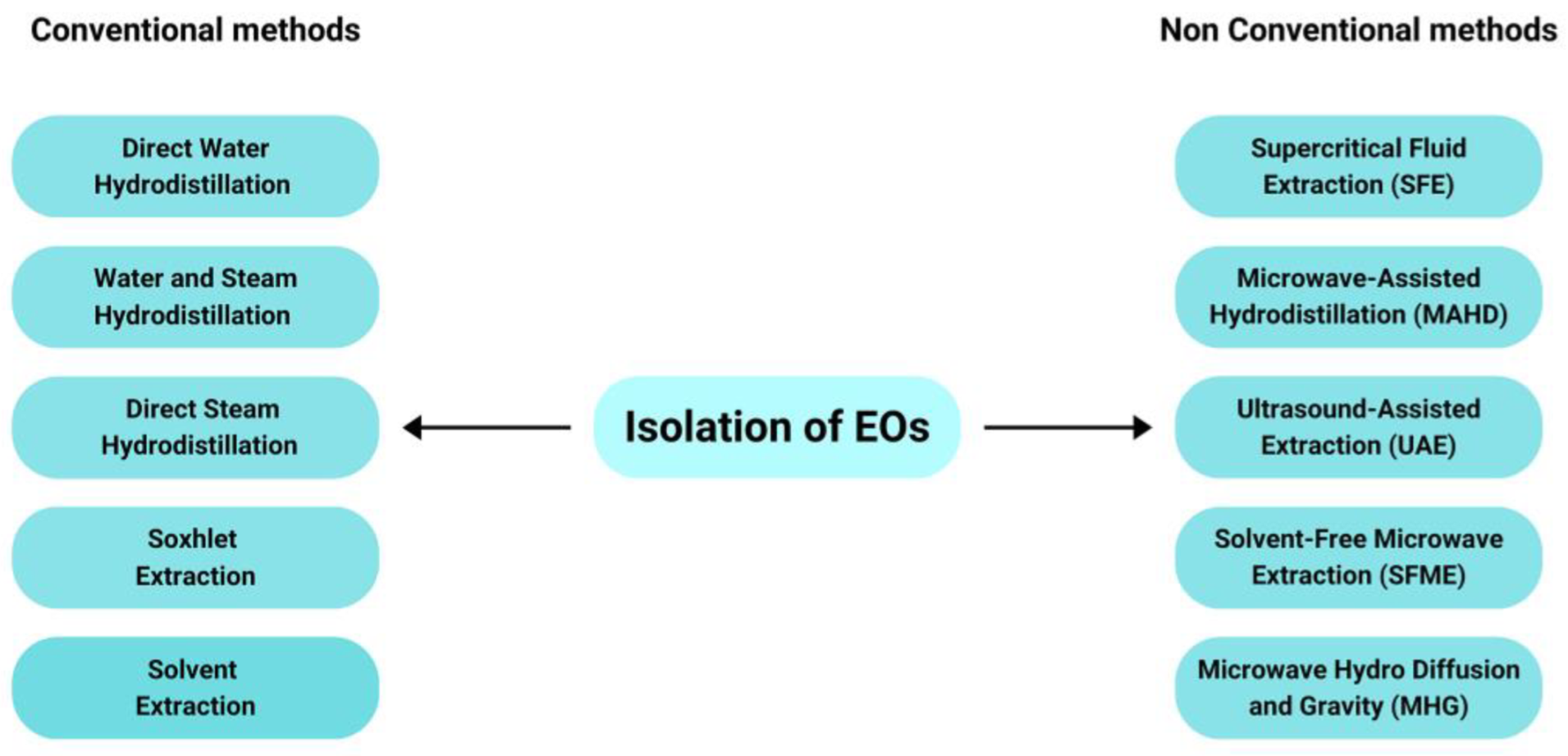
3. Isolation of the EOs
4. Chemical Composition of the Citrus EOs
| No. | Chemical Components | C. aurantifolia | C. nobilis | C. sinensis | C. limon | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [58] | [15] | [67] | [58] | [60] | [61] | [58] | [62] | [65] | [58] | [44] | [63] | ||
| 1. | δ-Limonene | 38.9 | 42.4 | 39.3 | 50.1 | 76.8 | 81.8 | 21.7 | 90.9 | 98.4 | 41.4 | 61.3 | 75.0 |
| 2. | trans-Limonene oxide | - | - | - | - | 0.3 | - | 0.01 | - | - | 0.2 | - | |
| 3. | β-Myrcene | 0.9 | 1.9 | - | 1.0 | 2.4 | 4.0 | - | 1.9 | 1.1 | 2.4 | 1.4 | - |
| 4. | β-Pinene | 26.7 | 12.6 | 28.4 | 3.7 | 0.8 | - | 15.4 | - | 0.03 | 14.2 | 9.7 | - |
| 5. | α-Pinene | - | 3.1 | 1.5 | - | 1.1 | 2.1 | 0.8 | - | 0.4 | - | 1.5 | - |
| 6. | α-Terpineol | 8.3 | 1.6 | 2.4 | 4.1 | - | 0.2 | 5.4 | 0.1 | - | 1.7 | 0.4 | - |
| 7. | 1-Terpinenol | - | 0.04 | - | - | - | - | - | - | - | - | - | - |
| 8. | 4-Terpineol | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| 9. | Terpinene-4-ol | 4.3 | - | 2.0 | 1.5 | 0.7 | 0.5 | 1.8 | - | - | - | - | - |
| 10. | Terpinolene | - | - | - | - | 0.7 | 0.4 | - | 0.1 | - | - | 0.2 | - |
| 11. | α-Terpinene | - | 0.37 | - | - | - | - | - | - | - | - | - | - |
| 12. | γ-Terpinene | - | 15.4 | 0.8 | - | 8.2 | 6.1 | - | 1.2 | - | 16.8 | 3.8 | - |
| 13. | Geranial | - | - | 2.1 | - | - | - | - | 0.1 | - | - | - | - |
| 14. | Geranyl acetate | 2.6 | 0.6 | 0.6 | - | - | 0.2 | 1.2 | - | - | 1.7 | - | 0.3 |
| 15. | Geraniol | 1.3 | 0.6 | 7.5 | 0.8 | - | - | - | - | - | 1.3 | - | - |
| 16. | Citronellal | - | - | - | 2.2 | - | - | 1.8 | 0.1 | - | - | 0.3 | - |
| 17. | β -Citronellal | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 18. | Citronellol | - | - | - | 1.9 | - | - | - | - | - | - | 0.6 | - |
| 20. | Citral | 3.6 | - | - | 0.6 | - | - | - | - | - | 2.7 | 4.2 | 8.1 |
| 21. | Z-Citral | - | 2.0 | - | - | - | - | - | - | - | - | - | - |
| 22. | E-Citral | - | 1.8 | - | - | - | - | - | - | - | - | - | 4.4 |
| 23. | p-Cymene | - | - | - | - | - | - | 1.4 | - | - | - | 0.1 | - |
| 24. | β- Ocymene | - | 0.3 | - | - | - | - | - | - | - | - | 0.1 | - |
| 25. | o-Ocymene | 1.9 | 1.3 | - | - | - | - | - | 0.3 | - | 2.3 | - | - |
| 26. | α-Phellandrene | 0.9 | 0.1 | - | - | - | - | - | - | - | 1.4 | - | - |
| 27. | Neral | - | - | 5.3 | - | - | - | - | 0.1 | - | - | - | - |
| 28. | Nerol | 0.9 | - | - | - | - | - | - | - | - | - | - | - |
| 29. | Z-Nerodilol | - | - | 0.6 | - | - | - | - | - | - | - | - | - |
| 30. | Neryl acetate | - | 2.2 | - | - | - | - | - | 0.02 | - | - | - | 1.4 |
| 31. | β-Bisabolen | 1.0 | - | - | - | - | - | - | - | - | 1.7 | - | 0.6 |
| 38. | Linalool | - | 0.6 | - | - | 0.3 | 0.9 | - | 0.9 | - | - | 0.4 | - |
| 40. | Linalool oxide | - | - | - | - | - | - | - | - | - | - | - | 0.4 |
| 41. | Trans-linalool oxide | - | - | - | - | - | - | - | - | - | - | - | 0.4 |
| 43. | 3-Carene | - | 0.5 | 0.5 | - | - | - | - | 0.1 | - | - | - | - |
| 44. | Carvone | - | - | - | - | - | 0.3 | - | - | - | - | - | - |
| 45. | α-Farnesene | - | - | - | - | 0.51 | 0.3 | - | - | - | - | - | - |
| 46. | (Z)-β-Farnesene | - | 0.1 | 0.4 | - | - | - | - | - | - | - | - | - |
| 47. | (E)-β-Farnesene | - | - | 1.5 | - | - | - | - | - | - | - | - | - |
| 48. | α-Thujene | - | 1.0 | - | - | 0.2 | 0.4 | - | - | - | - | 0.2 | - |
| 49. | Sabinene | - | 2.1 | - | - | - | 1.2 | 0.5 | - | 0.07 | - | - | - |
| 50. | δ-elemene | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| 51. | β-Elemene | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| 52. | γ-Elemene | - | 0.1 | - | - | 0.4 | 0.3 | 1.2 | - | - | - | - | 2.2 |
| 53. | Humulene | - | 0.1 | 0.1 | - | - | - | 1.2 | - | - | - | - | - |
| 54. | trans-Carveol | - | - | - | 0.7 | - | - | - | - | - | - | - | - |
| 63. | Germacrene-B | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 64. | Germacrene D | - | 0.2 | - | - | - | 0.1 | - | 0.1 | - | - | - | 0.2 |
| 67. | Methyl chavicol | - | - | - | - | 3.7 | - | - | - | - | - | - | - |
| 68. | δ-Cadinene | - | - | - | - | - | 0.3 | - | - | - | - | - | - |
| 70. | Camphor | - | 0.01 | - | - | - | - | - | - | - | - | - | 0.26 |
| 71. | Trans-carveol | - | - | - | - | - | - | - | - | - | - | - | 0.20 |
| 72. | Camphene | - | 0.14 | - | - | - | - | - | - | - | - | - | - |
| 73. | Cis-Carveol | - | - | - | - | - | - | - | - | - | - | - | 0.20 |
| 77. | α-caryophyllene | - | - | - | - | - | - | - | - | - | - | - | - |
| 78. | Trans-caryophyllene | - | 0.9 | - | - | - | - | - | - | - | - | - | - |
| 79 | Trans-α-bergamotene | - | 1.4 | 0.4 | - | - | - | - | - | - | - | - | 0.4 |
| 81. | Myristicin | - | - | - | - | - | - | - | - | - | - | - | 0.8 |
| Isolation Method | HD | HD | HD | HD | HD | HD | HD | - | MAHD | HD | HD | HD | |
| Recollection place (Country) | IDN | TWN | MYS | IDN | TUR | IRN | IDN | CHN | VNM | IDN | DZA | IDN | |
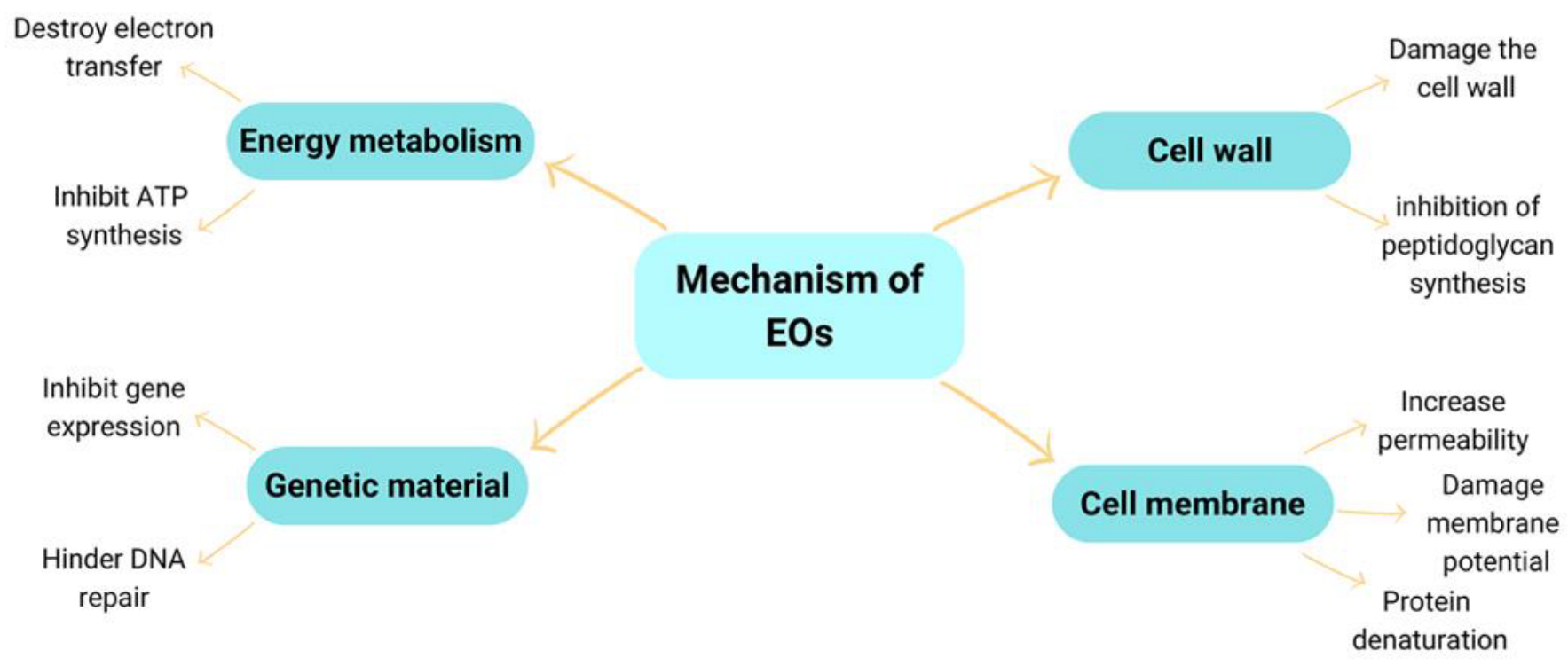
5. The Mechanism of Antimicrobial Action of the EOs
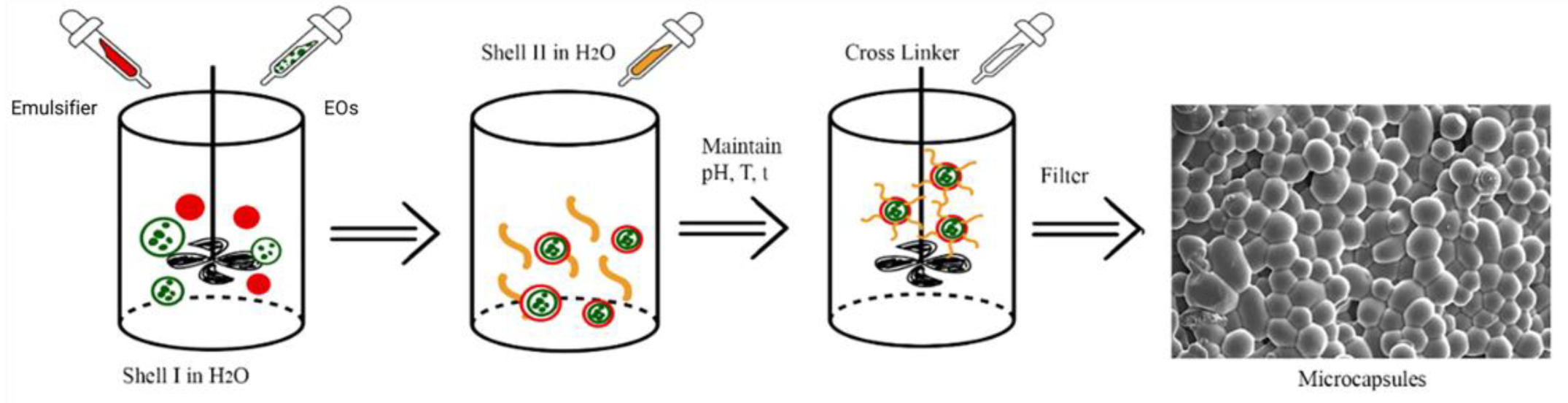
6. Complex Coacervation Methods
7. Microcapsule Shell
8. Cross-Binder
9. Emulsifier
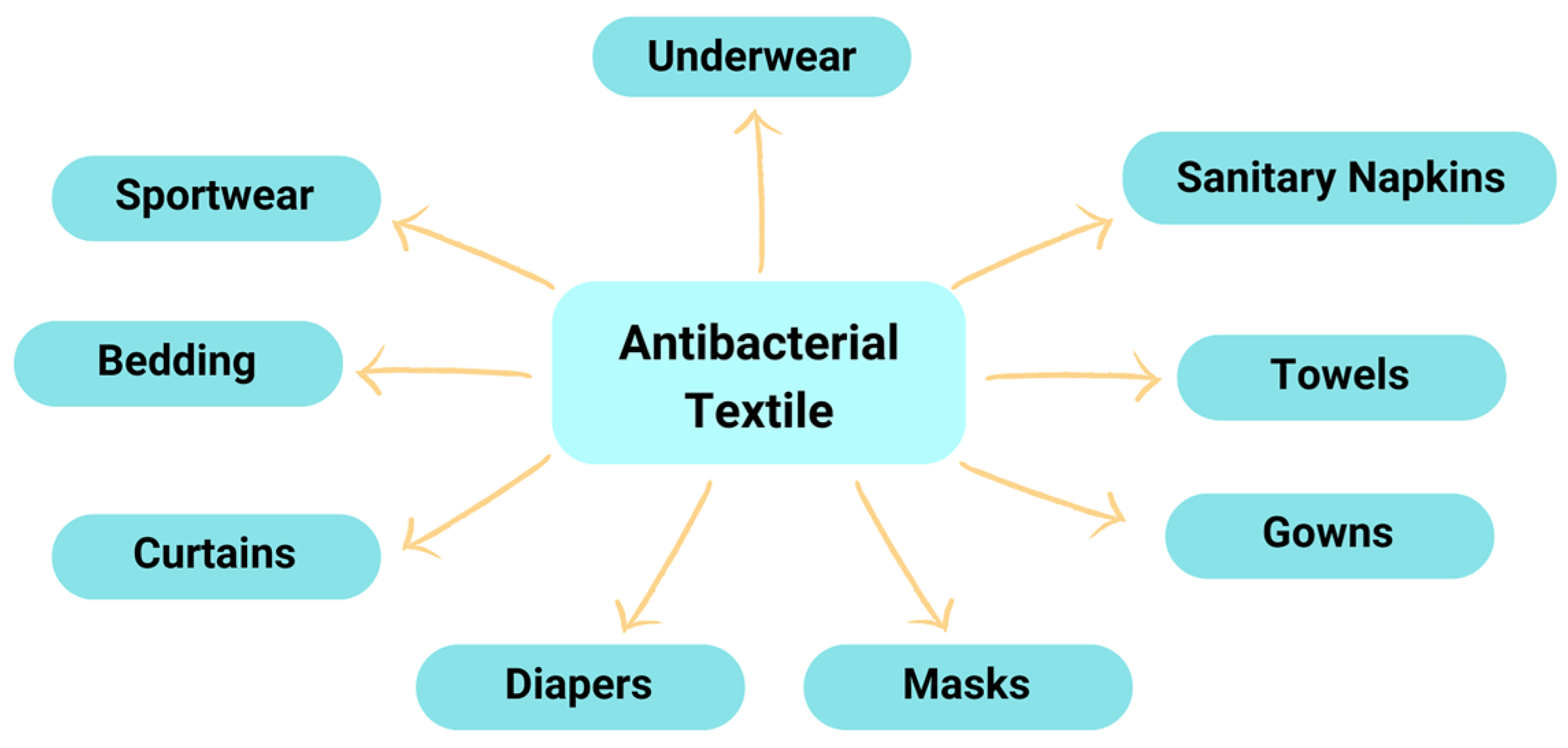
10. Microcapsules Immobilization onto Textile Materials
11. Future Prospects
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P.; Bora, H.; Kamle, M. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Coile, G.A. Textbook of Limnology, 4th ed.; Wavel. Press Inc.: New York, NY, USA, 1994. [Google Scholar]
- Ndiaye, E.H.B.; Gueye, M.T.; Ndiaye, I. Chemical composition of distilled essential oils and hydrosols of four senegalese citrus and enantiomeric characterization of chiral compounds. J. Essent. Oil-Bear. Plants 2017, 20, 820–834. [Google Scholar] [CrossRef]
- Spadaro, F.; Costa, R.; Circosta, C.; Occhiuto, F. Volatile composition and biological activity of key lime Citrus aurantifolia essential oil. Nat. Prod. Commun. 2012, 7, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Kamal, G.M.; Anwar, F.; Hussain, A.I. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011, 18, 1275–1282. [Google Scholar]
- Al Namani, J.; Baqir, E.; Al Abri, A. Phytochemical screening, phenolic content and antioxidant activity of Citrus aurantifolia L. Leaves grown in two regions of oman. Iran. J. Pharm. Sci. 2018, 14, 27–34. [Google Scholar]
- Obidi, O.; Adelowotan, A.; Ayoola, G. Antimicrobial activity of orange oil on selected pathogens. Int. J. Biotechnol. 2013, 2, 113–122. [Google Scholar]
- Torimiro, N.; Adegun, B.R.; Abioye, O.E.; Omole, R.K. Antibacterial activity of essential oil from Citrus aurantifolia (Christm.) Swingle peels against multidrug-resistant bacterial isolates. Adv. Microbiol. 2020, 10, 214–223. [Google Scholar] [CrossRef]
- Costa, R.; Bisignano, C.; Filocamo, A. Antimicrobial activity and chemical composition of Citrus aurantifolia (Christm.) Swingle essential oil from Italian organic crops. J. Essent. Oil Res. 2014, 26, 400–408. [Google Scholar] [CrossRef]
- Karsheva, M.; Kirova, E.; Alexandrova, S.; Georgieva, S. Comparison of citrus peels as a source of valuable components- polyphenols and antioxidants. J. Chem. Technol. Metall. 2013, 48, 475–478. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Giovagnoli-Vicuña, C.; Briones-Labarca, V.; Romero, M.S. Effect of extraction methods and in vitro bio-accessibility of microencapsulated lemon extract. Molecules 2022, 27, 4166. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Chang, H.-T.; Chang, S.-T. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Gamarra, F.M.C.; Sakanaka, L.S.; Tambourgi, E.B.; Cabrai, F.A. Influence on the quality of essential lemon (Citrus aurantifolia) oil by distillation process. Braz. J. Chem. Eng. 2006, 23, 147–151. [Google Scholar] [CrossRef]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in eastern oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Maji, T.K.; Baruah, I.; Dube, S.; Hussain, M.R. Microencapsulation of Zanthoxylum limonella oil (ZLO) in glutaraldehyde crosslinked gelatin for mosquito repellent application. Bioresour. Technol. 2007, 98, 840–844. [Google Scholar]
- Chanthaphon, S.; Chanthachum, S.; Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. Against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008, 30, 125–131. [Google Scholar]
- Ozkan, G.; Franco, P.; De Marco, I. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Lazarova-zdravkova, N.; Terzieva, T.; Peshev, D.; Georgieva, N. Antibacterial activity of microencapsulated via spouted-bed hydro-alcoholic rosemary extracts. J. Chem. Technol. Metall. 2020, 63, 40–44. [Google Scholar]
- Li, Y.; Li, X.; Liang, Z.P. Progress of microencapsulated phycocyanin in food and pharma industries: A review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The role of microencapsulation in food application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.R.A.; Jai, J.; Raslan, R.; Subuki, I. Microencapsulation of essential oils application in textile: A review. Adv. Mater. Res. 2015, 1113, 346–351. [Google Scholar]
- Valle, J.A.B.; Valle, R.D.C.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Lis, A.M.J. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, 50482. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, Á.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Jain, S.K.; Jain, N.K. Multiparticulate carriers for sun-screening agents. Int. J. Cosmet. Sci. 2010, 32, 89–98. [Google Scholar] [CrossRef]
- Özyildiz, F.; Karagönlü, S.; Basal, G. Micro-encapsulation of ozonated red pepper seed oil with antimicrobial activity and application to nonwoven fabric. Lett. Appl. Microbiol. 2013, 56, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Petrulis, D.; Petrulyte, S. Potential use of microcapsules in manufacture of fibrous products: A review. J. Appl. Polym. Sci. 2019, 136, 47066. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef]
- Wang, Y.; Ghosh, S.; Nickerson, M.T. Microencapsulation of flaxseed oil by lentil protein. Molecules 2022, 27, 3195. [Google Scholar] [CrossRef]
- Gokarneshan, N.; Nagarajan, V.B.; Viswanath, S. Developments in antimicrobial textiles-some insights on current research trends. Biomed. J. Sci. Tech. Res. 2017, 1, 230–233. [Google Scholar]
- Sharkawy, A.; Fernandes, I.P.; Barreiro, M.F. Aroma-loaded microcapsules with antibacterial activity for eco-friendly textile application: Synthesis, characterization, release, and green grafting. Ind. Eng. Chem. Res. 2017, 56, 5516–5526. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) essential oils: Volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, S.; Oyekanmi, B.; Ovaleye, O.; Oa, A. In vitro antibacterial activity of crude extract of Citrus aurantifolia L. and Tithonia diversifolia poacheae on clinicaln bacteria isolates. Int. J. Med. 2007, 4, 113–117. [Google Scholar]
- Jafari, S.; Esfahani, S.; Fazeli, M.R. Antimicrobial activity of lime essential oil against food-borne pathogens isolated from cream-filled cakes and pastries. Int. J. Biol. Chem. 2011, 5, 258–265. [Google Scholar] [CrossRef]
- Julaeha, E.; Nurzaman, M.; Eddy, D.R. Preparation and characterization of Citrus aurantifolia lime oils microcapsules by complex coacervation technique. Rev. Chim. 2021, 71, 146–155. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M.; Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- de Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R. Microencapsulation of sweet orange essential oil (Citrus aurantium var. dulcis) by liophylization using maltodextrin and maltodextrin/gelatin mixtures: Preparation, characterization, antimicrobial and antioxidant activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef]
- Julaeha, E.; Eddy, D.R.; Wahyudi, T. Coacervate microcapsules of Citrus aurantifolia essential oil (LOs): Optimization and their antibacterial activity study. ChemistrySelect 2022, 7, e202200187. [Google Scholar] [CrossRef]
- Atolani, O.; Adamu, N.; Oguntoye, O.S. Chemical characterization, antioxidant, cytotoxicity, anti-toxoplasma gondii and antimicrobial potentials of the Citrus sinensis seed oil for sustainable cosmeceutical production. Heliyon 2020, 6, e03399. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Himed, L.; Merniz, S.; Barkat, M. Chemical composition of Citrus limon (Eureka variety) essential oil and evaluation of its antioxidant and antibacterial activities. Afr. J. Biotechnol. 2018, 17, 356–361. [Google Scholar] [CrossRef]
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [PubMed]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Dănilă, E.; Moldovan, Z.; Popa, M. Chemical composition, antimicrobial and antibiofilm efficacy of C. limon and L. angustifolia EOs and of their mixtures against Staphylococcus epidermidis clinical strains. Ind. Crops Prod. 2018, 122, 483–492. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Zara, S. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT-Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Shi, H.; Xin, J. Cosmetic textiles: Concepts, application and prospects. Inst. Text. Cloth. 2007, 5, 21134–21138. [Google Scholar]
- Cerempei, A.; Muresan, E.I.; Cimpoesu, N. Biomaterials with controlled release of geranium essential oil. J. Essent. Oil Res. 2014, 26, 267–273. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Darjazi, B.B. The effect of geographical location on mexican lime (Citrus aurantifolia) peel components. Int. J. Agric. Innov. Res. 2014, 3, 1473–2319. [Google Scholar]
- Ben Salha, G.; Abderrabba, M.; Labidi, J. A status review of terpenes and their separation methods. Rev. Chem. Eng. 2019, 37, 433–447. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils Science, Technology, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ruberto, G. Analysis of Volatile Components of Citrus Fruit Essential Oils, Analysis of Taste and Aroma; Springer: Berlin, Germany, 2002. [Google Scholar]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Essential oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytotherapy Research. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef]
- Aripin, D.; Julaeha, E.; Dardjan, M.; Cahyanto, A. Chemical composition of Citrus spp. and oral antimicrobial effect of Citrus spp. peels essential oils against Streptococcus mutans. Padjadjaran J. Dent. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Wahyudi, T.; Mulyawan, A.S.; Kasipah, C. Pembuatan mikrokapsul minyak jeruk (Citrus aurantifolia) untuk aplikasi pada penyempurnaan tekstil. Arena Tekst. 2017, 32, 1–8. [Google Scholar] [CrossRef][Green Version]
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluation of the chemical composition and antioxidant activity of the peel oil of Citrus nobilis. Int. J. Food Prop. 2010, 13, 983–991. [Google Scholar] [CrossRef]
- Asgarpanah, J. Volatile composition of the peel and leaf essential oils of Citrus nobilis Lour. var. deliciosa Swingle. Afr. J. Biotechnol. 2012, 11, 6364–6367. [Google Scholar] [CrossRef]
- Qiao, Y.; Bi, J.X.; Zhang, Y.; Zhang, Y.; Fan, G.; Xiao, L.Y.; Si, Y.P.; Qiao, Y.; Bi, J.X. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef]
- Mahdani, F.Y.; Parmadiati, A.E.; Ernawati, D.S. Citrus limon peel essential oil–induced type iv hypersensitivity reaction. J. Exp. Pharmacol. 2020, 12, 213–220. [Google Scholar] [CrossRef]
- Othman, H.M.; Nahar, L.; Basar, N.; Jamil, S.; Sarker, S.M.S. Essential oils from the malaysian Citrus (Rutaceae) medicinal plants. Medicines 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Toan, T.Q.; Truc, T.T.; Le, X.T. Study on extraction process and analysis of components in essential oils of Vietnamese orange peel (Citrus sinensis) by microwave assisted hydrodistillation extraction. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020. [Google Scholar]
- Hien, T.T.; Quyen, N.T.C.; Minh, P.T.H.; Le, X.T. Determine the components of kaffir lime oil (Citrus hystrix DC.) in the microwave-assisted extraction process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020. [Google Scholar]
- Salehi, B.; Upadhyay, S.; Orhan, I.E. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Julaeha, E.; Puspita, P.; Rakhmawaty, D.E.; Wahyudi, T.; Nurzaman, M.; Nugraha, J.; Herlina, T.; Al Anshori, J. Microencapsulation of lime (Citrus aurantifolia) oil for antibacterial finishing of cotton fabric. RSC Adv. 2021, 11, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, H.; Liu, K. The role of JrPPOs in the browning of walnut explants. BMC Plant Biol. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.J.T.; de Sousa, J.P.; Lima, C.N.F. Phytochemical characterization of the Baccharis dracunculifolia DC (Asteraceae) essential oil and antibacterial activity evaluation. Ind. Crops Prod. 2018, 122, 591–595. [Google Scholar] [CrossRef]
- Williams, B.E.T.; Julius, B.; Timothy, N. Phytochemicals, elemental, proximate analysis and anti-nutrient composition of Citrus aurantifolia seeds. GJMR 2020, 20, 7–12. [Google Scholar]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Renard, D. A novel method of oil encapsulation in core-shell alginate microcapsules by dispersion-inverse gelation technique. Reactive and Functional Polymers. React. Funct. Polym. 2017, 114, 49–57. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Bhadauria, S.; Mahour, K. Formation of highly bioactive silver nanoparticles and their antibacterial activity. PhOL 2009, 3, 437–446. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, A.; Singh, S.K. Microencapsulation techniques applicable to food flavours research and developement: A comprehensive review. Int. J. Food Nutr. Sci. 2015, 4, 119–124. [Google Scholar]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J.P., Gun’ko, V.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 23–24. [Google Scholar]
- Silva, M.; Martins, I.M.; Barreiro, M.F. Functionalized textiles with PUU/limonene microcapsules: Effect of finishing methods on fragrance release. J. Text. Inst. 2017, 108, 361–367. [Google Scholar] [CrossRef]
- Campelo-Felix, F.H.; Souza, H.J.B.; Figueiredo, J.A. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Fernandes, I.; Martins, I.M. Microencapsulation of limonene for textile application. Ind. Eng. Chem. Res. 2008, 47, 4142–4147. [Google Scholar] [CrossRef]
- Badulescu, R.; Vivod, V.; Jausovec, D.; Voncina, B. Treatment of cotton fabrics with ethyl cellulose microcapsules. In Medical and Healthcare Textiles; Elsivier Ltd.: Sawston, UK, 2010; pp. 226–235. [Google Scholar]
- Jun-xia, X.; Hai-yan, Y.; Jian, Y. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011, 125, 1267–1272. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Y.; Wang, X. Microencapsulation of sweet orange oil terpeneless using the orifice method. J. Food Eng. 2012, 110, 390–394. [Google Scholar] [CrossRef]
- Teixeira, C.S.N.R.; Martins, I.M.D.; Mata, V.L.G. Characterization and evaluation of commercial fragrance microcapsules for textile application. J. Text. Inst. 2012, 103, 269–282. [Google Scholar]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D. Properties and controlled release of chitosan microencapsulated limonene oil. Rev. Bras. Farmacogn. 2014, 24, 691–698. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wu, T.; Wang, L.; Chen, S.; Ding, T.; Hu, Y.Y. Preparation and characterization of Citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Wijesirigunawardana, P.B.; Perera, B.G.K. Development of a cotton smart textile with medicinal properties using lime oil microcapsules. Acta Chim. Slov. 2018, 65, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, H.; Dou, H. Microcapsule of sweet orange essential oil changes gut microbiota in diet-induced obese rats. Biochem. Biophys. Res. Commun. 2018, 505, 991–995. [Google Scholar] [CrossRef]
- Lim, P.; Setthayanond, J. Factors affecting release of microencapsulated essential oils from finished silk fabric for automotive and home textile products. Int. J. Eng. Adv. Technol. 2019, 8, 501–504. [Google Scholar]
- Ramos, F.D.M.; Júnior, V.S.; Prata, A.S. Physical aspects of orange essential oil-contaning particles after vacuum spray drying processing. Food Chem. X 2021, 12, 100142. [Google Scholar] [CrossRef]
- Devi, N.; Kakati, D.K.; Devi, N.; Kakati, D.K. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J. Food Eng. 2013, 117, 193–204. [Google Scholar] [CrossRef]
- Devi, N.; Hazarika, D.; Deka, C.; Kakati, D.K. Study of complex coacervation of gelatin a and sodium alginate for microencapsulation of olive oil. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 936–945. [Google Scholar] [CrossRef]
- Shinde, U.A.; Nagarsenker, M.S. Characterization of gelatin-sodium alginate complex coacervation system. Indian J. Pharm. Sci. 2009, 71, 313–317. [Google Scholar]
- Yan, C.; Zhang, W. Coacervation processes. In Microencapsulation in the Food Industry, 1st ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Toma, A.; Deyno, S. Overview on mechanisms of antibacterial resistance. Int. J. Res. Pharm. Biosci. 2015, 2, 27–36. [Google Scholar]
- Stangierski, J.; Baranowska, H.M.; Rezler, R.; Kawecki, K. The effect of packaging methods, storage time and the fortification of poultry sausages with fish oil and microencapsulated fish oil on their rheological and water-binding properties. Molecules 2022, 27, 5235. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shao, S.; Han, X.; Zhang, Y. Preparation and characterization of methyl jasmonate microcapsules and their preserving effects on postharvest potato tuber. Molecules 2022, 27, 4728. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, W.; Zhu, G. Production and characterization of multinuclear microcapsules encapsulating lavender oil by complex coacervation. Flavour Fragr. J. 2014, 29, 166–172. [Google Scholar]
- Espinosa-andrews, H.; Ba, J.G.; Cruz-sosa, F.; Vernon-carter, E.J. Gum arabic-chitosan complex coacervation. Biomacromolecules 2007, 8, 1313–1318. [Google Scholar] [CrossRef]
- Omer, E.A.; Al-Omari, A.A.; Elgamidy, A.H. The emulsifying stability of gum arabic using the local sesame oil obtained from al-baha area. Int. J. Eng. Res. Technol. 2015, 4, 1172–1175. [Google Scholar]
- Allen, M.R.; Burr, D.B. Bone modeling and remodeling. In Basic Applied Bone Biology, 1st ed.; Burr, D.B., Allen, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 75–90. [Google Scholar]
- Manjanna Shivakumar, B.; Kumar, T.M.P. Microencapsulation: An acclaimed novel drug-delivery system for nsaids in arthritis. Crit. Rev. Ther. Drug Carr. Syst. 2012, 27, 509–545. [Google Scholar] [CrossRef]
- Alvim, I.D.; Grosso, C.R.F. Microparticles obtained by complex coacervation: Influence of the type of reticulation and the drying process on the release of the core material. Ciência E Tecnol. Aliment. 2010, 30, 1069–1076. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. A Comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. In Proceeding of the 4th WSEAS International Conference on Engineering Mechanics, Structures, Engineering Geology, Rhodes Island, Greece, 2011; pp. 384–389. [Google Scholar]
- Dong Xia, S.Q.; Hue, S.; Hayat, K.Z.J.; Zhang, S.Y.; XM, U.X. Optimization of cross-linking parameters during the production of transglutaminase hardened spherical multinuclear microcapsules by complex coacervation. Colloids Surf. Biointerfaces 2008, 63, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Noppakundilograt, S.; Pheatcharat, N.; Kiatkamjornwong, S. Multilayer-coated NPK compound fertilizer hydrogel with controlled nutrient release and water absorbency. J. Appl. Polym. Sci. 2014, 132, 1–11. [Google Scholar] [CrossRef]
- Bansode, S.; Banarjee, S.; Gaikwad, D. Microencapsulation: A review. Int. J. Pharma Bio Sci. 2010, 1, 38–43. [Google Scholar]
- Ferrándiz, C.L.; García, D.; Bonet, M.Á.M. Application of antimicrobial microcapsules on agrotextiles. J. Agric. Chem. Environ. 2017, 6, 62–82. [Google Scholar] [CrossRef]
- Julaeha, E.; Puspita, S.; Wahyudi, T. Mikroenkapsulasi minyak asiri jeruk nipis dengan koaservasi kompleks yang beraktivitas antibakteri untuk aplikasi pada bahan tekstil. Arena Tekst. 2020, 35, 67–76. [Google Scholar] [CrossRef]
| No. | Microencapsulation | Immobilization | Activity | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Core Material | Shell Material | Crosslinker | Emulsifier | Method | Fabric | Binder | Method | |||
| 1. | Limonene oil | Polyurethane-urea | PEG 400, EDTA Hydrazine | Polyvinylalcohol | Interfacial polymerization | Wool/polyester | Baypret USV | Foulard | - | [83] |
| 2. | Limonene oil | ethyl cellulose | - | - | Simple coacervation | Cotton | 1,2,3,4-butanetetracarboxylicacid (BTCA) | grafting | - | [84] |
| 3. | Sweet orange oil | Soybean protein isolate-gum Arabic | - | PEG 2000 PEG 4000 Maltodextrin Sucrose | Complex coacervation | - | - | - | - | [85] |
| 4. | Sweet orange oil | Chitosan- sodium alginate | CaCl2 | - | Complex coacervation | - | - | - | - | [86] |
| 5. | Lemon fragrance | - | - | - | Commercial microcapsules | 60% wool, 38% poly-ester, and 2% elastane. | polyacrylate | Pad-Dry-Cure | - | [87] |
| 6. | Limonene oil | Chitosan | - | Lutensol ON 30 (BASF) | Simple coacervation | Cellulose non-woven | - | Padding | - | [88] |
| 7. | Limonene and vanillin | Chitosan-gum Arabic | Tannic acid | PGPR 4150 Span 85 | Complex coacervation | Cotton | Citric acid | Grafting | S. aureusE. coli | [32] |
| 8. | Lime oil | Whey protein- maltodextrin | - | - | Orifice | - | - | - | Antioxidant | [82] |
| 9. | Citrus oil | Chitosan | Tween 20, 40,60 Tween 20/Span 80 (1:1) Tween 20/SDBS (1:1) Span 80 | - | Emulsion-ionic gelation | - | - | - | - | [89] |
| 10. | Lime oil | Chitosan-gum arabic | - | - | Complex coacervation | Cotton | Succinic acid | Dipped | E. coli, B. cereus, S. typhimurium, and S. aureus | [90] |
| 11. | Sweet orange oil (C. sinensis) | β-cyclodextrin | - | - | Inclusion encapsulation | - | - | - | diet-induced obese | [91] |
| 12. | Lemon oil (C. limon) | melamine-formaldehyde | - | - | Complex coacervation | woven silk | acrylic | pad-dry-cure | - | [92] |
| 13. | Lime oil | Gelatin-sodium alginate | Glutaraldehyde | Tween 80 | Complex coacervation | Cotton | Citric acid | Pad-Dry-Cure | S. aureusS. epidermidis E. coliK. pneumoniae | [37] |
| 14. | Orange oil | Maltodextrin-modified starch | - | - | Vacuum spray drying Conventional spray drying, | - | - | - | - | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julaeha, E.; Nurzaman, M.; Wahyudi, T.; Nurjanah, S.; Permadi, N.; Anshori, J.A. The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review. Molecules 2022, 27, 8090. https://doi.org/10.3390/molecules27228090
Julaeha E, Nurzaman M, Wahyudi T, Nurjanah S, Permadi N, Anshori JA. The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review. Molecules. 2022; 27(22):8090. https://doi.org/10.3390/molecules27228090
Chicago/Turabian StyleJulaeha, Euis, Mohamad Nurzaman, Tatang Wahyudi, Sarifah Nurjanah, Nandang Permadi, and Jamaludin Al Anshori. 2022. "The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review" Molecules 27, no. 22: 8090. https://doi.org/10.3390/molecules27228090
APA StyleJulaeha, E., Nurzaman, M., Wahyudi, T., Nurjanah, S., Permadi, N., & Anshori, J. A. (2022). The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review. Molecules, 27(22), 8090. https://doi.org/10.3390/molecules27228090