Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone
Abstract
1. Introduction
2. Results and Discussion
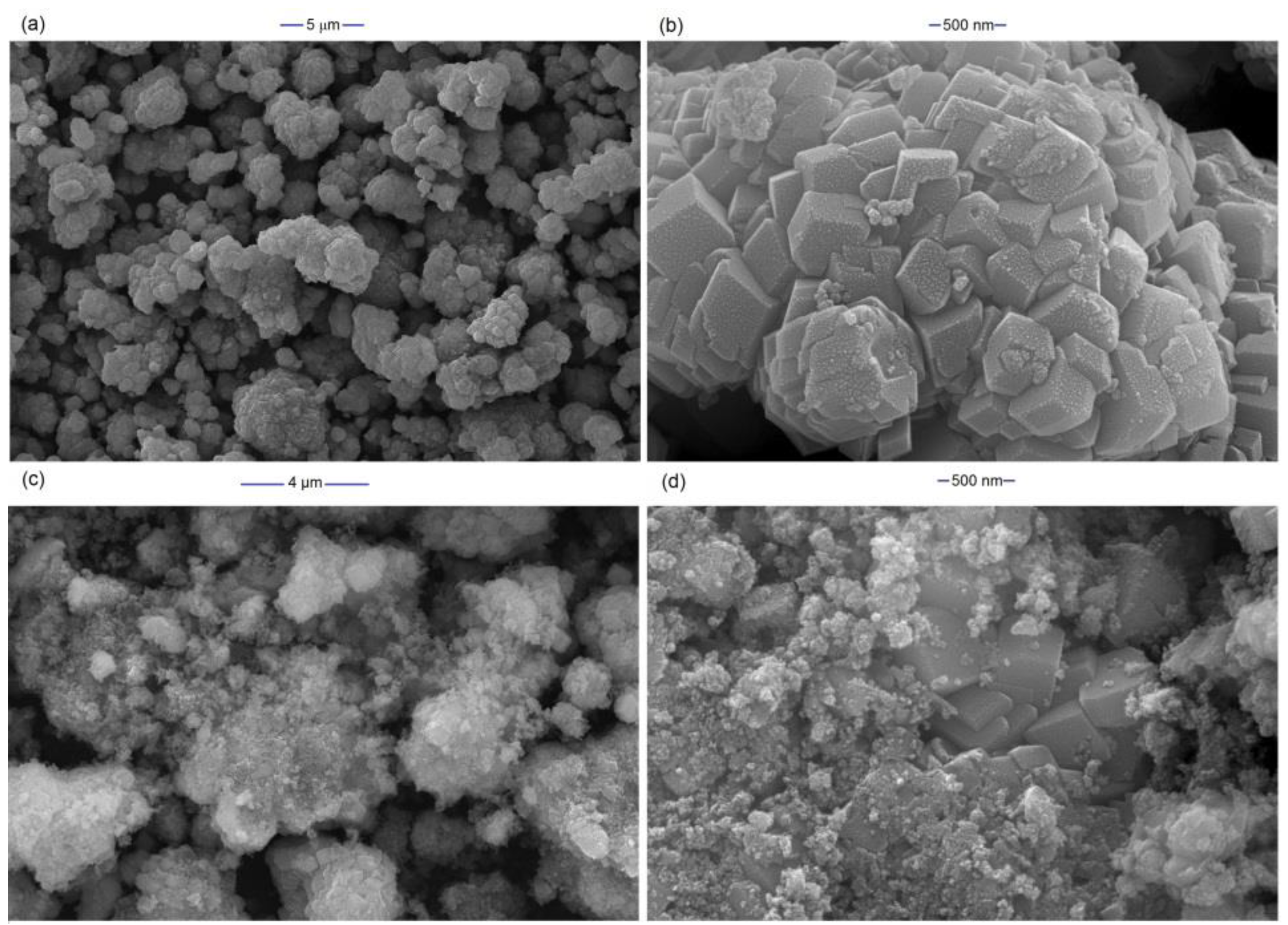
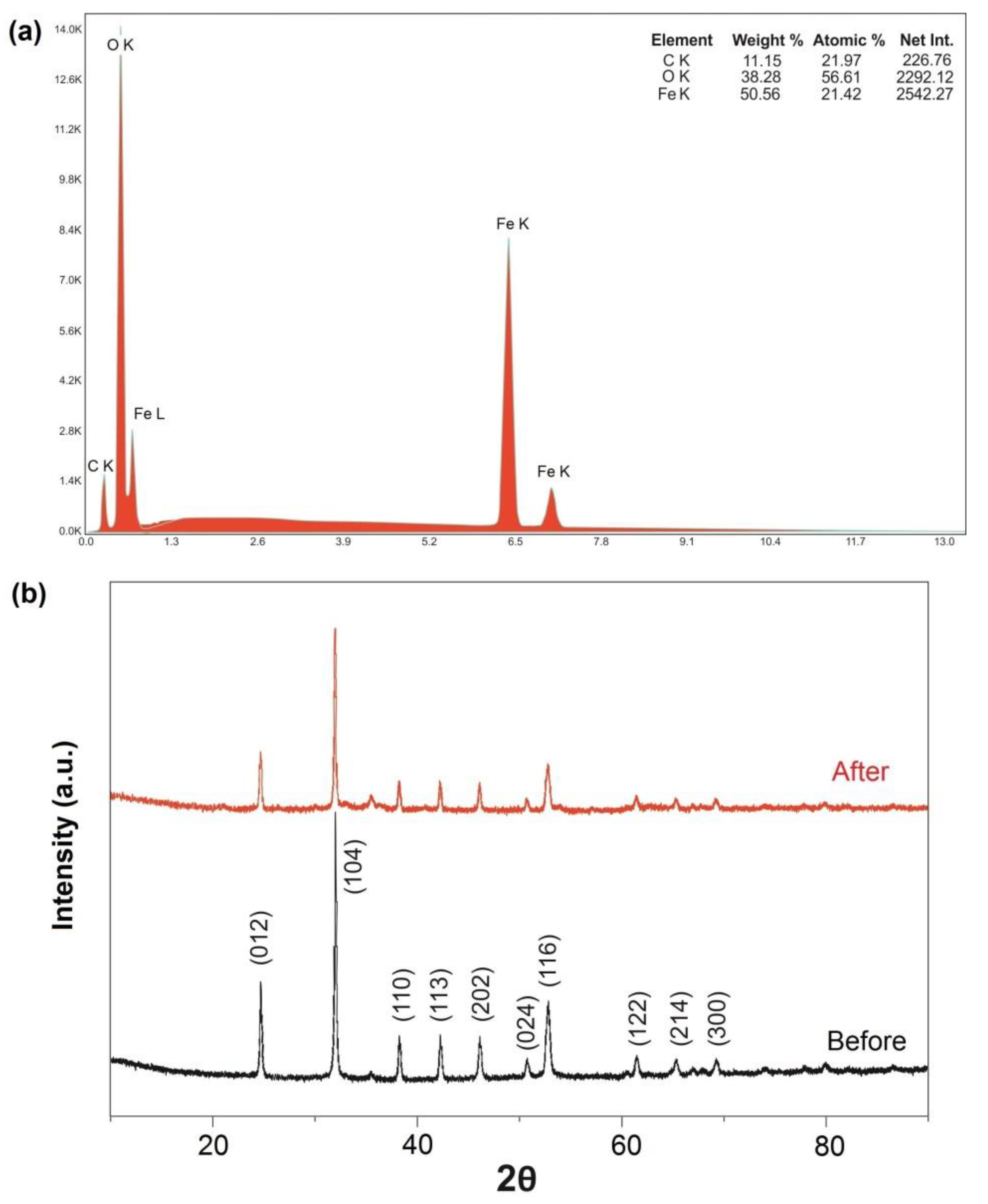
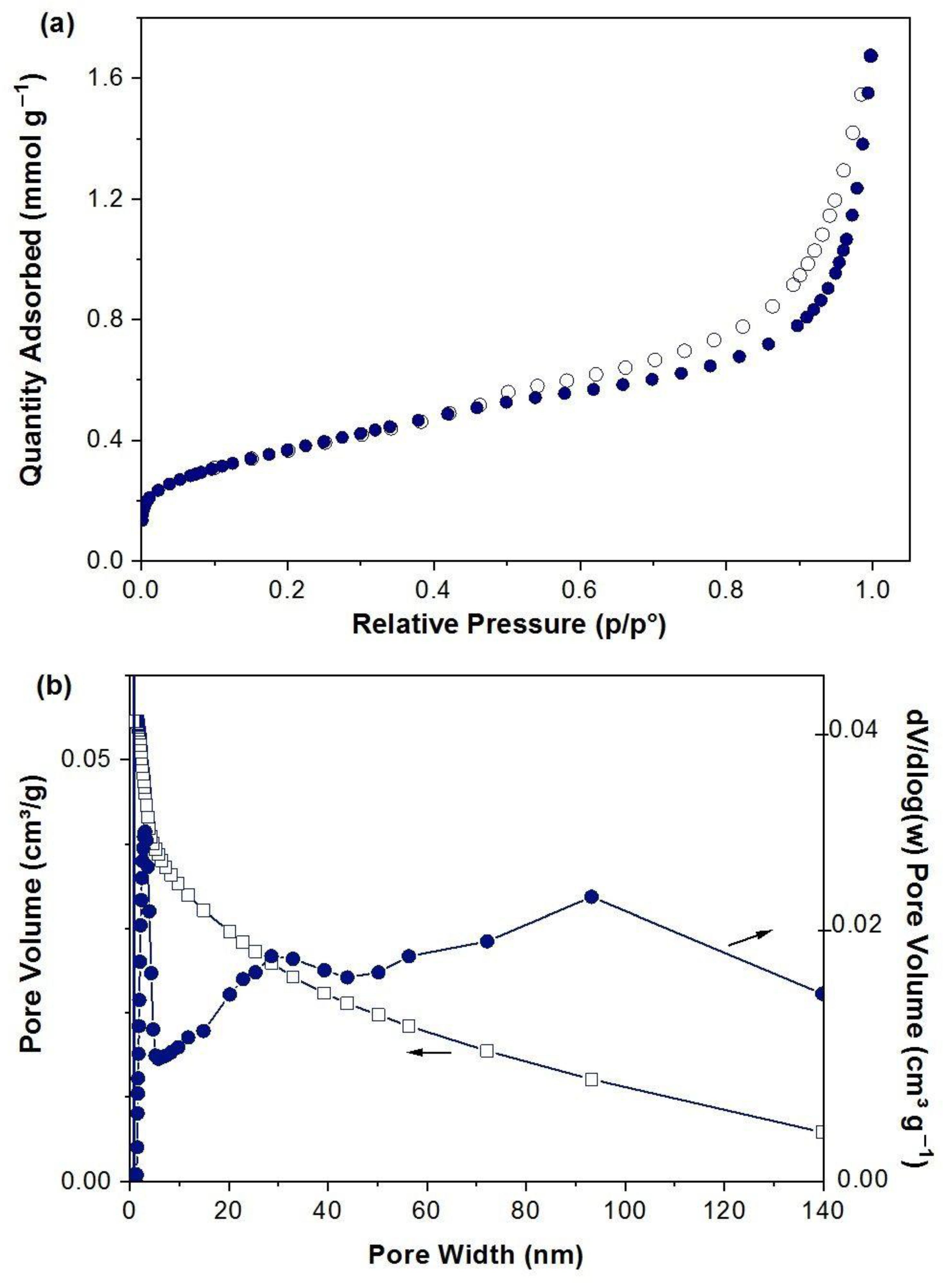
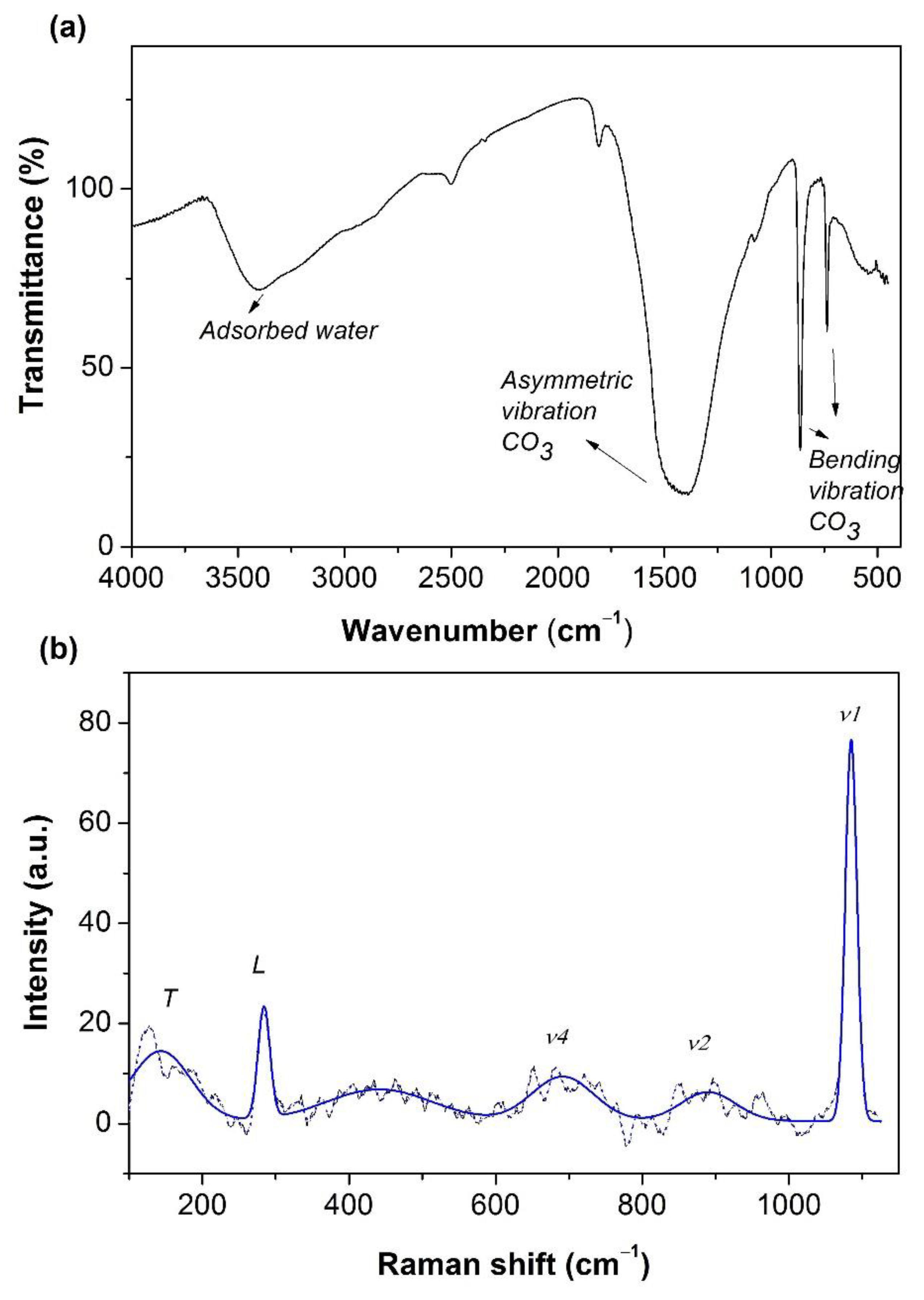
2.1. Characterization of FeCO3 Catalyst
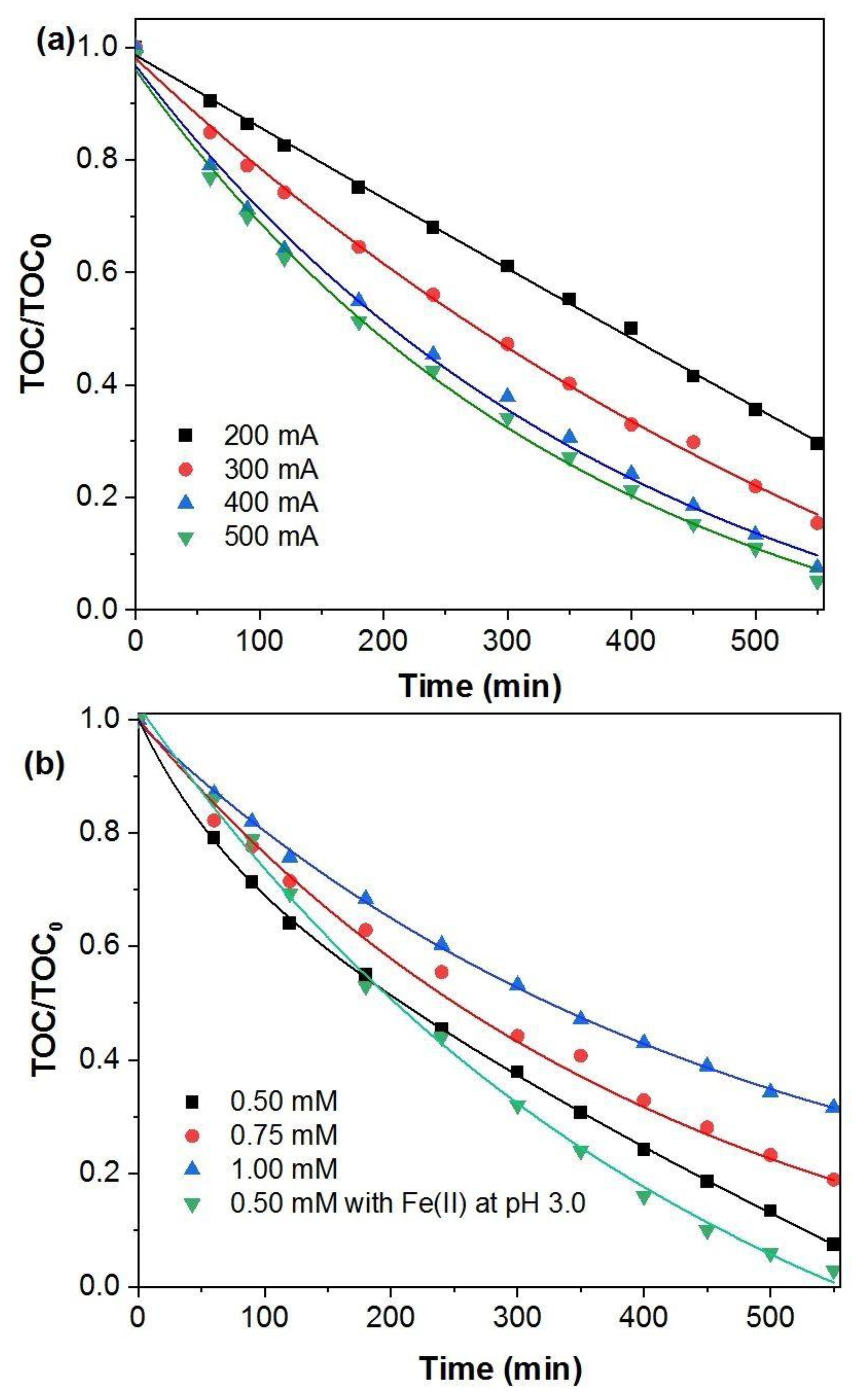
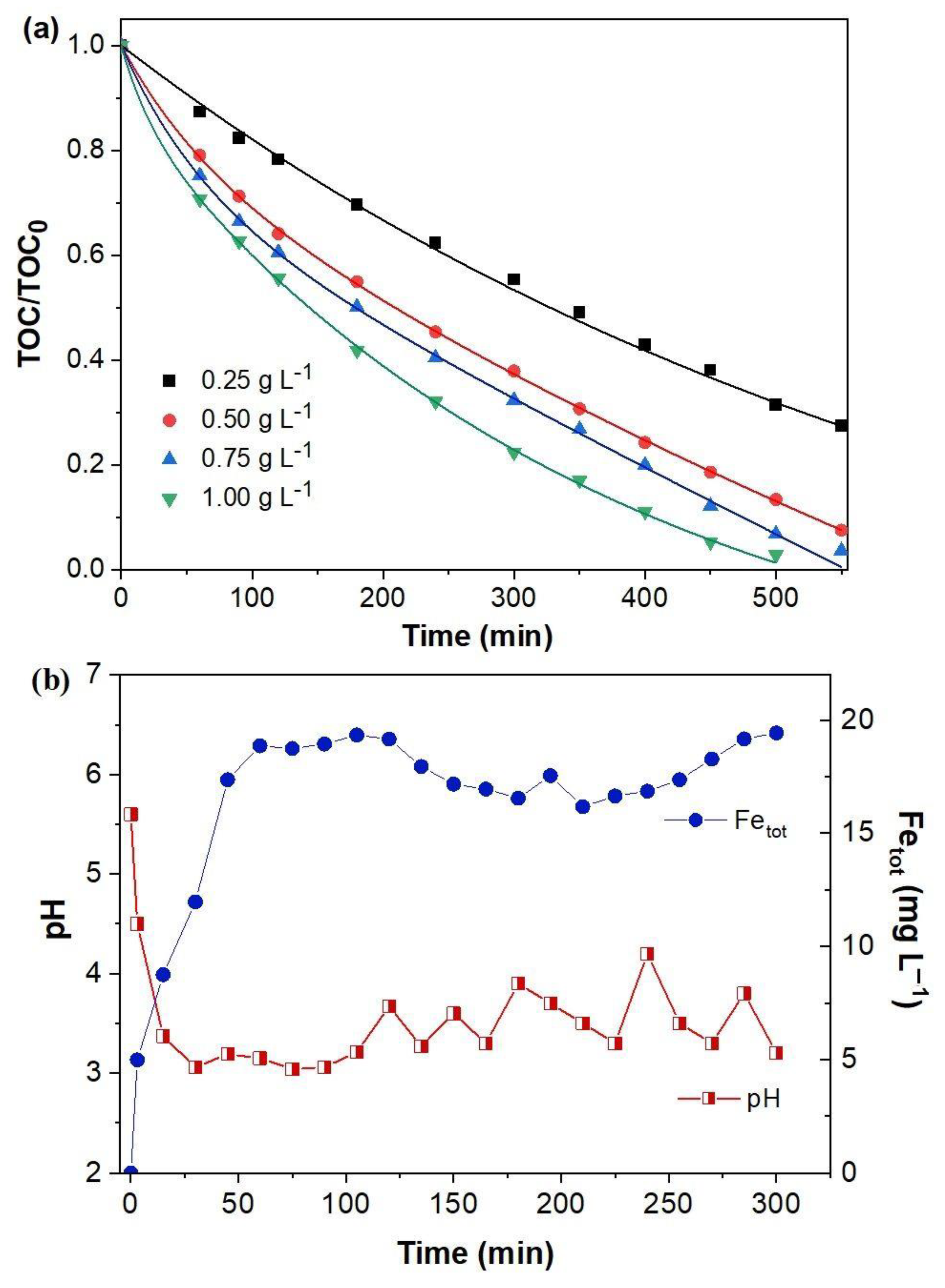
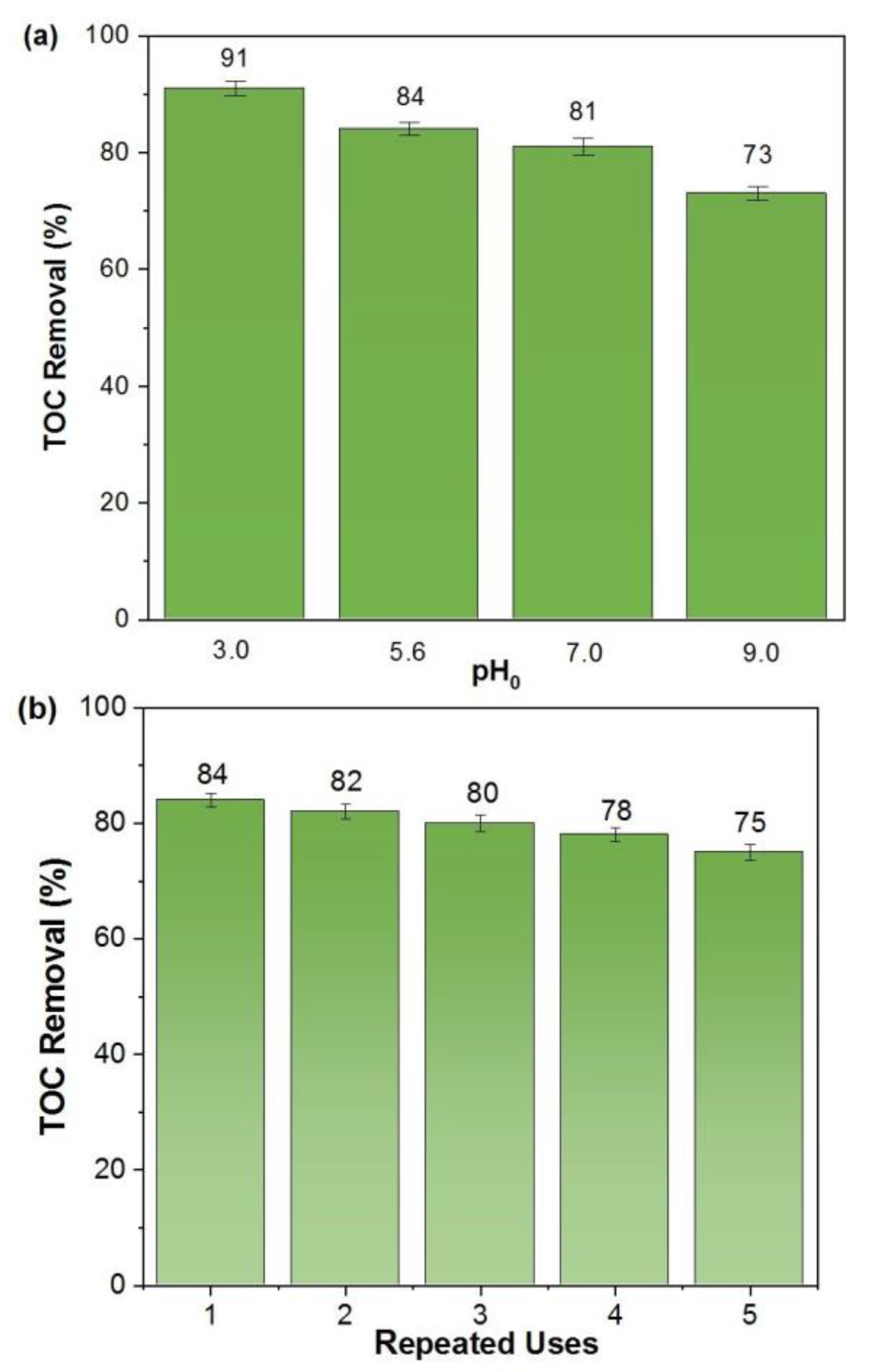
2.2. Oxidation of p-Benzoquinone by the Electro-Fenton Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis of FeCO3 Catalyst
3.3. Characterization of FeCO3
3.4. Electro-Fenton Experiments and Analytical Procedures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yu, L.; Xue, J.; Zhang, L.; Tang, C.; Guo, Y. Fabrication of a stable Ti/Pb-TiOxNWs/PbO2 anode and its application in benzoquinone degradation. Electrochim. Acta 2021, 368, 137532. [Google Scholar] [CrossRef]
- Ou, B.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z. Reuse of PANI wastewater treated by anodic oxidation/electro-Fenton for the preparation of PANI. Chemosphere 2020, 245, 125689. [Google Scholar] [CrossRef]
- Qiu, Z.; Fang, C.; He, N.; Bao, J. An oxidoreductase gene ZMO1116 enhances the p-benzoquinone biodegradation and chiral lactic acid fermentability of Pediococcus acidilactici. J. Biotechnol. 2020, 323, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sirés, I.; Oturan, N.; Özcan, A.; Oturan, M.A. Electrochemical Treatment of the Antibiotic Sulfachloropyridazine: Kinetics, Reaction Pathways, and Toxicity Evolution. Environ. Sci. Technol. 2012, 46, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Chakrabarty, S.; Choudhury, D.; Chakrabarti, G. 1,4-Benzoquinone (PBQ) induced toxicity in lung epithelial cells is mediated by the disruption of the microtubule network and activation of caspase-3. Chem. Res. Toxicol. 2010, 23, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Jensen, N.; O’Brien, P.J. Structure–activity relationships for thiol reactivity and rat or human hepatocyte toxicity induced by substituted p-benzoquinone compounds. J. Appl. Toxicol. 2008, 28, 608–620. [Google Scholar] [CrossRef] [PubMed]
- EPA. TRI Chemical List For RY 2014 (including Toxic Chemical Categories). Available online: https://www.epa.gov/toxics-release-inventory-tri-program/tri-chemical-list-ry-2014-including-toxic-chemical-categories (accessed on 10 November 2022).
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-Fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Sillanpaa, M. (Ed.) Advanced Water Treatment: Advanced Oxidation Processes; Elsevier: Amsterdam, The Netherlands, 2020; p. 464. ISBN 978-0-12-819225-2. [Google Scholar]
- Monteil, H.; Péchaud, Y.; Oturan, N.; Oturan, M.A. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Amali, S.; Zarei, M.; Ebratkhahan, M.; Khataee, A. Preparation of Fe@Fe2O3/3D graphene composite cathode for electrochemical removal of sulfasalazine. Chemosphere 2021, 273, 128581. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Le, T.X.H.; Bechelany, M.; Oturan, N.; Papirio, S.; Esposito, G.; van Hullebusch, E.; Cretin, M.; Oturan, M.A. Electrochemical mineralization of sulfamethoxazole over wide pH range using FeIIFeIII LDH modified carbon felt cathode: Degradation pathway, toxicity and reusability of the modified cathode. Chem. Eng. J. 2018, 350, 844–855. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manage. 2020, 267, 110629. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Yang, L.-M.; Chen, J.P.; Zheng, Y.-M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Padilla, J.A.; Xuriguera, E.; Brillas, E.; Sirés, I. Magnetic MIL(Fe)-type MOF-derived N-doped nano-ZVI@C rods as heterogeneous catalyst for the electro-Fenton degradation of gemfibrozil in a complex aqueous matrix. Appl. Catal. B-Environ. 2020, 266, 118604. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.; Theng, B.; Mora, M. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions-A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
- Expósito, E.; Sánchez-Sánchez, C.M.; Montiel, V. Mineral iron oxides as iron source in electro-Fenton and photoelectro-Fenton mineralization processes. J. Electrochem. Soc. 2007, 154, E116–E122. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Labiadh, L.; Oturan, M.A.; Panizza, M.; Hamadi, N.B.; Ammar, S. Complete removal of AHPS synthetic dye from water using new electro-Fenton oxidation catalyzed by natural pyrite as heterogeneous catalyst. J. Hazard. Mater. 2015, 297, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Labiadh, L.; Oturan, M.A.; Oturan, N.; Gadri, A.; Ammar, S.; Brillas, E. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process. Chemosphere 2015, 141, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Oturan, M.A.; Labiadh, L.; Guersalli, A.; Abdelhedi, R.; Oturan, N.; Brillas, E. Degradation of tyrosol by a novel electro-Fenton process using pyrite as a heterogeneous source of iron catalyst. Water Res. 2015, 74, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Heidari, Z.; Pelalak, R.; Alizadeh, R.; Oturan, N.; Shirazian, S.; Oturan, M.A. Application of Mmineral iron-based natural catalysts in electro-Fenton process: A comparative study. Catalysts 2021, 11, 57. [Google Scholar] [CrossRef]
- Hajiahmadi, M.; Zarei, M.; Khataee, A. Introducing an effective iron-based catalyst for heterogeneous electro-Fenton removal of Gemcitabine using three-dimensional graphene as cathode. J. Ind. Eng. Chem. 2021, 96, 254–268. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Sdiri, A.; Gálvez, M.E.; Zidi, H.; Da Costa, P.; Zina, M.B. Natural hematite and siderite as heterogeneous catalysts for an effective degradation of 4-chlorophenol via photo-Fenton process. Chem. Eng. 2018, 2, 29. [Google Scholar] [CrossRef]
- Acisli, O.; Khataee, A.; Soltani, R.D.C.; Karaca, S. Ultrasound-assisted Fenton process using siderite nanoparticles prepared via planetary ball milling for removal of reactive yellow 81 in aqueous phase. Ultrason. Sonochem. 2017, 35, 210–218. [Google Scholar] [CrossRef]
- Yan, N.; Liu, F.; Huang, W. Interaction of oxidants in siderite catalyzed hydrogen peroxide and persulfate system using trichloroethylene as a target contaminant. Chem. Eng. J. 2013, 219, 149–154. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B-Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Burg, A.; Shamir, D.; Shusterman, I.; Kornweitz, H.; Meyerstein, D. The role of carbonate as a catalyst of Fenton-like reactions in AOP processes: CO3− as the active intermediate. Chem. Commun. 2014, 50, 13096–13099. [Google Scholar] [CrossRef] [PubMed]
- King, D.W.; Farlow, R. Role of carbonate speciation on the oxidation of Fe(II) by H2O2. Mar. Chem. 2000, 70, 201–209. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Yang, T.; Huang, Z.; Liu, Y.; Fang, M.; Ouyang, X.; Hu, M. Controlled synthesis of porous FeCO3 microspheres and the conversion to α-Fe2O3 with unconventional morphology. Ceram. Int. 2014, 40, 11975–11983. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ahmed, I.S.; Mohamed, T.Y.; Khatab, M. A controlled, template-free, and hydrothermal synthesis route to sphere-like α-Fe2O3 nanostructures for textile dye removal. RSC Adv. 2016, 6, 20001. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, P.; Liu, J.; Zheng, Y.; Mustpha, N.A. Effects of low-molecular-weight organic acids/thiols on hydroxyl radical production from natural siderite oxidation. Chem. Geol. 2021, 584, 120537. [Google Scholar] [CrossRef]
- Li, G.; Guo, Y.; Jin, Y.; Tan, W.; Liu, F.; Yin, H. Intrinsic mechanisms of calcium sulfite activation by siderite for atrazine degradation. Chem. Eng. J. 2021, 426, 131917. [Google Scholar] [CrossRef]
- Shen, X.; Dong, W.; Wan, Y.; Feng, K.; Liu, Y.; Wei, Y. Influencing mechanisms of siderite and magnetite, on naphthalene biodegradation: Insights from degradability and mineral surface structure. J. Environ. Manag. 2021, 299, 113648. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, B.; Kim, Y.; Jang, H.; Lee, Y.J. Characterization of arsenite (As(III)) and arsenate (As(V)) sorption on synthetic siderite spherules under anoxic conditions: Different sorption behaviors with crystal size and arsenic species. J. Colloid Int. Sci. 2022, 613, 499–514. [Google Scholar] [CrossRef]
- Zhao, K.; Guo, H.; Zhou, X. Adsorption and heterogeneous oxidation of arsenite on modified granular natural siderite: Characterization and behaviors. Appl. Geochem. 2014, 48, 184–192. [Google Scholar] [CrossRef]
- Xing, B.; Graham, N.; Yu, W. Transformation of siderite to goethite by humic acid in the natural environment. Commun. Chem. 2020, 3, 38. [Google Scholar] [CrossRef]
- Michelin, A.; Burger, E.; Leroy, E.; Foy, E.; Neff, D.; Benzerara, K.; Dillmann, P.; Gin, S. Effect of iron metal and siderite on the durability of simulated archeological glassy material. Corros. Sci. 2013, 76, 403–414. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasasov, K.D.; Kupriyanov, I.N.; Ohtani, E.; Palalyanov, Y.N. Phase relations in the system FeCO3-CaCO3 at 6 GPa and 900–1700 °C and its relation to the system CaCO3-FeCO3-MgCO3. Am. Mineral. 2014, 99, 773–785. [Google Scholar] [CrossRef]
- Görmez, Ö.; Görmez, F.; Gözmen, B. Comparison of the heterogeneous GO-FePO4/electro-Fenton against the homogeneous Fe(II) ion and Fe(III)-oxalate complex/electro-Fenton for the degradation of metronidazole. J. Water Process. Eng. 2021, 43, 102265. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, C.; Zhang, D.; Tang, F. Degradation of 4-nitrophenol in aqueous medium by electro-Fenton method. J. Hazard. Mater. 2007, 145, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Brillas, E. Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode. Wat. Res. 2011, 45, 2975–2984. [Google Scholar] [CrossRef]
- Oturan, M.A.; Pimentel, M.; Oturan, N.; Sirés, I. Reaction sequence for the mineralization of the short-chain carboxylic acids usually formed upon cleavage of aromatics during electrochemical Fenton treatment. Electrochimica Acta 2018, 54, 173–182. [Google Scholar] [CrossRef]
- Haney, E.B.; Haney, R.L.; Hossner, L.R.; White, G.N. Neutralization potential determination of siderite (FeCO3) using selected oxidants. J. Environ. Qual. 2006, 35, 871–879. [Google Scholar] [CrossRef]
- Sun, F.; Liu, H.; Wang, H.; Shu, D.; Chen, T.; Zou, X.; Huang, F.; Chen, D. A novel discovery of a heterogeneous Fenton-like system based on natural siderite: A wide range of pH values from 3 to 9. Sci. Total Environ. 2020, 698, 134293. [Google Scholar] [CrossRef]
- Fosbøl, P.L.; Thomsen, K.; Stenby, E.H. Review and recommended thermodynamic properties of FeCO3. Corros. Eng. Sci. Technol. 2013, 45, 115–135. [Google Scholar] [CrossRef]
- Dong, C.; Ji, J.; Shen, B.; Xing, M.; Zhang, J. Enhancement of H2O2 decomposition by the co-catalytic effect of WS2 on the Fenton reaction for the synchronous reduction of Cr(VI) and remediation of phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Ruettimann, T.; Hug, S.J. pH dependence of Fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Environ. Sci. Technol. 2008, 42, 7424–7430. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/ peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Li, Q.; Yuan, B.; Ma, J. Sodium tetraborate simultaneously enhances the degradation of acetaminophen and reduces the formation potential of chlorinated by-products with heat-activated peroxymonosulfate oxidation. Wat. Res. 2022, 224, 119095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, W.; Chen, D.; Huang, J.; Yu, X.; Huang, X.; Fang, Y. One step hydrothermal synthesis of FeCO3 cubes for high performance lithium-ion battery anodes. Electrochim. Acta 2015, 182, 559–564. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görmez, Ö.; Saçlı, B.; Çağlayan, U.; Kalderis, D.; Gözmen, B. Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone. Molecules 2022, 27, 8056. https://doi.org/10.3390/molecules27228056
Görmez Ö, Saçlı B, Çağlayan U, Kalderis D, Gözmen B. Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone. Molecules. 2022; 27(22):8056. https://doi.org/10.3390/molecules27228056
Chicago/Turabian StyleGörmez, Özkan, Barış Saçlı, Uğur Çağlayan, Dimitrios Kalderis, and Belgin Gözmen. 2022. "Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone" Molecules 27, no. 22: 8056. https://doi.org/10.3390/molecules27228056
APA StyleGörmez, Ö., Saçlı, B., Çağlayan, U., Kalderis, D., & Gözmen, B. (2022). Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone. Molecules, 27(22), 8056. https://doi.org/10.3390/molecules27228056






