Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors
Abstract
1. Introduction
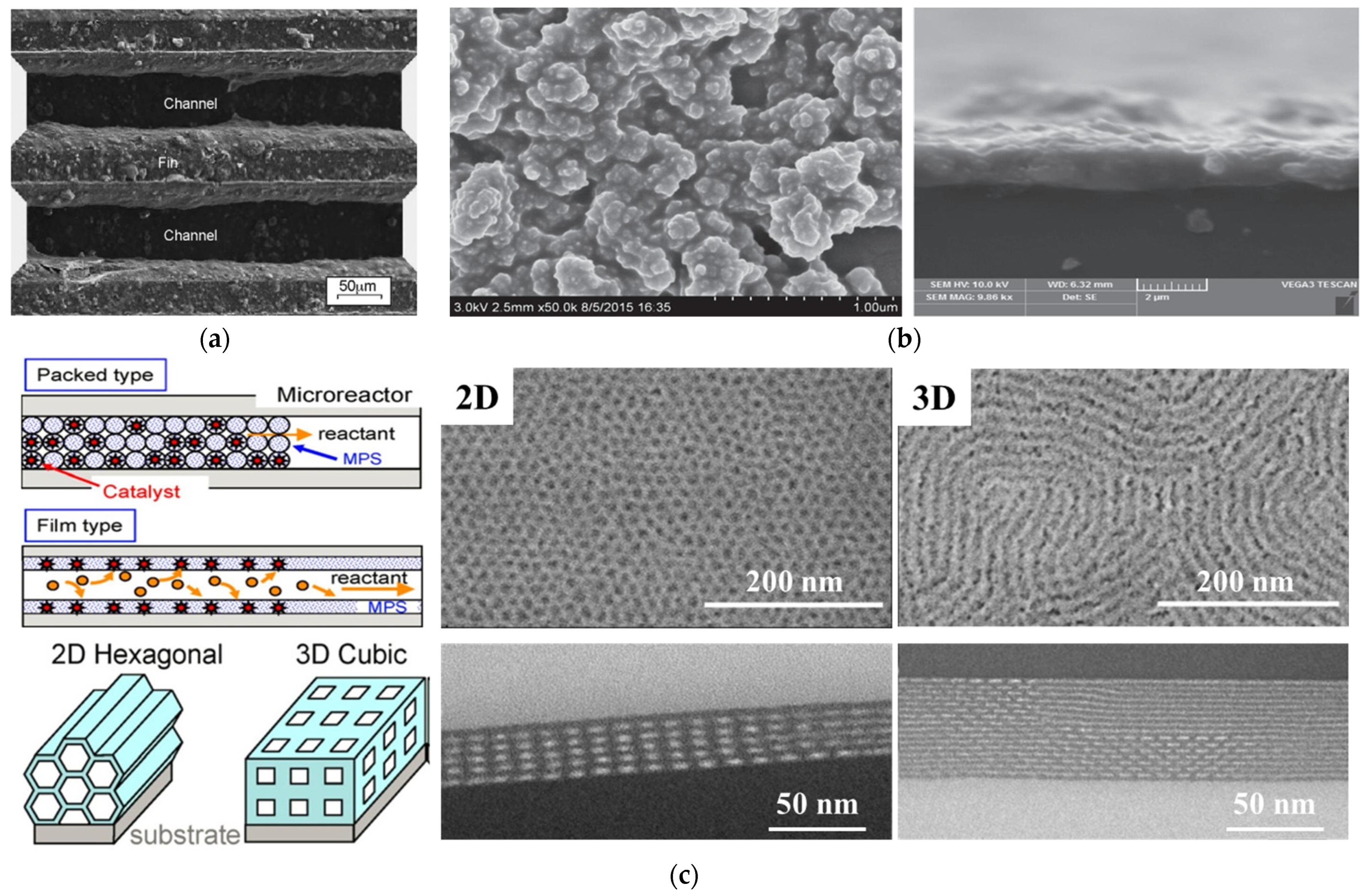
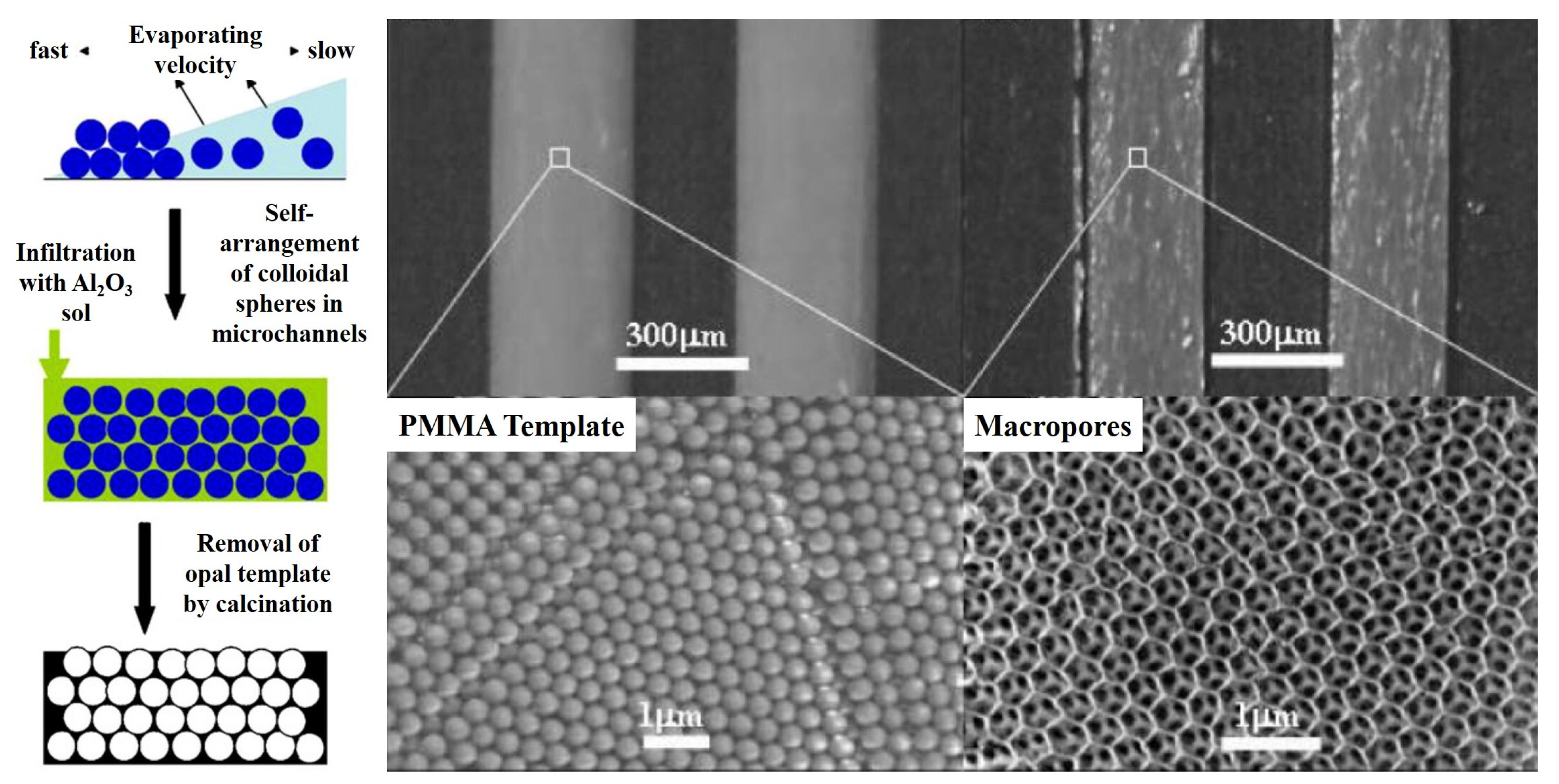
2. Catalysts Layer Preparation Based on the Sol–Gel Method
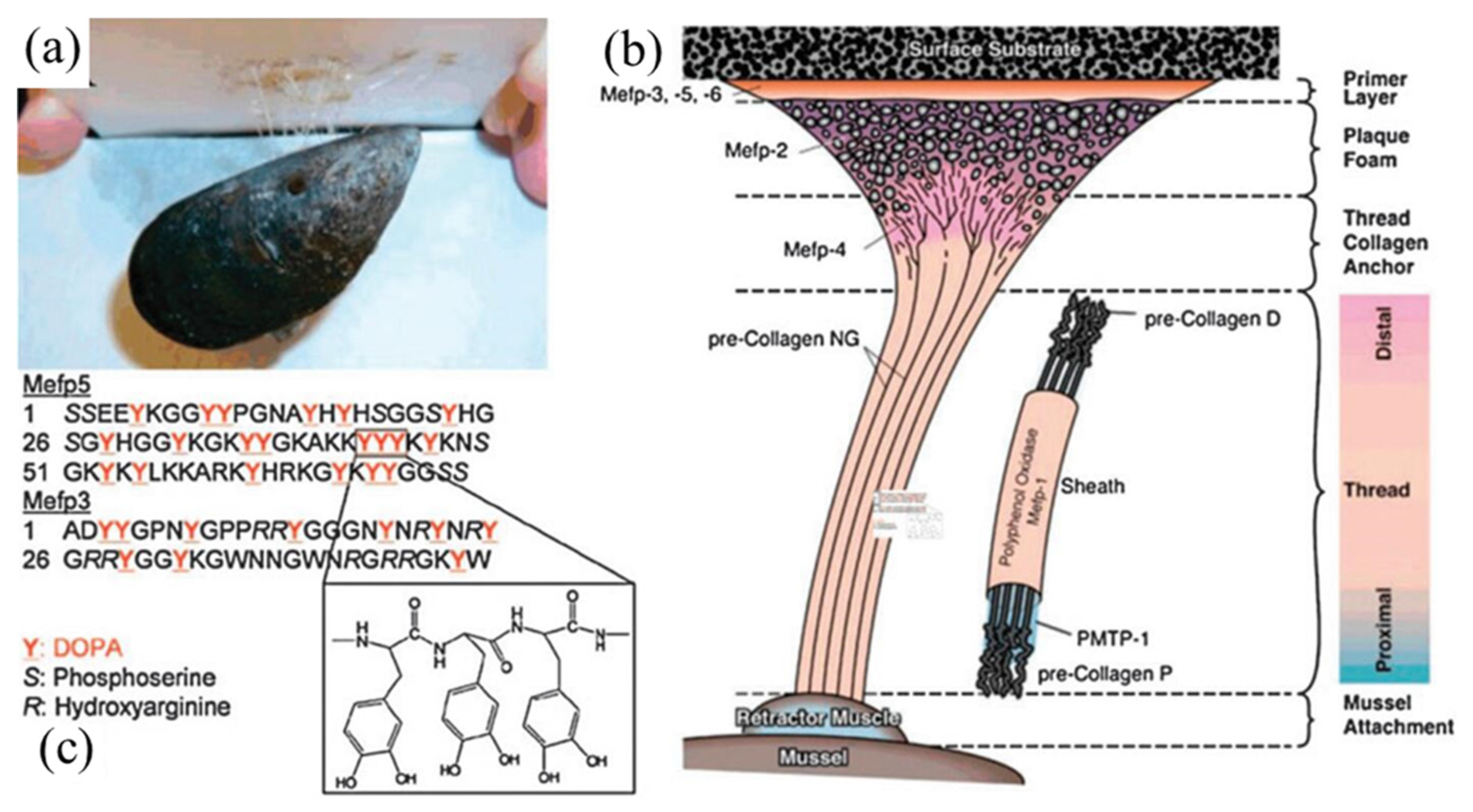
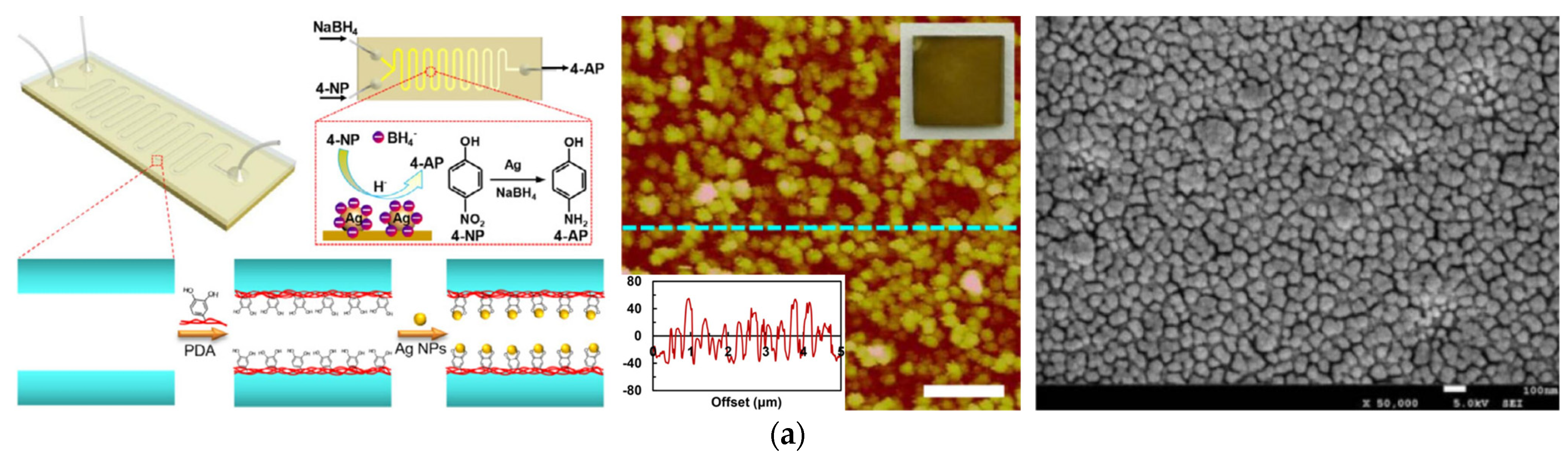
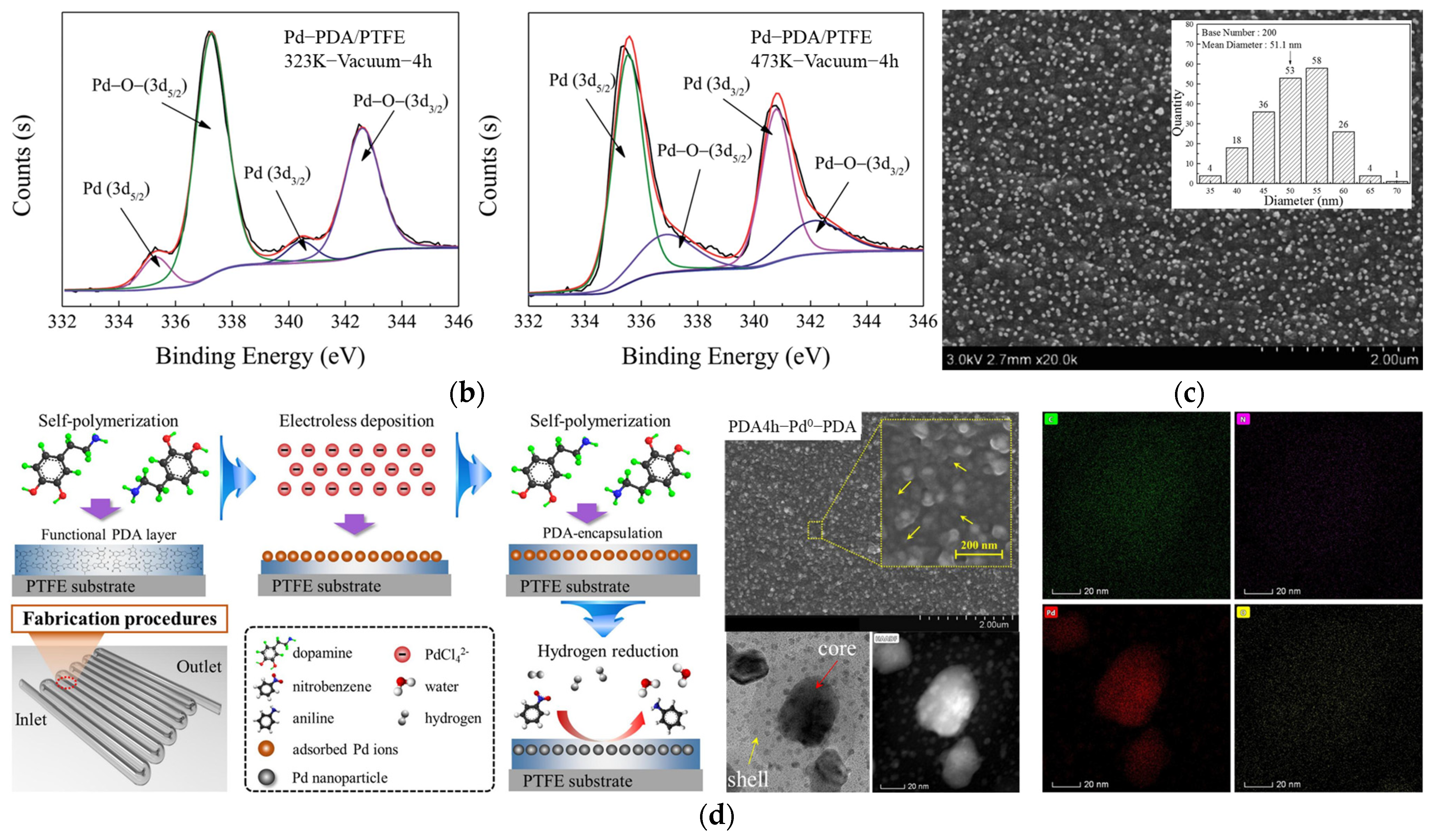
3. Bio–Inspired Electroless Deposition Method
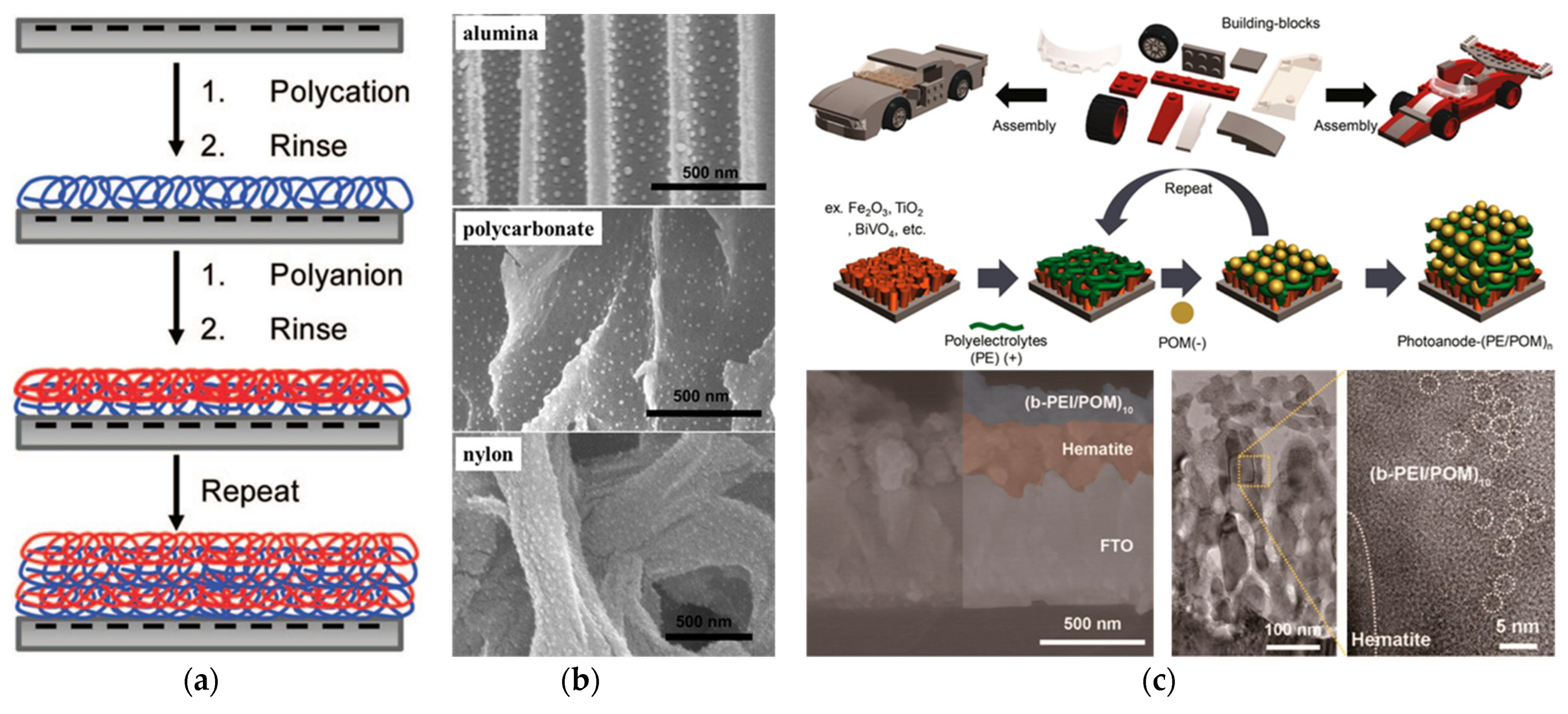
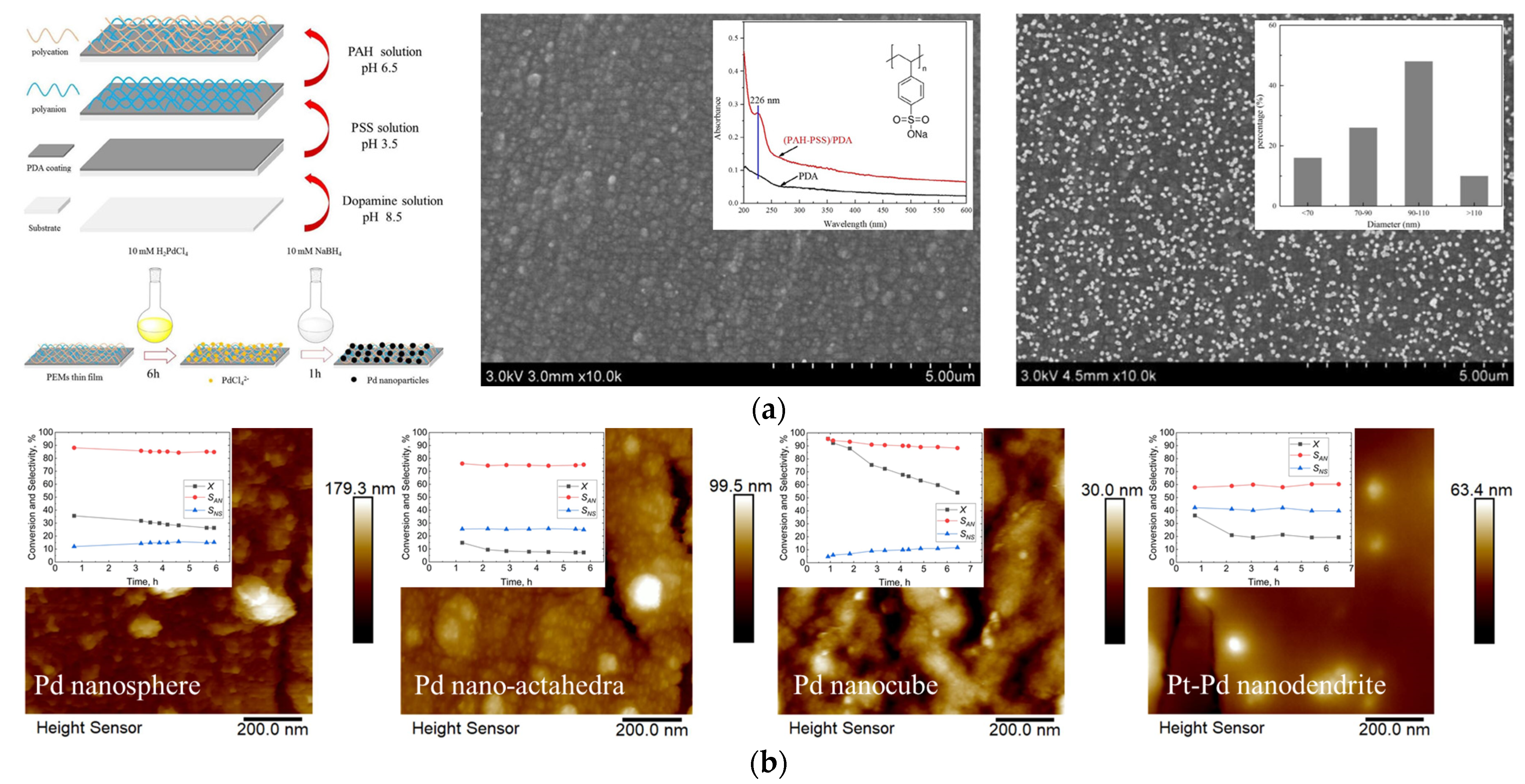
4. Layer–by–Layer Self–Assembly Method
5. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coyle, E.E.; Oelgemoller, M. Micro-photochemistry: Photochemistry in microstructured reactors. The new photochemistry of the future? Photochem. Photobiol. Sci. 2008, 7, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Kiwi-Minsker, L.; Renken, A. Microstructured reactors for catalytic reactions. Catal. Today 2005, 110, 2–14. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mori, Y.; Okamoto, K.; Akiyama, R.; Ueno, M.; Kitamori, T.; Kobayashi, S. A microfluidic device for conducting gas-liquid-solid hydrogenation reactions. Science 2004, 304, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, R. Residence Time Characteristics of Taylor Reacting Flow in a Microchannel Reactor during Long-Term Operation. ACS Sustain. Chem. Eng. 2022, 10, 4105–4113. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, X.; Chen, R.; Liao, Q. Gas consumption characteristics determined by gas-liquid two-phase flow coupled with catalytic reaction in a gas-liquid-solid microreactor. Int. J. Heat Mass Transf. 2019, 135, 897–906. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, J.; Wang, K.; Luo, G. Microreaction Technology for Synthetic Chemistry. Chin. J. Chem. 2019, 37, 161–170. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous manufacturing—The Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Lu, M.; Ozcelik, A.; Grigsby, C.L.; Zhao, Y.; Guo, F.; Leong, K.W.; Huang, T.J. Microfluidic hydrodynamic focusing for synthesis of nanomaterials. Nano Today 2016, 11, 778–792. [Google Scholar] [CrossRef]
- Jiao, L.; Wu, Y.; Hu, Y.; Wu, H.; Xu, Z.; Han, L.; Guo, Q.; Li, D.; Chen, R. Oil/Water Microreactor with a Core–Shell Wetting State on a SOB/OL-SHB/HL Multilevel Patterned Surface. J. Phys. Chem. C 2021, 125, 27771–27783. [Google Scholar] [CrossRef]
- Jiao, L.; Xu, Q.; Tong, J.; Liu, S.; Hu, Y.; Guo, Q.; Wu, H.; Li, W.; Zhao, Q.; Chen, R. Facile preparation of pliable superamphiphobic papers with high and durable liquid repellency for anti-corrosion and open surface microfluidics. Appl. Surf. Sci. 2022, 606, 154845. [Google Scholar] [CrossRef]
- Feng, H.; Liu, D.; Zhang, Y.; Shi, X.; Esan, O.C.; Li, Q.; Chen, R.; An, L. Advances and Challenges in Photoelectrochemical Redox Batteries for Solar Energy Conversion and Storage. Adv. Energy Mater. 2022, 12, 2200469. [Google Scholar] [CrossRef]
- Deng, K.; Zhang, Y.; Feng, H.; Liu, N.; Ma, L.; Duan, J.; Wang, Y.; Liu, D.; Li, Q. Efficient solar fuel production with a high-pressure CO2-captured liquid feed. Sci. Bull. 2022, 67, 1467–1476. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, X.; Chen, R.; Liao, Q.; Liu, J.; Li, L. High-performance gas-liquid-solid microreactor with polydopamine functionalized surface coated by Pd nanocatalyst for nitrobenzene hydrogenation. Chem. Eng. J. 2016, 306, 1017–1025. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Ye, C.; Yuan, Q. Gas–liquid two-phase flow in microchannel at elevated pressure. Chem. Eng. Sci. 2013, 87, 122–132. [Google Scholar] [CrossRef]
- Ahmad, H.; Kim, S.K.; Park, J.H.; Jung, S.Y. Development of two-phase flow regime map for thermally stimulated flows using deep learning and image segmentation technique. Int. J. Multiph. Flow 2022, 146, 103869. [Google Scholar] [CrossRef]
- Jin, N.; Yue, J.; Zhao, Y.; Lü, H.; Wang, C. Experimental study and mass transfer modelling for extractive desulfurization of diesel with ionic liquid in microreactors. Chem. Eng. J. 2021, 413, 127419. [Google Scholar] [CrossRef]
- Sobieszuk, P.; Pohorecki, R.; Cygański, P.; Grzelka, J. Determination of the interfacial area and mass transfer coefficients in the Taylor gas–liquid flow in a microchannel. Chem. Eng. Sci. 2011, 66, 6048–6056. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Du, P.; Heiszwolf, J.J.; Kapteijin, F.; Moulijn, J.A. Mass transfer characteristics of three-phase monolith reactors. Chem. Eng. Sci. 2001, 56, 6015–6023. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, X.; Chen, R.; Liao, Q.; Ye, D.; Zhang, B.; Liu, J.; Liu, M.; Chen, G.; Wang, K. pH response of zwitterionic polydopamine layer to palladium deposition in the microchannel. Chem. Eng. J. 2019, 356, 282–291. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Y.; Liu, D.; Chen, R. Residence time characteristic of Taylor reacting flow in a microchannel reactor. Chem. Eng. Sci. 2022, 253, 117575. [Google Scholar] [CrossRef]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renew. Sust. Energ. Rev. 2023, 171, 113017. [Google Scholar] [CrossRef]
- Shi, T.; Liu, D.; Feng, H.; Zhang, Y.; Li, Q. Evolution of Triple-Phase interface for enhanced electrochemical CO2 reduction. Chem. Eng. J. 2022, 431, 134348. [Google Scholar] [CrossRef]
- Shi, T.; Feng, H.; Liu, D.; Zhang, Y.; Li, Q. High-performance microfluidic electrochemical reactor for efficient hydrogen evolution. Appl. Energ. 2022, 325, 119887. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Haase, S.; Murzin, D.Y.; Salmi, T. Review on hydrodynamics and mass transfer in minichannel wall reactors with gas–liquid Taylor flow. Chem. Eng. Res. Des. 2016, 113, 304–329. [Google Scholar] [CrossRef]
- Mason, B.P.; Price, K.E.; Steinbacher, J.L.; Bogdan, A.R.; McQuade, D.T. Greener approaches to organic synthesis Using microreactor technology. Chem. Rev. 2007, 107, 2300–2318. [Google Scholar] [CrossRef]
- Elvira, K.S.; Casadevall i Solvas, X.; Wootton, R.C.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous Flow Upgrading of Selected C2–C6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lin, C.-Y.; Chen, J.-L.; Lu, K.-T.; Lee, J.-F.; Chen, J.-M. SBA-15-supported Pd catalysts: The effect of pretreatment conditions on particle size and its application to benzyl alcohol oxidation. J. Catal. 2017, 350, 21–29. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Weaver, D.W.; Ying, J.Y. Heterogeneous Heck Catalysis with Palladium-Grafted Molecular Sieves. J. Am. Chem. Soc. 1998, 120, 12289–12296. [Google Scholar] [CrossRef]
- Meille, V.; Pallier, S.; Santa Cruz Bustamante, G.V.; Roumanie, M.; Reymond, J.-P. Deposition of γ-Al2O3 layers on structured supports for the design of new catalytic reactors. Appl. Catal. A Gen. 2005, 286, 232–238. [Google Scholar] [CrossRef]
- Liguras, D.K.; Goundani, K.; Verykios, X.E. Production of hydrogen for fuel cells by catalytic partial oxidation of ethanol over structured Ni catalysts. J. Power Sources 2004, 130, 30–37. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Pfeifer, P.; Schubert, K.; Liauw, M.A.; Emig, G. PdZn catalysts prepared by washcoating microstructured reactors. Appl. Catal. A Gen. 2004, 270, 165–175. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, X.; Chen, R.; Liao, Q.; Feng, H.; Li, L. Catalytic membrane microreactor with Pd/γ-Al2O3 coated PDMS film modified by dopamine for hydrogenation of nitrobenzene. Chem. Eng. J. 2016, 301, 35–41. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Size control of palladium and their crystal structure. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Kataoka, S.; Endo, A.; Harada, A.; Inagi, Y.; Ohmori, T. Characterization of mesoporous catalyst supports on microreactor walls. Appl. Catal. A Gen. 2008, 342, 107–112. [Google Scholar] [CrossRef]
- Kataoka, S.; Takeuchi, Y.; Harada, A.; Yamada, M.; Endo, A. Microreactor with mesoporous silica support layer for lipase catalyzed enantioselective transesterification. Green Chem. 2010, 12, 331–337. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. Catalytic methane combustion in plate-type microreactors with different channel configurations: An experimental study. Chem. Eng. Sci. 2021, 236, 116517. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Luo, L.; Bellettre, J.; Yue, J. Preparation of Pt/γ-Al2O3 catalyst coating in microreactors for catalytic methane combustion. Chem. Eng. J. 2020, 380, 122424. [Google Scholar] [CrossRef]
- Kataoka, S.; Endo, A.; Harada, A.; Ohmori, T. Fabrication of mesoporous silica thin films inside microreactors. Mater. Lett. 2008, 62, 723–726. [Google Scholar] [CrossRef]
- Kataoka, S.; Endo, A.; Oyama, M.; Ohmori, T. Enzymatic reactions inside a microreactor with a mesoporous silica catalyst support layer. Appl. Catal. A Gen. 2009, 359, 108–112. [Google Scholar] [CrossRef]
- Kataoka, S.; Takeuchi, Y.; Harada, A.; Takagi, T.; Takenaka, Y.; Fukaya, N.; Yasuda, H.; Ohmori, T.; Endo, A. Microreactor containing platinum nanoparticles for nitrobenzene hydrogenation. Appl. Catal. A Gen. 2012, 427–428, 119–124. [Google Scholar] [CrossRef]
- Guan, G.; Kusakabe, K.; Taneda, M.; Uehara, M.; Maeda, H. Catalytic combustion of methane over Pd-based catalyst supported on a macroporous alumina layer in a microchannel reactor. Chem. Eng. J. 2008, 144, 270–276. [Google Scholar] [CrossRef]
- Guan, G.; Zapf, R.; Kolb, G.; Men, Y.; Hessel, V.; Loewe, H.; Ye, J.; Zentel, R. Low temperature catalytic combustion of propane over Pt-based catalyst with inverse opal microstructure in a microchannel reactor. Chem. Commun. 2007, 3, 260–262. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Perspectives on poly(dopamine). Chem. Sci. 2013, 4, 3796–3802. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, S.; Qiu, B.; Chen, J.; Qin, Y.; Wang, Y.; Xiao, Z.; Mai, Z.; Bai, K.; Liu, J. Enhanced Catalytic Performance of a Membrane Microreactor by Immobilizing ZIF-8-Derived Nano-Ag via Ion Exchange. Ind. Eng. Chem. Res. 2020, 59, 19553–19563. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, Y.H.; Seo, S.W.; Park, C.P.; Park, S.Y.; In, I. Mussel-Inspired Immobilization of Catalysts for Microchemical Applications. Adv. Mater. Interfaces 2015, 2, 1500174. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, H.; Chen, R.; Liao, Q.; Ye, D.; Zhang, B.; Liu, J.; Liu, M.; Chen, G. Experimental study on the durability of the polydopamine functionalized gas–liquid–solid microreactor for nitrobenzene hydrogenation. RSC Adv. 2018, 8, 5661–5669. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl. Catal. B-Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, Y.; Xie, R.; Ju, X.-J.; Wang, W.; Lin, L.; Chu, L.-Y. Facile immobilization of Ag nanoparticles on microchannel walls in microreactors for catalytic applications. Chem. Eng. J. 2016, 309, 691–699. [Google Scholar] [CrossRef]
- Ryoo, H.I.; Lee, J.S.; Park, C.B.; Kim, D.P. A microfluidic system incorporated with peptide/Pd nanowires for heterogeneous catalytic reactions. Lab Chip 2011, 11, 378–380. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, H.; Chen, R.; Liao, Q.; Ye, D.; Zhang, B. Core-shell structured Pd catalyst layer encapsulated by polydopamine for a gas-liquid-solid microreactor. Appl. Surf. Sci. 2019, 487, 416–425. [Google Scholar] [CrossRef]
- Xu, C.; Tian, M.; Liu, L.; Zou, H.; Zhang, L.; Wang, W. Fabrication and Properties of Silverized Glass Fiber by Dopamine Functionalization and Electroless Plating. J. Electrochem. Soc. 2012, 159, D217–D224. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, B.; Zhu, X.; Chen, R.; Liao, Q.; Ye, D.-d.; Liu, J.; Liu, M.; Chen, G. Multilayered Pd nanocatalysts with nano-bulge structure in a microreactor for multiphase catalytic reaction. Chem. Eng. Res. Des. 2018, 138, 190–199. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Bruening, M.L.; Dotzauer, D.M.; Jain, P.; Ouyang, L.; Baker, G.L. Creation of functional membranes using polyelectrolyte multilayers and polymer Brushes. Langmuir 2008, 24, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Kralik, M.; Biffis, A. Catalysis by metal nanoparticles supported on functional organic polymers. J. Mol. Catal. A Chem. 2001, 177, 113–138. [Google Scholar] [CrossRef]
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic Membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Herwig, G.; Hornung, C.H.; Peeters, G.; Ebdon, N.; Savage, G.P. Porous double-layer polymer tubing for the potential use in heterogeneous continuous flow reactions. ACS Appl. Mater. Interfaces 2014, 6, 22838–22846. [Google Scholar] [CrossRef]
- Kidambi, S.; Dai, J.; Li, J.; Bruening, M.L. Selective hydrogenation by Pd nanoparticles embedded in polyelectrolyte multilayers. J. Am. Chem. Soc. 2004, 126, 2658–2659. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical Applications of Layer-by-Layer Assembly: From Biomimetics to Tissue Engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Jeon, D.; Kim, H.; Lee, C.; Han, Y.; Gu, M.; Kim, B.S.; Ryu, J. Layer-by-Layer Assembly of Polyoxometalates for Photoelectrochemical (PEC) Water Splitting: Toward Modular PEC Devices. ACS Appl. Mater. Interfaces 2017, 9, 40151–40161. [Google Scholar] [CrossRef]
- Dotzauer, D.M.; Bhattacharjee, S.; Wen, Y.; Bruening, M.L. Nanoparticle-containing membranes for the catalytic reduction of nitroaromatic compounds. Langmuir 2009, 25, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- De León, A.S.; Garnier, T.; Jierry, L.; Boulmedais, F.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J. Enzymatic Catalysis Combining the Breath Figures and Layer-by-Layer Techniques: Toward the Design of Microreactors. ACS Appl. Mater. Interfaces 2015, 7, 12210–12219. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Paunovic, V.; Schouten, J.C.; Neira D’Angelo, M.F. Facile Synthesis of Catalytic AuPd Nanoparticles within Capillary Microreactors Using Polyelectrolyte Multilayers for the Direct Synthesis of H2O2. Nano Lett. 2017, 17, 6481–6486. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, X.; Liao, Q.; Chen, R.; Ye, D.; Chen, G.; Wang, K.; Song, S. Preparation of a Catalyst Layer by Layer-by-Layer Self-Assembly for Plate-Type Catalytic Membrane Microreactors. Ind. Eng. Chem. Res. 2020, 59, 15865–15874. [Google Scholar] [CrossRef]
- Khositanon, C.; Deepracha, S.; Assabumrungrat, S.; Ogawa, M.; Weeranoppanant, N. Simple Fabrication of a Continuous-Flow Photocatalytic Reactor Using Dopamine-Assisted Immobilization onto a Fluoropolymer Tubing. Ind. Eng. Chem. Res. 2022, 61, 1322–1331. [Google Scholar] [CrossRef]
- Chen, R.; Feng, H.; Zhu, X.; Liao, Q.; Ye, D.; Liu, J.; Liu, M.; Chen, G.; Wang, K. Interaction of the Taylor flow behaviors and catalytic reaction inside a gas-liquid-solid microreactor under long-term operation. Chem. Eng. Sci. 2018, 175, 175–184. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Liao, Q.; Chen, R.; Ye, D.; Feng, H.; Liu, M.; Chen, G. Layer-by-layer self-assembly of palladium nanocatalysts with polyelectrolytes grafted on the polydopamine functionalized gas-liquid-solid microreactor. Chem. Eng. J. 2017, 332, 174–182. [Google Scholar] [CrossRef]
- Liu, J.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; Feng, H.; Liu, M.; Chen, G.; Wang, K. In Situ Synthesis of a Multilayered (PSS-PAH-Pd)n Catalytic Hybrid Film Synthesized by the Layer-by-Layer Self-Assembly. Ind. Eng. Chem. Res. 2019, 58, 9038–9047. [Google Scholar] [CrossRef]
- Venezia, B.; Panariello, L.; Biri, D.; Shin, J.; Damilos, S.; Radhakrishnan, A.N.P.; Blackman, C.; Gavriilidis, A. Catalytic Teflon AF-2400 membrane reactor with adsorbed ex situ synthesized Pd-based nanoparticles for nitrobenzene hydrogenation. Catal. Today 2021, 362, 104–112. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, S.; Kim, M.; Sinha, A.K.; Lee, S.C.; Jung, E.; Chang, W.J.; Lee, K.-S.; Kim, J.H.; Cho, S.-P. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 2019, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Kan, E.; Chen, S.; Du, Z.; Liu, X.; Wang, T.; Zhu, W.; Huo, H.; Ma, J.; Liu, D.; et al. Enabling High Loading in Single-Atom Catalysts on Bare Substrate with Chemical Scissors by Saturating the Anchoring Sites. Small 2022, 18, 2200073. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Zhang, Y.; Liu, J.; Liu, D. Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors. Molecules 2022, 27, 8052. https://doi.org/10.3390/molecules27228052
Feng H, Zhang Y, Liu J, Liu D. Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors. Molecules. 2022; 27(22):8052. https://doi.org/10.3390/molecules27228052
Chicago/Turabian StyleFeng, Hao, Ying Zhang, Jian Liu, and Dong Liu. 2022. "Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors" Molecules 27, no. 22: 8052. https://doi.org/10.3390/molecules27228052
APA StyleFeng, H., Zhang, Y., Liu, J., & Liu, D. (2022). Towards Heterogeneous Catalysis: A Review on Recent Advances of Depositing Nanocatalysts in Continuous–Flow Microreactors. Molecules, 27(22), 8052. https://doi.org/10.3390/molecules27228052






