Control of Columnar Grain Microstructure in CSD LaNiO3 Films
Abstract
1. Introduction
2. Results and Discussion
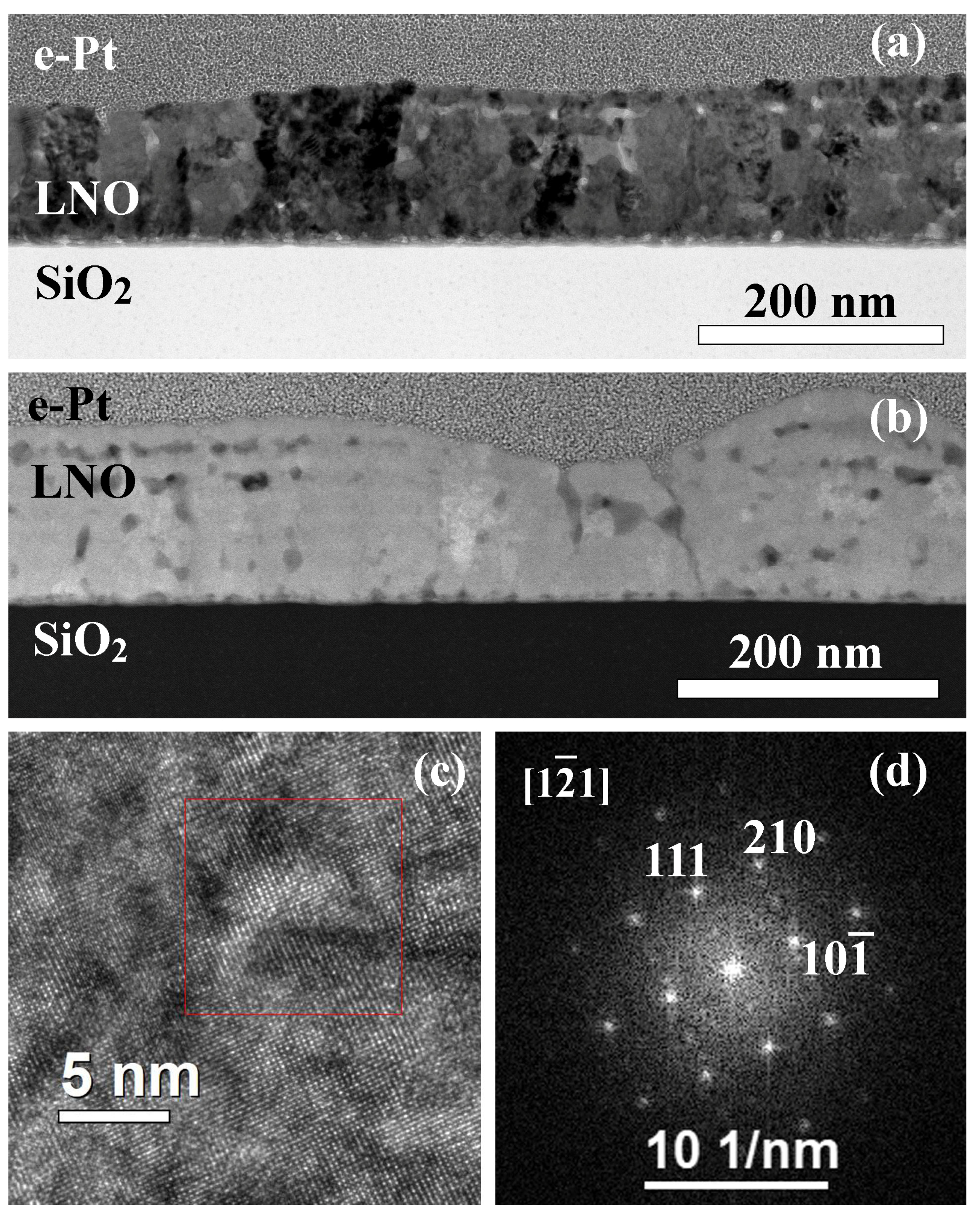
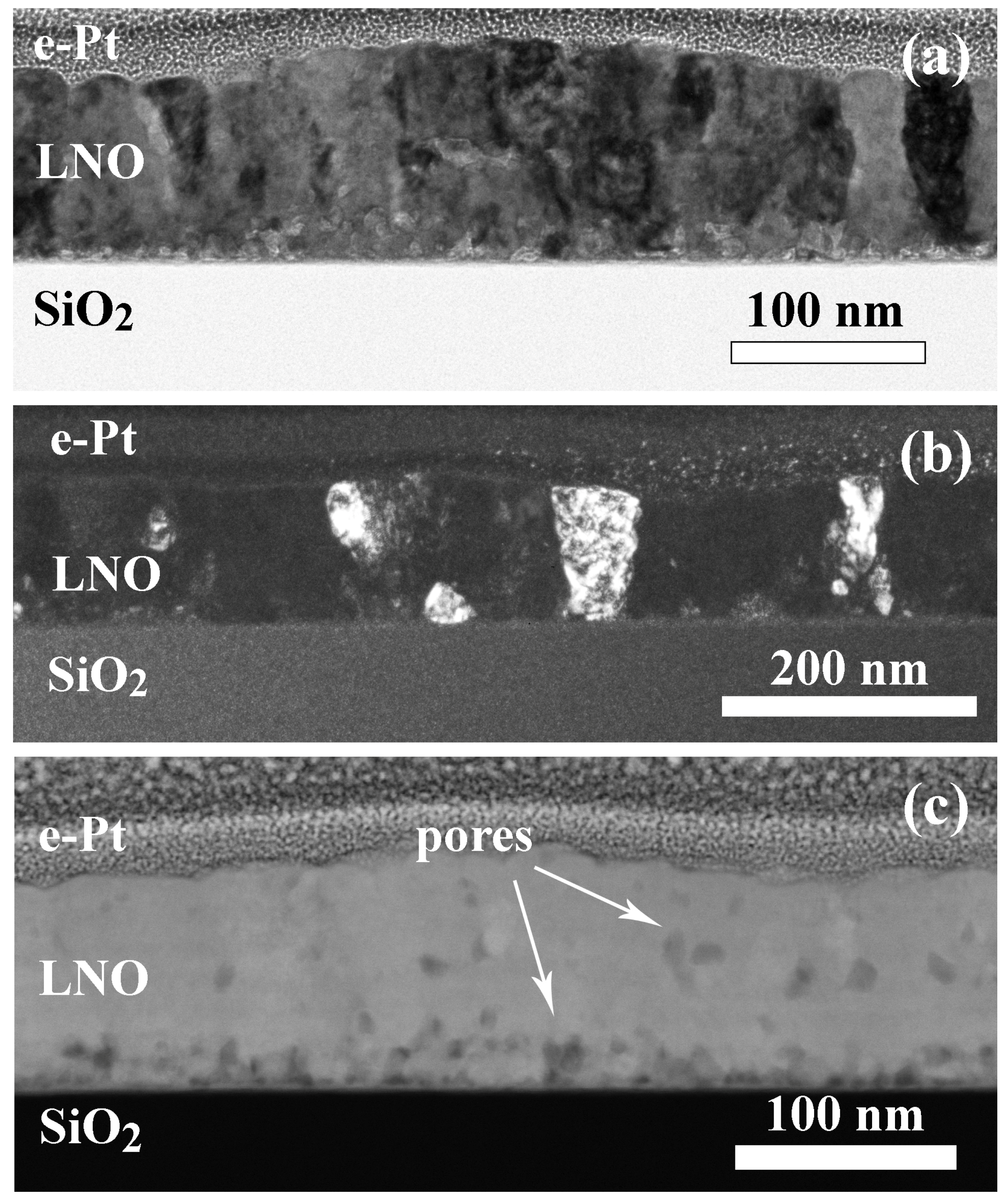
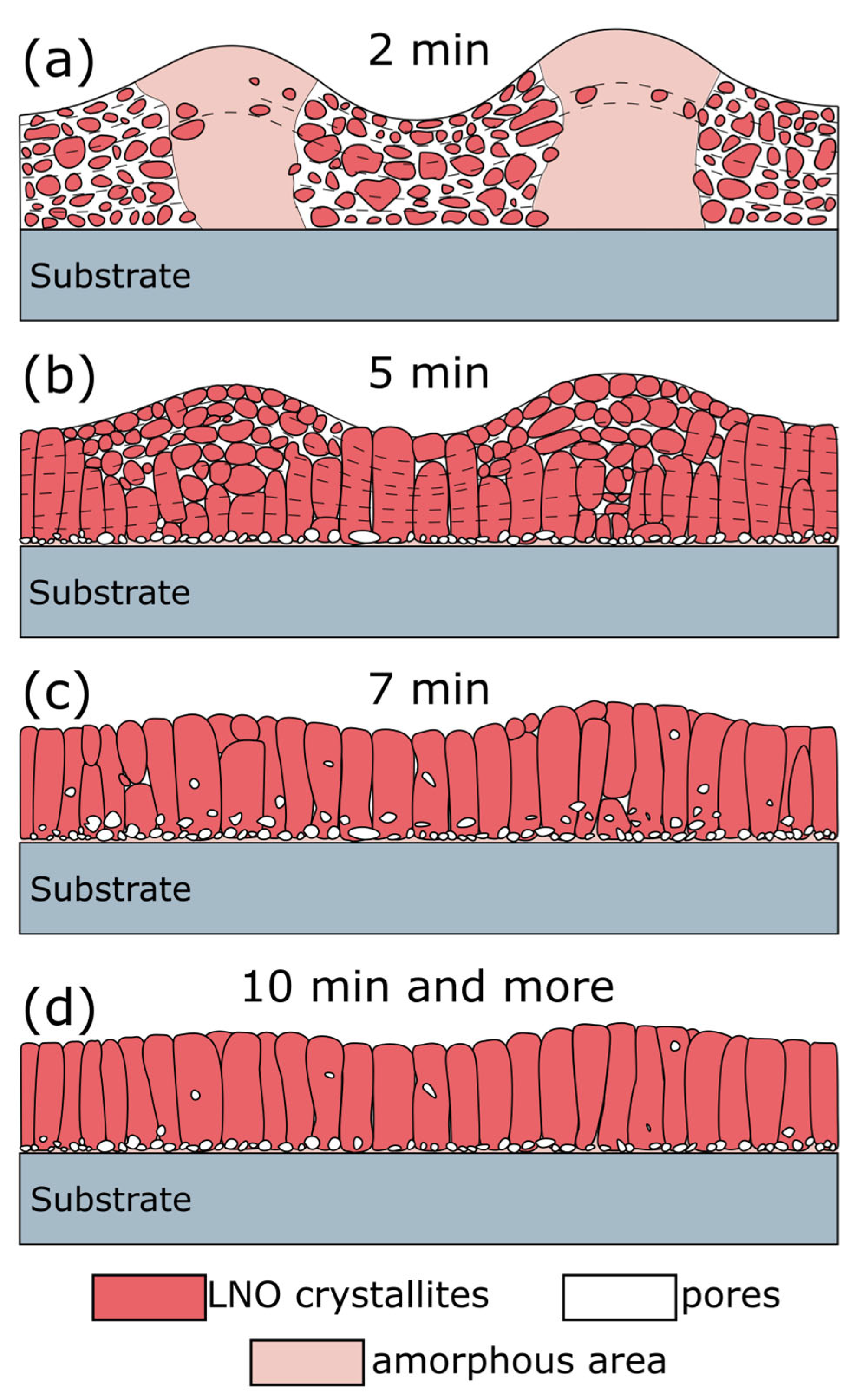
2.1. Effect of Annealing Time on the LNO Microstructure
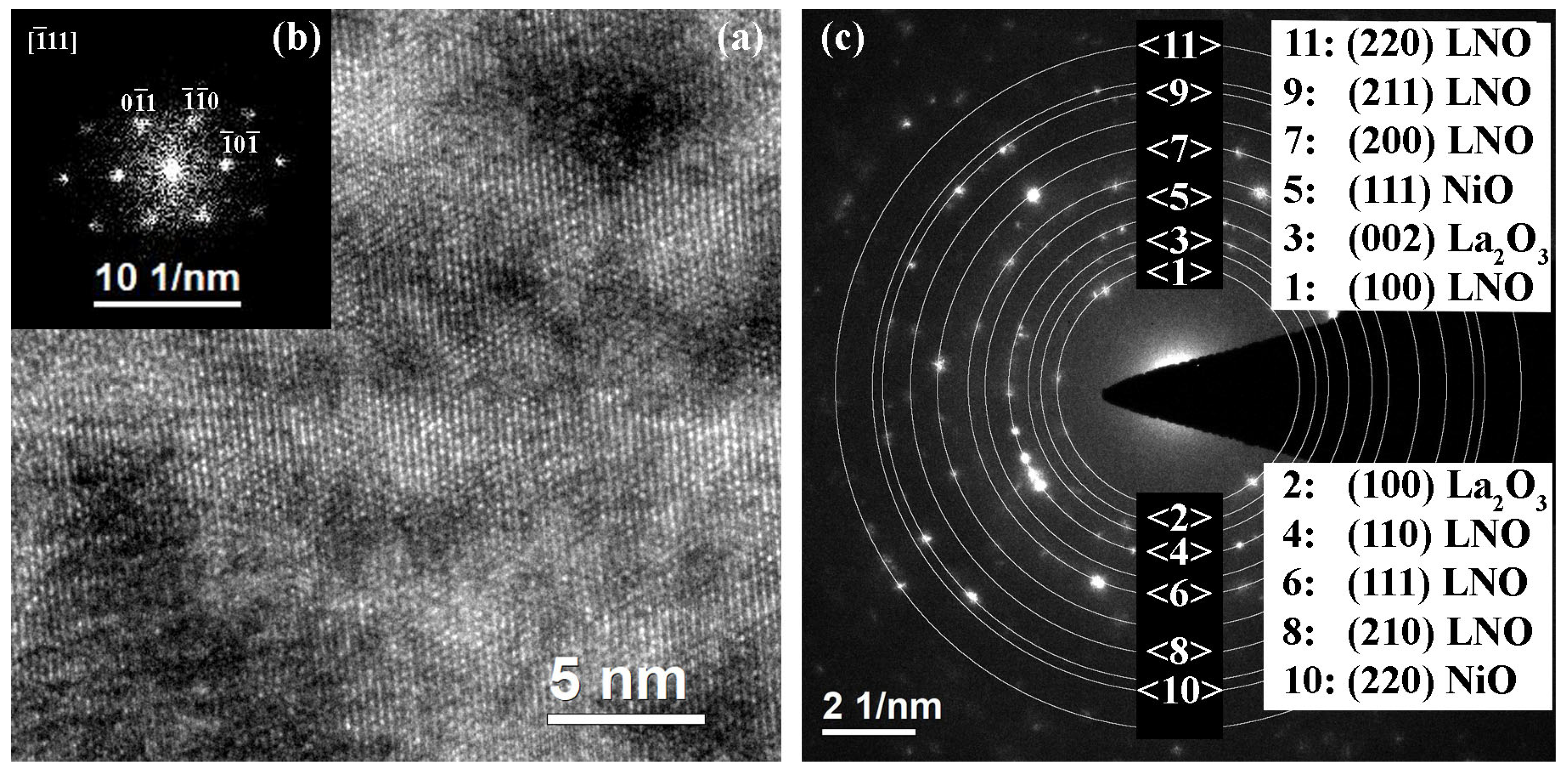
2.2. Structure of Samples with Additional Annealing
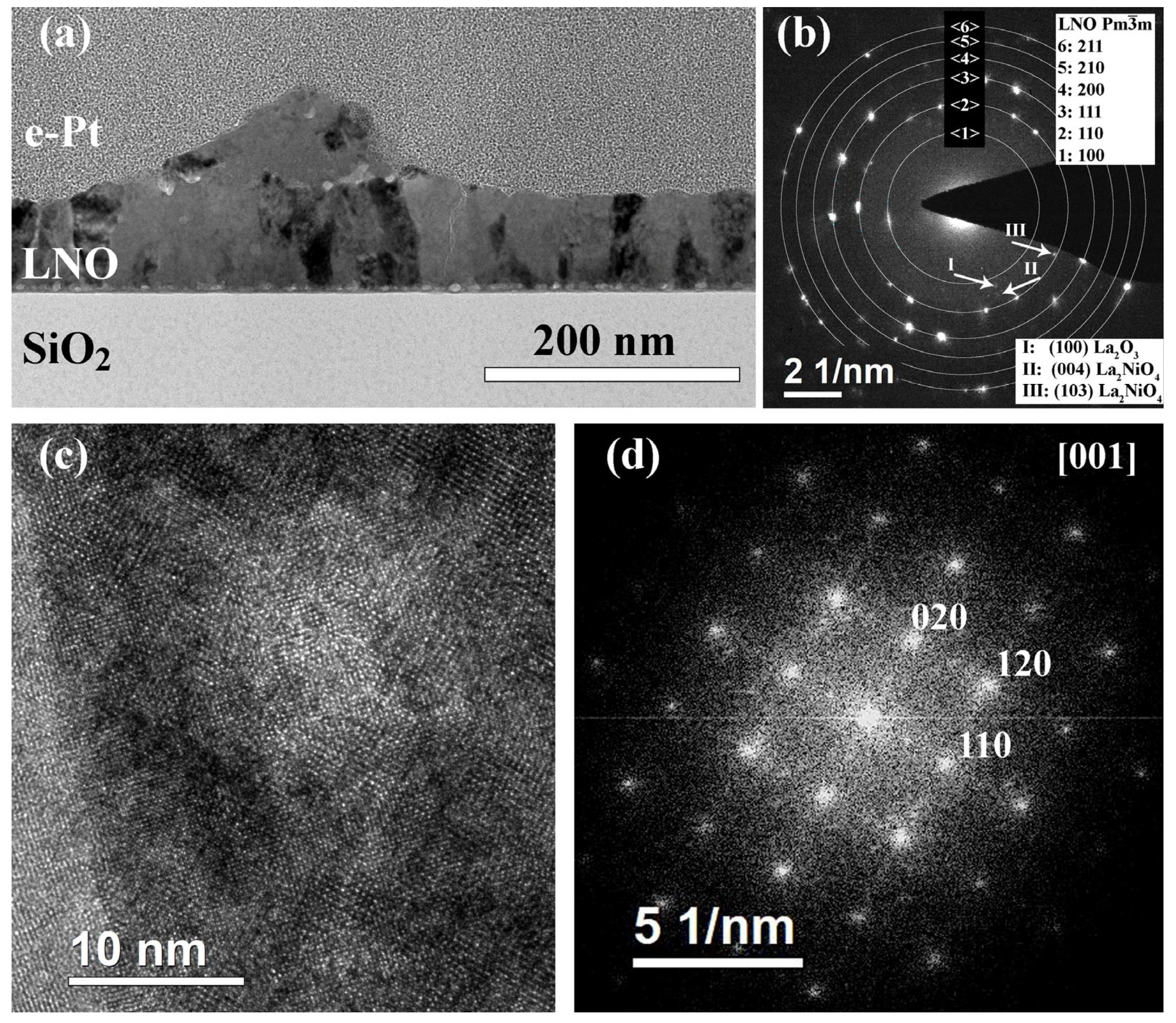
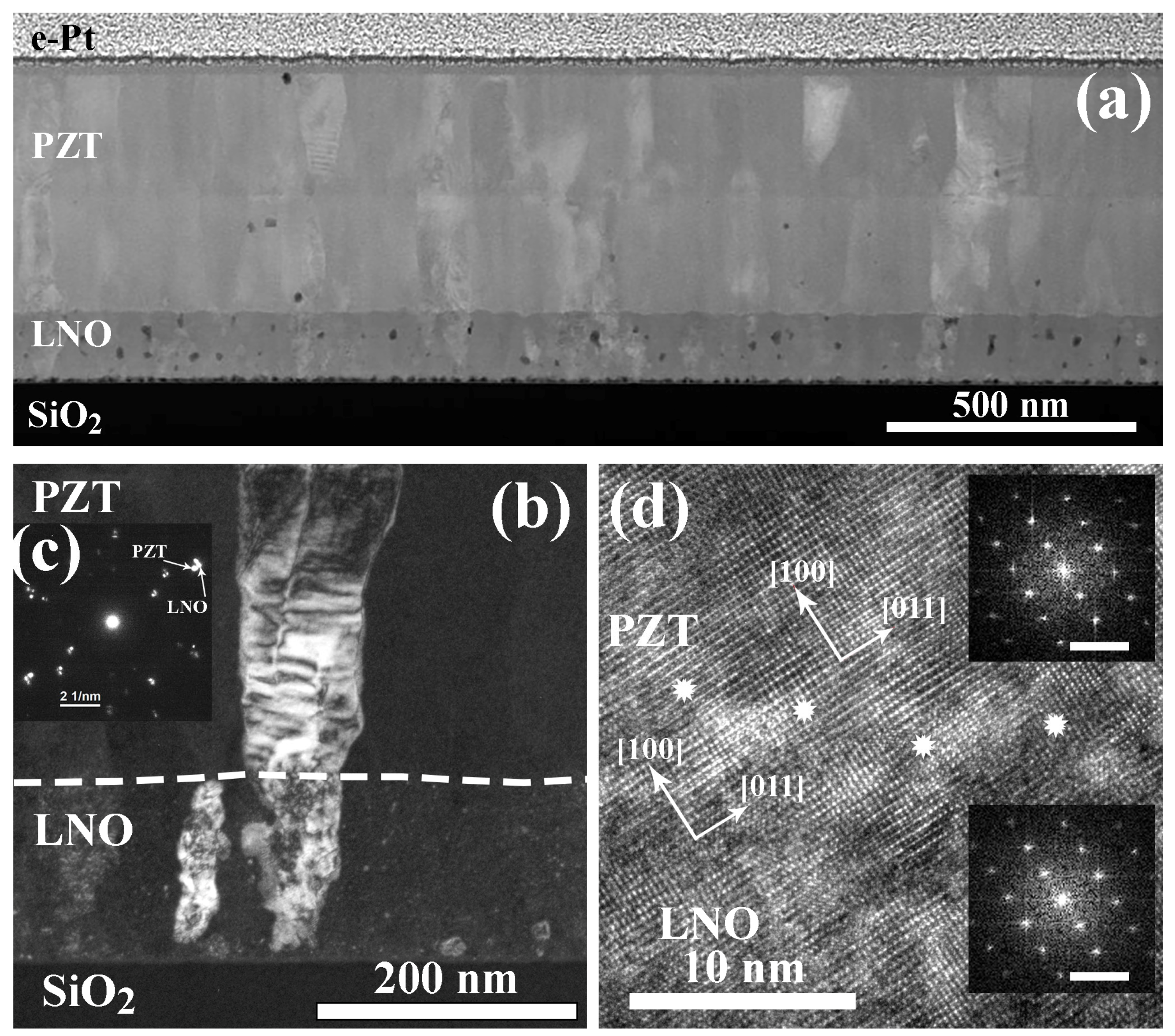
2.3. Structure of PZT-LNO-SiO2-Si Compositions
2.4. Mechanism of Crystallization
- Spin-on deposition of precursor’s solution. Solutions of La(CH3CO2)3·xH2O and (Ni(OCOCH3)2·4H2O dissolved in acetic acid CH3COOH are deposited on a silicon wafer. The liquid moves on the spinning wafer under an action of centrifugal force and viscous resisting force [65,66]. Film thinning causes a mass transfer increase via CH3COOH evaporation, which promotes interaction between acetate species. As a result of viscosity increase, liquid outflow stops, and further film thinning occurs only due to liquid evaporation. Acetic acid and water released from acetate molecules are partially evaporated (boiling point of acetic acid T = 118 °C). The film demonstrates a flat surface without any relief after spin-on deposition and after soft bake at 200 °C. Metal acetates LaAc3 and NiAc2 completely or partially lose water during the soft bake. Based on TGA data, Hussein shows two peaks at 130 °C and 180 °C due to the following LaAc3 dehydration reaction [67]:
- 2.
- Heat treatment at 300 °C. Note, that this is not a film processing step. However, this intermediate state is important to clarify why the films demonstrate wavy shape surface relief. As it was discussed earlier, an increase in temperature up to 300 °C leads to the formation of a pronounced relief with a regular wave structure (see Figure 3). After dehydration, LaAc3 have a tendency to association. For example, Hussein points to LaAc3 recrystallization at 210 °C [67]. NiAc2 dissociation starts in the same temperature range [70]. Regardless, we can consider the association of acetate and hydroxide precursors with the formation of small particles or clusters. We propose that the relief is formed as a result of self-assembly in the “solvent–particles” system caused by the evaporation of the solvent and interaction between particles (e.g., van der Waals interaction) [72,73]. The phenomenon of self-assembly in the dispersion of solid particles was first noticed during the drying of coffee drops, when the particles migrate to the edge of the drop and form a dark ring. Since then, this phenomenon has been shown for a wide range of surfaces, solvents, and solutes, and the organization mechanism has been shown to be caused by outward flow, which is driven by solvent loss through evaporation and geometric constraints [74,75]. It was shown that systems far from equilibrium can exhibit complex transitory structures, even under equilibrium fluctuations [72,73,76,77,78,79]. We suggest that a similar self-assembly process is the reason for the regular wavy shape surface in LNO films. More detailed investigation of this phenomenon may be useful in view of the creation of structures with special regular form.
- 3.
- 4.
- Deposition of the next layer. The upper layer is deposited under the same conditions as the previous one. An increase in annealing time leads to the coarsening and formation of column grains via crystallites coalescence. From a thermodynamic point of view, the driving force of coalescence is the reduction in the surface free energy. According to the pioneered Frenkel theory, the coalescence kinetics of two identical touching droplets is governed by viscous flowing and depends on their surface energy, viscosity, and size [82]. The characteristic time of coalescence 𝜏 followed the linear dependence on the initial particle radius 𝑟0: τ = η σ 𝑟0, where 𝜂 is the shear viscosity coefficient, and 𝜎 is the surface tension [82,83,84]. In our case, the processing temperature (650 °C) is lower than the melting point (~2200 °C), and the coalescence mechanism relates rather to the surface or grain boundary diffusion than to the hydrodynamic flows inside the droplet; however, Frenkel’s approach is still valid for rough process characterization [83]. Thus, sintering via coalescence of individual crystals is a time-dependent process and, in the case of LNO film, requires more than 5 min annealing of each layer (see Figure 16). As each deposited layer undergoes baking at 200 °C and annealing at 650 °C, the total annealing time of bottom layers will be significantly higher (35 min for the first deposited layer). As coalescence time τ is proportional to the crystallites size 𝑟0, the rate of coalescence is reduced with the crystallites growth and the coalescence of bigger crystals requires more time (10 min or more for each layer to complete column grain formation). It should be noted that sintering depends on the crystalline orientation of particles as the grain boundary energy relates to the degree of misorientation between two particles and gives an energy barrier for particle coalescence [83,85]. Reorientation takes some energy and time; for this reason, we have observed some disorientation inside the column grains as it was mentioned earlier.
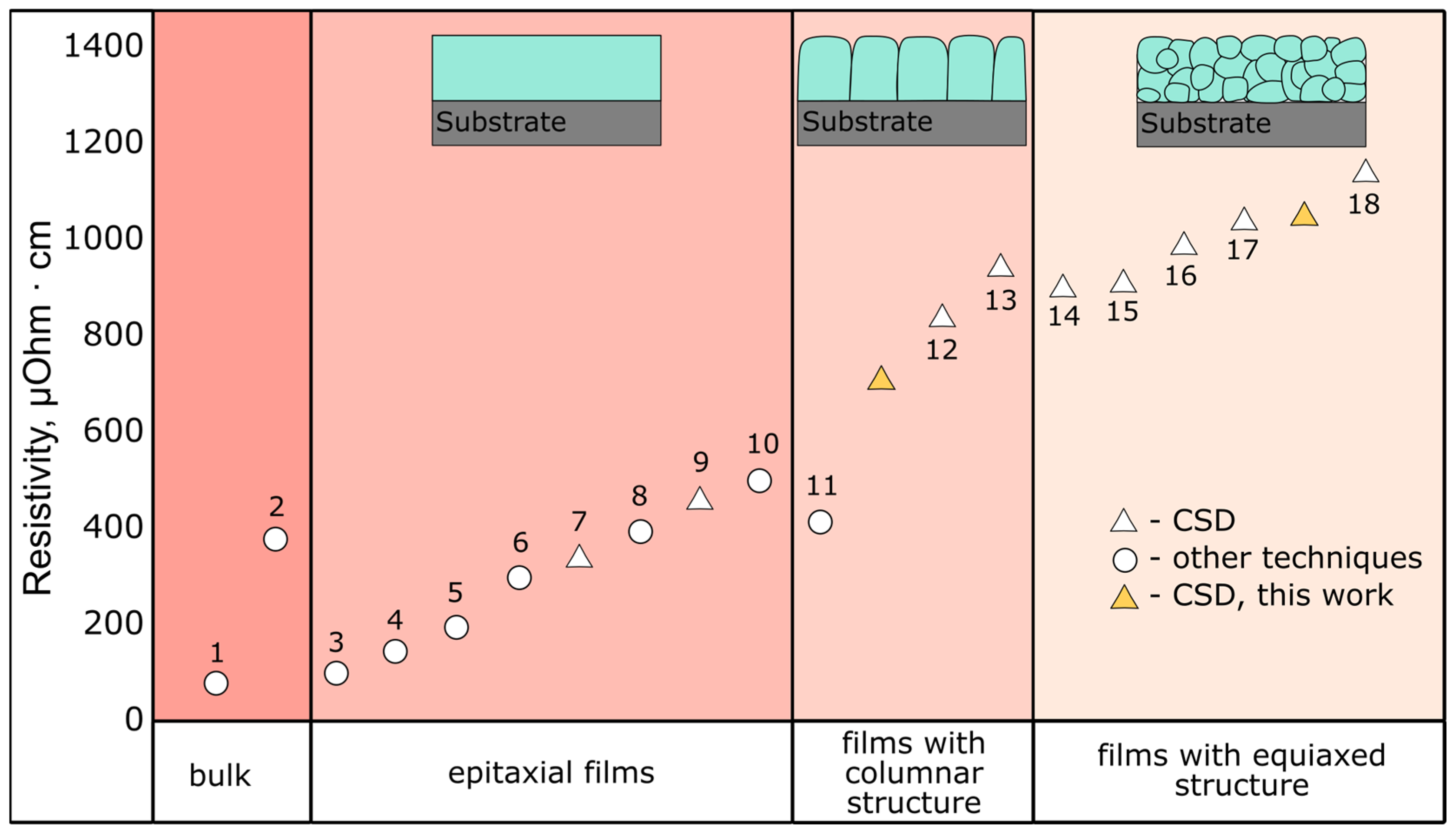
2.5. Electrical Properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Catalano, S.; Gibert, V.; Fowlie, J.; Íñiguez, J.; Triscone, J.-M.; Kreisel, J. Rare-Earth Nickelates RNiO3: Thin Films and Heterostructures. Rep. Prog. Phys. 2018, 81, 046501. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6549–6557. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Liu, Y.; Yan, Y.; Zhang, P. A Membraneless Direct Borohydride Fuel Cell Using LaNiO3-Catalysed Cathode. Fuel Cells 2008, 8, 394–398. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Zhu, J.; Zeng, Z.; Zhu, X. NiO/LaNiO3 Film Electrode with Binder-Free for High Performance Supercapacitor. Appl. Surf. Sci. 2016, 384, 92–98. [Google Scholar] [CrossRef]
- Chaloupka, J.; Khaliullin, G. Orbital Order and Possible Superconductivity in LaNiO3/LaMO3 Superlattices. Phys. Rev. Lett. 2008, 100, 16404. [Google Scholar] [CrossRef]
- Boris, A.V.; Matiks, Y.; Benckiser, E.; Frano, A.; Popovich, P.; Hinkov, V.; Wochner, P.; Castro-Colin, M.; Detemple, E.; Malik, V.K. Dimensionality Control of Electronic Phase Transitions in Nickel-Oxide Superlattices. Science 2011, 332, 937–940. [Google Scholar] [CrossRef]
- Gibert, M.; Viret, M.; Zubko, P.; Jaouen, N.; Tonnerre, J.-M.; Torres-Pardo, A.; Catalano, S.; Gloter, A.; Stéphan, O.; Triscone, J.-M. Interlayer Coupling through a Dimensionality-Induced Magnetic State. Nat. Commun. 2016, 7, 11227. [Google Scholar] [CrossRef]
- Hoffman, J.; Tung, I.C.; Nelson-Cheeseman, B.B.; Liu, M.; Freeland, J.W.; Bhattacharya, A. Charge Transfer and Interfacial Magnetism in (LaNiO3)n/(LaMnO3)2 Superlattices. Phys. Rev. B 2013, 88, 144411. [Google Scholar] [CrossRef]
- Piamonteze, C.; Gibert, M.; Heidler, J.; Dreiser, J.; Rusponi, S.; Brune, H.; Triscone, J.-M.; Nolting, F.; Staub, U. Interfacial Properties of LaMnO3/LaNiO3 Superlattices Grown along (001) and (111) Orientations. Phys. Rev. B 2015, 92, 14426. [Google Scholar] [CrossRef]
- Pontes, D.S.L.; Chiquito, A.J.; Pontes, F.M.; Longo, E.; Cdmf, L.; Federal, U.; Carlos, D.S.; Luiz, V.W.; Box, P.O.; Carlos, S.; et al. Structural, Dielectric, Ferroelectric and Optical Properties of PBCT, PBST and PCST Complex Thin Films on LaNiO3 Metallic Conductive Oxide Layer Coated Si Substrates by the CSD Technique. J. Alloys Compd. 2014, 609, 33–39. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Zhou, J. Orbital Ordering in Orthorhombic Perovskites. J. Mater. Chem. 2007, 17, 2394–2405. [Google Scholar] [CrossRef]
- Rajeev, K.P.; Shivashankar, G.V.; Raychaudhuri, A.K. Low-Temperature Electronic Properties of a Normal Conducting Perovskite Oxide (LaNiO3). Solid State Commun. 1991, 79, 591–595. [Google Scholar] [CrossRef]
- Amoresi, R.A.C.; Felix, A.A.; Lustosa, G.M.M.M.; Gasparotto, G.; Simões, A.Z.; Zaghete, M.A. Role of Morphological Characteristics on the Conductive Behavior of LaNiO3 Thin Films. Ceram. Int. 2016, 42, 16242–16247. [Google Scholar] [CrossRef]
- Xiao, J.; Tomczyk, M.; Reaney, I.M.; Vilarinho, P.M. Tailoring Ferroelectric Properties of 0.37BiScO3-0.63PbTiO3 Thin Films Using a Multifunctional LaNiO3 Interlayer. Cryst. Growth Des. 2018, 18, 4037–4044. [Google Scholar] [CrossRef]
- Duan, Z.; Cui, Y.; Yang, Z.; Li, K.; Wan, Y.; Lu, Z.; Xie, Y.; Zhang, J. Growth of Highly C-Axis Oriented LaNiO3 Films with Improved Surface Morphology on Si Substrate Using Chemical Solution Deposition and Rapid Heat Treatment Process. Ceram. Int. 2018, 44, 695–702. [Google Scholar] [CrossRef]
- Cheng, J.G.; Gabl, R.; Pitzer, D.; Primig, R.; Schreiter, M.; Wersing, W. Chemical Solution Deposition of Columnar-Grained Metallic Lanthanum Nitrate Thin Films. J. Am. Ceram. Soc. 2003, 86, 1786–1788. [Google Scholar] [CrossRef]
- Marshall, M.S.J.; Malashevich, A.; Disa, A.S.; Han, M.G.; Chen, H.; Zhu, Y.; Ismail-Beigi, S.; Walker, F.J.; Ahn, C.H. Conduction at a Ferroelectric Interface. Phys. Rev. Appl. 2014, 2, 051001. [Google Scholar] [CrossRef]
- Nozdrin, V.S.; Komandin, G.A.; Spektor, I.E.; Chernomyrdin, N.V.; Seregin, D.S.; Vishnevskiy, A.S.; Vorotilov, K.A. Optical Characteristics of LaNiO3 Thin Films in the Terahertz–Infrared Frequency Range. J. Appl. Phys. 2022, 131, 025305. [Google Scholar] [CrossRef]
- Zhu, M.W.; Wang, Z.J.; Chen, Y.N.; Zhang, Z.D. Microstructure and Transport Properties of Solgel Derived Highly (100)-Oriented Lanthanum Nickel Oxide Thin Films on SiO2/Si Substrate. J. Cryst. Growth 2011, 336, 44–49. [Google Scholar] [CrossRef]
- Tsubouchi, K.; Ohkubo, I.; Kumigashira, H.; Matsumoto, Y.; Ohnishi, T.; Lippmaa, M.; Koinuma, H.; Oshima, M. Epitaxial Growth and Surface Metallic Nature of LaNiO3 Thin Films. Appl. Phys. Lett. 2008, 92, 262109. [Google Scholar] [CrossRef]
- Escote, M.T.; Pontes, F.M.; Leite, E.R.; Varela, J.A.; Jardim, R.F.; Longo, E. Microstructural and Transport Properties of LaNiO3–δ Films Grown on Si (111) by Chemical Solution Deposition. Thin Solid Film. 2003, 445, 54–58. [Google Scholar] [CrossRef]
- Zhu, M.W.; Wang, Z.J.; Chen, Y.N.; Wang, H.L.; Zhang, Z.D. Effect of Grain Boundary on Electrical Properties of Polycrystalline Lanthanum Nickel Oxide Thin Films. Appl. Phys. A Mater. Sci. Process. 2013, 112, 1011–1018. [Google Scholar] [CrossRef]
- Li, A.D.; Ge, C.Z.; Wu, D.; Lü, P.; Zuo, Y.Q.; Yang, S.Z.; Ming, N. Ben Conductive Metallic LaNiO3 Films from Metallo-Organic Precursors. Thin Solid Film. 1997, 298, 165–169. [Google Scholar] [CrossRef]
- Vu, T.H.; Nguyen, D.M.; Vu, N.H. Synthesis and Characterisations of Sol-Gel-Derived LaNiO3 Thin-Film Electrodes on Si Substrates. Int. J. Nanotechnol. 2015, 12, 496–504. [Google Scholar]
- Sergeenkov, S.; Cichetto, L., Jr.; Zampieri, M.; Longo, E. Scaling like Behaviour of Resistivity Observed in LaNiO3 Thin Films Grown on SrTiO3 Substrate by Pulsed Laser Deposition. J. Phys. Condens. Matter 2015, 27, 485307. [Google Scholar] [CrossRef]
- Zhang, X.D.; Meng, X.J.; Sun, J.L.; Lin, T.; Ma, J.H.; Chu, J.H.; Kwon, D.Y.; Kim, C.W.; Kim, B.G. Preparation of LaNiO3 Thin Films with Very Low Room-Temperature Electrical Resistivity by Room Temperature Sputtering and High Oxygen-Pressure Processing. Thin Solid Film. 2008, 516, 919–924. [Google Scholar] [CrossRef]
- He, B.; Wang, Z. Effect of Substrate Temperature on Microstructure and Electrical Properties of LaNiO3 Films Grown on SiO2/Si Substrates by Pulsed Laser Deposition under a High Oxygen Pressure. Appl. Phys. A Mater. Sci. Process. 2016, 122, 905. [Google Scholar] [CrossRef]
- Zhu, M.; Komissinskiy, P.; Radetinac, A.; Vafaee, M.; Wang, Z.; Alff, L. Effect of Composition and Strain on the Electrical Properties of LaNiO3 Thin Films. Appl. Phys. Lett. 2013, 103, 141902. [Google Scholar] [CrossRef]
- Berini, B.; Noun, W.; Dumont, Y.; Popova, E.; Keller, N. High Temperature Ellipsometry of the Conductive Oxide LaNiO3. J. Appl. Phys. 2007, 101, 023529. [Google Scholar] [CrossRef]
- Sánchez, F.; Ferrater, C.; Guerrero, C.; García-Cuenca, M.V.; Varela, M. High-Quality Epitaxial LaNiO3 Thin Films on SrTiO3(100) and LaAlO3(100). Appl. Phys. A Mater. Sci. Process. 2000, 71, 59–64. [Google Scholar] [CrossRef]
- Haavik, C.; Rørvik, P.M. Conducting Oxide Thin Films. In Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Wien, Austria, 2013; pp. 621–654. [Google Scholar]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Wien, Austria, 2013; Volume 9783211993, ISBN 9783211993118. [Google Scholar]
- Turova, N.Y.; Turevskaya, E.P.; Kessler, V.G.; Yanovskaya, M.I.; Turova, N.Y. Oxoalkoxides. In The Chemistry of Metal Alkoxides; Springer: Boston, MA, USA, 2002; pp. 69–75. ISBN 978-0-306-47657-0. [Google Scholar]
- Vorotilov, K.A.; Yanovskaya, M.I.; Turevskaya, E.P.; Sigov, A.S. Sol-Gel Derived Ferroelectric Thin Films: Avenues for Control of Microstructural and Electric Properties. J. Sol-Gel Sci. Technol. 1999, 16, 109–118. [Google Scholar] [CrossRef]
- Zhang, T.; Fujisawa, K.; Zhang, F.; Liu, M.; Lucking, M.C.; Gontijo, R.N.; Lei, Y.; Liu, H.; Crust, K.; Granzier-Nakajima, T.; et al. Universal In Situ Substitutional Doping of Transition Metal Dichalcogenides by Liquid-Phase Precursor-Assisted Synthesis. ACS Nano 2020, 14, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Atanova, A.V.; Zhigalina, O.M.; Khmelenin, D.N.; Orlov, G.A.; Seregin, D.S.; Sigov, A.S.; Vorotilov, K.A. Microstructure Analysis of Porous Lead Zirconate–Titanate Films. J. Am. Ceram. Soc. 2022, 105, 639–652. [Google Scholar] [CrossRef]
- Varghese, J.; Ghoshal, T.; Deepak, N.; O’Regan, C.; Whatmore, R.W.; Morris, M.A.; Holmes, J.D. Fabrication of Arrays of Lead Zirconate Titanate (PZT) Nanodots via Block Copolymer Self-Assembly. Chem. Mater. 2013, 25, 1458–1463. [Google Scholar] [CrossRef]
- Porotnikov, D.; Zamkov, M. Progress and Prospects of Solution-Processed Two-Dimensional Semiconductor Nanocrystals. J. Phys. Chem. C 2020, 124, 21895–21908. [Google Scholar] [CrossRef]
- Surekha, P.; Varshney, A.D.; Jerusha, E.; Pant, B.; Rajesh, A.S. Optical Applications of Sol-Gel Nano-Composites. Mater. Today Proc. 2022, 62, 2034–2037. [Google Scholar] [CrossRef]
- Miyazaki, H.; Goto, T.; Miwa, Y.; Ohno, T.; Suzuki, H.; Ota, T.; Takahashi, M. Preparation and Evaluation of LaNiO3 Thin Film Electrode with Chemical Solution Deposition. J. Eur. Ceram. Soc. 2004, 24, 1005–1008. [Google Scholar] [CrossRef]
- Park, J.; Lim, Y.; Kong, S.; Lee, H.; Kim, Y.-B. Rapid Fabrication of Chemical Solution-Deposited Lanthanum Nickelate Thin Films via Intense Pulsed-Light Process Junghum. Coatings 2019, 9, 372. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wang, Z.J. Rapid Microwave Annealing of Amorphous Lead Zirconate Titanate Thin Films Deposited by Sol-Gel Method on La NiO3/SiO2/Si Substrates. J. Am. Ceram. Soc. 2013, 96, 90–95. [Google Scholar] [CrossRef]
- Hoffmann-Eifert, S.; Schneller, T. Orientation and Microstructure Design. In Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Wien, Austria, 2013; pp. 407–429. [Google Scholar]
- Zhigalina, O.M.; Burmistrova, P.V.; Vasiliev, A.L.; Roddatis, V.V.; Sigov, A.S.; Vorotilov, K.A. Microstructure of PZT Capacitor Structures. Ferroelectrics 2003, 286, 311–320. [Google Scholar] [CrossRef]
- Muralt, P. Polar Oxide Thin Films for MEMS Applications. In Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Wien, Austria, 2013; ISBN 9783211993118. [Google Scholar]
- Schwartz, R.W.; Clem, P.G.; Voigt, J.A.; Byhoff, E.R.; Van Stry, M.; Headley, T.J.; Missert, N.A. Control of Microstructure and Orientation in Solution-Deposited BaTiO3 and SrTiO3 Thin Films. J. Am. Ceram. Soc. 1999, 82, 2359–2367. [Google Scholar] [CrossRef]
- Zhigalina, O.M.; Vorotilov, K.A.; Sigov, A.S.; Kumskov, A.S. Influence of Crystallization Process on Structural State of CSD BST Thin Films. Ferroelectrics 2006, 335, 13–21. [Google Scholar] [CrossRef]
- Zhigalina, O.M.; Atanova, A.V.; Khmelenin, D.N.; Kotova, N.M.; Seregin, D.S.; Vorotilov, K.A. Structural Features and Mutual Influence of the Layers in PZT–LNO–SiOx–Si and PZT–LNO–Si Compositions. Crystallogr. Rep. 2019, 64, 961–967. [Google Scholar] [CrossRef]
- Miyazaki, T.; Imai, T.; Wakiya, N.; Sakamoto, N.; Fu, D.; Suzuki, H. Low-Temperature Crystallization of CSD-Derived PZT Thin Film with Laser Annealing. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 173, 89–93. [Google Scholar] [CrossRef]
- Milt, V.G.; Querini, C.A.; Miró, E.E. Thermal Analysis of K(x)/La2O3, Active Catalysts for the Abatement of Diesel Exhaust Contaminants. Thermochim. Acta 2003, 404, 177–186. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Tvardauskas, H.; Senulis, M.; Lisauskas, V.; Šliužiene, K.; Vengalis, B.; Orlowski, B.A.; Baškys, E. Surface Stability of Epitaxial LaM03-δ Thin Films. Lith. J. Phys. 2010, 50, 317–323. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Vengalis, B.; Šliužiene, K.; Orlowski, B.A.; Osinniy, V.; Drube, W. Investigation of Epitaxial LaNiO3-x Thin Films by High-Energy XPS. J. Alloys Compd. 2006, 423, 107–111. [Google Scholar] [CrossRef]
- Baškys, E.; Bondarenka, V.; Grebinskij, S.; Senulis, M.; Sereika, R. XPS Study of Sol-Gel Produced Lanthanum Oxide Thin Films. Lith. J. Phys. 2014, 54, 120–124. [Google Scholar] [CrossRef]
- De Asha, A.M.; Critchley, J.T.S.; Nix, R.M. Molecular Adsorption Characteristics of Lanthanum Oxide Surfaces: The Interaction of Water with Oxide Overlayers Grown on Cu(111). Surf. Sci. 1998, 405, 201–214. [Google Scholar] [CrossRef]
- De Asha, A.M.; Nix, R.M. Interaction of Carbon Dioxide with Oxidised La/Cu(111). J. Chem. Soc. Faraday Trans. 1995, 91, 3611–3617. [Google Scholar] [CrossRef]
- Dong, D.; Hu, W.C.; Zhan, W.L.; Liu, G.J. Surface Morphology of LaNiO3 Thin Films and Its Dependence on Annealing Temperature. Trans. Inst. Met. Finish. 2007, 85, 107–110. [Google Scholar] [CrossRef]
- Suzuki, H.; Naoe, T.; Miyazaki, H.; Ota, T. Deposition of Highly Oriented Lanthanum Nickel Oxide Thin Film on Silicon Wafer by CSD. J. Eur. Ceram. Soc. 2007, 27, 3769–3773. [Google Scholar] [CrossRef]
- Norman, A.K.; Morris, M.A. Preparation of the Single-Phase Perovskite LaNiO3. J. Mater. Process. Technol. 1999, 92–93, 91–96. [Google Scholar] [CrossRef]
- Gayathri, N.; Raychaudhuri, A.K.; Xu, X.Q.Q.; Peng, J.L.L.; Greene, R.L.L. Electronic Conduction in LaNiO3−δ: The Dependence on the Oxygen Stoichiometry δ. J. Phys. Condens. Matter 1998, 10, 1323–1338. [Google Scholar] [CrossRef]
- Holzlechner, G.; Kastner, D.; Slouka, C.; Hutter, H.; Fleig, J. Oxygen Vacancy Redistribution in PbZrxTi1−xO3 (PZT) under the Influence of an Electric Field. Solid State Ion. 2014, 262, 625–629. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Jia, Z.; Ren, T.-L. Effects of Electrodes on the Properties of Sol–Gel PZT Based Capacitors in FeRAM. Solid. State. Electron. 2009, 53, 473–477. [Google Scholar] [CrossRef]
- Podgorny, Y.V.; Antonovich, A.N.; Petrushin, A.A.; Sigov, A.S.; Vorotilov, K.A. Effect of Metal Electrodes on the Steady-State Leakage Current in PZT Thin Film Capacitors. J. Electroceramics 2022, 49, 15–21. [Google Scholar] [CrossRef]
- Podgorny, Y.V.; Vorotilov, K.A.; Sigov, A.S.; Scott, J.F. Dead Layer Thickness Estimation at the Ferroelectric Film-Metal Interface in PZT. Appl. Phys. Lett. 2019, 114, 132902. [Google Scholar] [CrossRef]
- Bergholz, W. Defect Engineering in Silicon Materials. In Defects and Impurities in Silicon Materials: An Introduction to Atomic-Level Silicon Engineering; Yoshida, Y., Langouche, G., Eds.; Springer: Tokyo, Japan, 2015; pp. 431–487. ISBN 978-4-431-55800-2. [Google Scholar]
- Bornside, D.E.; Macosko, C.W.; Scriven, L.E. Modeling of Spin Coating. J. Imaging Technol. 1987, 13, 122–130. [Google Scholar]
- Bornside, D.E.; Macosko, C.W.; Scriven, L.E. Spin Coating: One-dimensional Model. J. Appl. Phys. 1989, 66, 5185–5193. [Google Scholar] [CrossRef]
- Hussein, G.A.M. Spectrothermal Investigation of the Decomposition Course of Lanthanum Acetate Hydrate. J. Therm. Anal. 1994, 42, 1091–1102. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Shipochka, M.; Vitanov, P. Nickel Oxide Films Deposited by Sol-Gel Method: Effect of Annealing Temperature on Structural, Optical, and Electrical Properties. Materials 2022, 15, 1742. [Google Scholar] [CrossRef] [PubMed]
- Doremieux, J.L. Thermal Evolution of Nickel Acetate. I. Thermolysis in a Nitrogen Stream. Bull. Soc. Chim. Fr. 1969, 5, 1508. [Google Scholar]
- De Jesus, J.C.; González, I.; Quevedo, A.; Puerta, T. Thermal Decomposition of Nickel Acetate Tetrahydrate: An Integrated Study by TGA, QMS and XPS Techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Atanova, A.V.; Zhigalina, O.M.; Khmelenin, D.N.; Kotova, N.M.; Seregin, D.S.; Vorotilov, K.A. Chemical and Phase Inhomogeneity in LaNiO3 Electrodes Prepared by Chemical Solution Deposition. Ferroelectrics 2021, 574, 29–36. [Google Scholar] [CrossRef]
- Ge, G.; Brus, L. Evidence for Spinodal Phase Separation in Two-Dimensional Nanocrystal Self-Assembly. J. Phys. Chem. B 2000, 104, 41. [Google Scholar] [CrossRef]
- Rabani, E.; Reichman, D.R.; Geissler, P.L.; Brus, L.E. Drying-Mediated Self-Assembly of Nanoparticles. Nature 2003, 426, 271–274. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact Line Deposits in an Evaporating Drop. Phys. Rev. E—Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 756–765. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Gibert, M.; Puig, T.; Obradors, X. Growth of Strain-Induced Self-Assembled BaZrO3 Nanodots from Chemical Solutions. Surf. Sci. 2007, 601, 2680–2683. [Google Scholar] [CrossRef]
- Craster, R.V.; Matar, O.K. Dynamics and Stability of Thin Liquid Films. Rev. Mod. Phys. 2009, 81, 1131–1198. [Google Scholar] [CrossRef]
- Gelbart, W.M.; Sear, R.P.; Heath, J.R.; Chaney, S. Array Formation in Nano-Colloids: Theory and Experiment in 2D. Faraday Discuss. 1999, 112, 299–307. [Google Scholar] [CrossRef]
- Sear, R.P.; Chung, S.W.; Markovich, G.; Gelbart, W.M.; Heath, J.R. Spontaneous Patterning of Quantum Dots at the Air-Water Interface. Phys. Rev. E—Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1999, 59, R6255–R6258. [Google Scholar] [CrossRef]
- Da Rocha, S.M.R.; da Silva Queiroz, C.A.; Abrão, A. Synthesis and Characterization of Lanthanum Acetate for Application as a Catalyst. J. Alloys Compd. 2002, 344, 389–393. [Google Scholar] [CrossRef]
- Zhan, G.; Yu, J.; Xu, Z.; Zhou, F.; Chi, R. Kinetics of Thermal Decomposition of Lanthanum Oxalate Hydrate. Trans. Nonferrous Met. Soc. China 2012, 22, 925–934. [Google Scholar] [CrossRef]
- Frenkel, J.J. Viscous Flow of Crystalline Bodies under the Action of Surface Tension. J. Phys. 1945, 9, 385. [Google Scholar]
- Samsonov, V.M.; Alymov, M.I.; Talyzin, I.V.; Vasilyev, S.A. Size Dependence of the Melting Temperature and Mechanisms of the Coalescence/Sintering on the Nanoscale. J. Phys. Conf. Ser. 2019, 1352, 12044. [Google Scholar] [CrossRef]
- Samsonov, V.M.; Talyzin, I.V.; Vasilyev, S.A.; Alymov, M.I. On the Mechanisms of Coalescence of Nanodroplets and Sintering of Solid Particles. Colloid J. 2020, 82, 573–583. [Google Scholar] [CrossRef]
- Mu, Z.; Tang, R.; Liu, Z. Construction of Inorganic Bulks through Coalescence of Particle Precursors. Nanomaterials 2021, 11, 241. [Google Scholar] [CrossRef]
- Malashevich, A.; Ismail-Beigi, S. First-Principles Study of Oxygen-Deficient LaNiO3 Structures. Phys. Rev. B 2015, 92, 144102. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Direct Observation of Ni3+ and Ni2+ in Correlated LaNiO3−δ Films. Europhys. Lett. 2011, 93, 57002. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.W.; Zhao, L.; Hu, Z.; Chang, C.F.; Kuo, C.Y.; Schmidt, W.; Piovano, A.; Pi, T.W.; Sobolev, O.; et al. Antiferromagnetic Correlations in the Metallic Strongly Correlated Transition Metal Oxide LaNiO3. Nat. Commun. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Peng, J.L.; Li, Z.Y.; Ju, H.L.; Greene, R.L. Resisitivity, Thermopower, and Susceptibility of RNiO3 (R = La,Pr). Phys. Rev. B 1993, 48, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.R.; Payne, D.A. Solution Deposition and Heteroepitaxial Crystallization of LaNiO3 Electrodes for Integrated Ferroelectric Devices Solution Deposition and Heteroepitaxial Crystallization of LaNiO3 Electrodes for Integrated Ferroelectric Devices. Appl. Phys. Lett. 1997, 71, 3013–3015. [Google Scholar] [CrossRef]
- Cappelli, E.; Tromp, W.O.; McKeown Walker, S.; Tamai, A.; Gibert, M.; Baumberger, F.; Bruno, F.Y. A Laser-ARPES Study of LaNiO3 Thin Films Grown by Sputter Deposition. APL Mater. 2020, 8, 51102. [Google Scholar] [CrossRef]
- Son, J.; Moetakef, P.; Lebeau, J.M.; Ouellette, D.; Balents, L.; Allen, S.J.; Stemmer, S. Low-Dimensional Mott Material: Transport in Ultrathin Epitaxial LaNiO3 Films. Appl. Phys. Lett. 2010, 96, 22–25. [Google Scholar] [CrossRef]
- Kotova, N.M.; Vorotilov, K.A.; Seregin, D.S.; Sigov, A.S. Role of Precursors in the Formation of Lead Zirconate Titanate Thin Films. Inorg. Mater. 2014, 50, 612–616. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. The Piezoelectric Effect in Ceramics. In Piezoelectric Ceram; Elsevier: London, UK, 1971; pp. 7–21. [Google Scholar]
- Kozuka, H. Stress Evolution and Cracking in Sol-Gel-Derived Thin Films BT. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 275–311. ISBN 978-3-319-32101-1. [Google Scholar]
- Li, C.; Li, C.; Habler, G.; Baldwin, L.C.; Abart, R. An Improved FIB Sample Preparation Technique for Site-Specific Plan-View Specimens: A New Cutting Geometry. Ultramicroscopy 2017, 184, 310–317. [Google Scholar] [CrossRef]
- Valdes, L.B. Resistivity Measurements on Germanium for Transistors. Proc. IRE 1954, 42, 420–427. [Google Scholar] [CrossRef]
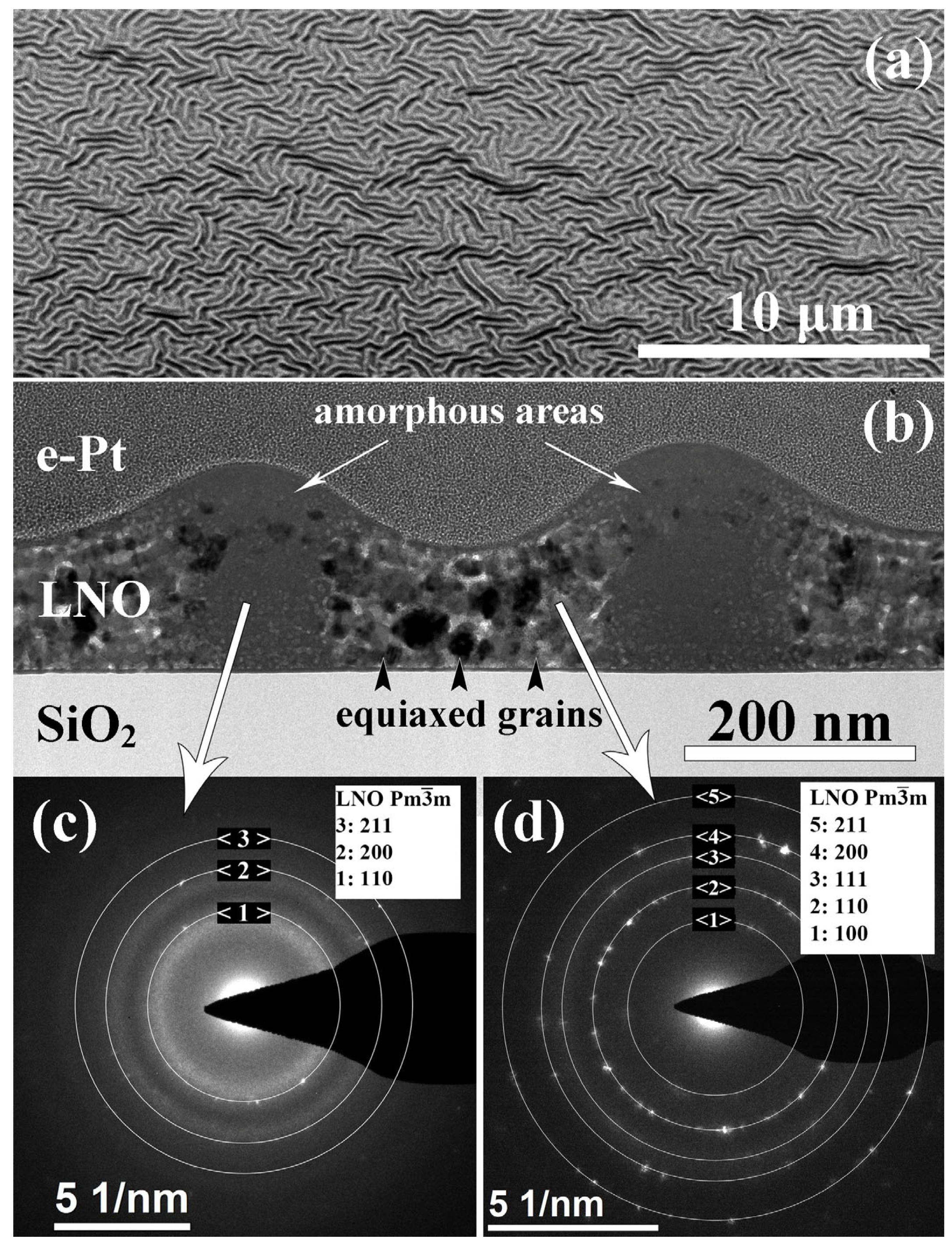
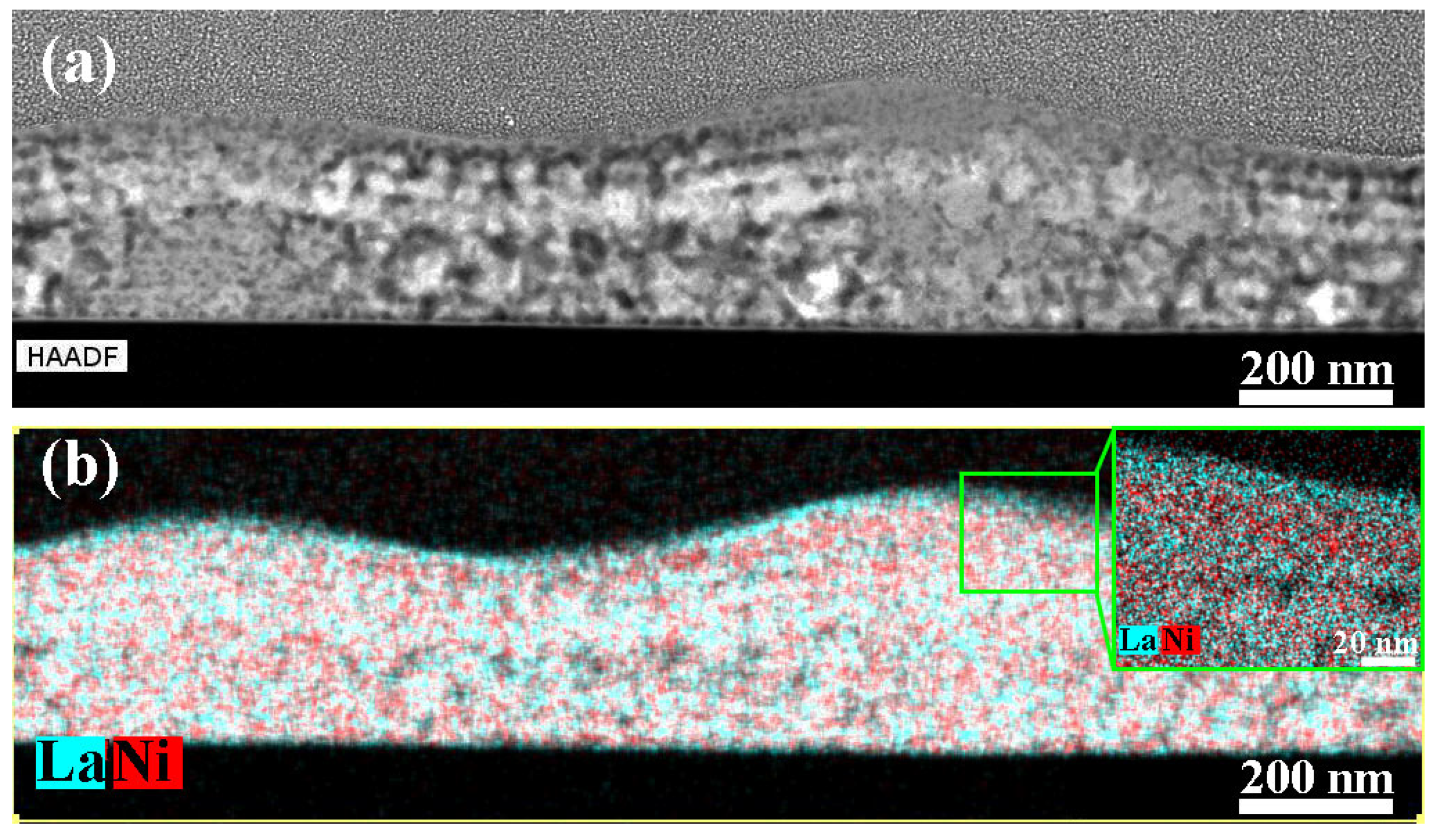
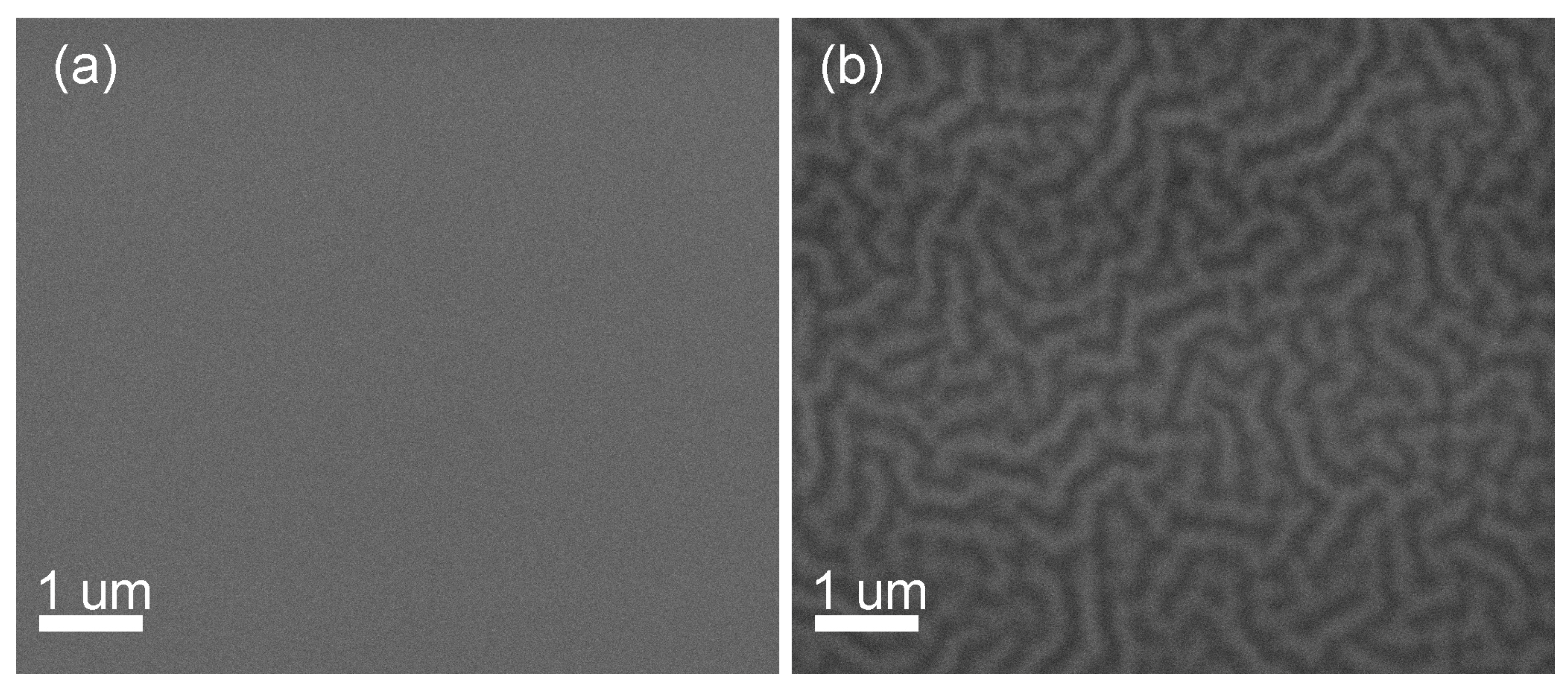
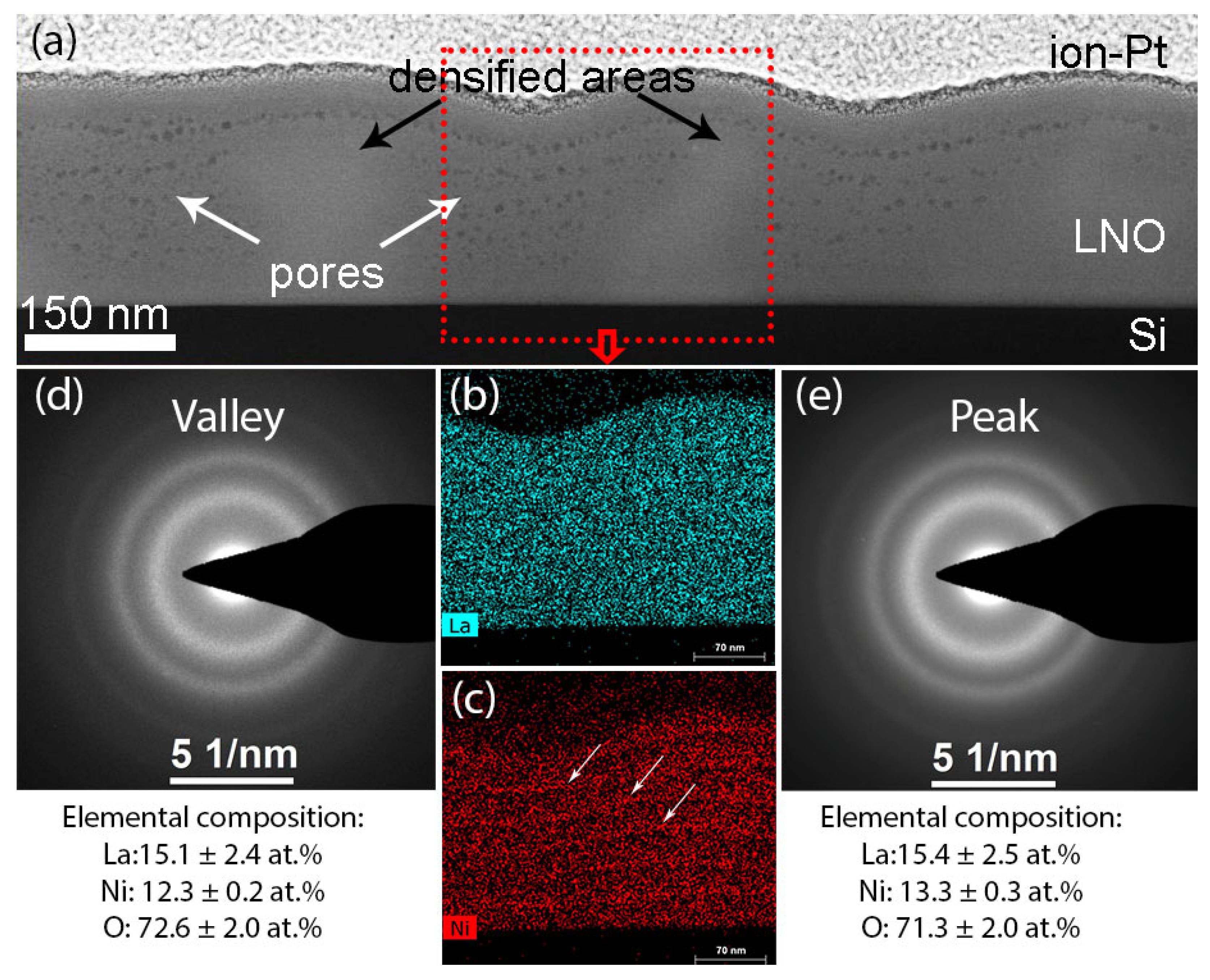
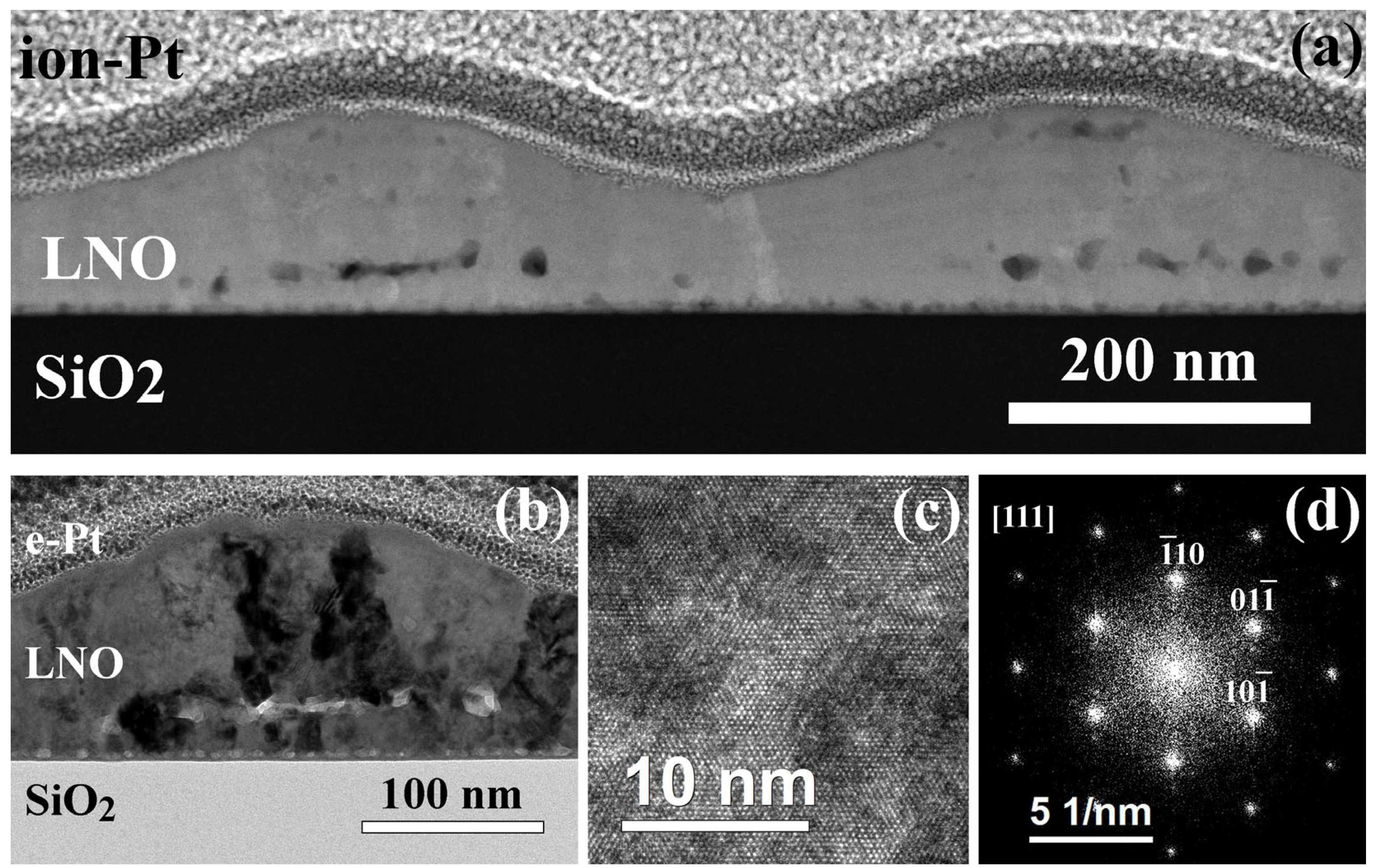
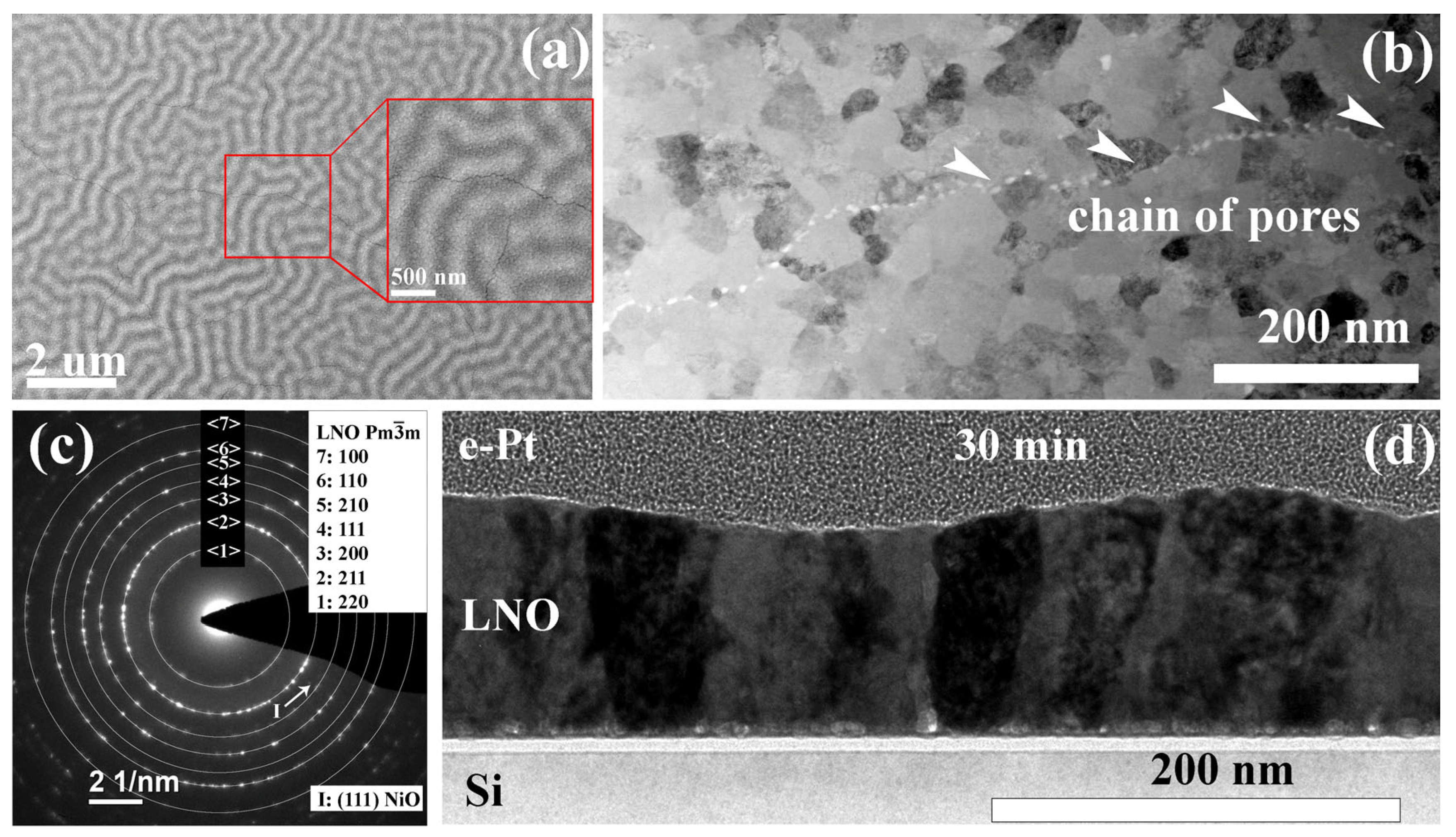
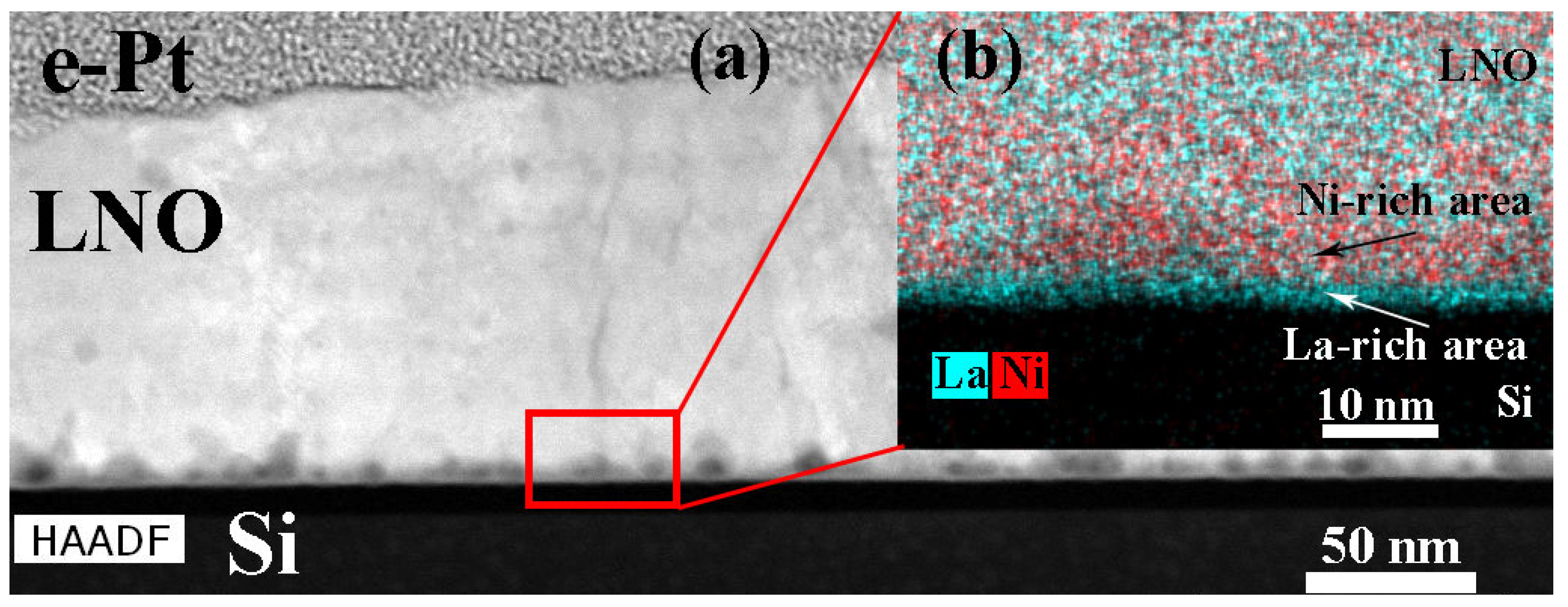
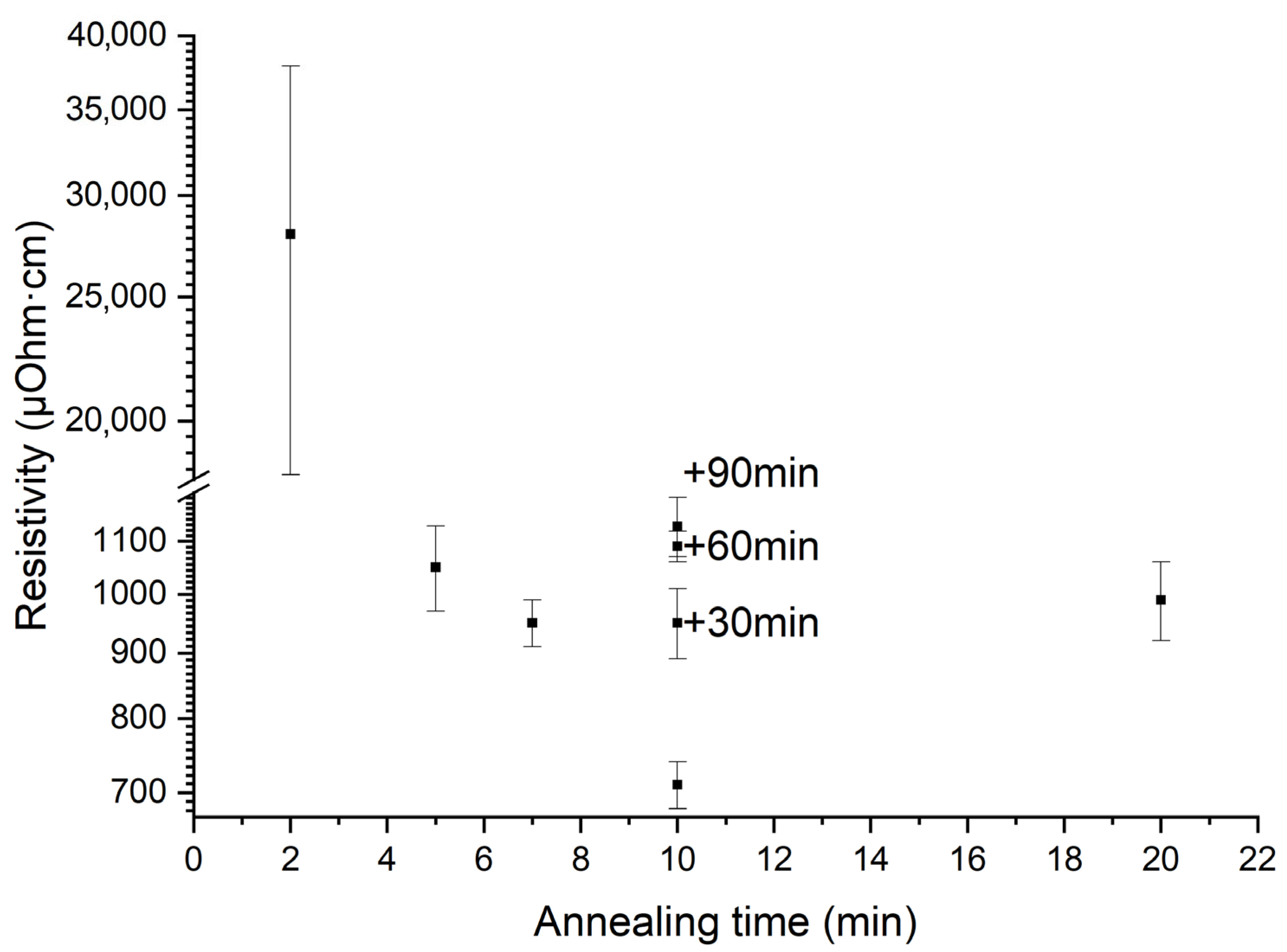


| Sample | Crystallization Time, min | Additional Annealing, min | Grain Structure | Main Phase | Resistivity, μOhm·cm |
|---|---|---|---|---|---|
| LNO-cryst-2 min | 2 | no | Equiaxed grains of 6–22 nm and amorphous hillocks. Pores are located between equiaxed grains. | ) | 18,000–30,000 |
| LNO-cryst-5 min | 5 | no | Equiaxed grains of 7–19 nm, columnar grains of 25–63 nm in width, germinated partially or over the entire width of the film. The pores are located mainly between equiaxed grains, as well as at the LNO-SiO2 boundary. | ) | 970–1130 |
| LNO-cryst-7 min | 7 | no | Predominantly columnar grains of 28–48 nm in width, as well as a small amount of equiaxed grains of 8–18 nm. Pores in the bulk of the film and at the LNO-SiO2 interface. | ) | 900–990 |
| LNO-cryst-10 min | 10 | no | Columnar grains of 22–38 nm in width in all areas, except of hillocks. Agglomeration of pores locally destroys columnar structure (top part of the film). | ) | 680–730 |
| LNO-cryst-20 min | 20 | no | Columnar grains of 21–43 nm in width. Agglomeration of pores locally destroys columnar structure (bottom part of the film). | ) | 920–1100 |
| LNO-anneal-(30–90) min | 10 | 30–90 | Close to ideal columnar grains of 18–72 nm in size. Inclusions of some single pores and chain of pores. | ) | 890–1010, 1060–1120, 1070–1190 at 30, 60, and 90 min. annealing, respectively |
| Structure | P(E) | ε(E) | ||||||
|---|---|---|---|---|---|---|---|---|
| E(−), kV/cm | Pr(−), µC/cm2 | E(+), kV/cm | Pr(+), µC/cm2 | E(−), kV/cm | ε max(−) | E(+), kV/cm | ε max(+) | |
| PZT-Pt | −84.4 | −25.2 | 68.4 | 27.2 | −54 | 656 | 30 | 595 |
| PZT-LNO | −65 | −25 | 65 | 25.4 | −42 | 668 | 30 | 669 |
| Experimental Group | Sample Designation | Preparation Description |
|---|---|---|
| LNO-SiO2(500 nm)-Si Film with variation of annealing time. | LNO-cryst-2 min | Each of the seven LNO layers was soft-baked at 200 °C, 4 min and annealed at T = 650 °C for 2, 5, 7, 10, or 20 min. |
| LNO-cryst-5 min | ||
| LNO-cryst-7 min | ||
| LNO-cryst-10 min | ||
| LNO-cryst-20 min | ||
| LNO-SiO2(10 nm)-Si Film with additional annealing after deposition of all layers. | LNO-anneal-30 min | Each of the seven layers of LNO was soft-baked at 200 °C, 4 min and annealed at 650 °C, 10 min. Then, the sample was divided into three parts and each one was additionally annealed at 650 °C for 30, 60, and 90 min, respectively. |
| LNO-anneal-60 min | ||
| LNO-anneal-90 min | ||
| PZT-LNO-SiO2(500 nm)-Si Ferroelectric film with bottom conducting LNO electrode. | PZT-LNO-SiO2-Si | Each of the seven layers of LNO was soft-baked at 200 °C, 4 min and annealed at 650 °C, 10 min, except of the last layer annealed for 30 min. After that, 10 layers of PZT film were deposited. Each layer was dried at 200 °C, 4 min and at 400 °C, 10 min. After deposition of every fifth layer, the film was annealed at 650 °C, 10 min. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanova, A.V.; Seregin, D.S.; Zhigalina, O.M.; Khmelenin, D.N.; Orlov, G.A.; Turkina, D.I.; Sigov, A.S.; Vorotilov, K.A. Control of Columnar Grain Microstructure in CSD LaNiO3 Films. Molecules 2023, 28, 1938. https://doi.org/10.3390/molecules28041938
Atanova AV, Seregin DS, Zhigalina OM, Khmelenin DN, Orlov GA, Turkina DI, Sigov AS, Vorotilov KA. Control of Columnar Grain Microstructure in CSD LaNiO3 Films. Molecules. 2023; 28(4):1938. https://doi.org/10.3390/molecules28041938
Chicago/Turabian StyleAtanova, Aleksandra V., Dmitry S. Seregin, Olga M. Zhigalina, Dmitry N. Khmelenin, Georgy A. Orlov, Daria I. Turkina, Alexander S. Sigov, and Konstantin A. Vorotilov. 2023. "Control of Columnar Grain Microstructure in CSD LaNiO3 Films" Molecules 28, no. 4: 1938. https://doi.org/10.3390/molecules28041938
APA StyleAtanova, A. V., Seregin, D. S., Zhigalina, O. M., Khmelenin, D. N., Orlov, G. A., Turkina, D. I., Sigov, A. S., & Vorotilov, K. A. (2023). Control of Columnar Grain Microstructure in CSD LaNiO3 Films. Molecules, 28(4), 1938. https://doi.org/10.3390/molecules28041938






