A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates
Abstract
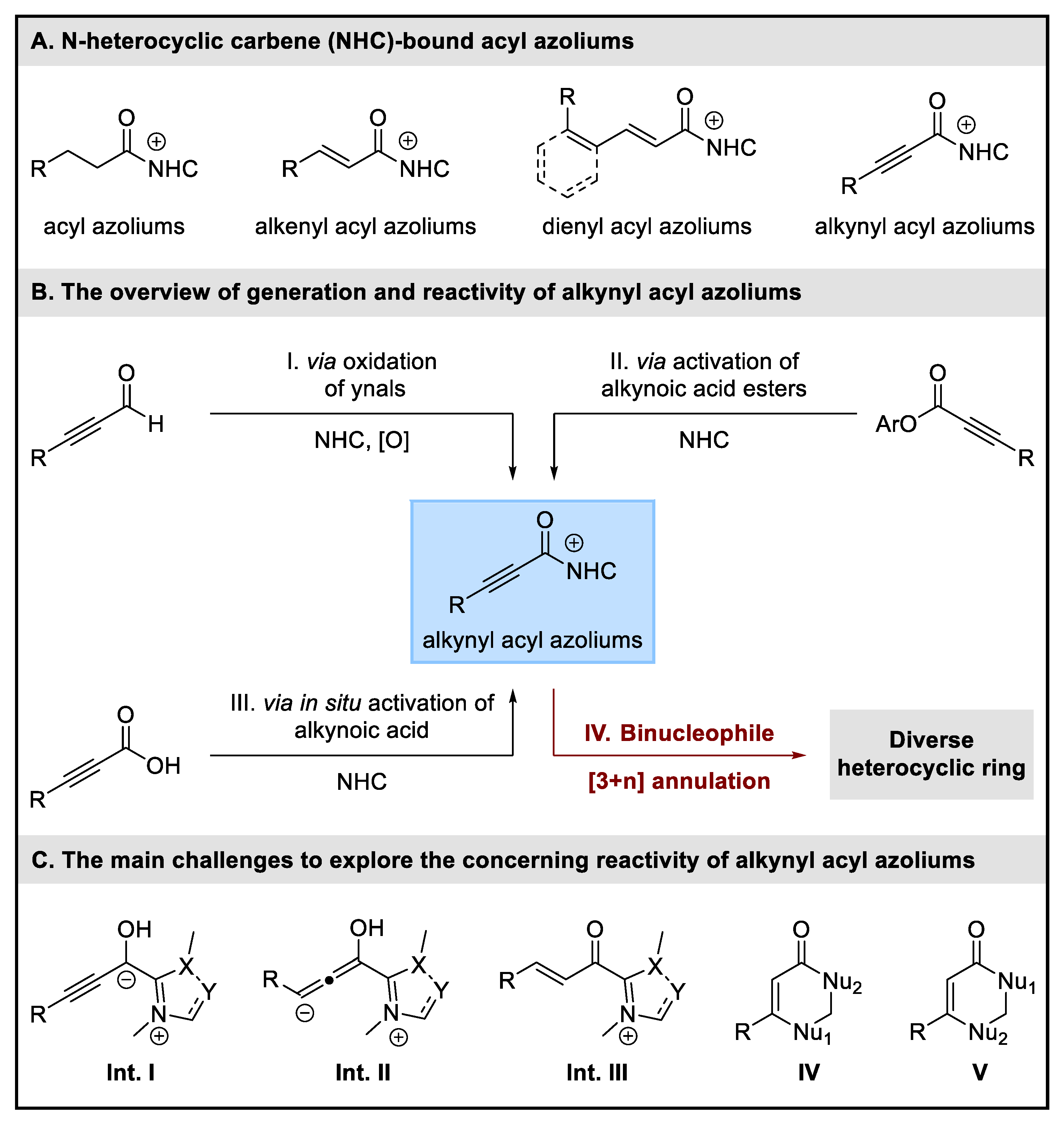
1. Introduction
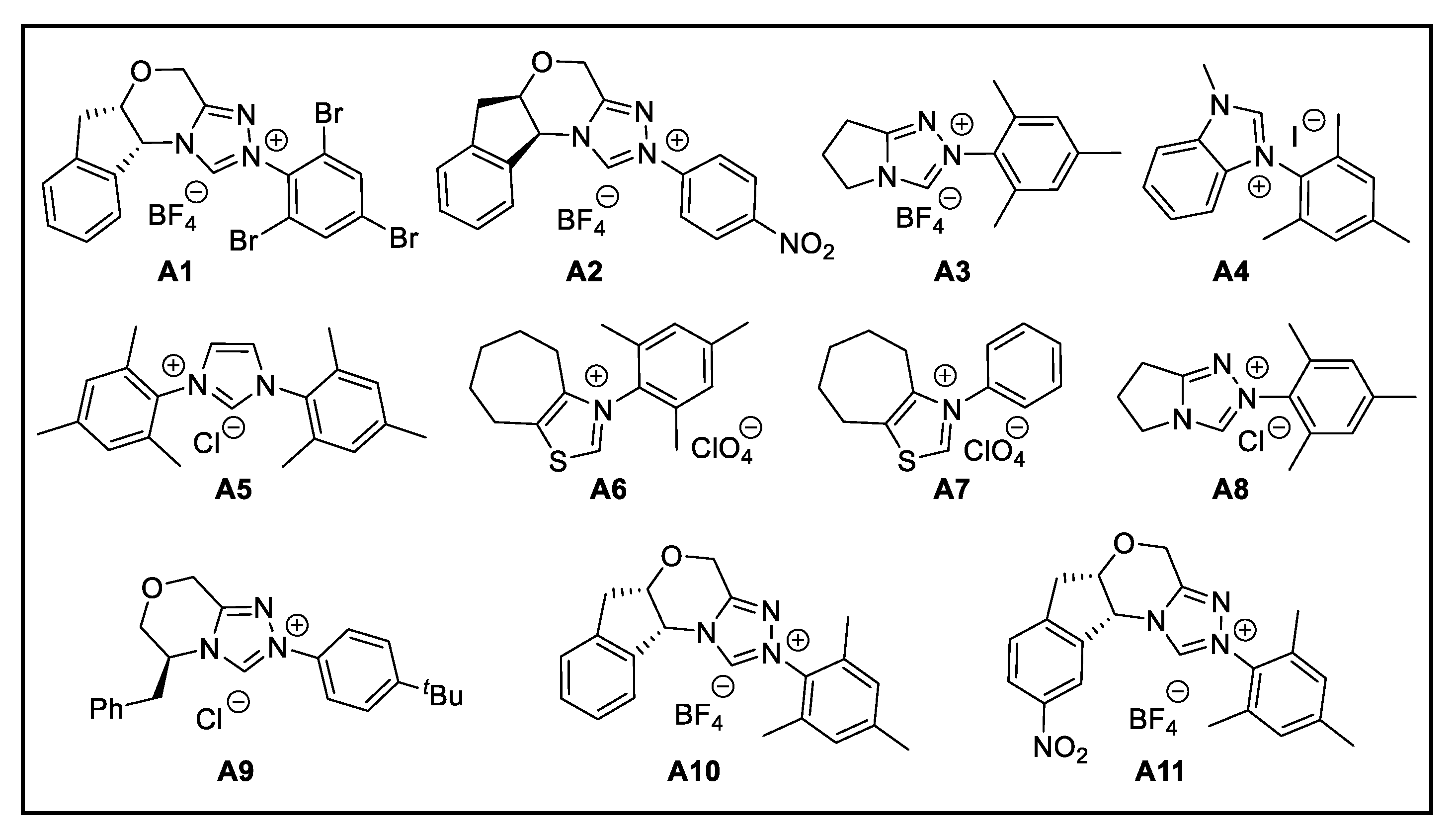
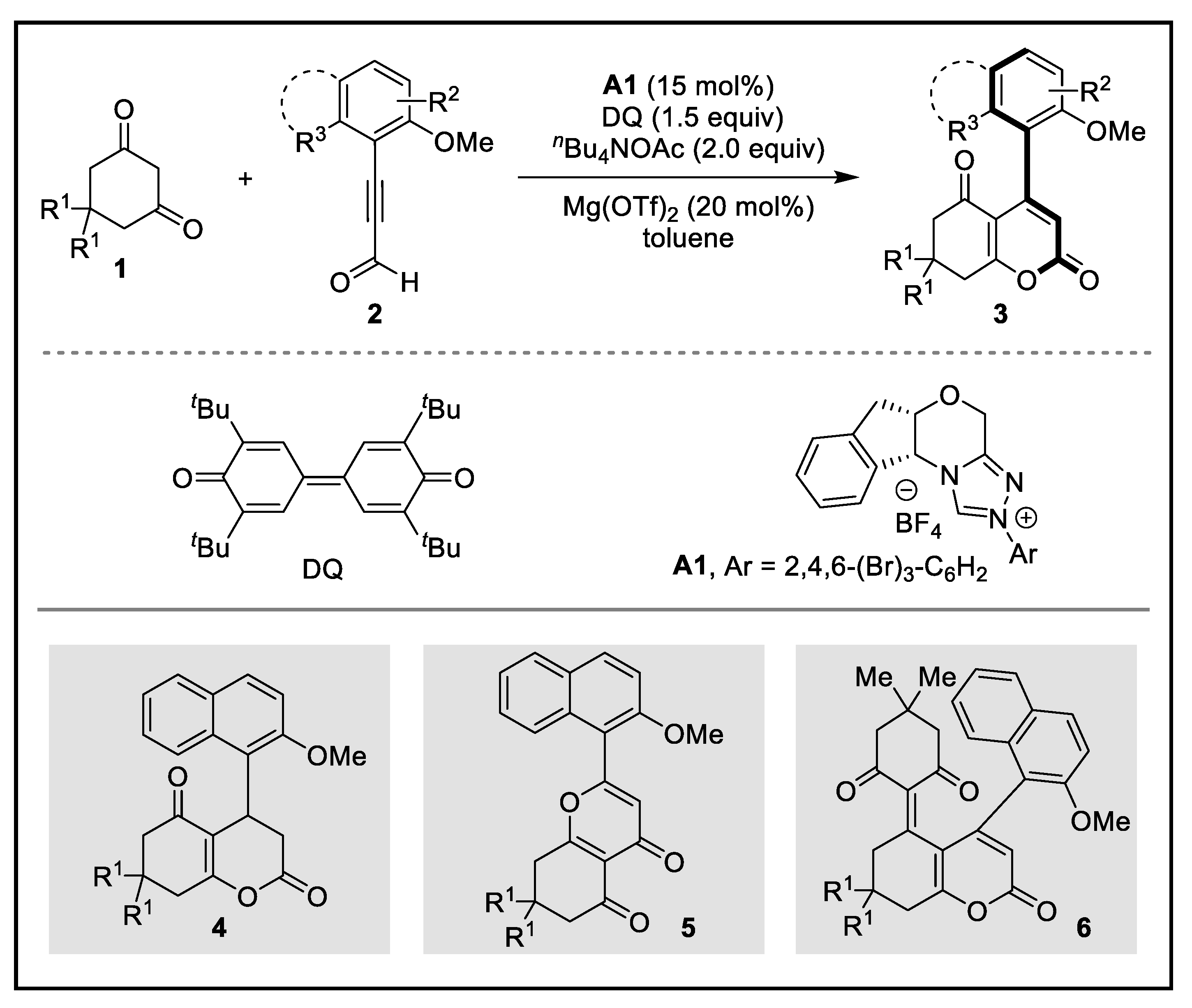
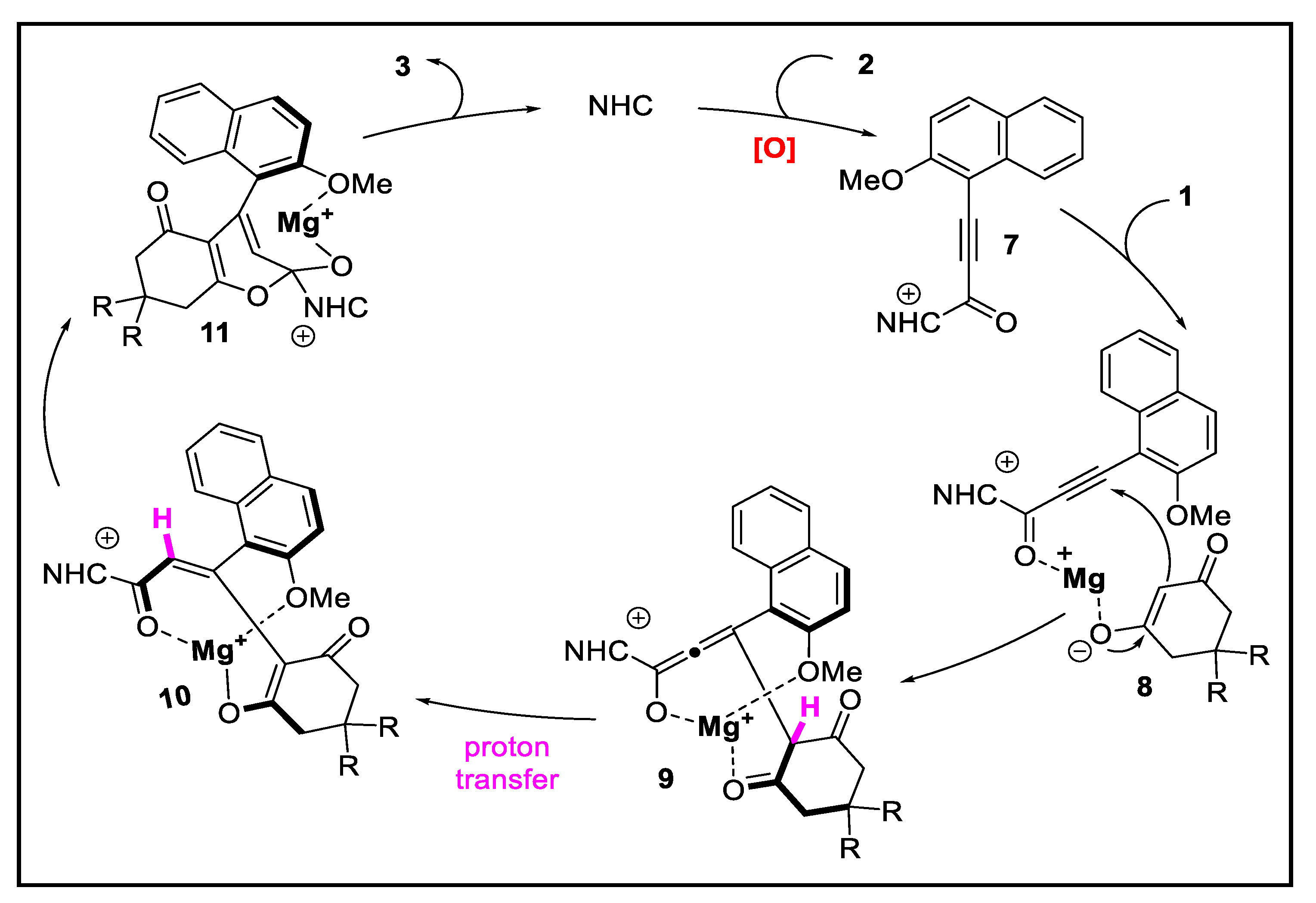
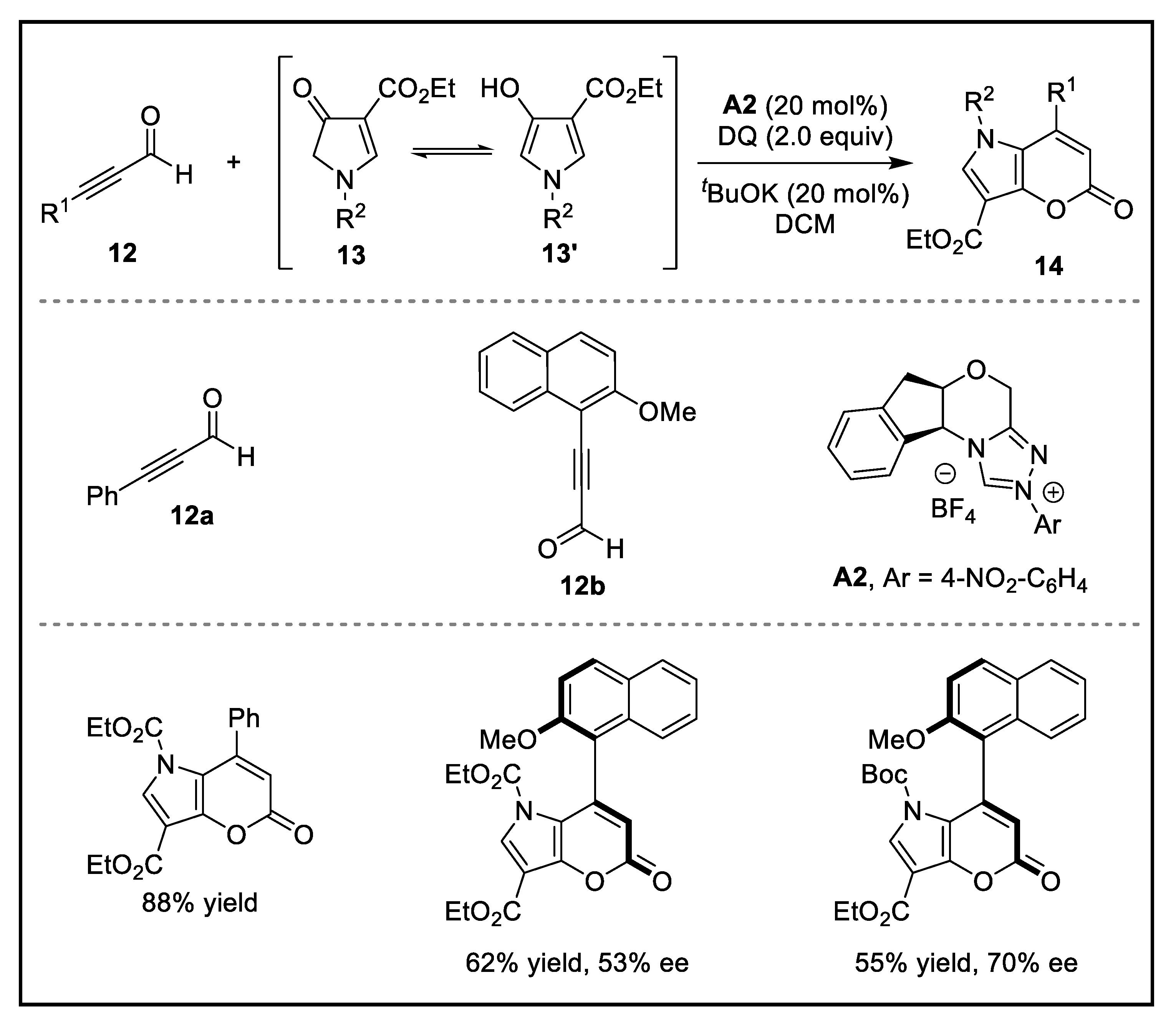
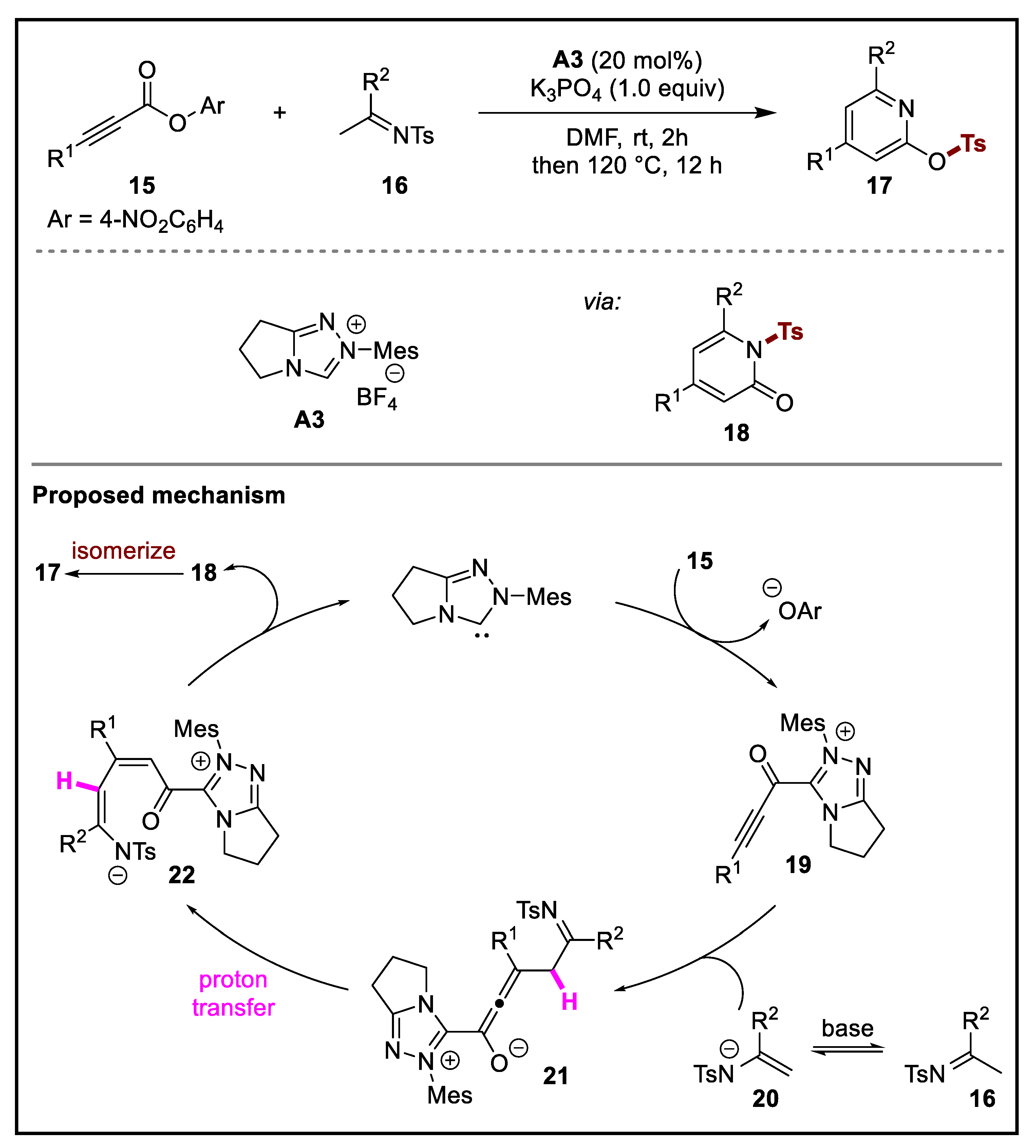
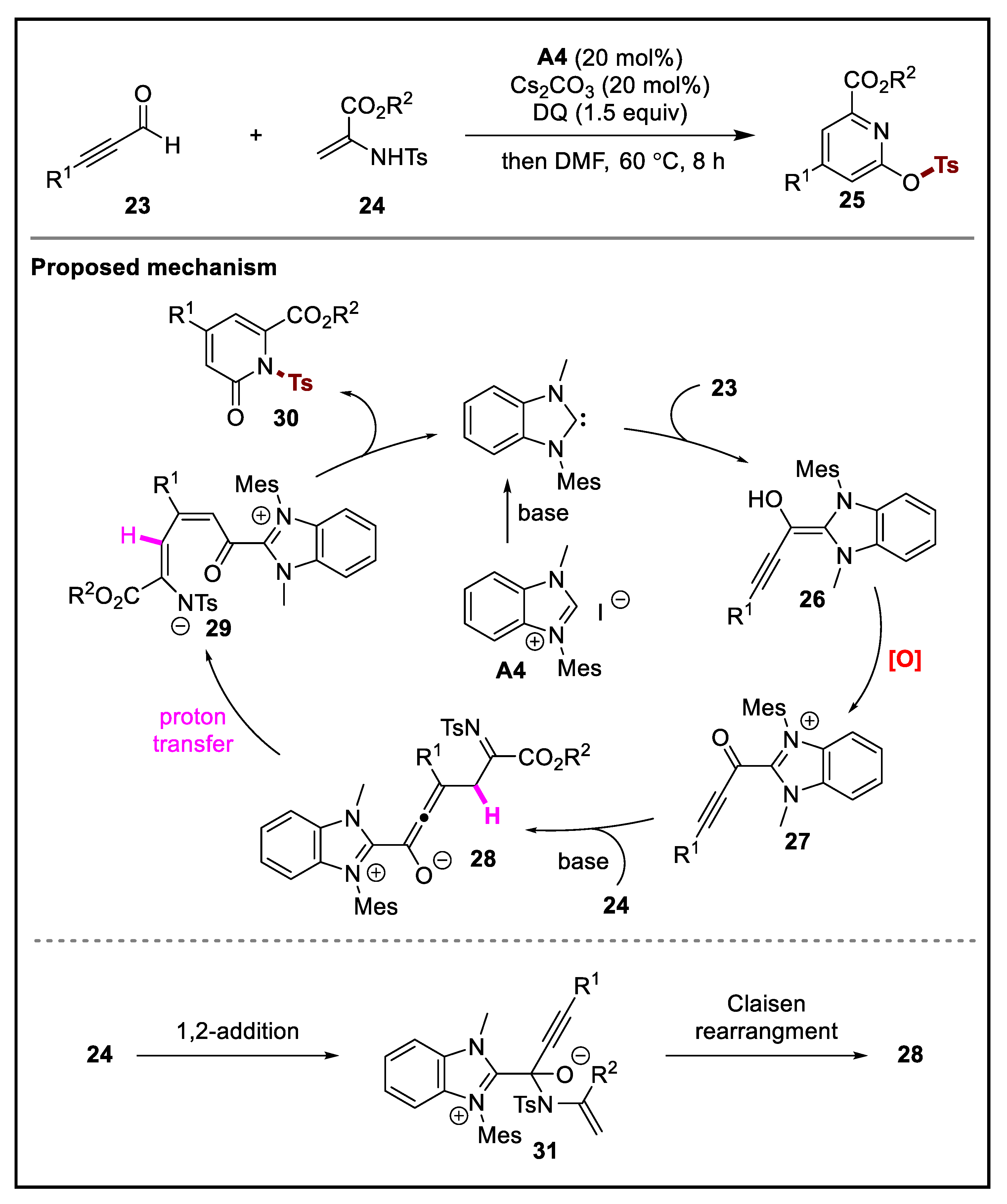
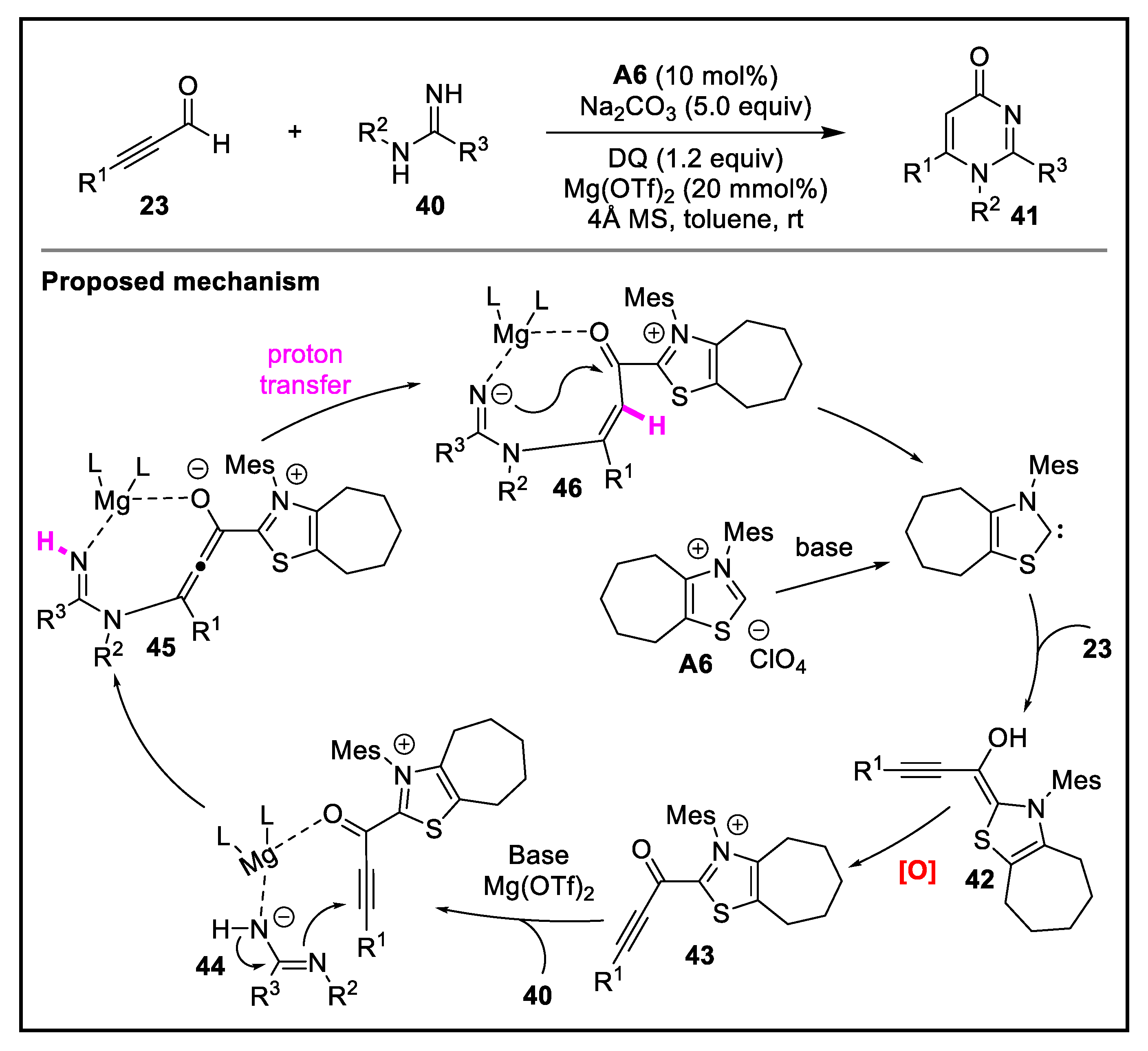
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ukai, T.; Tanaka, R.; Dokawa, T. A new catalyst for acyloin condensation. J. Pharm. Soc. Jpn. 1943, 63, 296–300. [Google Scholar]
- Breslow, R. On the mechanism of thiamine action. Iv.1 evidence from studies on model systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-heterocyclic carbene complexes in C–H activation reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.-S.; Szostak, M. Bian-NHC ligands in transition-metal-catalysis: A perfect union of sterically encumbered, electronically tunable N-heterocyclic carbenes? Chem. Eur. J. 2021, 27, 4478–4499. [Google Scholar] [CrossRef] [PubMed]
- Nahra, F.; Cazin, C.S.J. Sustainability in Ru- and Pd-based catalytic systems using N-heterocyclic carbenes as ligands. Chem. Soc. Rev. 2021, 50, 3094–3142. [Google Scholar] [CrossRef] [PubMed]
- Voloshkin, V.A.; Tzouras, N.V.; Nolan, S.P. Recent advances in the synthesis and derivatization of N-heterocyclic carbene metal complexes. Dalton Trans. 2021, 50, 12058–12068. [Google Scholar] [CrossRef] [PubMed]
- Neshat, A.; Mastrorilli, P.; Mousavizadeh Mobarakeh, A. Recent advances in catalysis involving bidentate N-heterocyclic carbene ligands. Molecules 2022, 27, 95. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-heterocyclic carbene complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Wang, J. Recent progress toward the construction of axially chiral molecules catalyzed by an N-heterocyclic carbene. ACS Catal. 2021, 11, 12520–12531. [Google Scholar] [CrossRef]
- Ohmiya, H. N-heterocyclic carbene-based catalysis enabling cross-coupling reactions. ACS Catal. 2020, 10, 6862–6869. [Google Scholar] [CrossRef]
- Pareek, M.; Reddi, Y.; Sunoj, R.B. Tale of the breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: Then and now. Chem. Sci. 2021, 12, 7973–7992. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ghosh, A.; Biju, A.T. N-heterocyclic carbene (NHC)-catalyzed transformations involving azolium enolates. Chem. Rec. 2022, 22, e202200054. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon–carbon bond-forming reactions. Chem. Soc. Rev. 2015, 44, 5040–5052. [Google Scholar] [CrossRef]
- Gao, J.; Feng, J.; Du, D. Generation of azolium dienolates as versatile nucleophilic synthons via N-heterocyclic carbene catalysis. Org. Chem. Front. 2021, 8, 6138–6166. [Google Scholar] [CrossRef]
- Chen, K.-Q.; Sheng, H.; Liu, Q.; Shao, P.-L.; Chen, X.-Y. N-heterocyclic carbene-catalyzed radical reactions. Sci. China Chem. 2021, 64, 7–16. [Google Scholar] [CrossRef]
- Mahatthananchai, J.; Bode, J.W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 2014, 47, 696–707. [Google Scholar] [CrossRef]
- Zhang, C.; Hooper, J.F.; Lupton, D.W. N-heterocyclic carbene catalysis via the α,β-unsaturated acyl azolium. ACS Catal. 2017, 7, 2583–2596. [Google Scholar] [CrossRef]
- Mondal, S.; Yetra, S.R.; Mukherjee, S.; Biju, A.T. NHC-catalyzed generation of α,β-unsaturated acylazoliums for the enantioselective synthesis of heterocycles and carbocycles. Acc. Chem. Res. 2019, 52, 425–436. [Google Scholar] [CrossRef]
- Zhao, C.; Blaszczyk, S.A.; Wang, J. Asymmetric reactions of N-heterocyclic carbene (NHC)-based chiral acyl azoliums and azolium enolates. Green Syn. Catal. 2021, 2, 198–215. [Google Scholar] [CrossRef]
- Zhu, T.; Mou, C.; Li, B.; Smetankova, M.; Song, B.-A.; Chi, Y.R. N-heterocyclic carbene-catalyzed δ-carbon lumo activation of unsaturated aldehydes. J. Am. Chem. Soc. 2015, 137, 5658–5661. [Google Scholar] [CrossRef] [PubMed]
- Gillard, R.M.; Fernando, J.E.M.; Lupton, D.W. Enantioselective N-heterocyclic carbene catalysis via the dienyl acyl azolium. Angew. Chem. Int. Ed. 2018, 57, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, W.; Zhu, S.; Zhu, T. Atroposelective arene formation by carbene-catalyzed formal [4 + 2] cycloaddition. Angew. Chem. Int. Ed. 2019, 58, 17625–17630. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Wu, J.; Huang, Z.; Sun, J.; Jin, Z.; Chi, Y.R. Carbene-catalyzed lumo activation of alkyne esters for access to functional pyridines. Chem. Commun. 2017, 53, 13359–13362. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, K.; Dong, S.; Lu, T.; Dong, Y.; Du, D. Esters as alkynyl acyl ammonium and azolium precursors: A formal [2 + 3] annulation with amidomalonates via Lewis base/Lewis acid cooperative catalysis. Org. Lett. 2017, 19, 6724–6727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, D.; Munkerup, K.; Huang, K.-W.; Li, F.; Wang, J. Enantioselective [3 + 3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat. Commun. 2018, 9, 611. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Zhang, R.; Duan, X.-Y.; Yu, H.-F.; Sun, B.-Y.; Qi, J. Access to dihydropyrano[3,2-b]pyrrol-5-ones skeletons by N-heterocyclic carbene-catalyzed [3 + 3] annulations. Chem. Commun. 2020, 56, 9854–9857. [Google Scholar] [CrossRef]
- Li, J.-H.; Duan, X.-Y.; Tian, Z.-H.; Zheng, Y.-F.; Qi, J. N-heterocyclic carbene-catalyzed activation of ynals for the construction of functional pyridines. Asian J. Org. Chem. 2020, 9, 385–390. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J. N-heterocyclic carbene-catalyzed annulation of ynals with amidines: Access to 1,2,6-trisubstituted pyrimidin-4-ones. Chem. Commun. 2018, 54, 4597–4600. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Gao, Z.-H.; Song, C.-Y.; Zhang, C.-L.; Wang, Z.-X.; Ye, S. N-heterocyclic carbene catalyzed cyclocondensation of α,β-unsaturated carboxylic acids: Enantioselective synthesis of pyrrolidinone and dihydropyridinone derivatives. Angew. Chem. Int. Ed. 2014, 53, 11611–11615. [Google Scholar] [CrossRef]
- Lee, A.; Younai, A.; Price, C.K.; Izquierdo, J.; Mishra, R.K.; Scheidt, K.A. Enantioselective annulations for dihydroquinolones by in situ generation of azolium enolates. J. Am. Chem. Soc. 2014, 136, 10589–10592. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Jiang, K.; Fu, Z.; Torres, J.; Zheng, P.; Yang, S.; Song, B.-A.; Chi, Y.R. Nucleophilic β-carbon activation of propionic acid as a 3-carbon synthon by carbene organocatalysis. Chem. Eur. J. 2015, 21, 9360–9363. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.-Q.; Zhang, H.-M.; Zhang, C.-L.; Gao, Z.-H.; Ye, S. N-heterocyclic carbene-catalyzed [4 + 2] annulation of α,β-unsaturated carboxylic acids: Enantioselective synthesis of dihydropyridinones and spirocyclic oxindolodihydropyridinones. Org. Chem. Front. 2016, 3, 77–81. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, C.; Li, T.; Wang, Y.; Lu, Y.; Wang, W.; Yao, C. N-heterocyclic carbene-catalyzed [4 + 2] cyclization of α,β-unsaturated carboxylic acids bearing γ-H with isatins: An enantioselective synthesis of spirocyclic oxindole–dihydropyranones. Org. Biomol. Chem. 2016, 14, 1485–1491. [Google Scholar] [CrossRef]
- Mondal, S.; Mukherjee, S.; Das, T.K.; Gonnade, R.; Biju, A.T. N-heterocyclic carbene-catalyzed aldol-lactonization of ketoacids via dynamic kinetic resolution. ACS Catal. 2017, 7, 3995–3999. [Google Scholar] [CrossRef]
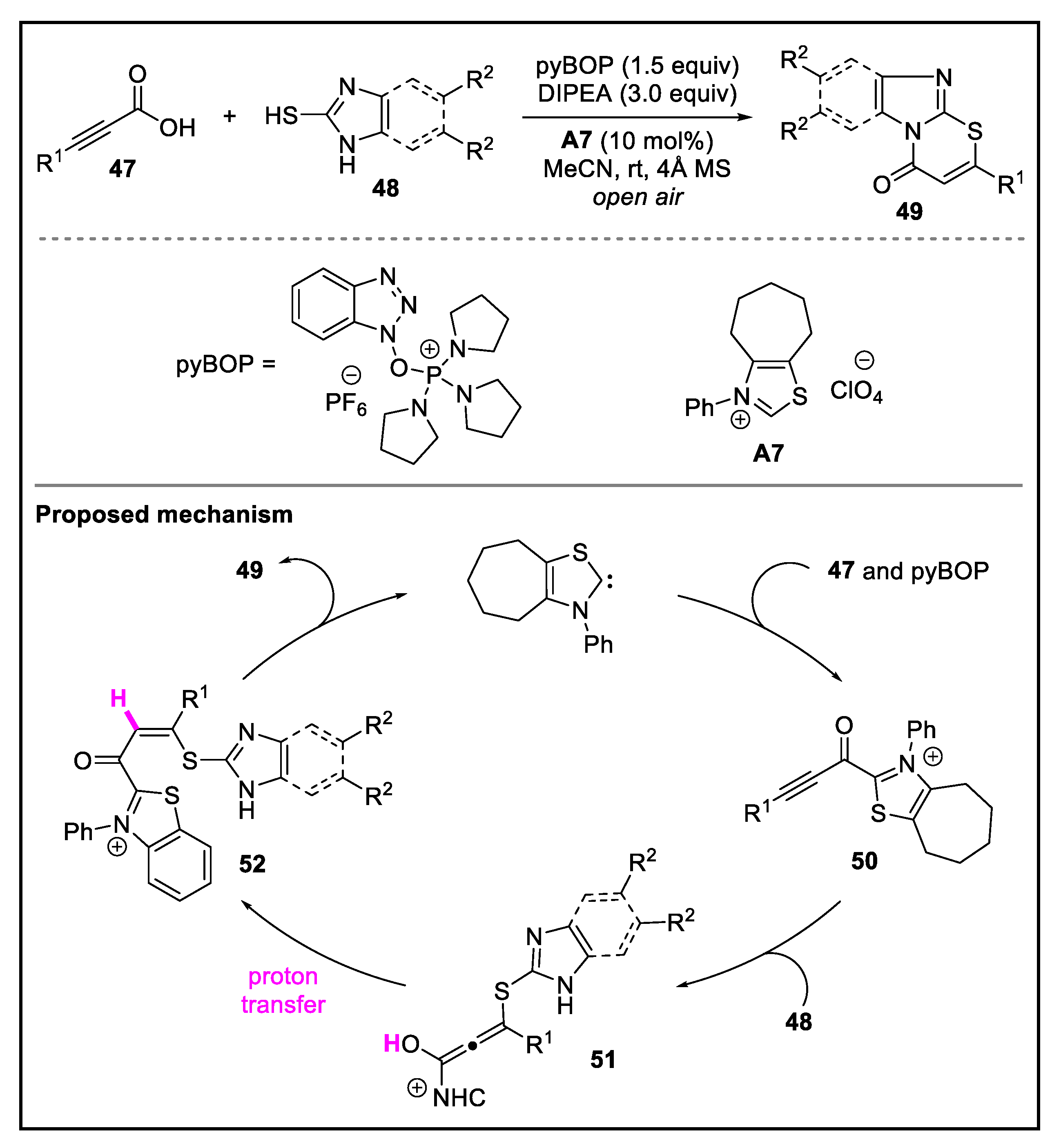
- Sun, K.; Jin, S.; Zhu, J.; Zhang, X.; Gao, M.; Zhang, W.; Lu, T.; Du, D. N-heterocyclic carbene-catalyzed in situ activation of alkynyl acids for C−S bond formation: Access to imidazo[2,1-b][1,3]thiazinones. Adv. Syn. Catal. 2018, 360, 4515–4522. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, W.; Zhang, Y.; Du, D.; Lu, T. N-heterocyclic carbene-catalyzed annulations of enals and ynals with indolin-3-ones: Synthesis of 3,4-dihydropyrano[3,2-b]indol-2-ones. Adv. Syn. Catal. 2013, 355, 321–326. [Google Scholar]
- Ni, Q.; Song, X.; Raabe, G.; Enders, D. N-heterocyclic carbene-catalyzed enantioselective annulation of indolin-3-ones with bromoenals. Chem. Asian J. 2014, 9, 1535–1538. [Google Scholar] [CrossRef]
- Sun, K.; Jin, S.; Fang, S.; Ma, R.; Zhang, X.; Gao, M.; Zhang, W.; Lu, T.; Du, D. N-heterocyclic carbene-catalyzed formal [3 + 3] annulation of alkynoic acid esters with indolin-3-ones: Access to functionalized pyrano[3,2-b]indol-2-ones. Org. Chem. Front. 2019, 6, 2291–2295. [Google Scholar] [CrossRef]
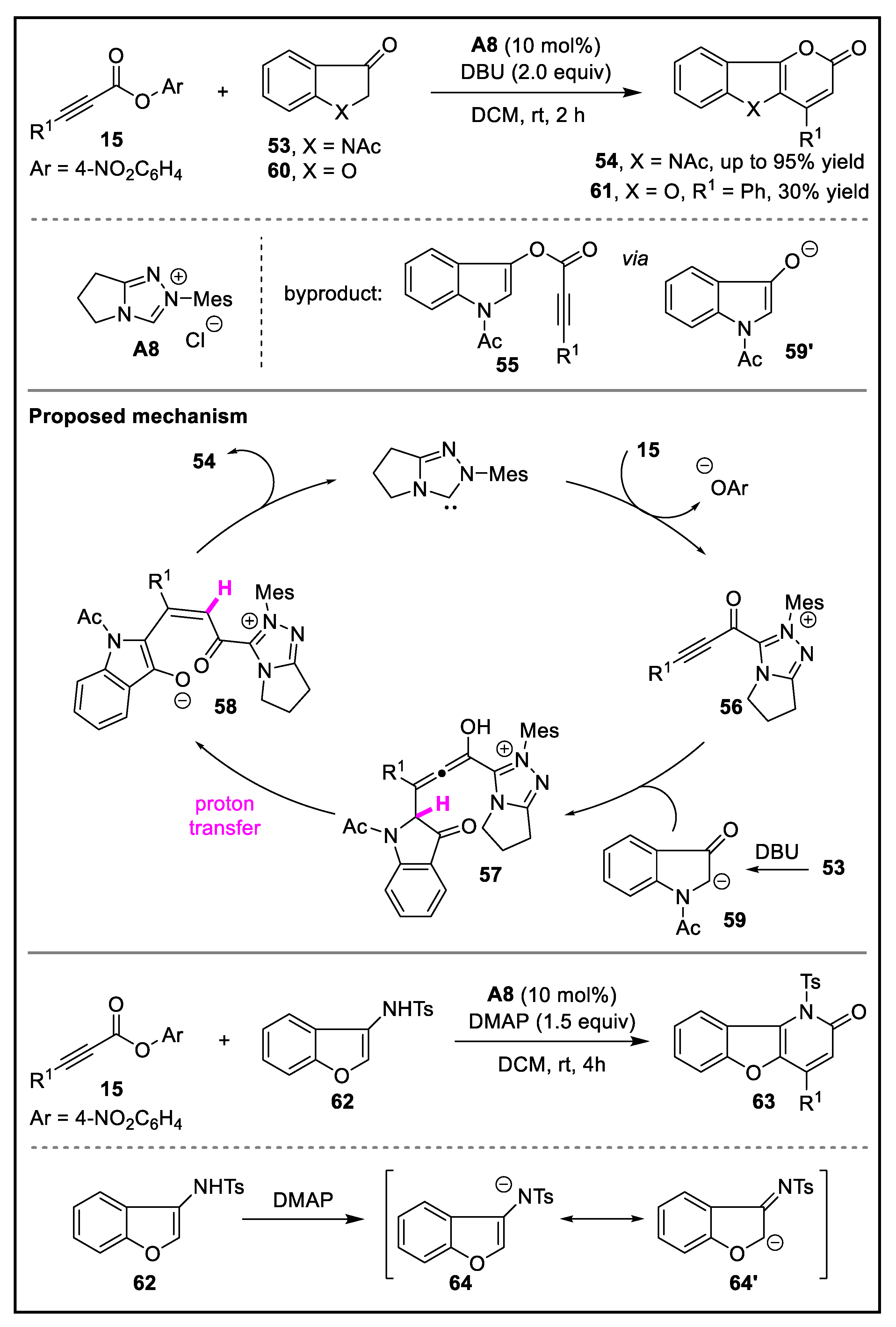
- Wang, X.; Shao, Y.; Zhang, S.; Lu, T.; Du, D. N-heterocyclic carbene-catalyzed formal [3 + 3] annulation of alkynyl acylazoliums for the synthesis of benzofuro[3,2-b]pyridin-2-ones. J. Org. Chem. 2021, 86, 12336–12343. [Google Scholar] [CrossRef]
- Ma, R.; Wang, X.; Zhang, Q.; Chen, L.; Gao, J.; Feng, J.; Wei, D.; Du, D. Atroposelective synthesis of axially chiral 4-aryl α-carbolines via N-heterocyclic carbene catalysis. Org. Lett. 2021, 23, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mou, C.; Qi, P.; Peng, X.; Jiang, S.; Hao, G.; Xue, W.; Yang, S.; Hao, L.; Chi, Y.R.; et al. N-heterocyclic carbene-catalyzed atroposelective annulation for access to thiazine derivatives with C−N axial chirality. Angew. Chem. Int. Ed. 2021, 60, 9362–9367. [Google Scholar] [CrossRef] [PubMed]
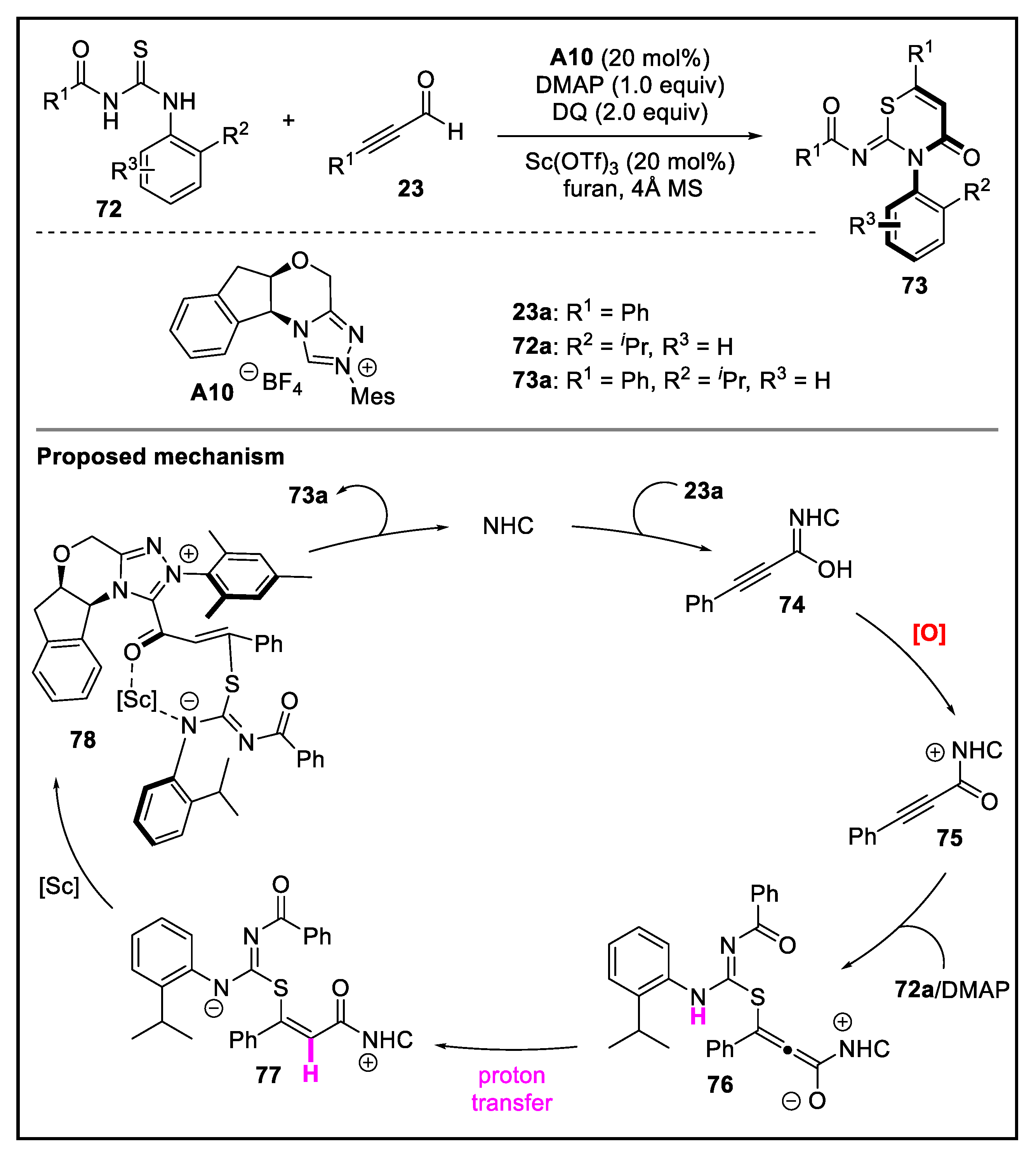
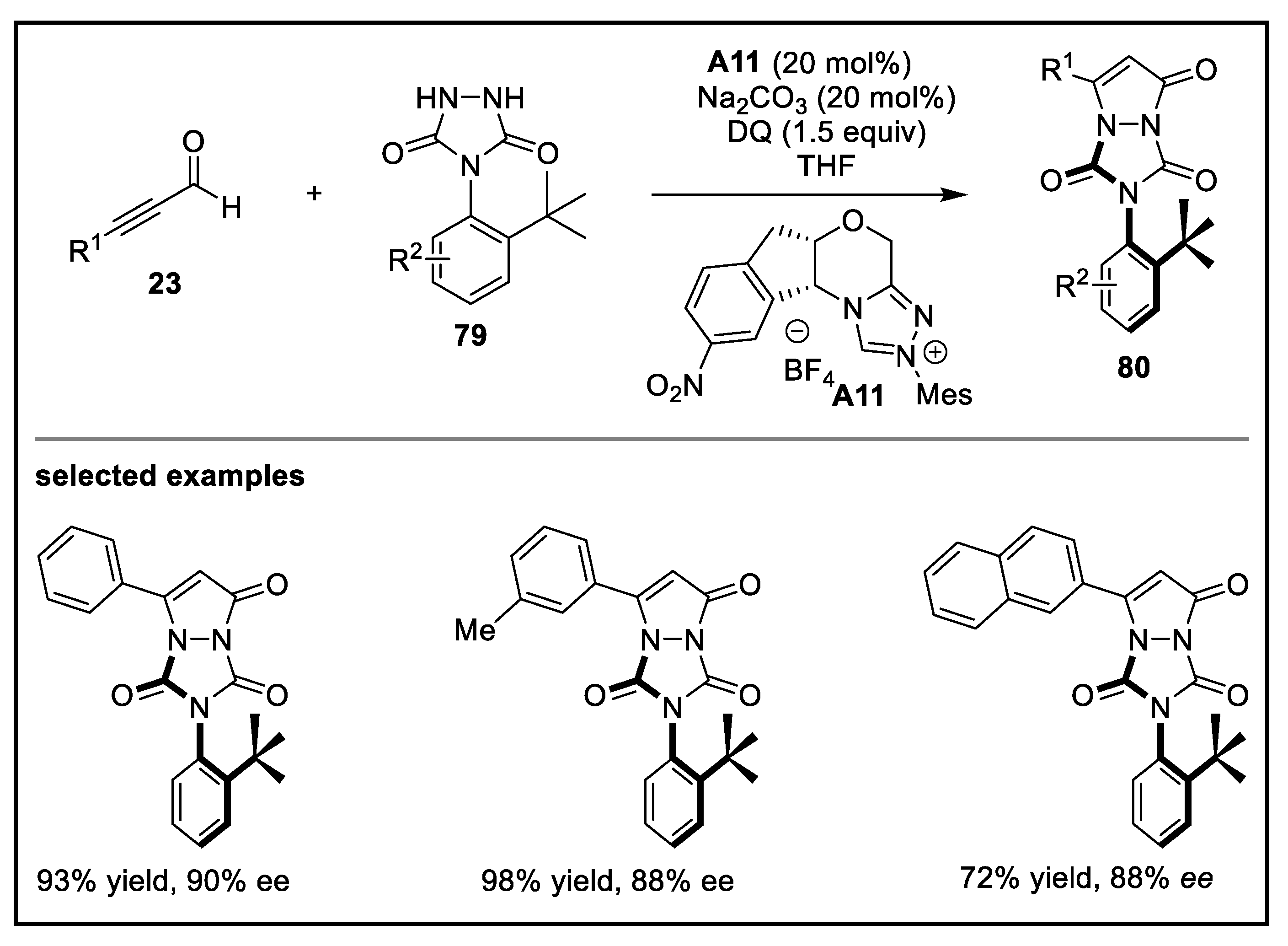
- Jin, J.; Huang, X.; Xu, J.; Li, T.; Peng, X.; Zhu, X.; Zhang, J.; Jin, Z.; Chi, Y.R. Carbene-catalyzed atroposelective annulation and desymmetrization of urazoles. Org. Lett. 2021, 23, 3991–3996. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Jiang, C.; Zhao, C. A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates. Molecules 2022, 27, 7990. https://doi.org/10.3390/molecules27227990
Dong Z, Jiang C, Zhao C. A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates. Molecules. 2022; 27(22):7990. https://doi.org/10.3390/molecules27227990
Chicago/Turabian StyleDong, Ziyang, Chengming Jiang, and Changgui Zhao. 2022. "A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates" Molecules 27, no. 22: 7990. https://doi.org/10.3390/molecules27227990
APA StyleDong, Z., Jiang, C., & Zhao, C. (2022). A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates. Molecules, 27(22), 7990. https://doi.org/10.3390/molecules27227990






