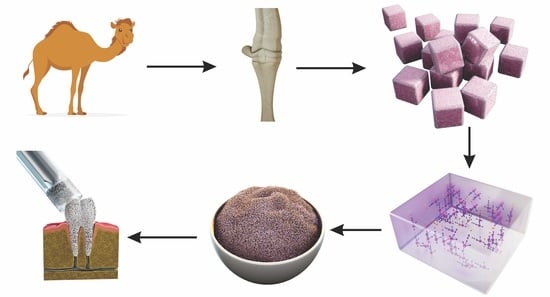
Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Camel Bone Preparation
2.2. Defatting and Deproteinization
2.3. Characterization
2.3.1. Fourier-Transform Infra-Red Spectroscopy (FTIR)
2.3.2. Micro-Raman Analysis
2.3.3. XRD Analysis
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Inductively Coupled Plasma Mass Spectrometry (ICPMS)
2.3.6. Micro CT Analysis
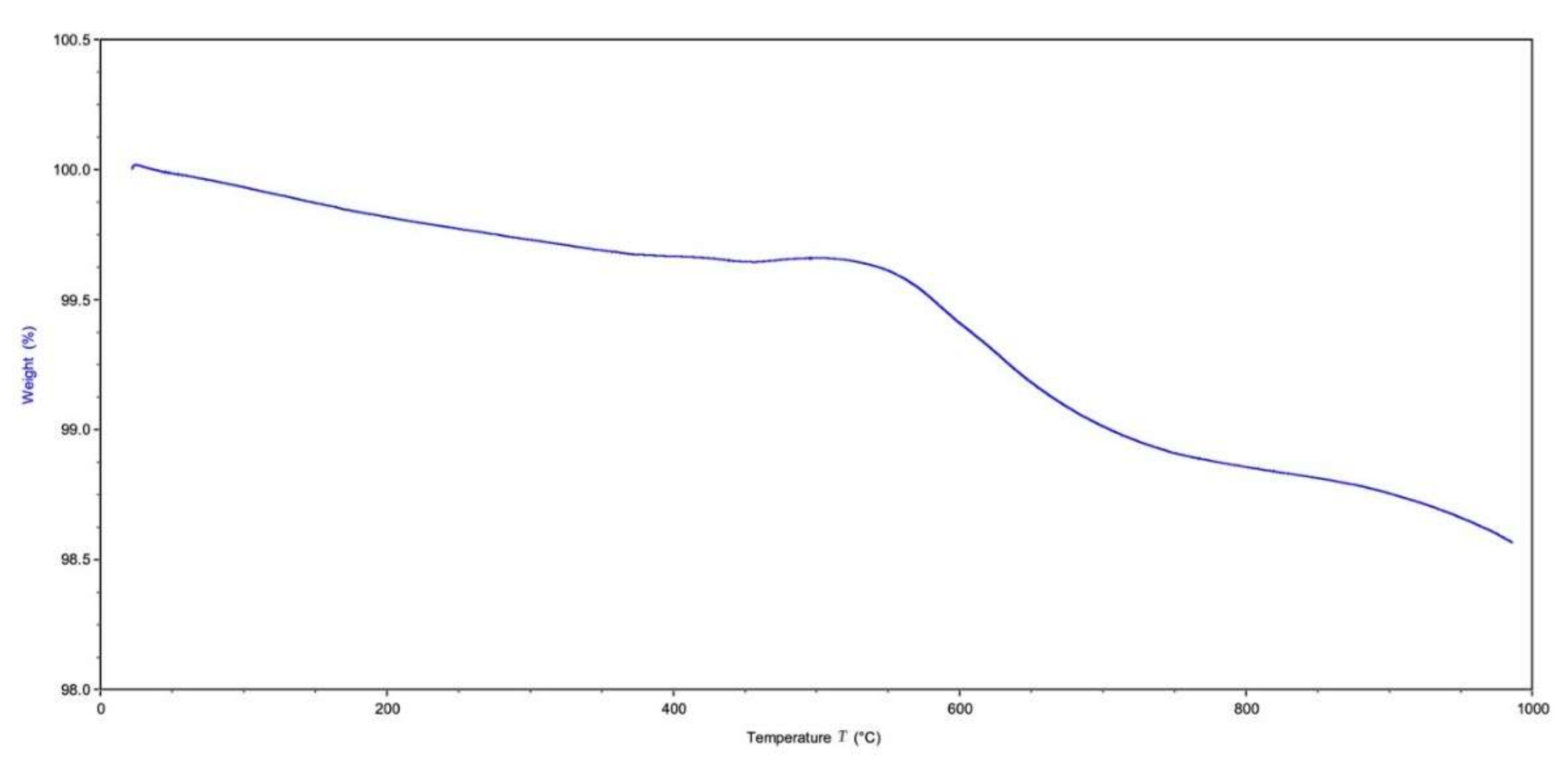
2.3.7. Thermogravimetric Analysis (TGA)
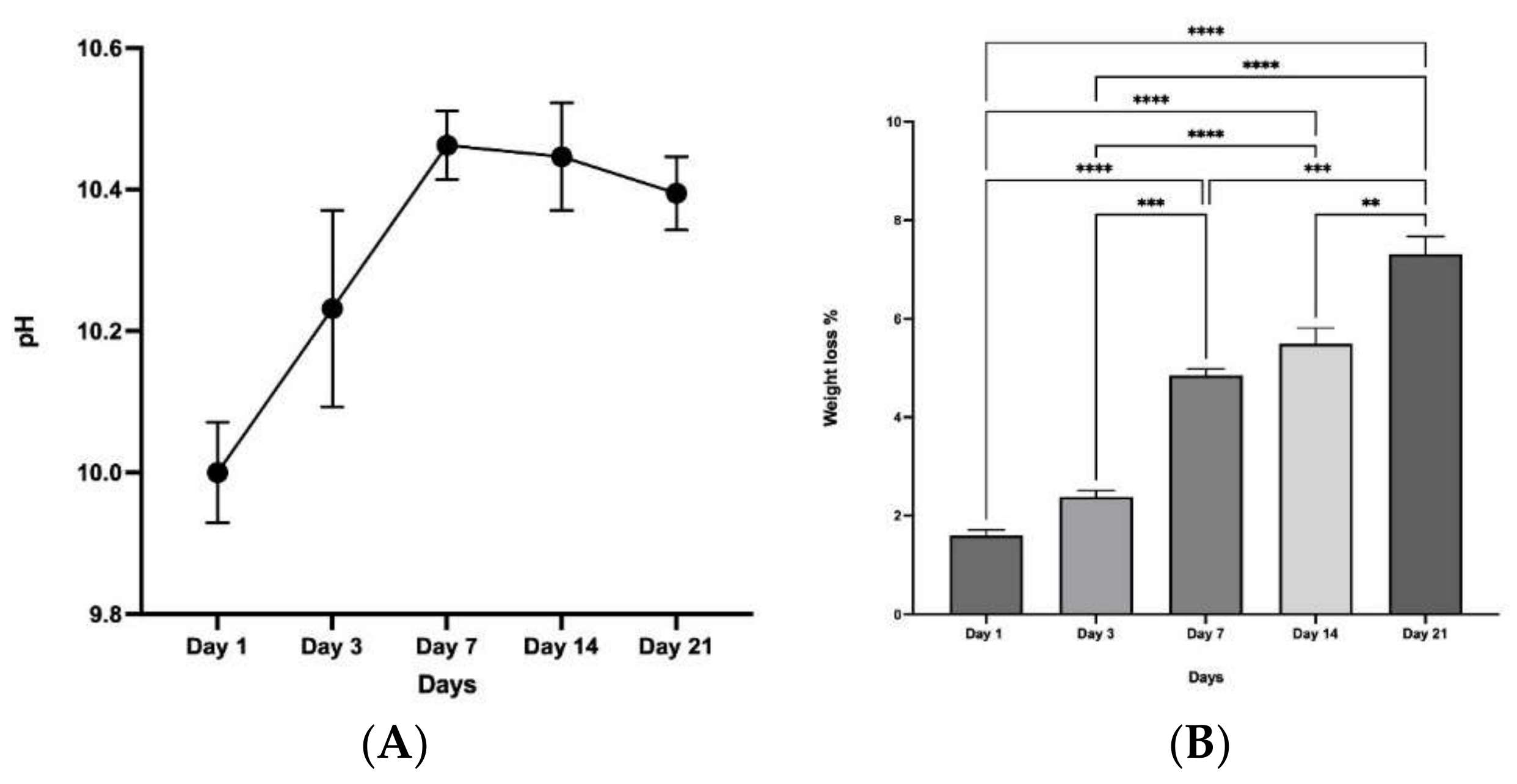
2.3.8. Chemical Stability and Biodegradation in Simulated Body Fluid (SBF)
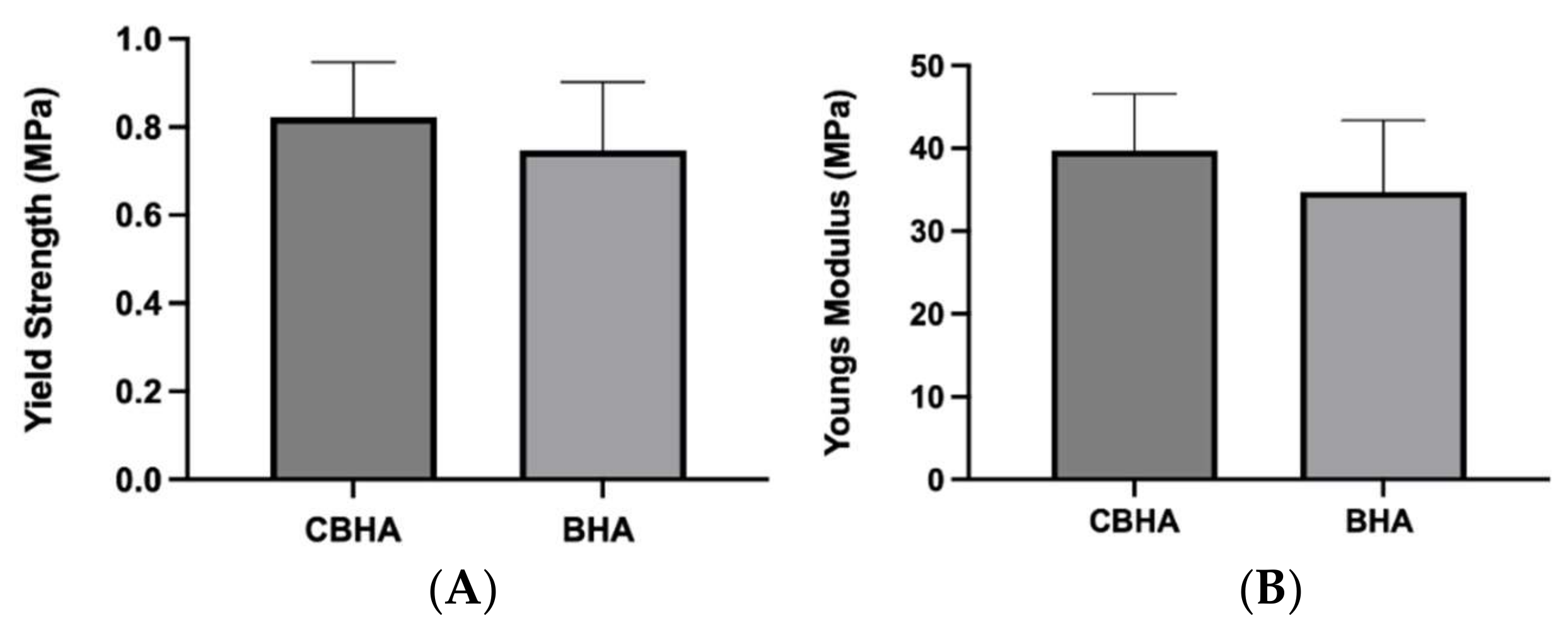
2.3.9. Mechanical Properties
3. Results
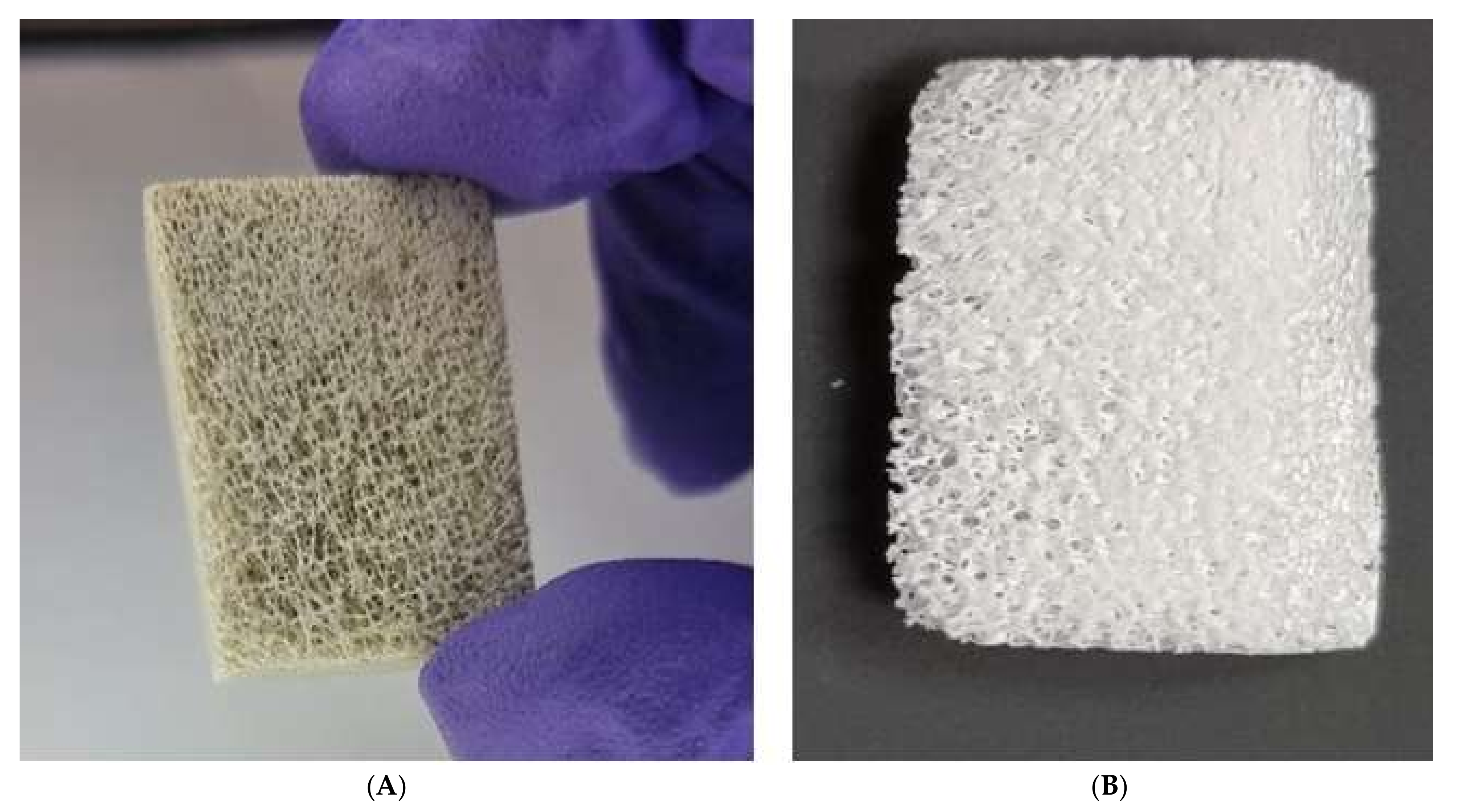
3.1. Bone Processing
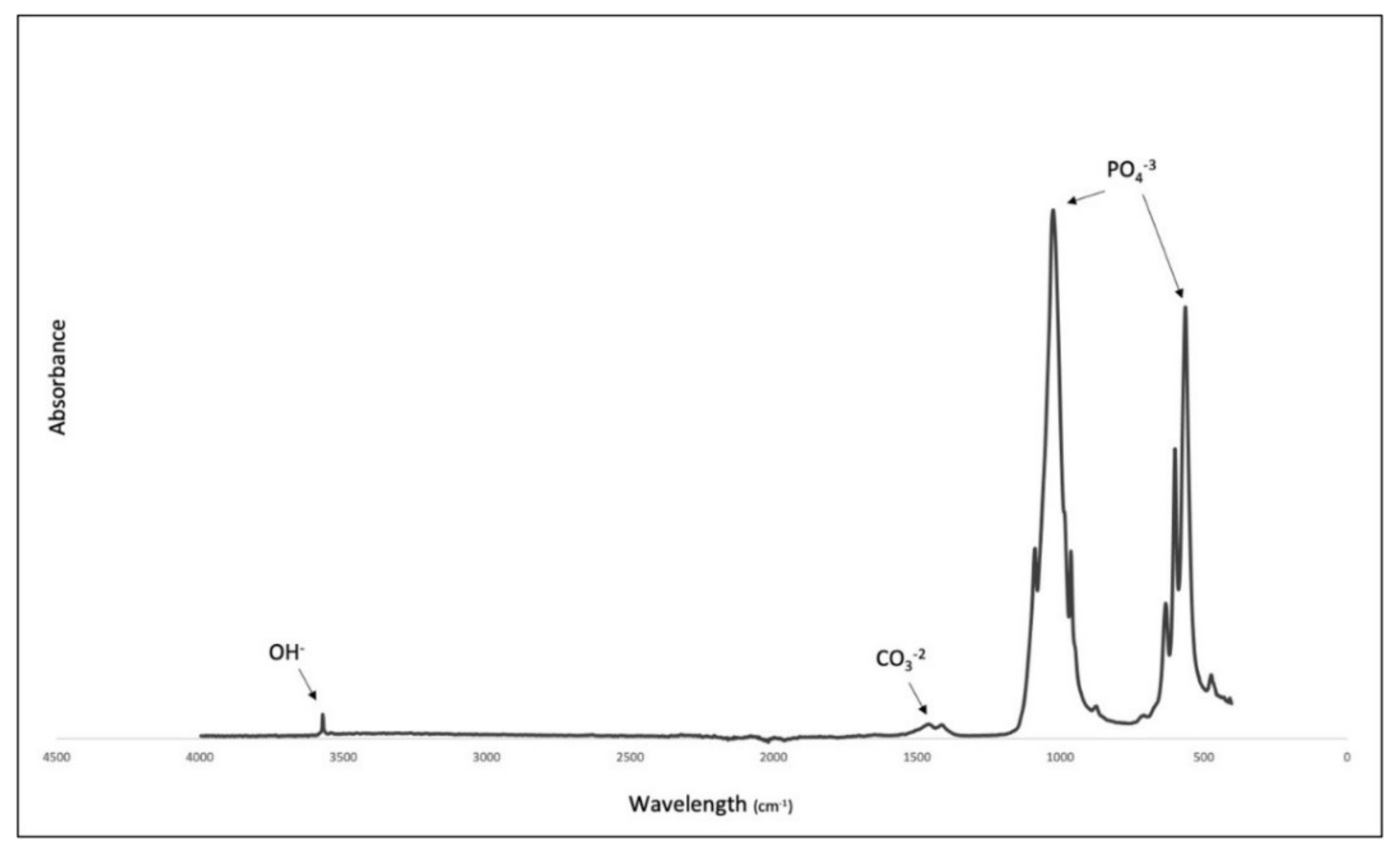
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. X-ray Diffraction (XRD)
3.4. Chemical Compositional Analysis by Micro-Raman Spectroscopy
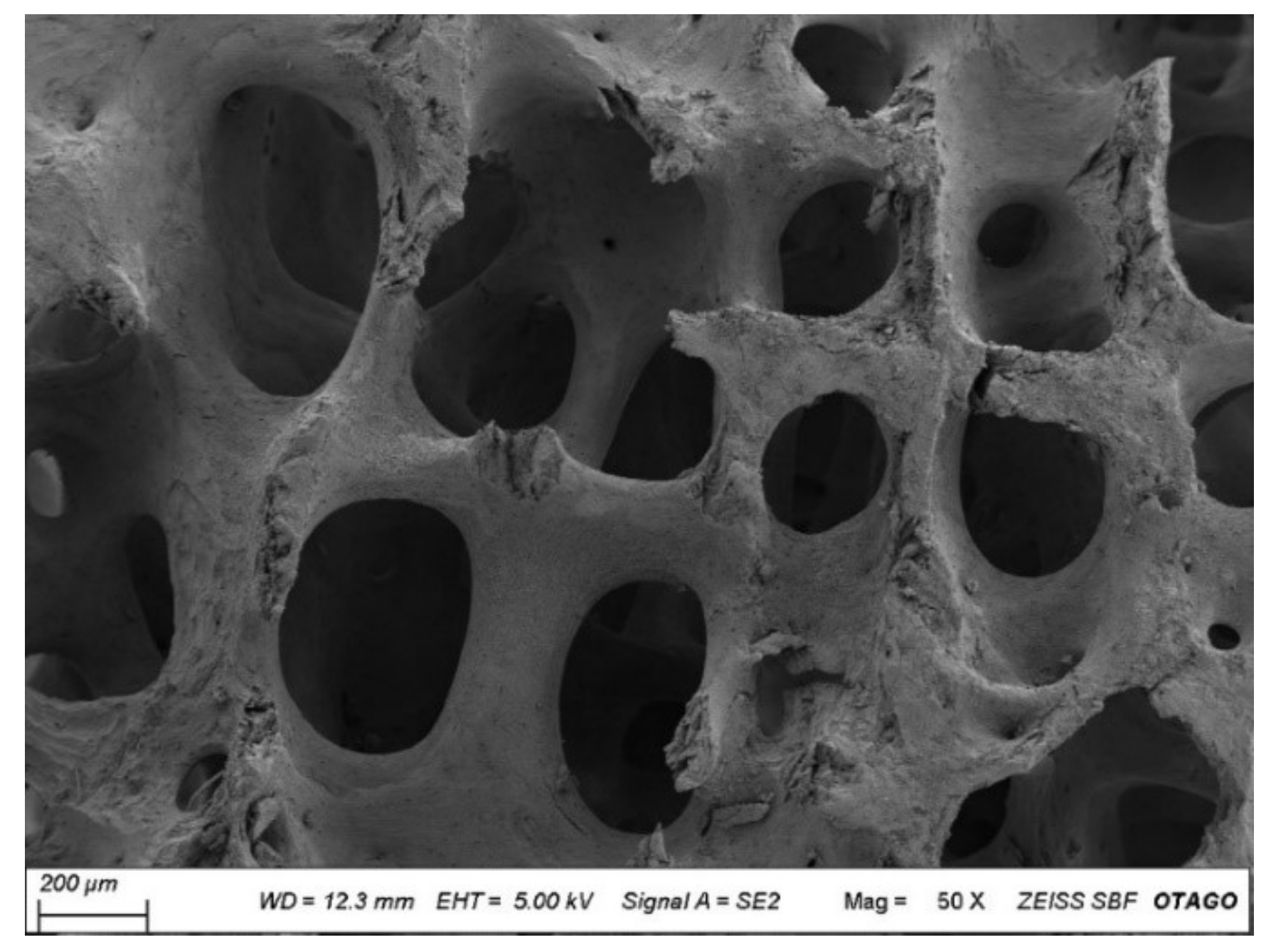
3.5. Scanning Electron Microscopy
3.6. Micro-Architectural Analysis of the CBHA Scaffold
3.7. Energy Dispersive X-ray (EDX) and Inductive Coupled Mass Analysis
3.8. Thermal Gravimetric Analysis
3.9. Chemical Stability and Degradation
3.10. Mechanical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.M. Autogenous bone: Is it still the gold standard? Implant Dent. 2010, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeldt, D.; Rubin, C. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 2001, 10, S86–S95. [Google Scholar] [CrossRef]
- Jaber, H.L.; Hammood, A.S.; Parvin, N. Synthesis and characterization of hydroxyapatite powder from natural Camelus bone. J. Aust. Ceram. Soc. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef]
- Bahrololoom, M.; Javidi, M.; Javadpour, S. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J. Ceram. Process. Res. 2009, 10, 129–138. [Google Scholar]
- Barakat, N.A.M.; Khil, M.S.; Omran, A.M.; Sheikh, F.A.; Kim, H.Y. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mater. Process. Technol. 2009, 209, 3408–3415. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- GAS General Information about The Kingdom of Saudi Arabia; General Authority for Statistics: Riyadh, Saudi Arabia, 2018; p. 51.
- Al-Kanhal, M.A.; Al-Mohizea, I.S.; Al-Othaimeen, A.I.; Khan, M.A. Nutritive value of various rice based dishes in Saudi Arabia. Ecol. Food Nutr. 1999, 38, 223–235. [Google Scholar] [CrossRef]
- Kurtu, M.Y. An Assessment of the Productivity for Meat and the Carcass Yield of Camels (Camelus dromedarius) and of the Consumption of Camel Meat in the Eastern Region of Ethiopia. Trop. Anim. Health Prod. 2004, 36, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. Simulated Body Fluid (SBF) as a Standard Tool to Test the Bioactivity of Implants. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Wiley: Hoboken, NJ, USA, 2008; Volume 3, pp. 97–109. [Google Scholar] [CrossRef]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Timlin, J.A.; Carden, A.; Morris, M.D.; Rajachar, R.M.; Kohn, D.H. Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone. Anal. Chem. 2000, 72, 2229–2236. [Google Scholar] [CrossRef]
- Sofronia, A.M.; Baies, R.; Anghel, E.M.; Marinescu, C.A.; Tanasescu, S. Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater. Sci. Eng. C 2014, 43, 153–163. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Ramirez-Gutierrez, C.F.; del Real, A.; Rubio-Rosas, E.; Rodriguez-García, M.E. Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J. Mater. Sci. 2016, 51, 4431–4441. [Google Scholar] [CrossRef]
- Johnson, G.S.; Mucalo, M.R.; Lorier, M.A. The processing and characterization of animal-derived bone to yield materials with biomedical applications. Part 1: Modifiable porous implants from bovine condyle cancellous bone and characterization of bone materials as a function of processing. J. Mater. Sci. Mater. Med. 2000, 11, 427–441. [Google Scholar] [CrossRef]
- Rogers, K.D.; Daniels, P. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 2002, 23, 2577–2585. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; De Gruyter: Berlin, Germany, 2016; pp. 1–29. [Google Scholar] [CrossRef]
- Teixeira, S.; Ferraz, M.P.; Monteiro, F.J. Biocompatibility of highly macroporous ceramic scaffolds: Cell adhesion and morphology studies. J. Mater. Sci. Mater. Med. 2008, 19, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Joschek, S.; Nies, B.; Krotz, R.; Göpferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.R.; Mellonig, J.T.; Brunsvold, M.A.; McDonnell, H.T.; Cochran, D.L. Clinical evaluation of Bio-Oss®: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J. Clin. Periodontol. 1999, 26, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.B.; Castro-Silva, I.I.; Da Rocha Coutinho, L.A.C.; Lenharo, A.; Granjeiro, J.M. Osteoconduction and Bioresorption of Bone Allograft versus Anorganic Bovine Bone Xenograft: A Histomorphometric Study in Humans. J. Biomim. Biomater. Tissue Eng. 2013, 18, 85–95. [Google Scholar] [CrossRef]
- Renders, G.A.P.; Mulder, L.; van Ruijven, L.J.; van Eijden, T.M.G.J. Porosity of human mandibular condylar bone. J. Anat. 2007, 210, 239–248. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef]
- Chu, T.M.G.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Ravi, N.D.; Balu, R.; Sampath Kumar, T.S. Strontium-Substituted Calcium Deficient Hydroxyapatite Nanoparticles: Synthesis, Characterization, and Antibacterial Properties. J. Am. Ceram. Soc. 2012, 95, 2700–2708. [Google Scholar] [CrossRef]
- Orlovskii, V.P.; Komlev, V.S.; Barinov, S.M. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg. Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Murugan, E.; Akshata, C.R.; Ilangovan, R.; Mohan, M. Evaluation of quaternization effect on chitosan-HAP composite for bone tissue engineering application. Colloids Surf. B Biointerfaces 2022, 218, 112767. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]





| Sample | Na | Mg | P | Ca | Zn | Sr | Cd | As | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxyapatite (mg/kg) | 2.90 × 104 | 6.73 × 103 | 1.58 × 105 | 3.6 × 105 | 198 | 880 | <0.25 | <0.25 | 3.20 |
| ASTM Maximum Limits (F1185-03) | 5 | 211 | 30 | ||||||
| Ca: P (SEM-EDX) Ca: P (ICP-MS) | 1.79 1.76 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, Z.; Alfarhan, M.F.; Mazher, J.; Bayan, Y.; Cooper, P.R.; Dias, G.J.; Adanir, N.; Ratnayake, J. Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules 2022, 27, 7946. https://doi.org/10.3390/molecules27227946
Khurshid Z, Alfarhan MF, Mazher J, Bayan Y, Cooper PR, Dias GJ, Adanir N, Ratnayake J. Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules. 2022; 27(22):7946. https://doi.org/10.3390/molecules27227946
Chicago/Turabian StyleKhurshid, Zohaib, Mohammed Farhan Alfarhan, Javed Mazher, Yasmin Bayan, Paul R. Cooper, George J. Dias, Necdet Adanir, and Jithendra Ratnayake. 2022. "Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application" Molecules 27, no. 22: 7946. https://doi.org/10.3390/molecules27227946
APA StyleKhurshid, Z., Alfarhan, M. F., Mazher, J., Bayan, Y., Cooper, P. R., Dias, G. J., Adanir, N., & Ratnayake, J. (2022). Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules, 27(22), 7946. https://doi.org/10.3390/molecules27227946