Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater
Abstract
1. Introduction
2. Experiment
2.1. Chemicals
2.2. Synthesis of CdS Photocatalyst
2.3. Characterization
2.4. Photocatalytic Degradation of the Toxic Pollutants
3. Discussion
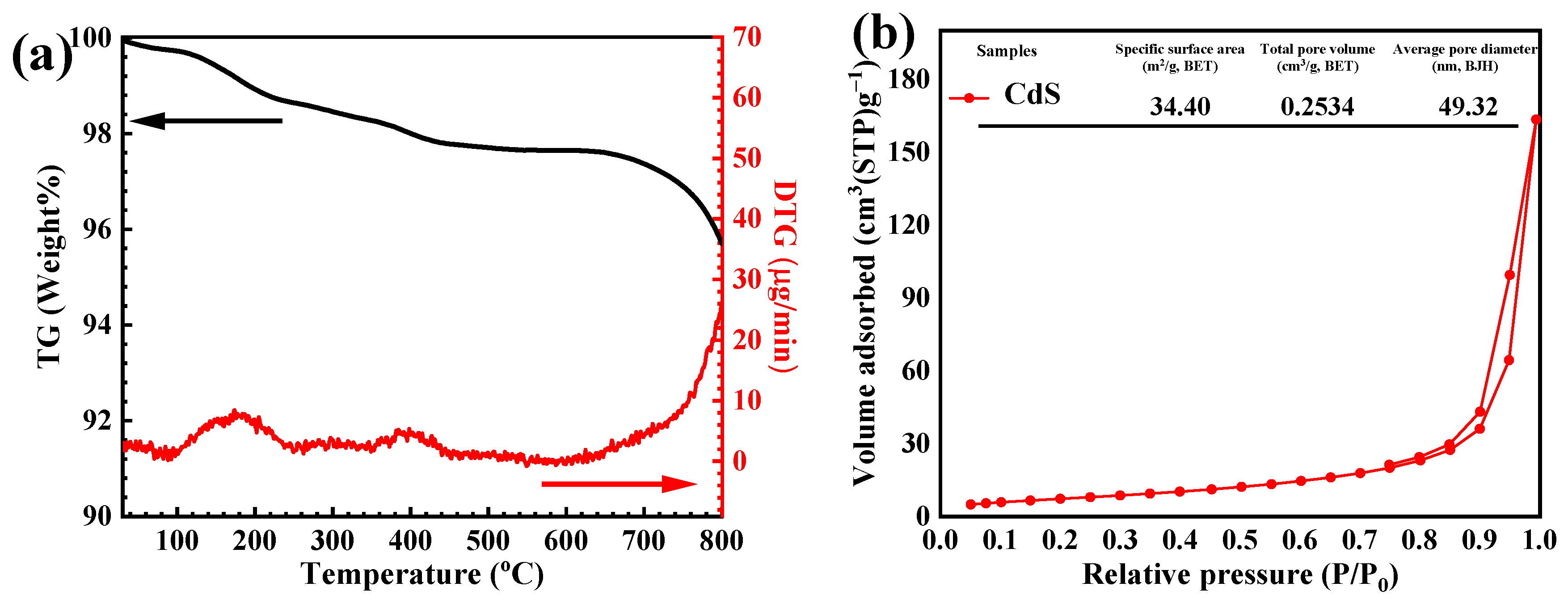
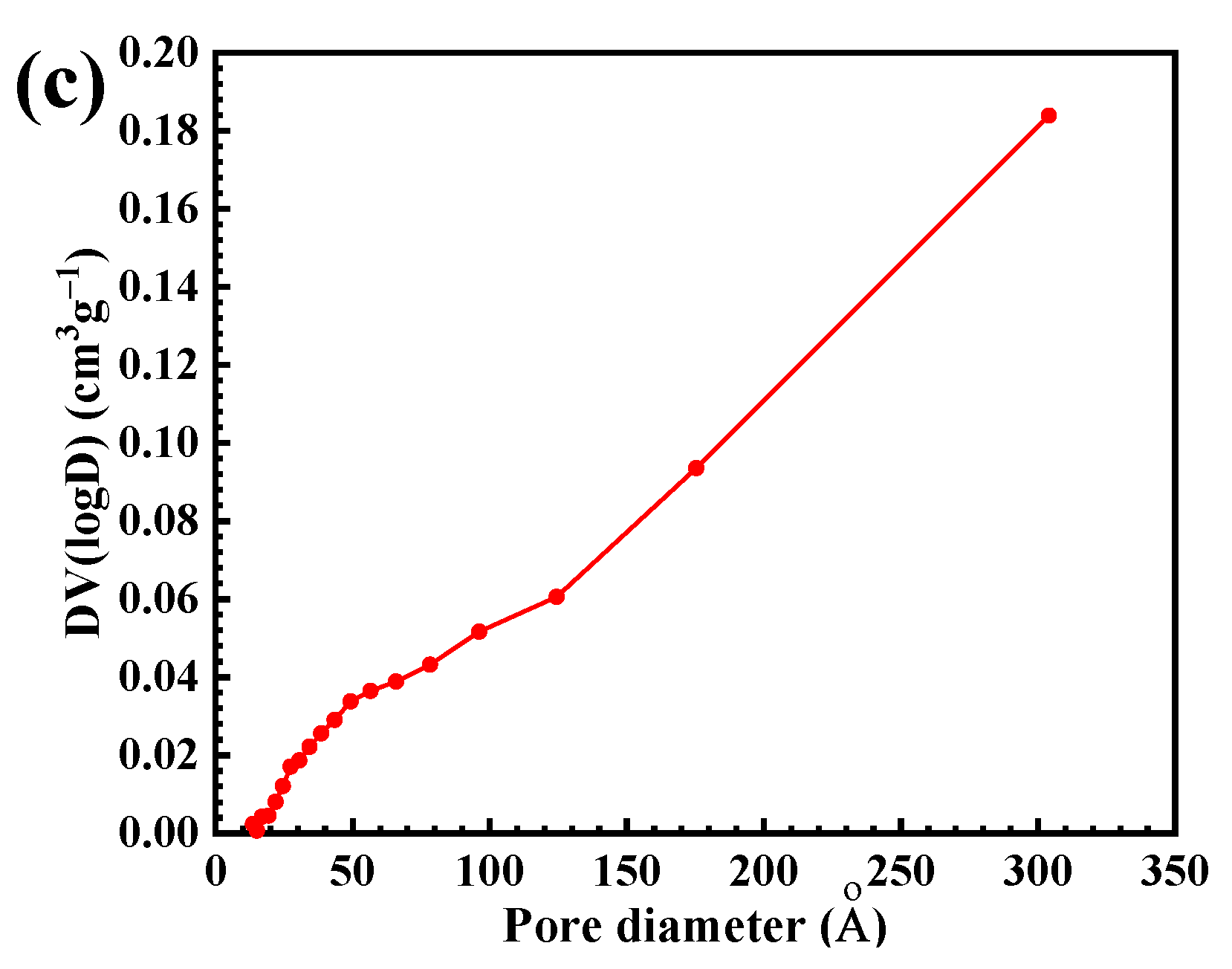
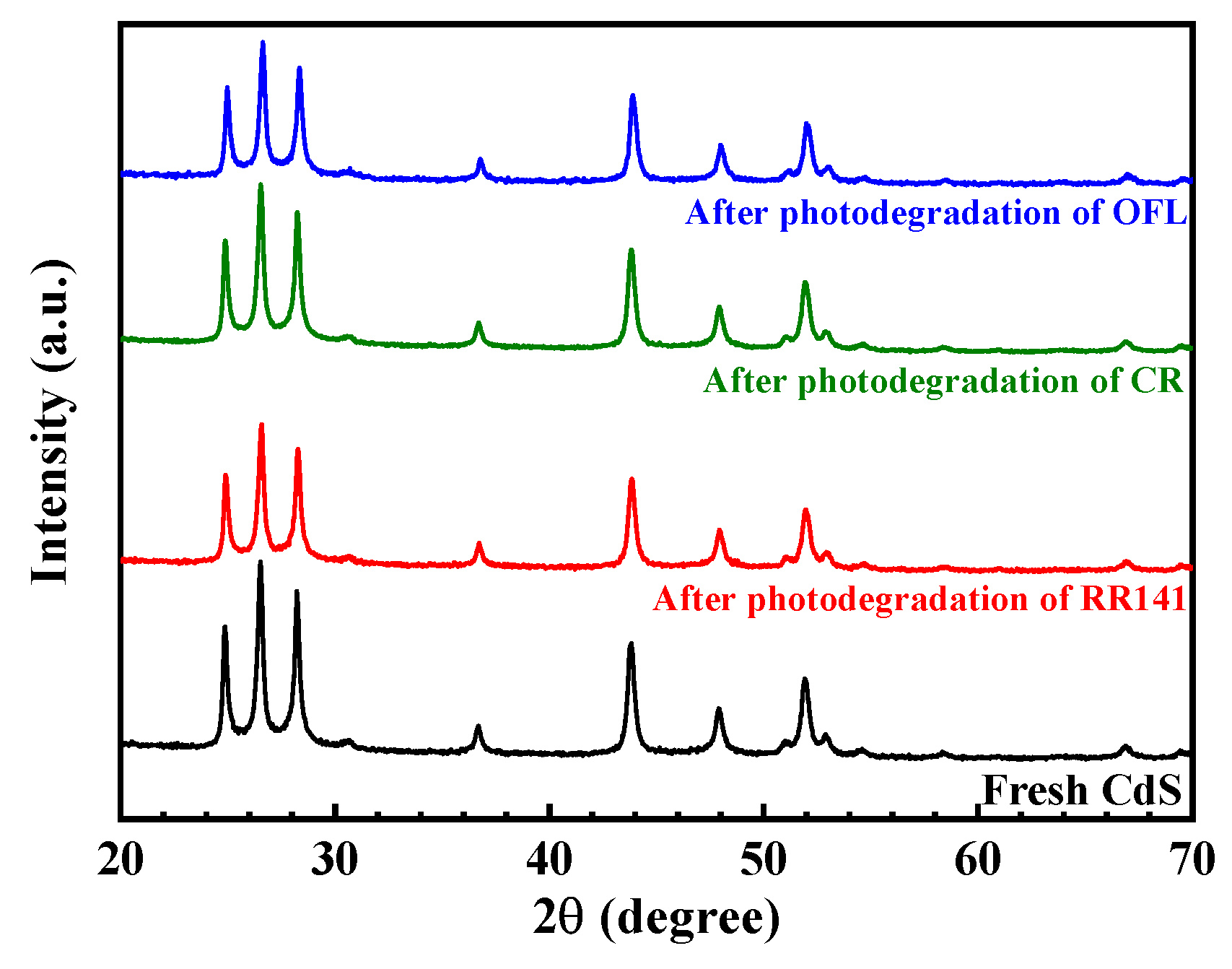
3.1. Characterization of the CdS Catalyst
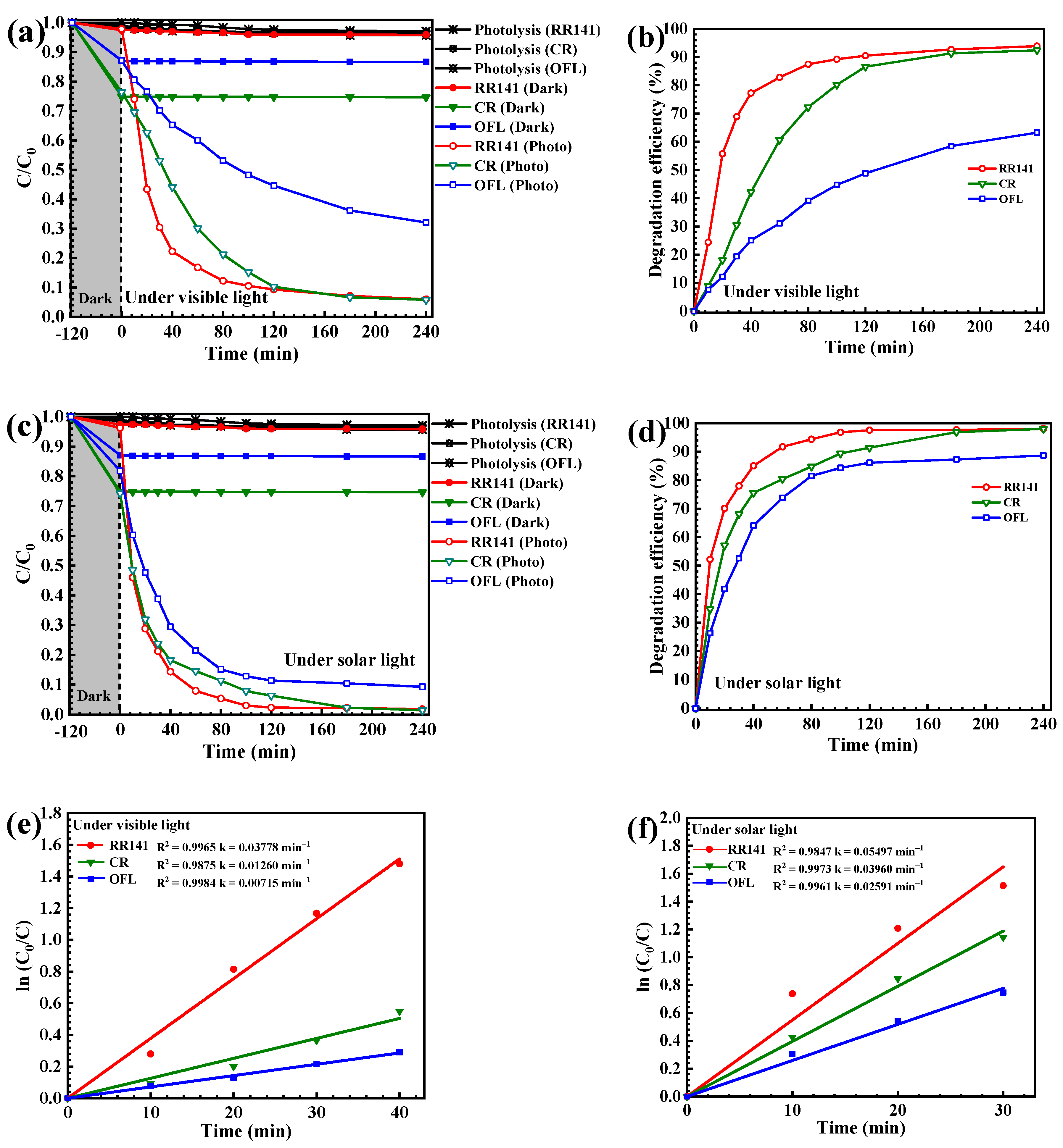
3.2. Photodegradation Study
3.2.1. Photodegradation of Pollutants
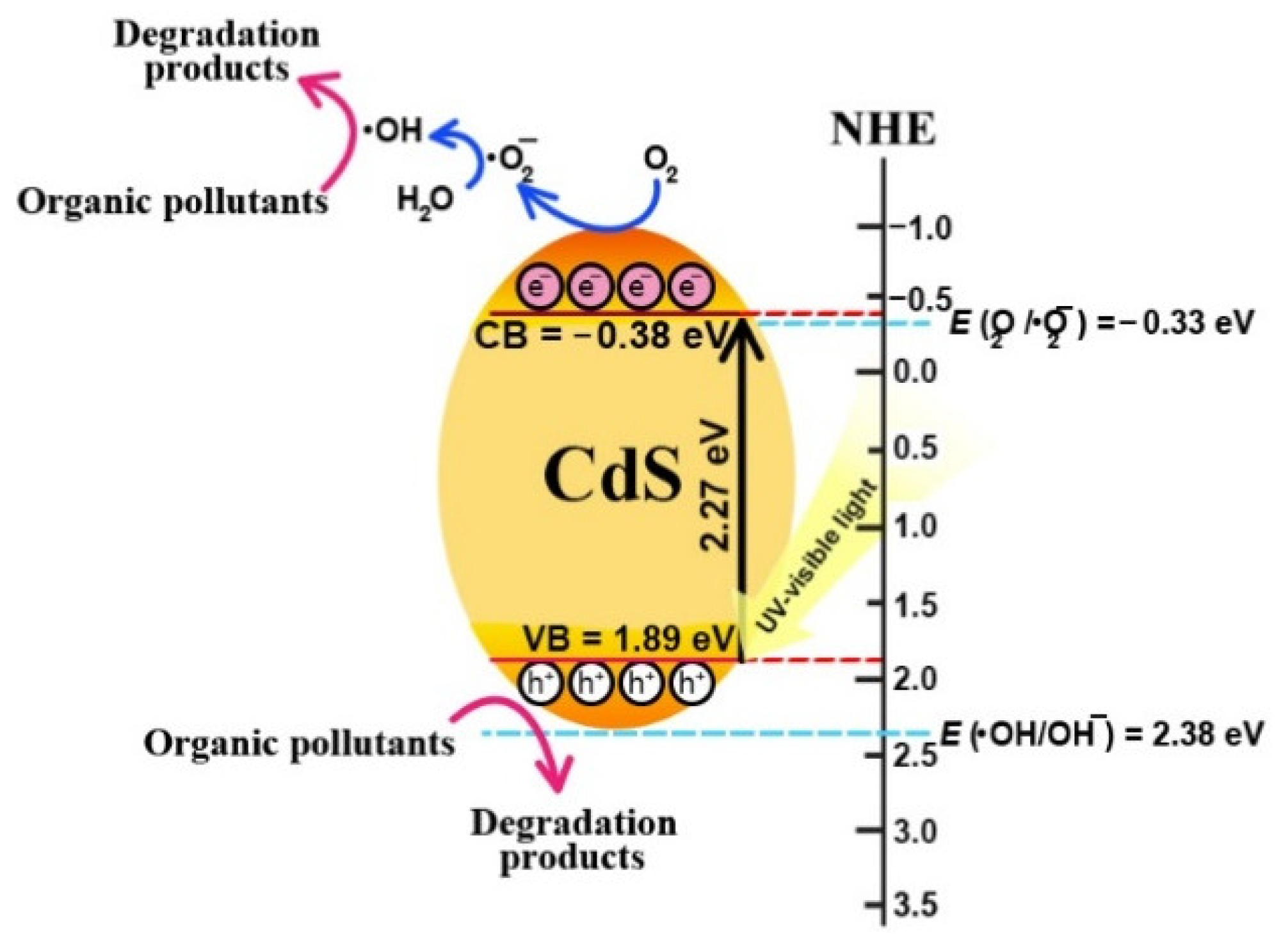
3.2.2. Photocatalytic Degradation Mechanism and Cycling Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chankhanittha, T.; Nanan, S. Hydrothermal synthesis, characterization and enhanced photocatalytic performance of ZnO toward degradation of organic azo dye. Mater. Lett. 2018, 226, 79–82. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Visible-light-responsive photocatalyst based on ZnO/CdS nanocomposite for photodegradation of reactive red azo dye and ofloxacin antibiotic. Mater. Sci. Semicond. Process. 2021, 123, 105558. [Google Scholar] [CrossRef]
- Kakarndee, S.; Nanan, S. SDS capped and PVA capped ZnO nanostructures with high photocatalytic performance toward photodegradation of reactive red (RR141) azo dye. J. Environ. Chem. Eng. 2018, 6, 74–94. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Piyavarakorn, V.; Nanan, S. Performance of solvothermally grown Bi2MoO6 photocatalyst toward degradation of organic azo dyes and fluoroquinolone antibiotics. Mater. Lett. 2020, 258, 126764. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Nanan, S. Solar light-driven photocatalyst based on bismuth molybdate (Bi4MoO9) for detoxification of anionic azo dyes in wastewater. J. Mater. Sci. Mater. Electron. 2021, 32, 1977–1991. [Google Scholar] [CrossRef]
- Wang, X.; Mu, B.; Hui, A.; Wang, A. Comparative study on photocatalytic degradation of Congo red using different clay mineral/CdS nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 5383–5392. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, X.; Wang, J.; Jia, W.; Zhu, G.; Qu, F. Facile template-free synthesis and visible-light driven photocatalytic performances of dendritic CdS hierarchical structures. Dalton Trans. 2013, 42, 4633–4638. [Google Scholar] [CrossRef]
- Rani, M.; Keshu; Shanker, U. Efficient visible light photocatalytic organic colorants elimination performance induced by biosynthesized titanium dioxide coupled cadmium sulfide nanostructures. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. Visible light driven photocatalytic degradation of ofloxacin and malachite green dye using cadmium sulphide nanoparticles. J. Environ. Chem. Eng. 2018, 6, 3631–3639. [Google Scholar] [CrossRef]
- Vaizoğullar, A.I. Ternary CdS/MoS2/ZnO Photocatalyst: Synthesis, Characterization and Degradation of Ofloxacin Under Visible Light Irradiation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4129–4141. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018, 360, 34–43. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Cao, Z.; Xu, J.; Hu, J.; Huang, Y.; Cui, C.; Liu, H.; Wang, H. Simultaneous Enhancements of Light-Harvesting and Charge Transfer in UiO-67/CdS/RGO Composites toward Ofloxacin Photo-Degradation. Chem. Eng. J. 2020, 381, 122771–122781. [Google Scholar] [CrossRef]
- Li, J.; Xia, Z.; Ma, D.; Liu, G.; Song, N.; Xiang, D.; Xin, Y.; Zhang, G.; Chen, Q. Improving photocatalytic activity by construction of immobilized Z-scheme CdS/Au/TiO2 nanobelt photocatalyst for eliminating norfloxacin from water. J. Colloid Interface Sci. 2021, 586, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, X.; Wang, Y.; Zhu, B.; Yang, J. Fabrication of Magnetically Recoverable Fe3O4/CdS/g-C3N4 Photocatalysts for Effective Degradation of Ciprofloxacin under Visible Light. Ceram. Int. 2020, 46, 20974–20984. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Senasu, T.; Hemavibool, K.; Nanan, S. Hydrothermally grown CdS nanoparticles for photodegradation of anionic azo dyes under UV-visible light irradiation. RSC Adv. 2018, 8, 22592–22605. [Google Scholar] [CrossRef]
- Senasu, T.; Nijpanich, S.; Juabrum, S.; Chanlek, N.; Nanan, S. CdS/BiOBr heterojunction photocatalyst with high performance for solar-light-driven degradation of ciprofloxacin and norfloxacin antibiotics. Appl. Surf. Sci. 2021, 567, 150850. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Z. A Solvothermal Synthesis of the CdS Nanorods Doped with Cr and Investigation of Ferromagnetic and Optical Properties at Room Temperature. Integr. Ferroelectr. 2022, 225, 282–296. [Google Scholar] [CrossRef]
- Pei, H.; Jia, Q.; Guo, R.; Zhang, T.; Liu, N.; Mo, Z. Flower-like CeO2/CdS Quantum Dots Heterojunction Nanocomposites with High Photocatalytic Activity for RhB Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129256–129266. [Google Scholar] [CrossRef]
- Jothibas, M.; Sankar, M.; Muthuvel, A.; Srinivasan, S.; Elayaraja, M. Enhanced sunlight irradiated photocatalytic activity of Sn doped CdS nanoparticles for the degradation of organic pollutants. Inorg. Chem. Commun. 2022, 136, 109149–109160. [Google Scholar] [CrossRef]
- Kong, X.; Yu, F.; Zhang, H.; Lv, F.; Wang, Y.; Yin, L.; Huang, J.; Feng, Q. Synthesis and study of morphology regulation, formation mechanism and photocatalytic performance of CdS. Appl. Surf. Sci. 2022, 576, 151817–151824. [Google Scholar] [CrossRef]
- Yi, L.; Fan, Y.; Yang, R.; Zhu, R.; Zhu, Z.; Hu, J. Fabrication and optimization of CdS photocatalyst using nature leaf as biological template for enhanced visible-light photocatalytic hydrogen evolution. Catal. Today 2022, 402, 241–247. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Kolaei, M.; Tayebi, M.; Masoumi, Z.; Lee, B.-K. A novel approach for improving photoelectrochemical water splitting performance of ZnO-CdS photoanodes: Unveiling the effect of surface roughness of ZnO nanorods on distribution of CdS nanoparticles. J. Alloys Compd. 2022, 906, 164314–164326. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Solvothermal synthesis of BiOBr photocatalyst with an assistant of PVP for visible-light-driven photocatalytic degradation of fluoroquinolone antibiotics. Catal. Today 2022, 384–386, 209–227. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Photiwat, T.; Hemavibool, K.; Nanan, S. Preparation, characterization, and photocatalytic study of solvothermally grown CTAB-capped Bi2WO6 photocatalyst toward photodegradation of Rhodamine B dye. Opt. Mater. 2021, 117, 111183. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, B.; Qu, F.; Wu, X. Synthesis of self-assembled CdS nanospheres and their photocatalytic activities by photodegradation of organic dye molecules. Chem. Eng. J. 2014, 258, 203–209. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, P.; Duan, M.; Xu, J.; Liu, M.; Luo, D. Synthesis and Characterization of Successive Z-Scheme CdS/Bi2MoO6/BiOBr Heterojunction Photocatalyst with Efficient Performance for Antibiotic Degradation. J. Alloys Compd. 2021, 870, 159385–159399. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced graphene oxide-CdS heterostructure: An efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl. Catal. B Environ. 2019, 245, 143–158. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.; Jin, L.; Wu, K. Combination Mechanism and Enhanced Visible-Light Photocatalytic Activity and Stability of CdS/g-C3N4 Heterojunctions. J. Mater. Sci. Technol. 2017, 33, 30–38. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Nazari, S.; Joshaghani, M.; Zinadini, S.; Sibali, L.; Feyzi, M. Highly Efficient Azo Dye Degradation in a Photocatalytic Rotating Disc Reactor with Deposited L-Histidine-TiO2-CdS. Mater Sci. Semicond Process. 2022, 152, 107071–107083. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Yenjai, C.; Nanan, S. Utilization of formononetin and pinocembrin from stem extract of Dalbergia parviflora as capping agents for preparation of ZnO photocatalysts for degradation of RR141 azo dye and ofloxacin antibiotic. Catal. Today 2022, 384–386, 279–293. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Komchoo, N.; Senasu, T.; Piriyanon, J.; Youngme, S.; Hemavibool, K.; Nanan, S. Silver decorated ZnO photocatalyst for effective removal of reactive red azo dye and ofloxacin antibiotic under solar light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127034. [Google Scholar] [CrossRef]
- Khan, H.R.; Murtaza, G.; Choudhary, M.A.; Ahmed, Z.; Malik, M.A. Photocatalytic removal of carcinogenic reactive red S3B dye by using ZnO and Cu doped ZnO nanoparticles synthesized by polyol method: A kinetic study. Sol. Energy 2018, 173, 875–881. [Google Scholar] [CrossRef]
- Gnanamozhi, P.; Rajivgandhi, G.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Pandiyan, V.; Li, W.-J. Enhanced antibacterial and photocatalytic degradation of reactive red 120 using lead substituted ZnO nanoparticles prepared by ultrasonic-assisted co-precipitation method. Ceram. Int. 2020, 46, 19593–19599. [Google Scholar] [CrossRef]
- Vaizoğullar, A.I. Facile preparation and characterization of NiO/Ni2O3-decorated nanoballs and mixed phase CdS nano rods (CdS&NiO/Ni2O3) for effective photocatalytic decomposition of Congo red under visible light irradiation. J. Dispers. Sci. Technol. 2021, 42, 1408–1418. [Google Scholar] [CrossRef]
- Habibi, M.H.; Rahmati, M.H. The Effect of Operational Parameters on the Photocatalytic Degradation of Congo Red Organic Dye Using ZnO-CdS Core-Shell Nano-Structure Coated on Glass by Doctor Blade Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 160–164. [Google Scholar] [CrossRef]

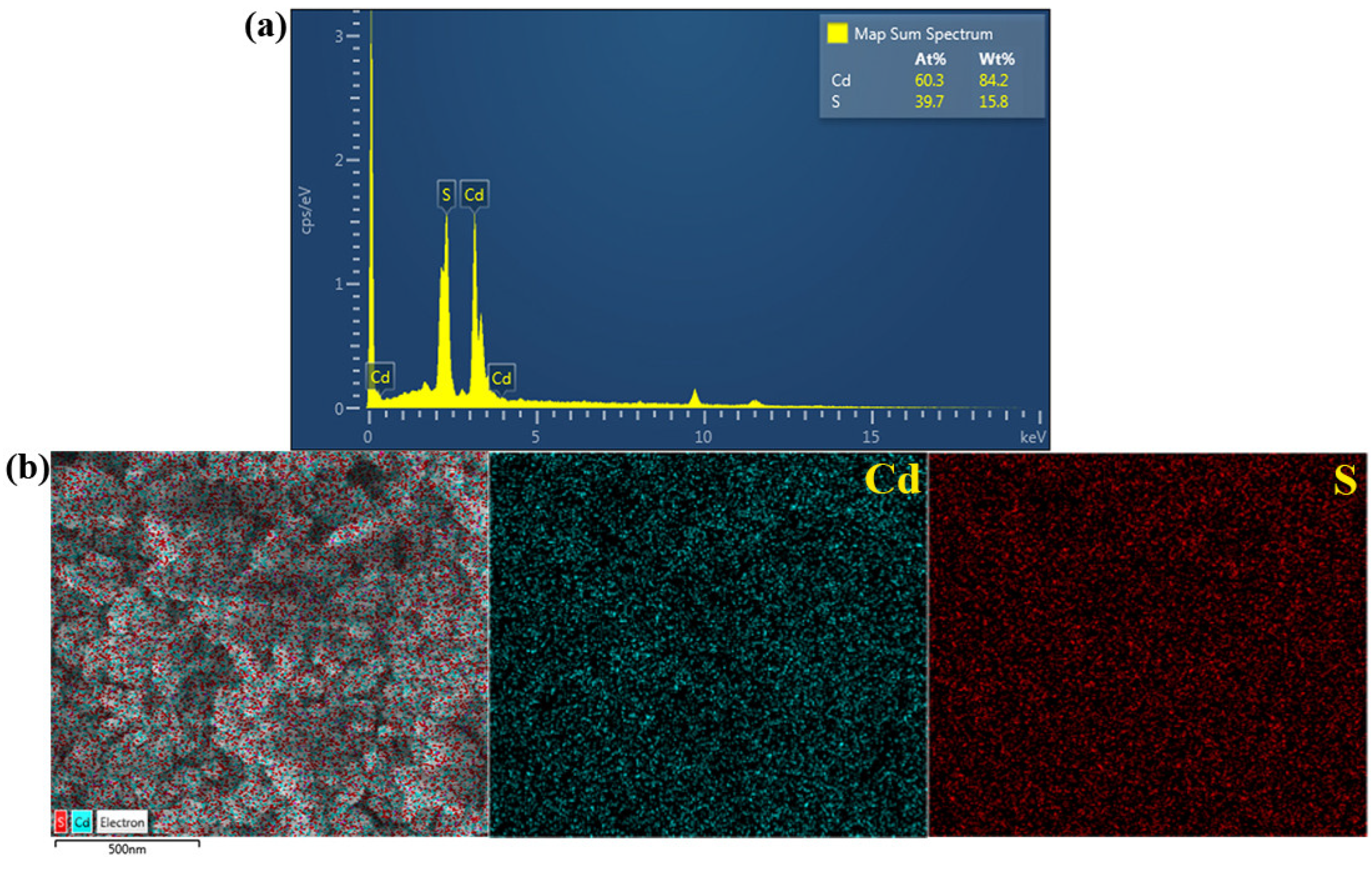
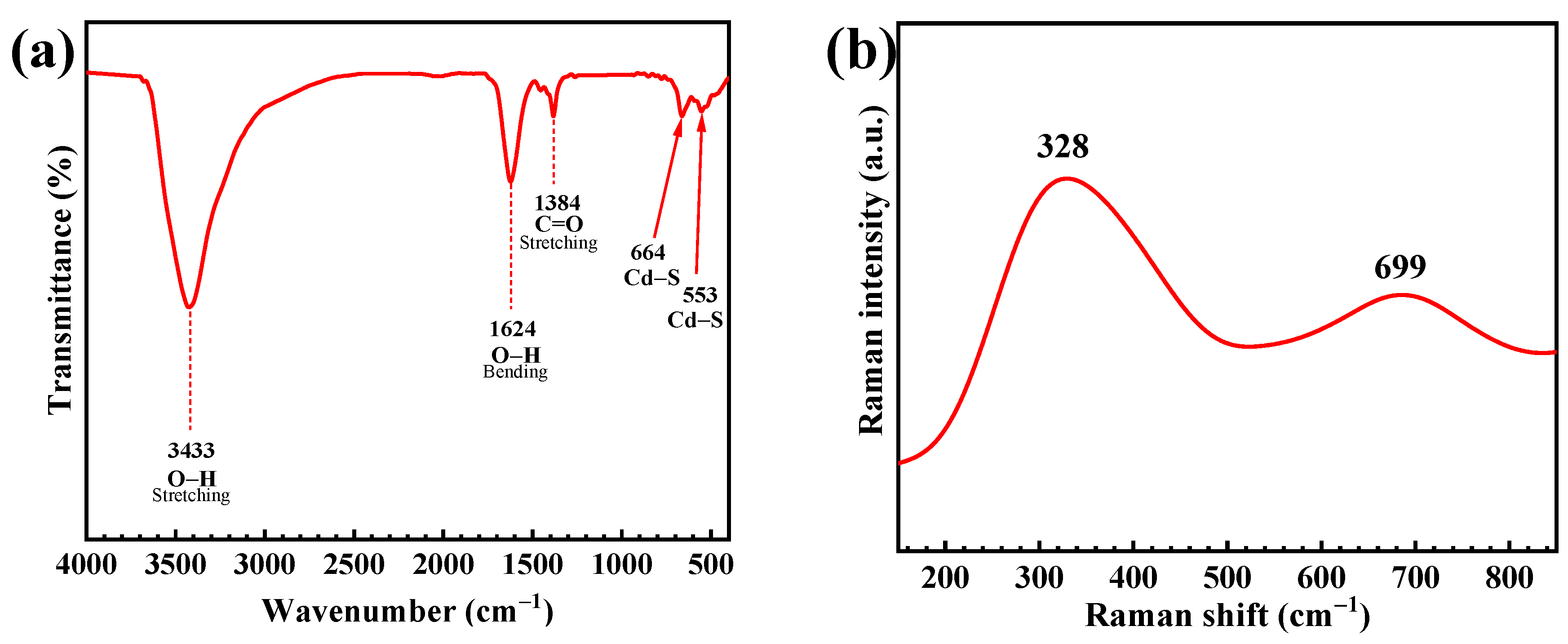
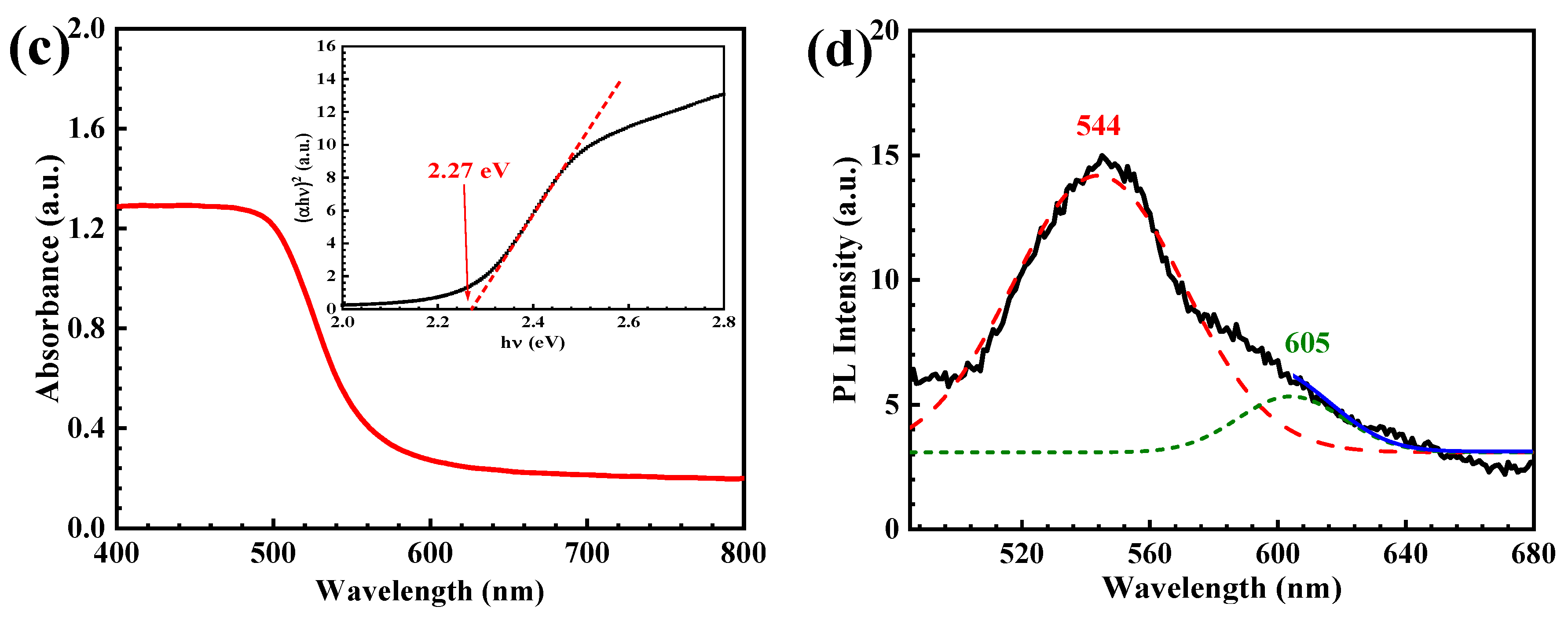
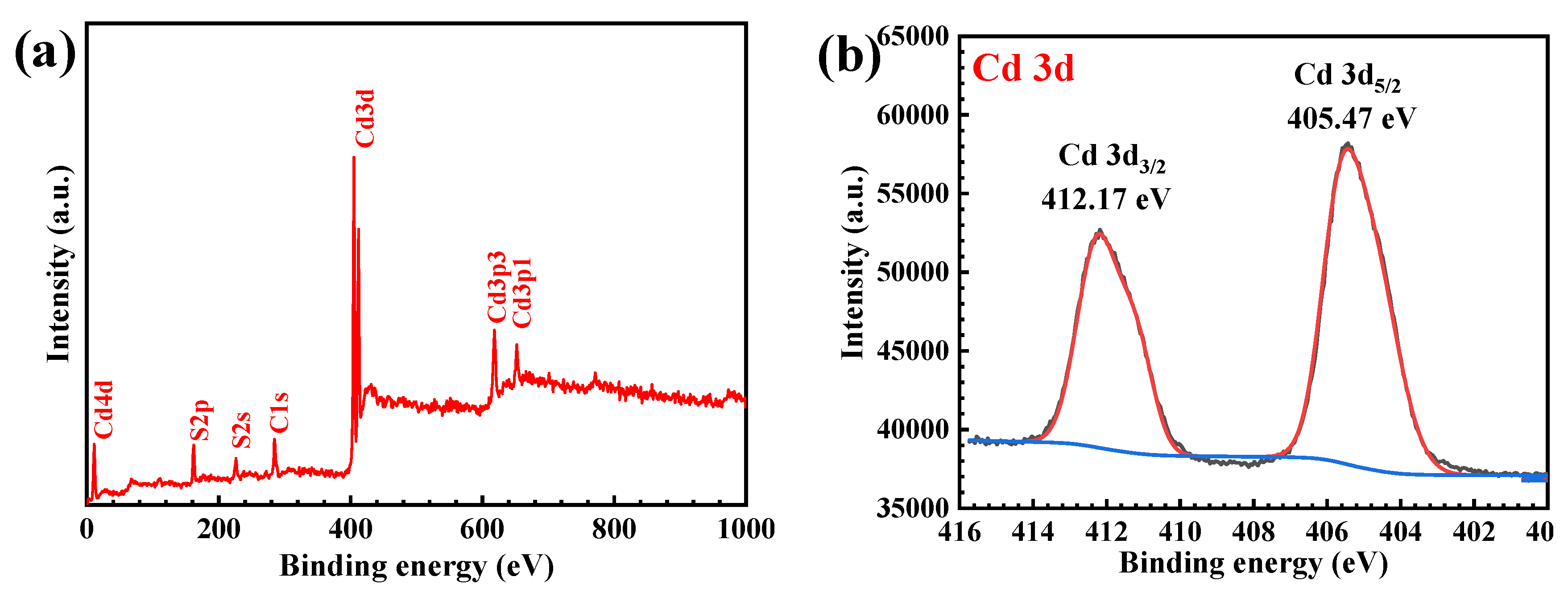



| Catalyst | Conc. | Catalyst Loading | Light Source | Lamp | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Photodegradation of Reactive Red 141 (RR141) azo dye | |||||||
| ZnO | 10 mgL−1 | 50 mg | UV | 125 W Hg lamp | 240 | 95 | [1] |
| ZnO | 10 mgL−1 | 50 mg | UV | 125 W Hg lamp | 240 | 98 | [2] |
| SDS capped ZnO | 10 mgL−1 | 50 mg | UV | 125 W Hg lamp | 240 | 95 | [3] |
| SDS capped ZnO | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 60 | [3] |
| Cu-ZnO | 50 mgL−1 | 100 mg | UV | - | 120 | 89 | [36] |
| 3% Pb-ZnO | 30 mgL−1 | 30 mg | UV | - | 120 | 96 | [37] |
| ZnO/CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 120 | 80 | [2] |
| Bi2MoO6 | 10 mgL−1 | 50 mg | UV | 125 W Hg lamp | 240 | 37 | [4] |
| Bi2MoO6 | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 45 | [4] |
| Bi4MoO9 | 10 mgL−1 | 50 mg | UV | 125 W Hg lamp | 240 | 68 | [5] |
| Bi4MoO9 | 10 mgL−1 | 50 mg | Sunlight | - | 240 | 70 | [5] |
| CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 93 | This work |
| CdS | 10 mgL−1 | 50 mg | Sunlight | - | 240 | 98 | This work |
| Photodegradation of Congo Red (CR) dye | |||||||
| CdS | 30 mgL−1 | 30 mg | Visible | 300 W xenon lamp | 80 | 90 | [6] |
| CdS | 10 mgL−1 | 50 mg | Visible | 800 W xenon lamp | 60 | 91 | [7] |
| CdS | 10 mgL−1 | 250 mg | Sunlight | - | 120 | 31 | [38] |
| CdS | 25 mgL−1 | 15 mg | Sunlight | - | 300 | 85 | [8] |
| TiO2-CdS | 25 mgL−1 | 15 mg | Sunlight | - | 300 | 95 | [8] |
| ZnO-CdS | 10 mgL−1 | - | UV | 250 W Hg lamp | 100 | 88 | [39] |
| CdS&NiO/Ni2O3 | 10 mgL−1 | 250 mg | Sunlight | - | 120 | 82 | [38] |
| CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 180 | 91 | This work |
| CdS | 10 mgL−1 | 50 mg | Sunlight | - | 180 | 97 | This work |
| Photodegradation of Ofloxacin (OFL) antibiotic | |||||||
| CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 70 | [2] |
| CdS | 10 mgL−1 | 25 mg | Visible | 85 W | 80 | 79 | [9] |
| ZnO/CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 73 | [2] |
| CdS/MoS2 | 10 mgL−1 | 100 mg | Visible | 400 W xenon lamp | 90 | 61 | [10] |
| CdS/TiO2 | 10 mgL−1 | 450 mg | Visible | 85 W | 180 | 86 | [11] |
| CdS | 10 mgL−1 | 50 mg | Visible | 15 W | 240 | 63 | This work |
| CdS | 10 mgL−1 | 50 mg | Sunlight | - | 240 | 89 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senasu, T.; Ruengchai, N.; Khamdon, S.; Lorwanishpaisarn, N.; Nanan, S. Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater. Molecules 2022, 27, 7944. https://doi.org/10.3390/molecules27227944
Senasu T, Ruengchai N, Khamdon S, Lorwanishpaisarn N, Nanan S. Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater. Molecules. 2022; 27(22):7944. https://doi.org/10.3390/molecules27227944
Chicago/Turabian StyleSenasu, Teeradech, Nattakarn Ruengchai, Sarawoot Khamdon, Narubeth Lorwanishpaisarn, and Suwat Nanan. 2022. "Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater" Molecules 27, no. 22: 7944. https://doi.org/10.3390/molecules27227944
APA StyleSenasu, T., Ruengchai, N., Khamdon, S., Lorwanishpaisarn, N., & Nanan, S. (2022). Hydrothermal Synthesis of Cadmium Sulfide Photocatalyst for Detoxification of Azo Dyes and Ofloxacin Antibiotic in Wastewater. Molecules, 27(22), 7944. https://doi.org/10.3390/molecules27227944





