Theoretical and Experimental Insights of Benzimidazole Catalyzed by the Epoxy–Acrylic Acid Reaction
Abstract
:1. Introduction
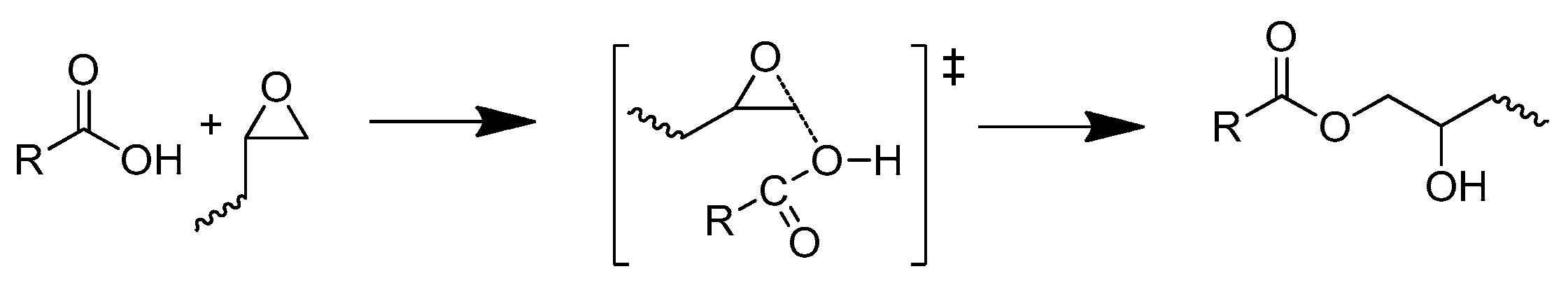
- (1)
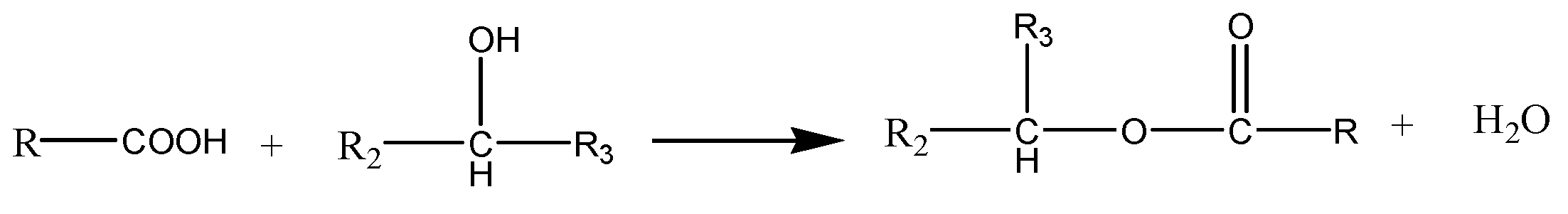
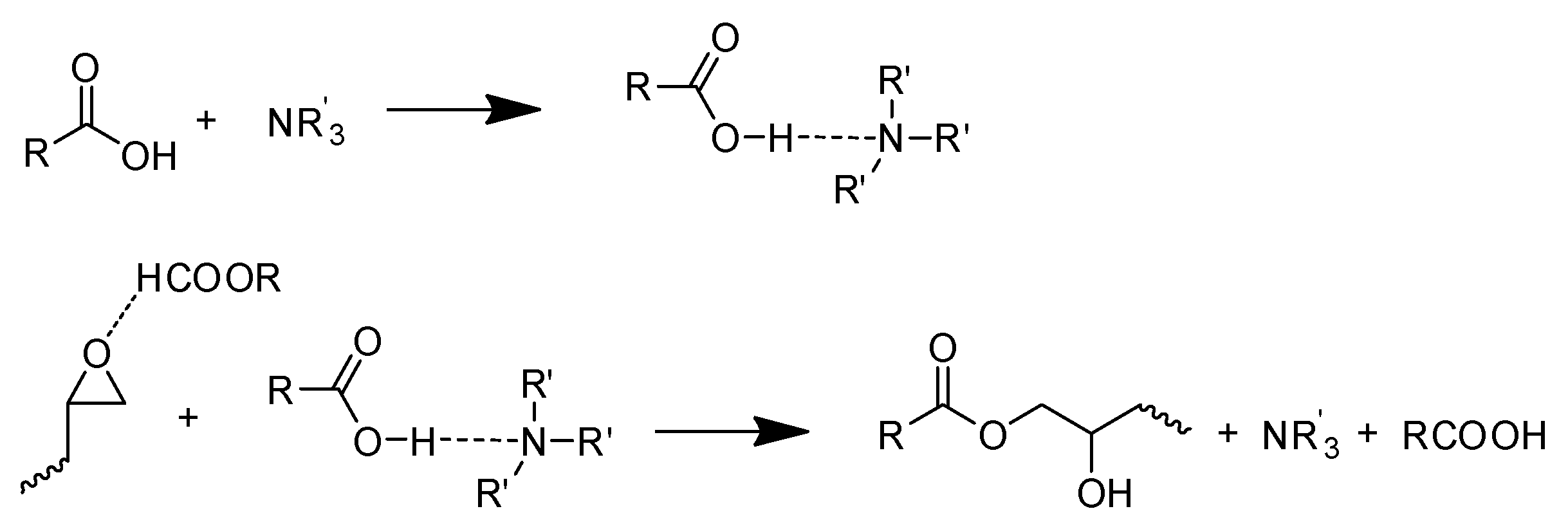
- The reaction between the carboxylic group and the epoxide group.
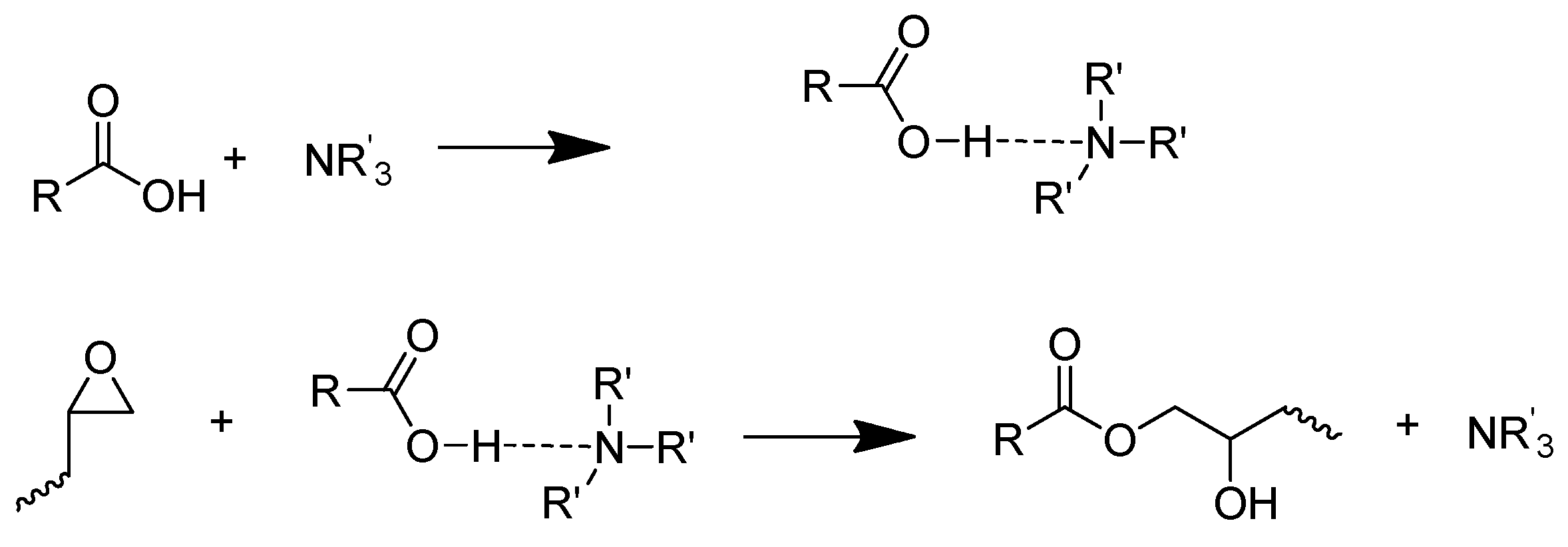
- (2)
- The reaction between carboxylic and –OH groups on epoxy resin chains.
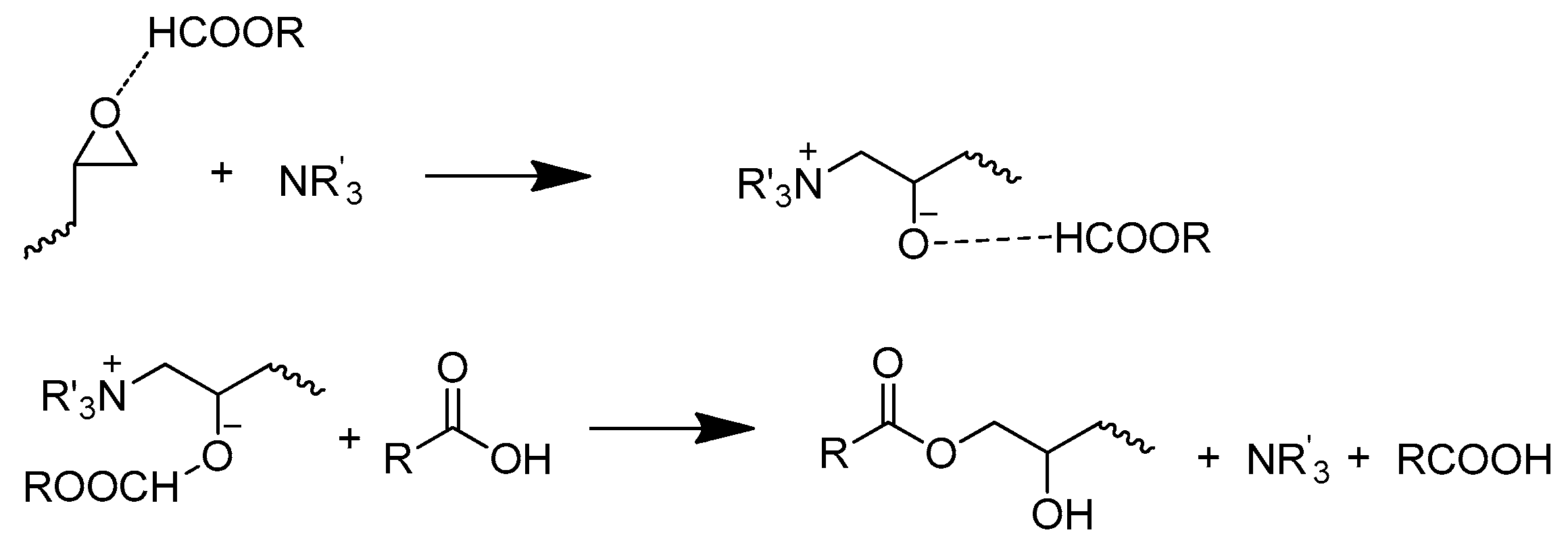
- (3)
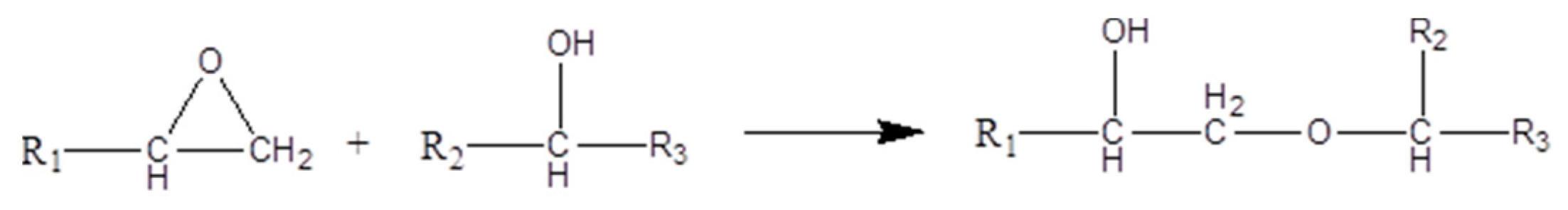
- The reaction between epoxide and –OH groups.
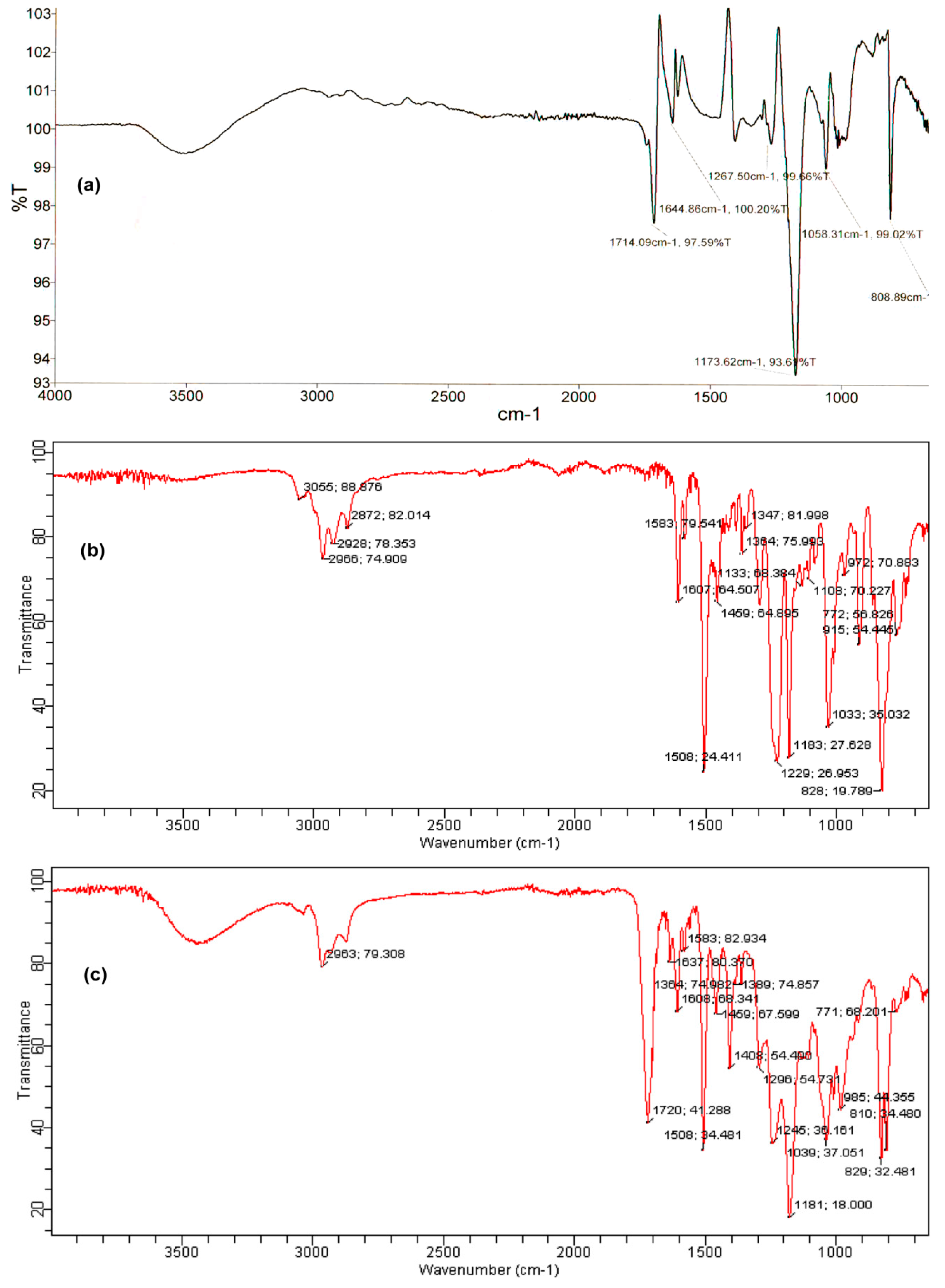
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Method
3.3. Computational Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clay, J.; Armstrong, S.; Leotsakos, G. High Performance Water-Based Adhesion Compositions and Applications. U.S. Patent No. 10,167,416, 1 January 2019. [Google Scholar]
- Olson, D.; Lin, W.; Seeger, G. Adhesive Formulations for Bonding Composite Materials. U.S. Patent No. 9,290,631, 22 March 2016. [Google Scholar]
- Jaswal, S.; Gaur, B. New Trends in Vinyl Ester Resins. Rev. Chem. Eng. 2014, 30, 567–581. [Google Scholar] [CrossRef]
- Alía, C.; Jofre-Reche, J.; Suárez, J. Influence of Post-Curing Temperature on the Structure, Properties, and Adhesion of Vinyl Ester Adhesive. J. Adhes. 2015, 29, 518–531. [Google Scholar] [CrossRef]
- Taillemite, S.; Pauer, R. Bright Future for Vinyl Ester Resins in Corrosion Applications. Reinf. Plast. 2009, 53, 34–37. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Dhakal, H.N.; Zhang, Z.Y.; Materials, A. Water Absorption Behavior, Mechanical and Thermal Properties of Vinyl Ester Matrix Nanocomposites Based on Layered Silicate. Polym. Technol. 2013, 7, 3–10. [Google Scholar] [CrossRef]
- Guo, Z.; Shin, K.; Karki, A.B.; Young, D.P.; Kaner, R.B.; Hahn, H.T. Fabrication and Characterization of Iron Oxide Nanoparticles Filled Polypyrrole Nanocomposites. J. Nanoparticle Res. 2009, 11, 1441–1452. [Google Scholar] [CrossRef]
- Guo, Z.; Liang, X.; Pereira, T.; Scaffaro, R.; Thomas Hahn, H. CuO Nanoparticle Filled Vinyl-Ester Resin Nanocomposites: Fabrication, Characterization and Property Analysis. Compos. Sci. Technol. 2007, 67, 2036–2044. [Google Scholar] [CrossRef]
- Alhuthali, A.; Low, I.M.; Dong, C. Characterisation of the Water Absorption, Mechanical and Thermal Properties of Recycled Cellulose Fibre Reinforced Vinyl-Ester Eco-Nanocomposites. Compos. Part B Eng. 2012, 43, 2772–2781. [Google Scholar] [CrossRef]
- Gawdzik, B.; Matynia, T. Synthesis and Modification of Epoxy-based Divinyl Ester Resin. J. Appl. Polym. Sci. 2001, 81, 2062–2067. [Google Scholar] [CrossRef]
- Firdous, H.; Bajpai, M. UV Curable Heat Resistant Epoxy Acrylate Coatings. Chem. Chem. Technol. 2010, 4, 205–216. [Google Scholar]
- Srivastava, A.; Agrawal, S.; Rai, J.S.P. Kinetics of Esterification of Cycloaliphatic Epoxies with Methacrylic Acid. J. Appl. Polym. Sci. 2002, 86, 3197–3204. [Google Scholar] [CrossRef]
- Pal, N.; Srivastava, A.; Rai, J.S.P. Synthesis of Vinyl Ester Resins in the Presence of Monoepoxies: A Kinetic Study. Polym. Plast. Technol. Eng. 2003, 42, 105–122. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Tanaka, Y. Study of Epoxy Compounds. VII. 1,2 Base-Catalyzed Reaction of Substituted Phenyl Glycidyl Ethers with Benzoic Acid. J. Org. Chem. 1966, 31, 1559–1564. [Google Scholar] [CrossRef]
- Oldring, P.K.T.; Hayward, G. A Manual for Resin for Surface Coatings. Sita Technol 1987, 1, 177–185. [Google Scholar]
- Madec, P.-J.; Maréchal, E. Kinetics and Mechanisms of Polyesterifications II. Reactions of Diacids with Diepoxides. In Analysis/Reactions/Morphology; Springer: Berlin/Heidelberg, Germany, 1985; pp. 153–228. ISBN 978-3-540-39435-8. [Google Scholar]
- Odian, G. Principles of Polymerization; 4th, illustrat; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN1 0471274003. ISBN2 9780471274001. [Google Scholar]
- Srivastava, A.; Pal, N.; Agarwal, S.; Rai, J.S.P. Kinetics and Mechanism of Esterification of Epoxy Resin with Methacrylic Acid in the Presence of Tertiary Amines. Adv. Polym. Technol. 2005, 24, 1–13. [Google Scholar] [CrossRef]
- Sultania, M.; Rai, J.S.P.; Srivastava, D. Kinetic Modeling of Esterification of Cardanol-Based Epoxy Resin in the Presence of Triphenylphosphine for Producing Vinyl Ester Resin: Mechanistic Rate Equation. J. Appl. Polym. Sci. 2010, 118, 1979–1989. [Google Scholar] [CrossRef]
- Lebedev, M.; Guskov, K. The Reactions of A-Oxides. IV. Investigation of Acid Catalyst and Intermediate Products during Reaction of Ethylene Oxide with Carboxylic Acids. Kinet. Katal 1964, 5, 446–453. [Google Scholar]
- Mareš, F.; Hetflejš, J.; Bažant, V. Reactions of Carboxylic Acids with Ethylene Oxide. I. Kinetics of the Reaction of Terephthalic Acid with Ethylene Oxide. Collect. Czechoslov. 1969, 34, 3086–3097. [Google Scholar] [CrossRef]
- Samsonova, T.I.; Mikhailov, G.D.; Malykh, V.A.; Chegolya, A.S. Production of Bis(β-Ethoxy)Terephthalate from Terephthalic Acid and Ethylene Oxide. Fibre Chem. 1977, 9, 25–29. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the Epoxy-Carboxyl Reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Shvets, V.F.; Lebedev, N.N.; Tyukova, O.A. Kinetics and Stereochemistry of Alph Epoxide Reactions with Alcohols and Carboxylic Acids without Catalysts. Zhurnal Org. Khimii 1971, 7, 1851. [Google Scholar]
- Sorokin, M.D.; Gershanov, E.L. Kinetics and Mechanisms of Polyesterifications II. Reactions of Diacids with Diepoxides. Kinet. Katal. 1967, 8, 512. [Google Scholar]
- Ogg, C.L.; Porter, W.L.; Willits, C.O. Determining the Hydroxyl Content of Certain Organic Compounds Macro-and Semimicromethods. Ind. Eng. Chem. Anal. Ed. 1945, 17, 394–397. [Google Scholar] [CrossRef]

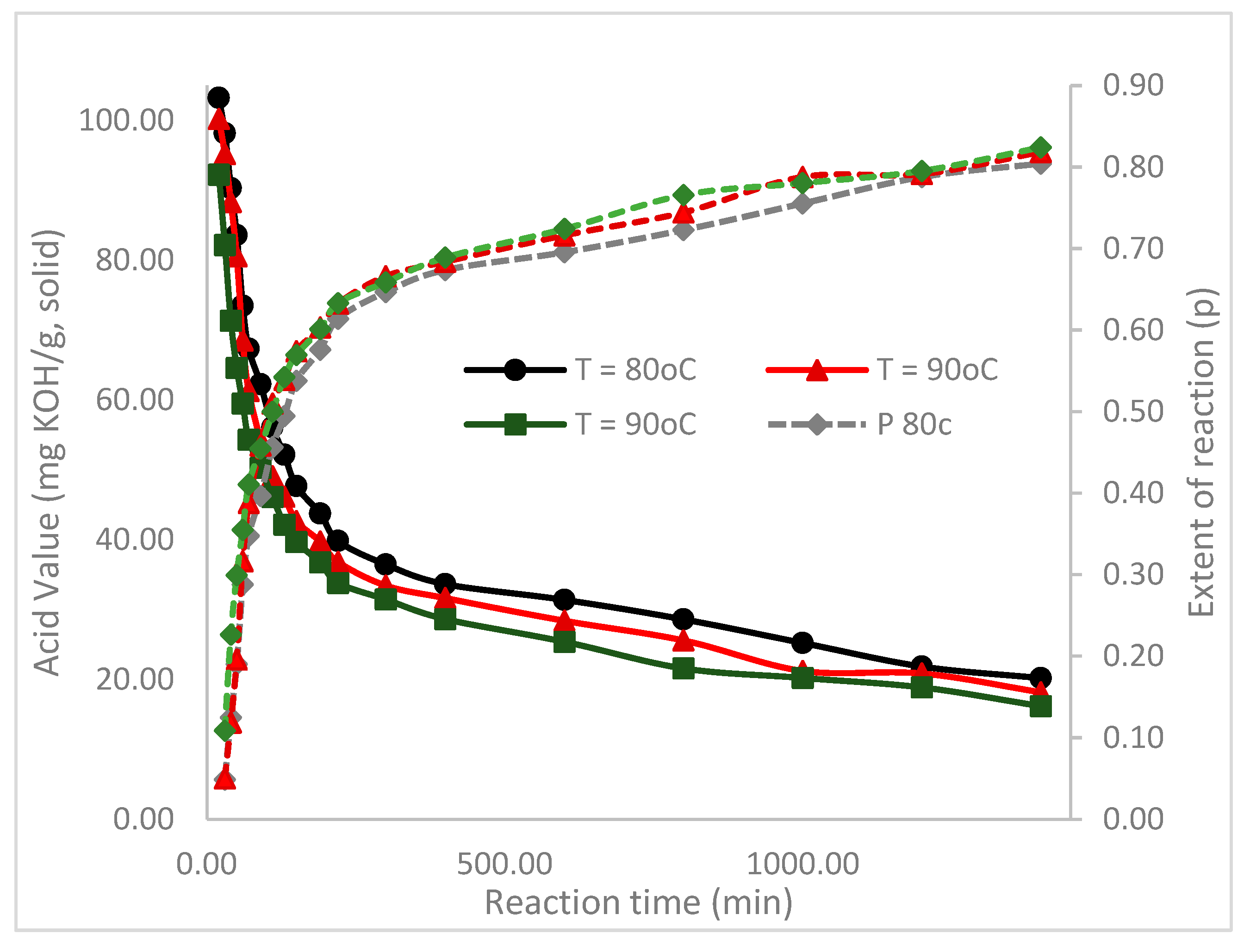


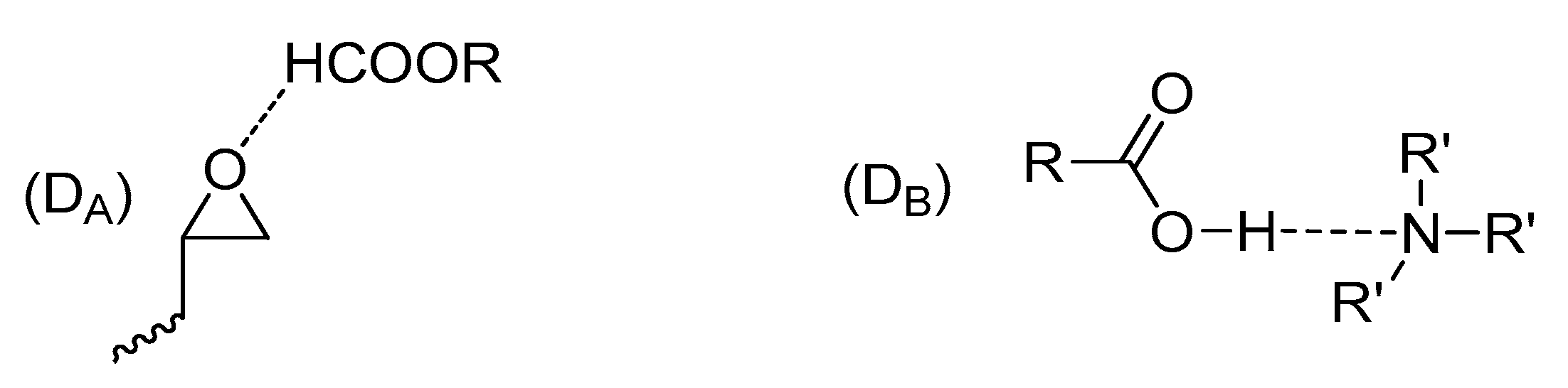


| Time (min) | 80 °C | 90 °C | 100 °C | |||
|---|---|---|---|---|---|---|
| p | p | p | ||||
| 30.00 | 0.05 | 1.05 | 0.05 | 1.05 | 0.11 | 1.12 |
| 40.00 | 0.12 | 1.14 | 0.12 | 1.13 | 0.23 | 1.29 |
| 50.00 | 0.19 | 1.23 | 0.20 | 1.24 | 0.30 | 1.43 |
| 60.00 | 0.29 | 1.40 | 0.32 | 1.46 | 0.35 | 1.55 |
| 70.00 | 0.35 | 1.53 | 0.39 | 1.63 | 0.41 | 1.70 |
| 90.00 | 0.40 | 1.66 | 0.46 | 1.85 | 0.45 | 1.83 |
| 110.00 | 0.46 | 1.84 | 0.51 | 2.04 | 0.50 | 2.00 |
| 130.00 | 0.49 | 1.98 | 0.54 | 2.17 | 0.54 | 2.19 |
| 150.00 | 0.54 | 2.16 | 0.57 | 2.35 | 0.57 | 2.32 |
| 190.00 | 0.58 | 2.36 | 0.60 | 2.52 | 0.60 | 2.51 |
| 220.00 | 0.61 | 2.59 | 0.63 | 2.72 | 0.63 | 2.73 |
| 300 | 0.65 | 2.83 | 0.67 | 2.99 | 0.66 | 2.93 |
| 400 | 0.67 | 3.07 | 0.68 | 3.17 | 0.69 | 3.22 |
| 600 | 0.70 | 3.29 | 0.72 | 3.53 | 0.72 | 3.63 |
| 800 | 0.72 | 3.61 | 0.74 | 3.91 | 0.77 | 4.27 |
| 1000 | 0.76 | 4.09 | 0.79 | 4.72 | 0.78 | 4.56 |
| 1200 | 0.79 | 4.72 | 0.79 | 4.80 | 0.80 | 4.88 |
| 1400 | 0.80 | 5.11 | 0.82 | 5.51 | 0.82 | 5.69 |
| T | First Order Rate Equation (6) | |
|---|---|---|
| K | R | |
| 80 °C | 0.0015 | 0.778 |
| 90 °C | 0.0016 | 0.770 |
| 100 °C | 0.00162 | 0.813 |
| Pathway | Compound | ΔE (kJ/mol) |
|---|---|---|
| Ri | TSi | 119 |
| Ri-hb | TSi-hb | 76 |
| Pi-hb | −46 | |
| Zi | TSzi | 140 |
| Pzi | 117 | |
| Zi-hb | TS1zi-hb | 64 |
| P1zi-hb | 29 | |
| Raa | TSaa | 189 |
| Paa | −49 | |
| Raa-hb | TSaa-hb | 113 |
| Paa-hb | −46 |
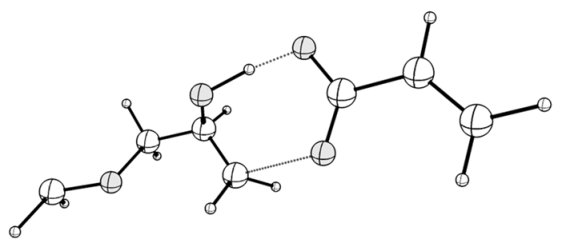 | 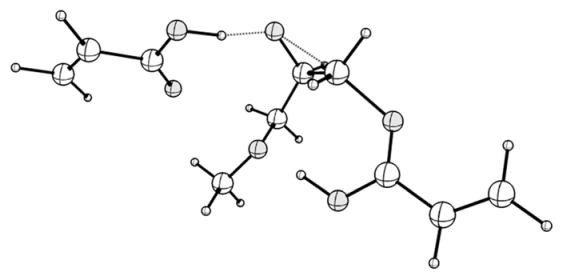 |
| TS_i: <O1-C1-C2 = 88.42, C2-O2 = 2.286, ν (i, cm−1) = −771 | TS_i_hb: <O1-C1-C2 = 91.84, C2-O2 = 1.757, ν (i, cm−1) = −402 |
 | 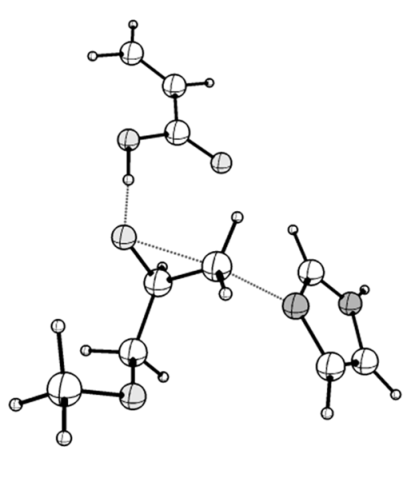 |
| TS_1z: <O1-C1-C2 = 91.69, C2-N1 = 1.799, ν (i, cm−1) = −442 | TS_1z_hb: <O1-C1-C2 = 82.87, C2-N1 = 2.030, ν (i, cm−1) = −456 |
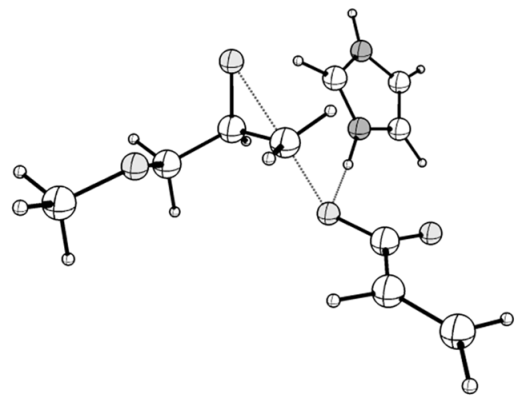 | 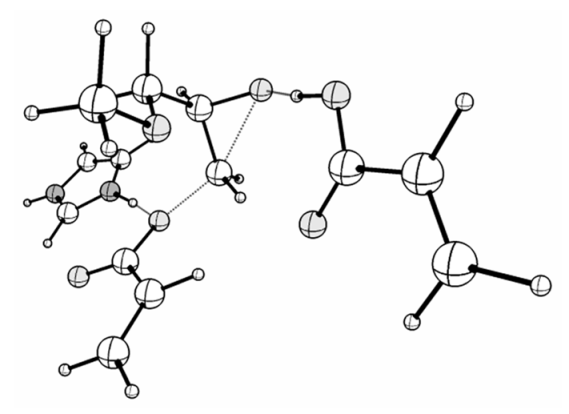 |
| TS_Raa: <O1-C1-C2 = 90.38, C2-O2 = 1.899, ν (i, cm−1) = −449 | TS_Raa-hb: <O1-C1-C2 = 89.53, C2-O2 = 1.837, ν (i, cm−1) = −482 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saif, M.J.; Fazal-ur-Rehman; Abrar, S.; Hameed, A.; Idrees, N.; Asif, M. Theoretical and Experimental Insights of Benzimidazole Catalyzed by the Epoxy–Acrylic Acid Reaction. Molecules 2022, 27, 7900. https://doi.org/10.3390/molecules27227900
Saif MJ, Fazal-ur-Rehman, Abrar S, Hameed A, Idrees N, Asif M. Theoretical and Experimental Insights of Benzimidazole Catalyzed by the Epoxy–Acrylic Acid Reaction. Molecules. 2022; 27(22):7900. https://doi.org/10.3390/molecules27227900
Chicago/Turabian StyleSaif, Muhammad Jawwad, Fazal-ur-Rehman, Shazia Abrar, Arruje Hameed, Nazeran Idrees, and Muhammad Asif. 2022. "Theoretical and Experimental Insights of Benzimidazole Catalyzed by the Epoxy–Acrylic Acid Reaction" Molecules 27, no. 22: 7900. https://doi.org/10.3390/molecules27227900
APA StyleSaif, M. J., Fazal-ur-Rehman, Abrar, S., Hameed, A., Idrees, N., & Asif, M. (2022). Theoretical and Experimental Insights of Benzimidazole Catalyzed by the Epoxy–Acrylic Acid Reaction. Molecules, 27(22), 7900. https://doi.org/10.3390/molecules27227900






