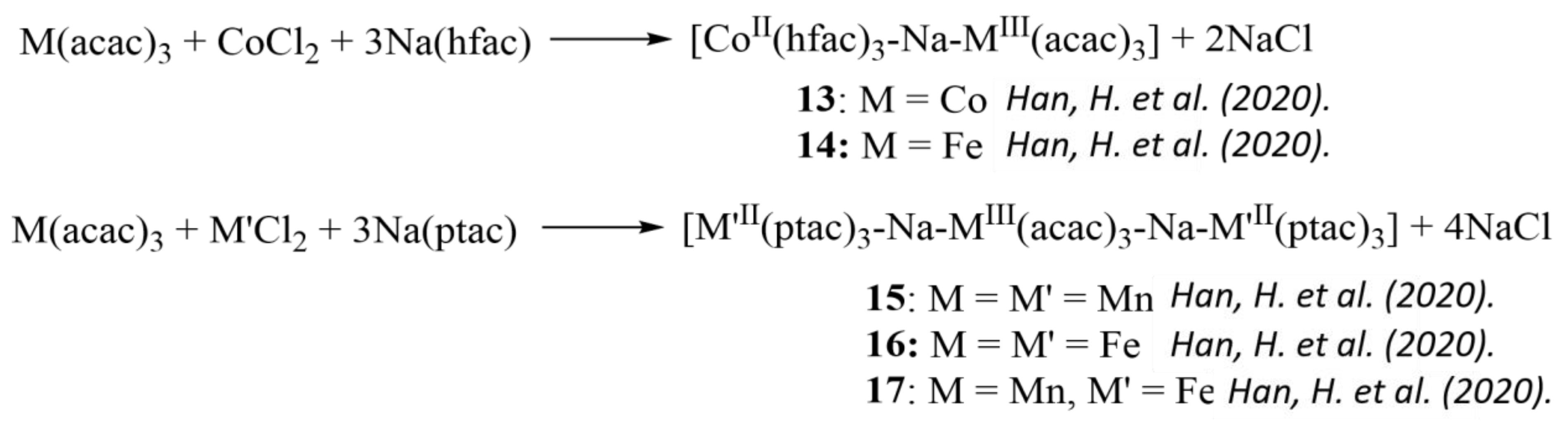Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands
Abstract
1. Introduction
2. Overview of Fluorinated β-Diketones Used as Ligands
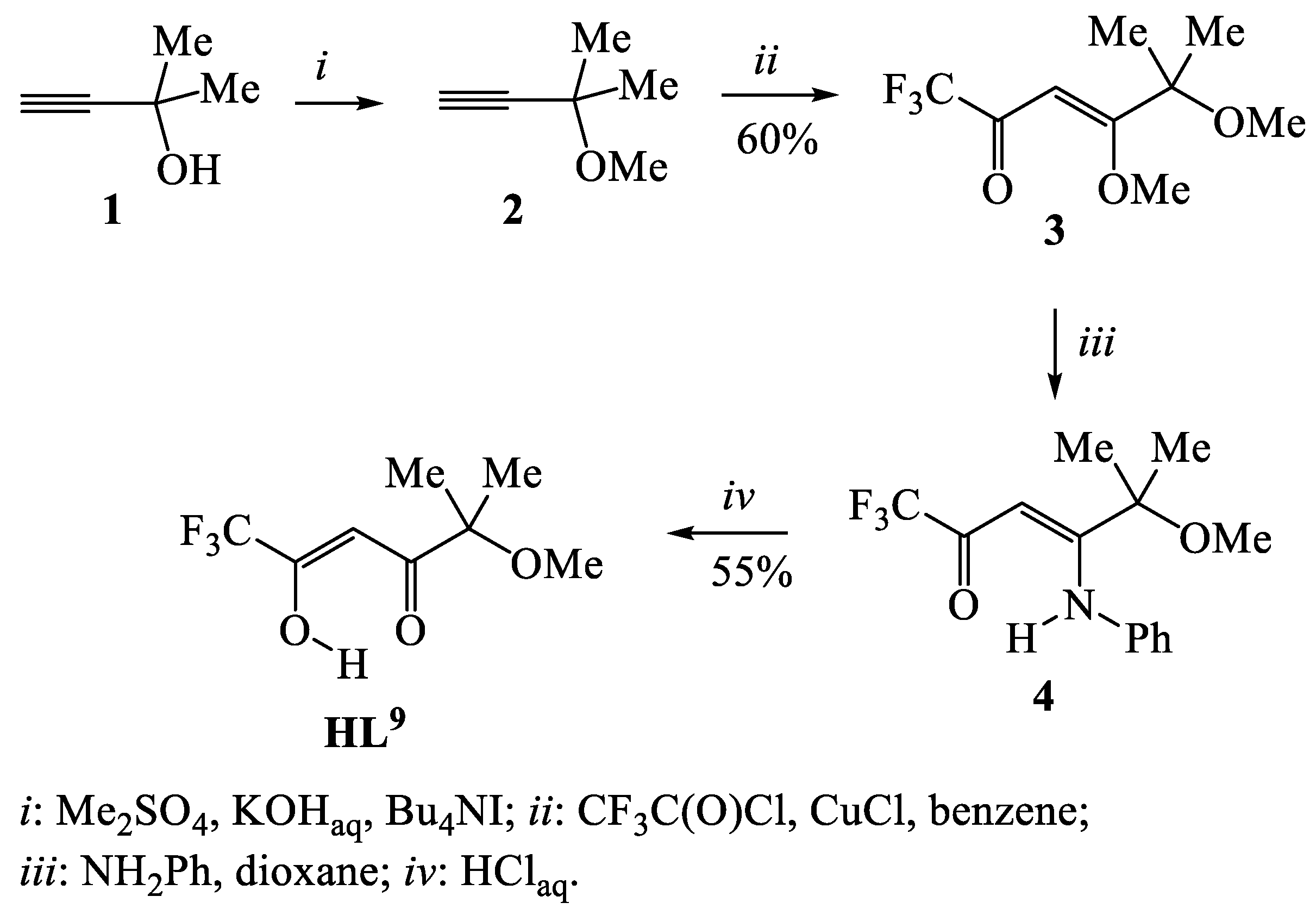
3. The Synthesis of Fluorinated Ligands, Containing the β-Dicarbonyl Fragment
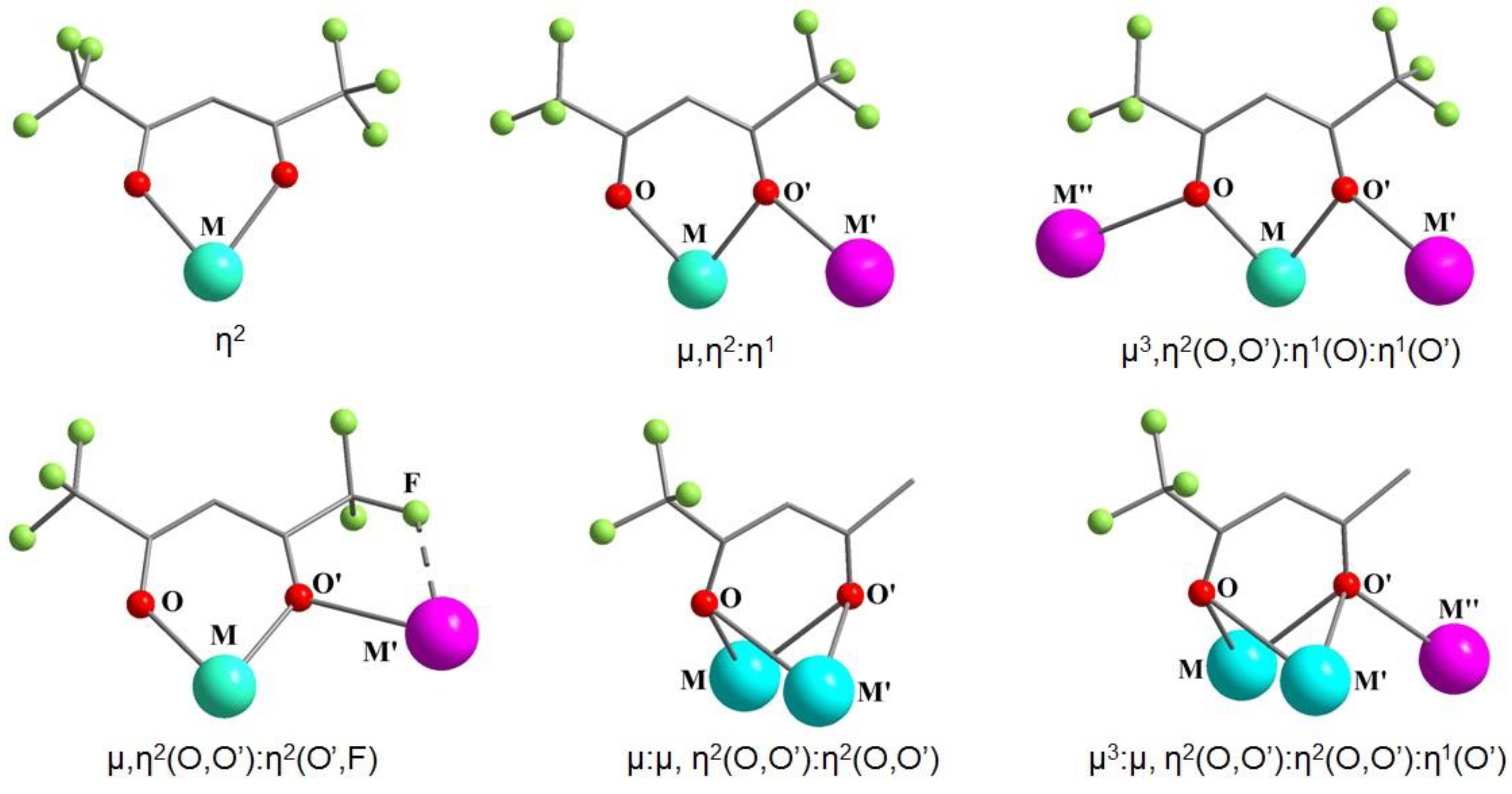
4. Coordination Modes of Fluorinated β-Diketonates
5. Heterometallic Complexes Containing Bridging β-Diketonate Anions
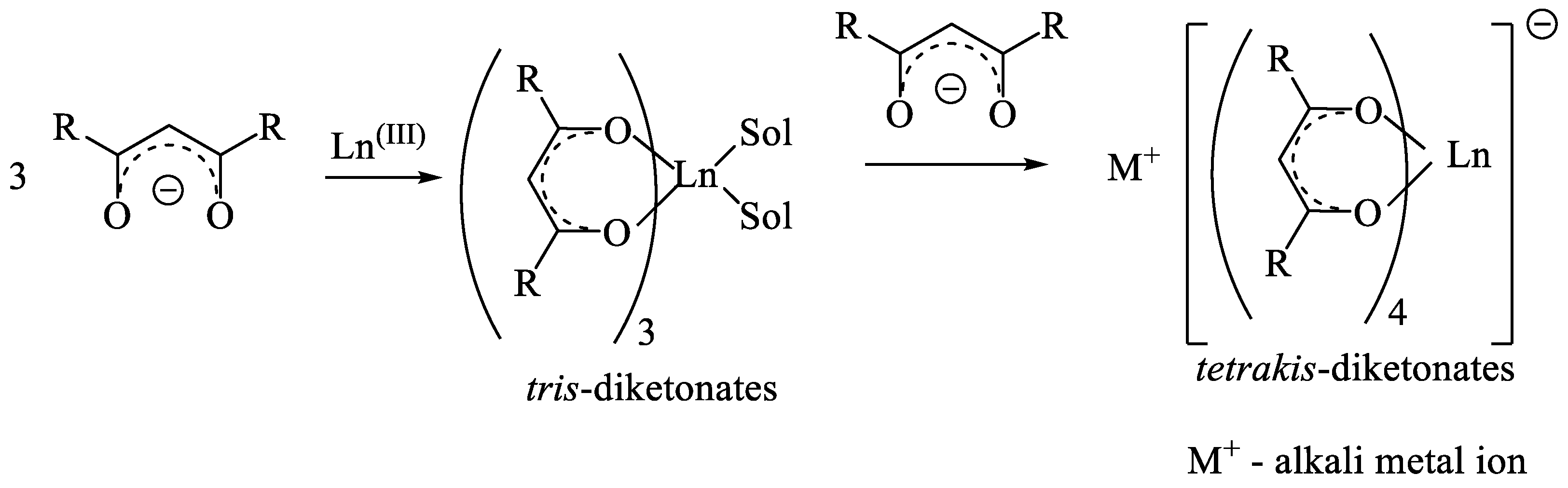
6. Lanthanide(III) tetrakis-β-Diketonates
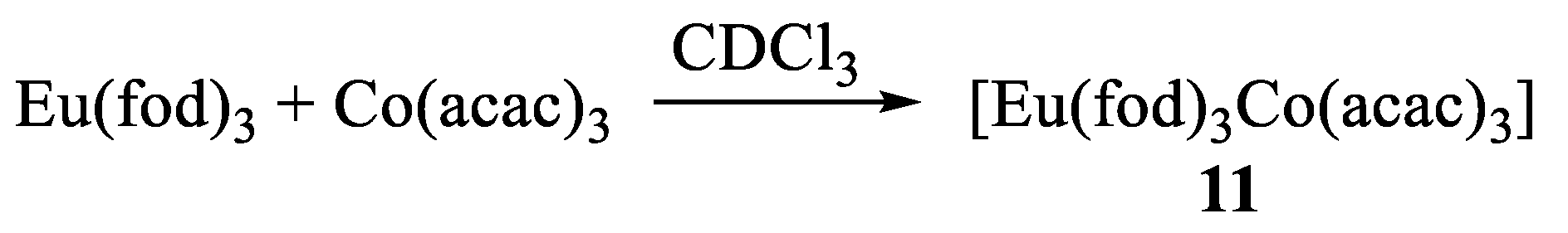
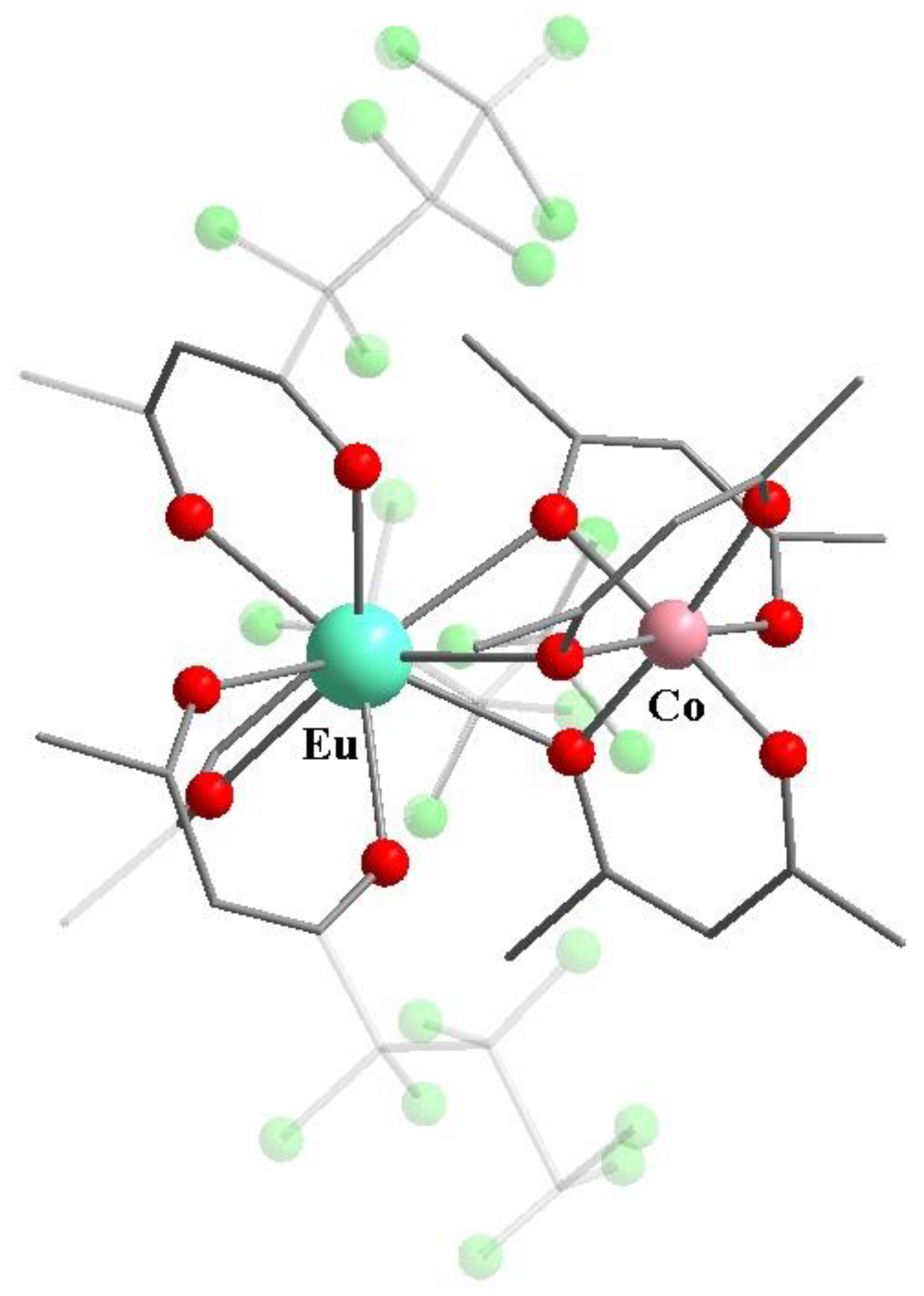
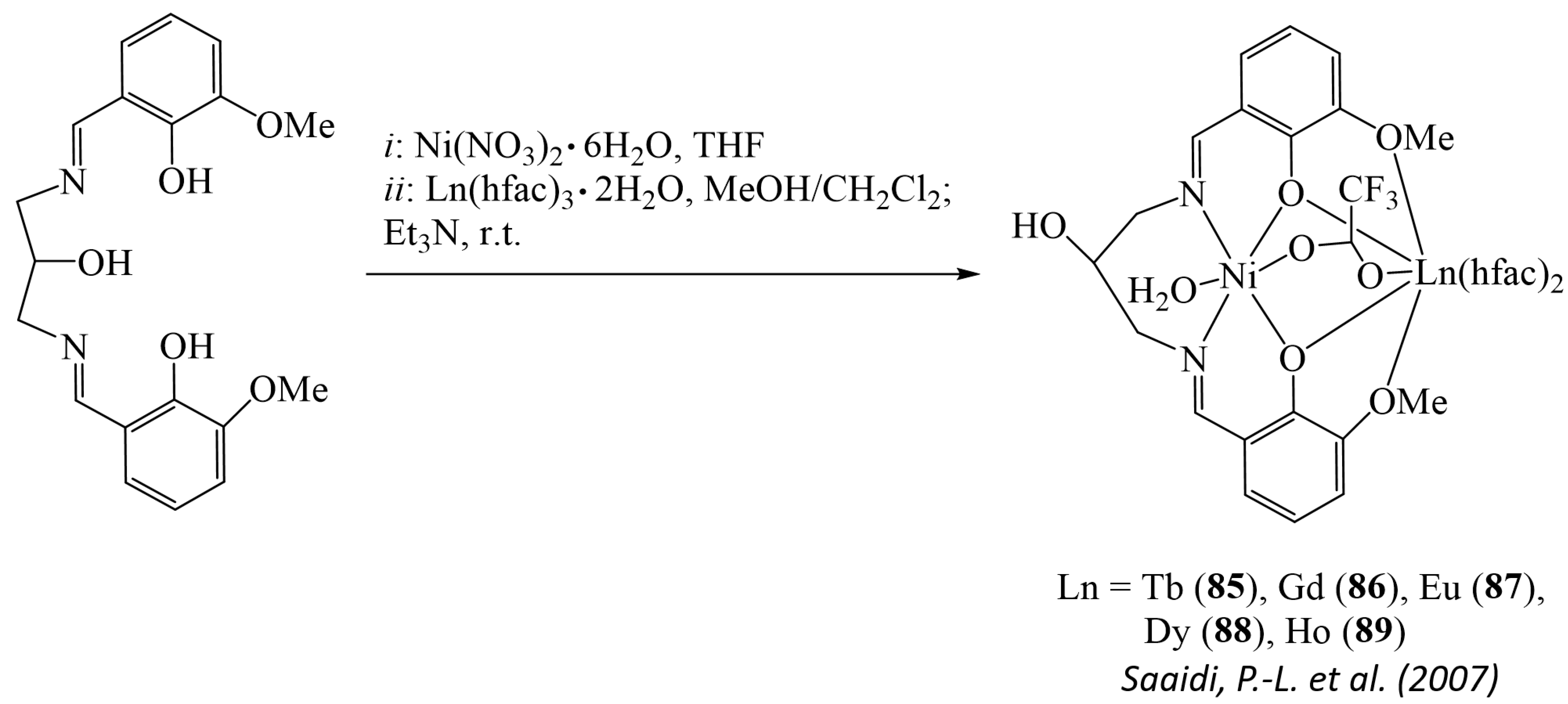
7. Heteronuclear Complexes with Isolated Metal Centers
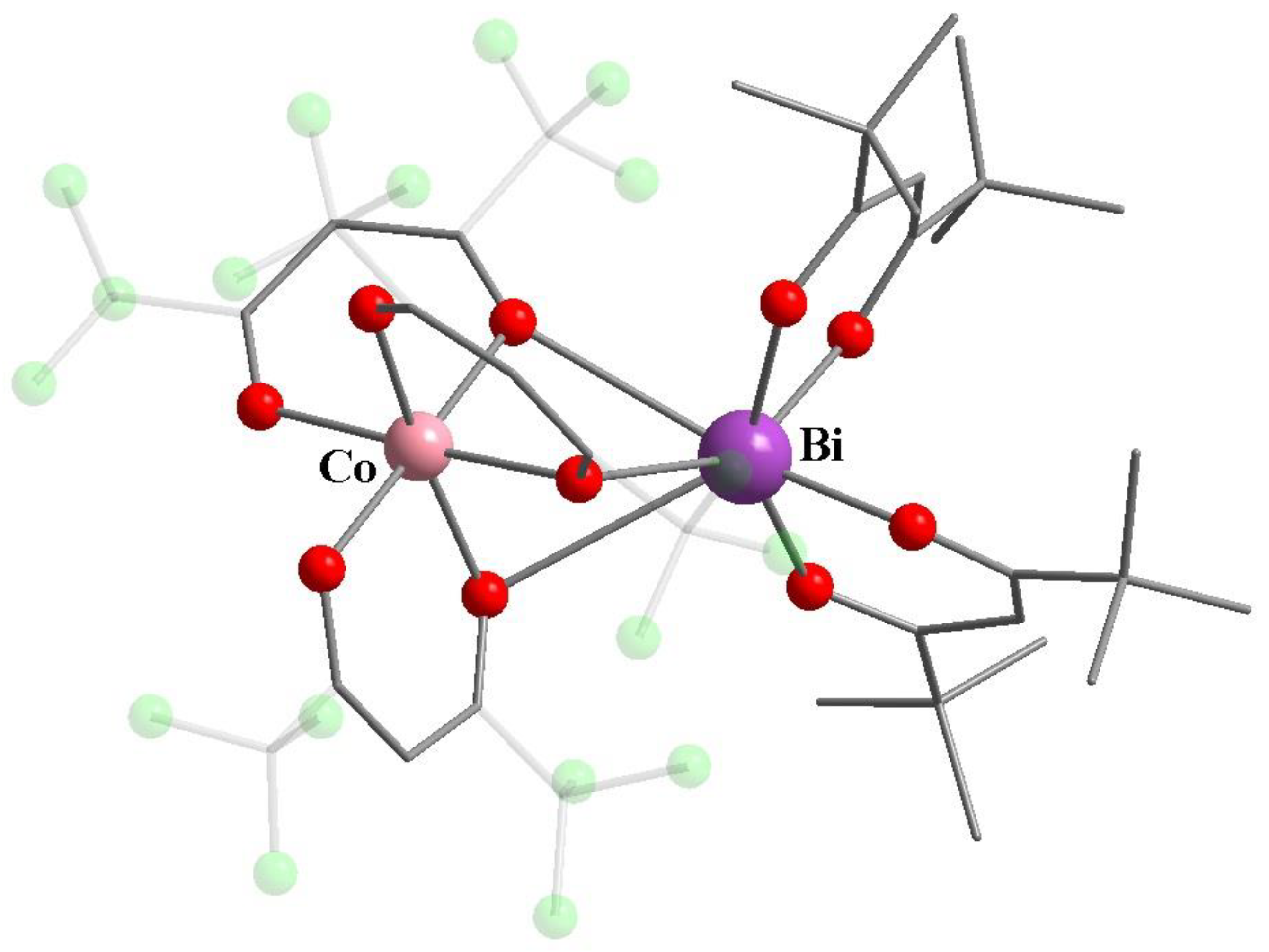
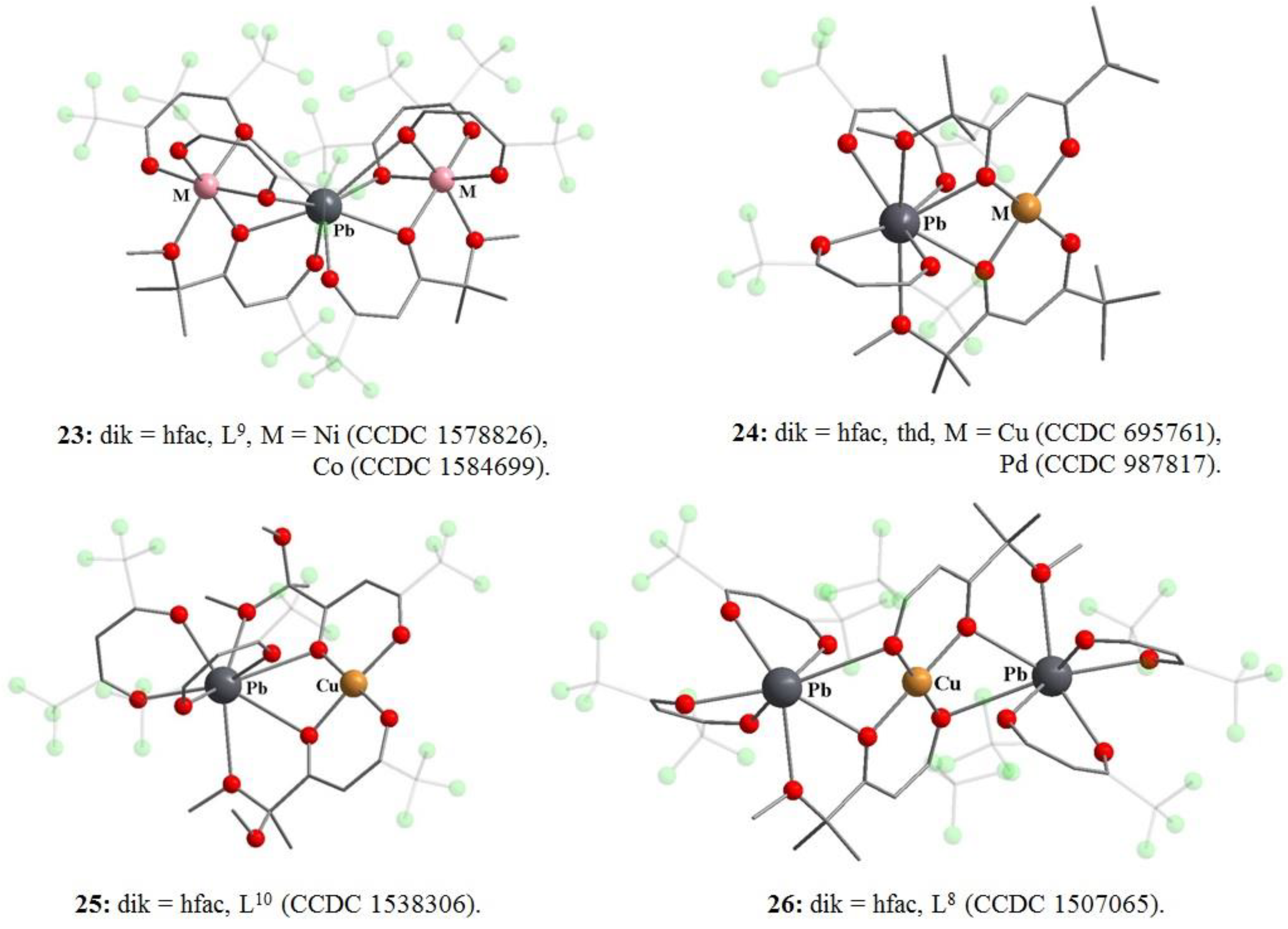
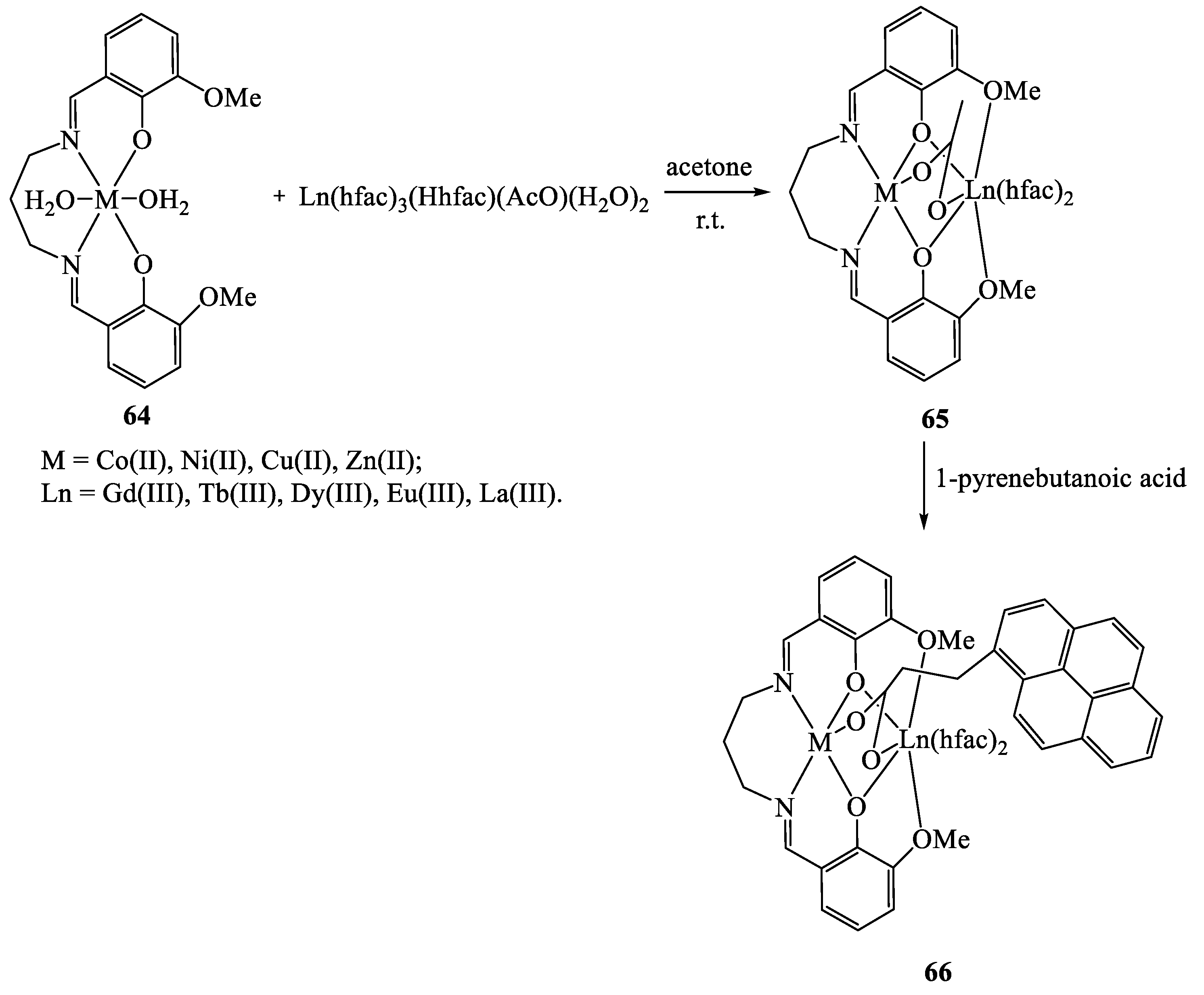
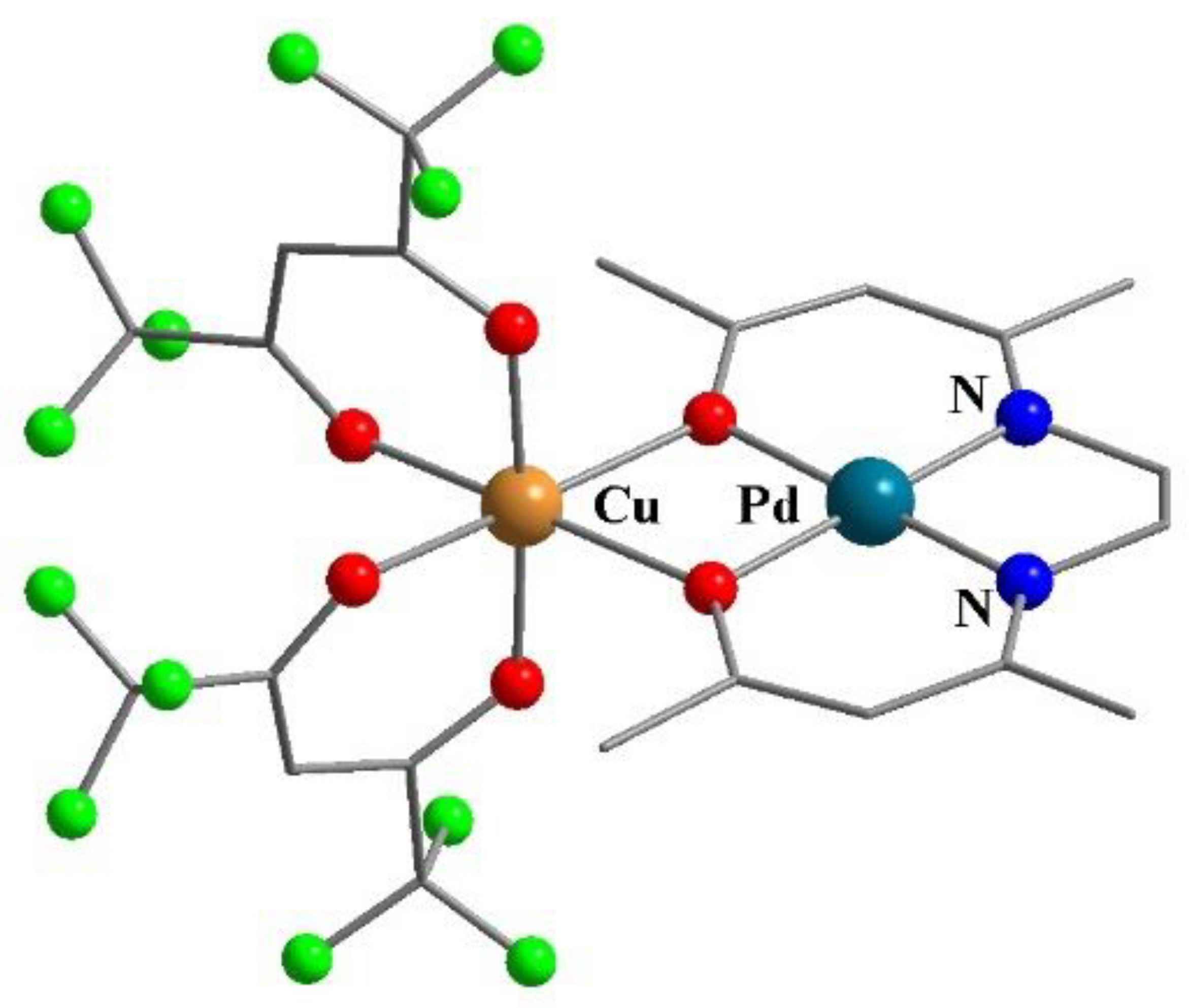
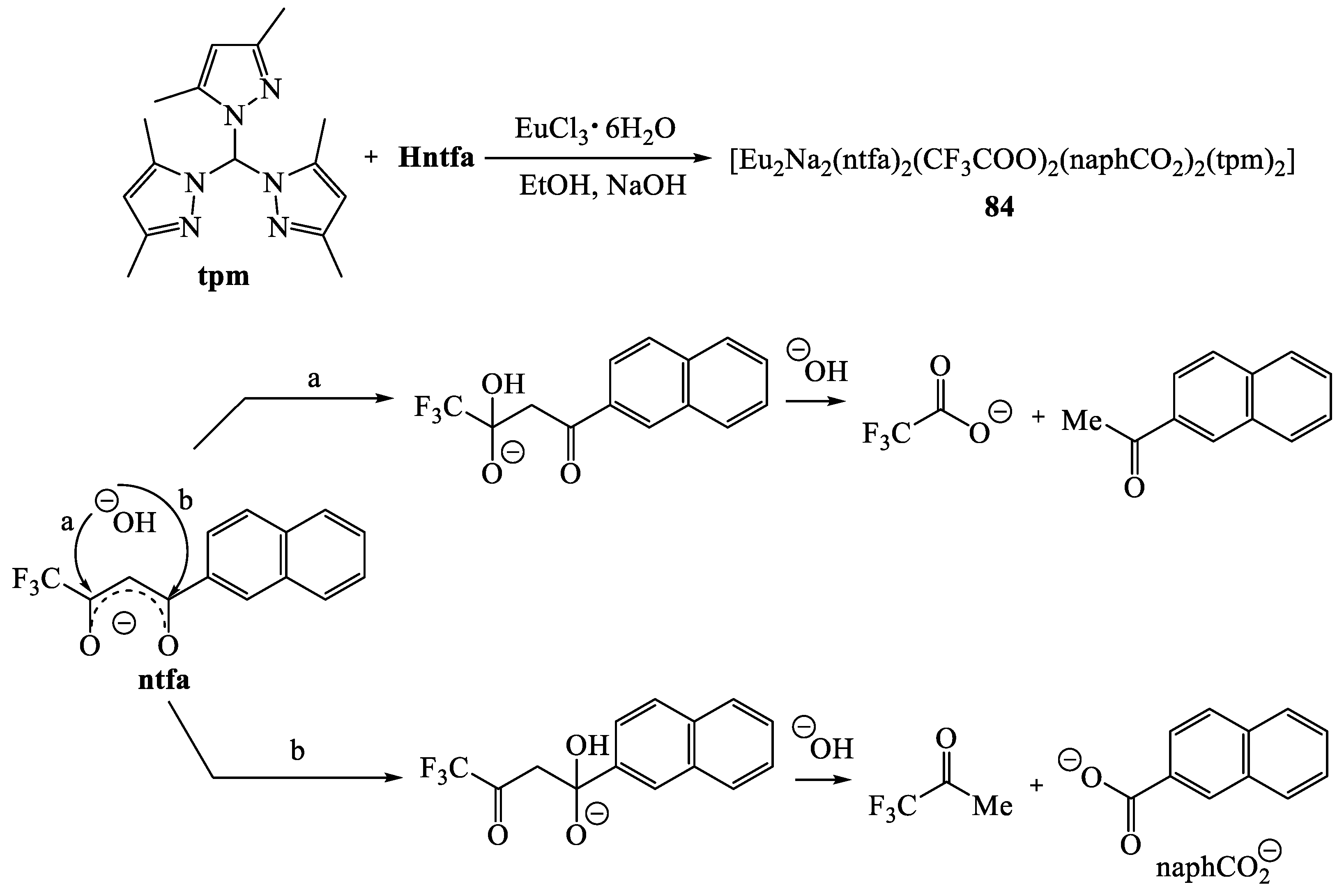
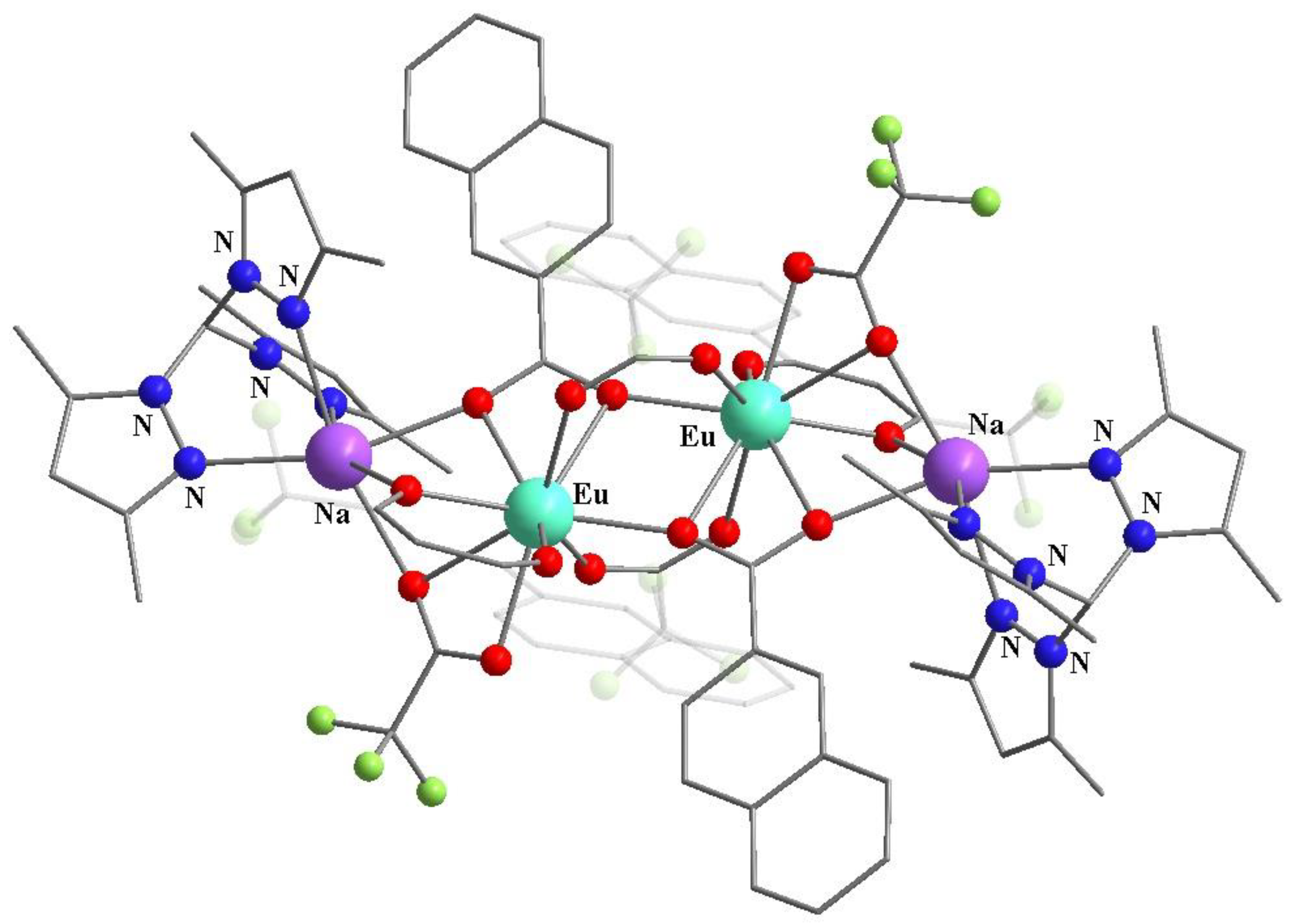
8. Binuclear Complexes Based on β-Diketones with an Additional Chelating Cavity
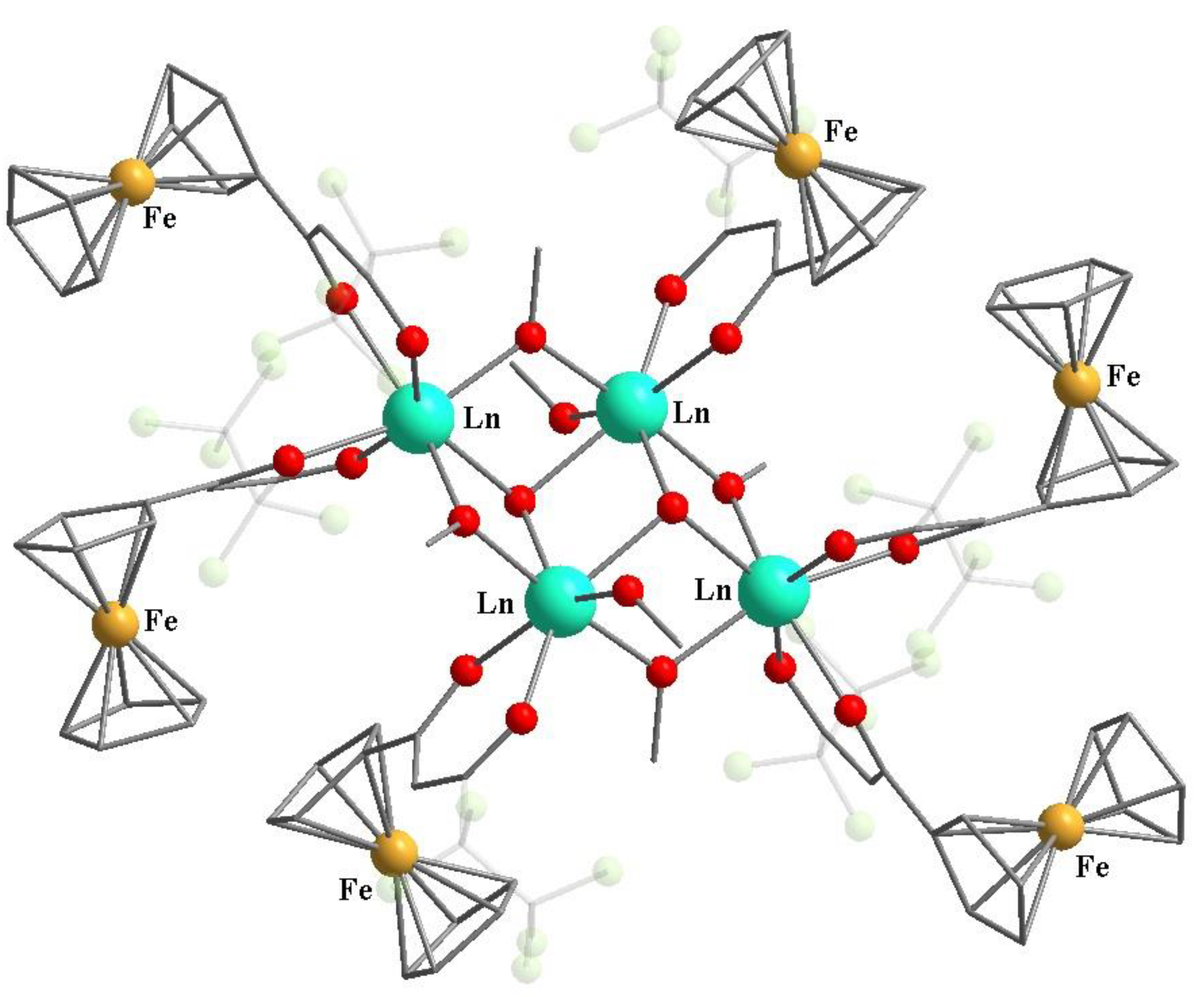
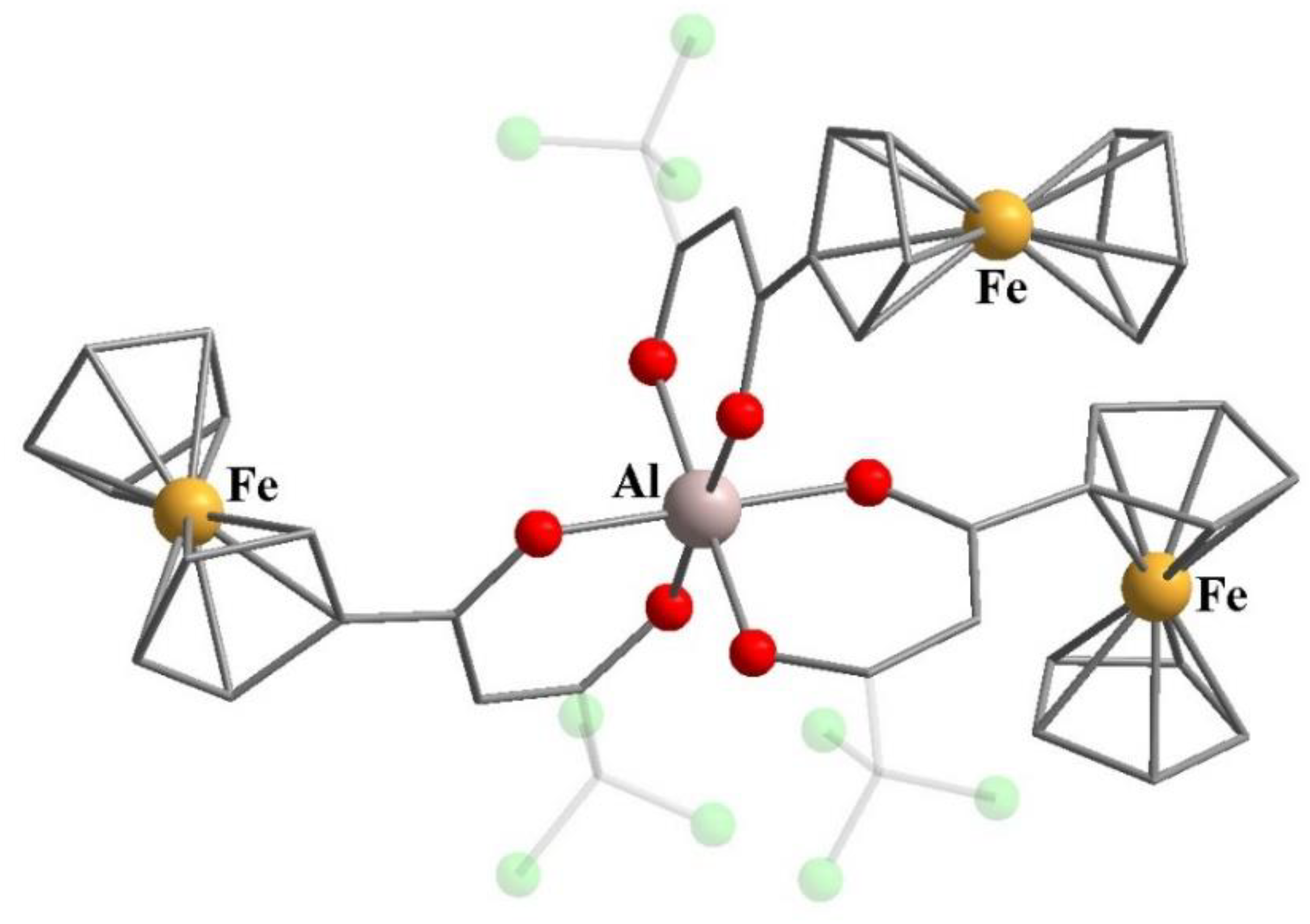
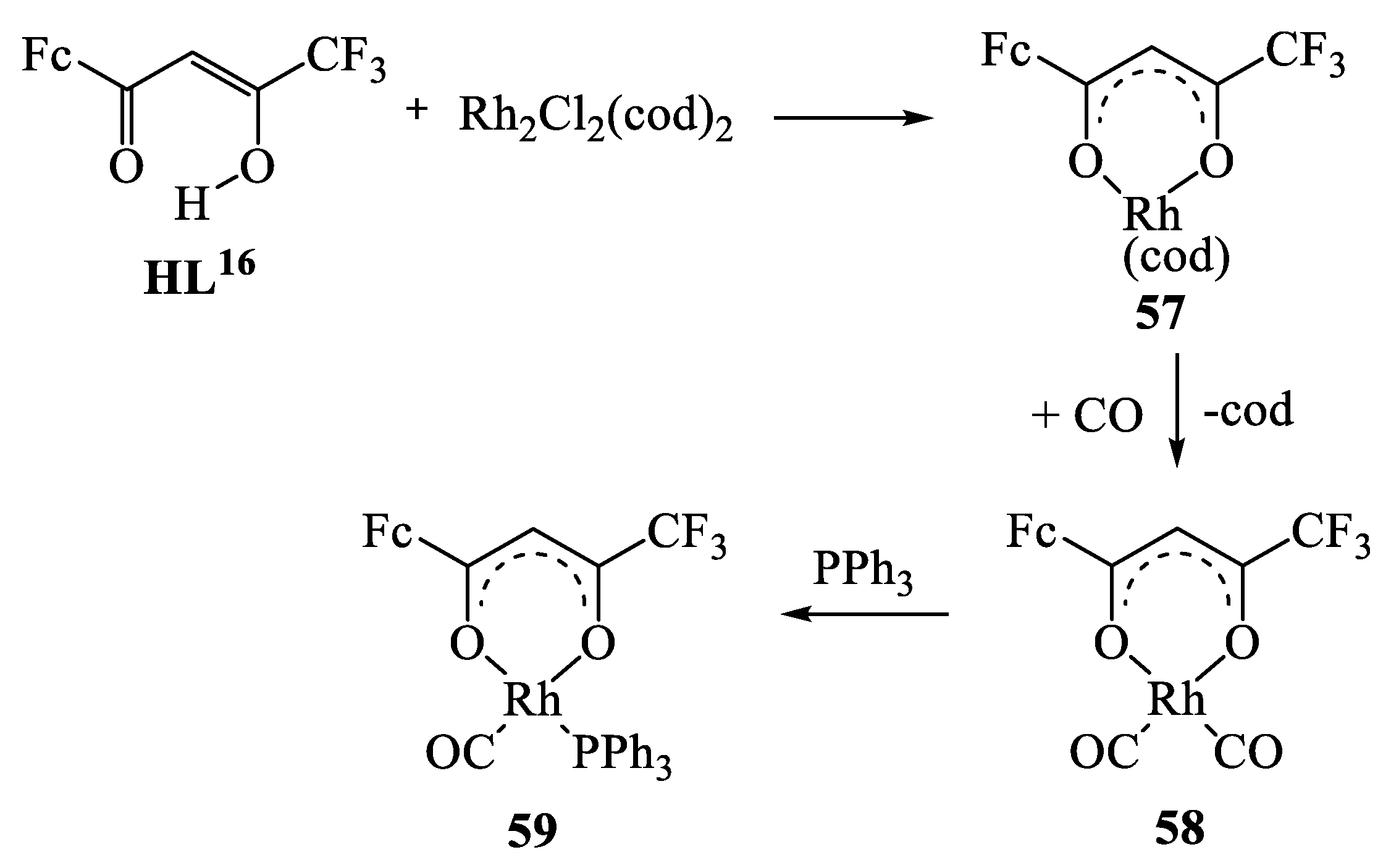
9. Metallocene-Containing Heterometallic β-Diketonates
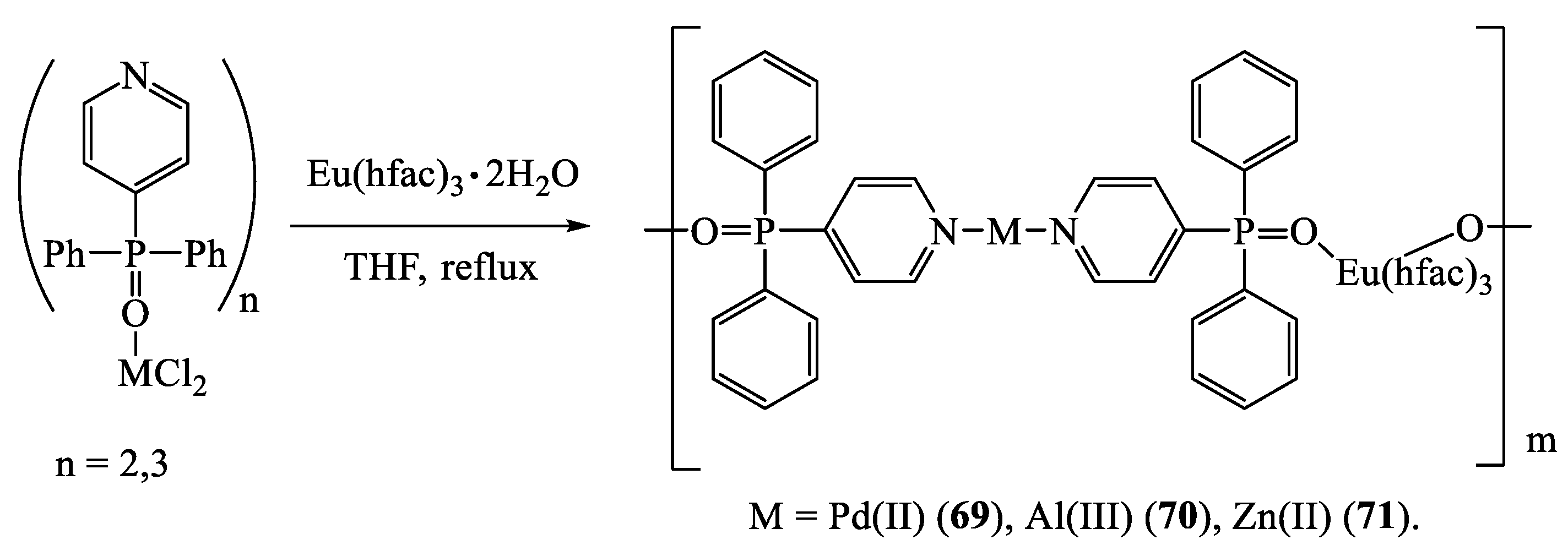
10. Heteroleptic Complexes Involving β-Diketonates and Polytopic Coligands
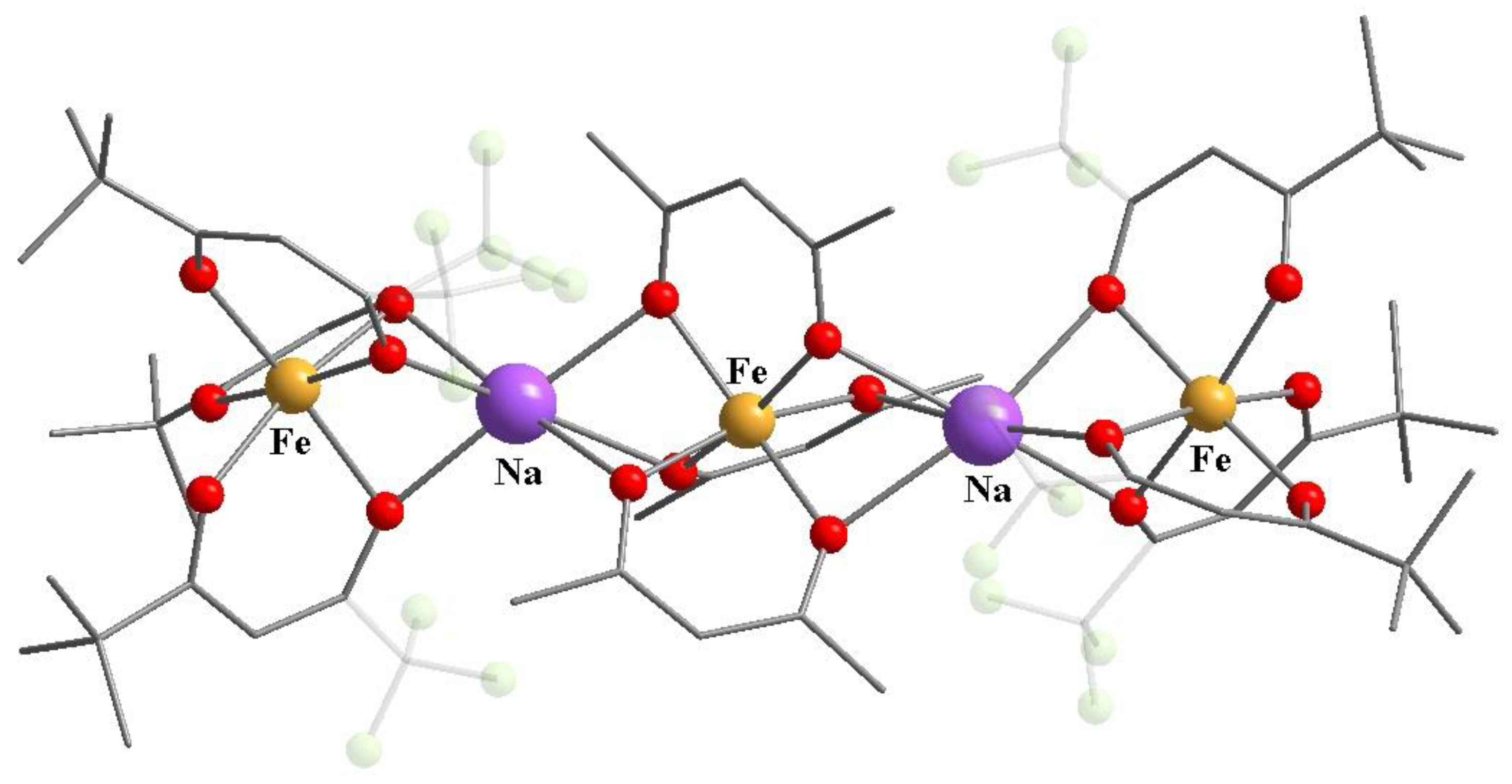
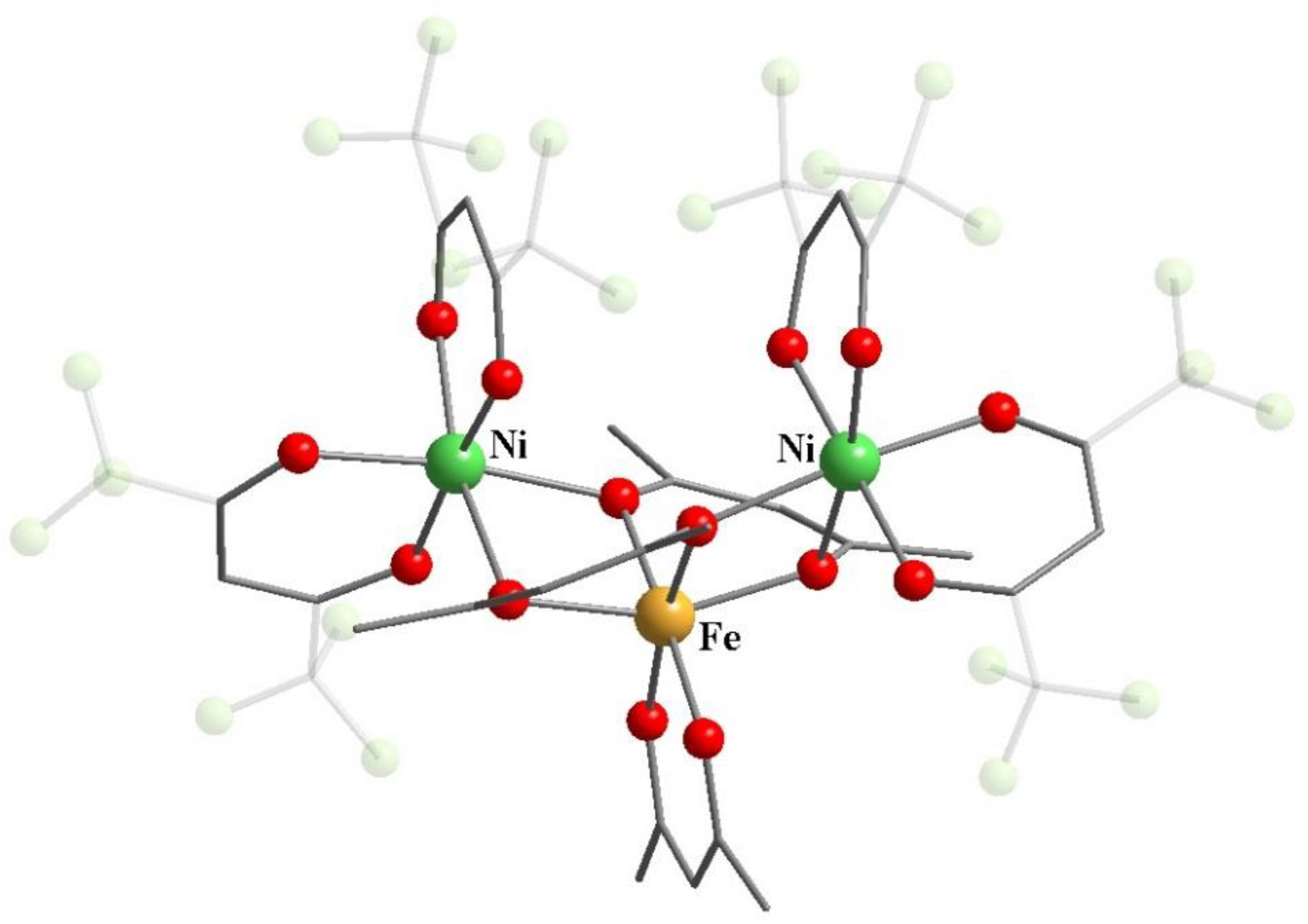
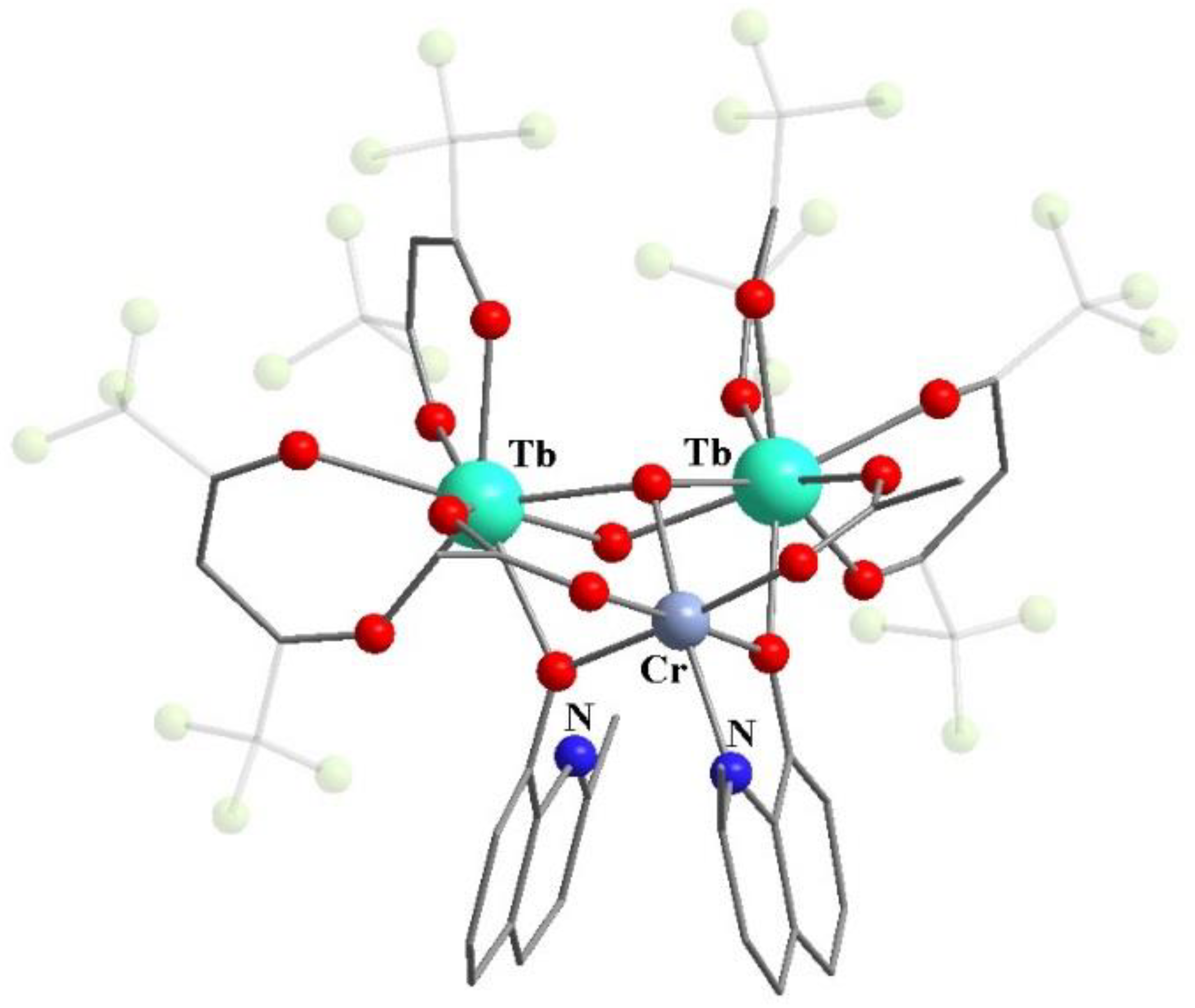
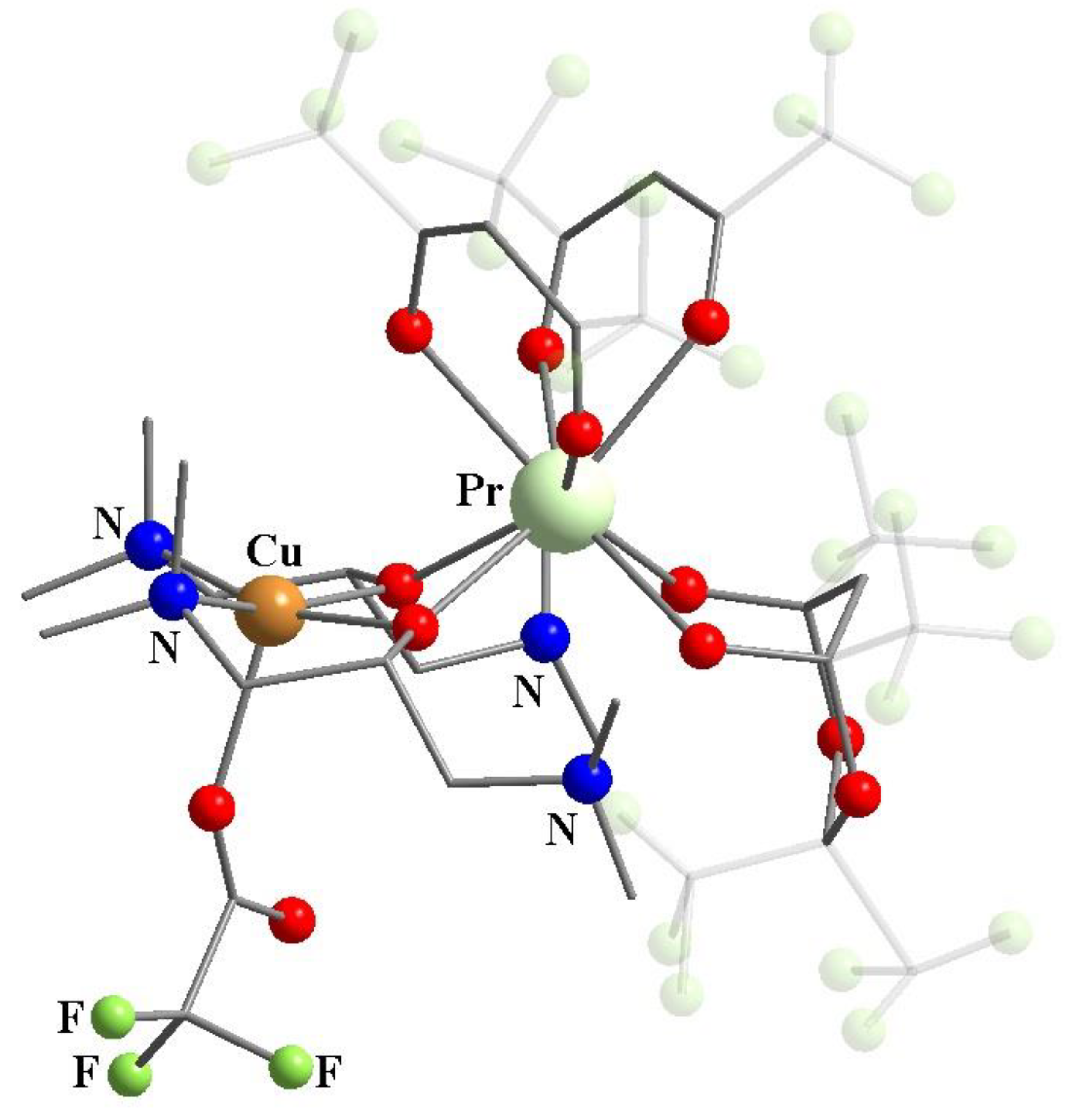
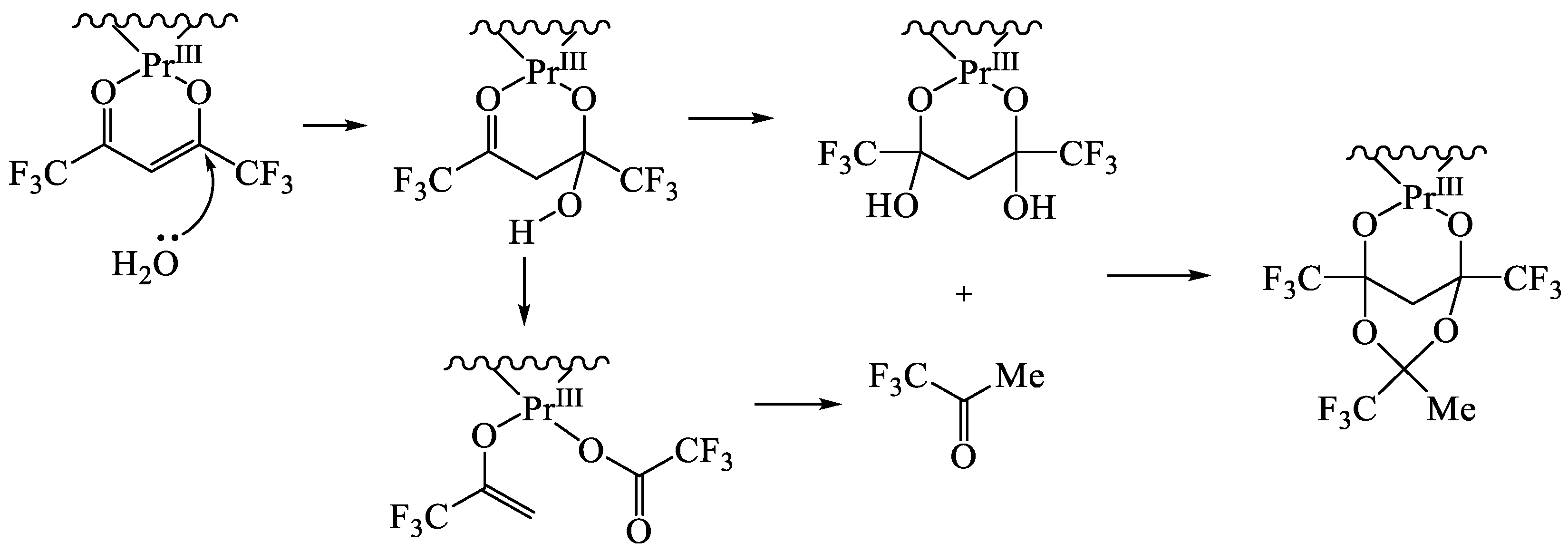
11. Heterometallic Complex Synthesis Based on hfac Transformations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
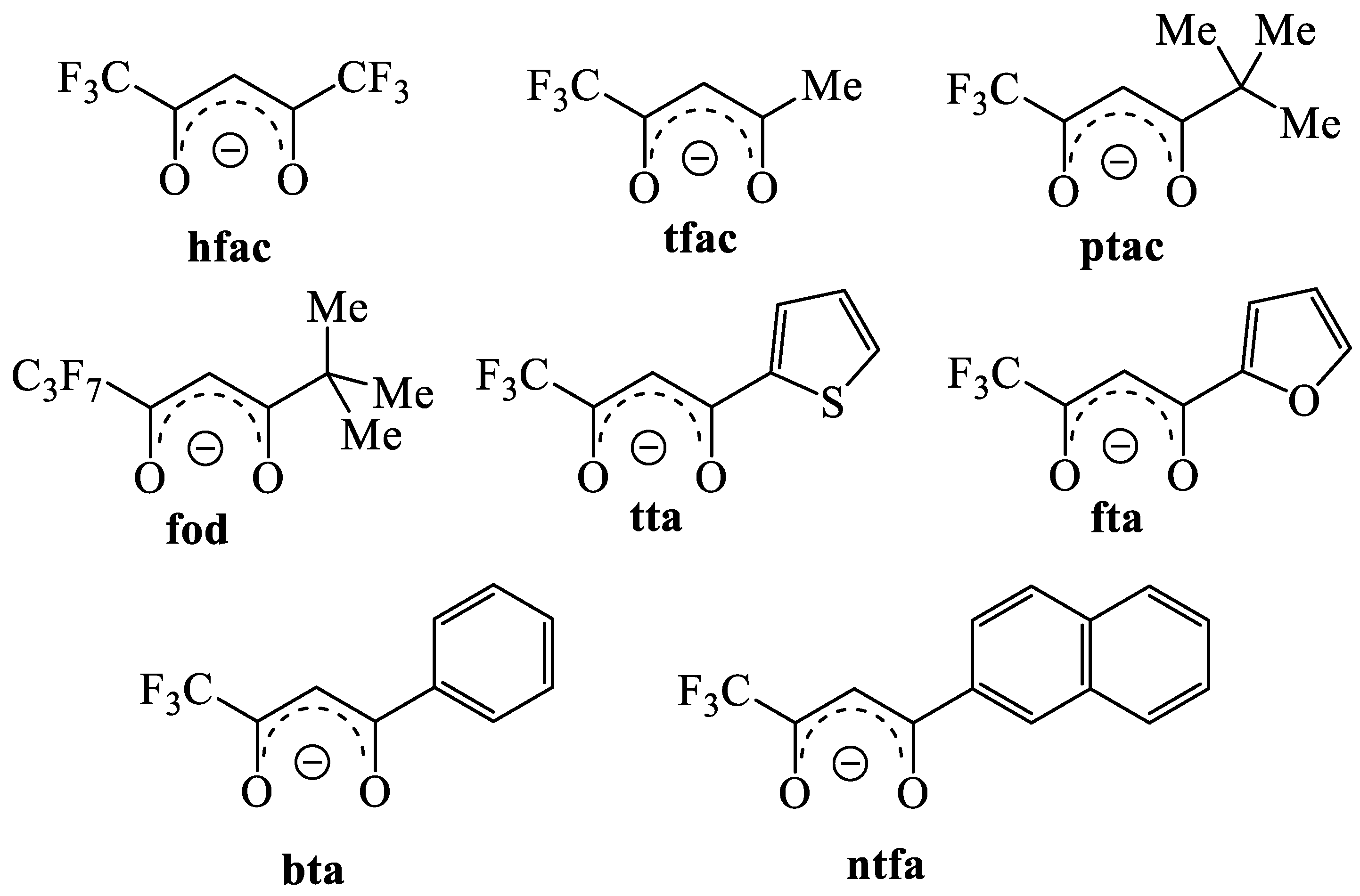
Abbreviations
| MOCVD | metal organic chemical vapor deposition; |
| hfac | 1,1,1,5,5,5-hexafluoro-2,4-pentanedionate; |
| tfa | trifluoroacetate; |
| tfac | 1,1,1-trifluoro-2,4-pentanedionate; |
| ptac | 1,1,1-trifluoro-5,5-dimethyl-2,4-hexanedionate; |
| fod | 6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedionate; |
| tta | thenoyltrifluoroacetylacetonate; |
| fta | furanoyltrifluoroacetylacetonate; |
| bta | benzoyltrifluoroacetone; |
| ntfa | 4,4,4-trifluoro-1-(2-naphtyl)-1,3-butanedionate; |
| Na2EDTA | ethylenediaminetetraacetic acid disodium salt; |
| PTSA | p-toluenesulfonic acid; |
| dik | β-diketonate; |
| acac | acetylacetonate; |
| thd | 2,2,6,6-tetramethyl-3,5-heptanedionate; |
| pfbm | bis(pentafluorobenzoyl)methane; |
| Cp | cyclopentadienyl; |
| Fc | ferrocene; |
| DME | 1,2-dimethoxyethane; |
| DMF | dimethylformamide; |
| Et | ethyl; |
| LDA | lithium di(isopropyl)amide; |
| Me | methyl; |
| THF | tetrahydrofuran; |
| ttpt | tris-CF3-tetrahydropyran-2,4,6-triol; |
| bptz | 3,6-bis(2-pyridyl)-1,2,4,5-tetrazine; |
| phen | 1,10-phenantroline; |
| bdmap | 1,3-bis(dimethylamino)-2-propanol. |
References
- Jain, A. Multifunctional, heterometallic ruthenium-platinum complexes with medicinal applications. Coord. Chem. Rev. 2019, 401, 213067. [Google Scholar] [CrossRef]
- Liu, J.; Xue, J.; Yang, G.-P.; Dang, L.-L.; Ma, L.-F.; Li, D.-S.; Wang, Y.-Y. Recent advances of functional heterometallic-organic framework (HMOF) materials: Design strategies and applications. Coord. Chem. Rev. 2022, 463, 214521. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Fang, W.-H.; Zhang, L.; Zhang, J. Recent advances in heterometallic polyoxotitanium clusters. Coord. Chem. Rev. 2020, 404, 213099. [Google Scholar] [CrossRef]
- Wang, J.; Feng, M.; Akhtar, M.N.; Tong, M.-L. Recent advance in heterometallic nanomagnets based on TMxLn4 cubane subunits. Coord. Chem. Rev. 2019, 387, 129–153. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C-H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII–LnIII complexes: Single molecule magnets and magnetic refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Gil-Rubio, J.; Vicente, J. The coordination and supramolecular chemistry of gold metalloligands. Chem. Eur. J. 2018, 24, 32–46. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-diketonate complexes as energy-efficient emissive materials: A review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Clegg, J.K.; Li, F.; Lindoy, L.F. Oligo-β-diketones as versatile ligands for use in metallo-supramolecular chemistry: Recent progress and perspectives. Coord. Chem. Rev. 2022, 455, 214355. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Malandrino, G.; Fragalà, I.L. Engineering of molecular architectures of β-diketonate precursors toward new advanced materials. Coord. Chem. Rev. 2007, 251, 1931–1950. [Google Scholar] [CrossRef]
- Gromilov, S.A.; Baidina, I.A. Regularities of Crystal Structures of Cu(II) β-Diketonates. J. Struct. Chem. 2004, 45, 1031–1081. [Google Scholar] [CrossRef]
- Steinborn, D. The unique chemistry of platina-β-diketones. Dalton Trans. 2005, 16, 2664. [Google Scholar] [CrossRef] [PubMed]
- Zolotareva, N.V.; Semenov, V.V. β-Diketones and their derivatives in sol–gel processes. Russ. Chem. Rev. 2013, 82, 964–987. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015, 7, e223. [Google Scholar] [CrossRef]
- Malandrino, G.; Pellegrino, A.L.; Lucchini, G.; Speghini, A. Energy conversion systems: Molecular architecture engineering of metal precursors and their applications to vapor phase and solution routes. J. Mater. Res. 2020, 35, 2950–2966. [Google Scholar] [CrossRef]
- Fromm, K.M.; Gueneau, E.D. Structures of alkali and alkaline earth metal clusters with oxygen donor ligands. Polyhedron 2004, 23, 1479–1504. [Google Scholar] [CrossRef]
- Kaizaki, S. Coordination effects of nitroxide radicals in transition metal and lanthanide complexes. Coord. Chem. Rev. 2006, 250, 1804–1818. [Google Scholar] [CrossRef]
- Minkin, V.I.; Starikova, A.A. Molecular design of the valence tautomeric mixed-ligand adducts of CoII diketonates with redox-active ligands. Mendeleev Commun. 2015, 25, 83–92. [Google Scholar] [CrossRef]
- Malandrino, G.; Fragalà, I.L. Lanthanide “second-generation” precursors for MOCVD applications: Effects of the metal ionic radius and polyether length on coordination spheres and mass-transport properties. Coord. Chem. Rev. 2006, 250, 1605–1620. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Aromí, G.; Gamez, P.; Reedijk, J. Poly beta-diketones: Prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 2008, 252, 964–989. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Di Nicola, C.; Pettinari, C. Half-sandwich metal complexes with β-diketone-like ligands and their anticancer activity. Eur. J. Inorg. Chem. 2018, 2018, 3521–3536. [Google Scholar] [CrossRef]
- Kljun, J.; Turel, I. β-Diketones as scaffolds for anticancer drug design—From organic building blocks to natural products and metallodrug components. Eur. J. Inorg. Chem. 2017, 2017, 1655–1666. [Google Scholar] [CrossRef]
- Brock, A.J.; Clegg, J.K.; Li, F.; Lindoy, L.F. Recent developments in the metallo-supramolecular chemistry of oligo-β-diketonato ligands. Coord. Chem. Rev. 2018, 375, 106–133. [Google Scholar] [CrossRef]
- Clegg, J.K.; Li, F.; Lindoy, L.F. Di-, tri- and oligometallic platforms: Versatile components for use in metallo-supramolecular chemistry. Coord. Chem. Rev. 2013, 257, 2536–2550. [Google Scholar] [CrossRef]
- Hansen, P.E. Structural studies of β-diketones and their implications on biological effects. Pharmaceuticals 2021, 14, 1189. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Metal–organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef]
- Devi, A. ‘Old Chemistries’ for new applications: Perspectives for development of precursors for MOCVD and ALD applications. Coord. Chem. Rev. 2013, 257, 3332–3384. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Lin, L.-R.; Wang, X.; Wei, G.-N.; Tang, H.-H.; Zhang, H.; Ma, L.-H. Azobenzene-derived tris-β-diketonate lanthanide complexes: Reversible trans-to-cis photoisomerization in solution and solid state. Dalton Trans. 2016, 45, 14954–14964. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Yan, P.; Chen, P.; Hou, G.; Li, G. Synthesis, Crystal Structure, and Luminescent Properties of 2-(2,2,2-Trifluoroethyl)-1-indone Lanthanide Complexes. Inorg. Chem. 2012, 51, 5050–5057. [Google Scholar] [CrossRef] [PubMed]
- Ambili Raj, D.B.; Francis, B.; Reddy, M.L.P.; Butorac, R.R.; Lynch, V.M.; Cowley, A.H. Highly luminescent poly(methyl methacrylate)-incorporated europium complex supported by a carbazole-based fluorinated β-diketonate ligand and a 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene oxide co-ligand. Inorg. Chem. 2010, 49, 9055–9063. [Google Scholar] [CrossRef]
- Francis, B.; Heering, C.; Freire, R.O.; Reddy, M.L.P.; Janiak, C. Achieving visible light excitation in carbazole based Eu3+–β-diketonate complexes via molecular engineering. RSC Adv. 2015, 5, 90720. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Kreshchenova, Y.M.; Dolotova, E.P. A convenient and practical synthesis of β-diketones bearing linear perfluorinated alkyl groups and a 2-thienyl moiety. Beilstein J. Org. Chem. 2018, 14, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, H.; Chen, P.; Sun, W.; Yan, P. A series of triple-stranded lanthanide(III) helicates: Syntheses, structures and single molecular magnets. Polyhedron 2017, 126, 1–7. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Sun, W.; Chen, P. Crystallization and single molecule magnetic behavior of quadruple-stranded helicates: Tuning the anisotropic axes. Dalton Trans. 2020, 49, 2843–2849. [Google Scholar] [CrossRef]
- Shi, J.; Hou, Y.; Chu, W.; Shi, X.; Gu, H.; Wang, B.; Sun, Z. Crystal structure and highly luminescent properties studies of bis-β-diketonate lanthanide complexes. Inorg. Chem. 2013, 52, 5013–5022. [Google Scholar] [CrossRef]
- Tan, Y.B.; Yamada, M.; Katao, S.; Nishikawa, Y.; Asanoma, F.; Yuasa, J.; Kawai, T. Self-assembled tetranuclear EuIII complexes with D2- and C2h symmetrical square scaffold. Inorg. Chem. 2020, 59, 12867–12875. [Google Scholar] [CrossRef]
- Chen, P.; Li, H.; Sun, W.; Tang, J.; Zhang, L.; Yan, P. Crystallization of triple- and quadruple-stranded dinuclear bis-β-diketonate-Dy(III) helicates: Single molecule magnetic behavior. CrystEngComm 2015, 17, 7227–7232. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Y.; Gao, T.; Li, H.; Yan, P. The role of ancillary ligand on regulating photoluminescence properties of Eu(III) helicates. Inorg. Chim. Acta 2021, 525, 120495. [Google Scholar] [CrossRef]
- Yao, Z.; Zhou, Y.; Gao, T.; Yan, P.; Li, H. Ancillary ligand modulated stereoselective selfassembly of triple-stranded Eu(III) helicate featuring circularly polarized luminescence. RSC Adv. 2021, 11, 10524–10531. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-F.; Yan, P.-F.; Chen, P.; Wang, Y.; Xu, H.; Li, G.-M. Highly luminescent bis-diketone lanthanide complexes with triple-stranded dinuclear structure. Dalton Trans. 2012, 41, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Khamidullina, L.A.; Puzyrev, I.S.; Glukhareva, T.V.; Shatunova, S.A.; Slepukhin, P.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fan, Z.; Kalinina, T.A.; et al. Synthesis, characterization, DFT calculations, and biological activity of copper(II) complexes with 1,1,1-trifluoro-4-(2-methoxyphenyl)butan-2,4-dione. J. Mol. Struct. 2019, 1176, 515–528. [Google Scholar] [CrossRef]
- Saloutin, V.I.; Burgart, Y.V.; Edilova, Y.O.; Slepukhin, P.A.; Kudyakova, Y.S.; Bazhin, D.N. Synthesis and structure of homoleptic copper complexes based on fluorinated functionalized 1,3-diketones. Russ. Chem. Bull. 2021, 70, 839–846. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Burgart, Y.V.; Saloutin, V.I. Intramolecular cyclization of lithium 4,4-dimethoxy-1-(perfluoroalkyl)pentane-1,3-dionates on treatment with boron trifluoride diethyl etherate. Russ. Chem. Bull. 2018, 68, 497–499. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Onoprienko, A.Y.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Effect of the nature of a fluorinated substituent on the synthesis of functionalized 1,3-diketones. Russ. Chem. Bull. 2021, 70, 745–752. [Google Scholar] [CrossRef]
- Slepukhin, P.A.; Boltacheva, N.S.; Filyakova, V.I.; Charushin, V.N. Synthesis and structure of lithium 3-trifluoromethyl-1,3-diketonates containing pyridyl substituents. Russ. Chem. Bull. 2019, 68, 1213–1218. [Google Scholar] [CrossRef]
- Rigamonti, L.; Piccioli, M.; Nava, A.; Malavolti, L.; Cortigiani, B.; Sessoli, R.; Cornia, A. Structure, magnetic properties and thermal sublimation of fluorinated Fe4 single-molecule magnets. Polyhedron 2017, 128, 9–17. [Google Scholar] [CrossRef]
- Yu, T.; Cao, Y.; Su, W.; Zhang, C.; Zhao, Y.; Fan, D.; Huang, M.; Yue, K.; Cheng, S.Z.D. Synthesis, structure, photo- and electroluminescence of an iridium(III) complex with a novel carbazole functionalized β-diketone ligand. RSC Adv. 2014, 4, 554–562. [Google Scholar] [CrossRef]
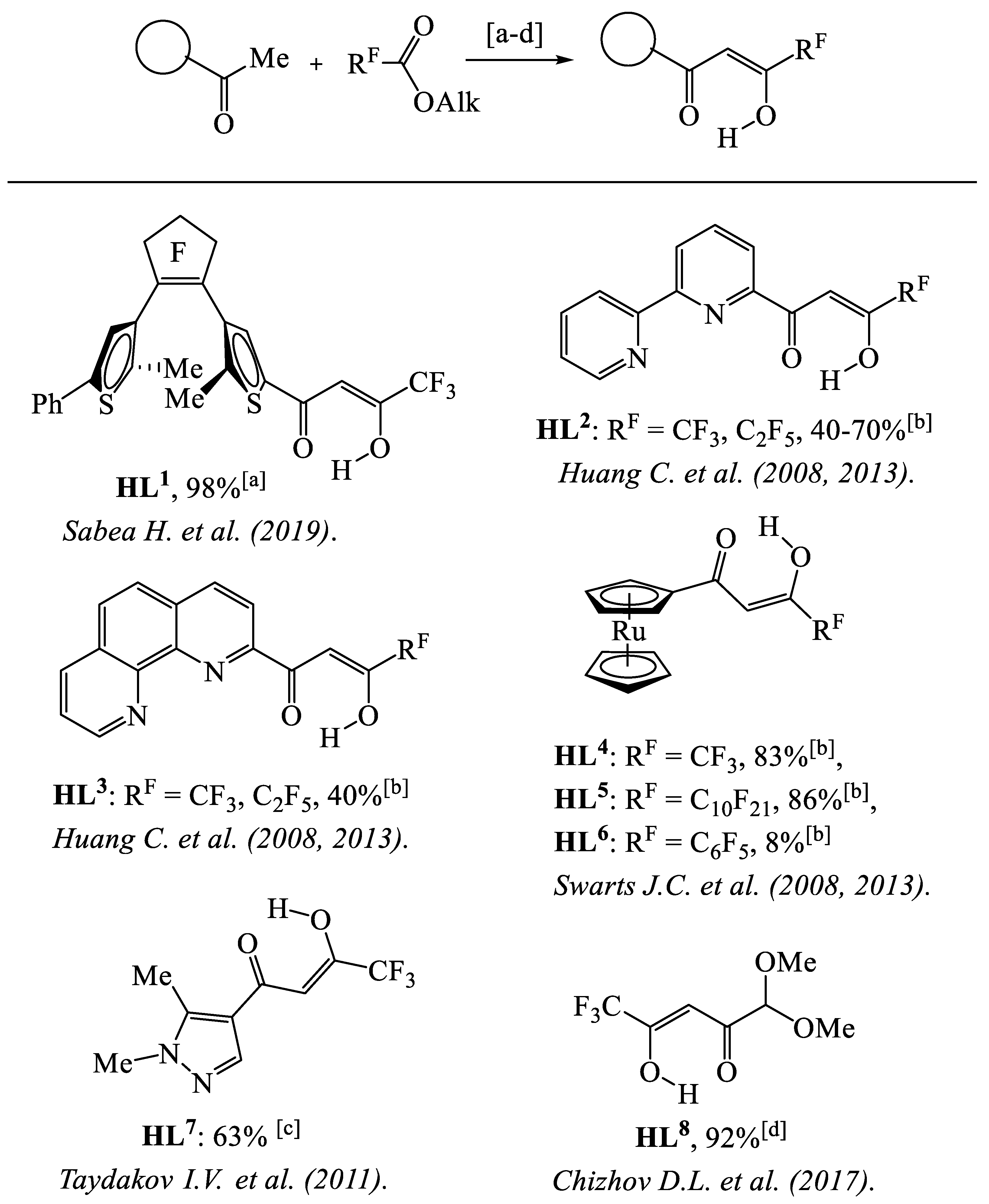
- Sabea, H.; Norel, L.; Galangau, O.; Hijazi, H.; Métivier, R.; Roisnel, T.; Maury, O.; Bucher, C.; Riobé, F.; Rigaut, S. Dual light and redox control of NIR luminescence with complementary photochromic and organometallic antennae. J. Am. Chem. Soc. 2019, 141, 20026–20030. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Xing, Y.; Chen, F.; Han, R.; Wang, J.; Bian, Z.; Fu, L.; Liu, Z.; Ai, X.; Zhang, J.; et al. Energy-transfer mechanisms in IrIII–EuIII bimetallic complexes. ChemPlusChem 2013, 78, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Bian, Z.-Q.; Liu, Z.-W.; Nie, D.-B.; Chen, Z.-Q.; Huang, C.-H. Highly efficient sensitized red emission from europium (III) in Ir-Eu bimetallic complexes by 3MLCT energy transfer. Inorg. Chem. 2008, 47, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.C.; Fourie, E.; Conradie, J.; Swarts, J.C. Ruthenocene-containing-diketones: Synthesis, pKa′ values, keto–enol isomerization kinetics, and electrochemical aspects. Organometallics 2008, 27, 353–362. [Google Scholar] [CrossRef]
- Erasmus, E.; Swarts, J.C. Intramolecular communication and electrochemical observation of the 17-electron ruthenocenium cation in fluorinated ruthenocene-containing β-diketones; polymorphism of C10H21 and C10F21 derivatives. New J. Chem. 2013, 37, 2862–2873. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Krasnoselsky, S.S. Modified method for the synthesis of isomeric N-substituted (1H-pyrazolyl)-propane-1,3-diones. Chem. Heterocycl. Comp. 2011, 47, 695. [Google Scholar] [CrossRef]
- Chizhov, D.L.; Belyaev, D.V.; Yachevskii, D.S.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Efficient and scalable synthesis of 3-(polyfluoroacyl)pyruvaldehydes dimethyl acetals: A novel functionalized fluorinated building-block. J. Fluor. Chem. 2017, 199, 39–45. [Google Scholar] [CrossRef]
- Soldatov, D.V.; Ripmeester, J.A.; Shergina, S.I.; Sokolov, I.E.; Zanina, A.S.; Gromilov, S.A.; Dyadin, Y.A. α- and β-Bis(1,1,1-trifluoro-5,5-dimethyl-5-methoxyacetylacetonato)copper(II): Transforming the dense polymorph into a versatile new microporous framework. J. Am. Chem. Soc. 1999, 121, 4179–4188. [Google Scholar] [CrossRef]
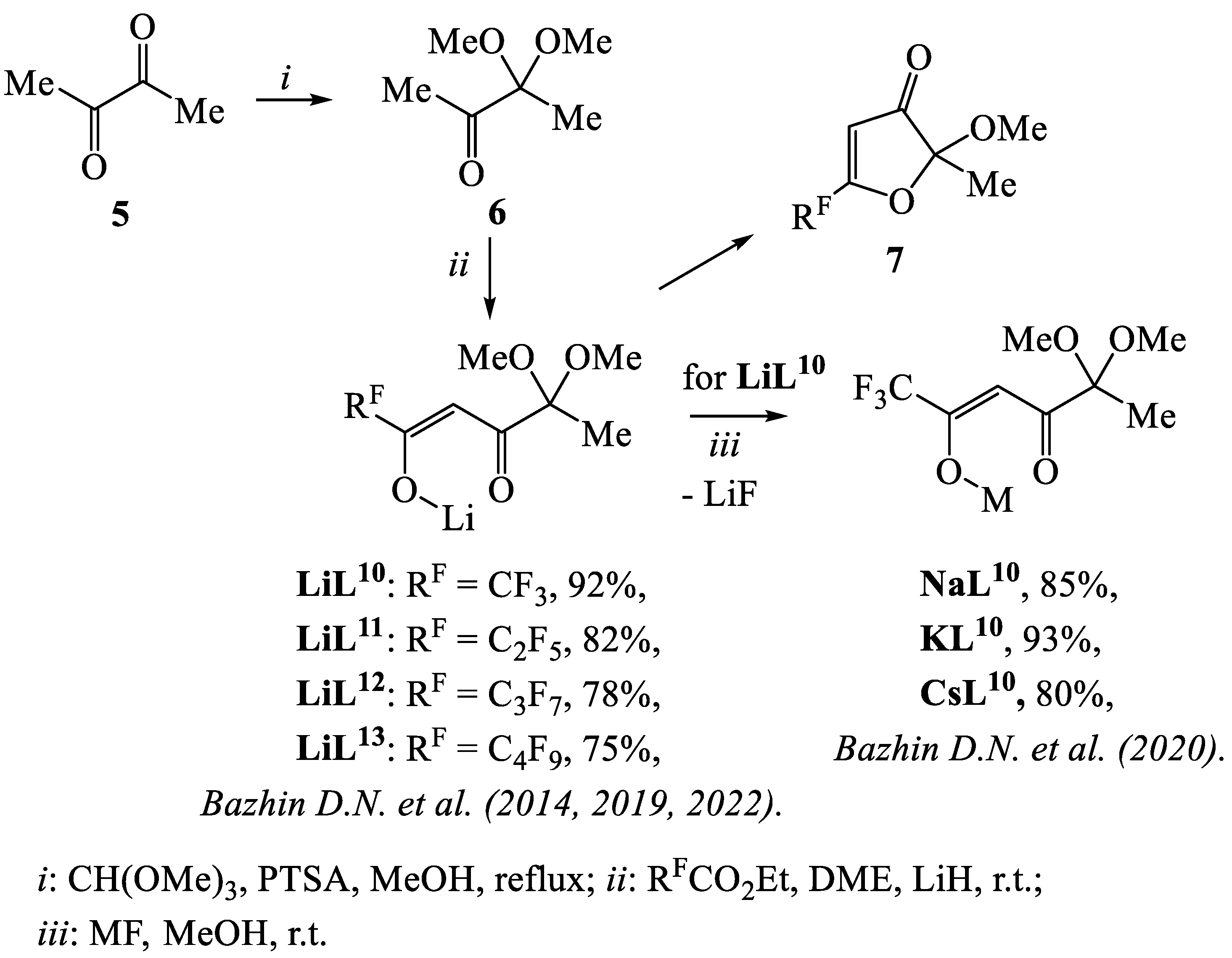
- Bazhin, D.N.; Chizhov, D.L.; Röschenthaler, G.-V.; Kudyakova, Y.S.; Burgart, Y.V.; Slepukhin, P.A.; Saloutin, V.I.; Charushin, V.N. A concise approach to CF3-containing furan-3-ones, (bis)pyrazoles from novel fluorinated building blocks based on 2,3-butanedione. Tetrahedron Lett. 2014, 55, 5714–5717. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Onoprienko, A.Y.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Fluorine-containing furan-3(2H)-ones in reactions with binucleophiles: CF3 vs C2F5. Chem. Heterocycl. Comp. 2019, 55, 517–522. [Google Scholar] [CrossRef]
- Edilova, Y.O.; Kudyakova, Y.S.; Kiskin, M.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Expanding 1,2,4-triketone toolbox for use as fluorinated building blocks in the synthesis of pyrazoles, pyridazinones and β-diketohydrazones. J. Fluor. Chem. 2022, 253, 109932. [Google Scholar] [CrossRef]
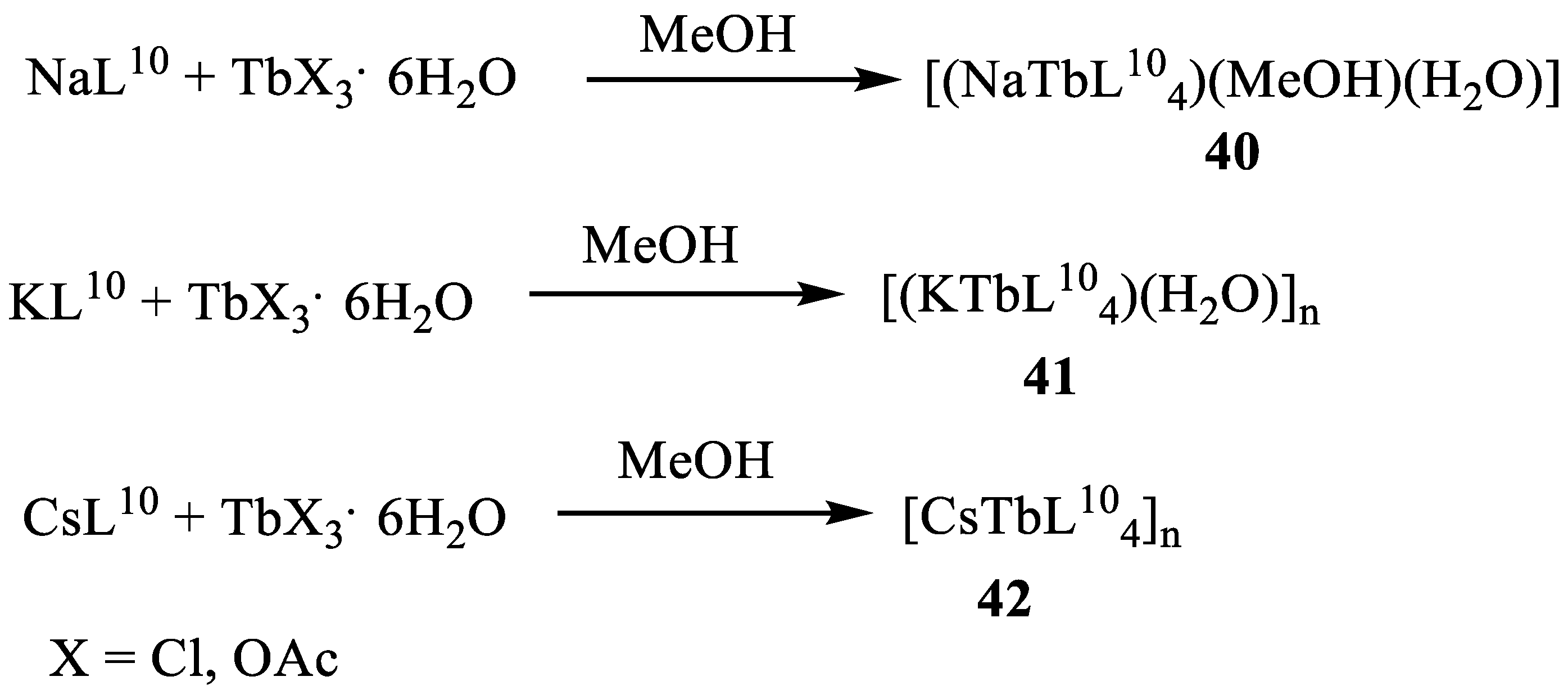
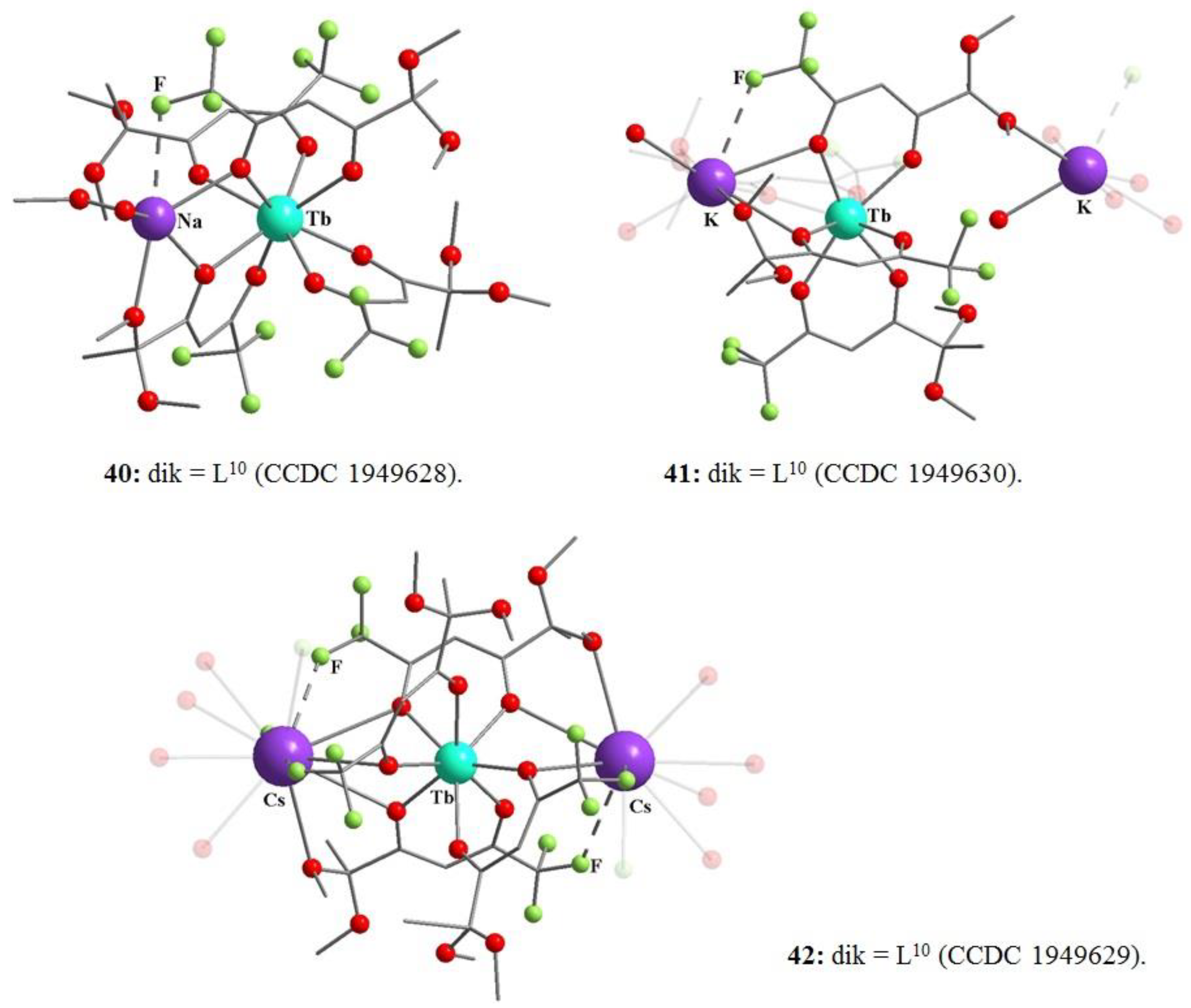
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. The impact of the alkali metal ion on the crystal structure and (mechano)luminescence of terbium(III) tetrakis(β-diketonates). Eur. J. Inorg. Chem. 2020, 2020, 523. [Google Scholar] [CrossRef]
- Hori, A.; Shinohe, A.; Takatani, S.; Miyamoto, T.K. Synthesis and crystal structures of fluorinated β-diketonate metal (Al3+, Co2+, Ni2+, and Cu2+) complexes. Bull. Chem. Soc. Jpn. 2009, 82, 96–98. [Google Scholar] [CrossRef]
- Crowder, J.M.; Han, H.; Wei, Z.; Dikarev, E.V.; Petrukhina, M.A. Unsolvated homo- and heterometallic highly fluorinated β-diketonate complexes of copper(II). Polyhedron 2019, 157, 33–38. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Lip, H.C.; Louie, H.W.; Drew, M.G.B.; Hudson, M.J. Interaction of lanthanide shift reagents with co-ordination complexes; direct observation of nuclear magnetic resonance signals for free and complexed tris(pentane-2,4-dionato)cobalt(III) at ambient temperature, and X-ray crystal and molecular structure of the 1:1 adduct formed. Chem. Commun. 1977, 21, 778–780. [Google Scholar] [CrossRef]
- Han, H.; Wei, Z.; Barry, M.C.; Filatov, A.S.; Dikarev, E.V. Heterometallic molecular precursors for a lithium–iron oxide material: Synthesis, solid state structure, solution and gas-phase behaviour, and thermal decomposition. Dalton Trans. 2017, 46, 5644–5649. [Google Scholar] [CrossRef]
- Han, H.; Carozza, J.C.; Colliton, A.P.; Zhang, Y.; Wei, Z.; Filatov, A.S.; Chen, Y.-S.; Alkan, M.; Rogachev, A.Y.; Dikarev, E.V. Heterotrimetallic mixed-valent molecular precursors containing Periodic table neighbors: Assignment of metal positions and oxidation states. Angew. Chem. Int. Ed. 2020, 59, 9624–9630. [Google Scholar] [CrossRef]
- Han, H.; Carozza, J.C.; Zhou, Z.; Zhang, Y.; Wei, Z.; Abakumov, A.M.; Filatov, A.S.; Chen, Y.-S.; Santa Lucia, D.J.; Berry, J.F.; et al. Heterotrimetallic Precursor with 2:2:1 Metal Ratio Requiring at Least a Pentanuclear Molecular Assembly. J. Am. Chem. Soc. 2020, 142, 12767–12776. [Google Scholar] [CrossRef]
- Lieberman, C.M.; Filatov, A.S.; Wei, Z.; Rogachev, A.Y.; Abakumov, A.M.; Dikarev, E.V. Mixed-valent, heteroleptic homometallic diketonates as templates for the design of volatile heterometallic precursors. Chem. Sci. 2015, 6, 2835–2842. [Google Scholar] [CrossRef]
- Lieberman, C.M.; Wei, Z.; Filatov, A.S.; Dikarev, E.V. Mixed-Ligand Approach to Changing the Metal Ratio in Bismuth–Transition Metal Heterometallic Precursors. Inorg. Chem. 2016, 55, 3946–3951. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Urkasym Kyzy, S.; Rybalova, T.V.; Korolkov, I.V.; Sysoev, S.V.; Koretskaya, T.P.; Kuchumov, B.M.; Turgambaeva, A.E. Volatile trinuclear heterometallic beta-diketonates: Structure and thermal properties related to the chemical vapor deposition of composite thin films. Polyhedron 2020, 191, 114806. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Tkachev, S.V.; Baidina, I.A.; Korolkov, I.V.; Turgambaeva, A.E.; Igumenov, I.K. Volatile Pd–Pb and Cu–Pb heterometallic complexes: Structure, properties, and trans-to-cis isomerization under cocrystallization of Pd and Cu β-diketonates with Pb hexafluoroacetylacetonate. J. Coord. Chem. 2015, 68, 1890–1902. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Urkasym Kyzy, S.; Rybalova, T.V.; Baidina, I.A.; Korolkov, I.V.; Chizhov, D.L.; Bazhin, D.N.; Kudyakova, Y.S. Isomerization as a tool to design volatile heterometallic complexes with methoxy-substituted β-diketonates. J. Coord. Chem. 2018, 71, 2194–2208. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Baidina, I.A.; Basova, T.V.; Bulusheva, L.G.; Igumenov, I.K. Self-Assembly of Coordination Polymers from Volatile PdII and PbII β-Diketonate Derivatives through Metallophilic Interactions. Eur. J. Inorg. Chem. 2013, 2013, 5738–5745. [Google Scholar] [CrossRef]
- Hirai, Y.; Nakanishi, T.; Kitagawa, Y.; Fushimi, K.; Seki, T.; Ito, H.; Hasegawa, Y. Luminescent Europium(III) Coordination Zippers Linked with Thiophene-Based Bridges. Angew. Chem. Int. Ed. 2016, 55, 12059–12062. [Google Scholar] [CrossRef]
- Francis, B.; Nolasco, M.M.; Brandão, P.; Ferreira, R.A.S.; Carvalho, R.S.; Cremona, M.; Carlos, L.D. Efficient Visible-Light-Excitable Eu3+ Complexes for Red Organic Light-Emitting Diodes. Eur. J. Inorg. Chem. 2020, 2020, 1260–1270. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chen, L.; Wang, X.; Lin, L.-R. Synthesis of Bis-β-Diketonate Lanthanide Complexes with an Azobenzene Bridge and Studies of Their Reversible Photo/Thermal Isomerization Properties. ACS Omega 2019, 4, 15530–15538. [Google Scholar] [CrossRef]
- Barry, M.C.; Wei, Z.; He, T.; Filatov, A.S.; Dikarev, E.V. Volatile Single-Source Precursors for the Low-Temperature Preparation of Sodium-Rare Earth Metal Fluorides. J. Am. Chem. Soc. 2016, 138, 8883–8887. [Google Scholar] [CrossRef]
- Mara, D.; Artizzu, F.; Laforce, B.; Vincze, L.; Van Hecke, K.; Van Deun, R.; Kaczmarek, A.M. Novel tetrakis lanthanide β-diketonate complexes: Structural study, luminescence properties and temperature sensing. J. Lumin. 2019, 213, 343–355. [Google Scholar] [CrossRef]
- Battiato, S.; Rossi, P.; Paoli, P.; Malandrino, G. Heterobimetallic sodium rare-earth complexes: “Third-generation” MOCVD precursors for the deposition of NaREF4 (RE = Y, Gd) films. Inorg. Chem. 2018, 57, 15035–15039. [Google Scholar] [CrossRef]
- Zeng, D.; Ren, M.; Bao, S.-S.; Zheng, L.-M. Tuning the Coordination Geometries and Magnetic Dynamics of [Ln(hfac)β]-through Alkali Metal Counterions. Inorg. Chem. 2014, 53, 795–801. [Google Scholar] [CrossRef]
- Taidakov, I.V.; Krasnosel’skii, S.S.; Lobanov, A.N.; Vitukhnovskii, A.G.; Starikova, Z.A. Cesium Tetrakis(1(1,5-Dimethyl-1H-Pyrazol-4-yl)-4,4,4-Trifluorobutane-1,3-diono)europiate(III): Synthesis, Crystal Structure, and Luminescence Properties. Russ. J. Coord. Chem. 2013, 39, 680–684. [Google Scholar] [CrossRef]
- Dinh, T.-H.; Nguyen, H.-H.; Nguyen, M.-H. A structural and spectroscopic study on heterometallic sodium(I)-europium(III) β-diketonate complexes. Inorg. Chem. Comm. 2021, 130, 108674. [Google Scholar] [CrossRef]
- Catalano, M.R.; Pellegrino, A.L.; Rossi, P.; Paoli, P.; Cortelletti, P.; Pedroni, M.; Speghini, A.; Malandrino, G. Upconverting Er3+,Yb3+ activated β-NaYF4 thin films: A solution route using a novel sodium β-diketonate polyether adduct. New J. Chem. 2017, 41, 4771–4775. [Google Scholar] [CrossRef]
- Jimenez, G.L.; Rosales-Hoz, M.J.; Leyva, M.A.; Reyes-Rodriguez, J.L.; Galindo-Garcia, U.; Falcony, C. Structural analysis of an europium-sodium complex containing 2-thenoyltrifluoroacetone and succinimide as ligands, a highly photoluminescent material. J. Mol. Struct. 2021, 1228, 129778. [Google Scholar] [CrossRef]
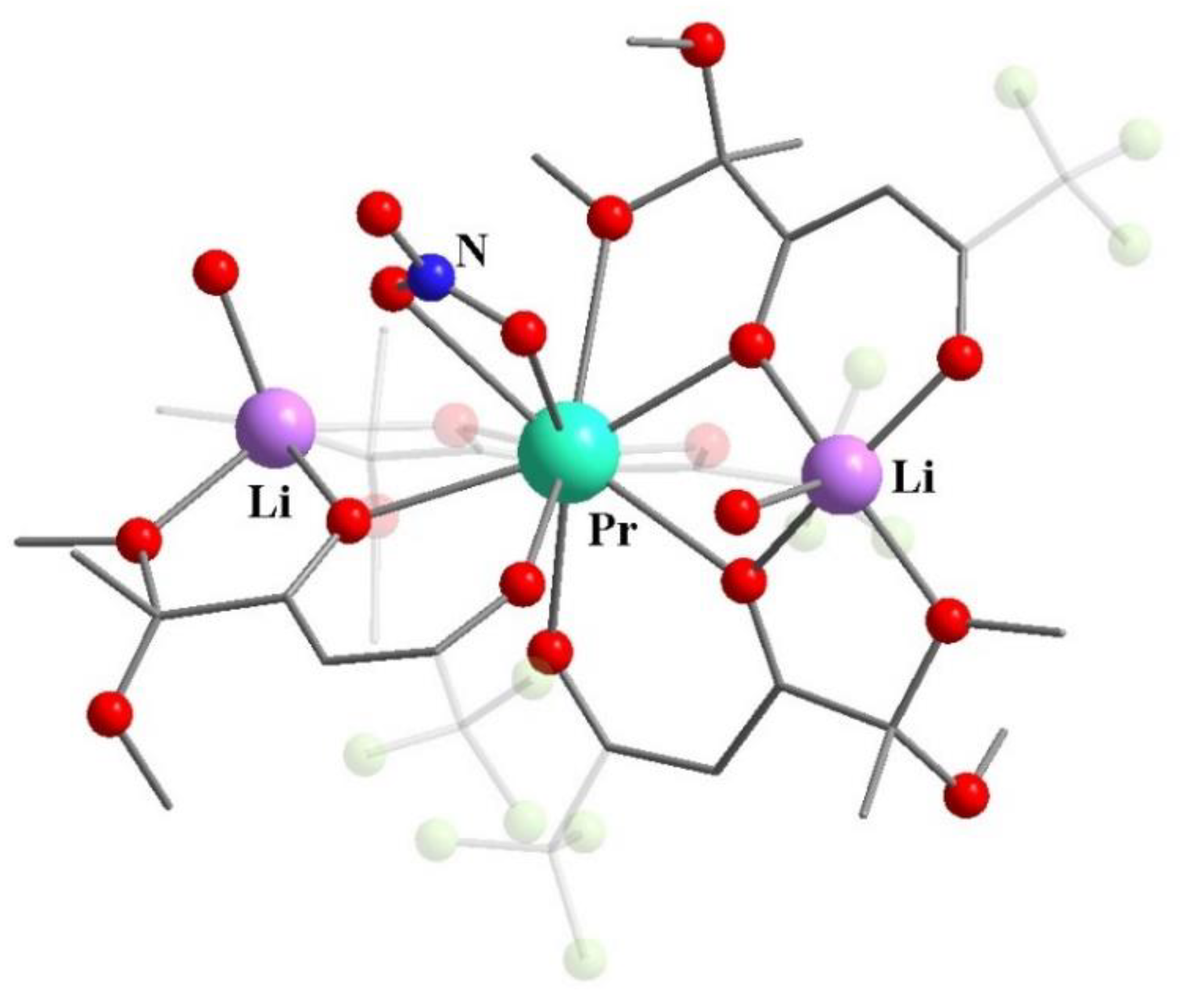
- Kudyakova, Y.S.; Slepukhin, P.A.; Ganebnykh, I.N.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Lithium β-Diketonate in the Synthesis of Homo- and Heteronuclear Complexes of Rare-Earth Metals. Russ. J. Coord. Chem. 2021, 47, 280–295. [Google Scholar] [CrossRef]
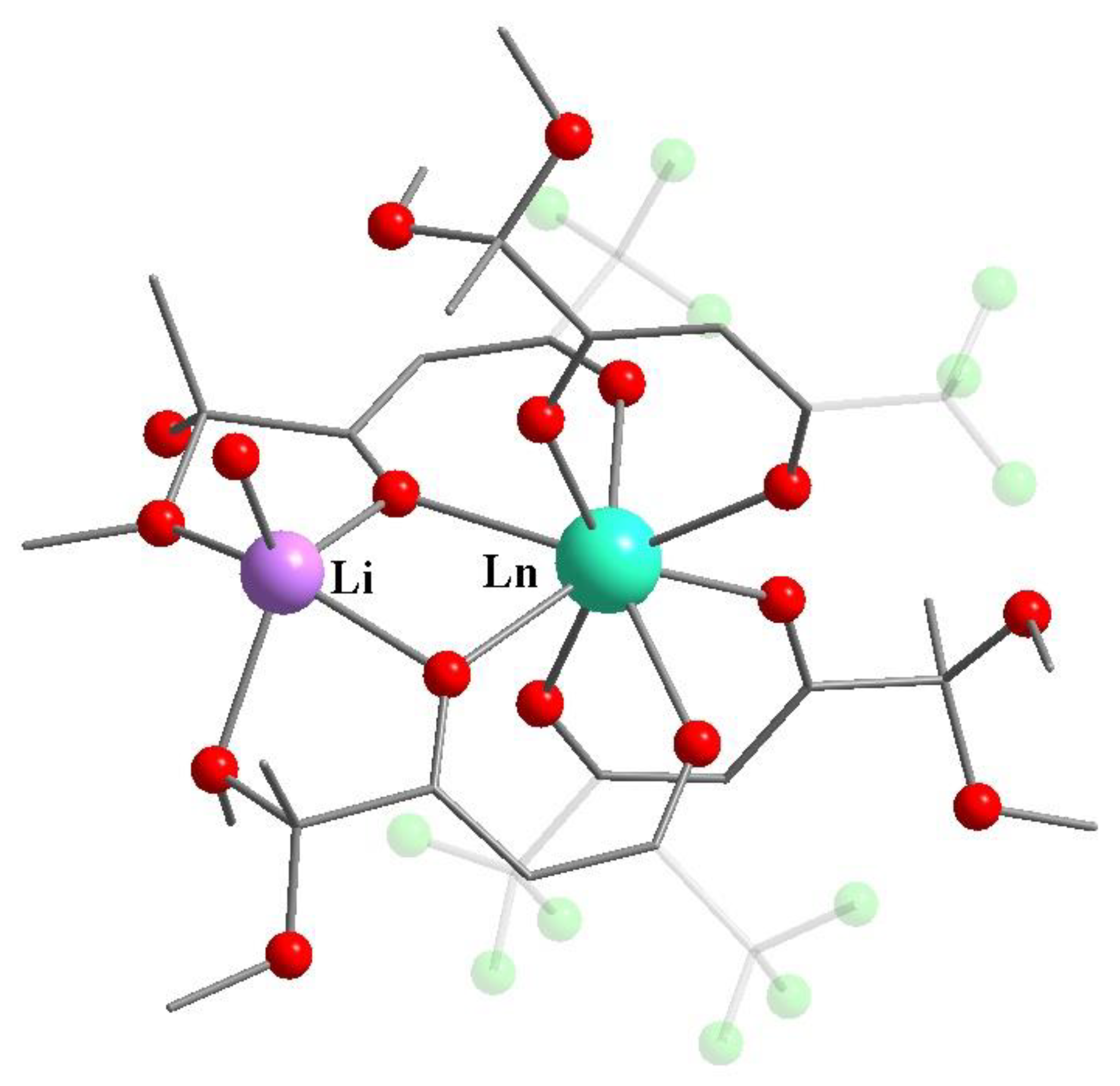
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Functionalized Trifluoromethyl-Containing A Rare Example of Discrete Lanthanide–Lithium Tetrakis-β-Diketonates: Synthesis, Structures, and Luminescence Properties. Russ. J. Coord. Chem. 2020, 46, 545–552. [Google Scholar] [CrossRef]
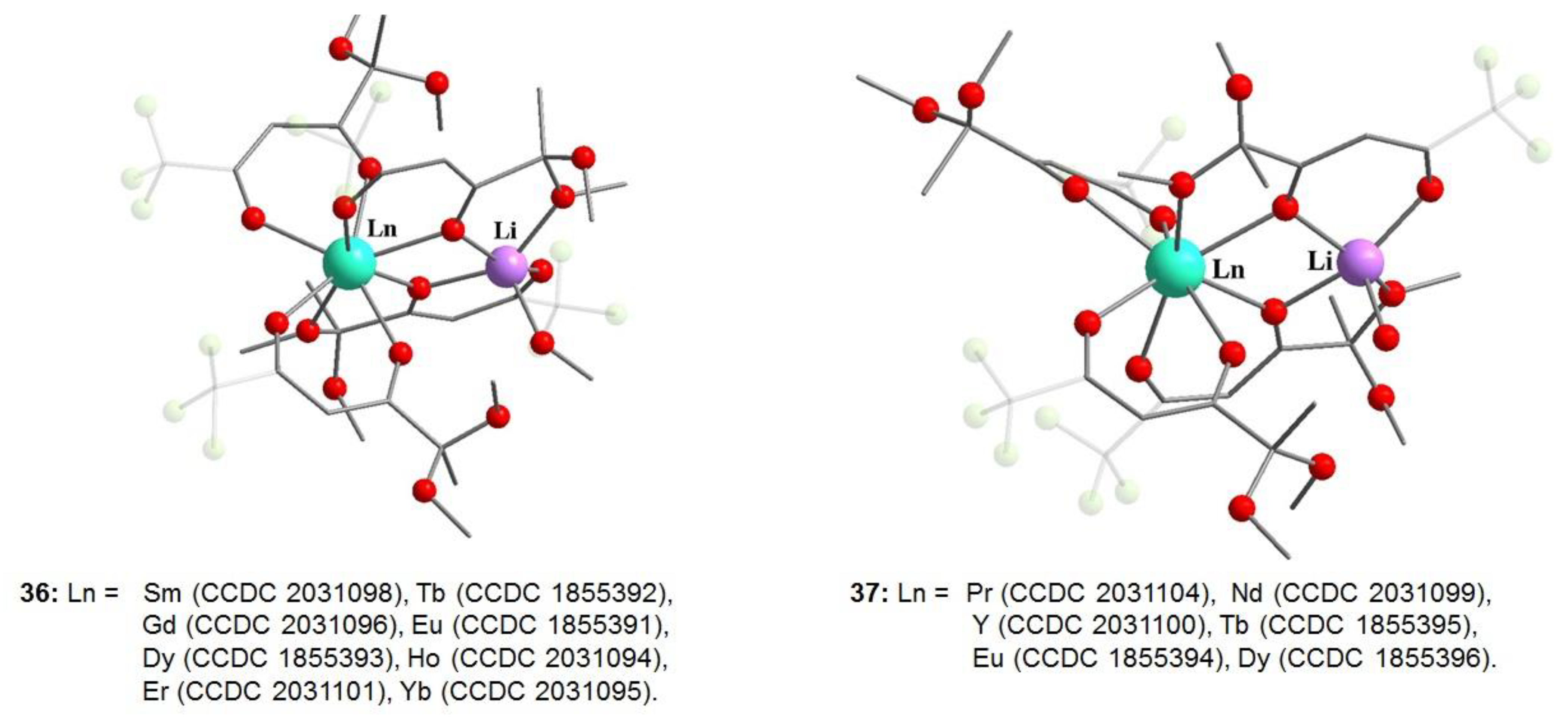
- Bazhin, D.N.; Kudyakova, Y.S.; Bogomyakov, A.S.; Slepukhin, P.A.; Kim, G.A.; Burgart, Y.V.; Saloutin, V.I. Dinuclear lanthanide–lithium complexes based on fluorinated β-diketonate with acetal group: Magnetism and effect of crystal packing on mechanoluminescence. Inorg. Chem. Front. 2019, 6, 40–49. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Role of alkyl substituents in the structure and luminescence properties of discrete terbium(III)-lithium(I) β-diketonates. J. Mol. Struct. 2021, 1226, 129331. [Google Scholar] [CrossRef]
- Lia, X.-L.; Lia, J.; Wanga, A.; Liub, C.-M.; Cuia, M.; Zhang, Y.-Q. Observation of field-induced single-ion magnet behavior in a mononuclear DyIII complex by co-crystallization of a square-planar CuII complex. Inorg. Chim. Acta 2020, 510, 119718. [Google Scholar] [CrossRef]
- Barry, M.C.; Lieberman, C.M.; Wei, Z.; Clérac, R.; Filatov, A.S.; Dikarev, E.V. Expanding the Structural Motif Landscape of Heterometallic β Diketonates: Congruently Melting Ionic Solids. Inorg. Chem. 2018, 57, 2308–2313. [Google Scholar] [CrossRef]
- Lieberman, C.M.; Vreshch, V.D.; Filatov, A.S.; Dikarev, E.V. Synthesis and characterization of {[CuI3Sn2(OBut)6]+[CuII(hfac)3]}− A heterometallic cluster with unique triangular copper(I) core. Inorg. Chim. Acta 2015, 424, 156–161. [Google Scholar] [CrossRef]
- Li, D.; Chen, F.-F.; Bian, Z.-Q.; Liu, Z.-W.; Zhao, Y.-L.; Huang, C.-H. Sensitized near-infrared emission of YbIII from an IrIII–YbIII bimetallic complex. Polyhedron 2009, 28, 897–902. [Google Scholar] [CrossRef]
- Chen, F.-F.; Bian, Z.-Q.; Lou, B.; Ma, E.; Liu, Z.-W.; Nie, D.-B.; Chen, Z.-Q.; Bian, J.; Chen, Z.-N.; Huang, C.-H. Sensitised near-infrared emission from lanthanides using an iridium complex as a ligand in heteronuclear Ir2Ln arrays. Dalton Trans. 2008, 41, 5577–5583. [Google Scholar] [CrossRef]
- Horikoshi, R.; Tominaga, T.; Mochida, T. Mixed-Metal Coordination Polymers and Molecular Squares Based on a Ferrocene-Containing Multidentate Ligand 1,2-Di(4-pyridylthio)ferrocene. Cryst. Growth Des. 2018, 18, 5089–5098. [Google Scholar] [CrossRef]
- Dolinar, B.S.; Alexandropoulos, D.I.; Vignesh, K.R.; James, T.A.; Dunbar, K.R. Lanthanide Triangles Supported by Radical Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 908–911. [Google Scholar] [CrossRef]
- Kishore, P.V.V.N.; Rasamsetty, A.; Sañudo, E.C.; Baskar, V. Redox shield enfolding a magnetic core. Polyhedron 2015, 102, 361–365. [Google Scholar] [CrossRef]
- Jakob, A.; Joubert, C.C.; Rüffer, T.; Swarts, J.C.; Lang, H. Chemical and electrochemical oxidation studies on new copper(I) ferrocenyl-functionalised β-diketonates. Inorg. Chim. Acta 2014, 411, 48–55. [Google Scholar] [CrossRef]
- Gericke, H.J.; Muller, A.J.; Swarts, J.C. Electrochemical Illumination of Intramolecular Communication in Ferrocene-Containing tris-β-Diketonato Aluminum(III) Complexes; Cytotoxicity of Al(FcCOCHCOCF3)3. Inorg. Chem. 2012, 51, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Maseme, M.R.; Buitendach, B.E.; Erasmus, E.; Swarts, J.C. The chemistry of spin-coated rhodium-ferrocenyl complexes supported on silanol-capped silicon wafers. Polyhedron 2021, 204, 115277. [Google Scholar] [CrossRef]
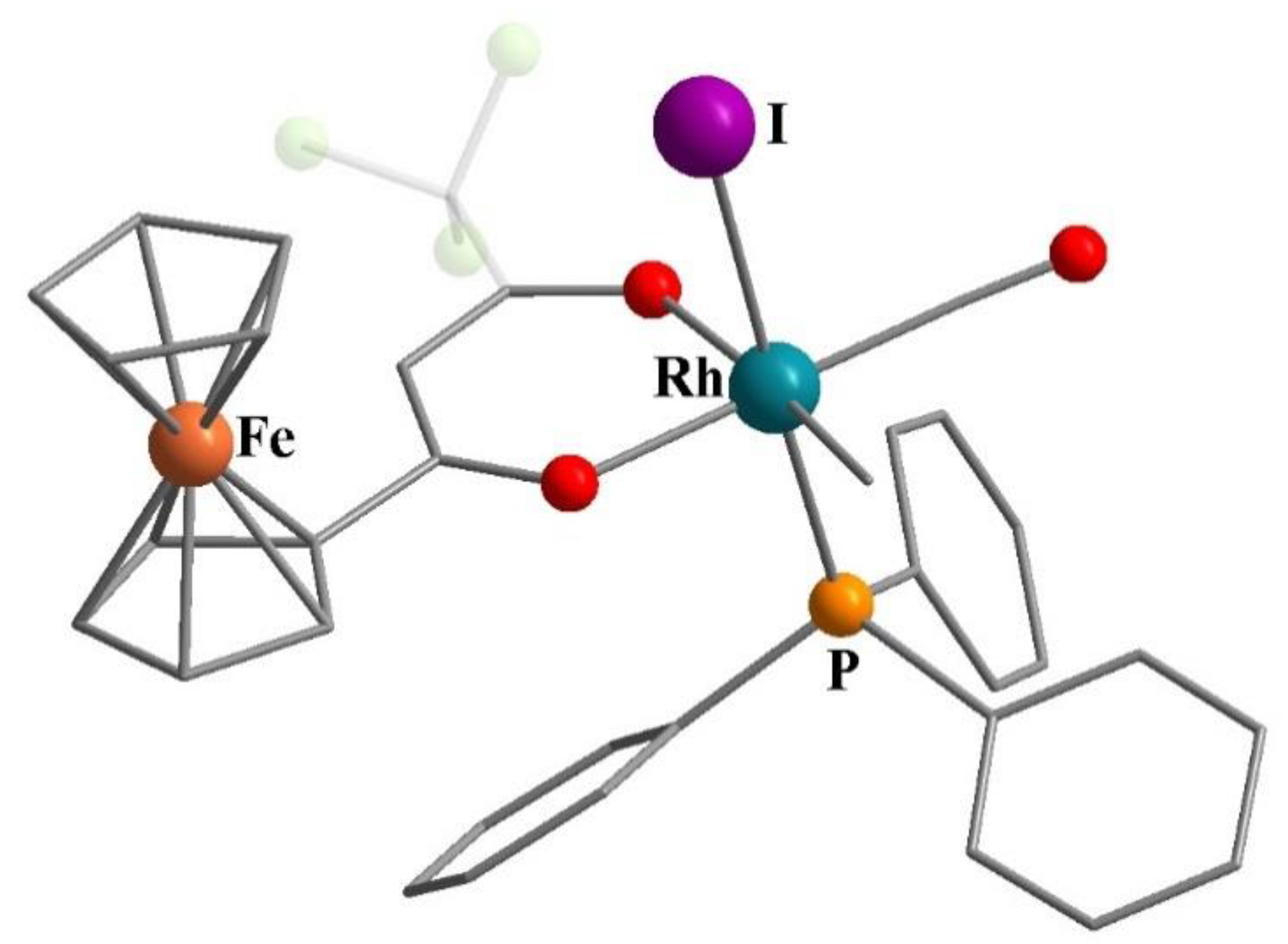
- Conradie, J.; Lamprecht, G.J.; Roodt, A.; Swarts, J.C. Kinetic study of the oxidative addition reaction between methyl iodide and [Rh(FcCOCHCOCF3)(CO)(PPh3)]: Structure of [Rh(FcCOCHCOCF3)(CO)(PPh3)(CH3)(I)]. Polyhedron 2007, 26, 5075–5087. [Google Scholar] [CrossRef]
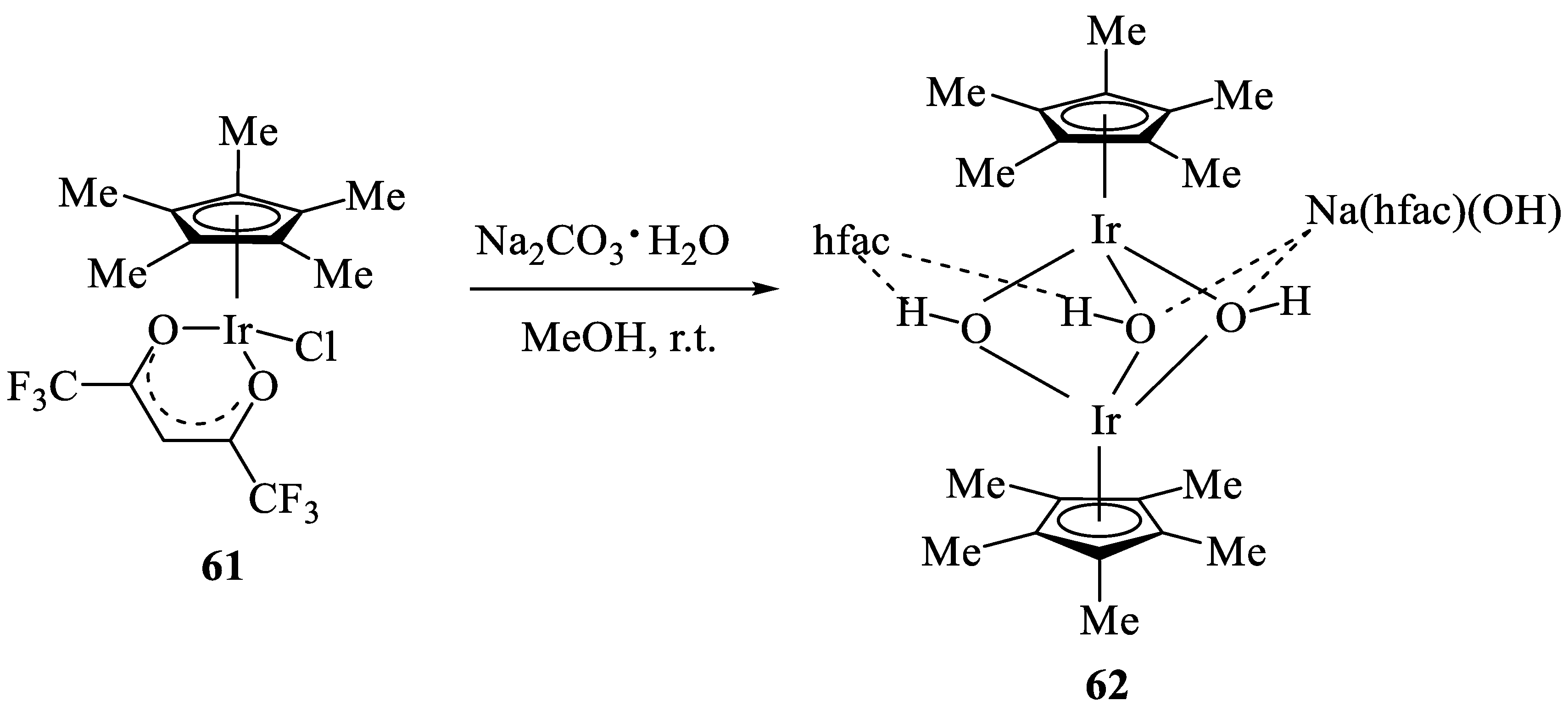
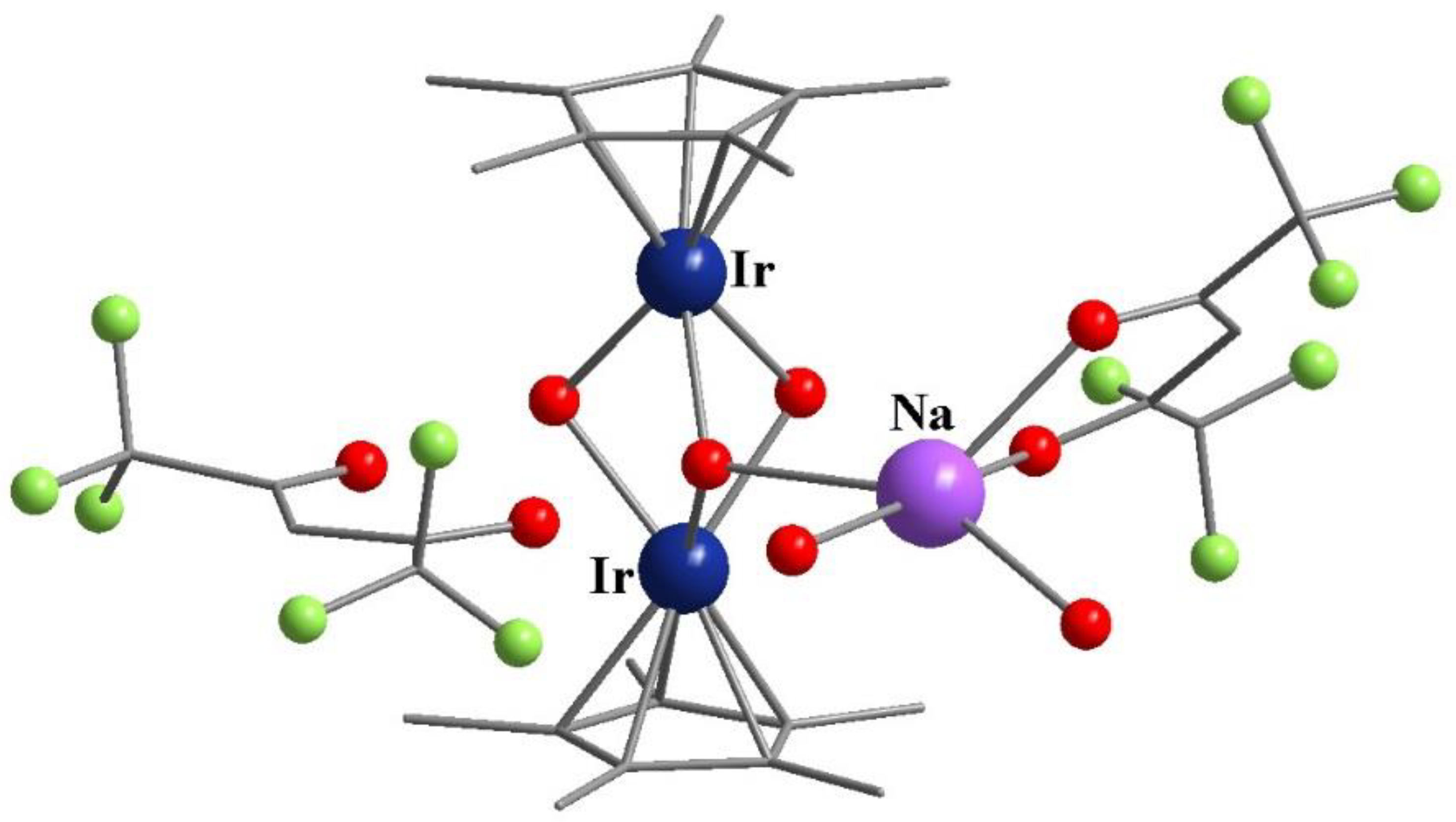
- DuChane, C.M.; Merola, J.S. Hexafluoroacetylacetonate (hfac) as ligand for pentamethylcyclopentadienyl (Cp∗) rhodium and iridium complexes: Some surprising results, including an Ir3Na1O4 cubane structure. J. Organomet. Chem. 2020, 929, 121552. [Google Scholar] [CrossRef]
- Towatari, M.; Nishi, K.; Fujinami, T.; Matsumoto, N.; Sunatsuki, Y.; Kojima, M.; Mochida, N.; Ishida, T.; Re, N.; Mrozinski, J. Syntheses, Structures, and Magnetic Properties of Acetato- and Diphenolato-Bridged 3d–4f Binuclear Complexes [M(3-MeOsaltn)(MeOH)x(ac)Ln(hfac)2] (M = ZnII, CuII, NiII, CoII; Ln = LaIII, GdIII, TbIII, DyIII; 3-MeOsaltn = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato; ac = Acetato; hfac = Hexafluoroacetylacetonato; x = 0 or 1). Inorg. Chem. 2013, 52, 6160–6178. [Google Scholar] [CrossRef]
- Apostol, A.A.; Mihalache, I.; Mocanu, T.; Tutunaru, O.; Pachiu, C.; Gavrila, R.; Maxim, C.; Andruh, M. Luminescent [ZnIILnIII] complexes anchored on graphene: Synthesis and crystal structures of [ZnIIEuIII] and [ZnIITbIII] complexes decorated with pyrene groups. Appl. Organomet. Chem. 2020, 35, e6126. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Nikolaeva, N.S.; Krasnov, P.O.; Sukhikh, A.A.; Smolentsev, A.I.; Kovaleva, E.A.; Morozova, N.B.; Basova, T.V. Synthesis, structural, vibrational and DFT investigation of new binuclear molecular Pd-Cu and Cu-Cu complexes formed by Schiff base and hexafluoroacetylacetonate building blocks. J. Mol. Struct. 2020, 1216, 128341. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Shi, L.; Chen, Z. Sensitized luminescence in dinuclear lanthanide(III) complexes of bridging 8-hydroxyquinoline derivatives with different electronic properties. Dalton Trans. 2011, 40, 5549–5556. [Google Scholar] [CrossRef]
- Wang, W.-M.; Liu, S.-Y.; Xu, M.; Bai, L.; Wang, H.-Q.; Wen, X.; Zhao, X.-Y.; Qiao, H.; Wu, Z.-L. Structures and magnetic properties of phenoxo-O-bridged dinuclear lanthanide(III) compounds: Single molecule magnet behavior and magnetic refrigeration. Polyhedron 2018, 145, 114–119. [Google Scholar] [CrossRef]
- Xu, H.-B.; Li, J.; Zhang, L.-Y.; Huang, X.; Li, B.; Chen, Z.-N. Structures and Photophysical Properties of Homo- and Heteronuclear Lanthanide(III) Complexes with Bridging 2-Methyl-8-hydroxyquinoline (HMq) in the m-Phenol Mode. Cryst. Growth. Des. 2010, 10, 4101–4108. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakanishi, T.; Kitagawa, Y.; Seki, T.; Ito, H.; Fushimi, K.; Hasegawa, Y. Synthesis and Photophysical Properties of Eu(III) Complexes with Phosphine Oxide Ligands including Metal Ions. Bull. Chem. Soc. Jpn. 2018, 91, 6–11. [Google Scholar] [CrossRef]
- Shi, J.Y.; Ma, Z.L.; Tian, L. A new mononuclear terbium and copper cocrystalline nitronyl nitroxide complex: Synthesis, structure and magnetic properties. J. Mol. Struct. 2021, 1224, 129012. [Google Scholar] [CrossRef]
- Patrascu, A.A.; Briganti, M.; Soriano, S.; Calancea, S.; Allao Cassaro, R.A.; Totti, F.; Vaz, M.G.F.; Andruh, M. SMM Behavior Tuned by an Exchange Coupling LEGO Approach for Chimeric Compounds: First 2p-3d-4f Heterotrispin Complexes with Different Metal Ions Bridged by One Aminoxyl Group. Inorg. Chem. 2019, 58, 13090–13101. [Google Scholar] [CrossRef] [PubMed]
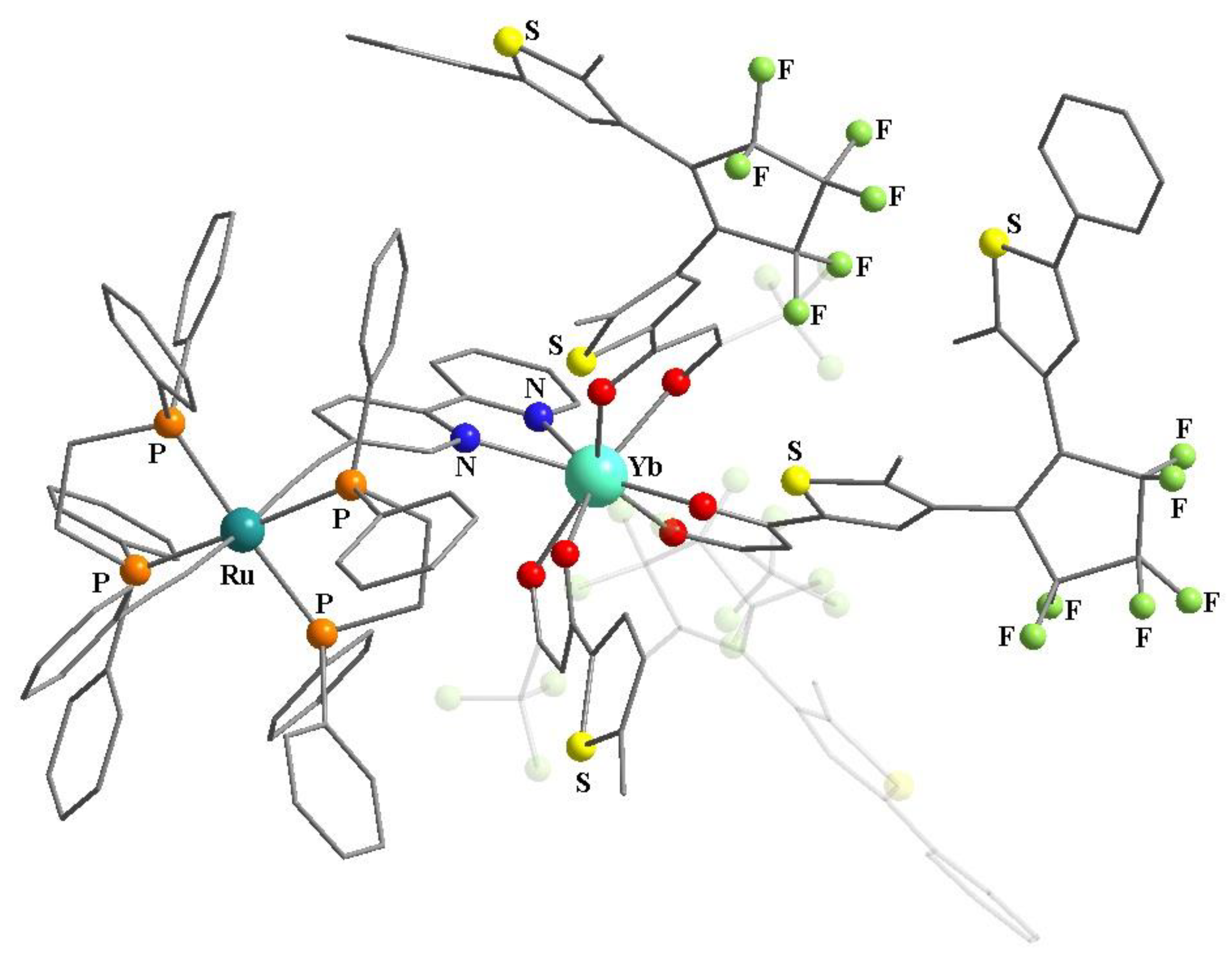
- Souza, M.S.; Briganti, M.; Reis, S.G.; Stinghen, D.; Bortolot, C.S.; Cassaro, R.A.A.; Guedes, G.P.; Silva, F.C.; Ferreira, V.F.; Novak, M.A.; et al. Magnetic Cationic Copper(II) Chains and a Mononuclear Cobalt(II) Complex Containing [Ln(hfac)4]− Blocks as Counterions. Inorg. Chem. 2019, 58, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jing, P.; Lu, J.; Xi, L.; Wang, Q.; Ding, L.; Wang, W.-M.; Song, Z. Multifunctional properties of {CuII2LnIII2} systems involving nitrogen-rich nitronyl nitroxide: Single-molecule magnet behavior, luminescence, magnetocaloric effects and heat capacity. Dalton Trans. 2021, 50, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Xi, L.; Lu, J.; Han, J.; Huang, X.; Jin, C.; Xie, J.; Li, L. Regulating Spin Dynamics of Nitronyl Nitroxide Biradical Lanthanide Complexes through Introducing Different Transition Metals. Chem. Asian J. 2021, 16, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liang, X.; Zhang, Y.; Ouyang, Z.; Dong, W. A family of 3d-4f Cu-Ln ladder-like complexes: Synthesis, structures and magnetic properties. Polyhedron 2020, 180, 114435. [Google Scholar] [CrossRef]
- Xi, L.; Sun, J.; Wang, K.; Lu, J.; Jing, P.; Li, L. Slow magnetic relaxation in CoII-LnIII heterodinuclear complexes achieved through a functionalized nitronyl nitroxide biradical. Dalton Trans. 2020, 49, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Chen, P.Y.; Wu, M.Z.; Tian, L.; Liu, Z.Y. Synthesis of a series of hetero-multi-spin Ln2Cu3 complexes based on a methyl-pyrazole nitronyl nitroxide radical with slow magnetic relaxation behaviors. Dalton Trans. 2019, 48, 9187–9193. [Google Scholar] [CrossRef]
- Hu, P.; Wu, Y.-N.; Zhao, Y.-H.; Cao, J.-F.; Lai, J.-L.; Ma, D.-Y.; Zhu, L.-L.; Xiao, F.-P. Structure changes from radical-3d ring dimer to radical-3d-4f 1D chain with different magnetic properties. Inorg. Chim. Acta 2020, 512, 119867. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Yang, M.; Sun, Z.; Tang, J.; Ma, Y.; Li, L. Functionalized Nitronyl Nitroxide Biradicals for the Construction of 3d-4f Heterometallic Compounds. Inorg. Chem. 2018, 57, 9757–9765. [Google Scholar] [CrossRef]
- Romanenko, G.V.; Kuznetsova, O.V.; Fursova, E.Y.; Letyagin, G.A.; Ovcharenko, V.I. The structure of multinuclear copper (II) hexafluoroacetylacetone. J. Struct. Chem. 2019, 60, 275–278. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Gyurkac, M.; Fischer, R.C.; Torvisco, A.; Massoud, S.S.; Vicente, R. Synthesis and characterization of Lanthanum(III) complexes containing 4,4,4-trifluoro-1-(naphthalen-2yl)butane-1,3-dionate. Polyhedron 2020, 179, 114384. [Google Scholar] [CrossRef]
- Wong, H.-Y.; Chan, W.T.K.; Law, G.-L. Assembly of Lanthanide(III) Cubanes and Dimers with Single-Molecule Magnetism and Photoluminescence. Inorg. Chem. 2018, 57, 6893–6902. [Google Scholar] [CrossRef] [PubMed]
- Zaim, A.; Favera, N.D.; Guenee, L.; Nozary, H.; Hoang, T.N.Y.; Eliseeva, S.V.; Petoud, S.; Piguet, C. Lanthanide hexafluoroacetylacetonates vs. nitrates for the controlled loading of luminescent polynuclear single-stranded oligomers. Chem. Sci. 2013, 4, 1125–1136. [Google Scholar] [CrossRef]
- Filyakova, T.I.; Saloutina, L.V.; Slepukhin, P.A.; Saloutin, V.I.; Chupakhin, O.N. Synthesis and crystal structures of the heptafluoroacetylacetonatocopper(II) and 4-iminoheptafluoropent-2-ene-2-aminatocopper(II) complexes with triphenylphosphine oxide and pyridine. Russ. Chem. Bull. 2009, 58, 1132–1138. [Google Scholar] [CrossRef]
- Chen, L.; Tan, Y.; Xu, H.; Wang, K.; Chen, Z.-H.; Zheng, N.; Li, Y.-Q.; Lin, L.-R. Enhanced E/Z-photoisomerization and luminescence of stilbene derivative co-coordinated in di-β-diketonate lanthanide complexes. Dalton Trans. 2020, 49, 16745–16761. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.M.; Ananias, D.; Almeida, P.; Filipe, A.; Pillinger, M.; Valente, A.A.; Carlos, L.D.; Goncalves, I.S. Crystal structure and temperature-dependent luminescence of a heterotetranuclear sodium-europium(III) β-diketonate complex. Dalton Trans. 2015, 44, 488–492. [Google Scholar] [CrossRef]
- Lin, J.; Yue, L.; Xin, L.; Jinlei, T.; Shiping, Y. Three series of heterometallic NiII-LnIII Schiff base complexes: Synthesis, crystal structures and magnetic characterization. Dalton Trans. 2017, 46, 12558–12573. [Google Scholar] [CrossRef]
- Kogane, T.; Harada, K.; Hirota, R.; Urushiyama, A. Structures and characterization of reaction products of bis(1,1,1,5,5,5-hexafluoropentane-2,4-dionato)copper(II) with 3-methyl- and 3,5-dimethyl-1H-pyrazoles. Polyhedron 1996, 15, 4093–4101. [Google Scholar] [CrossRef]
- Saaidi, P.-L.; Jeanneau, E.; Bouchu, D.; Hasserodt, J. The case of a Cd2Cu2 complex containing an apparently C3-symmetric ligand: Erroneous ligand-structure assignment by X-ray diffraction data analysis. Polyhedron 2007, 26, 1191–1198. [Google Scholar] [CrossRef]
- Chappidi, D.Y.; Gordon, M.N.; Ashberry, H.M.; Huang, J.; Labedis, B.M.; Cooper, R.E.; Cooper, B.J.; Carta, V.; Skrabalak, S.E.; Dunbar, K.R.; et al. Mechanochemical Syntheses of Ln(hfac)3(H2O)x (Ln = La-Sm, Tb): Isolation of 10-, 9-, and 8-Coordinate Ln(hfac)n Complexes. Inorg. Chem. 2022, 61, 12197–12206. [Google Scholar] [CrossRef]
- Wang, S.; Pang, Z.; Smith, K.D.L.; Hua, Y.-S.; Deslippe, C.; Wagner, M.J. Decomposition and Cycloaddition Reactions of hfacac, and Syntheses and Structures of LnIII2(hfacac)4(bdmap)2(H2O)2(THF)2, LnIIICuII(bdmapH)2(hfacac)2(O2CCF3)L, LnIIICuII2(hfacac)(bdmap)3(O2CCH3)2(O2CCF3)(hfacacH) (Ln = Y, Pr, Nd; hfacac = hexafluoroacetylacetonato; bdmap = 1,3-bis(dimethylamino)-2-propanolato; L = 2-methyl-2,4,6-tris(trifluoromethyl)-1,3-dioxane-4,6-diolato). Inorg. Chem. 1995, 34, 908–917. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I. Fluorinated 1,2,4-triketone analogs: New prospects for heterocyclic and coordination chemistry. Russ. Chem. Bull. 2022, 71, 1321–1341. [Google Scholar] [CrossRef]

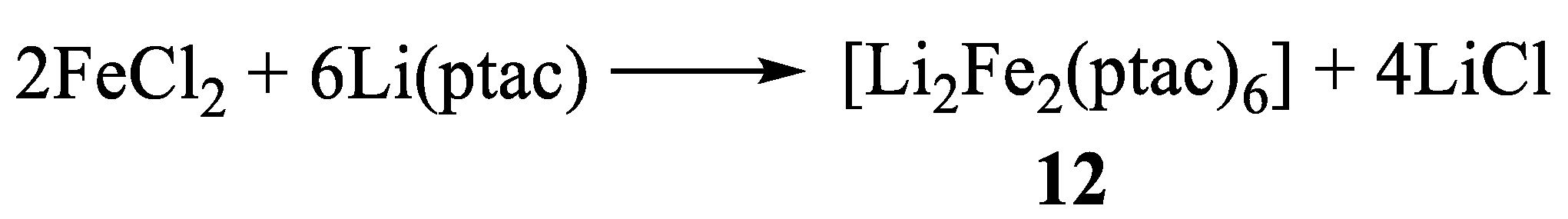





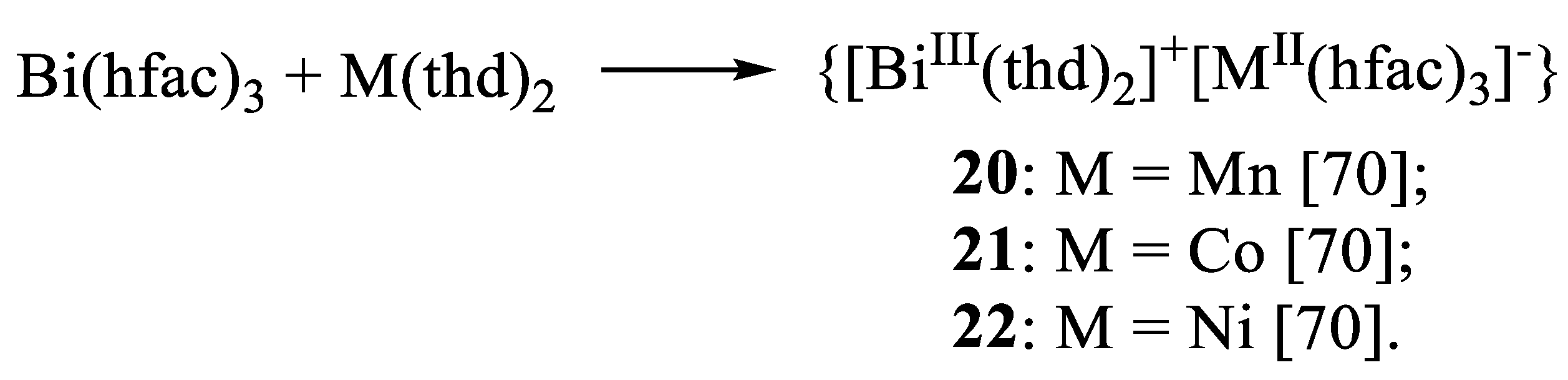
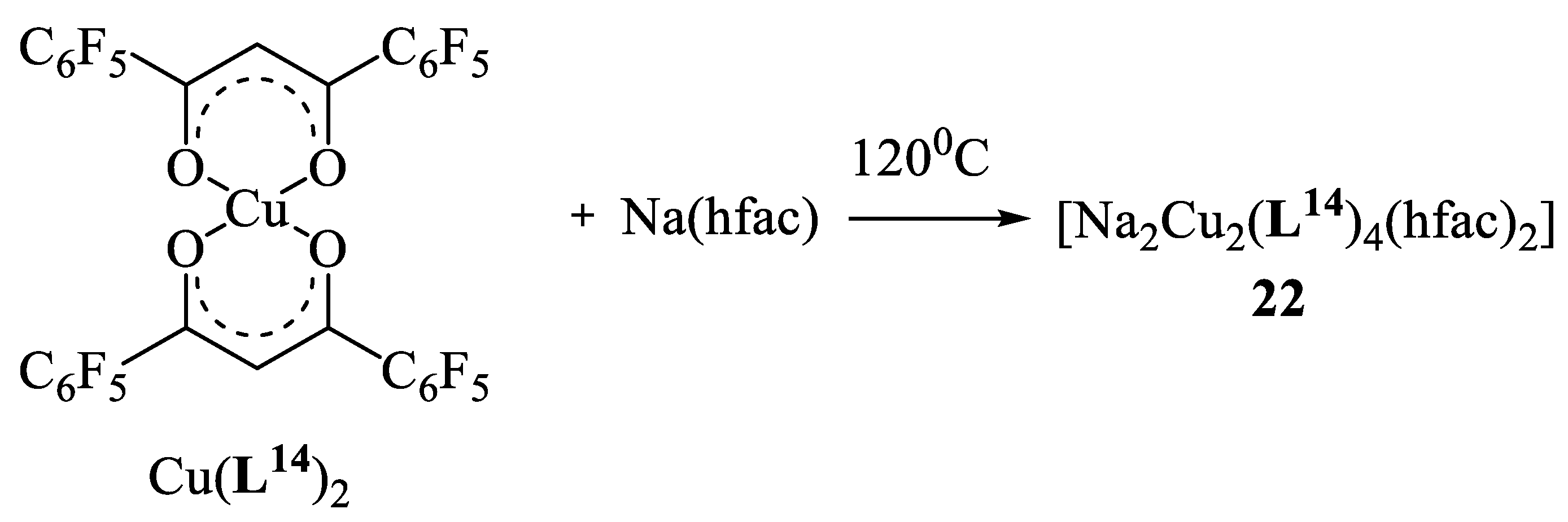

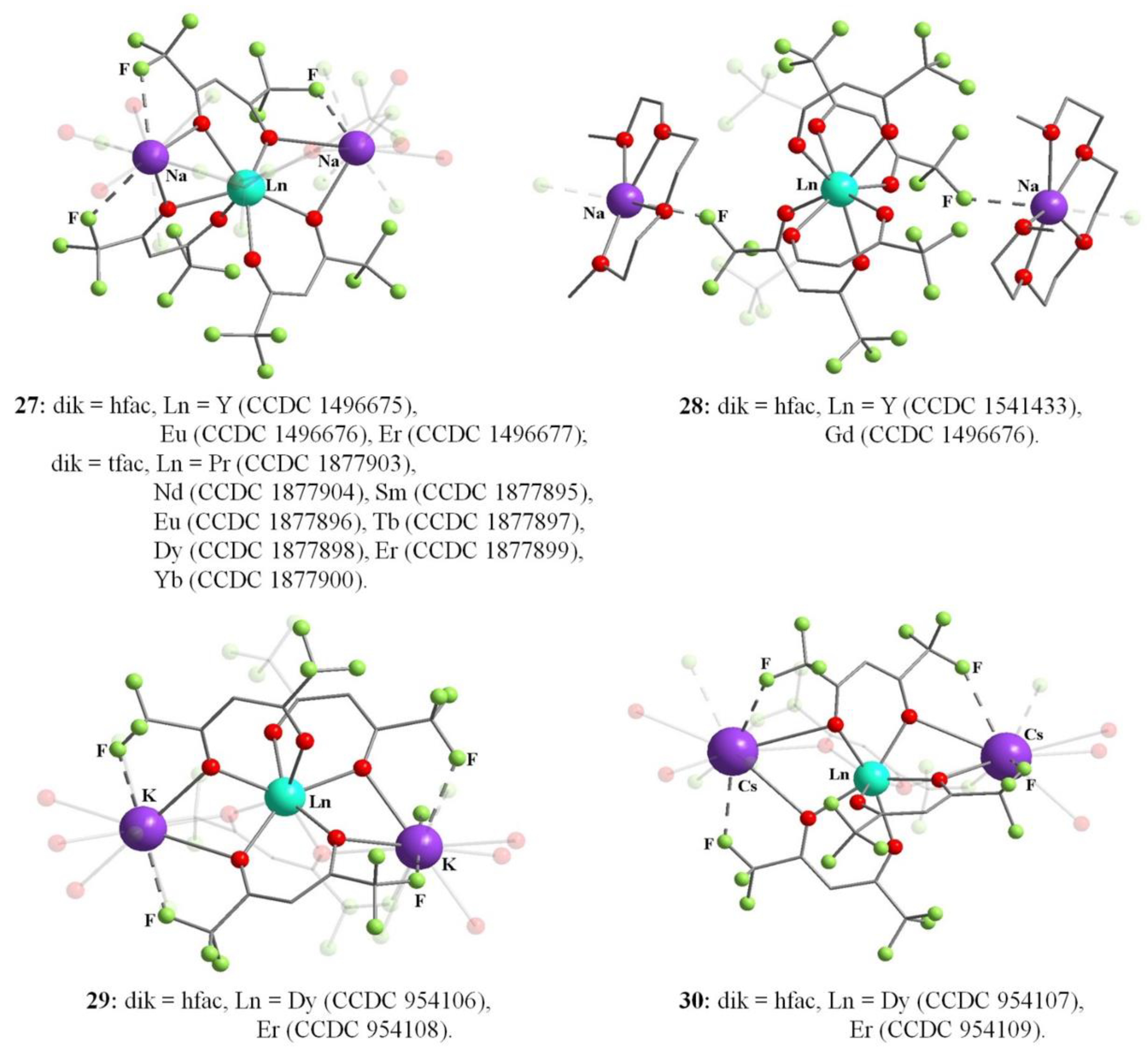
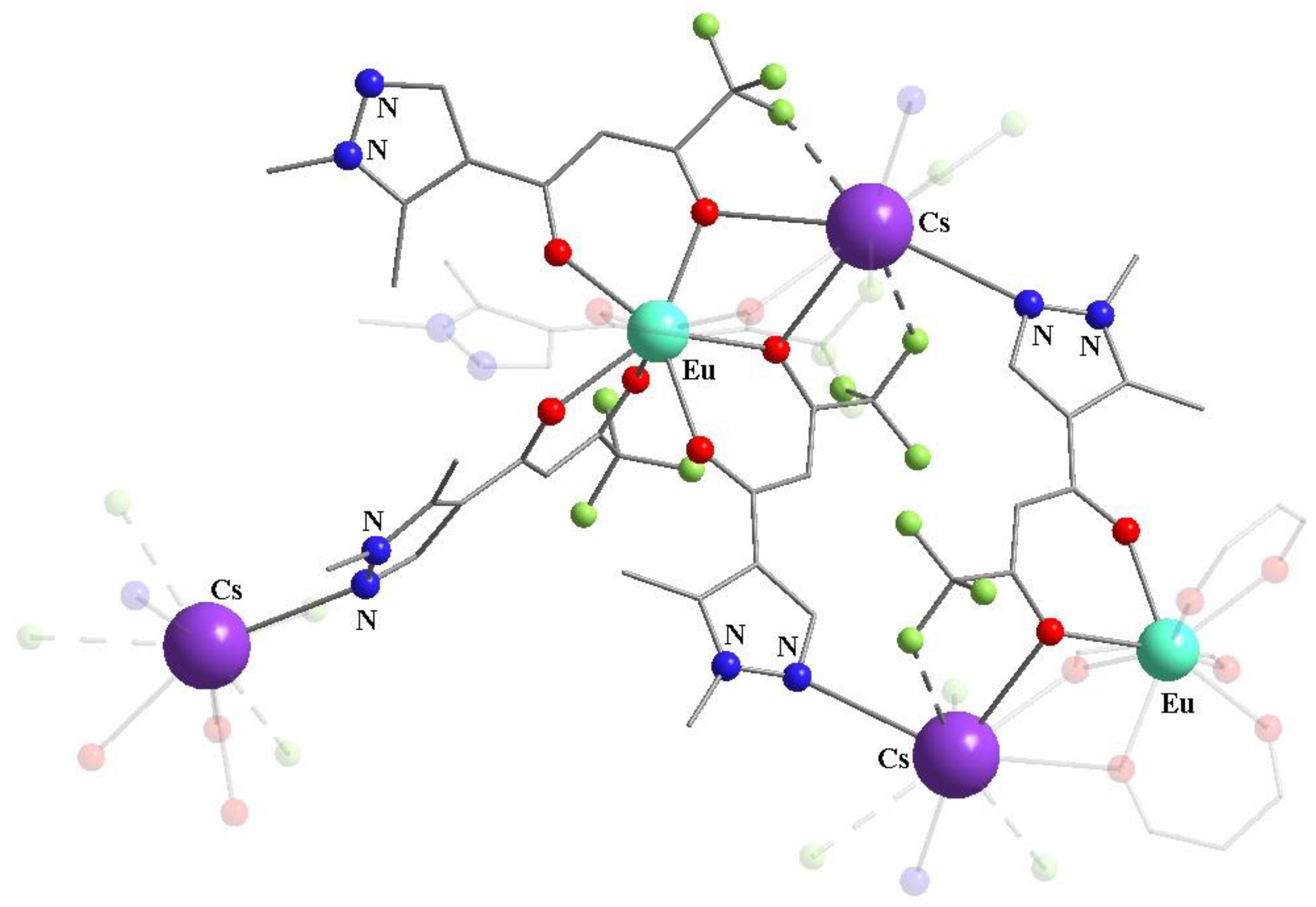

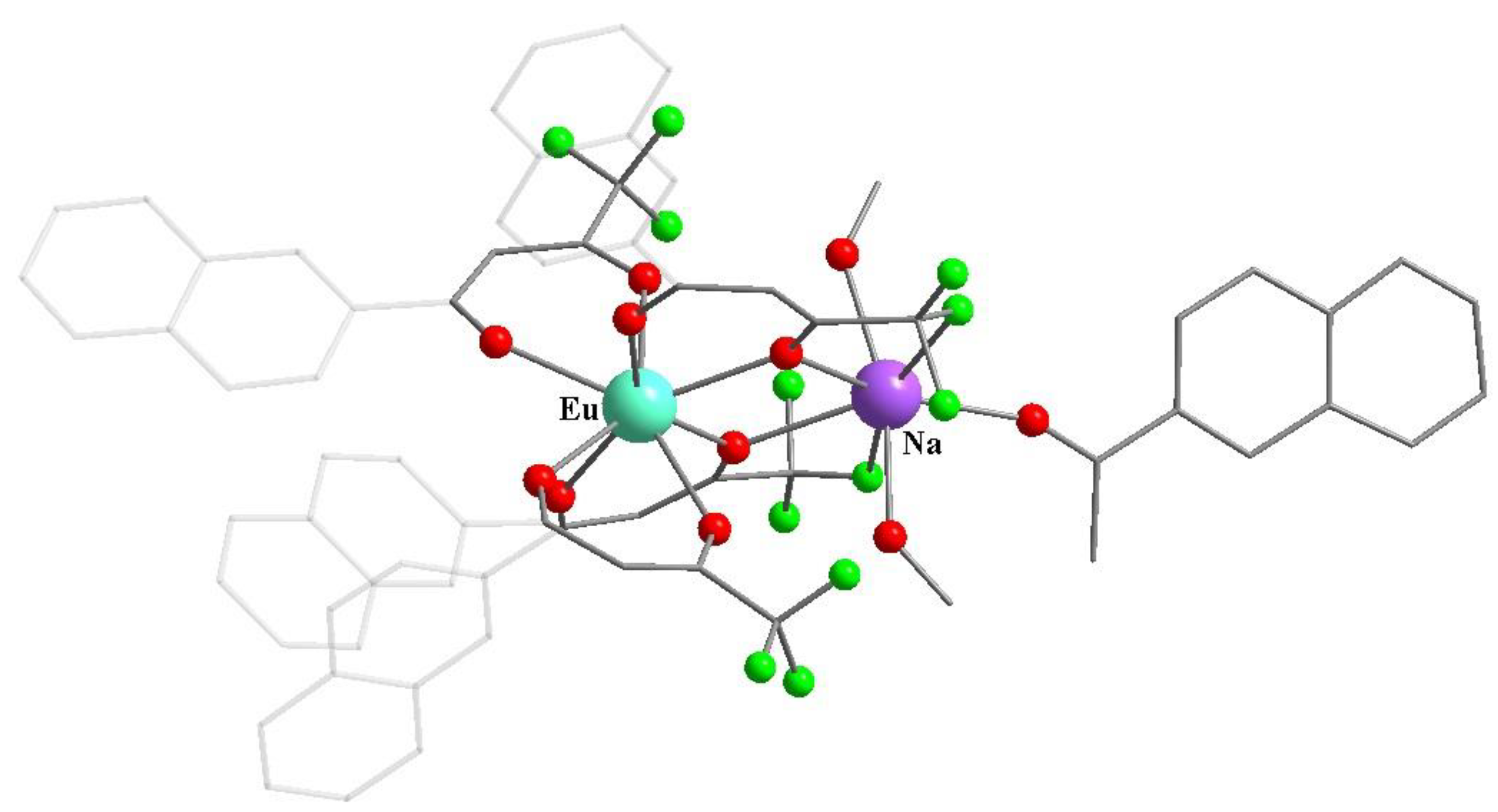

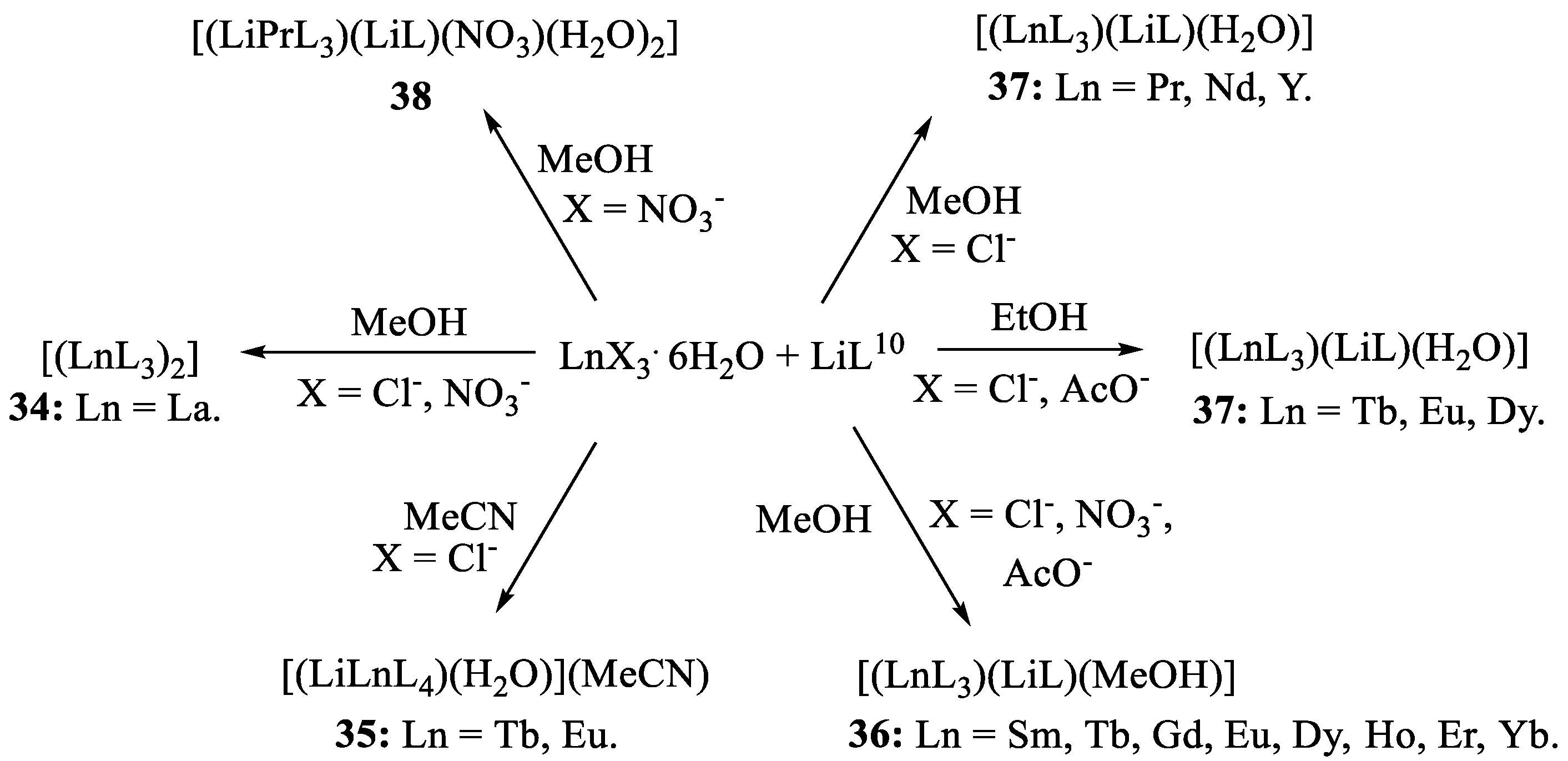


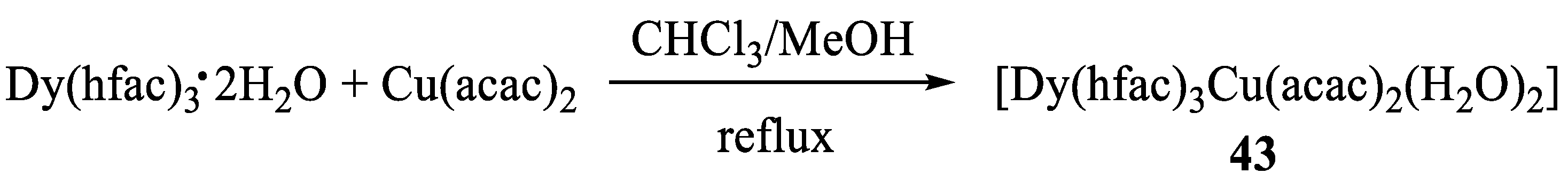

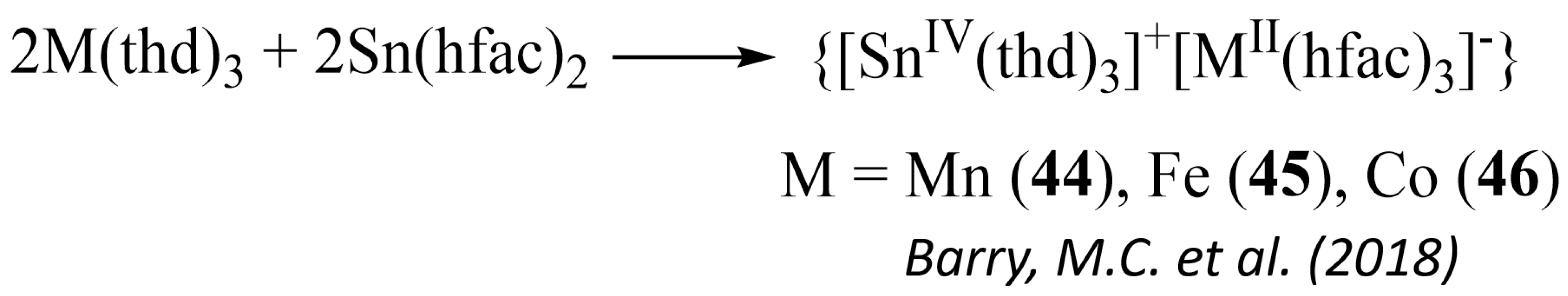

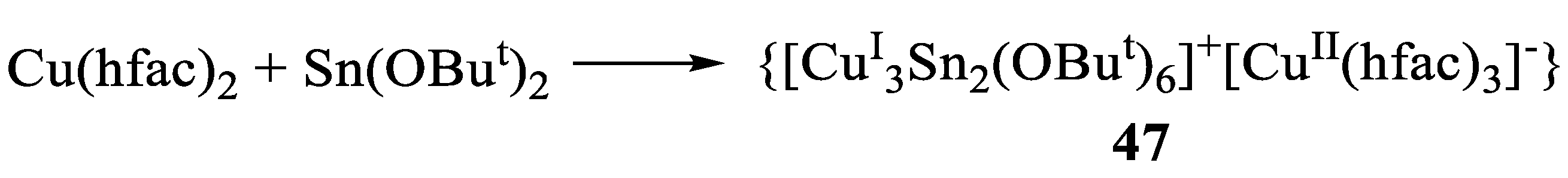

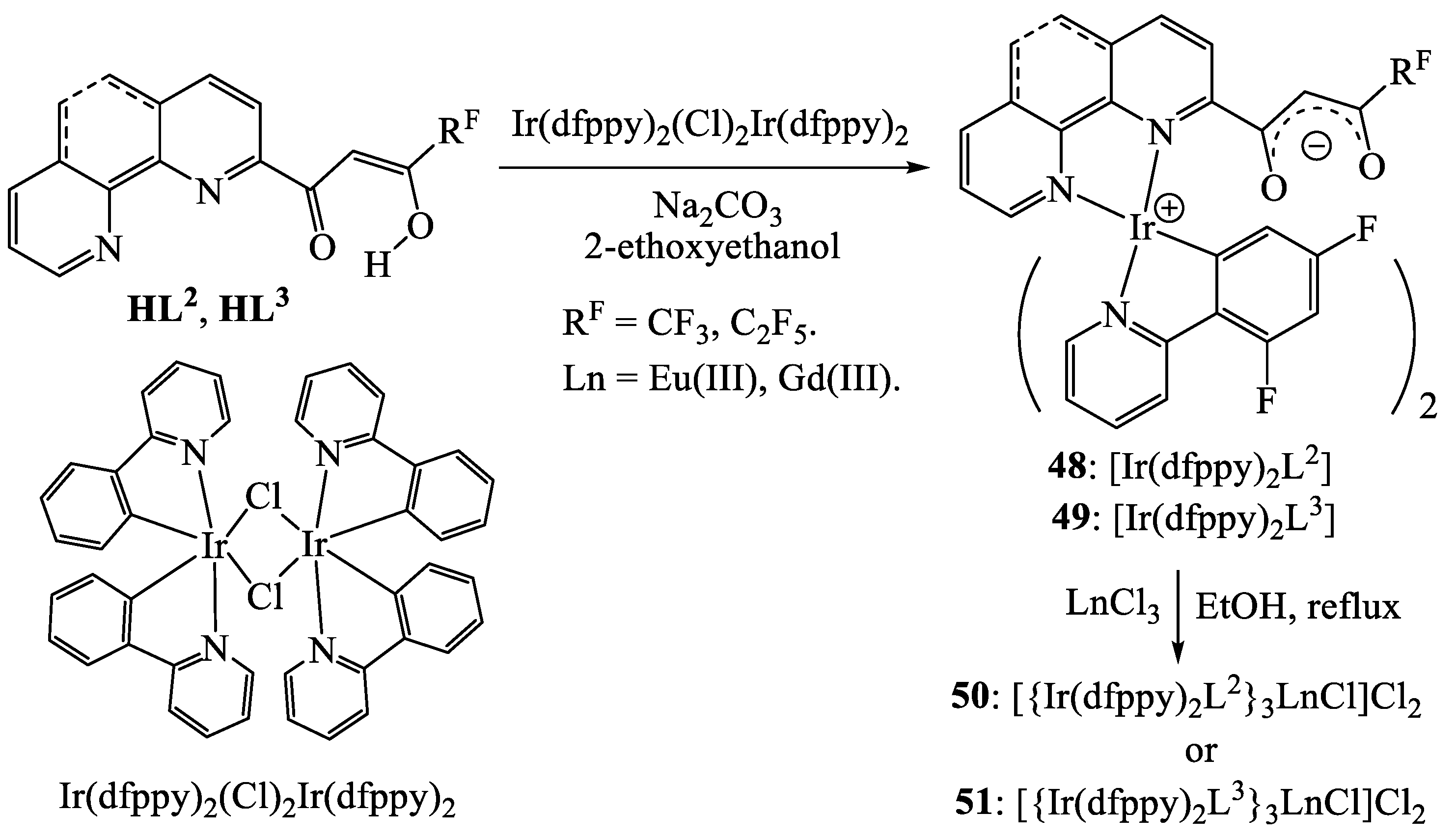
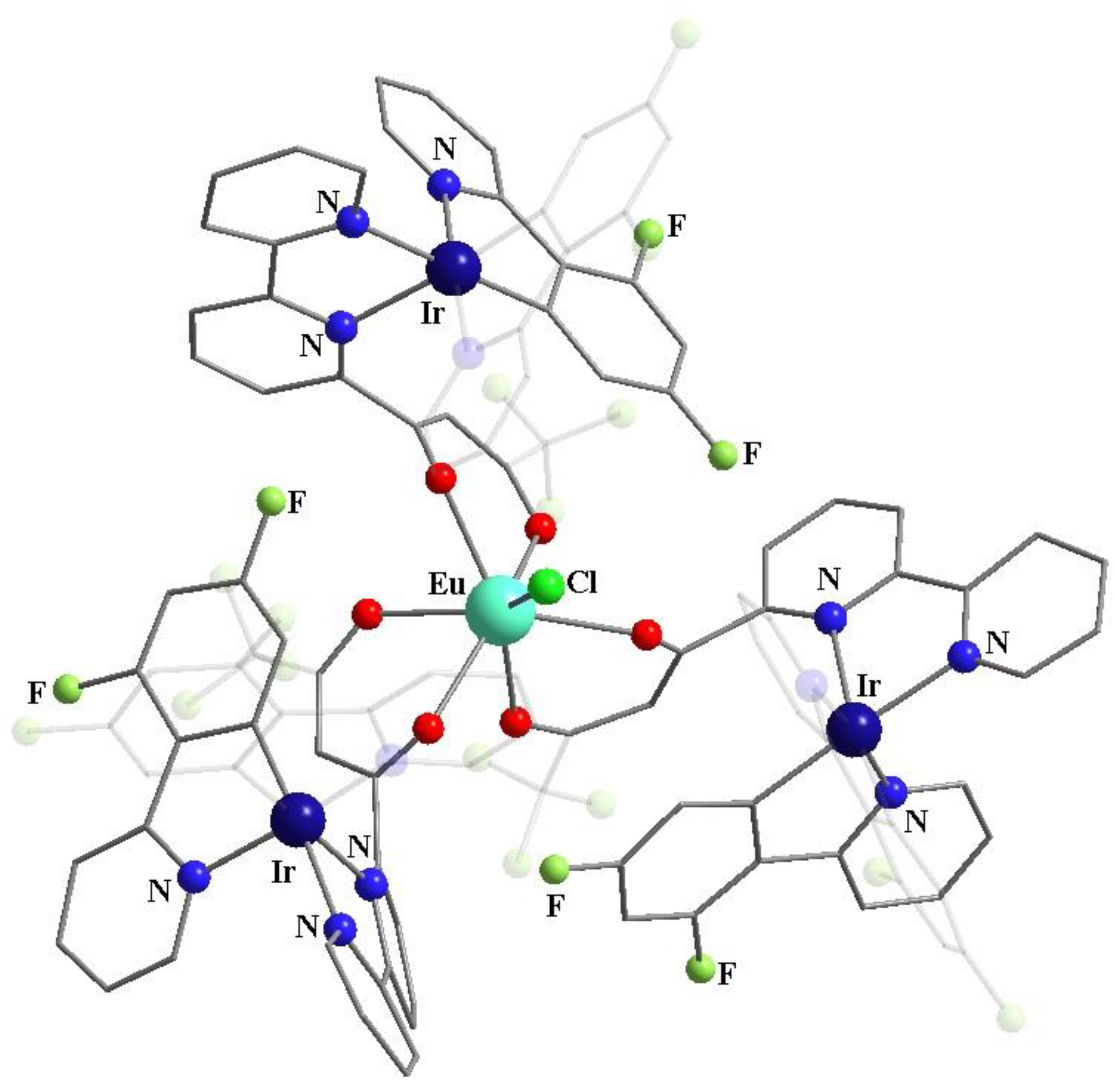
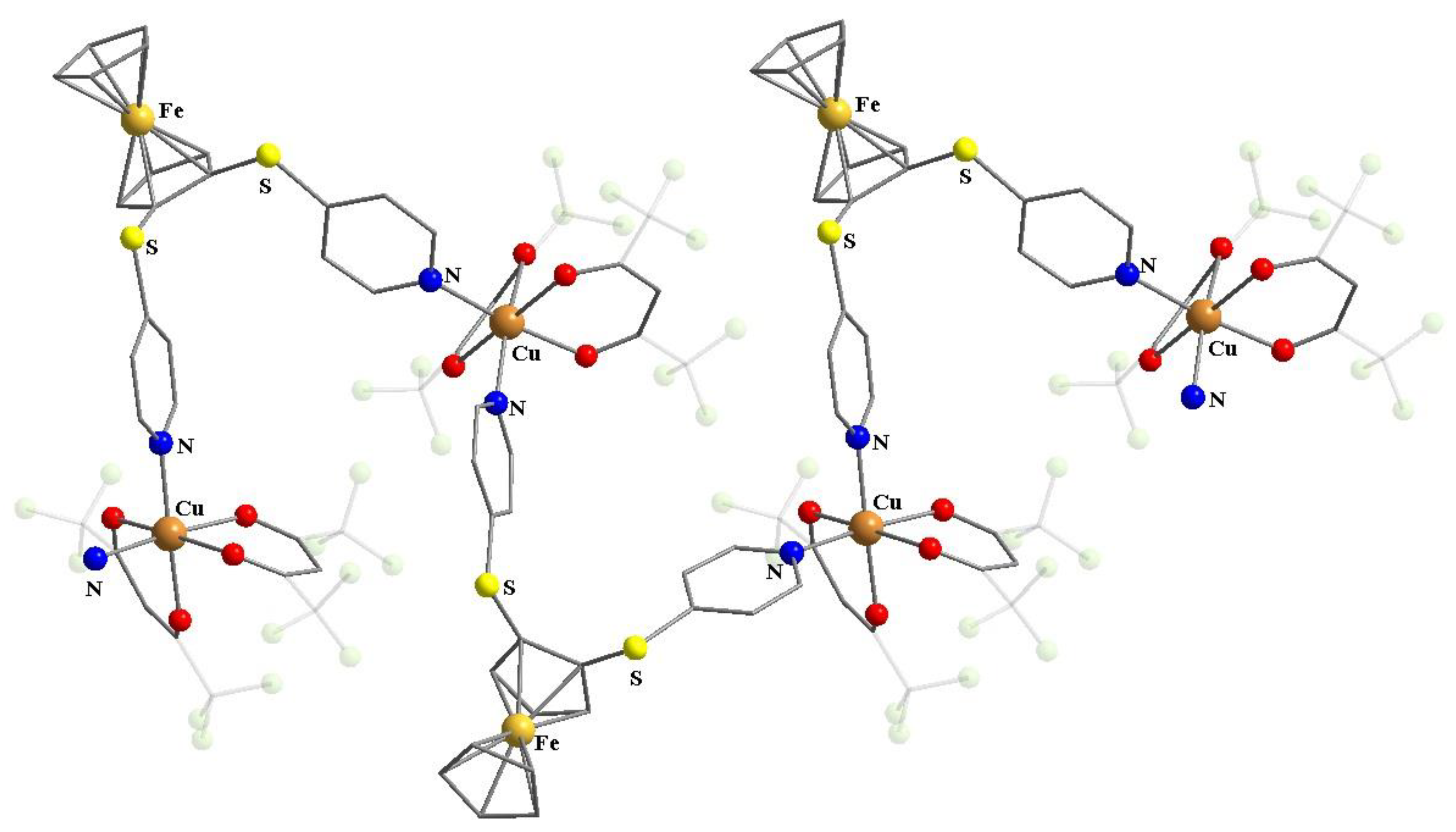

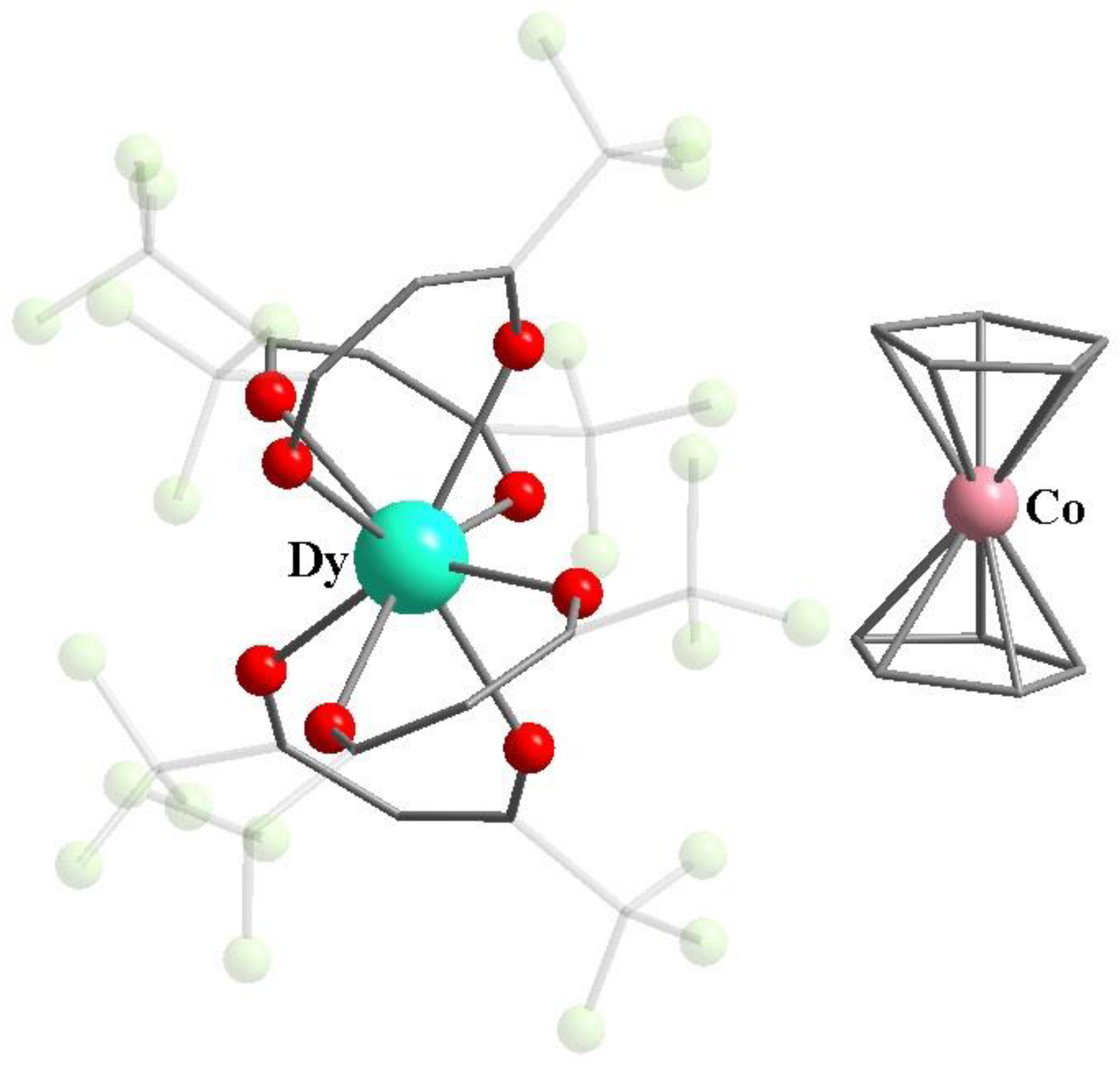



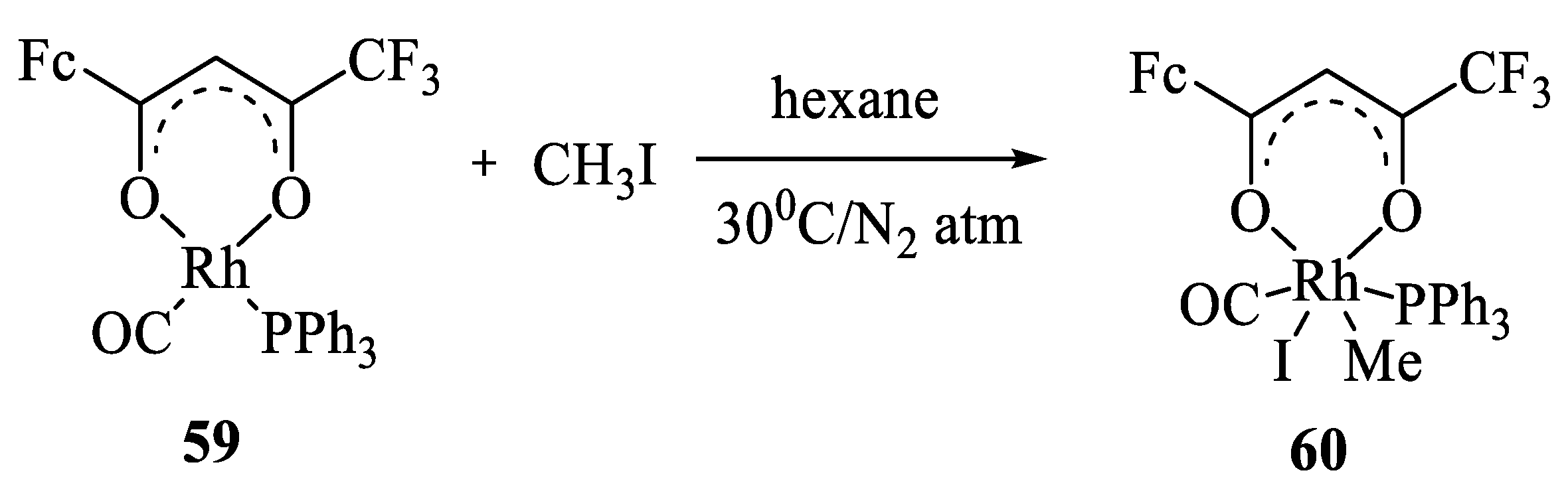

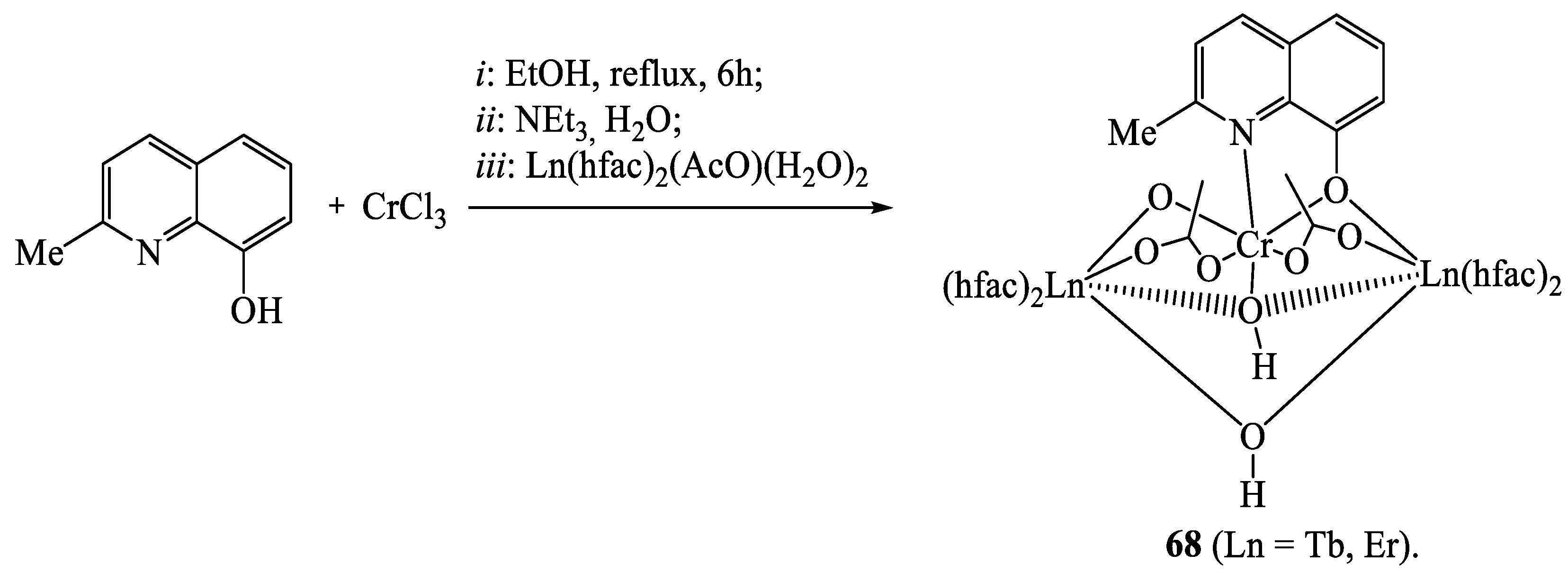



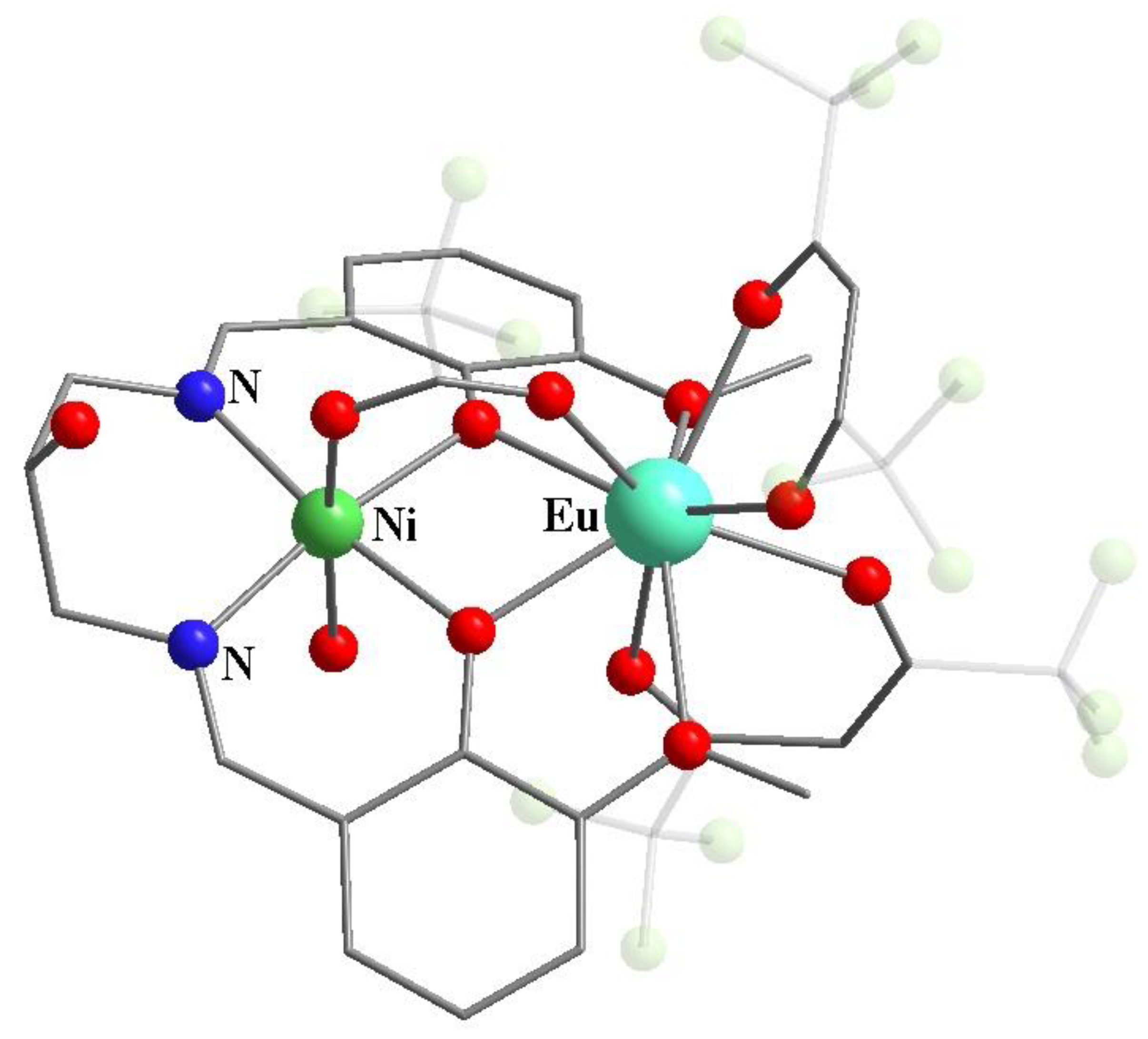
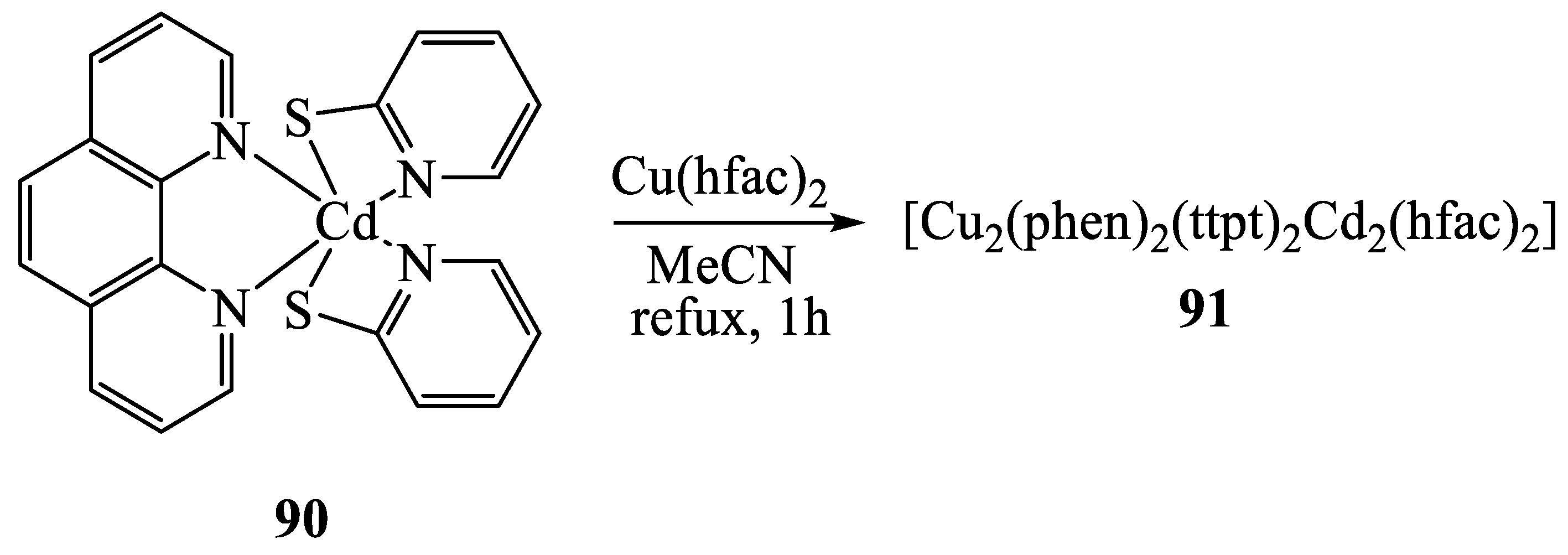
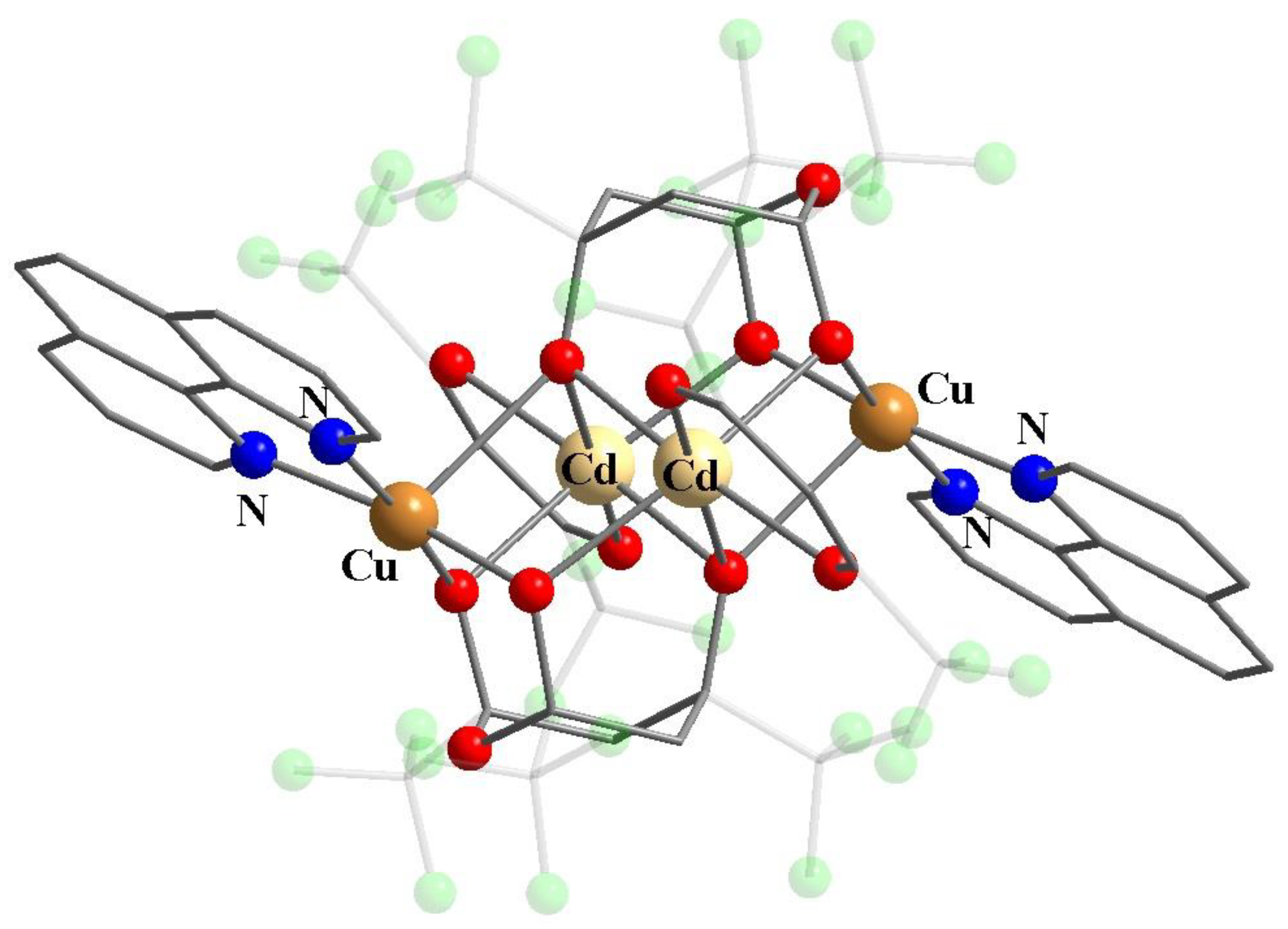

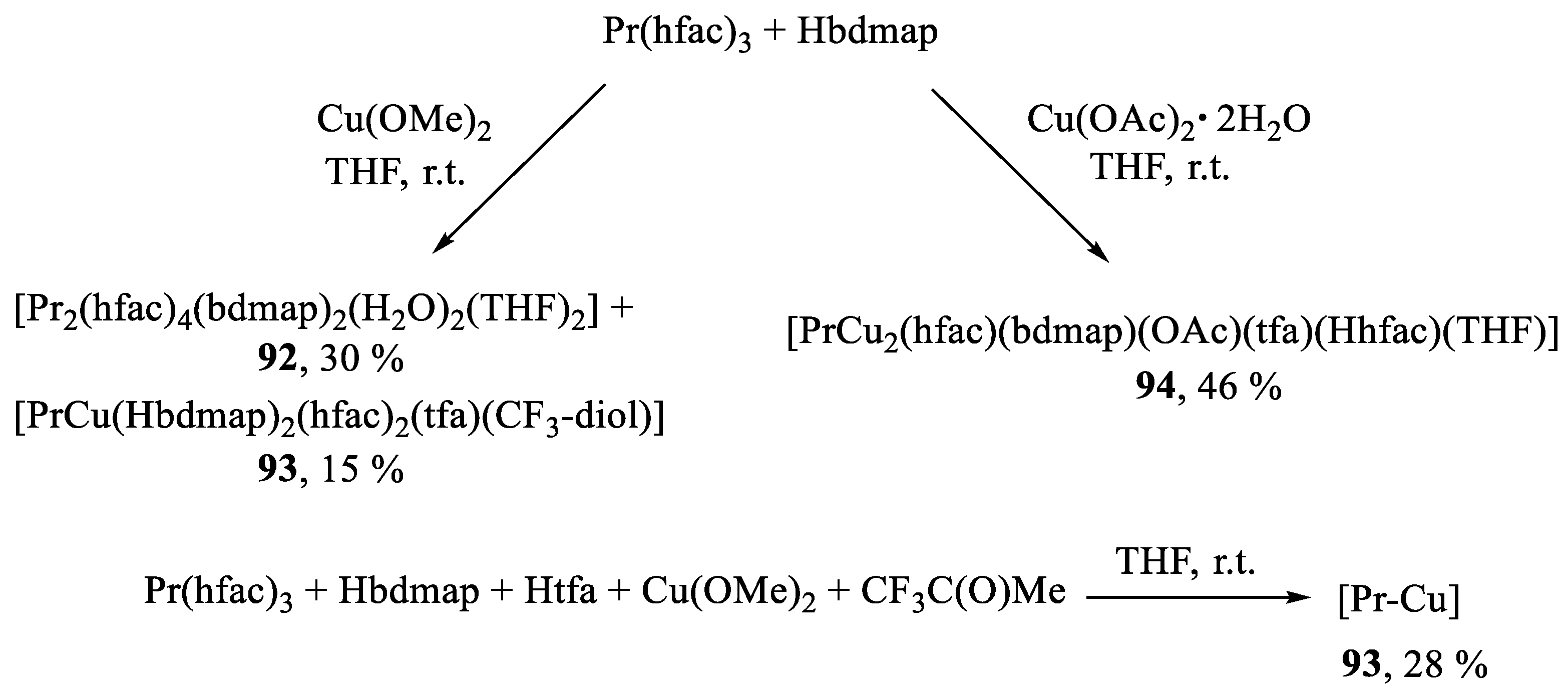
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saloutin, V.I.; Edilova, Y.O.; Kudyakova, Y.S.; Burgart, Y.V.; Bazhin, D.N. Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands. Molecules 2022, 27, 7894. https://doi.org/10.3390/molecules27227894
Saloutin VI, Edilova YO, Kudyakova YS, Burgart YV, Bazhin DN. Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands. Molecules. 2022; 27(22):7894. https://doi.org/10.3390/molecules27227894
Chicago/Turabian StyleSaloutin, Viktor I., Yulia O. Edilova, Yulia S. Kudyakova, Yanina V. Burgart, and Denis N. Bazhin. 2022. "Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands" Molecules 27, no. 22: 7894. https://doi.org/10.3390/molecules27227894
APA StyleSaloutin, V. I., Edilova, Y. O., Kudyakova, Y. S., Burgart, Y. V., & Bazhin, D. N. (2022). Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands. Molecules, 27(22), 7894. https://doi.org/10.3390/molecules27227894