Comparative Study of Antimicrobial Activity of Silver, Gold, and Silver/Gold Bimetallic Nanoparticles Synthesized by Green Approach
Abstract
1. Introduction
2. Results
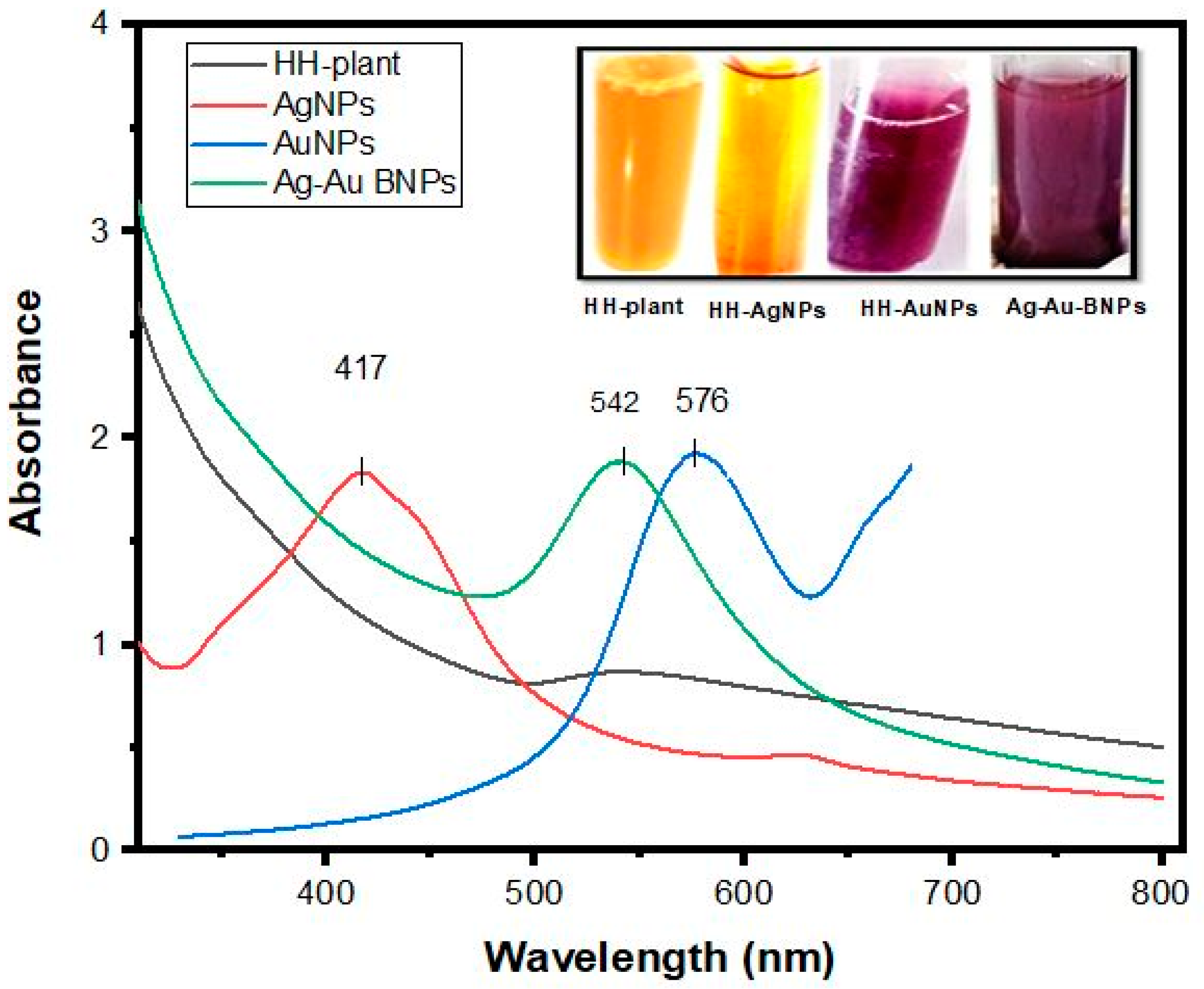
2.1. UV-Visible Spectrophotometric Analysis of NPs
2.2. Factors Effecting Synthesis of NPs
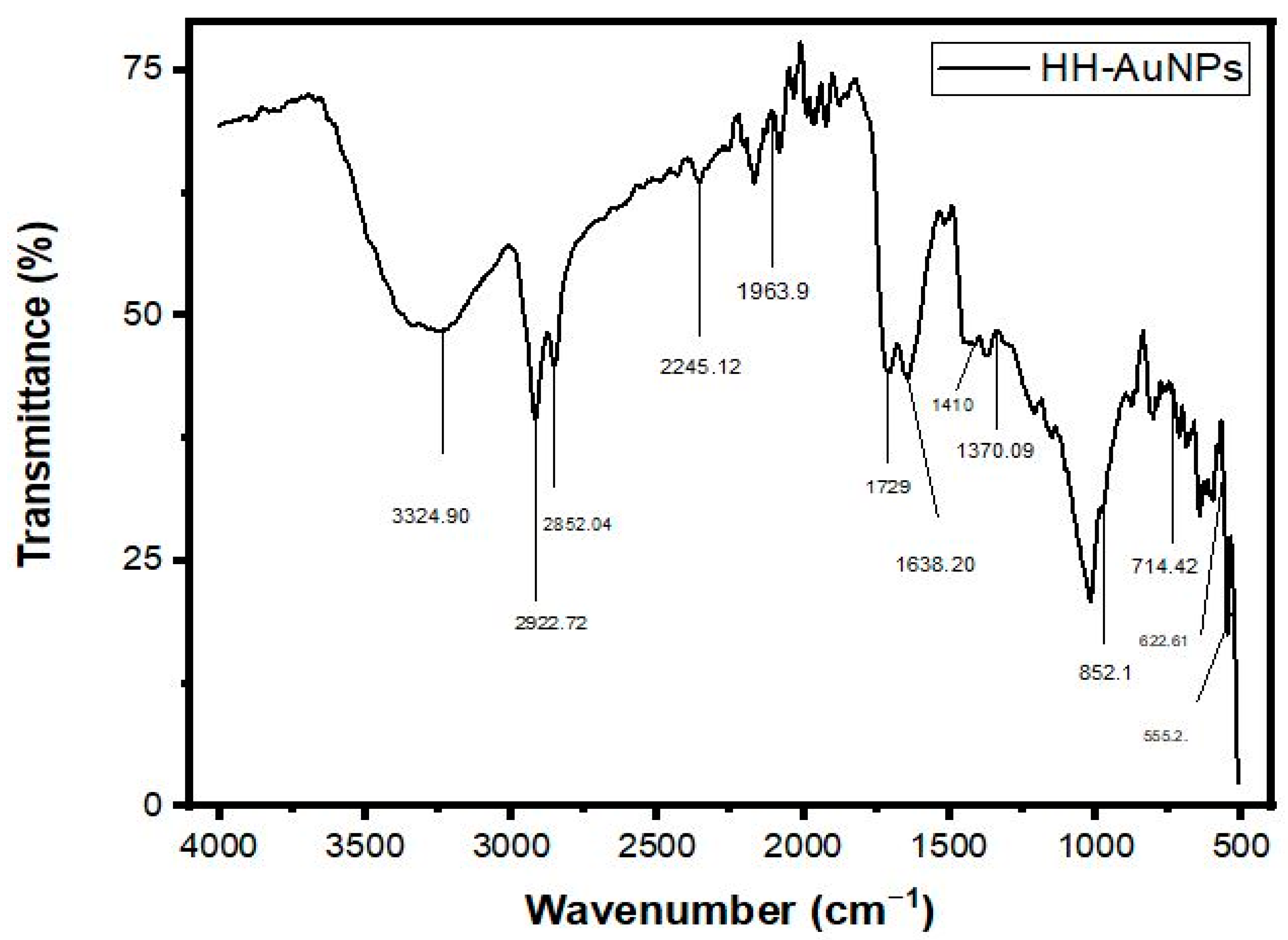
2.3. FT-IR Analysis of NPs
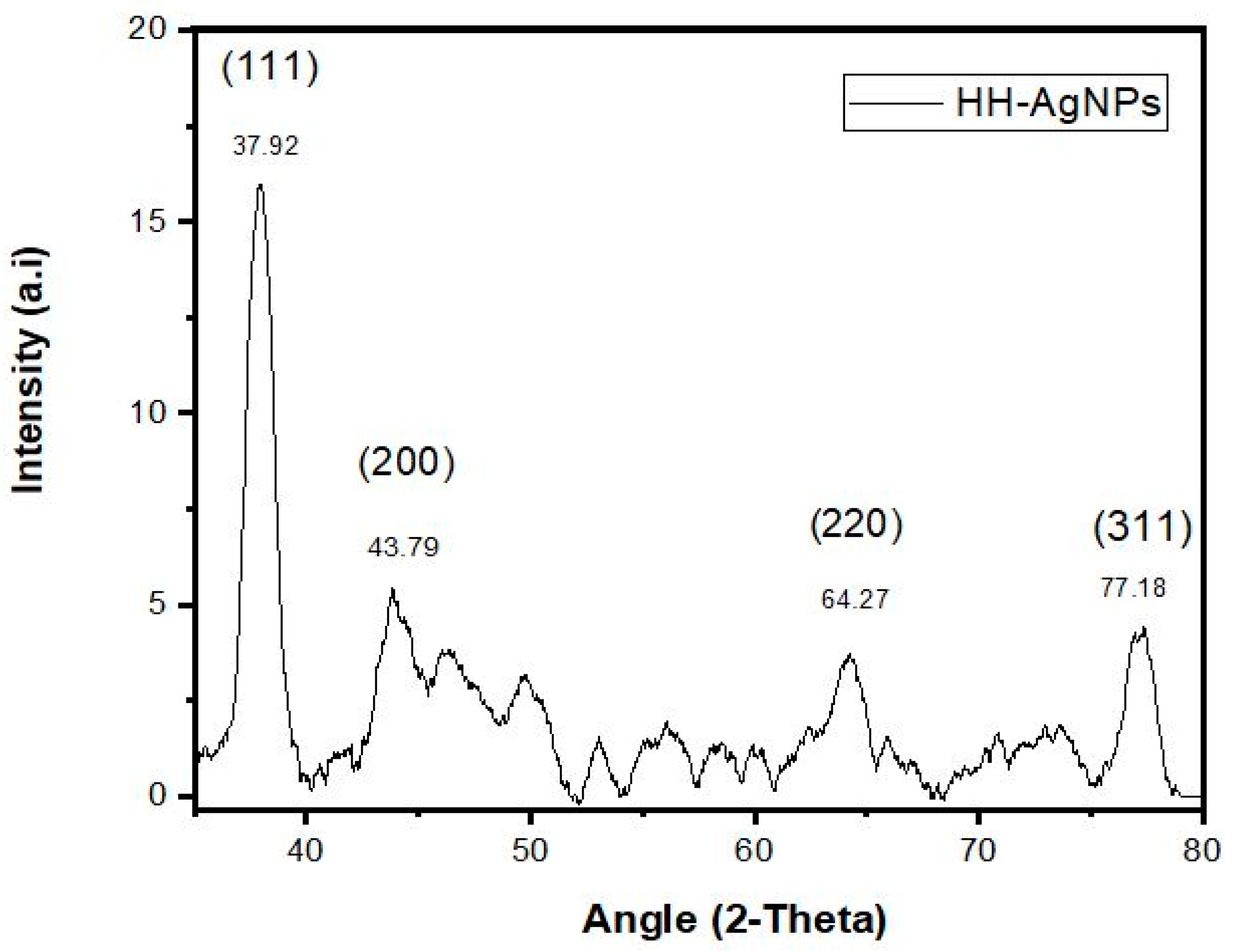
2.4. XRD Analysis of NPs
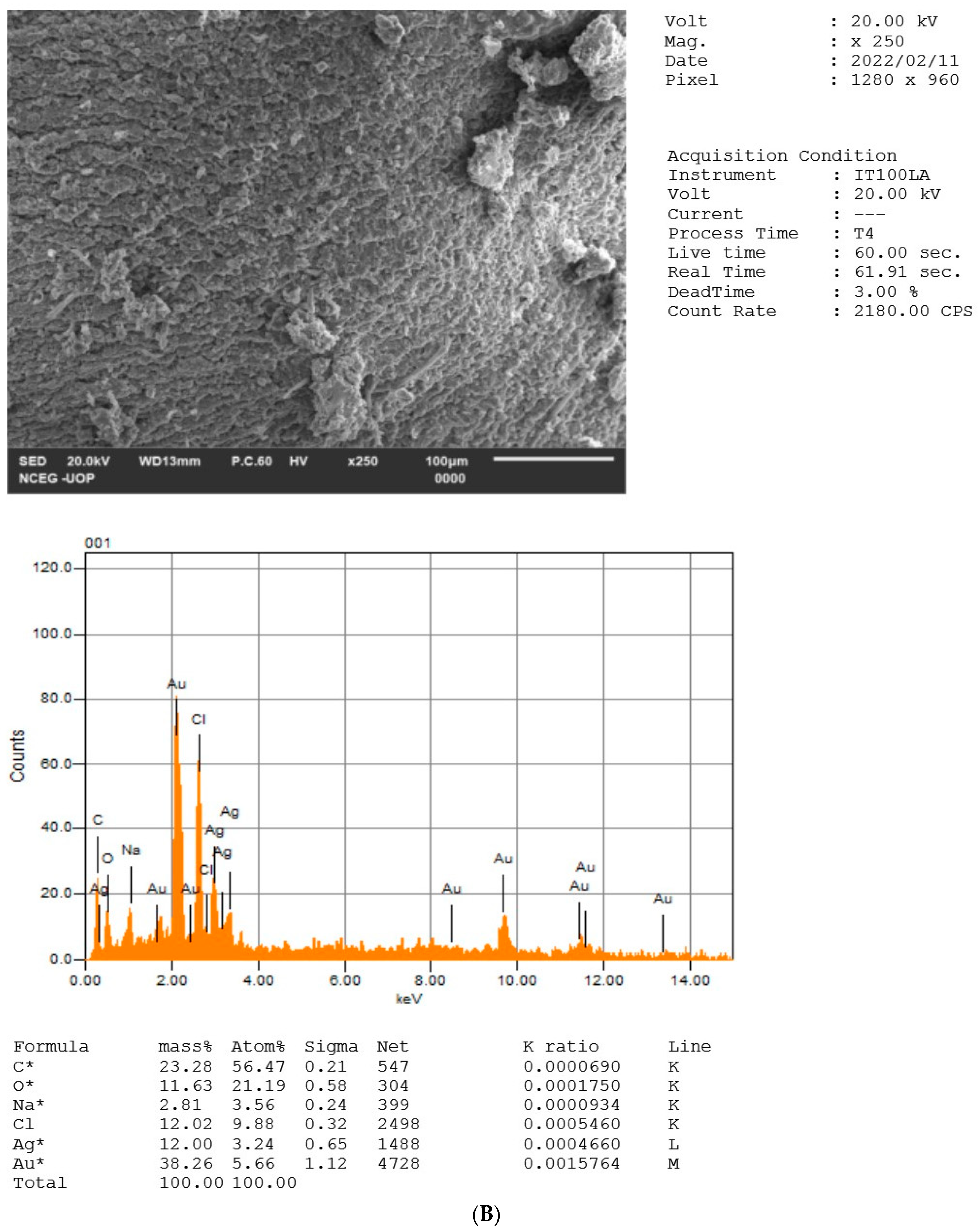
2.5. EDX Analysis of NPs
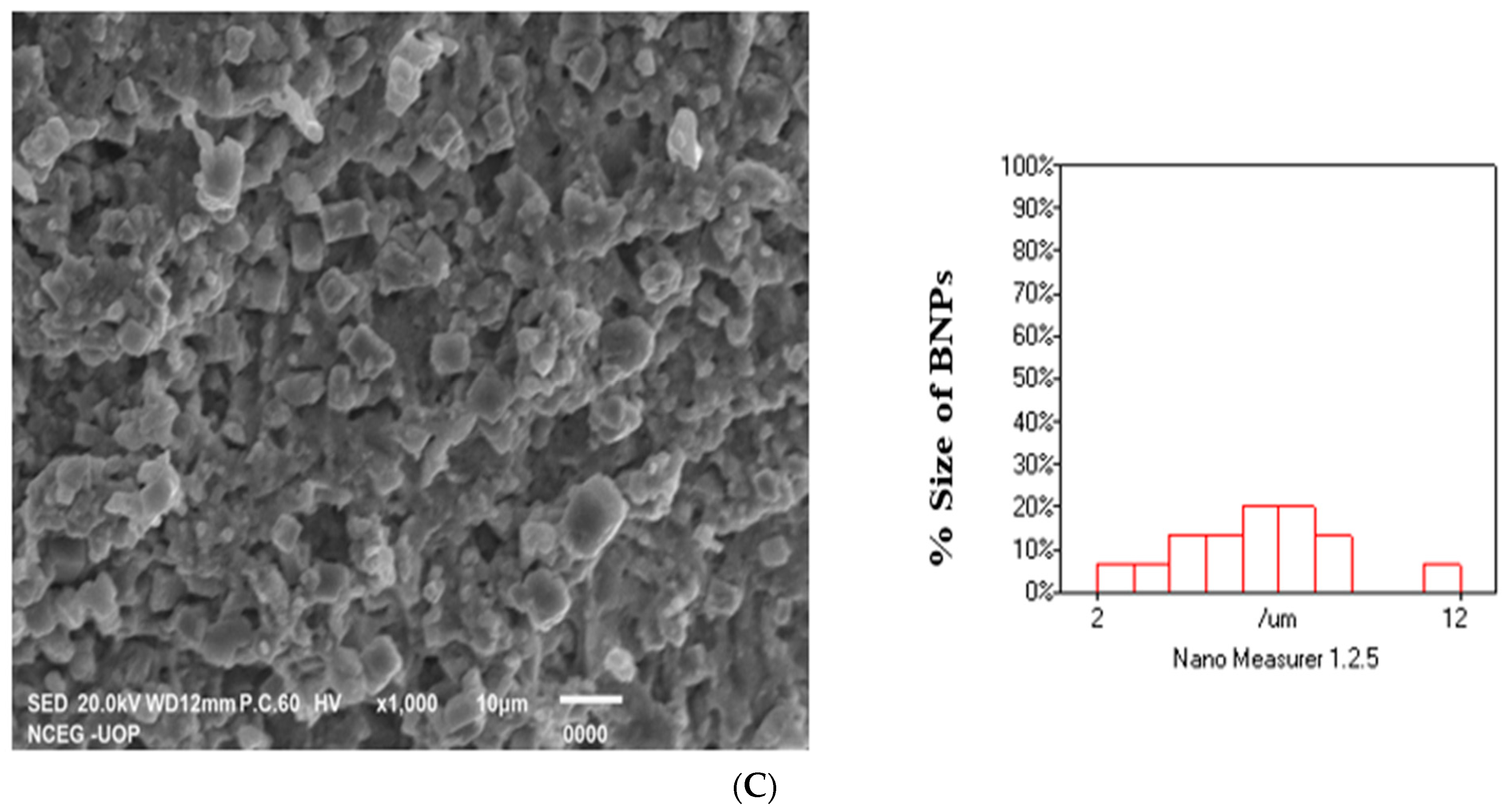
2.6. SEM Analysis of Ag, Au, MNPs and Ag-Au BNPs
2.7. Antibacterial Activity
2.8. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Plant’s Extraction
3.3. Synthesis of Ag, Au, and Ag/Au BNPs
3.4. Factors Affecting Synthesis Rate, Size, and Shape of NPs
3.5. Characterization
3.6. Antibacterial Activity (Agar Diffusion Method)
3.7. Antifungal Bio-Assay
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dinesh, M.G.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B Biointerfaces 2013, 106, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013, 461–462, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anticancer evaluation. J. Photochem. Photobiol. B 2013, 125, 63–69. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.L.; Sudha, D.; Kalpana, M.; Lakshmi, P.T. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef]
- Gopinath, K.; Gowri, S.; Karthika, V.; Arumugam, A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba. J. Nanostruct. Chem. 2014, 4, 115. [Google Scholar] [CrossRef]
- Inbakandan, D.; Kumar, C.; Abraham, L.S.; Kirubagaran, R.; Venkatesan, R.; Khan, S.A. Silver nanoparticles with anti microfouling effect: A study against marine biofilm forming bacteria. Colloids Surf. B Biointerfaces 2013, 111, 636–643. [Google Scholar] [CrossRef]
- Patil, R.S.; Kokate, M.R.; Kolekar, S.S. Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 234–238. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Padma, M.; Samadanam, I.D.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Dorobăţ, O.M.; Moisoiu, A.; Tălăpan, D. Incidence and resistance patterns of pathogens from lower respiratory tract infections (LRTI). Pneumologia 2007, 56, 7–15. [Google Scholar]
- Apte, M.; Girme, G.; Nair, R.; Bankar, A.; Kumar, A.; Zinjarde, S. Melanin mediated synthesis of gold nanoparticles by Yarrowia lipolytica. Mater. Lett. 2013, 95, 149–152. [Google Scholar] [CrossRef]
- Barapatre, A.; Aadil, K.R.; Jha, H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour. Bioprocess. 2016, 3, 8. [Google Scholar] [CrossRef]
- Dhandapani, P.; Maruthamuthu, S.; Rajagopal, G. Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effect on aquatic biofilm. J. Photochem. Photobiol. B 2012, 110, 43–49. [Google Scholar] [CrossRef]
- Prabhawathi, V.; Sivakumar, P.M.; Doble, M. Green Synthesis of Protein Stabilized Silver Nanoparticles Using Pseudomonas fluorescens, a Marine Bacterium, and Its Biomedical Applications When Coated on Polycaprolactam. Ind. Eng. Chem. Res. 2012, 51, 5230–5239. [Google Scholar] [CrossRef]
- Lokina, S.; Suresh, R.; Giribabu, K.; Stephen, A.; Lakshmi Sundaram, R.; Narayanan, V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Sher, N.; Ahmed, M.; Mushtaq, N.; Khan, R.A. Calligonum polygonoides reduced nanosilver: A new generation of nanoproduct for medical applications. Eur. J. Integr. Med. 2020, 33, 101042. [Google Scholar] [CrossRef]
- Maqbool, Q.; Czerwinska, N.; Giosue, C.; Sabbatini, S.; Ruello, M.L.; Tittarelli, F. New waste-derived TiO2 nanoparticles as a potential photocatalytic additive for lime based indoor finishings. J. Clean. Prod. 2022, 373, 133853. [Google Scholar] [CrossRef]
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon—Cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Margarita, V.; Fais, G.; Pantaleo, A.; Manca, A.; Concas, A.; Rappelli, P.; Fiori, P.L.; Cao, G. Characterization of nanomaterials synthesized from Spirulina platensis extract and their potential antifungal activity. PLoS ONE 2022, 17, e0274753. [Google Scholar] [CrossRef]
- Stevens, P.F. Asparagales: Amaryllidoideae. Angiosperm Phylogeny Website Version; Missouri Botanical Garden: St. Louis, MO, USA, 2016. [Google Scholar]
- Wang, Y.; Chen, D.; He, X.; Shen, J.; Xiong, M.; Wang, X.; Zhou, D.; Wei, Z. Revealing the complex genetic structure of cultivated amaryllis (Hippeastrum hybridum) using transcriptome-derived microsatellite markers. Sci. Rep. 2018, 8, 10645. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Christmastime Plants. In Small Animal Toxicology; Saunders: St. Louis, MO, USA, 2006; pp. 643–663. [Google Scholar]
- Prasad, K.S.; Savithramma, N. Biosynthesis and Validation of Silver Nanoparticles from Nymphaea caerulea. Am. J. Adv. Drug Deliv. 2015, 3, 149–159. [Google Scholar]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol. 2009, 123, 494–498. [Google Scholar] [CrossRef]
- Sheny, D.S.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Tamuly, C.; Hazarika, M.; Borah, S.C.; Das, M.R.; Boruah, M.P. In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: Green chemistry approach. Colloids Surf. B 2013, 102, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess. Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Armendariz, V.; Herrera, I.; Peralta-videa, J.R.; Jose-yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanoparticle Res. J. Recent Sci. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Sreedhara Reddy, P. Enhanced antimicrobial activity of silver nanoparticles with controlled particle size by pH variation. Powder Technol. 2015, 269, 110–117. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir ACS J. Surf. Colloids 2006, 22, 736–741. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-protected metal nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 885–890. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Zamiri, R.; Zak, A.K.; Abdullah, A.H.; Ibrahim, N.A. Time-dependent effect in green synthesis of silver nanoparticles. Int. J. Nanomed. 2011, 6, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Balavandy, S.K.; Shameli, K.; Biak, D.R.; Abidin, Z.Z. Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem. Cent. J. 2014, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, Q.; Su, Q.; Liu, R. Green Synthesis of Silver Nanoparticles at Room Temperature Using Kiwifruit Juice. Spectrosc. Lett. 2014, 47, 790–795. [Google Scholar] [CrossRef]
- Baer, D.R. Surface Characterization of Nanoparticles: Critical needs and significant challenges. J. Surf. Anal. 2011, 17, 163–169. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef]
- Devi, T.S.R.; Gayathri, S. FTIR and FT-Raman spectral analysis of paclitaxel drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 2, 106–110. [Google Scholar]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Syed, B.; Bisht, N.; Prithvi, S.B.; Karthik, R.N.; Prasad, A.; Dhananjaya, B.L.; Satish, S.; Prasad, H.; Prasad, M.N.N. Phytogenic synthesis of nanoparticles from Rhizophora mangle and their bactericidal potential with DNA damage activity. Nano-Struct. Nano-Objects 2017, 10, 112–115. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef]
- Syed, B.; Karthik, N.; Bhat, P.; Bisht, N.; Prasad, A.; Satish, S.; Prasad, M.N.N. Phyto-biologic bimetallic nanoparticles bearing antibacterial activity against human pathogens. J. King Saud Univ.-Sci. 2019, 31, 798–803. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O. A novel zerovalent manganese for removal of copper ions: Synthesis, characterization and adsorption studies. Appl. Water Sci. 2017, 7, 1409–1427. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O. Kinetics and equilibrium models for sorption of Cu(II) onto a novel manganese nano-adsorbent. J. Dispers. Sci. Technol. 2016, 37, 119–133. [Google Scholar] [CrossRef]
- Dada, A.O.; Inyinbor, A.A.; Idu, E.I.; Bello, O.M.; Oluyori, A.P.; Adelani-Akande, T.A.; Okunola, A.A.; Dada, O. Effect of operational parameters, characterization and antibacterial studies of green synthesis of silver nanoparticles using Tithonia diversifolia. PeerJ 2018, 6, e5865. [Google Scholar] [CrossRef] [PubMed]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Zahoor, M.; Jalal, A.; Rahman, A.U. Green Synthesis of Silver Nanoparticles by Using Ziziphus nummularia Leaves Aqueous Extract and Their Biological Activities. J. Nanomater. 2016, 2016, 8026843. [Google Scholar] [CrossRef]
- Gustavsson, J.F. Global Food Losses and Food Waste: Extent, Causes and Prevention: Study Conducted for the International Congress “Save Food!” at Interpack 2011 Dusseldorf, Germany; FAO: Rome, Italy; SP Group: Singapore, 2011. [Google Scholar]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. 1—Microbiological Spoilage of Foods and Beverages. In The Stability and Shelf Life of Food; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–42. [Google Scholar]
- Säde, E.; Penttinen, K.; Björkroth, J.; Hultman, J. Exploring lot-to-lot variation in spoilage bacterial communities on commercial modified atmosphere packaged beef. Food Microbiol. 2017, 62, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.P.; Baral, P.; Aryal, P.; Ghimire, K.R.; Neupane, S.; Dahal, N.; Singh, A.; Ghimire, L.; Shrestha, K. Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Biomed Res. Int. 2015, 2015, 265425. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Malik, S.; Ahmed, J. Antibiotic susceptibility pattern and ESBL prevalence in nosocomial Escherichia coli from urinary tract infections in Pakistan. Afr. J. Biotechnol. 2009, 8, 3921–3926. [Google Scholar]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag–Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Danziger-Isakov, L.A.; Worley, S.; Arrigain, S.; Aurora, P.; Ballmann, M.; Boyer, D.; Conrad, C.; Eichler, I.; Elidemir, O.; Goldfarb, S.; et al. Increased mortality after pulmonary fungal infection within the first year after pediatric lung transplantation. J. Heart Lung Transplant. 2008, 27, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Ker, C.C.; Hung, C.C.; Huang, S.Y.; Chen, M.Y.; Hsieh, S.M.; Lin, C.C.; Chang, S.C.; Luh, K.T. Comparison of bone marrow studies with blood culture for etiological diagnosis of disseminated mycobacterial and fungal infection in patients with acquired immunodeficiency syndrome. J. Microbiol. Immunol. Infect. 2002, 35, 89–93. [Google Scholar] [PubMed]
- al-Sogair, S.M.; Moawad, M.K.; al-Humaidan, Y.M. Fungal infection as a cause of skin disease in the eastern province of Saudi Arabia: Cutaneous candidosis. Mycoses 1991, 34, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, W.E. Introduction to Antifungal Drugs. Clininfedise Clin. Infect. Dis. 2000, 30, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.B.H.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Almansob, A.; Bahkali, A.H.; Ameen, F. Efficacy of Gold Nanoparticles against Drug-Resistant Nosocomial Fungal Pathogens and Their Extracellular Enzymes: Resistance Profiling towards Established Antifungal Agents. Nanomaterials 2022, 12, 814. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Jogee, P.S.; Ingle, A.P.; Rai, M. Isolation and identification of toxigenic fungi from infected peanuts and efficacy of silver nanoparticles against them. JFCO Food Control 2017, 71, 143–151. [Google Scholar] [CrossRef]
- Fakhri, A.; Tahami, S.; Naji, M. Synthesis and characterization of core-shell bimetallic nanoparticles for synergistic antimicrobial effect studies in combination with doxycycline on burn specific pathogens. J. Photochem. Photobiol. B Biol. 2017, 169, 21–26. [Google Scholar] [CrossRef] [PubMed]



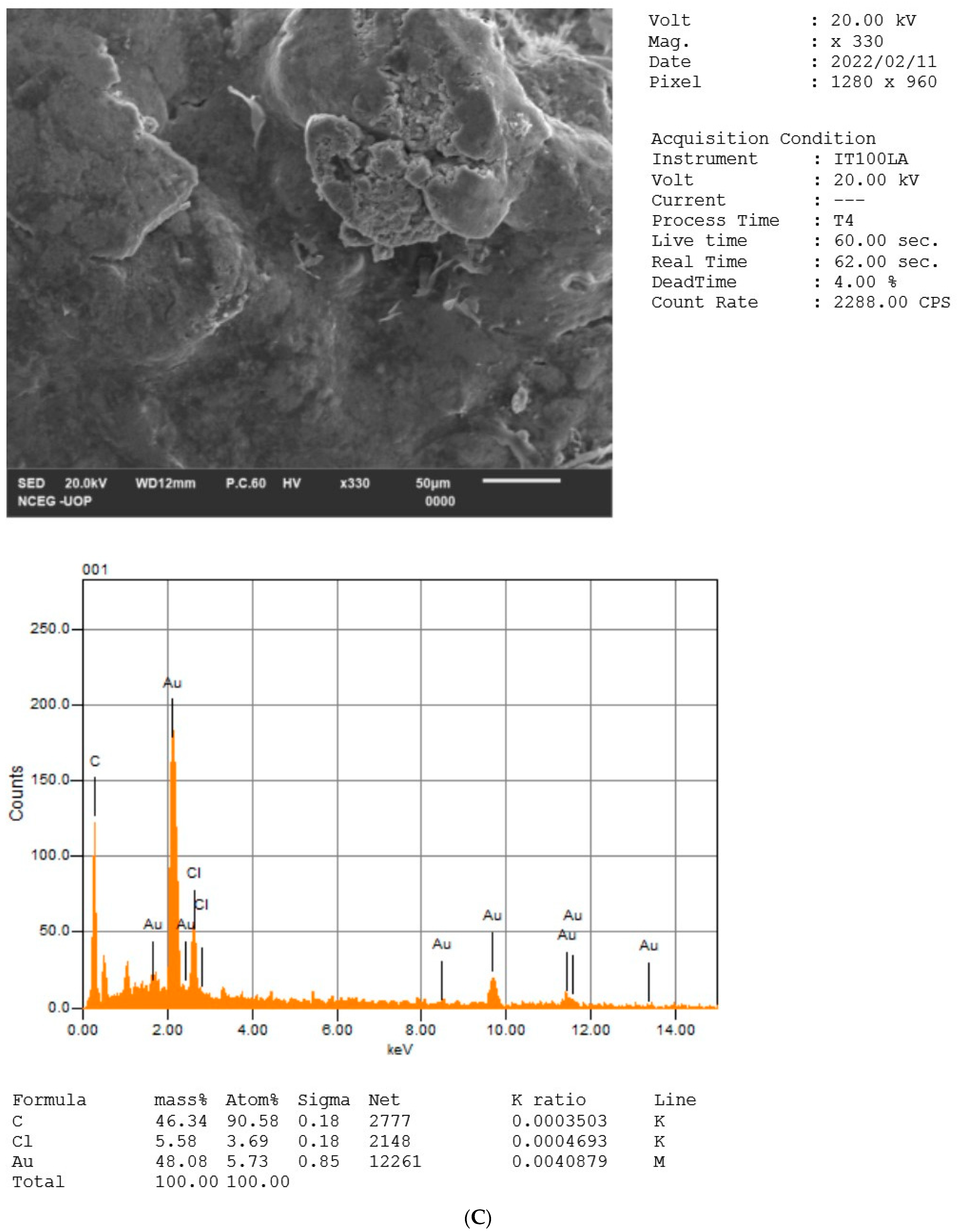

| S. No | Wave Number cm−1 [Reference Article] | Wave Number cm−1 [HH-Plant] | Wave Number cm−1 [HH-AgNPs] | Wave Number cm−1 [HH-AuNPs] | Wave Number cm−1 [HH-BNPs] | Functional Group Assignment | Phyto Compounds Identified |
|---|---|---|---|---|---|---|---|
| 1 | 3400–3200 | 3266.48 | 3352.52 | 3324.90 | 3338.71 | O-H stretch | Poly hydroxy compound |
| 2 | 2935–2915 | 2917.21 | 2915.41 | 2922.72 | 2915.41 | Asymmetric stretching of –CH (CH2) vibration | Saturated aliphatic compound lipids |
| 3 | 2865–2845 | 2855.98 | 2858.54 | 2852.04 | 2852 | Symmetric stretching of –CH (CH2) vibration | Lipids, protein |
| 4 | 2260–2100 | 2259.95 | 2258.93 | 2245.12 | 2252.43 | Carbon-carbon triple bond | Terminal alkynes |
| 5 | 2100–1800 | 1985.34 | 1977.009 | 1963.9 | 1963.19 | Carbonyl compound frequency | Transition metal carbonyls |
| 6 | 1740–1725 | 1740.10 | 1730.01 | 1729 | 1737.32 | C=O stretch | Aldehyde compound |
| 7 | 1650–1600 | 1608.02 | 1617.08 | 1638.20 | 1624.39 | C=O stretching vibration, Ketone group | Ketone compound |
| 8 | 1410–1310 | 1410 | 1412.34 | 1410 | 1410 | O-H bend, alcoholic group | Phenol or tertiary alcohol |
| 9 | 1340–1250 | 1290.95 | 1277 | 1250 | 1251 | CN stretch | Aromatic primary amine |
| 10 | 995–850 | 852.68 | 850.01 | 852.1 | 850 | P-O-C stretch | Aromatic phosphates |
| 11 | 800–700 | 743.11 | 728.2 | 714.42 | 735.66 | C-Cl stretch | Aliphatic chloro compound |
| 12 | 700–600 | 676.003 | 664.86 | 622.61 | 664.86 | C-Br stretch | Aliphatic bromo compounds |
| 13 | 690–550 | 571.91 | 580.36 | 555.2 | 573.05 | Halogen compounds (bromo compounds) | Aliphatic bromo compounds |
| HH-AgNPs | |||||
| S. no | 2θ Value | Element | Plane | Interplanar spacing (d) | Lattice constants (a0) |
| 1 | 37.92 | Ag | 1 1 1 | 2.36 Å | 4.08 Å |
| 2 | 43.79 | Ag | 2 0 0 | 2.06 Å | 4.12 Å |
| 3 | 64.27 | Ag | 2 2 0 | 1.44 Å | 4.07 Å |
| 4 | 77.18 | Ag | 3 1 1 | 1.23 Å | 4.07 Å |
| HH-AuNPs | |||||
| S. no | 2θ Value | Element | Plane | Interplanar spacing (d) | Lattice constants (a0) |
| 1 | 38.02 | Au | 1 1 1 | 2.36 Å | 4.08 Å |
| 2 | 44.29 | Au | 2 0 0 | 2.06 Å | 4.08 Å |
| 3 | 64.37 | Au | 2 2 0 | 1.44 Å | 4.07 Å |
| 4 | 77.58 | Au | 3 1 1 | 1.22 Å | 4.04 Å |
| HH-BNPs | |||||
| S. no | 2θ Value | Element | Plane | Interplanar spacing (d) | Lattice constants (a0) |
| 1 | 38.12 | Ag-Au | 1 1 1 | 2.36 Å | 4.08 Å |
| 2 | 44.09 | Ag-Au | 2 0 0 | 2.06 Å | 4.12 Å |
| 3 | 64.76 | Ag-Au | 2 2 0 | 1.44 Å | 4.07 Å |
| 4 | 77.48 | Ag-Au | 3 1 1 | 1.23 Å | 4.07 Å |
| S. no | Weight % | Atomic % | ||||
|---|---|---|---|---|---|---|
| Elements | HH-AgNPs | HH-AuNPs | HH-BNPs | HH-AgNPs | HH-AuNPs | HH-BNPs |
| C | 34.23 | 46.34 | 23.28 | 51.44 | 90.58 | 56.47 |
| O | 35.12 | nill | 11.63 | 39.62 | nill | 21.19 |
| Al | 2.09 | nill | nill | 1.40 | nill | nill |
| Si | 5.82 | nill | nill | 3.74 | nill | nill |
| Cl | nill | 5.58 | 12.02 | nill | 3.69 | 9.88 |
| Na | nill | nill | 2.81 | nill | nill | 3.56 |
| Ag | 22.75 | nill | 12 | 3.81 | nill | 3.24 |
| Au | nill | 48.08 | 38.26 | nill | 5.73 | 5.66 |
| A. HH plant extract zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A. meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 ± 0.04 | 0 | 0 |
| 60 | 0 | 1.5 ± 0.13 | 1 ± 0.011 | 0 | 2 ± 0.012 | 0 | 0 | 5.5 ± 0.012 | 0 | 0 |
| 90 | 8.5 ± 0.04 | 2.5 ± 0.11 | 3.5 ± 0.012 | 0 | 4.5 ± 0.011 | 3.5 ± 0.012 | 5.5 ± 0.01 | 6.5 ± 0.011 | 7 ± 0.014 | 4 ± 0.011 |
| Levofloxacin | 13 ± 0.14 | 14.5 ± 0.006 | 11.5 ± 0.03 | 19 ± 0.011 | 15.5 ± 0.013 | 12 ± 0.011 | 9.5 ± 0.012 | 13 ± 0.011 | 11 ± 0.013 | 10.5 ± 0.008 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. HH-AgNPs zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A. meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 0 | 0 | 2 ± 0.03 | 0 | 8.5 ± 0.11 | 6.5 ± 0.06 | 0 | 1 ± 0.11 | 2 ± 0.03 | 0 |
| 60 | 5.5 ± 0.02 | 2 ± 0.21 | 7.4 ± 0.11 | 8 ± 0.41 | 3 ± 0.17 | 7 ± 0.03 | 0 | 8.5 ± 0.22 | 3.5 ± 0.21 | 0 |
| 90 | 10.5 ± 0.03 | 5.5 ± 0.1 | 9.5 ± 0.1 | 6 ± 23 | 6.5 ± 0.17 | 3.5 ± 0.12 | 7 ± 0.03 | 6 ± 0.21 | 8.5 ± 0.23 | 8.5 ± 0.11 |
| Levofloxacin | 13.5 ± 0.01 | 11 ± 0.3 | 18.5 ± 0.3 | 32 ± 22 | 11 ± 0.11 | 11.5 ± 0.13 | 9.5 ± 0.11 | 17 ± 0.03 | 11 ± 0.05 | 11 ± 0.03 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. HH-AuNPs zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A. meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 0.5 ± 0.22 | 0 | 2.5 ± 0.12 | 0 | 3 ± 0.11 | 0.5 ± 0.14 | 0 | 0.8 ± 0.13 | 0 | 0 |
| 60 | 9 ± 0.21 | 3.5 ± 0.11 | 5.5 ± 0.06 | 3.5 ± 0.12 | 5.4 ± 0.21 | 5.5 ± 0.11 | 0.5 ± 0.22 | 3.1 ± 0.4 | 6 ± 0.5 | 0 |
| 90 | 14 ± 0.22 | 9 ± 0.21 | 6.5 ± 0.05 | 9 ± 0.23 | 7.5 ± 0.12 | 7 ± 0.15 | 2.5 ± 0.11 | 7.5 ± 0.13 | 8 ± 0.11 | 5.5 ± 0.11 |
| Levofloxacin | 16 ± 0.12 | 15.5 ± 0.32 | 12 ± 0.11 | 18 ± 0.12 | 10 ± 0.22 | 12.5 ± 0.21 | 10 ± 0.14 | 16 ± 0.12 | 9.5 ± 0.13 | 10 ± 0.14 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D. HH-BNPs zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A. meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 1.2 ± 0.21 | 3.5 ± 0.13 | 2.5 ± 0.13 | 2 ± 0.11 | 3.6 ± 0.12 | 4 ± 0.13 | 0 | 1.5 ± 0.21 | 3 ± 0.32 | 0 |
| 60 | 10 ± 0.11 | 4 ± 0.11 | 6 ± 0.28 | 4.3 ± 0.07 | 5.9 ± 0.11 | 12 ± 0.23 | 9 ± 0.11 | 6 ± 0.31 | 6.5 ± 0.23 | 0.4 ± 0.11 |
| 90 | 15 ± 0.12 | 9.6 ± 0.12 | 11.5 ± 0.11 | 10 ± 0.08 | 7.8 ± 0.01 | 12.6 ± 0.11 | 9.5 ± 0.13 | 8.5 ± 0.11 | 10 ± 0.34 | 9 ± 0.11 |
| Levofloxacin | 11 ± 0.31 | 12.5 ± 0.21 | 14 ± 0.11 | 13 ± 0.18 | 13 ± 0.03 | 14 ± 0.11 | 15.5 ± 0.15 | 12.5 ± 0.15 | 14 ± 0.11 | 14.5 ± 0.13 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. AgNO3 salt zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A.meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 0 | 0 | 0 | 1.5 ± 0.14 | 1.1 ± 0.11 | 0.5 ± 0.11 | 0 | 0 | 0 | 0 |
| 60 | 0 | 0 | 2 ± 0.11 | 2 ± 0.2 | 4 ± 0.11 | 1 ± 0.21 | 0 | 0 | 3.5 ± 0.11 | 0 |
| 90 | 0 | 0 | 3 ± 0.11 | 3 ± 0.11 | 5.5 ± 0.11 | 2 ± 0.13 | 0 | 0.2 ± 0.11 | 4 ± 0.11 | 0 |
| Levofloxacin | 14 ± 0.11 | 15 ± 0.06 | 11.5 ± 0.21 | 10 ± 0.18 | 14.5 ± 0.03 | 11 ± 0.21 | 10 ± 0.11 | 21 ± 0.12 | 15.5 ± 0.11 | 11.5 ± 0.09 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F. HAuCl4·3H2O salt zone inhibition (mm) | ||||||||||
| Concentration (µg) | S. aureus | K. pneumonia | A. meriye | S. pyogen | E. coli | S. marcescens | B. cereus | MRSA | M. luteus | S. pneumonia |
| 30 | 0 | 0 | 0 | 1.5 ± 0.14 | 0 | 0 | 0 | 0 | 0 | 0 |
| 60 | 0 | 0 | 0 | 0.5 ± 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 90 | 0 | 0 | 0 | 2 ± 0.11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Levofloxacin | 11 ± 0.18 | 16.5 ± 0.16 | 9 ± 0.23 | 13.5 ± 0.12 | 12 ± 0.13 | 14 ± 0.13 | 10 ± 0.14 | 21.5 ± 0.11 | 10 ± 0.14 | 9.5 ± 0.19 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. HH-plant extract inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 15 ± 0.13 | 53 ± 0.12 | 55 ± 0.11 | 58 ± 0.1 | 67 ± 0.13 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 43 ± 0.13 | 64 ± 0.14 | 75 ± 0.08 | 64 ± 0.12 | 70 ± 0.11 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 0 | 32 ± 0.05 | 58 ± 0.02 | 70 ± 0.11 | 80 ± 0.08 | 145 ± 0.14 | 0 |
| B. HH-AgNPs inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 54 ± 0.11 | 56.5 ± 0.09 | 62 ± 0.21 | 67 ± 0.11 | 75 ± 0.13 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 78 ± 0.21 | 91 ± 0.15 | 96 ± 0.23 | 97 ± 0.22 | 111 ± 0.15 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 65 ± 07 | 74 ± 0.12 | 79 ± 0.11 | 83.5 ± 0.21 | 92.5 ± 0.13 | 145 ± 0.14 | 0 |
| C. HH-AuNPs inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 57.5 ± 0.11 | 67.5 ± 0.13 | 69.5 ± 0.15 | 77.5 ± 0.11 | 82.5 ± 0.18 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 65 ± 0.12 | 80 ± 0.09 | 85 ± 0.18 | 88 ± 0.13 | 94.5 ± 0.21 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 45 ± 0.13 | 63 ± 0.06 | 70 ± 0.12 | 77 ± 0.09 | 92 ± 0.04 | 145 ± 0.14 | 0 |
| D. HH-BNPs inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 65 ± 0.11 | 81 ± 0.13 | 86 ± 0.15 | 89 ± 0.11 | 93 ± 0.18 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 69 ± 0.12 | 85 ± 0.09 | 92 ± 0.18 | 97 ± 0.13 | 114 ± 0.21 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 67.5 ± 0.13 | 71.5 ± 0.06 | 85 ± 0.12 | 89 ± 0.09 | 112 ± 0.04 | 145 ± 0.14 | 0 |
| E. AgNO3 salt inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 0 | 10 ± 0.13 | 19 ± 0.11 | 23 ± 0.12 | 15 ± 0.11 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 7 ± 0.12 | 15 ± 0.22 | 22 ± 0.18 | 24 ± 0.14 | 31 ± 011 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 6 ± 0.11 | 10.5 ± 0.26 | 21.5 ± 0.12 | 28 ± 0.12 | 33 ± 0.14 | 145 ± 0.14 | 0 |
| F. HAuCl4·3H2O salt inhibition (mm) | ||||||||
| 20 µg | 40 µg | 60 µg | 80 µg | 100 µg | Terbinafine | DMSO | ||
| 1 | A. niger | 0 | 0 | 0 | 5 ± 0.11 | 11 ± 0.21 | 145 ± 0.12 | 0 |
| 2 | A. fumigatus | 10 ± 0.21 | 16 ± 0.23 | 25 ± 0.11 | 31 ± 0.09 | 37 ± 04 | 145 ± 0.09 | 0 |
| 3 | A. flavus | 4 ± 0.11 | 10 ± 0.17 | 15 ± 0.13 | 21 ± 0.22 | 27 ± 0.13 | 145 ± 0.14 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, N.; Alkhalifah, D.H.M.; Ahmed, M.; Mushtaq, N.; Shah, F.; Fozia, F.; Khan, R.A.; Hozzein, W.N.; Aboul-Soud, M.A.M. Comparative Study of Antimicrobial Activity of Silver, Gold, and Silver/Gold Bimetallic Nanoparticles Synthesized by Green Approach. Molecules 2022, 27, 7895. https://doi.org/10.3390/molecules27227895
Sher N, Alkhalifah DHM, Ahmed M, Mushtaq N, Shah F, Fozia F, Khan RA, Hozzein WN, Aboul-Soud MAM. Comparative Study of Antimicrobial Activity of Silver, Gold, and Silver/Gold Bimetallic Nanoparticles Synthesized by Green Approach. Molecules. 2022; 27(22):7895. https://doi.org/10.3390/molecules27227895
Chicago/Turabian StyleSher, Naila, Dalal Hussien M. Alkhalifah, Mushtaq Ahmed, Nadia Mushtaq, Faridullah Shah, Fozia Fozia, Rahmat Ali Khan, Wael N. Hozzein, and Mourad A. M. Aboul-Soud. 2022. "Comparative Study of Antimicrobial Activity of Silver, Gold, and Silver/Gold Bimetallic Nanoparticles Synthesized by Green Approach" Molecules 27, no. 22: 7895. https://doi.org/10.3390/molecules27227895
APA StyleSher, N., Alkhalifah, D. H. M., Ahmed, M., Mushtaq, N., Shah, F., Fozia, F., Khan, R. A., Hozzein, W. N., & Aboul-Soud, M. A. M. (2022). Comparative Study of Antimicrobial Activity of Silver, Gold, and Silver/Gold Bimetallic Nanoparticles Synthesized by Green Approach. Molecules, 27(22), 7895. https://doi.org/10.3390/molecules27227895







