Abstract
Ten new differently substituted 3-benzyl-5-aryl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidin-4,6,11-triones 3 were synthesized by a simple and cost-efficient procedure in a one-pot, three-component reaction from readily available ethyl 2-amino-4-aryl-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene-3-carboxylates, benzylamine and triethyl orthoformate under solvent- and catalyst-free conditions. All the new compounds were screened for their antiproliferative activity against two colorectal-cancer-cell lines. The results showed that the compounds 3-benzyl-5-phenyl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3a) and 3-benzyl-5-(3-hydroxyphenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3g) exhibited the most potent balanced inhibitory activity against human LoVo and HCT-116 cancer cells.
1. Introduction
Cancer is inherently a genetic disease. The accumulation of hereditary and/or acquired defects in genes that regulate cell proliferation and survival are responsible for the development of cancer [1]. Cancer is one of the leading causes of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths [2]. The most common cancers are breast, lung, colorectal, and prostate. Colorectal cancer (CRC) is the most frequently diagnosed cancer in Europe and the US, and the second leading cause of cancer-related death [3].
Despite the availability of developed drugs, including targeted tumor therapies, the World Health Organization has announced that it is quite possible that the global burden of cancer will continue to increase in the coming years without real effective responses [4]. Therefore, the development of new anti-cancer drugs is a major goal and challenge for modern medicinal chemistry.
Benzo[g]chromenes bearing the naphthoquinone structural moiety occur in a variety of natural products that show a broad spectrum of biological activities [5,6,7,8]. On the other hand, several synthetic benzo[g]chromene derivatives have received growing interest in the pharmaceutical industry and in the study of organic synthesis due to their various biological and pharmacological properties, including antimicrobial [9,10], antileishmanial [11,12], and anticancer activities [13,14,15,16,17]. Pyrimidines and fused pyrimidines are also known to be preferred structures with diverse biological activities, and many of them are used as antimicrobial [18,19,20,21], anti-inflammatory [22,23], and anticancer therapeutic agents [24,25,26,27,28,29].
The association of these two scaffolds in a single molecule could create a synergistic effect in terms of activity and better drug-likeness properties.
This type of fused system has already been reported in the literature as an anti-Alzheimer’s [30,31], antioxidant [32], or antibacterial agent [33,34,35].
In light of the above considerations, herein, we describe the synthesis of novel 3-benzyl-5-aryl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidin-4,6,11-triones 3 (Scheme 1) bearing both biologically active benzo[g]chromene and pyrimidine functional motifs, by a simple and cost-effective procedure, in a one-pot, three-component reaction. All the synthesized products were evaluated as inhibitors of colon-cancer-cell proliferation.
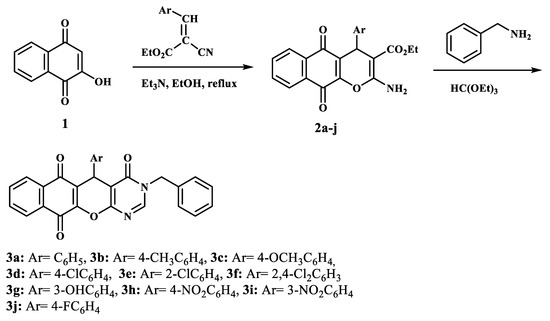
Scheme 1.
Synthesis of benzochromenopyrimidines 3a–j.
2. Results
2.1. Synthesis
The synthesis of racemic benzochromenopyrimidine derivatives 3 was achieved by using a synthetic route shown in Scheme 1. In the first step, the preparation of the precursor ethyl 2-amino-4-aryl-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene-3-carboxylates was performed by mixing commercial 2-hydroxy-1,4-naphthoquinone 1 with ethyl 2-cyano-3-arylacrylates in the presence of a catalytic amount of triethylamine, at reflux [36,37,38,39]. Next, the one-pot condensation between compounds 2a–j, benzylamine, and triethyl orthoformate, which were used as both reagent and solvent, at reflux, led to the corresponding 3-benzyl-5-aryl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-triones 3a–j (Table 1). The desired new compounds were obtained from modest-to-good yields (37–60%), and their analytical and spectroscopic data were in good agreement with their structures. In particular, the 1H NMR spectra showed the appearance of two doublets attributed to the methylene group protons –CH2–Ph between 4.95 and 5.15 ppm, with coupling constants for a typical AB system due to the magnetic non-equivalence of the methylene-group protons (see Supplementary Data).

Table 1.
Synthesis of benzo[6,7]chromeno[2,3-d]pyrimidine derivatives under one-pot solvent- and catalyst-free conditions.
Interestingly, we can note that the nature of the aryl group can have a significant impact on the yield of the reaction.
Indeed, a chlorine in position 2 of the aryl group decreased the yield from 59 to 43% compared to the absence of substituent. This effect was enhanced when the aryl group bore a chlorine in position 2 and another in position 4, where the yield decreased, this time to 37%.
On the other hand, no significant effect was observed when the different substituents were in position 3 or 4, including chlorine.
2.2. Biological Evaluation
2.2.1. Cytotoxicity Test
The ten newly synthesized benzochromenopyrimidines 3a–j were evaluated for their in vitro antiproliferative activity against two representative cell lines of human colon cancer, LoVo and HCT-116, using the standard 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. The cytotoxicity of each compound was assessed at different concentrations of 100 µM, 50 µM, 25 µM, 12.5 µM, 6.25 µM, 3.12 µM, 1.5 µM, 0.8 µM, and 0.4 µM. Oxaliplatin and 5-FU, the most common chemotherapy drugs used to treat colorectal cancer, were used as standards. The curve of the cell survival of LoVo and HCT-116 after treatment was obtained by the relation plotting of surviving fraction and drug concentration. All the results showed that the concentration of the cytotoxic compounds 3a–j was inversely proportional to the percentage of cell viability. The value of concentration required to inhibit 50% of the cell viability (IC50) was determined and compared to those of the standard drugs, as shown in Table 2.

Table 2.
Results of in vitro cytotoxic activity of the synthesized compounds on human-colon-cancer-cell lines (LoVo and HCT-116) a.
Several benzochromenopyrimidines showed good activities compared to the oxaliplatin and 5-FU against both types of cancer-cell line.
Concerning the LoVo cell lines, four compounds (3a: IC50 = 14.99 µM, 3b: IC50 = 19.55 µM, 3e: IC50 = 18.49 µM and 3g: IC50 = 11.79 µM) showed activity that was better than or comparable to those of the two standards. In particular, compound 3g, which showed an IC50 equal to 11.79 µM, was 1.7 times more active than the oxaliplatin and 5-FU. Thus, from the point of view of the structure–activity relationship (SAR), several conclusions can be drawn: (i) It is clear that the cytotoxic effect was related to the nature of the substituent in the aryl group. (ii) With the exception of compound 3b, the substitution in position 4 of the aryl group did not enhance the activity. (iii) The two most promising compounds were the chlorine in position 2 and the hydroxy in position 3. This suggests that for better activity, a polar group in position 2 or 3 is necessary.
For the HCT-116 lines, six compounds showed better activity than the 5-FU (3a: IC50 = 15.92 µM, 3e: IC50 = 13.70 µM, 3g: IC50 = 13.61 µM, 3h: IC50 = 16.45 µM, 3i: IC50 = 7.15 µM and 3j: IC50 = 23.85 µM), of which three (3e: IC50 = 13.70 µM, 3g: IC50 = 13.61 µM and 3i: IC50 = 7.15 µM) also showed activity that was better than or comparable to that of oxaliplatin (IC50 = 13.48 µM).
In this case, the SAR was also related to the nature of the aryl group. The comparison of the results of these compounds with oxaliplatin showed that the substitution in position 4 did not favor the activity. This unfavorable effect was related to the nature of the substituent (CH3 > OCH3 > Cl > F > NO2).
According to these results, the most balanced compounds were 3a and 3g, with an IC50 equal to 14.99 µM and 11.79 µM, respectively, against the Lovo cell lines, and 15.92µM and 13.61 µM, respectively, against the HCT-116 cell lines. Compounds 3a and 3g were, thus, more active than oxaliplatin and 5-FU against the LoVo cell lines and showed activities that were almost comparable to those of the oxaliplatin and two-fold more active than those of the 5-FU against the HCT-116 cell line.
2.2.2. ADME Studies
Next, the physicochemical properties of the synthesized compounds were investigated by Data Warrior software, a chemical- and biological-data-visualization-and-analysis tool developed by Actelion/Idorsia Pharmaceuticals Ltd. (Table 3). This software utilizes different parameters of Lipinski’s rule of five (molecular weight, LogP, LogS, H-acceptors, H-donors, topological polar surface (TPSA) for the analysis of drug-like properties. All the compounds showed suitable MW values (MW < 500) for the pharmacokinetics of a drug in the human body with the exception of compound 3f, which had a slightly higher value, 515.351 g/mol.

Table 3.
Physicochemical properties of the synthesized compounds calculated by Data Warrior.
Lipophilicity is one of the properties of compounds that determine whether a molecule will cross the biological membrane, of which Log P (less than 5) is an important physiochemical example. Interestingly most of the compounds showed good lipophilicity, with Log P values between 3.3154 and 4.843. Only compound 3f, bearing two chlorines on the aromatic ring, showed a Log P higher than 5, with a value equal to 5.449. This value remained lower than 6.5, which is the upper limit for druggable compounds, indicating that 3f was slightly lipophilic. Since lipophilicity plays a crucial role in determining the solubility of drug candidates in biological systems, we also calculated the Log S values of these compounds. All the compounds showed low aqueous solubility, suggesting reduced bioavailability. Structural modifications could be considered by introducing more polar groups to improve hydrophilicity and, therefore, duggability. Introducing an additional hydroxyl in position 2 of the aryl group of compound 3g could be an option to enhance the solubility without a loss of activity. Indeed, the SAR showed that the presence of a polar group in 2 and 3 seems to be a necessary condition for the activity. Nevertheless, we can introduce polar groups, such as halogens or hydroxyls, on the aromatic rings of benzyl or benzochromene and study their impact on drugability and biological activity.
The number of donor and acceptor hydrogen bonds was also in agreement with Lipinski’s rule of five. Indeed, for all the compounds, the number of donor hydrogen bonds was lower than 5 and the number of acceptors was lower than 10. It can be noted, however, that compounds 3h and 3i had slightly more hydrogen acceptors than their analogues.
Data Warrior also calculates drug-likeness as a qualitative concept to predict whether synthesized compounds are drug-like. This parameter is calculated by using several data, such as LogP, LogS, and molar mass, as well as other parameters, such as the presence of structures with specific pharmacological properties (such as enones, which can be mutagenic and carcinogenic). It can be noted that all the compounds had an interesting drug-likeness prediction, except for compounds 3h and 3i, for which it was <0.
The TPSA corresponded to the Van der Waals surface of the molecules’ polar atoms (usually oxygen and nitrogen) and their attached hydrogens. The polar surface area was no greater than 140 Å2, as suggested by Veber’s Rule. Interestingly, all the compounds had a TPSA < 100 Å2, except for compounds 3h and 3i, for which it was 121.86 Å2.
3. Materials and Methods
Melting points (°C) were determined with a Kofler hot bench and were uncorrected. Analytical thin-layer chromatography (TLC) on silica-gel-precoated aluminum sheets (Type 60 F254, 0.25-mm thickness; from Merck, Darmstadt, Germany) was employed to follow the progress of the reactions and to check the purity and homogeneity of the synthesized products. Nuclear-magnetic-resonance spectra (NMR) were recorded on a Brucker DRX-400 Avance spectrometer (at 400 MHz for 1H and 100 MHz for 13C), using dimethylsulfoxide (DMSO-d6) as the solvent and tetramethylsilane (TMS) as internal standard. The chemical shifts are expressed in parts per million (ppm) and the multiplicities of 1H NMR signals were designated as follows: s: singlet; d: doublet; t: triplet; q: quartet; and m: multiplet. Coupling constants were expressed in hertz (Hz). High-resolution mass spectra (HRMS) were carried out by using a Bruker micrOTOF-Q II spectrometer (Bruker Daltonics) in positive electrospray ionization time-of-flight at UCA Clermont Ferrand, France.
3.1. Synthesis of Compounds 2a–j
3.1.1. General Procedure for the Synthesis of Ethyl 2-Amino-4-(3-hydroxyphenyl)-5,10-dioxo-5,10-dihy dro-4H-benzo[g]chromene-3-carboxylate (2a–j)
An equimolar mixture of ethyl 2-cyano-3-arylacrylates and 2-hydroxy-1,4-naphthoquinone 1 was dissolved in 30 mL of ethanol in the presence of triethylamine as a catalyst. The reaction mixture was heated at reflux for 2 h. The resulting precipitate was collected by filtration and recrystallized from ethanol.
All compounds 2a–j were previously described in the following studies: 2a–c and 2i–j [40], 2d [37], 2e [41], 2f [38] and 2g [42].
3.1.2. General Procedure for the Synthesis of 3-Benzyl-5-aryl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidin-4,6,11-triones (3a–j)
A mixture of ethyl 2-amino-4-aryl-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene-3-carboxylates 2a–j (0.5 g, 1.33 mmol), benzylamine (0.14 g, 1.33 mmol), and triethyl orthoformate (3 mL) was heated under catalyst-free and solvent-free conditions. The completion of the reaction required a time of 3 to 5 h, as highlighted by TLC analysis, leading to the formation of a precipitate, which was collected by filtration, washed with ethanol, and dried. The obtained products 3a–j were characterized by spectroscopic analysis (NMR and HRMS), which showed good agreement with the desired structure.
3-Benzyl-5-phenyl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3a)
Light-yellow solid; yield: 59%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H, CHpyrimidine), 8.10–8.07 (m, 1H), 7.93–7.91 (m, 1H), 7.89–7.85 (m, 2H), 7.38 (d, J = 7.4 Hz, 2H), 7.32–7.24 (m, 7H), 7.19–7.15 (m, 1H), 5.14 (d, J = 14.7 Hz, 1H, CH2), 5.13 (s, 1H, CHpyran), 4.98 (d, J = 14.7 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.5, 160.4, 159.7, 152.2, 150.1, 142.4, 136.4, 135.1, 134.7, 131.5, 131.1, 129.1 (2C), 129.0 (2C), 128.7 (2C), 128.3, 128.2 (2C), 127.5, 126.6, 126.3, 123.3, 103.7, 49.8, 34.8. HRMS (ESI, M + H+) Calcd for C28H19N2O4: 447.1345. Found: 447.1339.
3-Benzyl-5-(p-tolyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3b)
Olive-green solid; yield: 53%; mp 252–4 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.71 (s, 1H, CHpyrimidine), 8.09–8.07 (m, 1H), 7.90–7.86 (m, 3H), 7.30–7.24 (m, 7H), 7.05 (d, J = 7.1 Hz, 2H), 5.12 (d, J = 14.7 Hz, 1H, CH2), 5.08 (s, 1H, CHpyran), 4.98 (d, J = 14.7 Hz, 1H, CH2), 2.20 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.5, 160.4, 159.6, 152.1, 149.9, 139.5, 136.8, 136.4, 135.1, 134.7, 131.5, 131.0, 129.3 (2C), 129.1 (2C), 129.0 (2C), 128.3, 128.2 (2C), 126.6, 126.3, 123.5, 100.8, 49.8, 34.3, 21.0. HRMS (ESI, M + H+) Calcd for C29H21N2O4: 461.1501. Found: 461.1494.
3-Benzyl-5-(4-methoxyphenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3c)
Ochre-yellow solid; yield: 60%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.71 (s, 1H, CHpyrimidine), 8.09–8.07 (m, 1H), 7.92–7.86 (m, 3H), 7.31 (m, 7H), 6.80 (d, J = 7.6 Hz, 2H), 5.12 (d, J = 14.2 Hz, 1H, CH2), 5.07 (s, 1H, CHpyran), 4.98 (d, J = 14.2 Hz, 1H, CH2), 3.68 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) δ 183.1, 177.5, 160.4, 159.5, 158.7, 152.0, 149.8, 136.4, 135.1, 134.7, 134.6, 131.5, 131.1, 130.2 (2C), 129.1 (2C), 128.3, 128.2 (2C), 126.6, 126.3, 123.4, 114.1 (2C), 103.8, 55.4, 49.8, 33.9. HRMS (ESI, M + H+) Calcd for C29H21N2O5: 477.1450. Found: 477.1443.
3-Benzyl-5-(4-chlorophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3d)
Pale yellow solid; yield: 55%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H, CHpyrimidine), 8.08 (m, 1H), 7.90–7.86 (m, 3H), 7.42 (d, J = 6.7 Hz, 2H), 7.31 (m, 7H), 5.14–5.11 (m, 2H, CH2, CHpyran), 4.98 (d, J = 14.6 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.4, 160.4, 159.6, 152.3, 150.2, 141.4, 136.4, 135.0, 134.7, 132.1 (2C), 131.4, 131.1 (2C), 129.0 (2C), 128.6 (2C), 128.3, 128.2 (2C), 126.6, 126.3, 122.6, 103.2, 49.8, 34.5. HRMS (ESI, M + H+) Calcd for C28H1835ClN2O4: 481.0955. Found: 481.0948.
3-Benzyl-5-(2-chlorophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3e)
Red solid brick; yield: 43%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H, CHpyrimidine), 8.08–8.06 (m, 1H), 7.88–7.84 (m, 3H), 7.43–7.41 (m, 2H), 7.34–7.18 (m, 7H), 5.48 (s, 1H, CHpyran), 5.10 (d, J = 14.6 Hz, 1H, CH2), 4.95 (d, J = 14.6 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 182.9, 177.5, 160.1, 159.8, 152.4, 150.2, 139.8, 136.4, 135.2, 134.7, 133.9, 132.7, 131.4, 130.8, 129.8 (2C), 129.1 (2C), 128.3, 128.0 (2C), 127.6, 126.5, 126.4, 122.5, 102.9, 49.7, 33.5. HRMS (ESI, M + H+) Calcd for C28H1835ClN2O4: 481.0955. Found: 481.0948.
3-Benzyl-5-(2,4-dichlorophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3f)
Ochre-yellow solid; yield: 37%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H, CHpyrimidine), 8.08–8.06 (m, 1H), 7.88–7.85 (m, 3H), 7.49–7.44 (m, 2H), 7.33–7.25 (m, 6H), 5.46 (s, 1H, CHpyran), 5.11 (d, J = 14.7 Hz, 1H, CH2), 4.96 (d, J = 14.7 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.4, 160.1, 159.7, 152.4, 150.2, 139.1, 136.2, 135.3, 134.9, 134.8, 133.7, 132.7, 131.3, 130.7, 129.1 (2C), 129.0, 128.3, 128.0 (2C), 127.7, 126.6, 126.4, 122.0, 102.6, 49.7, 33.1. HRMS (ESI, M + H+) Calcd for C28H1735Cl2N2O4: 515.0565. Found: 515.0559.
3-Benzyl-5-(3-hydroxyphenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3g)
Red solid brick; yield: 57%; mp 200–2 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.37 (s, 1H, OH), 8.74 (s, 1H, CHpyrimidine), 8.07 (m, 1H), 7.93–7.85 (m, 3H), 7.34–7.31 (m, 5H), 7.05–7.02 (m, 1H), 6.80–6.76 (m, 2H), 6.56 (d, J = 7.9 Hz, 1H), 5.15 (d, J = 14.6 Hz, 1H, CH2), 5.04 (s, 1H, CHpyran), 5.00 (d, J = 14.6 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.5, 160.4, 159.7, 157.6, 152.1, 149.9, 143.6, 136.4, 135.1, 134.7, 131.4, 131.0, 129.7, 129.1 (2C), 128.3, 128.2 (2C), 126.6, 126.4, 123.5, 119.7, 116.1, 114.6, 103.7, 49.8, 34.5. HRMS (ESI, M + H+) Calcd for C28H19N2O5: 463.1294. Found: 463.1286.
3-Benzyl-5-(4-nitrophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3h)
Ochre-yellow solid; yield: 50%; mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H, CHpyrimidine), 8.10–8.08 (m, 3H), 7.87–7.81 (m, 3H), 7.70 (d, J = 8.5 Hz, 2H), 7.32–7.27 (m, 5H), 5.21 (s, 1H, CHpyran), 5.09 (d, J = 14.7 Hz, 1H, CH2), 5.47 (d, J = 14.7 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.3, 160.4, 159.7, 152.6, 150.4, 149.7, 146.9, 136.2, 135.1, 134.8, 131.3, 131.0, 130.8 (2C), 129.1 (2C), 128.4, 128.2 (2C), 126.6, 126.3, 123.7 (2C), 122.0, 102.6, 49.9, 35.3. HRMS (ESI, M + H+) Calcd for C28H18N3O6: 492.1196. Found: 492.1192.
3-Benzyl-5-(3-nitrophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3i)
Orange solid; yield: 52%; mp 258–260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H, CHpyrimidine), 8.24 (s, 1H), 8.06–8.04 (m, 2H), 7.89–7.85 (m, 4H), 7.56 (m, 1H), 7.29 (m, 5H), 5.25 (s, 1H, CHpyran), 5.12 (d, J = 14.7 Hz, 1H, CH2), 4.98 (d, J = 14.7 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.0, 177.3, 160.4, 159.7, 152.7, 150.5, 148.0, 144.4, 136.3, 136.1, 135.0, 134.7, 131.4, 131.2, 130.1, 129.0 (2C), 128.3, 128.2 (2C), 126.6, 126.3, 124.0, 122.6, 121.8, 102.8, 49.9, 35.2. HRMS (ESI, M + H+) Calcd for C28H18N3O6: 492.1196. Found: 492.1190.
3-Benzyl-5-(4-fluorophenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3j)
Ochre-yellow solid, yield: 51%, mp > 260 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H, CHpyrimidine), 8.09–8.07 (m, 1H), 7.93–7.83 (m, 3H), 7.45–7.42 (m, 2H), 7.33–7.26 (m, 5H), 7.09–7.05 (m, 2H), 5.13 (d, J = 14.7 Hz, 1H, CH2), 5.12 (s, 1H, CHpyran), 4.99 (d, J = 14.7 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 183.1, 177.4, 160.4, 159.6, 152.3, 150.1, 138.6, 136.4, 135.0, 134.7, 131.4 (2C), 131.2, 131.1, 129.0 (2C), 128.3, 128.2 (2C), 126.6, 126.3, 122.9, 115.5, 115.3, 103.5, 49.8, 34.2. HRMS (ESI, M + H+) Calcd for C28H18FN2O4: 465.1251. Found: 465.1244.
3.2. Biological Evaluation
3.2.1. Materials and Methods
Two colon-cancer-cell lines (LoVo and HCT-116) were used in this study. Cells were cultured in RPMI-1640 media supplemented with 10% FBS and penicillin streptomycin. They were grown in a humidified incubator with 5% of CO2 at 37 °C.
3.2.2. Cytotoxic Activity by MTT Assay
The synthesized compounds were solubilized in the DMSO as stock solutions (100 mM) and serial dilutions were prepared with cell-culture media just prior to use.
A 96-well plate was taken and seeded with 5000 cells/well, after which it was incubated overnight in an incubator at 37 °C with 5% of CO2. Next, the treatments were performed in triplicates and the plate was placed back in the incubator for 48h. After 48 h, the medium was removed and 100 µL of MTT was added into each well and incubated for 24 h. Subsequently, the MTT containing medium was removed from the wells. A total of 100 µL of SDS 10% was added into each well to dissolve the formazan crystals from the cells. Next, the plate was analyzed on micro plate reader (Varioskan Thermo Fisher) after 4 h. The absorbance was measured for each well with a wavelength of 570 nm. The IC50 values were then calculated.
3.3. Statistical Data Analyses
All experiments were performed in triplicate. Data were exposed as mean ± SD. Statistical analyses were performed by Student’s test. The normality and Leven’s test for homogeneity of variances were applied prior to one-way analysis of variance (ANOVA) and multiple mean comparisons were performed with Duncan’s test at p values ≤ 0.05 to investigate the significance differences in factors between synthesized compounds and standards (oxaliplatin and 5-FU) at a confidence level of 95%.
4. Conclusions
In the present study, a new series of benzochromenopyrimidine derivatives 3 was synthesized in a single step, by reacting ethyl 2-amino-4-aryl-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene-3-carboxylates 2 with benzylamine and triethyl orthoformate, both of which were readily available, without a solvent or catalyst.
The evaluation of the newly synthesized compounds for antitumor activity against the human-colon-cancer-cell lines LoVo and HCT-116 exhibited good results. Among the tested compounds, 3-benzyl-5-(3-hydroxyphenyl)-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3g) showed strong activity against the LoVo cell line with an IC50 value equal to 11.79 μM, and 3-benzyl-5-phenyl-3,5-dihydro-4H-benzo[6,7]chromeno[2,3-d]pyrimidine-4,6,11-trione (3a) also exhibited high antitumor activity against towards the LoVo cell line, with an IC50 value of 14.99 μM, comparing very well with standards, oxaliplatin and 5-FU. In addition, ligands 3a and 3g showed good activities against the HCT-116 cell line, with IC50 equal to 15.92 μM and 13.61 μM, respectively.
Interestingly, both compounds showed suitable physicochemical properties according to the drug-likeness score for druggability predicted by the Data Warrior software.
In summary, this preliminary study revealed that compounds 3a and 3g may be promising agents for further research into the treatment of colon cancer. It should be noted that products 3a and 3g were obtained in racemic form and that their antiproliferative activities could be attributed to one of the enantiomers.
Therefore, work is currently underway in our laboratories to develop analogues with better pharmacological profiles by identifying the contribution of each enantiomer to the biological activity. The results will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227878/s1, Supplementary Data S1: The NMR and HRMS spectras.
Author Contributions
E.C. carried out the synthesis of the molecules. F.E. and D.M. performed the biological study. P.J.B. provided the Physicochemical properties. S.A. supervised the biological assays and edited the manuscript. F.C. and J.M.-C. supervised the project and edited the manuscript and L.I. supervised the project and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Regional Council of Franche-Comté (2022Y-13659 and 13660 ACCURATE PROJECT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henidi, H.A.; Al-Abd, A.M.; Al-Abbasi, F.A.; BinMahfouz, H.A.; El-Deeb, I.M. Design and Synthesis of Novel Phenylaminopyrimidines with Antiproliferative Activity against Colorectal Cancer. RSC Adv. 2019, 9, 21578–21586. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Ranjan Dwivedi, A.; Kumar, V.; Kaur, H.; Kumar, N.; Prakash Yadav, R.; Poduri, R.; Baranwal, S.; Kumar, V. Anti-Proliferative Potential of Triphenyl Substituted Pyrimidines against MDA-MB-231, HCT-116 and HT-29 Cancer Cell Lines. Bioorg. Med. Chem. Lett. 2020, 30, 127468. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.who.int/health-topics/cancer (accessed on 27 September 2022).
- Siripong, P.; Kanokmedakul, K.; Piyaviriyagul, S.; Yahuafai, J.; Chanpai, R.; Ruchirawat, S.; Oku, N. Antiproliferative Naphthoquinone Esters from Rhinacanthus nasutus Kurz. Roots on Various Cancer Cells. J. Tradit. Med. 2006, 23, 166–172. [Google Scholar] [CrossRef]
- Sperry, J.; Lorenzo-Castrillejo, I.; Brimble, M.A.; Machín, F. Pyranonaphthoquinone Derivatives of Eleutherin, Ventiloquinone L, Thysanone and Nanaomycin A Possessing a Diverse Topoisomerase II Inhibition and Cytotoxicity Spectrum. Bioorg. Med. Chem. 2009, 17, 7131–7137. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.; Kazufumi, T.; Ishibashi, M. New Pyranonaphthoquinones and a Phenazine Alkaloid Isolated from Streptomyces Sp. IFM 11307 with TRAIL Resistance-Overcoming Activity. J. Antibiot. 2011, 64, 729–734. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, J.; Ding, K.; Chen, D.; Cen, S.; Ge, M. Phomonaphthalenone A: A Novel Dihydronaphthalenone with Anti-HIV Activity from Phomopsis Sp. HCCB04730. Phytochem. Lett. 2013, 6, 257–260. [Google Scholar] [CrossRef]
- Jardosh, H.H.; Patel, M.P. Microwave-Induced CAN Promoted Atom-Economic Synthesis of 1H-Benzo[b]Xanthene and 4H-Benzo[g]Chromene Derivatives of N-Allyl Quinolone and Their Antimicrobial Activity. Med. Chem. Res. 2013, 22, 2954–2963. [Google Scholar] [CrossRef]
- Tangeti, V.; Vasundhara, D.; Kumar, M.; Mylapalli, H.; Kumar, K. Synthesis, Characterization and Cytotoxic Investigations of Novel C3-Dihydrofuran Substituted 1H-Benzo[g]Chromene-2,5,10-Triones besides Antimicrobial Study. Asian J. Chem. 2017, 29, 503–511. [Google Scholar] [CrossRef]
- Guimarães, T.T.; Pinto, M.d.C.F.R.; Lanza, J.S.; Melo, M.N.; do Monte-Neto, R.L.; de Melo, I.M.M.; Diogo, E.B.T.; Ferreira, V.F.; Camara, C.A.; Valença, W.O.; et al. Potent Naphthoquinones against Antimony-Sensitive and -Resistant Leishmania Parasites: Synthesis of Novel α- and nor-α-Lapachone-Based 1,2,3-Triazoles by Copper-Catalyzed Azide–Alkyne Cycloaddition. Eur. J. Med. Chem. 2013, 63, 523–530. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Jentzsch, J.; Shaikh, A.; Singh Shuveksh, P.; Koko, W.S.; Khan, T.A.; Ahmed, K.; Schobert, R.; Ersfeld, K.; Biersack, B. New Pyrano-4H-Benzo[g]Chromene-5,10-Diones with Antiparasitic and Antioxidant Activities. Chem. Biodivers. 2021, 18, e2000839. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malachowski, W.P.; DuHadaway, J.B.; LaLonde, J.M.; Carroll, P.J.; Jaller, D.; Metz, R.; Prendergast, G.C.; Muller, A.J. Indoleamine 2,3-Dioxygenase Is the Anticancer Target for a Novel Series of Potent Naphthoquinone-Based Inhibitors. J. Med. Chem. 2008, 51, 1706–1718. [Google Scholar] [CrossRef][Green Version]
- da Rocha, D.R.; de Souza, A.C.G.; Resende, J.A.L.C.; Santos, W.C.; dos Santos, E.A.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Ferreira, V.F. Synthesis of New 9-Hydroxy-α- and 7-Hydroxy-β-Pyran Naphthoquinones and Cytotoxicity against Cancer Cell Lines. Org. Biomol. Chem. 2011, 9, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Magedov, I.V.; Kireev, A.S.; Jenkins, A.R.; Evdokimov, N.M.; Lima, D.T.; Tongwa, P.; Altig, J.; Steelant, W.F.A.; Van Slambrouck, S.; Antipin, M.Y.; et al. Structural Simplification of Bioactive Natural Products with Multicomponent Synthesis. 4. 4H-Pyrano-[2,3-b]Naphthoquinones with Anticancer Activity. Bioorg. Med. Chem. Lett. 2012, 22, 5195–5198. [Google Scholar] [CrossRef]
- da Cruz, E.H.G.; Silvers, M.A.; Jardim, G.A.M.; Resende, J.M.; Cavalcanti, B.C.; Bomfim, I.S.; Pessoa, C.; de Simone, C.A.; Botteselle, G.V.; Braga, A.L.; et al. Synthesis and Antitumor Activity of Selenium-Containing Quinone-Based Triazoles Possessing Two Redox Centres, and Their Mechanistic Insights. Eur. J. Med. Chem. 2016, 122, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Subramaniyan, S.A.; Kim, S.-Y.; Kim, J.-S.; Park, B.-H.; Shim, K.S.; Yoo, D.J. Synthesis and Anticancer Evaluation of 1,4-Naphthoquinone Derivatives Containing a Phenylaminosulfanyl Moiety. ChemMedChem 2019, 14, 532–544. [Google Scholar] [CrossRef]
- Patel, R.B.; Desai, P.S.; Desai, K.R.; Chikhalia, K.H. Synthesis of Pyrimidine Based Thiazolidinones and Azetidinones: Antimicrobial and Antitubercular Agents. IJC-B 2006, 45B, 747–751. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; Ashour, H.M.A.; Abd El Razik, H.A. Synthesis and Biological Evaluation of Some Novel Polysubstituted Pyrimidine Derivatives as Potential Antimicrobial and Anticancer Agents. Archiv. Pharm. 2009, 342, 299–310. [Google Scholar] [CrossRef]
- Garavito, M.F.; Narváez-Ortiz, H.Y.; Zimmermann, B.H. Pyrimidine Metabolism: Dynamic and Versatile Pathways in Pathogens and Cellular Development. J. Genet. Genom. 2015, 42, 195–205. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Wolska, A.; Mikołajczyk, A.; Bryndal, I.; Cieplik, J.; Lis, T.; Matera-Witkiewicz, A. A New Pyrimidine Schiff Base with Selective Activities against Enterococcus Faecalis and Gastric Adenocarcinoma. Molecules 2021, 26, 2296. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Singh, N.; Johar, M.; Kumar, A. Synthesis, Anti-Inflammatory and Analgesic Activities Evaluation of Some Mono, Bi and Tricyclic Pyrimidine Derivatives. Bioorg. Med. Chem. 2005, 13, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of Some New Pyrimido[2′,1′:2,3]Thiazolo[4,5-b]Quinoxaline Derivatives as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2010, 45, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.A.; Abdelall, E.K.A.; Abdelgawad, M.A.; Ahmed, R.R.; Bakr, R.B. Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]Pyrimidin-4-One Derivatives. Molecules 2014, 19, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Shyyka, O.; Pokhodylo, N.; Finiuk, N.; Matiychuk, V.; Stoika, R.; Obushak, M. Anticancer Activity Evaluation of New Thieno[2,3-d]Pyrimidin-4(3H)-Ones and Thieno[3,2-d]Pyrimidin-4(3H)-One Derivatives. Sci. Pharm. 2018, 86, 28. [Google Scholar] [CrossRef]
- Fouad, M.M.; El-Bendary, E.R.; Suddek, G.M.; Shehata, I.A.; El-Kerdawy, M.M. Synthesis and in Vitro Antitumor Evaluation of Some New Thiophenes and Thieno[2,3-d]Pyrimidine Derivatives. Bioorg. Chem. 2018, 81, 587–598. [Google Scholar] [CrossRef]
- Cherukupalli, S.; Chandrasekaran, B.; Aleti, R.R.; Sayyad, N.; Hampannavar, G.A.; Merugu, S.R.; Rachamalla, H.R.; Banerjee, R.; Karpoormath, R. Synthesis of 4,6-Disubstituted Pyrazolo[3,4-d]Pyrimidine Analogues: Cyclin-Dependent Kinase 2 (CDK2) Inhibition, Molecular Docking and Anticancer Evaluation. J. Mol. Struct. 2019, 1176, 538–551. [Google Scholar] [CrossRef]
- Kilic, A.; Beyazsakal, L.; Işık, M.; Türkeş, C.; Necip, A.; Takım, K.; Beydemir, Ş. Mannich Reaction Derived Novel Boron Complexes with Amine-Bis(Phenolate) Ligands: Synthesis, Spectroscopy and in Vitro/in Silico Biological Studies. J. Organomet. Chem. 2020, 927, 121542. [Google Scholar] [CrossRef]
- Malki, A.; Ashour, H.M.A.; Elbayaa, R.Y.; Issa, D.A.E.; Aziz, H.A.; Chen, X. Novel 1,5-Diphenyl-6-Substituted 1H-Pyrazolo[3,4-d]Pyrimidin-4(5H)-Ones Induced Apoptosis in RKO Colon Cancer Cells. J. Enzym. Inhib. Med. Chem. 2016, 31, 1286–1299. [Google Scholar] [CrossRef]
- Dgachi, Y.; Martin, H.; Bonet, A.; Chioua, M.; Iriepa, I.; Moraleda, I.; Chabchoub, F.; Marco-Contelles, J.; Ismaili, L. Synthesis and Biological Assessment of Racemic Benzochromenopyrimidinetriones as Promising Agents for Alzheimer’s Disease Therapy. Future Med. Chem. 2017, 9, 715–721. [Google Scholar] [CrossRef]
- Cherif, M.; Horchani, M.; Al-Ghamdi, Y.O.; Almalki, S.G.; Alqurashi, Y.E.; Ben Jannet, H.; Romdhane, A. New Pyrano-1,2,3-Triazolopyrimidinone Derivatives as Anticholinesterase and Antibacterial Agents: Design, Microwave-Assisted Synthesis and Molecular Docking Study. J. Mol. Struct. 2020, 1220, 128685. [Google Scholar] [CrossRef]
- Khurana, J.M.; Lumb, A.; Chaudhary, A.; Nand, B. Synthesis and in Vitro Evaluation of Antioxidant Activity of Diverse Naphthopyranopyrimidines, Diazaanthra[2,3-d][1,3]Dioxole-7,9-Dione and Tetrahydrobenzo[a]Xanthen-11-Ones. RSC Adv. 2013, 3, 1844–1854. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Mosleh, T.; Hamta, A. Synthesis of Some Novel Chromenopyrimidine Derivatives and Evaluation of Their Biological Activities. Iran J. Pharm. Res. 2014, 13, 873–879. [Google Scholar] [PubMed]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.-H.; Han, M.-K.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. Chem. Open 2019, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Khurana, J.M.; Nand, B.; Saluja, P. DBU: A Highly Efficient Catalyst for One-Pot Synthesis of Substituted 3,4-Dihydropyrano[3,2-c]Chromenes, Dihydropyrano[4,3-b]Pyranes, 2-Amino-4H-Benzo[h]Chromenes and 2-Amino-4H Benzo[g]Chromenes in Aqueous Medium. Tetrahedron 2010, 66, 5637–5641. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, H.; Li, X. An Improved Procedure for the Three-Component Synthesis of Benzo[g]Chromene Derivatives Using Basic Ionic Liquid. J. Heterocycl. Chem. 2011, 48, 1264–1268. [Google Scholar] [CrossRef]
- Khurana, J.M.; Magoo, D.; Chaudhary, A. Efficient and Green Approaches for the Synthesis of 4H-Benzo[g]Chromenes in Water, Under Neat Conditions, and Using Task-Specific Ionic Liquid. Synth. Commun. 2012, 42, 3211–3219. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M.; Maleki, A. Potassium Phthalimide-N-Oxyl: A Novel, Efficient, and Simple Organocatalyst for the One-Pot Three-Component Synthesis of Various 2-Amino-4H-Chromene Derivatives in Water. Tetrahedron 2013, 69, 1074–1085. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Tajbakhsh, M.; Daraie, M.; Mohammadi, M. Dysprosium–Balsalazide Complex Trapped between the Functionalized Halloysite and g-C3N4: A Novel Heterogeneous Catalyst for the Synthesis of Annulated Chromenes in Water. Appl. Organomet. Chem. 2022, 36, e6829. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Bateni, F.-S.; Babaei, P. CeO2/CuO@N-GQDs@NH2 Nanocomposite as a High-Performance Catalyst for the Synthesis of Benzo[g]Chromenes. Appl. Organomet. Chem. 2020, 34, e5657. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Enayat-Mehri, N.; Eshteghal, F. 4-(4′-Diamino-Di-Phenyl)-Sulfone Supported on Hollow Magnetic Mesoporous Fe3O4@SiO2 NPs: As a Reusable and Efficient Catalyst for the Synthesis of Ethyl 2-Amino-5,10-Dihydro-5,10-Dioxo-4-Phenyl-4H Benzo[g]Chromene-3-Carboxylates. J. Saudi Chem. Soc. 2018, 22, 485–495. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).