Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health
Abstract
1. Introduction
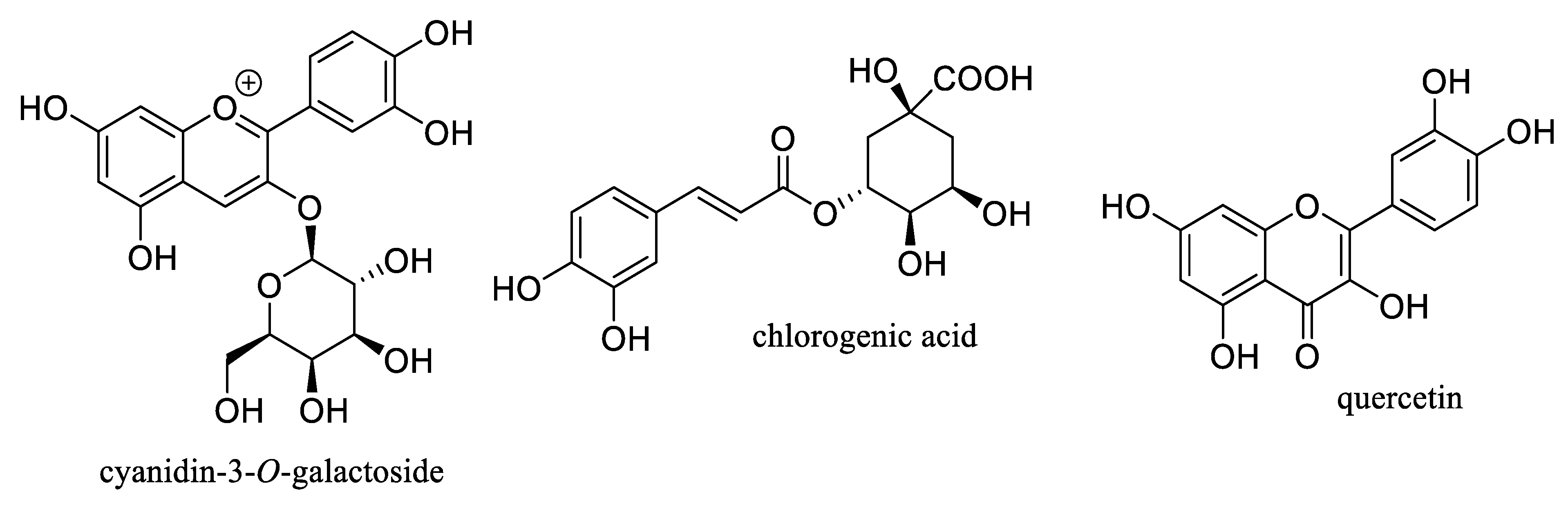
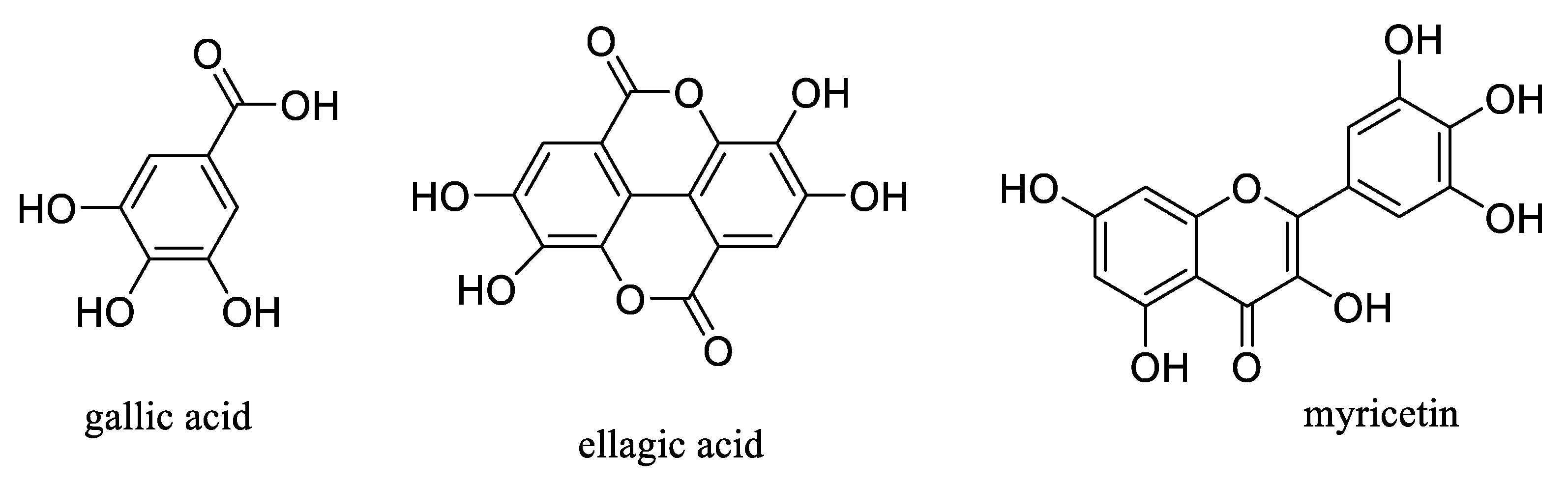
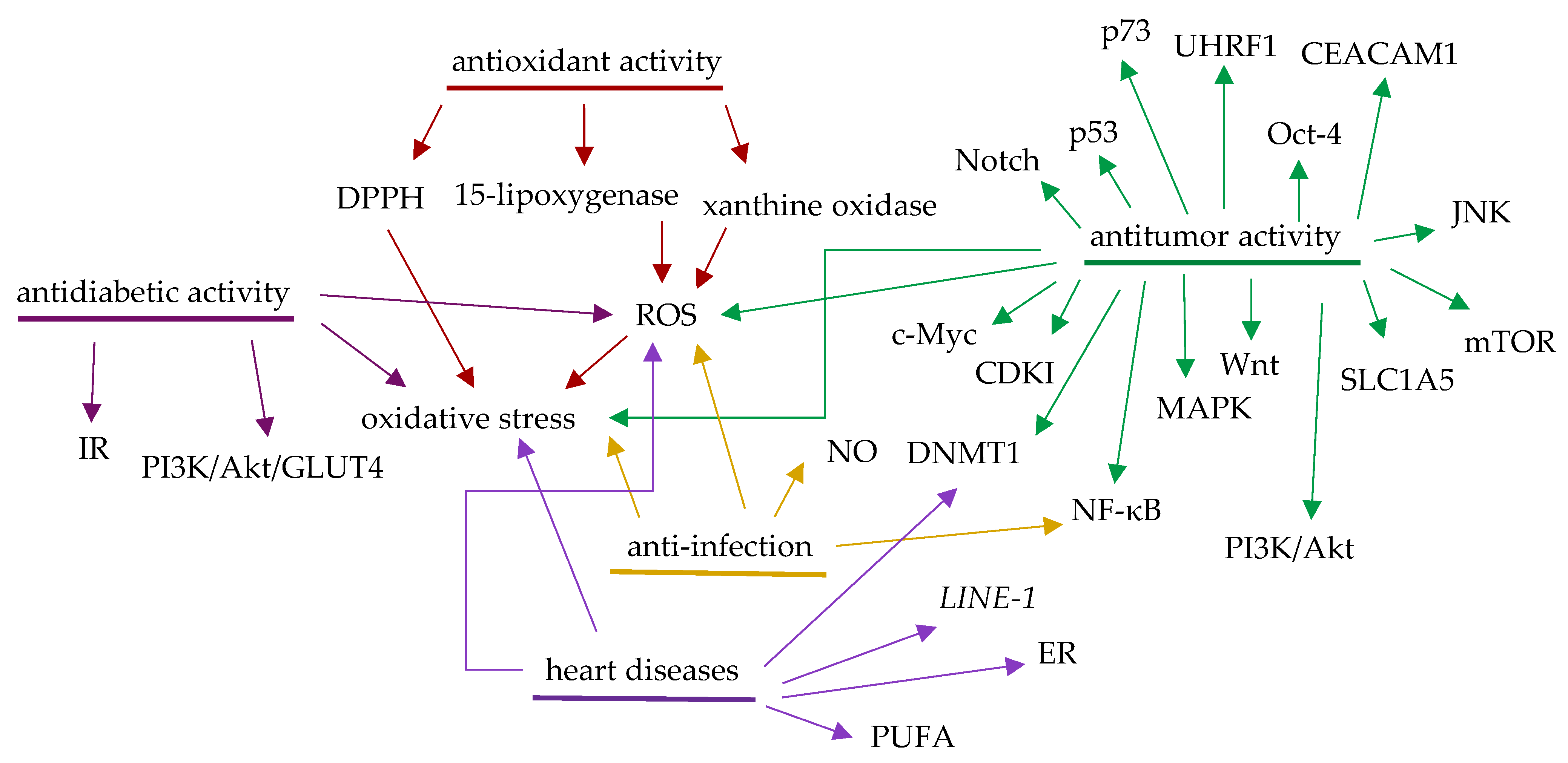
2. Antioxidant Activity of Aronia Berries
3. Potential Antitumor Activity of Aronia Berries
4. Potential Anti-Infective Activity of Aronia Berries
5. Potential Benefits to the Prevention and Treatment of Cardiovascular Diseases of Aronia Berries
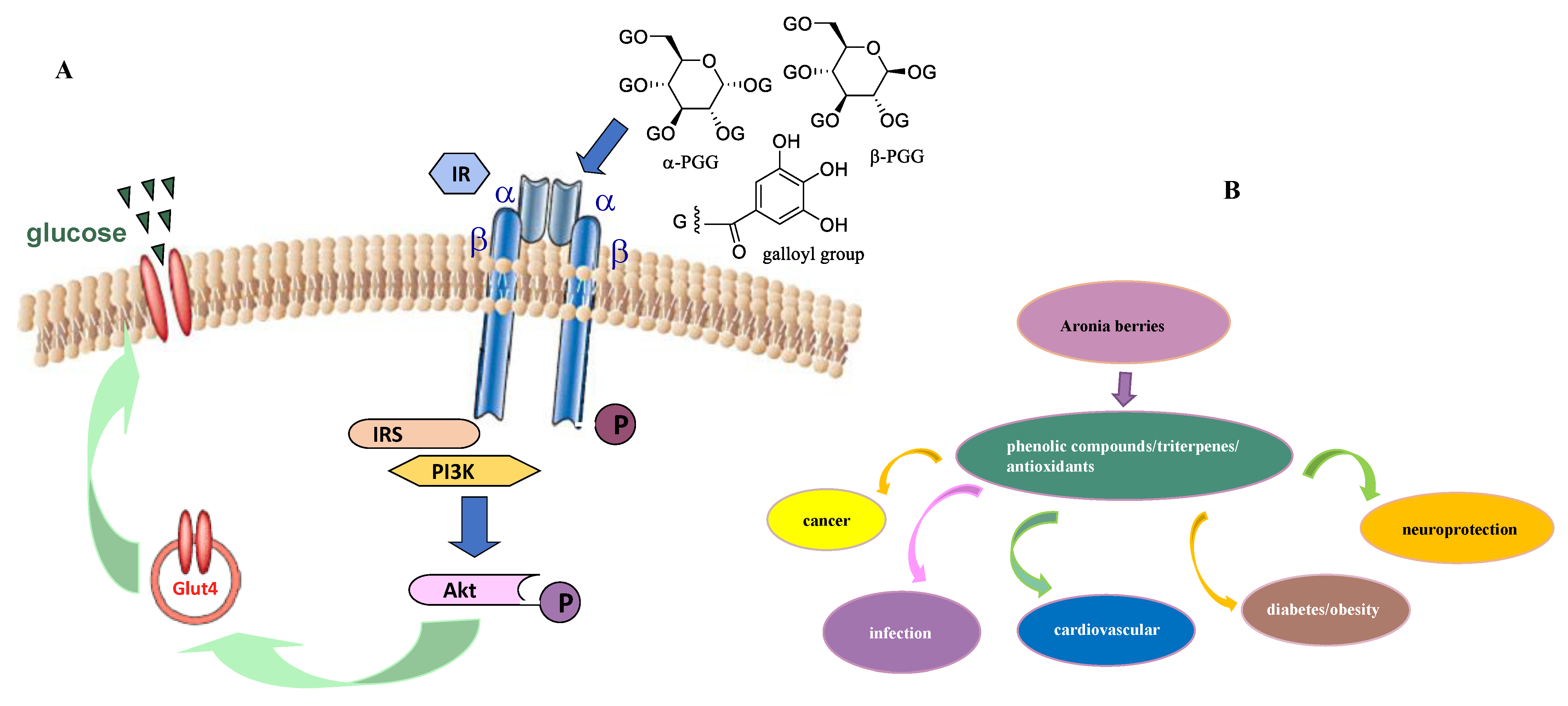
6. Potential Antidiabetic Activity of Aronia Berries
7. Miscellaneous Bioactivities of Aronia Berries
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sidor, A.; Drożdżyńska, A.; Gramza-Michalowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors–an overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Wang, D.; Gao, Y.-X. Advances in studies on the function and application of Aronia melanocarpa. Food Res. Dev. 2021, 42, 206–213. [Google Scholar]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Naman, C.B.; Li, J.; Moser, A.; Hendrycks, J.M.; Benatrehina, P.A.; Chai, H.; Yuan, C.; Keller, W.J.; Kinghorn, A.D. Computer-assisted structure elucidation of black chokeberry (Aronia melanocarpa) fruit juice isolates with a new fused pentacyclic flavonoid skeleton. Org. Lett. 2015, 17, 2988–2991. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Safety 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-M.; Zhou, X.; Zhang, J.-L.; Li, T. Research progress of anthocyanin antioxidant function in Aronia melanocarpa. Food Res. Dev. 2017, 38, 220–224. [Google Scholar]
- Sidor, A.; Gramza-Michalowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Y.; Yuan, C.; Pan, L.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Antioxidant and quinone reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012, 60, 11551–11559. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Benatrehina, P.A.; Rague, A.L.; Pan, L.; Kinghorn, A.D.; Naman, C.B. Isolation and analysis of antioxidant phytochemicals from black chokeberry, maqui, and goji berry dietary supplements. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS Symposium Series; American Chemical Society: Washington, WA, USA, 2018; Volume 1286, pp. 3–19. [Google Scholar]
- Nowak, D.; Grabczewska, Z.; Gośliński, M.; Obońska, K.; Dabrowska, A.; Kubica, J. Effect of chokeberry juice consumption on antioxidant capacity, lipids profile and endothelial function in healthy people: A pilot study. Czech J. Food Sci. 2016, 34, 39–46. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.; Cieślicka, M.; Kujawski, S.; Piskorska, E.; Kowalik, T.; Korycka, J.; Skarpańska-Stejnborn, A. Effects of antioxidant supplementation on oxidative stress balance in young footballer—A randomized double-blind trial. J. Int. Soc. Sports Nutr. 2021, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Ruginǎ, D.; Sconta, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Wang, Y.; Jiao, X.; Chou, S.; Li, E.; Li, B. Preparative purification of polyphenols from Aronia melanocarpa (chokeberry) with cellular antioxidant and antiproliferative activity. Molecules 2018, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer effects of extracts from three different chokeberry species. Nutr. Cancer 2021, 73, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef]
- Bermứdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gao, J.; Hao, R.; Zhang, C.; Liu, H.; Fan, J.; Wei, J. Aronia melanocarpa Elliot anthocyanins inhibit colon cancer by regulating glutamine metabolism. Food Biosci. 2021, 40, 100910. [Google Scholar] [CrossRef]
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa induce apoptosis in Caco-2 cells through Wnt/β-catenin signaling pathway. Chem. Biodiversity 2020, 17, e2000654. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, P.; Vandenakker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: An updated overview. Nutrients 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in colorectal cancer prevention review. Antioxidants 2021, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Stambouli, M.; Burrus, B.; Emhemmed, F.; Dandache, I.; Auger, C.; Etienne-Selloum, N.; Schini-Kerth, V.B.; Fuhrmann, G. The polyphenolic-rich Aronia melanocarpa juice kills teratocarcinomal cancer stem-like cells, but not their differentiated counterparts. J. Funct. Foods 2013, 5, 1244–1252. [Google Scholar] [CrossRef]
- Abdullah Thani, N.A.; Keshavarz, S.; Lwaleed, B.A.; Cooper, A.J.; Rooprai, H.K. Cytotoxicity of gemcitabine enhanced by polyphenolics from Aronia melanocarpa in pancreatic cancer cell line AsPC-1. J. Clin. Pathol. 2014, 67, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-galactoside: A natural compound with multiple health benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in osteosarcoma cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anticancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Shriwas, P.; Chen, X.; Kinghorn, A.D.; Ren, Y. Plant-derived glucose transport inhibitors with potential antitumor activity. Phytother. Res. 2020, 34, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Maejima, K. Aronia extract for supporting an active life. Food Style 21 2014, 18, 67–69. [Google Scholar]
- Makanae, Y.; Ato, S.; Kido, K.; Fujita, S. Dietary Aronia melanocarpa extract enhances mTORC1 signaling, but has no effect on protein synthesis and protein breakdown-related signaling, in response to resistance exercise in rat skeletal muscle. J. Int. Soc. Sports Nutr. 2019, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Shaikh, N.; Sawant, G.; Dixit, N. A review on ursolic acid: A naturally obtained pentacyclic triterpene. Int. J. Pharm. Pharm. Sci. 2021, 13, 1–5. [Google Scholar] [CrossRef]
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in anti-inflammatory activity, mechanism and therapeutic application of ursolic acid. Mini Rev. Med. Chem. 2022, 22, 422–436. [Google Scholar]
- Mioc, M.; Milan, A.; Malita, D.; Mioc, A.; Prodea, A.; Racoviceanu, R.; Ghiulai, R.; Cristea, A.; Căruntu, F.; Soica, C. Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part I). Int. J. Mol. Sci. 2022, 23, 7740. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-Y.; Yun, B.-S.; Lee, D.-S. Triterpene acid (3-O-p-coumaroyltormentic acid) isolated from Aronia extracts inhibits breast cancer stem cell formation through downregulation of c-Myc protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, M.; Malinowska, J.; Kontek, B.; Kolodziejczyk-Czepas, J.; Czernek, U.; Potemski, P.; Piekarski, J.; Jeziorski, A.; Olas, B. Chemotherapy modulates the biological activity of breast cancer patients plasma: The protective properties of black chokeberry extract. Food Chem. Toxicol 2013, 53, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Daoutidou, M.; Plessas, S.; Alexopoulos, A.; Mantzourani, I. Assessment of antimicrobial activity of pomegranate, cranberry, and black chokeberry extracts against foodborne pathogens. Foods 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguepi Tsopmejio, I.S.; Diao, Z.; Xiao, H.; Wang, X.; Jin, Z.; Song, H. Aronia melanocarpa (Michx.) Elliott attenuates dextran sulfate sodium-induced inflammatory bowel disease via regulation of inflammation-related signaling pathways and modulation of the gut microbiota. J. Ethnopharmacol. 2022, 292, 115190. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Kim, M.-B.; Park, Y.-K.; Bae, M.; Kang, H.; Hu, S.; Pham, T.X.; Carpenter, R.; Lee, J.; Lee, O.-H.; et al. Anthocyanin-rich Aronia berry extract mitigates high-fat and high-sucrose diet-induced adipose tissue inflammation by inhibiting nuclear factor-κB activation. J. Med. Food 2021, 24, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT Food Sci. Technol. 2021, 150, 112018. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes–asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Handeland, M.; Grude, N.; Torp, T.; Slimestad, R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term—A pilot study. Nutr. Res. 2014, 34, 518–525. [Google Scholar] [CrossRef]
- Gramza-Michalowska, A.; Sidor, A.; Kulczyński, B. Berries as a potential anti-influenza factor—A review. J. Funct. Foods 2017, 37, 116–137. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ochnik, M.; Franz, D.; Sobczyński, M.; Naporowski, P.; Banach, M.; Orzechowska, B.; Sochocka, M. Inhibition of human respiratory influenza A virus and human betacoronavirus-1 by the blend of double-standardized extracts of Aronia melanocarpa (Michx.) Elliott and Sambucus nigra L. Pharmaceuticals 2022, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Hejrati, A.; Nurzadeh, M.; Roham, M. Association of coronavirus pathogencity with the level of antioxidants and immune system. J. Family Med. Prim. Care 2021, 10, 609–614. [Google Scholar] [PubMed]
- Ho, G.T.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating activity of Aronia melanocarpa polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef] [PubMed]
- Gajić, D.; Saksida, T.; Koprivica, I.; Šenerović, L.; Morić, I.; Šavikin, K.; Menković, N.; Pejnović, N.; Stojanović, I. Immunomodulatory activity and protective effects of chokeberry fruit extract on Listeria monocytogenes infection in mice. Food Funct. 2020, 11, 7793–7803. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Negm, W.A.; Alexiou, A.; Batiha, G.E.-S. Ursolic acid and SARS-CoV-2 infection: A new horizon and perspective. Inflammopharmacology 2022, 30, 1493–1501. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotla, E.; Oniszczuk, A. The efficacy of black chokeberry fruits against cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef] [PubMed]
- Stojković, L.; Jovanović, I.; Zivković, M.; Zec, M.; Djurić, T.; Zivotić, I.; Kuveljić, J.; Kolaković, A.; Kolić, I.; Djordjević, A.; et al. The effects of Aronia melanocarpa juice consumption on the mRNA expression profile in peripheral blood mononuclear cells in subjects at cardiovascular risk. Nutrients 2020, 12, 1484. [Google Scholar] [CrossRef]
- Stojković, L.; Zec, M.; Zivkovic, M.; Bundalo, M.; Bošković, M.; Glibetić, M.; Stankovic, A. Polyphenol-rich Aronia melanocarpa juice consumption affects LINE-1 DNA methylation in peripheral blood leukocytes in dyslipidemic women. Front. Nutr. 2021, 8, 689055. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with Aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: A meta analysis of controlled clinical trials. J. Diet. Suppl. 2021, 18, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chalubiński, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur. J. Nutr. 2012, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: A prospective controlled trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial effects of polyphenol-rich chokeberry juice consumption on blood pressure level and lipid status in hypertensive subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia berry (poly) phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Turner, C.G.; Wong, B.J.; Feresin, R.G. Berry-derived polyphenols in cardiovascular pathologies: Mechanisms of disease and the role of diet and sex. Nutrients 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic effects of Aronia melanocarpa and its other therapeutic properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Veličković Radovanović, R.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry juice supplementation in type 2 diabetic patients—Impact on health status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-D.; Kang, S.-H.; Moon, K.-H.; Lee, J.-H.; Kim, D.-G.; Kim, W.; Kim, J.-S.; Ahn, B.-Y.; Jin, J.-S. The effect of Aronia berry on type 1 diabetes in vivo and in vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (Michx.) Elliott)—A natural product for metabolic disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa on cardiometabolic diseases: A systematic review of quasi-design studies and randomized controlled trials. Rev. Diabetic Stud. 2022, 18, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Dragan, S.; Andrica, F.; Serban, M.-C.; Timar, R. Polyphenols-rich natural products for treatment of diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qie, X.; Quan, W.; Zeng, M.; Qin, F.; Chen, J.; Adhikari, B.; He, Z. Omnifarious fruit polyphenols: An omnipotent strategy to prevent and intervene diabetes and related complication? Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Polce, S.A.; Burke, C.; Franca, L.M.; Kramer, B.; de Andrade Paes, A.M.; Carrillo-Sepulveda, M.A. Ellagic acid alleviates hepatic oxidative stress and insulin resistance in diabetic female rats. Nutrients 2018, 10, 531. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.; Li, J.; Liu, F.; Liu, X.; Himmeldirk, K.; Ren, Y.; Wagner, T.E.; Chen, X. Natural antidiabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. 2005, 336, 430–437. [Google Scholar] [CrossRef]
- Ren, Y.; Himmeldirk, K.; Chen, X. Synthesis and structure-activity relationship study of antidiabetic penta-O-galloyl-D-glucopyranose and its analogs. J. Med. Chem. 2006, 49, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; Kim, J.; Ren, Y.; Himmeldirk, K.; Liu, Y.; Qian, Y.; Liu, F.; Chen, X. Orally efficacious novel small molecule 6-chloro-6-deoxy-1,2,3,4-tetra-O-galloyl-α-D-glucopyranose selectively and potently stimulates insulin receptor and alleviates diabetes. J. Mol. Endocrinol. 2013, 51, 15–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borowska, S.; Brzóska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds—Implications for health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef] [PubMed]
- Meżyńska, M.; Brzóska, M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019, 39, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Marazova, K.; Krasnaliev, I.; Galunska, B.; Borisova, P.; Belcheva, A. Effect of Aronia melanocarpa fruit juice on indomethacin-induced gastric mucosal damage and oxidative stress in rats. Exp. Toxicol. Pathol. 2005, 56, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Weon, J.B.; Ryu, G.; Yang, W.S.; Kim, N.Y.; Kim, M.K.; Ma, C.J. Neuroprotective effect of Aronia melanocarpa extract against glutamate-induced oxidative stress in HT22 cells. BMC Compl. Altern. Med. 2017, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, E.; Delchev, S.; Topolov, M.; Dimitrova, S.; Uzunova, Y.; Valcheva-Kuzmanova, S.; Kratchanova, M.; Vladimirova-Kitova, L.; Denev, P. Aronia melanocarpa (Michx.) Elliot fruit juice reveals neuroprotective effect and improves cognitive and locomotor functions of aged rats. Food Chem. Toxicol. 2019, 132, 110674. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of neuroprotective anthocyanins from black chokeberry (Aronia melanocarpa) against amyloid-β-induced cognitive impairment. Foods 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.; Shi, A.; Wang, N.; Wang, L.; Wei, Y. Oleanolic acid and ursolic acid: Therapeutic potential in neurodegenerative diseases, neuropsychiatric diseases and other brain disorders. Nutr. Neurosci. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.-S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, L.N.; Burdette, J.E.; Carcache de Blanco, E.J.; Coss, C.C.; Eustaquio, A.S.; Fuchs, J.R.; Kinghorn, A.D.; MacFarlane, A.; Mize, B.K.; Oberlies, N.H.; et al. Discovery of anticancer agents of diverse natural origin. J. Nat. Prod. 2022, 85, 702–719. [Google Scholar] [CrossRef]
- Ren, Y.; Elkington, B.G.; Henkin, J.M.; Sydara, K.; Kinghorn, A.D.; Soejarto, D.D. Bioactive small-molecule constituents of Lao plants. J. Med. Plants Res. 2021, 15, 540–559. [Google Scholar]
- Güler, O.; Polat, R.; Karaköse, M.; Cakılcıoğlu, U.; Akbulut, S. An ethnoveterinary study on plants used for the treatment of livestock diseases in the province of Giresun (Turkey). S. Afr. J. Bot. 2021, 142, 53–62. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; da Silva Campos, R.A.; Borguini, M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef]
- Ren, Y.; Anaya-Eugenio, G.D.; Czarnecki, A.A.; Ninh, T.N.; Yuan, C.; Chai, H.-B.; Soejarto, D.D.; Burdette, J.E.; Carcache de Blanco, E.J.; Kinghorn, A.D. Cytotoxic and NF-κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi-synthetic derivatives. Bioorg. Med. Chem. 2018, 26, 4452–4460. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Emerging avenues linking inflammation and cancer. Free Rad. Biol. Med. 2012, 52, 2013–2037. [Google Scholar] [CrossRef]
- Anari, F.; Ramamurthy, C.; Zibelman, M. Impact of tumor microenvironment composition on therapeutic responses and clinical outcomes in cancer. Future Oncol. 2018, 14, 1409–1421. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. https://doi.org/10.3390/molecules27227823
Ren Y, Frank T, Meyer G, Lei J, Grebenc JR, Slaughter R, Gao YG, Kinghorn AD. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules. 2022; 27(22):7823. https://doi.org/10.3390/molecules27227823
Chicago/Turabian StyleRen, Yulin, Tyler Frank, Gunnar Meyer, Jizhou Lei, Jessica R. Grebenc, Ryan Slaughter, Yu G. Gao, and A. Douglas Kinghorn. 2022. "Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health" Molecules 27, no. 22: 7823. https://doi.org/10.3390/molecules27227823
APA StyleRen, Y., Frank, T., Meyer, G., Lei, J., Grebenc, J. R., Slaughter, R., Gao, Y. G., & Kinghorn, A. D. (2022). Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules, 27(22), 7823. https://doi.org/10.3390/molecules27227823






