Abstract
Essential oils (EOs) are chemical substances, mostly produced by aromatic plants in response to stress, that have a history of medicinal use for many diseases. In the last few decades, EOs have continued to gain more attention because of their proven therapeutic applications against the flu and other infectious diseases. Influenza (flu) is an infectious zoonotic disease that affects the lungs and their associated organs. It is a public health problem with a huge health burden, causing a seasonal outbreak every year. Occasionally, it comes as a disease pandemic with unprecedentedly high hospitalization and mortality. Currently, influenza is managed by vaccination and antiviral drugs such as Amantadine, Rimantadine, Oseltamivir, Peramivir, Zanamivir, and Baloxavir. However, the adverse side effects of these drugs, the rapid and unlimited variabilities of influenza viruses, and the emerging resistance of new virus strains to the currently used vaccines and drugs have necessitated the need to obtain more effective anti-influenza agents. In this review, essential oils are discussed in terms of their chemistry, ethnomedicinal values against flu-related illnesses, biological potential as anti-influenza agents, and mechanisms of action. In addition, the structure-activity relationships of lead anti-influenza EO compounds are also examined. This is all to identify leading agents that can be optimized as drug candidates for the management of influenza. Eucalyptol, germacrone, caryophyllene derivatives, eugenol, terpin-4-ol, bisabolene derivatives, and camphecene are among the promising EO compounds identified, based on their reported anti-influenza activities and plausible molecular actions, while nanotechnology may be a new strategy to achieve the efficient delivery of these therapeutically active EOs to the active virus site.
1. Introduction
Natural products (NPs) are chemical substances in the form of primary and secondary metabolites which are produced by living organisms such as plants, animals, marine organisms, bacteria, and fungi [1,2]. They are mostly referred to as small molecules or secondary metabolites, representing a unique scaffold of compounds that are distributed in a broad variety of chemical classes, such as alkaloids, cardiac glycosides, flavonoids, phenolics, saponins, sterols, and terpenoids [3]. Essential oil compounds form part of these classes as volatile and non-volatile aromatic substances, comprised mostly of terpenoids and some phenylpropanoid derivatives [4,5].
Chemically, terpenoids form the largest group of essential oils, such as monoterpenes (C10) and sesquiterpenes (C15) and a few diterpenes and phenylpropanoids [6]. Many of them have functional groups such as aldehyde, ether, ester, alcohol, carboxylic acid, phenol, amines, and amides [6].
Essential oils are naturally distributed in many higher plants and are most abundant in aromatic plant families, including Lamiaceae (Peppermint family), Myrtaceae (Eucalyptus family), Rutaceae (Citrus family), and Zingiberaceae (Ginger family) [7]. They have been reported in the seeds, fruits, peels, flowers, buds, leaves, young stems, barks, resins, woods, bulbs, roots, and rhizomes of many plants [8] and are extracted by methods of hydro-distillation, steam distillation, hydro-diffusion, solvent extraction, maceration, cold-press extraction, supercritical fluid (CO2) extraction, sub-critical liquid extraction, microwave-assisted extraction, and enfleurage [4,9].
The use of EOs predates modern history. They are one of the most important NPs that have ever been utilized by many cultures, for many centuries, around the world for domestic (cosmetics, perfumery, food, and beverages) and medicinal purposes [10]. Essential oils are popularly known to help relieve the airways during the cold (winter) or flu season, and evidence has emerged of their medicinal importance in the amelioration of respiratory tract infectious diseases, such as the common cold, pneumonia, and influenza [11]. The flu (influenza) is a disease that often occurs during the winter or cold season, and so can be referred to as seasonal influenza [12].
Influenza is an infectious zoonotic respiratory disease caused by the influenza A, B, C, and D viruses, where A and B are the common virus type that causes seasonal flu [13,14]. Influenza is characterized by the sudden onset of fever, dry cough, sore throat, runny nose, headache, and joint pains [12]. It is a major public health problem, accounting for about 3–5 million cases worldwide, with about a 10% annual death rate [12]. It is responsible for high hospitalization and deaths among high-risk individuals, such as the elderly and those with co-morbidities [15].
Vaccination remains a primary strategy to prevent and control influenza, due to the waning immunity that is associated with it [16]. So far, an effort made to reduce the burden of influenza through the administration of vaccines has yielded promising results, with more expectations for success. Vaccine selectivity is still a major issue because the currently available influenza vaccines are virus-type- and sub-type-specific [17]. In addition, many people are still in doubt about the harmlessness of vaccines due to cultural beliefs, myths, and conspiracy theories, thus causing vaccine hesitancy [18]. Therefore, there is a need for deliberate and continuous vaccine education and advocacy to help bring influenza to a halt.
Currently, four FDA-approved antiviral drugs of three different classes are recommended for use against the recently circulating influenza viruses, Baloxavir marboxil, Oseltamivir, Peramivir, and Zanamivir [19]. Despite the availability of these drugs, influenza is still on the rampage, taking its toll on human health and well-being. In fact, the problem is further exacerbated because the available drugs still lack strong antiviral activity against all the influenza virus strains; in addition, there is always an emergence of new resistant strains and the resurgence of old virulent strains that were not adequately reduced in past influenza outbreaks [20].
Therefore, there is an urgent need to identify lead antiviral agents that can be developed into new anti-influenza drugs. This review explores essential oils because of their age-long ethnomedicinal use across many cultures for the management of flu and other airway diseases. The chemical compositions of EOs are discussed with respect to their anti-influenza potentials. The mechanism of action and structure-activity relationship of the lead antiviral compounds are also highlighted in this study to identify the leading EO compounds that can be optimized as anti-influenza drug candidates.
2. Methodology
The study was carried out by an extensive, open (no duration set) literature review of articles in the BioMedCentral (BMC), Elsevier, Google Scholar, Hindawi, PubMed, Nature, ScienceDirect, Scopus, and Springer Nature databases, amongst others. The keywords for the search included “essential oils”, “essential oil compounds”, “essential oils and flu”, “influenza viruses”, “anti-influenza essential oils”, “anti-influenza essential oil compounds”, “mechanism of action of anti-influenza essential oil compounds”, and “structure-activity relationship of anti-influenza essential oil compounds”. Some inclusive criteria such as “natural volatile compounds”, “terpenes and terpenoids”, “aromatic terpenes”, “phenylpropanoid derivatives”, “antiviral essential oils”, “anti-flu essential oils”, and “anti-influenza terpenes”, were also used in the literature search. Compounds other than EO compounds, and diseases other than influenza or flu were excluded from the literature search. All the chemical structures were drawn with a Chem Draw Ultra® 7.0 Software application, licensed by CambridgeSoft Corporation (Cambridge, MA 02140, USA), while diagrammatic representations were prepared with Microsoft PowerPoint Version 365 licensed by ©Microsoft Corporation (Johannesburg, South Africa).
To the best of our knowledge, this article gives a comprehensive and up-to-date account of the anti-influenza potentials of essential oils and their compounds and will add to the repository of anti-influenza essential oil compounds.
3. Essential Oils as an Integral Part of Natural Products
3.1. Natural Products
Natural products (NPs) are chemical substances that originate from plants, animals, marine organisms, fungi, and bacteria [21]. They are mostly secondary metabolites produced by living organisms and used by them for defense and adaptation purposes [22]. NPs represent a large group of diverse chemical entities with a broad spectrum of biological activities that have found many applications, notably in humans and veterinary medicines, food and agriculture, and cosmetics [22,23].
Natural products are a diversified group of natural substances, mostly secondary metabolites, discovered to provide a variety of health benefits in humans [24]. Evidence has emerged of the various biological and/or pharmacological activities of NPs [25,26]. Thus, they are regarded as drug leads because many known drugs are inspired by or developed from them [27]. For instance, the anti-influenza drugs, Favipiravir, Oseltamivir, Peramivir, and Zanamivir are nature-inspired [28].
Plants produce an enormous variety of NPs with diverse chemical structures [29]. These chemical entities fall into seven major categories: alkaloids, carbohydrates, specialized amino acids and peptides, flavonoids, polyketides and fatty acids, terpenoids and steroids, and phenylpropanoids [30] (Figure 1). A group of volatile and non-volatile, low molecular weight aromatic compounds, termed essential oils, can be found in the last three categories [4,5].
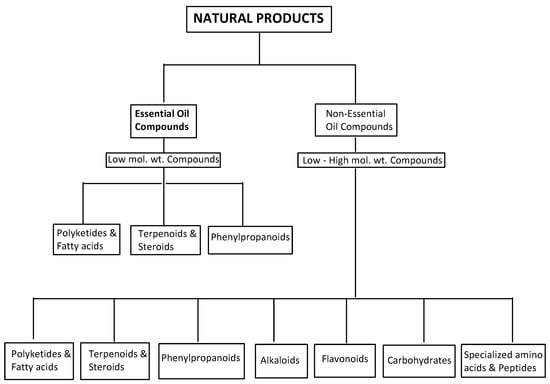
Figure 1.
An overview of natural products, showing the essential oil compounds.
3.2. Plant-Derived Essential Oils
The International Organization for Standardization (ISO) defined plant-derived essential oils as products obtained from aromatic plants through specialized extraction methods without any significant change to their chemical compositions [31]. They are volatile and aromatic chemical substances, which are a mixture of fragrant and odorless compounds, mostly confined in the plant cytoplasm in the form of tiny droplets between cells and named after the plants from which they are derived [31,32].
Plant-derived EOs are produced in the glandular trichomes and other specialized secretory organs of plants, which include flowers, fruits, seeds, leaves, bark, and roots in the forms of bulbs and rhizomes [33]. They are usually plants’ secondary metabolites and are known to perform major ecological and physiological functions in plants, such as defense against herbivores and microbial pathogens (irritants and repellents), reduction in abiotic stress, allelopathy, inter-plant signaling, defense against pathogenic microorganisms (antimicrobial agents), and attraction of plant pollinators and seed dispersers for the purpose of plant reproduction and survival [34,35].
Well over 3000 EOs have been identified from about 2000 plant species belonging to aromatic families such as Asteraceae, Lamiaceae, Myrtaceae, Poaceae, Rosaceae, Rutaceae, Umbelliferae, and Zingiberaceae, amongst others [36], as presented in Table 1.

Table 1.
Some Essential Oil-Bearing Plant Families with their Major Essential Oil Compounds.
3.3. Biosynthetic Routes of Essential Oil Compounds
Essential oils are complex mixtures of hydrocarbons and their oxygenated and nitrogenous derivatives, derived from two different isoprenoid pathways in the secretory cells of plants [33], the acetate-mevalonate pathway for the formation of isoprenoids from isopentenyl pyrophosphate and dimethylallyl pyrophosphate in the cytoplasm, and the pyruvate pathway for the synthesis of 2-C-methyl-D-erythritol-4-phosphate in the plastids [76,77,78]. Thus, both pathways are mediated by different enzymatic reactions [76], as presented in Figure 2.
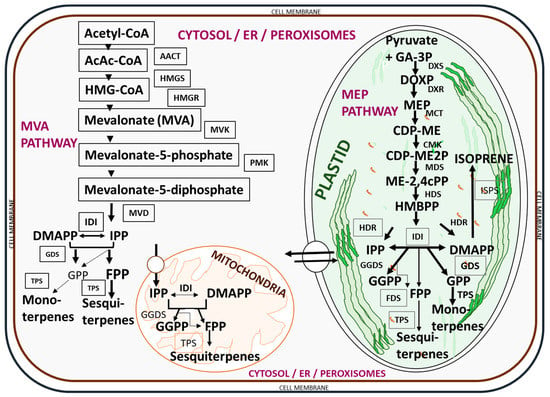
Figure 2.
Biosynthetic pathways for essential oil compounds in plants. A list of enzymes involved in the biosynthesis includes acetoacetyl-CoA (AcAc-CoA), acetoacetyl-CoA thiolase (AACT), CDP-ME kinase (CMK), DOXP reducto-isomerase (DXR), DOXP synthase (DXS), farnesyl diphosphate synthase (FDS), geranyl diphosphate synthase (GDS), geranyl geranyl diphosphate synthase (GGDS), (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR), (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HDS), 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), HMG-CoA reductase (HMGR), HMG-CoA synthase (HMGS), isopentenyl diphosphate isomerase (IDI), isoprene synthase (ISPS), 2-C-methyl-D-erythritol-4-phosphate cytidylyltransferase (MCT), 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (MDS), mevalonate diphosphate decarboxylase (MVD), mevalonate kinase (MVK), phosphomevalonate kinase (PMK), and terpene synthase (TPS). The key intermediates (compounds) involved in the biosynthetic process include: 4-(cytidine 50-diphospho)-2-C-methyl-D-erythritol (CDP-ME), 4-(cytidine 50-diphospho)-2-C-methyl-D-erythritol phosphate (CDP-ME2P), isopentenyl diphosphate (IPP), dimethylallyl diphosphate (DMAPP), farnesyl diphosphate (FPP), geranyl geranyl diphosphate (GGPP), geranyl diphosphate (GPP), glyceraldehyde-3-phosphate (GA-3P), 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (ME-2,4Cpp), 2-C-methyl-D-erythritol-4-phosphate (MEP), (E)-4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP), and 1-deoxy-D-xylulose 5-phosphate (DOXP). The pathways were adapted from Nagegowda [76] and redrawn with copyright permission by the Federation of European Biochemical Societies © 2010 Elsevier B.V. Publication.
3.4. Classes of Essential Oils
Essential oils are comprised mostly of a mixture of low molecular weight, lipophilic, and sparingly polar organic compounds, and it is these properties that contribute to their high level of volatility [5]. Based on their chemical properties, they are structurally categorized as isoprenoids (terpenoids), a major EO group, while other minor groups include the phenylpropanoid derivatives [79].
Structurally, an isoprene unit (2-methylbutan-1,3-diene) is the building block upon which all isoprenoids are formed, according to the isoprene rule propounded by Breitmaier [80]. The isoprene unit (hemiterpene, C5 unit) through head-to-tail and tail-to-tail condensations, forms seven classes of terpenoids, monoterpenes (C10 isoprenoids), sesquiterpenes (C15 isoprenoids), diterpenes (C20 isoprenoids), sesterterpenes (C25 isoprenoids), triterpenes (C30 isoprenoids), tetraterpenes (C40 isoprenoids), and polyterpenes (at least C45 isoprenoids) [75,79,80]. However, many of the low molecular weight terpenoids (C5, C10, and C15 isoprenoids) and a few others (C20 and C25 isoprenoids) are naturally found to be EO compounds [77,81,82,83]. Essential oil compounds can be grouped into acyclic and cyclic isoprenoids, while the cyclic group is further sub-grouped into monocyclic, bicyclic, tricyclic, etc., based on the number of cyclic rings [5,84,85,86].
3.5. Medicinal Applications of Plant-Derived Essential Oils
For centuries, essential oils have continued to find applications in many cultures around the world for different purposes, such as foods, beverages, perfumes and cosmetics, and medicines [10]. Aromatherapy is an ancient but popular traditional medicine practice that uses EOs as the major therapeutic agent to treat many diseases [87]. Among the early accounts for the medicinal application of EOs was the practice of aromatherapy by the ancient Egyptians in 4500 BC, traditional Chinese medicine (TCM) in 3000 BC, and the ancient Indian Ayurvedic medicine around 2000 BC [10]. Later, ancient Greek scientists provided the first documented evidence for the medicinal application of EOs, such as cumin, peppermint, saffron, and thyme, around 450 BC [7]. An infusion of EO from the 16th-century rosemary plant (Salvia rosemarinus) later became a form of medicine after it was used by Queen Isabell to cure rheumatism in the court of King Louis XIV [88].
Essential oils have continued to find usefulness in disease prevention and management to date. Evidence has emerged concerning the medicinal application of aromatic herbs, their EO compounds, and other bioactive plant secondary metabolites for the management of common respiratory diseases (RDs), such as asthma, chronic obstructive pulmonary disease (COPD), pneumonia, and influenza, to mention a few [89]. The last few decades witnessed the development of herbal pharmacopoeias and monographs, some of which have validated the ethnomedicinal claims of aromatic herbs and their EOs as remedies for chronic RDs such as flu (influenza) and its associated ailments [90,91]. Therefore, there is a need for a better understanding of influenza and the biological potential of natural EOs against this malady.
4. Influenza (Flu)
Influenza is a contagious viral disease that affects the upper and lower respiratory tract [92]. Influenza viruses can be found in humans and some animals such as Aves and cattle, and are generally categorized as type A, B, C, and D influenza viruses [93,94]. The common types are the influenza A and B viruses, which affect humans and are mostly characterized as the seasonal flu [92]. Influenza A viruses are largely implicated in the flu pandemic and are a common cause of zoonotic infections, often characterized by virulent infections in humans [14,95]. The influenza C viruses are predominantly responsible for mild illness in animals and are rarely implicated in human epidemics [14,95]. Influenza D viruses mostly affect animals, with rare cases of human-to-human transmission [93]. Symptoms associated with influenza virus infections include a fever, sore throat, runny nose, cough, fatigue, and headache, owing to disease of the upper respiratory tract, while the lower respiratory tract may present with severe or acute pneumonia [96].
The influenza disease caused by the type A influenza virus of zoonotic origin, is a major public health concern, as it is responsible for both the common seasonal influenza epidemic (seasonal flu) and the sporadic and unpredictable (10–50 years of occurrence) global influenza pandemic outbreaks [97]. Seasonal influenza outbreaks typically occur during the winter season in temperate regions (Europe, Southern Africa), due to favorable conditions of low humidity and low temperatures [97,98]. However, in tropical countries, it is characterized by a complex pattern of occurrences due to an interplay of climatic factors such as temperature levels, hours of sunshine, and the level of rainfall [98].
On the other hand, pandemic influenza is characterized by a fast spread of the influenza A virus from the virus origin to the rest of the world in several waves over a short period, as witnessed in the first influenza pandemic of 1918 by the influenza A H1N1 virus strain, and subsequent pandemics of 1957, 1968, and 2009, caused by the Influenza A H2N2, H3N2, and H1N1 virus strains, respectively [97,99].
According to the World Health Organization, up to 1 billion influenza virus infection cases are reported annually, with about 4 million of the cases leading to severe illness, and around 400,000 reported deaths [95]. The most vulnerable groups are often infants between the ages of 0–9 months and adults 65-years-old and above [100].
Vaccination remains an effective means to reduce the burden of influenza [101]. The National Advisory Committee on Immunization (NACI) recommended the use of two classes of influenza vaccines, the Inactivated Influenza Vaccines (IIVs) and the Live Attenuated Influenza Vaccines (LAIVs) [102]. Just prior to the COVID-19 pandemic, it was reported that vaccination prevented an estimated 3.7 million cases of influenza, 105,000 influenza-related hospitalizations, and 6300 influenza-associated deaths worldwide [103]. However, more success in flu vaccination is still desired. Some key issues to be addressed include the complexity involved in predicting the pattern of seasonal influenza, reduced vaccine efficacy based on repeated annual immunization, an antigenic mismatch between the developed vaccines and the circulating virus strains, an age difference of the different cohorts involved in vaccination, and the issue of variability in the virulence level of different seasonal strains of the virus [104,105,106].
Even though vaccination is the most effective means of reducing the burden of influenza, antiviral drugs can be very useful in delaying the spread of new pandemic viruses, and they have also been found useful for the treatment of critically ill influenza patients [107]. There have been significant strides in the development of influenza antiviral drugs (IADs), and there are currently three classes of FDA-approved IADs: M2 proton channel antagonists, neuraminidase inhibitors, and polymerase acidic endonuclease inhibitors [108]. The drugs Amantadine and Rimantadine, are M2 proton channel antagonists, which used to be effective for the treatment of influenza A virus infection but have lost their efficacies over the years due to the emergence of more virulent strains of the type A virus, such as the 2009 H1N1 influenza A virus [19]. The FDA-approved neuraminidase inhibitors such as Oseltamivir, Peramivir, and Zanamivir are more efficacious and less toxic for the management of influenza than the M2 proton channel antagonists [109]. However, these drugs are associated with adverse effects, such as skin rash, diarrhea, anaphylactic reaction, headache, nausea, vomiting, cough, and gastritis [108]. Baloxavir is a cap-dependent, polymerase acidic endonuclease inhibitor which is similar in potency to neuraminidase inhibitors, except for the fact that it is newer and offers a different mechanism of action from the earlier developed neuraminidase inhibitory drugs [108,110].
Based on the rapid and unlimited variabilities of influenza viruses and the emerging resistance of new influenza virus strains to the currently used drugs, there is a dire need to discover more lead anti-influenza agents with a novel mechanism of action and develop (synthesize and optimize) more effective analogs from the already existing ones [109]. Natural products, including EOs, continue to offer an inexhaustible reservoir of bioactive compounds as lead therapeutic agents for the management of diseases. Some EOs and their compounds have been reported to demonstrate remarkable biological activities against a wide range of viruses, including influenza viruses [111]. It is against this backdrop that the anti-influenza potentials of EOs and their compounds were discussed, vis a viz the mechanism of action and structure-activity relationships of lead antiviral compounds, to source newer anti-influenza agents.
5. Essential Oils as Potential Anti-Influenza Agents
5.1. Anti-Influenza Properties of Plant-Derived Essential Oils and Their Compounds
Evidence has emerged on the anti-influenza potentials of many aromatic plants that are used for the treatment of flu and flu symptoms (cold, cough, sore throat, bronchitis, and pneumonia) by various ethnomedicines [112].
For instance, the EOs of Cynanchum stauntonii roots demonstrated an in vitro activity against Influenza A/NWS/33 (H1N1) virus at an IC50 value of 64 µg/mL and selectivity index of 8, with the main EOs used comprising (E,E)-2,4-decadienal, 3-ethyl-4-methypentanol, 5-pentyl-3H-furan-2-one, (E,Z)-2,4-decadienal, 2(3H)-furanone,dihydro-5-pentyl, and caryophyllene oxide [113]. Further investigation revealed considerable inhibitory effects on influenza-induced deaths with 40, 70, and 100% survival rates when administered 50, 150, and 300 mg/kg doses of the EO, respectively, in male albino mice [113].
The leaf EOs of Melaleuca alternifolia (tea tree oil) contain terpinen-4-ol, terpinolene, and α-terpineol, which showed considerable in vitro activity against the influenza A virus in MDCK cells by interference with acidification of intra-lysosomal compartment [114]. Mosla dianthera is an aromatic herb used in the TCM to treat colds, coughs, nasal congestion, bronchitis, fever, and headache [115]. The EOs derived from the aerial part of M. dianthera exhibited significant in vivo inhibitory activity against the influenza A virus at 90–360 mg/kg body weight in mice, with elemicin, thymol, β-caryophyllene, iso-elemicin, asarone, and α-caryophyllene implicated as the major active ingredients [112].
The in vitro antiviral activities of Citrus bergamia and Eucalyptus globulus EOs against the influenza A H1N1 virus have also been reported, in which E. globulus vapor EOs reduced viral infection by 78%, with no cytotoxicity, while that of C. bergamia reduced the viral cytopathic effect with no cytotoxicity [116]. The major EOs characterized in the former are limonene, linalyl acetate, and linalool, while the latter contained 1,8-cineole, γ-terpinene, p-cymene, and α-thujene [116]. In addition, thujone- and α-pinene rich Cedar (Thuja plicata) EO vapor demonstrated over 90% inhibitory activities against some membrane-containing influenza A H1N1 and H3N2 and B viruses within 10 min of exposure to the viruses in vitro [117]. Other anti-influenza aromatic plants and their major EO components are presented in Table 2, while the structures of some anti-influenza EO compounds are shown in Figure 3.

Table 2.
Some plant-derived essential oil compounds and their anti-influenza properties.
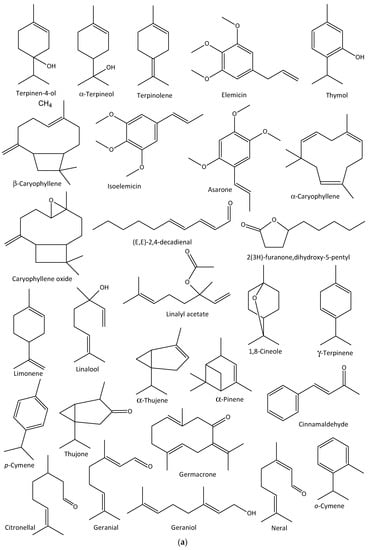
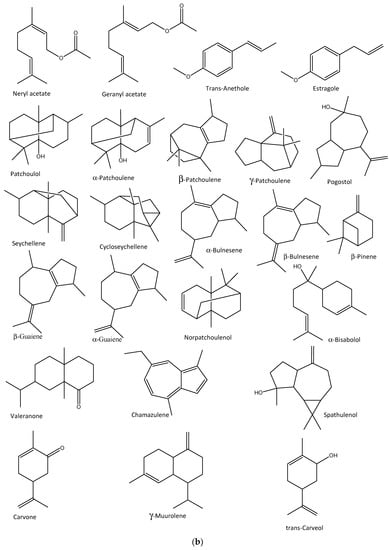
Figure 3.
Structures of some bioactive essential oil compounds (a,b) that have been implicated against some influenza viruses.
5.2. Mechanisms of Action and Structure-Activity Relationships of Some Lead Anti-Influenza Essential Oil Compounds
Basically, the molecular mechanisms of action of lead anti-influenza agents can be summed up under two major categories: those agents that target influenza virus proteins or genes and those that target the various components within the hosts for replication and propagation [107]. These mechanisms can be used to further categorize anti-influenza agents (virus inhibitors). First, entry and attachment (fusion) inhibitors, which are commonly used as an adjuvant in the preparation of anti-influenza vaccines [132,133]. The aerial EO of Melaleuca alternifolia, known as tea tree oil (TTO), has been shown in an in silico simulation study to interfere with the entry and fusion activities of the influenza virus [116]. The anti-influenza activity has been attributed to its hydroxylated monoterpenes, terpinen-4-ol, and α-terpineol [114]. Other known groups are hemagglutinin inhibitors [134], M2 ion channel protein inhibitors [135], endosomal and lysosomal inhibitors, also implicated in the TTO [125], protease inhibitors [136], RNA polymerase inhibitors [137], pathway inhibitors [138], neuraminidase inhibitors [139], non-structural protein inhibitors [107], caspase inhibitors [140], glycoprotein/glycosylation inhibitors [141], phospholipase inhibitors [142], release inhibitors [143,144], and autophagy [145]. Natural antiviral agents including EO compounds can act as inhibitors during the influenza virus activity stages of binding, penetration, uncoating, genome replication, assembly, and release of the virus (Figure 4); thus, they may offer considerable protection and efficacy as anti-influenza agents [146].
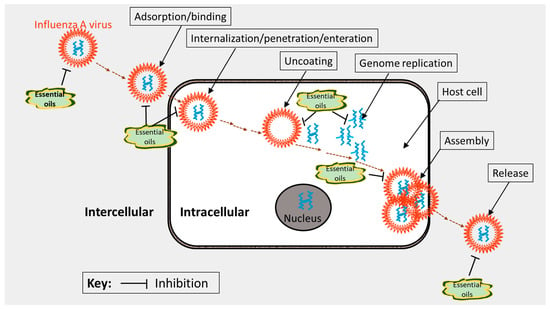
Figure 4.
Plausible target sites (mechanisms of action) of essential oils during the influenza virus lifecycle. The illustration was adapted from Ma and Yao [146] and redrawn with copyright permission by ©MDPI, Basel, 2020.
Three major compounds, curdione, curcumol, and germacrone, were implicated in the antiviral EO components of the TCM Zedoary oil [147]. The compounds impaired influenza A (H1N1) virus replication in vitro and in vivo, with germacrone exhibiting the highest anti-H1N1 effect [147]. Germacrone was shown to activate the transcription of interferon genes and protect the peripheral cells from influenza virus infections [147]. It also showed a marked decrease in the expression of antiviral proteins, RIG-I, IFNs, OAS, IRF3/7, MX, and EIF2AK2/PKR, viral replication, and viral load, with increased TAP1 expression, inhibited TAK1 phosphorylation, and consequently inhibited the NF-κB signaling and intrinsic antiviral responses (Figure 5) [147,148]. The biological properties of germacrone have been linked to its ketone and non-conjugated double bonds (Figure 6) [149].
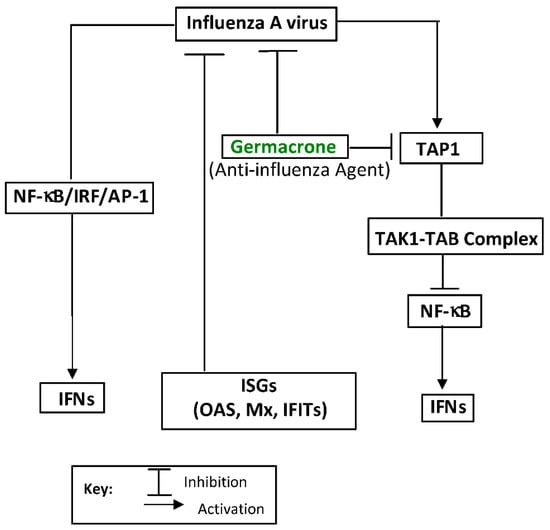
Figure 5.
Inhibition of TAP1 expression (pathway inhibition) by germacrone, a lead antiviral essential oil compound against the influenza A (H1N1) virus, and its replication: a mechanism of action. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), interferons (IFNs), and interferon regulatory factors (IRFs) regulate many aspects of innate and adaptive immune responses, including driving anti-viral responses. Interferon stimulating genes (ISGs) are critical effectors of IFN response to viral infection. Transcription factor (AP-1) regulates the inflammatory gene expression in response to viral infections. The transporter associated with antigen processing 1 (TAP1), transforming growth factor-β-activated kinase 1 (TAK1), and TAK1 binding protein (TAB) are also involved. The illustration was adapted from Li et al. [147] and redrawn with copyright permission by © Elsevier BV, The Netherlands, 2020.

Figure 6.
Structures of some lead anti-influenza essential oil compounds showing their active moieties (colored).
Some anti-influenza active bisabolane-type sesquiterpenoids from turmeric oil (Curcuma longa) have also been reported [150]. Generally, turmeric oil is used in ethnomedicine for the treatment of flu-related and/or airway inflammatory diseases, such as bronchitis, pneumonia, and influenza [151]. The compounds significantly acted as pathway inhibitors against the influenza A/PR/8/34 (H1N1) virus replication in MDCK and A549 cells in vitro [150]. The compounds act by inhibiting the expression of pro-inflammatory cytokines (IL-6, IL-8, IP-10, and TNF-α), and regulating the activity of the NF-κB/MAPK and RIG-1/STAT-1/2 signaling pathways [150]. The presence of ketone, α, β-unsaturation, and presence of an electron-withdrawing group (OH, OCH3, NH2, SH, and halogens) have been reported to influence the bioactivity of this group of compounds (Figure 6) [152].
Eucalyptol (1,8-cineole), a monoterpenoid principally from Eucalyptus plants, is another lead anti-influenza agent to discuss [153]. Eucalyptus oil is used in traditional medicines as a remedy for respiratory ailments [154]. Eucalyptol is a major EO component of the oil, and it has been shown to exert considerable protection against influenza viral infection in vivo [155]. The oil efficiently decreased the levels of cytokines, IL-4, IL-5, IL-10, and MCP-1 in nasal lavage fluids, as well as the levels of IL-1β, IL-6, and necrosis factors TNF-α and IFN-γ in the lung tissues of mice infected with the influenza A virus [153]. It also reduced the expression of the inflammatory response, NF-kB, p65, intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1 in lung tissues [154]. The findings thus suggest that eucalyptol is capable of augmenting protection against influenza virus infection in mice by inhibiting pulmonary inflammatory responses in the tissues [154].
In another study, eucalyptol (12.5 mg/kg) demonstrated lower viral titers, less pulmonary edema, less weight loss, less inflammation, a lower mortality rate, and a longer survival time when it was co-administered with 0.2 µg of haemagglutinin influenza vaccine, compared to when the vaccine was administered alone [156]. The mechanism of action of eucalyptol has been reported to be an increase in the antiviral activity of IRF3 as well as the IκBα- and JNK-dependent inhibitory effect of IRF3 on the NF-κB p65 and NF-κB proinflammatory signaling pathways [156,157]. The presence of an epoxy functional group and the unique inter-atomic distance between the R1-C-O-C-R2 of eucalyptol have been linked to its remarkable biological effects (Figure 6) [158].
In a recent study, isocaryophyllene acetamides (ICAs) and some S-containing derivatives of caryophyllene oxide (caryophyllane thiosesquiterpenoid, CTS) were shown to inhibit the replication of rimantadine-resistant influenza virus A/California/07/09 (H1N1) pdm09 and influenza virus A/Puerto Rico/8/34 (H1N1) strains, respectively [159,160]. Due to the natural bicyclic framework of ICAs (Figure 6, they are known to show marked anti-influenza activity by blocking the M2 protein of the influenza virus and by inhibiting the cleavage of hemagglutinin [160]. This led to an aggregation of the virus and lysosomal vacuole membranes and virus inactivation [160]. Gyrdymova and others demonstrated the influence of the S-containing functional group on anti-influenza activity, showing that bisulfide-containing CTS compounds possess high virus-inhibitory activities and suggesting S-containing derivatives of caryophyllene oxide as promising substrates for the design of newer anti-influenza and/or antiviral agents [161].
Some caryophyllene derivatives (Ginsamides, GAs) were reported to demonstrate dose-dependent virus inhibition and subtype-specific virus-inhibiting activity (IC50 = 0.15 µM) against the influenza virus H1N1 and H1N1pdm09 strains in a pool of influenza virus A/Puerto Rico/8/34 (H1N1) strains in MDCK in vitro cell cultures [162]. Ginsamides showed considerable in vivo protective ability against the virus at 150 mg/kg/day and inhibited the fusogenic activity that is typical of influenza A/Puerto Rico/8/34 (H1N1) viruses [163]. According to the report, GAs can act as lead inhibitors against the viral infection of normal cells and may offer the host an opportunity to maintain a complete immune response [162,164]. Structurally, the bicyclic backbone and the amide functional group of GAs (Figure 6) are known to confer a high level of antiviral and anti-influenza activities [107,161,163].
Eugenol and citronellol are major EO compounds of Cinnamomum zeylanicum and Pelargonium graveolense, respectively [165,166]. The combined EOs demonstrated in vitro antiviral activity (MIC = 100 3.1 µL/mL) against the influenza A (H1N1) virus [167]. It acted by targeting the virus surface before and during the adsorption event in the viral lifecycle, thus making it a natural neuraminidase inhibitor [167]. Structurally, eugenol and citronellol contain phenolic hydroxyl and primary alcohol functional groups, respectively (Figure 6), which confer some biological properties, such as antioxidant, anti-inflammatory, and antiviral activities, amongst others [168,169].
A novel camphor-based anti-influenza agent, camphecene, has been reported to cause a significant decrease in the number of influenza virions fusing their envelopes with endosomal membranes [170]. This nitrogen-containing camphor derivative has been reported to possess unique chemical properties that bind it effectively to the active sites of hemagglutinin (HA), acting as an HA inhibitor, and thus causing a decrease in viral pathogenicity [170]. Based on the pharmacokinetic study, camphecene demonstrated a remarkable decrease in virus titer in the lungs and mortality at 7.5 mg/kg, following a 6 h dose regime in vivo [171]. It also demonstrated an additive effect with Tamiflu, a synthetic anti-influenza drug, which suggests it is an anti-influenza drug candidate [170,171]. Several analogs of this compound have been synthesized, and the structure-activity relationship analysis suggests that camphecene analogs should bear an oxygen atom with a short linker (C2–C4), either as a hydroxyl or ketone group, or as part of a heterocycle (Figure 6), for optimal anti-influenza activity [172].
6. Future Perspectives and Conclusions
As it has been shown in this review, there have been deliberate efforts by scientists to exploit the EOs of individual aromatic plants or groups of plants for their anti-influenza potentials, partly due to the emergence of more antigenic influenza viruses, the increased lethality of influenza disease outbreaks, the reduced effectiveness level of vaccines and drugs, and the ethnomedicinal consideration of natural products for alternative medicines. At best, Choi reported 62 plant EOs for in vitro antiviral activity against three selected influenza virus strains [128]. Vimalanathan and Hudson evaluated the in vitro anti-influenza activities of EO vapors obtained from nine aromatic plants [167], while a recent report by Wani and colleagues showed antiviral activities against the influenza A (H5N1) virus by EOs derived from bergamot, cinnamon, lavender, lemongrass, thyme, and citrus [119]. Therefore, a discussion of the various associated aromatic plants and their chemical products became necessary. The common botanical sources of EOs, their chemical classification and biogenetic routes, and the antiviral properties and molecular mechanism of action of some EO compounds were major items of discussion in our review to identify drug candidates that can be optimized to mitigate the ferocity of antigenically distant and vaccine-/drug resistant strains of the influenza viruses. Thus, this may be one of the very few reviews that extensively discusses sourcing anti-influenza agents specifically from EOs and their aromatic compounds.
It is our opinion that a multi-level approach should be put in place to resolve the serious health crisis caused by seasonal and pandemic flu outbreaks. First, there should be region-specific influenza vaccination programs in influenza virus-originating areas [98,173]. For instance, annual vaccination campaigns should be initiated about 5 months apart in Northern and Southern China, and influenza surveillance should be significantly improved in the mid-latitude provinces due to the complexity associated with seasonal patterns in these regions [98]. In addition, there is an urgent need to develop universal influenza vaccines that can offer protection against antigenically distant influenza viruses [174].
A novel antiviral approach, termed small interfering RNA (siRNA) vector technology, can be adopted to bring about a multiple-fold reduction in viral titer shed [175]. However, this method has only been validated in an in vitro assessment. The medicinal application of EOs and their lead compounds as anti-influenza agents or, simply put, for therapeutic use, has generated much interest in recent times. Currently, the U.S. FDA indicates EOs for use as cosmetic and food supplements or drugs [176]. Therefore, there is a need to validate the herbal raw materials, including ascertaining the reputation of their sources and standardizing both the extraction process and the final products (EOs), for quality assurance purposes [176,177,178].
Some EO compounds, including 1,8-cineole, eugenol, germacrone, thiol- and amide derivatives of caryophyllene oxide, curcumol, terpinen-4-ol, and bisabolane-type sesquiterpenoids, have all shown considerable potential as influenza drug candidates in this study. However, there are more comprehensive mechanistic studies as well as detailed clinical evaluations on these lead EO compounds. For instance, the exact effects of germacrone on the influenza virus life cycle need to be critically evaluated to provide a proof-of-concept for the development of novel influenza virus inhibitors [107].
In addition, there are conflicts regarding the role that individual compounds play in the overall EO antiviral activity. For instance, Eucalyptus globulus and Salvia officinalis both contain 1,8-cineole (eucalyptol) as the major component. However, the former plant oil was reported to have a strong activity (IC50 < 3.1 μg/mL) against the influenza (H1N1) virus, while the latter was poorly active [167]. Therefore, the various compounds making up an EO should be evaluated for their individual anti-influenza properties in both in vitro and in vivo settings so that the probable biological role of each compound can be determined.
A milestone achievement worthy of mention is the use of newer formulation strategies, such as nanobiotechnology, to offer a site-specific and target-oriented delivery approach to treating diseases [179]. This novel technology has also been adopted in recent times for the efficient delivery of therapeutically active EOs [180,181] and uses encapsulation strategies to develop lipid-based delivery systems, such as solid nanoparticles, nanostructured lipid carriers, liposomes, and micro- and nano-emulsions [176]. The nano formulation techniques reduce volatility and increase bioavailability while improving chemical stability and reducing toxicity, thus overcoming the limitations of high volatility, hydrophobicity, instability, and the risk of toxicity associated with the pharmaceutical application of EOs [176].
Despite the beneficial attributes offered by nanobiotechnology, the half-lives of nano-formulated bioactive EOs need to be improved while their pharmacokinetic parameters need to be optimized [181]. This can be achieved by searching for other chemical derivatives with a prolonged period of anti-influenza activity and by optimizing the application schedule, as was achieved in the synthesis of novel anti-influenza camphecene analogs from camphor [171,172].
In conclusion, essential oils are an integral part of natural products with medicinal potential for the management of illnesses such as influenza (flu) and other respiratory diseases. There is an urgent need to exploit nature for more novel anti-influenza agents, vis a viz conducting preclinical and clinical evaluations on established antiviral EO compounds, for the development of newer influenza drugs. This will require collaborative research efforts for health solutions so that good health and well-being can be attained in real-time.
Author Contributions
A.O.O. (Ayodeji Oluwabunmi Oriola) conceptualized and wrote the original manuscript draft; A.O.O. (Adebola Omowunmi Oyedeji) provided resources, supervised, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Directorate of Research Development and Innovation, Walter Sisulu University (WSU), South Africa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Directorate of Research Development and Innovation, WSU, South Africa, for funding support. Obafemi Awolowo University, Ile-Ife, Nigeria is appreciated, for the study leave permission granted to the corresponding author to utilize a Postdoctoral Research Fellowship at WSU, South Africa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruce, O.S.; Onyegbule, A.F. Biosynthesis of Natural Products. In Bioactive Compounds-Biosynthesis, Characterization and Applications; Zepka, L.Q., Nascimento, T.C.d., Jacob-Lopes, E., Eds.; IntechOpen: London, UK, 2021; 304p, Available online: https://www.intechopen.com/books/10290 (accessed on 10 October 2022).
- Ntie-Kang, F.; Svozil, D. An Enumeration of Natural Products from Microbial, Marine and Terrestrial Sources. Phys. Sci. Rev. 2020, 5, 20180121. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as Important Bioactive Constituents of Essential Oils. Essent. Oils—Bioact. Compd. New Perspect. Appl. 2020. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Butchbauer, G. Handbook of Essential Oils: Science, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2009; p. 978042914. [Google Scholar] [CrossRef]
- Medicinal Plant Research in Africa: Pharmacology and Chemistry—Ghent University Library. Available online: https://lib.ugent.be/catalog/ebk01:2550000001064753 (accessed on 6 August 2022).
- Stratakos, A.C.; Koidis, A. Methods for Extracting Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 31–38. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Horváth, G.; Ács, K. Essential Oils in the Treatment of Respiratory Tract Diseases Highlighting Their Role in Bacterial Infections and Their Anti-inflammatory Action: A Review. Flavour Fragr. J. 2015, 30, 331. [Google Scholar] [CrossRef]
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 August 2022).
- Key Facts about Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/about/keyfacts.htm (accessed on 6 August 2022).
- Types of Influenza Viruses|CDC. Available online: https://www.cdc.gov/flu/about/viruses/types.htm (accessed on 7 August 2022).
- Wu, P.; Presanis, A.M.; Bond, H.S.; Lau, E.H.Y.; Fang, V.J.; Cowling, B.J. A Joint Analysis of Influenza-Associated Hospitalizations and Mortality in Hong Kong, 1998–2013. Sci. Rep. 2017, 7, 929. [Google Scholar] [CrossRef]
- Senthur Nambi, P.; Suresh Kumar, D.; Gopalakrishnan, R. Influenza Vaccine. Indian J. Pract. Pediatr. 2022, 12, 372–377. [Google Scholar] [CrossRef]
- Selecting Viruses for the Seasonal Influenza Vaccine|CDC. Available online: https://www.cdc.gov/flu/prevent/vaccine-selection.htm (accessed on 7 August 2022).
- Ullah, I.; Khan, K.S.; Tahir, M.J.; Ahmed, A.; Harapan, H. Myths and Conspiracy Theories on Vaccines and COVID-19: Potential Effect on Global Vaccine Refusals. Vacunas 2021, 22, 93. [Google Scholar] [CrossRef]
- Influenza (Flu) Antiviral Drugs and Related Information|FDA. Available online: https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information (accessed on 7 August 2022).
- Yin, H.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, A.; Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural Sources as Potential Anti-Cancer Agents: A Review. Int. J. Phytomed. 2011, 3, 9–26. [Google Scholar]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural Products for Human Health: An Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The Influence of Natural Products upon Drug Discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Shibata, M.A.; Khan, I.A.; Iinuma, M.; Shirai, T. Natural Products for Medicine. J. Biomed. Biotechnol. 2012, 2012, 147120. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.M.; Szopa, A. Biological Activities of Natural Products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Omrani, M.; Keshavarz, M.; Nejad Ebrahimi, S.; Mehrabi, M.; McGaw, L.J.; Ali Abdalla, M.; Mehrbod, P. Potential Natural Products Against Respiratory Viruses: A Perspective to Develop Anti-COVID-19 Medicines. Front. Pharmacol. 2021, 11, 2115. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products: Synthesis, Function, and Application; Springer: New York, NY, USA, 2009; pp. 3–50. [Google Scholar] [CrossRef]
- Jabeen, S.; Hanif, M.A.; Khan, M.M.; Waseem, R.; Qadri, K. Natural Products Sources and Their Active Compounds on Disease Prevention: A Review. IJCBS 2014, 6, 76–83. [Google Scholar]
- Ríos, J.L. Essential Oils: What They Are and How the Terms Are Used and Defined. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Sonwa, M.M. Isolation and Structure Elucidation of Essential Oil Constituents : Comparative Study of the Oils of Cyperus Alopecuroides, Cyperus Papyrus, and Cyperus Rotundus. Ph.D. Thesis, Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky, Hamburg, Germany, 2000. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C. Trease and Evans’ Pharmacognosy: Sixteenth Edition; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009; pp. 1–603. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. A Review of the Composition of the Essential Oils and Biological Activities of Angelica Species. Sci. Pharm. 2017, 85, 33. [Google Scholar] [CrossRef]
- Marzouki, H.; Khaldi, A.; Falconieri, D.; Piras, A.; Marongiu, B.; Molicotti, P.; Zanetti, S. Essential Oils of Daucus carota Subsp. Carota of Tunisia Obtained by Supercritical Carbon Dioxide Extraction. NPC Nat. Prod. Commun. 2010, 5, 1955–1958. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and Antimicrobial Activity of the Essential Oil and Methanol Extracts of Achillea millefolium Subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Orav, A.; Arak, E.; Raal, A. Phytochemical Analysis of the Essential Oil of Achillea millefolium L. from Various European Countries. Nat. Prod. Res. 2006, 20, 1082–1088. [Google Scholar] [CrossRef]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An Experimental and Theoretical Study on Essential Oil of Aethionema sancakense: Characterization, Molecular Properties and RDG Analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef]
- Morales-López, J.; Centeno-Álvarez, M.; Nieto-Camacho, A.; López, M.G.; Pérez-Hernández, E.; Pérez-Hernández, N.; Fernández-Martínez, E. Evaluation of Antioxidant and Hepatoprotective Effects of White Cabbage Essential Oil. Pharm. Biol. 2016, 55, 233–241. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Al-Saidi, S. Phytochemical Analysis of the Essential Oil from Botanically Certified Oleogum Resin of Boswellia sacra (Omani Luban). Molecules 2008, 13, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical Composition of the Essential Oil and Supercritical CO2 Extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef] [PubMed]
- Ljaljević Grbić, M.; Unković, N.; Dimkić, I.; Janaćković, P.; Gavrilović, M.; Stanojević, O.; Stupar, M.; Vujisić, L.; Jelikić, A.; Stanković, S.; et al. Frankincense and Myrrh Essential Oils and Burn Incense Fume against Micro-Inhabitants of Sacral Ambients. Wisdom of the Ancients? J. Ethnopharmacol. 2018, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.A.; Adam, M.E.; Hassan, S.M.; Albalawi, A.R. Chemical Composition, Antimicrobial and Antibiofilm Activity of the Essential Oil and Methanol Extract of the Mediterranean Cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L. The Anxiolytic Effect of Juniperus virginiana L. Essential Oil and Determination of Its Active Constituents. Physiol. Behav. 2018, 189, 50–58. [Google Scholar] [CrossRef]
- Sakai, A.; Yoshimura, H. Monoterpenes of Salvia leucophylla. Curr. Bioact. Compd. 2012, 8, 90–100. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical Composition of the Essential Oil from Basil (Ocimum basilicum Linn.) and Its in Vitro Cytotoxicity against HeLa and HEp-2 Human Cancer Cell Lines and NIH 3T3 Mouse Embryonic Fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Med. 2003, 69, 413–419. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; de Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.C. Chemical Composition and Antifungal Activity of Rosemary (Rosmarinus officinalis L.) Oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Ben Jabeur, M.; Somai-Jemmali, L.; Hamada, W. Thyme Essential Oil as an Alternative Mechanism: Biofungicide-Causing Sensitivity of Mycosphaerella graminicola. J. Appl. Microbiol. 2017, 122, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical Composition of Essential Oils from Cinnamomum camphora and Their Insecticidal Activity against the Stored Product Pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid. Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; de Martino, L.; Fratianni, F.; Coppola, R.; de Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Bel Haj Salah, K.; Aouni, M.; Khouja, M.L.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules 2012, 17, 3044–3057. [Google Scholar] [CrossRef] [PubMed]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical Composition and Antibacterial Activities of Seven Eucalyptus species Essential Oils Leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical Composition of Myrtle (Myrtus communis L.) Berries Essential Oils as Observed in a Collection of Genotypes. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Tea Tree Oil: Contact Allergy and Chemical Composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef]
- Stevanovic, F.; Francezon, N.; Stevanovic, T. Spruce Bark Oil. Bioresources 2017, 12, 2635–2645. [Google Scholar]
- Samadi, N.; Manayi, A.; Vazirian, M.; Samadi, M.; Zeinalzadeh, Z.; Saghari, Z.; Abadian, N.; Mozaffarian, V.O.A.; Khanavi, M. Chemical Composition and Antimicrobial Activity of the Essential Oil of Anthemis altissima L. Var. altissima. Nat. Prod. Res. 2012, 26, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, T.; Kafil, H.S.; Asnaashari, S.; Farajnia, S.; Delazar, A.; Baek, S.C.; Hamishehkar, H.; Kim, K.H. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Pinus eldarica Grown in Northwestern Iran. Molecules 2019, 24, 3203. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon Grass (Cymbopogon citratus) Essential Oil as a Potent Anti-Inflammatory and Antifungal Drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.S.; Luthra, R. Biotransformation of Geranyl Acetate to Geraniol during Palmarosa (Cymbopogon martinii, Roxb. Wats. Var. Motia) Inflorescence Development. Phytochemistry 2001, 57, 675–680. [Google Scholar] [CrossRef]
- de Toledo, L.G.; dos Santos Ramos, M.A.; Spósito, L.; Castilho, E.M.; Pavan, F.R.; de Oliveira Lopes, É.; Zocolo, G.J.; Silva, F.A.N.; Soares, T.H.; dos Santos, A.G.; et al. Essential Oil of Cymbopogon nardus (L.) Rendle: A Strategy to Combat Fungal Infections Caused by Candida species. Int. J. Mol. Sci. 2016, 17, 1252. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Miya, G.; Nyalambisa, M.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Profiling, Toxicity and Anti-Inflammatory Activities of Essential Oils from Three Grapefruit Cultivars from KwaZulu-Natal in South Africa. Molecules 2021, 26, 3387. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, N.; Chen, H.; Zhong, B.; Yang, A.; Kuang, F.; Ouyang, Z.; Chun, J. Fumigant Activity of Sweet Orange Essential Oil Fractions Against Red Imported Fire Ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2017, 110, 1556–1562. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An Evaluation of Antioxidant, Anti-Inflammatory, and Antinociceptive Activities of Essential Oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.D.; Saptami, K.; Caputo, L.; de Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E.; et al. Chemical and Biological Evaluation of Essential Oils from Cardamom species. Molecules 2018, 23, 2818. [Google Scholar] [CrossRef]
- Tarfaoui, K.; Brhadda, N.; Ziri, R.; Oubihi, A.; Imtara, H.; Haida, S.; al kamaly, O.M.; Saleh, A.; Parvez, M.K.; Fettach, S.; et al. Chemical Profile, Antibacterial and Antioxidant Potential of Zingiber officinale Roscoe and Elettaria cardamomum (L.) Maton Essential Oils and Extracts. Plants 2022, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber officinale Roscoe. Biomed. Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Food Reviews International Biosynthesis of Essential Oils in Aromatic Plants: A Review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New Insights into Plant Isoprenoid Metabolism. Mol. Plant 2012, 5, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Masango, P. Cleaner Production of Essential Oils by Steam Distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Arroo, R. Eberhard Breitmaier. Terpenes—Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH, 2006, 214 Pp ISBN 3-527-31786-4. Appl. Organomet. Chem. 2007, 21, 377. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.H.; Schmidt, A.; Wang, G.D.; Sun, G.L.; Grant, M.; Kuang, C.; Yang, M.J.; Jing, S.X.; Li, C.H.; et al. A Geranylfarnesyl Diphosphate Synthase Provides the Precursor for Sesterterpenoid (C25) Formation in the Glandular Trichomes of the Mint Species Leucosceptrum canum. Plant Cell 2016, 28, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.M.; Behzad, S.; Asgari, S.; Khankandi, H.P.; Farzaei, M.H. Sesterterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–391. [Google Scholar] [CrossRef]
- Islam, M.T.; da Mata, A.M.O.F.; de Aguiar, R.P.S.; Paz, M.F.C.J.; de Alencar, M.V.O.B.; Ferreira, P.M.P.; de Carvalho Melo-Cavalcante, A.A. Therapeutic Potential of Essential Oils Focusing on Diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond. Adv. Biochem. Eng. Biotechnol. 2015, 148, 355–389. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography 2015, 2, 410–421. [Google Scholar] [CrossRef]
- Sarup, P.; Bala, S.; Kamboj, S. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu). Scientifica 2015, 2015, 138039. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceição, E.C.; et al. Anti-Inflammatory Activity of Nanoemulsions of Essential Oil from Rosmarinus officinalis L.: In Vitro and in Zebrafish Studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Oriola, A.O.; Oyedeji, A.O. Plant-Derived Natural Products as Lead Agents against Common Respiratory Diseases. Molecules 2022, 27, 3054. [Google Scholar] [CrossRef]
- Liao, B.; Hu, H.; Xiao, S.; Zhou, G.; Sun, W.; Chu, Y.; Meng, X.; Wei, J.; Zhang, H.; Xu, J.; et al. Global Pharmacopoeia Genome Database Is an Integrated and Mineable Genomic Database for Traditional Medicines Derived from Eight International Pharmacopoeias. Sci. China Life Sci. 2022, 65, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef]
- Boktor, S.W.; Hafner, J.W. Influenza. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Glezen, W.P. Emerging Infections: Pandemic Influenza. Epidemiol. Rev. 1996, 18, 64–76. [Google Scholar] [CrossRef]
- Yang, L.; Chan, K.P.; Wong, C.M.; Chiu, S.S.S.; Magalhaes, R.J.S.; Thach, T.Q.; Peiris, J.S.M.; Clements, A.C.A.; Hu, W. Comparison of Influenza Disease Burden in Older Populations of Hong Kong and Brisbane: The Impact of Influenza and Pneumococcal Vaccination. BMC Infect. Dis. 2019, 19, 162. [Google Scholar] [CrossRef]
- Hagey, R.J.; Elazar, M.; Pham, E.A.; Tian, S.; Ben-Avi, L.; Bernardin-Souibgui, C.; Yee, M.F.; Moreira, F.R.; Rabinovitch, M.V.; Meganck, R.M.; et al. Programmable Antivirals to Target Conserved Essential Shapes in Pandemic Viral Genomes. Nat. Med. 2022, 28, 1944–1955. [Google Scholar] [CrossRef]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer, W.A. The Hidden Burden of Influenza: A Review of the Extra-Pulmonary Complications of Influenza Infection. Influenza Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Alonso, W.J.; Feng, L.; Tan, Y.; Shu, Y.; Yang, W.; Viboud, C. Characterization of Regional Influenza Seasonality Patterns in China and Implications for Vaccination Strategies: Spatio-Temporal Modeling of Surveillance Data. PLoS Med. 2013, 10, e1001552. [Google Scholar] [CrossRef] [PubMed]
- Palese, P.; Tumpey, T.M.; Garcia-Sastre, A. What Can We Learn from Reconstructing the Extinct 1918 Pandemic Influenza Virus? Immunity 2006, 24, 121–124. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Global Influenza Programme. Available online: https://www.who.int/teams/global-influenza-programme/vaccines (accessed on 13 September 2022).
- Young, K.; Gemmill, I.; Harrison, R. Summary of the NACI Seasonal Influenza Vaccine Statement for 2020–2021. Can. Commun. Dis. Rep. 2020, 46, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Seasonal Flu Vaccines|CDC. Available online: https://www.cdc.gov/flu/prevent/flushot.htm (accessed on 13 September 2022).
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing Seasonal Influenza—The Need for a Universal Influenza Vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Komori, K.; Suzuki, M.; Morimoto, K.; Kishikawa, T.; Yasaka, T.; Ariyoshi, K. Negative Impact of Prior Influenza Vaccination on Current Influenza Vaccination among People Infected and Not Infected in Prior Season: A Test-Negative Case-Control Study in Japan. Vaccine 2017, 35, 687–693. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Denis, M.; Plotkin, S. Seasonal Influenza Vaccine Efficacy and Its Determinants in Children and Non-Elderly Adults: A Systematic Review with Meta-Analyses of Controlled Trials. Vaccine 2012, 31, 49–57. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.L.; Bragazzi, N.L.; Panatto, D. Compounds with Anti-Influenza Activity: Present and Future of Strategies for the Optimal Treatment and Management of Influenza Part II: Future Compounds against Influenza Virus. J. Prev. Med. Hyg. 2014, 55, 109. [Google Scholar]
- Świerczyńska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef] [PubMed]
- Doll, M.K.; Winters, N.; Boikos, C.; Kraicer-Melamed, H.; Gore, G.; Quach, C. Safety and Effectiveness of Neuraminidase Inhibitors for Influenza Treatment, Prophylaxis, and Outbreak Control: A Systematic Review of Systematic Reviews and/or Meta-Analyses. J. Antimicrob. Chemother. 2017, 72, 2990–3007. [Google Scholar] [CrossRef]
- Hayden, F.G.; Shindo, N. Influenza Virus Polymerase Inhibitors in Clinical Development. Curr. Opin. Infect. Dis. 2019, 32, 176. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; al Zarzour, R. COVID-19 and Therapy with Essential Oils Having Antiviral, Anti-Inflammatory, and Immunomodulatory Properties. Inflammopharmacology 2020, 28, 1153. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.F.; Wang, W.; Dai, X.Y.; Wang, Z.Y.; Shen, Z.H.; Ying, H.Z.; Yu, C.H. Chemical Compositions and Anti-Influenza Activities of Essential Oils from Mosla dianthera. J. Ethnopharmacol. 2012, 139, 668–671. [Google Scholar] [CrossRef]
- Zai-Chang, Y.; Bo-Chu, W.; Xiao-Sheng, Y.; Qiang, W. Chemical Composition of the Volatile Oil from Cynanchum stauntonii and Its Activities of Anti-Influenza Virus. Colloids Surf. B Biointerfaces 2005, 43, 198–202. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (Tea Tree) Oil on Influenza Virus A/PR/8: Study on the Mechanism of Action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.H.; Eun, J.S.; Shin, T.Y. Mosla dianthera Inhibits Mast Cell-Mediated Allergic Reactions through the Inhibition of Histamine Release and Inflammatory Cytokine Production. Toxicol. Appl. Pharmacol. 2006, 216, 479–484. [Google Scholar] [CrossRef]
- Madia, V.N.; Toscanelli, W.; de Vita, D.; de Angelis, M.; Messore, A.; Ialongo, D.; Scipione, L.; Tudino, V.; D’auria, F.D.; di Santo, R.; et al. Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils. Molecules 2022, 27, 3718. [Google Scholar] [CrossRef]
- Selvarani, V.; James, H. The Activity of Cedar Leaf Oil Vapor against Respiratory Viruses: Practical Applications. J. Appl. Pharm. Sci. 2013, 3, 11–15. [Google Scholar] [CrossRef]
- Hayashi, K.; Imanishi, N.; Kashiwayama, Y.; Kawano, A.; Terasawa, K.; Shimada, Y.; Ochiai, H. Inhibitory Effect of Cinnamaldehyde, Derived from Cinnamomi Cortex, on the Growth of Influenza A/PR/8 Virus in Vitro and in Vivo. Antivir. Res. 2007, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An Updated and Comprehensive Review of the Antiviral Potential of Essential Oils and Their Chemical Constituents with Special Focus on Their Mechanism of Action against Various Influenza and Coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.M.; Al-Mahdy, D.A.; Abd El Aziz, O.M.; Kandil, A.M.; Tantawy, M.A.; el Alfy, T.S.M. Chemical Composition and Antiviral Activity of Essential Oils from Citrus reshni Hort. Ex Tanaka (Cleopatra Mandarin) Cultivated in Egypt. J. Essent. Oil-Bear. Plants 2018, 21, 264–272. [Google Scholar] [CrossRef]
- Liao, Q.; Qian, Z.; Liu, R.; An, L.; Chen, X. Germacrone Inhibits Early Stages of Influenza Virus Infection. Antivir. Res. 2013, 100, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; de Filippis, A.; Fiorito, F.; de Martino, L.; Fratini, F. Antimicrobial Activity of Some Essential Oils against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Pseudintermedius-Associated Pyoderma in Dogs. Animals 2020, 10, 1782. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Lai, Y.; Liao, S.; Liu, N.; Xu, P. Intranasal Co-Administration of 1,8-Cineole with Influenza Vaccine Provide Cross-Protection against Influenza Virus Infection. Phytomedicine 2017, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Hawary, S.S.; Mohammed, M.M.D.; Farid, M.A.; Abdel-Wahed, N.A.M.; Ali, M.A.; El-Abd, E.A.W. Chemical Composition, Antiviral against Avian Influenza (H5N1) Virus and Antimicrobial Activities of the Essential Oils of the Leaves and Fruits of Fortunella margarita, Lour. Swingle, Growing in Egypt. J. Appl. Pharm. Sci. 2015, 5, 6–12. [Google Scholar] [CrossRef][Green Version]
- Garozzo, A.; Timpanaro, R.; Bisignano, B.; Furneri, P.M.; Bisignano, G.; Castro, A. In Vitro Antiviral Activity of Melaleuca alternifolia Essential Oil. Lett. Appl. Microbiol. 2009, 49, 806–808. [Google Scholar] [CrossRef]
- Shabby, A.S.; El-Gengaihi, S.; Khattab, M. Oil of Melissa officinalis L., as Affected by Storage and Herb Drying. J. Essent. Oil Res. 1995, 7, 667–669. [Google Scholar] [CrossRef]
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral Activity of the Oseltamivir and Melissa officinalis L. Essential Oil against Avian Influenza A Virus (H9N2). VirusDisease 2016, 27, 170–178. [Google Scholar] [CrossRef]
- Choi, H.J. Chemical Constituents of Essential Oils Possessing Anti-Influenza A/WS/33 Virus Activity. Osong Public Health Res. Perspect. 2018, 9, 348. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Peng, S.Z.; Chen, H.M.; Zhang, F.X.; Xu, P.P.; Xie, J.H.; He, J.J.; Chen, J.N.; Lai, X.P.; Su, Z.R. Oral Administration of Patchouli Alcohol Isolated from Pogostemonis herba Augments Protection against Influenza Viral Infection in Mice. Int. Immunopharmacol. 2012, 12, 294–301. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Lu, Y.; Ling, L.; Peng, N.; Zhong, Y. Essential Oil Composition and Bioactivities of Waldheimia glabra (Asteraceae) from Qinghai-Tibet Plateau. Molecules 2017, 22, 460. [Google Scholar] [CrossRef]
- Scherließ, R.; Ajmera, A.; Dennis, M.; Carroll, M.W.; Altrichter, J.; Silman, N.J.; Scholz, M.; Kemter, K.; Marriott, A.C. Induction of Protective Immunity against H1N1 Influenza A(H1N1)Pdm09 with Spray-Dried and Electron-Beam Sterilised Vaccines in Non-Human Primates. Vaccine 2014, 32, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Torri, A.; Harte, W.E.; Danetz, S.; Cianci, C.; Tiley, L.; Day, S.; Mullaney, D.; Yu, K.L.; Ouellet, C.; et al. Molecular Mechanism Underlying the Action of a Novel Fusion Inhibitor of Influenza A Virus. J. Virol. 1997, 71, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, J.; Kakui, M.; Iwasaki, H.; Fujiwara, T.; Sugimoto, H.; Hattori, N. Identification of a Novel HA Conformational Change Inhibitor of Human Influenza Virus. Arch. Virol. 1999, 144, 865–878. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Ma, C.; Fiorin, G.; Wang, J.; Pinto, L.H.; Lamb, R.A.; Klein, M.L.; DeGrado, W.F. Structure and Inhibition of the Drug-Resistant S31N Mutant of the M2 Ion Channel of Influenza A Virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1315–1320. [Google Scholar] [CrossRef]
- Bahgat, M.M.; Bazejewska, P.; Schughart, K. Inhibition of Lung Serine Proteases in Mice: A Potentially New Approach to Control Influenza Infection. Virol. J. 2011, 8, 27. [Google Scholar] [CrossRef]
- Loregian, A.; Mercorelli, B.; Nannetti, G.; Compagnin, C.; Palù, G. Antiviral Strategies against Influenza Virus: Towards New Therapeutic Approaches. Cell. Mol. Life Sci. 2014, 71, 3659–3683. [Google Scholar] [CrossRef]
- Planz, O. Development of Cellular Signaling Pathway Inhibitors as New Antivirals against Influenza. Antivir. Res. 2013, 98, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Zhu, X.; He, Z.; Liu, S.; Li, M.; Pang, J.; Lin, Y. Studies on Synthesis and Structure-Activity Relationship (SAR) of Derivatives of a New Natural Product from Marine Fungi as Inhibitors of Influenza Virus Neuraminidase. Mar. Drugs 2011, 9, 1887–1901. [Google Scholar] [CrossRef]
- Feldman, T.; Kabaleeswaran, V.; Jang, S.B.; Antczak, C.; Djaballah, H.; Wu, H.; Jiang, X. A Class of Allosteric Caspase Inhibitors Identified by High-Throughput Screening. Mol. Cell 2012, 47, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Muniruzzaman, S.; Pan, Y.T.; Zeng, Y.; Atkins, B.; Izumori, K.; Elbein, A.D. Inhibition of Glycoprotein Processing by L-Fructose and L-Xylulose. Glycobiology 1996, 6, 795–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oguin, T.H.; Sharma, S.; Stuart, A.D.; Duan, S.; Scott, S.A.; Jones, C.K.; Daniels, J.S.; Lindsley, C.W.; Thomas, P.G.; Brown, H.A. Phospholipase D Facilitates Efficient Entry of Influenza Virus, Allowing Escape from Innate Immune Inhibition. J. Biol. Chem. 2014, 289, 25405–25417. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Lo, C.W.; Liu, C.H.; Lin, S.; Yen, H.R.; Lin, T.Y.; Horng, J.T. Mechanism by Which Ma-Xing-Shi-Gan-Tang Inhibits the Entry of Influenza Virus. J. Ethnopharmacol. 2012, 143, 57–67. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Yen, H.R.; Liu, C.H.; Lin, S.; Horng, J.T. Ching-Fang-Pai-Tu-San Inhibits the Release of Influenza Virus. J. Ethnopharmacol. 2012, 144, 533–544. [Google Scholar] [CrossRef]
- Dai, J.; Wang, G.; Li, W.; Zhang, L.; Yang, J.; Zhao, X.; Chen, X.; Xu, Y.; Li, K. High-Throughput Screening for Anti-Influenza A Virus Drugs and Study of the Mechanism of Procyanidin on Influenza A Virus-Induced Autophagy. J. Biomol. Screen. 2012, 17, 605–617. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Li, L.; Xie, Q.; Bian, G.; Zhang, B.; Wang, M.; Wang, Y.; Chen, Z.; Li, Y. Anti-H1N1 Viral Activity of Three Main Active Ingredients from Zedoary Oil. Fitoterapia 2020, 142, 104489. [Google Scholar] [CrossRef]
- Xia, Z.; Xu, G.; Yang, X.; Peng, N.; Zuo, Q.; Zhu, S.; Hao, H.; Liu, S.; Zhu, Y. Inducible TAP1 Negatively Regulates the Antiviral Innate Immune Response by Targeting the TAK1 Complex. J. Immunol. 2017, 198, 3690–3704. [Google Scholar] [CrossRef] [PubMed]
- Galisteo Pretel, A.; Pérez Del Pulgar, H.; Guerrero de León, E.; López-Pérez, J.L.; Olmeda, A.S.; Gonzalez-Coloma, A.; Barrero, A.F.; Quílez Del Moral, J.F. Germacrone Derivatives as New Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship. Molecules 2019, 24, 2898. [Google Scholar] [CrossRef] [PubMed]
- Ti, H.; Mai, Z.; Wang, Z.; Zhang, W.; Xiao, M.; Yang, Z.; Shaw, P. Bisabolane-Type Sesquiterpenoids from Curcuma longa L. Exert Anti-Influenza and Anti-Inflammatory Activities through NF-ΚB/MAPK and RIG-1/STAT1/2 Signaling Pathways. Food Funct. 2021, 12, 6697–6711. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Theiss, A.L.; Wang, X.; Goel, A. Essential Turmeric Oils Enhance Anti-Inflammatory Efficacy of Curcumin in Dextran Sulfate Sodium-Induced Colitis. Sci. Rep. 2017, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.; Martini, M.v.; de Oliveira, C.M.A.; Cunha, S.; de Carvalho, J.E.; Ruiz, A.L.T.G.; da Silva, C.C. Antitumor Activity of (-)-α-Bisabolol-Based Thiosemicarbazones against Human Tumor Cell Lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical Composition and Antimicrobial Activity of Essential Oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1,8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef]
- Juergens, U.R.; Stöber, M.; Vetter, H. Inhibition of Cytokine Production and Arachidonic Acid Metabolism by Eucalyptol (1.8-Cineole) in Human Blood Monocytes in Vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef]
- Müller, J.; Greiner, J.F.W.; Zeuner, M.; Brotzmann, V.; Schäfermann, J.; Wieters, F.; Widera, D.; Sudhoff, H.; Kaltschmidt, B.; Kaltschmidt, C. 1,8-Cineole Potentiates IRF3-Mediated Antiviral Response in Human Stem Cells and in an Ex Vivo Model of Rhinosinusitis. Clin. Sci. 2016, 130, 1339–1352. [Google Scholar] [CrossRef]
- Villecco, M.B.; Catalán, J.v; Vega, M.I.; Garibotto, F.M.; Enriz, R.D.; Catalán, C.A.N. Synthesis and Antibacterial Activity of Highly Oxygenated 1,8-Cineole Derivatives. Nat. Prod. Commun. 2008, 3, 1934578X0800300301. [Google Scholar] [CrossRef]
- Yarovaya, O.I.; Stro, A.A.; Orshanskaya, Y.R.; Zarubaev, V.V.; Khazanov, V.A.; Salakhutdinov, N.F. (1S,3aR,4R,7aS)-N-(2,2,4,7a-Tetramethyloctahydro-1,4-ethanoindene-3a-yl)-acetamide Application as a Influenza Virus Reproduction Inhibitor. RU2616255C1, 13 April 2017. [Google Scholar]
- Gyrdymova, Y.v.; Sinegubova, E.O.; Muryleva, A.S.; Zarubaev, V.v.; Rubtsova, S.A. Anti-Influenza Activity of Several Caryophyllane Hiosesquiterpenoids. Chem. Nat. Compd. 2019, 55, 1179–1181. [Google Scholar] [CrossRef]
- Scholtissek, C.; Quack, G.; Klenk, H.D.; Webster, R.G. How to Overcome Resistance of Influenza A Viruses against Adamantane Derivatives. Antivir. Res. 1998, 37, 83–95. [Google Scholar] [CrossRef]
- Volobueva, A.S.; Yarovaya, O.I.; Kireeva, M.v.; Borisevich, S.S.; Kovaleva, K.S.; Mainagashev, I.Y.; Gatilov, Y.v.; Ilyina, M.G.; Zarubaev, V.v.; Salakhutdinov, N.F. Discovery of New Ginsenol-Like Compounds with High Antiviral Activity. Molecules 2021, 26, 6794. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Pavia, A.T. Antiviral Management of Seasonal and Pandemic Influenza. J. Infect. Dis. 2006, 194, S119–S126. [Google Scholar] [CrossRef]
- Plotch, S.J.; O’Hara, B.; Morin, J.; Palant, O.; LaRocque, J.; Bloom, J.D.; Lang, S.A.; DiGrandi, M.J.; Bradley, M.; Nilakantan, R.; et al. Inhibition of Influenza A Virus Replication by Compounds Interfering with the Fusogenic Function of the Viral Hemagglutinin. J. Virol. 1999, 73, 140–151. [Google Scholar] [CrossRef]
- Rana, V.S.; Juyal, J.P.; Blazquez, M.A. Chemical Constituents of Essential Oil of Pelargonium graveolens Leaves. Int. J. Aromather. 2002, 4, 216–218. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wimalasena, S.; Jayatilake, G.S.; Jayawardena, A.L.; Senanayake, U.M.; Mubarak, A.M. A Comparison of Essential Oil Constituents of Bark, Leaf, Root and Fruit of Cinnamon (Cinnamomum zeylanicum Blum) Grown in Sri Lanka. J. Natl. Sci. Found. 2001, 29, 147–153. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-Influenza Virus Activity of Essential Oils and Vapors. Am. J. Essent. Oils Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative Structure-Activity Relationship of Molecules Constituent of Different Essential Oils with Antimycobacterial Activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 15, 332. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure-Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Zarubaev, V.v.; Pushkina, E.A.; Borisevich, S.S.; Galochkina, A.v.; Garshinina, A.v.; Shtro, A.A.; Egorova, A.A.; Sokolova, A.S.; Khursan, S.L.; Yarovaya, O.I.; et al. Selection of Influenza Virus Resistant to the Novel Camphor-Based Antiviral Camphecene Results in Loss of Pathogenicity. Virology 2018, 524, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zarubaev, V.v.; Garshinina, A.v.; Volobueva, A.S.; Slita, A.v.; Yarovaya, O.I.; Bykov, V.v.; Leonov, K.A.; Motov, V.S.; Khazanov, V.A.; Salakhutdinov, N.F. Optimization of Application Schedule of Camphecene, a Novel Anti-Influenza Compound, Based on Its Pharmacokinetic Characteristics. Fundam Clin. Pharmacol. 2022, 36, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Artyushin, O.I.; Sharova, E.v.; Vinogradova, N.M.; Genkina, G.K.; Moiseeva, A.A.; Klemenkova, Z.S.; Orshanskaya, I.R.; Shtro, A.A.; Kadyrova, R.A.; Zarubaev, V.v.; et al. Synthesis of Camphecene Derivatives Using Click Chemistry Methodology and Study of Their Antiviral Activity. Bioorg. Med. Chem. Lett. 2017, 27, 2181–2184. [Google Scholar] [CrossRef]
- Wang, X.; Kulkarni, D.; Dozier, M.; Hartnup, K.; Paget, J.; Campbell, H.; Nair, H. Influenza Vaccination Strategies for 2020–21 in the Context of COVID-19. J. Glob. Health 2020, 10, 021102. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Tsvetnitsky, V.; Donnelly, J.J. Universal Influenza Vaccines: Shifting to Better Vaccines. Vaccine 2016, 34, 2926. [Google Scholar] [CrossRef]
- Linke, L.M.; Wilusz, J.; Pabilonia, K.L.; Fruehauf, J.; Magnuson, R.; Olea-Popelka, F.; Triantis, J.; Landolt, G.; Salman, M. Inhibiting Avian Influenza Virus Shedding Using a Novel RNAi Antiviral Vector Technology: Proof of Concept in an Avian Cell Model. AMB Express 2016, 6, 16. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Sievees, A.F. MPlKB Methods of Extracting Volatile Oils from Plant Material and the Production of Such Oils in the United States; US Department of Agriculture: Washington, DC, USA, 1928. [Google Scholar]
- Sachan, A.K.; Sachan, N.K.; Kumar, S.; Sachan, A.; Gangwar, S.S. Evaluation and Standardization of Essential Oils for Development of Alternative Dosage Forms. Eur. J. Sci. Res. 2010, 46, 194–203. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Campolo, O.; Cherif, A.; Ricupero, M.; Siscaro, G.; Grissa-Lebdi, K.; Russo, A.; Cucci, L.M.; di Pietro, P.; Satriano, C.; Desneux, N.; et al. Citrus Peel Essential Oil Nanoformulations to Control the Tomato Borer, Tuta absoluta: Chemical Properties and Biological Activity. Sci. Rep. 2017, 7, 13036. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid.-Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).