Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application
Abstract
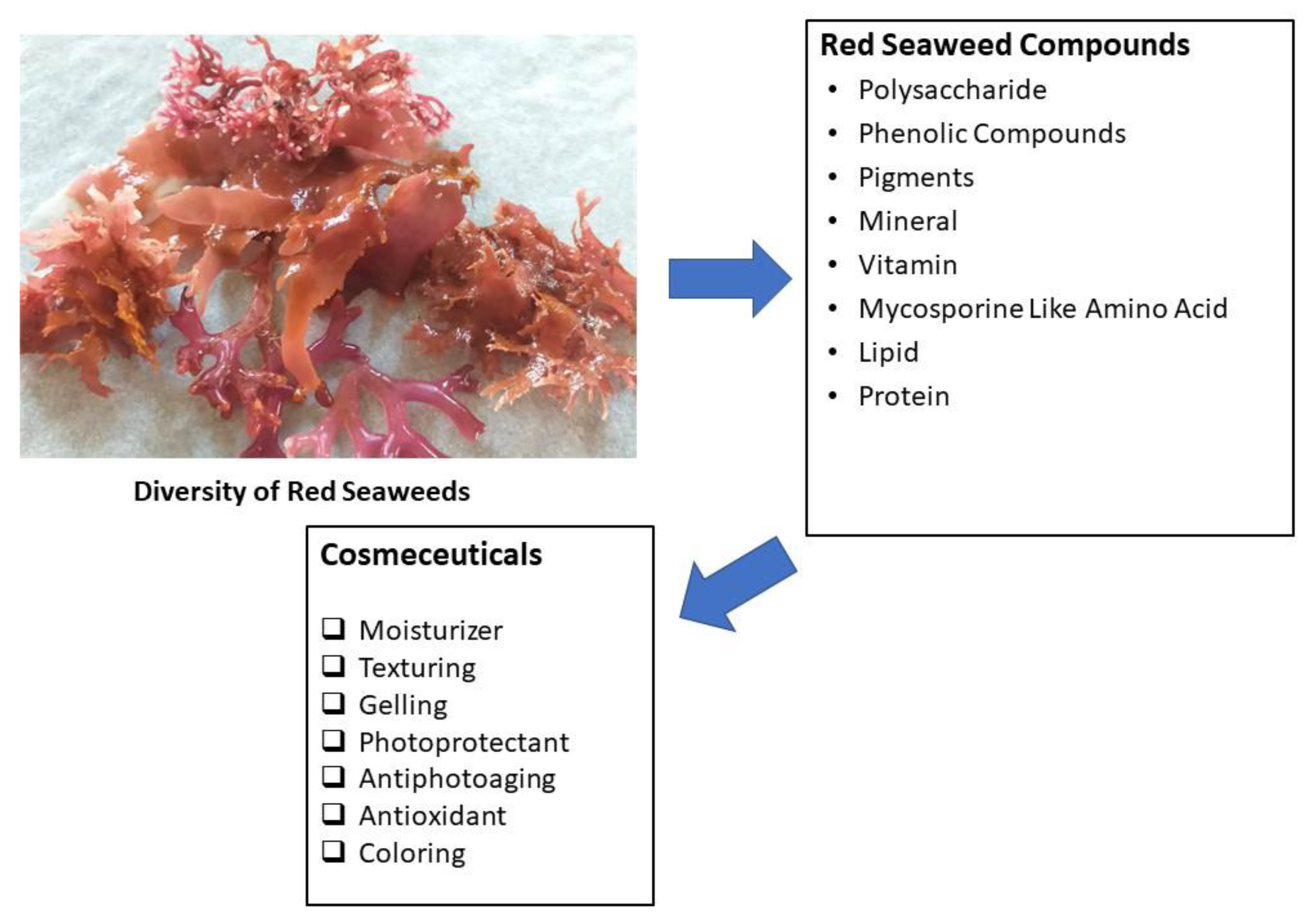
1. Introduction
2. Red Seaweeds (Rhodophyta)
3. Photoprotectant and Antiphotoaging Properties
3.1. Photoprotectant
3.2. Antiphotoaging
4. Hydrogel
4.1. Agar
4.2. Carrageenan
4.3. Porphyran
4.4. Galactan Sulfate
| Species | Polysaccharides | Cosmetic Benefits | References |
|---|---|---|---|
| Neopyropia yezoensis | Porphyran | Anti-inflammatory, antioxidant, antiaging | [149] |
| Halymenia durvillei | Sulfated polysaccharides | Antiaging and antiwrinkle | [110] |
| Gracilaria corticata | Sulfated polysaccharides | Antioxidant | [115] |
| Porphyrayezoensis | Porphyran | Anti-inflammatory | [146] |
| Eucheuma denticulatum | Carrageenan | Antioxidant, photoprotection | [143] |
| Acanthophora muscoides | Sulfated polysaccharides Carrageenan | Anticoagulant, antinociceptive, anti-inflammatory, gel agents | [150] |
| Chondrus crispus | Carrageenan | Gel and thickening agent, skin moisturizer | [151] |
| Gracilaria chouae, G. blodgetti | Agar | Antioxidant, thickeners antitumor, radiation protector, antiaging | [137] |
| Acanthophora muscoides | Carrageenan | Anticoagulant, anti-inflammatory, gelling agents | [32] |
| Pyropia yezoensis | Porphyran | Antioxidant and anti-inflammatory | [32] |
| Gigartina skottsbergii | Galactan sulfate | Antioxidant | [152] |
5. Prospects for Indonesia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. Available online: https://www.fda.gov/cosmetics (accessed on 1 September 2022).
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- GlobeNewswire. Available online: https://www.globenewswire.com/news-release/2021/10/05/2308851/0/en/Cosmetics-Market-Size-to-Worth-Around-US-480-4-Bn-by-2030.html (accessed on 1 September 2022).
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/skin-care-products-market (accessed on 5 October 2022).
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.; Simal-Gandara, J. Metabolites from macroalgae and its applications in the cosmetic industry: A circular economy approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Demont, A. The science behind the ingredients. Cosmet. Lab. 2020, 1–16. [Google Scholar]
- Thompson, C.C.; Kruger, R.H.; Thompson, F.L. Unlocking marine biotechnology in the developing world. Trends Biotechnol. 2017, 35, 1119–1121. [Google Scholar] [CrossRef]
- Pallela, R.; Na-Young, Y.; Kim, S. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An eco-sustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Chan, C.-X.; Ho, C.-L.; Phang, S.-M. Trends in seaweed research. Trends. Plant Sci. 2006, 11, 165–166. [Google Scholar] [CrossRef]
- Sathianeson, S.; Siddik, A.A.; Baakdah, M.; Al-Sofyani, A.A. An introduction to the ecological significance of seaweeds on coastal ecosystems. In Biotechnological Applications of Seaweeds; Nabti, E., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 1–14. [Google Scholar]
- Sudatti, D.B.; Duarte, H.M.; Soares, A.R.; Salgado, L.T.; Pereira, R.C. New ecological role of seaweed secondary metabolites as auto toxic and allelopathic. Front. Plant Sci. 2020, 25, 347. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharmacogn. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of seaweed. In Seaweed in Health and Disease Prevention; Fleurence, F., Levine, I., Eds.; Elsevier: Amsterdam, Netherlands, 2016; pp. 41–106. [Google Scholar]
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in mythology, folklore, poetry, and life. J. Appl. Phycol. 2020, 32, 3157–3182. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Sondak, C.F.; Beardall, J. The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 2017, 52, 495–505. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweed of the World; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–22. [Google Scholar]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetic. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Druehl, L.D.; Clarkston, B.E. Pacific Seaweeds: A Guide to Common Seaweeds of the West Coast; Harbour Publishing: Madeira Park, BC, Canada, 2016; pp. 33–157. [Google Scholar]
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland, 2nd ed.; Wild Nature Press: Plymouth, UK, 2020. [Google Scholar]
- Jha, B.; Reddy, C.; Thakur, M.C.; Rao, M.U. Seaweeds of India: The Diversity and Distribution of Seaweeds of Gujarat Coast; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mouritsen, O. Marine macroalgae benefit people culturally, industry, nutritionally, and ecologically. Am. Sci. 2013, 101, 458. [Google Scholar] [CrossRef]
- Gurgel, C.F.D.; Lopez-Bautista, J. Red algae. In Encyclopedia of Life Science; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–5. [Google Scholar]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliviera, P.; Chamorro, F.; Otero, P.; Lourenco-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2022, 1–32. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Barnes, P.W.; Williamson, C.E.; Lucas, R.M.; Robinson, S.A.; Madronich, S.; Paul, N.D.; Bornman, J.F.; Bais, A.F.; Sulzberger, B.; Wilson, S.R.; et al. Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat. Sustain. 2019, 2, 569–579. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; van Sluys, M.A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent progress in antioxidant active substances from marine biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; de Medeiros, L.S.; Jacinavicius, F.R.; Long, P.F.; Pinto, E. Development and validation of a rapid LC-MS/MS method for the quantification of mycosporines and mycosporine-like amino acids (MAAs) from cyanobacteria. Algal Res. 2020, 46, 101796. [Google Scholar] [CrossRef]
- Kim, B.K.; Park, M.O.; Min, J.O.; Kang, S.H.; Shin, K.H.; Yang, E.J.; Ha, S.Y. The interplay of mycosporine-like amino acids between phytoplankton groups and northern krill (Thysanoessa sp.) in a high-latitude Fjord (Kongsfjorden, Svalbard). Mar. Drugs 2022, 20, 238. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, contents, and types of mycosporine-like amino acids (MAAs) in marine macroalgae and a database for MAAs based on these characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lamar, Á.; González-Pumariega, M.; Fuentes-León, F.; Vernhes Tamayo, M.; Schuch, A.P.; Menck, C.F. Evaluation of genotoxic and DNA photo-protective activity of Bryothamnion triquetrum and Halimeda incrassata seaweeds extracts. Cosmetics 2017, 4, 23. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- David, S.R.; Baharulnizam, N.B.; Rajabalaya, R. A review on biological assays of red algae marine compounds: An insight into skin whitening activities. J. Herb. Med. 2022, 35, 100585. [Google Scholar] [CrossRef]
- La Barre, S.; Roullier, C.; Boustie, J. Mycosporine-like amino acids (MAAs) in biological photosystems. In Outstanding Marine Molecules; Wiley: Hoboken, NJ, USA, 2014; pp. 333–360. [Google Scholar] [CrossRef]
- Bedoux, G.; Pliego-Cortés, H.; Dufau, C.; Hardouin, K.; Boulho, R.; Freile-Pelegrin, Y.; Robledo, D.; Bourgougnon, N. Production and properties of mycosporine-like amino acids isolated from seaweeds. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 95, pp. 213–245. [Google Scholar] [CrossRef]
- Cardozo, K.H.; Marques, L.G.; Carvalho, V.M.; Carignan, M.O.; Pinto, E.; Marinho-Soriano, E.; Colepicolo, P. Analyses of photoprotective compounds in red algae from the Brazilian coast. Rev. Bras. Farmacogn. 2011, 21, 202–208. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, isolation and characterization of mycosporine-like amino acids from four species of red macroalgae. Mar. Drugs 2021, 19, 615. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient extraction and antioxidant capacity of mycosporine-like amino acids from red alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAAs from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6630. [Google Scholar] [CrossRef] [PubMed]
- Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Nguyen, N.H.; Karsten, U.; Ganzera, M. Bostrychines A–F, six novel mycosporine-like amino-acids and a novel betaine from the red alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. [Google Scholar] [CrossRef]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N.; Bonomi, J.; Álvarez-Gómez, F.; de la Coba, F. Mycosporine-like amino acids from red algae to develop natural UV sunscreens. In Sunscreens: Source, Formulation, Efficacy and Recommendations. Biochemistry Research Trends; Rastogi, R.P., Ed.; NOVA Science Publishers: New York, NY, USA, 2018; pp. 99–130. [Google Scholar]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an integrated multi-trophic aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Schmid, M.; Stengel, D.B. Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J. Phycol. 2015, 51, 25–36. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand–mycosporine-like amino acids and heterosides. J. Exp. Mar. Biol. Ecol. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Véliz, K.; Chandía, N.; Karsten, U.; Lara, C.; Thiel, M. Geographic variation in biochemical and physiological traits of the red seaweeds Chondracanthus chamissoi and Gelidium lingulatum from the south east Pacific coast. J. Appl. Phycol. 2019, 31, 665–682. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Chaves, P.; Álvarez-Gómez, F.; Korbee, N.; Bonomi-Barufi, J. Photoprotection properties of marine photosynthetic organisms grown in high ultraviolet exposure areas: Cosmeceutical applications. Algal Res. 2020, 49, 101956. [Google Scholar] [CrossRef]
- Zwerger, M.; Ganzera, M. Fast and efficient separation of eleven mycosporine-like amino acids by UHPLC-DAD and their quantification in diverse red algae. Mar. Drugs 2022, 20, 395. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Kamiya, M.; West, J.; Ganzera, M. Chemotaxonomic study of Bostrychia spp. (Ceramiales, Rhodophyta) based on their mycosporine-like amino acid content. Molecules 2020, 25, 3273. [Google Scholar] [CrossRef] [PubMed]
- Jofre, J.; Celis-Plá, P.S.; Figueroa, F.L.; Navarro, N.P. Seasonal variation of mycosporine-like amino acids in three subantarctic red seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environm. Res. 2014, 101, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, V.; Zuccarello, G.C.; Karsten, U. Seasonal changes in stress metabolites of native and introduced red algae in New Zealand. J. Appl. Phycol. 2021, 33, 1157–1170. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J. Appl. Phycol. 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- Barufi, J.B.; Mata, M.T.; Oliveira, M.C.; Figueroa, F.L. Nitrate reduces the negative effect of UV radiation on photosynthesis and pigmentation in Gracilaria tenuistipitata (Rhodophyta): The photoprotection role of mycosporine-like amino acids. Phycologia 2012, 51, 636–648. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) system. Mar. Biotech. 2017, 19, 246–254. [Google Scholar] [CrossRef]
- Navarro, N.P.; Korbee, N.; Jofre, J.; Figueroa, F.L. Short-term variations of mycosporine-like amino acids in the carrageenan-producing red macroalga Mazzaella laminarioides (Gigartinales, Rhodophyta) are related to nitrate availability. J. Appl. Phycol. 2021, 33, 2537–2546. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, W.K.; Kim, H.-I.; Paek, S.H.; Jang, S.J.; Jo, Y.; Choi, H.; Lee, J.H.; Moh, S.H. Transcriptome profiling of human follicle dermal papilla cells in response to porphyra-334 treatment by RNA-seq. Evid. Based Complement. Altern. Med. 2021, 2021, 6637513. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of prophyra-334 on UVA-induced photoaging in human skin fibroblast. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2019, 18, 35. [Google Scholar] [CrossRef]
- Lawrence, K.; Gacesa, R.; Long, P.; Young, A. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef]
- Gunn, D.A.; Christensen, K. Skin aging and health. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin, Germany, 2017; pp. 551–562. [Google Scholar]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- Rui, Y.; Zhaohui, Z.; Wenshen, S.; Bafang, L.; Hu, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Perez-Armada, L.; Dominguez, H. Cosmetics from marine sources. In Handbook of Marine Biotechnology, 1st ed.; Kim, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. [Google Scholar]
- Resende, D.I.S.P.; Ferreira, M.; Magalhaes, C.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Janarthanan, M.; Kumar, M.S. Ontogenesis of textile face mask using cotton fabric by treating with red seaweed extract for cosmetotextile applications. J. Text. Inst. 2019, 110, 959–971. [Google Scholar] [CrossRef]
- Dixit, D.; Reddy, C.R.K. Non-targeted secondary metabolite profile study for deciphering the cosmeceutical potential of red marine macro alga Jania rubens—An LCMS-based approach. Cosmetics 2017, 4, 45. [Google Scholar] [CrossRef]
- Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities of carrageenan. In Advances in Food and Nutrition Research, 1st ed.; Kim, S.-K., Ed.; Elsevier: Oxford, UK, 2014; Volume 71, pp. 113–124. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.K. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.; Gunawan, K.Y.; Widowati, W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef]
- Michalun, M.V.; Dinardo, J.C. Skin Care and Cosmetic Ingredients Dictionary, 4th ed.; Cengage Learning: New York, NY, USA, 2014. [Google Scholar]
- Wang, X.; Zhang, Z.; Zhou, H.; Sun, X.; Chen, X.; Xu, N. The anti-aging effects of Gracilaria lemaneiformis polysaccharide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2019, 140, 600–604. [Google Scholar] [CrossRef]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Maia Campos, P.M.B.G. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- La-Mer. Available online: https://www.la-mer.com/en/about-la-mer/algae-seaweed-and-more/ (accessed on 23 August 2022).
- SpecialChem—The Material Selection Platform: Cosmetics Ingredients. ExpoZen—GreenTech (specialchem.com). Available online: https://cosmetics.specialchem.com/product/i-greentech-expozen (accessed on 27 July 2022).
- ExpoZen. Available online: https://asharrison.com.au/expozen-gb/ (accessed on 5 October 2022).
- Ryu, B.; Qian, Z.; Kim, M.; Nam, K.W.; Kim, S. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Guinea, M.; Franco, V.; Araujo-Bazan, L.; Rodriguez-Martin, I.; Gonzales, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated Salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Al-Bader, T.; Byrne, A.; Gillbro, J.; Mitarotonda, A.; Metois, A.; Vial, F.; Rawlings, A.V.; Laloeuf, A. Effect of cosmetic ingredients as anticellulite agents: Synergistic action of actives with in vitro and in vivo efficacy. J. Cosmet. Dermatol. 2012, 11, 17–26. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian Coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 2020, 146, 725–729. [Google Scholar] [CrossRef]
- Kasanah, N.; Setyadi; Triyanto; Trialfhianty, T.I. Indonesian Seaweeds: Biodiversity of Seaweeds in Gunung Kidul, Yogyakarta, 1st ed.; UGM Press: Yogyakarta, Indonesia, 2018. [Google Scholar]
- Kasanah, N.; Ulfah, M.; Nugroho, A.; Wijnana, A.P.A.; dan Triyanto. Indonesian Seaweeds: Biodiversity of Seaweeds in East Nusa Tenggara, 1st ed.; UGM Press: Yogyakarta, Indonesia, 2020. [Google Scholar]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Gröniger, A.; Sinha, R.P.; Klisch, M.; Häder, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Niu, M.Y.; Zhang, Z.H.; Gao, M.; Bu, L.; Zhang, M.M. Optimization the extraction process of mycosporine-like amino acids from Eucheuma. Acad. Period. Farm Prod. Process. 2014, 7, 42–46. [Google Scholar]
- Quintano, E.; Celis-PláP, S.M.; Martínez, B.; Díez, I.; Muguerza, N.; Figueroa, F.L.; Gorostiaga, J.M. Ecophysiological responses of a threatened red alga to increased irradiance in an in situ transplant experiment. Mar. Environ. Res. 2019, 144, 166–177. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Israel, A.; Neori, A.; Martínez, B.; Malta, E.J.; Put, A.; Inken, S.; Marquardt, R.; Abdala-Díaz, R.; Korbee, N. Effect of nutrient supply on photosynthesis and pigmentation to short-term stress (UV radiation) in Gracilaria conferta (Rhodophyta). Mar. Pollut. Bull. 2010, 60, 1768–1778. [Google Scholar] [CrossRef]
- Jin, N.N.; Zhang, Z.H.; Li, B.F. The constitutes and extraction analysis of mycosporine-like amino acids (MAAs) in the Gracilariaceae. Mar. Sci. 2012, 36, 74–80. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Ferreira, M.J.P.; dos Santos, D.Y.A.C. Mycosporine-like amino acids from Gracilariopsis tenuifrons (Gracilariales, Rhodophyta) and its variation under high light. J. Appl. Phycol. 2016, 28, 2035–2040. [Google Scholar] [CrossRef]
- Parailloux, M.; Godin, S.; Fernandes, S.C.; Lobinski, R. Untargeted analysis for mycosporines and mycosporine-like amino acids by hydrophilic interaction liquid chromatography (HILIC)—Electrospray orbitrap MS2/MS3. Antioxidants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.; Schmitz, C.; Ramlov, F.; Maraschin, M.; Korbee, N.; et al. The influence of environmental features in the content of mycosporine-like amino acids in red marine algae along the Brazilian coast. J. Phycol. 2018, 54, 380–390. [Google Scholar] [CrossRef]
- Stein, E.M.; Marques, L.G.; Fujii, M.T.; Colepicolo, P. Mycosporine-like amino acids (MAAs): Qualitative analysis of photoprotective compounds in Laurencia complex seaweed species (Ceramiales, Rhodophyta) from Espírito Santo State, Brazil. Planta Med. 2013, 79, PI104. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Zhang, X.; Liu, Y.; Chen, X.Q.; Cheong, K.L. Quantification of 3, 6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2018, 39, 99–113. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. H-1 and C-13 high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Kasanah, N.; Seto, D.S.; Khotimah, H.; Triyanto. Edible Seaweed: Chemistry, Bioactivity, and Toxicity; UGM PRESS: Yogyakarta, Indonesia, 2022. [Google Scholar]
- Langford, A.; Zhang, J.; Waldron, S.; Julianto, B.; Siradjuddin, I.; Neish, I.; Nuryartono, N. Price analysis of the Indonesian carrageenan seaweed industry. Aquaculture 2022, 550, 737828. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Burlington, Canada, 2014; Chapter 10; Volume 73, pp. 197–220. [Google Scholar]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyop, N.H.; Abdullah, S.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Ren, S.W.; Li, J.; Wang, W.; Guan, H.S. Protective effects of kappa-ca3000+CP against ultraviolet-induced damage in HaCaT and MEF cells. J. Photochem. Photobiol. B 2010, 101, 22–30. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and oligo-porphyran originating from red algae Porphyra: Preparation, biological activities, and potential applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264. 7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Lee, W.K.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Sulfated galactans from red seaweeds and their potential applications. PJSRR 2018, 4, 2. [Google Scholar]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.G.; de Queiroz, I.N.L.; Quinderé, A.L.G.; Benevides, N.M.B.; Tovar, A.M.F.; de Souza Mourão, P.A. Extraction and structural properties of Acanthophora muscoides (Rhodophyceae) extracellular matrix sulfated polysaccharides and their effects on coagulation. Acta Sci. Technol. 2016, 38, 273–282. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Marine algae: A source of biomass for biotechnological applications. In Natural Products from Marine Algae—Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1308, pp. 1–37. [Google Scholar]
- Barahona, T.; Encinas, M.V.; Mansilla, A.; Matsuhiro, B.; Zúñiga, E.A. A sulfated galactan with antioxidant capacity from the green variant of tetrasporic Gigartina skottsbergii (Gigartinales, Rhodophyta). Carbohydrate Res. 2012, 347, 114–120. [Google Scholar] [CrossRef]
- Waters, T.J.; Lionata, H.; Prasetyo Wibowo, T.; Jones, R.; Theuerkauf, S.; Usman, S.; Amin, I.; Ilman, M. Coastal Conservation and Sustainable Livelihoods through Seaweed Aquaculture in Indonesia: A Guide for Buyers, Conservation Practitioners, and Farmers, 1st ed.; The Nature Conservancy: Arlington, VA, USA; Jakarta, Indonesia, 2019; pp. 1–47. [Google Scholar]
- Rimmer, M.A.; Sugama, K.; Rakhmawati, D.; Rofiq, R.; Habgood, R.H. A review and SWOT analysis of aquaculture development in Indonesia. Rev. Aquac. 2013, 5, 255–279. [Google Scholar] [CrossRef]
- Rimmer, M.A.; Larson, S.; Lapong, I.; Purnomo, A.H.; Pong-Masak, P.R.; Swanepoel, L.; Paul, N.A. Seaweed aquaculture in Indonesia contributes to social and economic aspects of livelihoods and community wellbeing. Sustainability 2021, 13, 10946. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/585522/global-value-cosmetics-market/ (accessed on 1 September 2022).


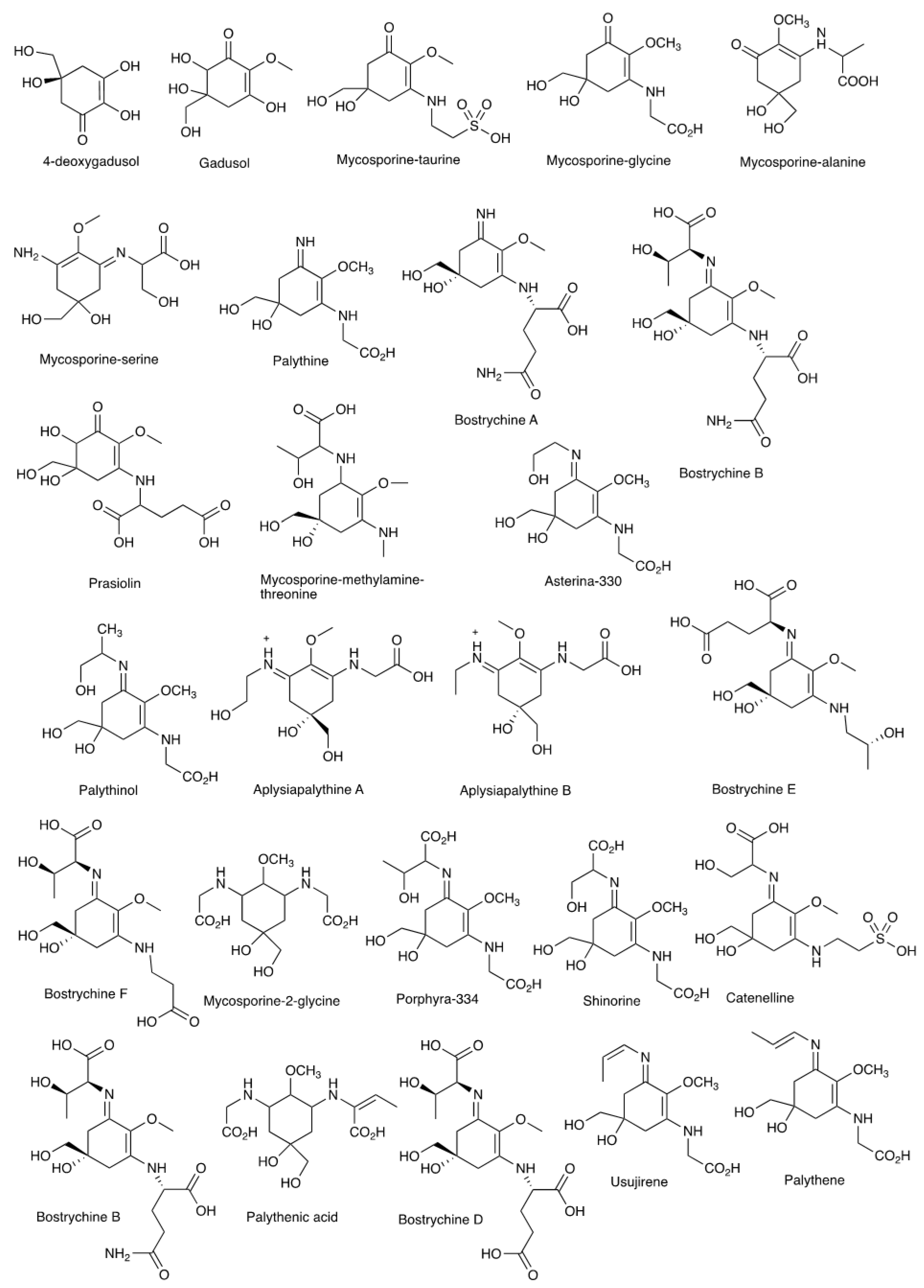





| MAA | λmax (nm) | m/z |
|---|---|---|
| 4-deoxygadusol | 268 | 174.05 |
| Gadusol | 269 | 205.07 |
| Mycosporine-taurine | 309 | 295.07 |
| Mycosporine-glycine | 310 | 245.09 |
| Mycosporine-alanine-glycine | 310 | 259.11 |
| Mycosporine-serine | 310 | 275.12 |
| Palythine | 320 | 244.11 |
| Bostrychines A | 322 | 315.14 |
| Bostrychines C | 322 | 316.13 |
| Prasiolin | 324 | 333.11 |
| Mycosporine-methylamine-threonine | 328 | 304.16 |
| Asterina-330 | 330 | 288.13 |
| Palythinol | 332 | 302.15 |
| Aplysiapalythine A | 332 | 289.14 |
| Aplysiapalythine B | 332 | 273.14 |
| Bostrychines F | 332 | 360.15 |
| Bostrychines E | 333 | 371.17 |
| Mycosporine-2-glycine | 334 | 306.14 |
| Porphyra-334 | 334 | 364.14 |
| Shinorine | 334 | 332.12 |
| Catenelline | 334 | 382.1 |
| Bostrychines B | 335 | 417.17 |
| Palythenic acid | 337 | 332.16 |
| Bostrychines D | 337 | 418.16 |
| Usujirene | 357 | 284.14 |
| Palythene | 360 | 284.14 |
| Lineage | Geography | MAAs |
|---|---|---|
| 1 | North America, South America, Australia | Unpurified MAAs detected at 332 and 308 nm |
| 2 | Australia, Southeast Asia | Palythine-threonine |
| 3 | Central and South America | Palythine-threonine, Porphyra-334 |
| Species | Compound | Activity | References |
|---|---|---|---|
| Gracilaria lemaneiformis | Polysaccharides | Antiaging | [107] |
| Porphyra yezoensis | PYP1–5; Porphyra-334 Extract | Antiphotoaging Remediate skin aging | [87] [57] |
| Porphyra umbilicalis | Extract; polyphenols | Antioxidant, Antiaging properties | [71,108,109] |
| Gelidium corneum | Ethanol:dH2O (4:1) extract | Antioxidant | [71] |
| Osmundea pinnatifida | Ethanol:dH2O (4:1) extract | Antioxidant | [71] |
| Porphyra tenera | Porphyra-334; shinorine | Antiphotoaging | [93] |
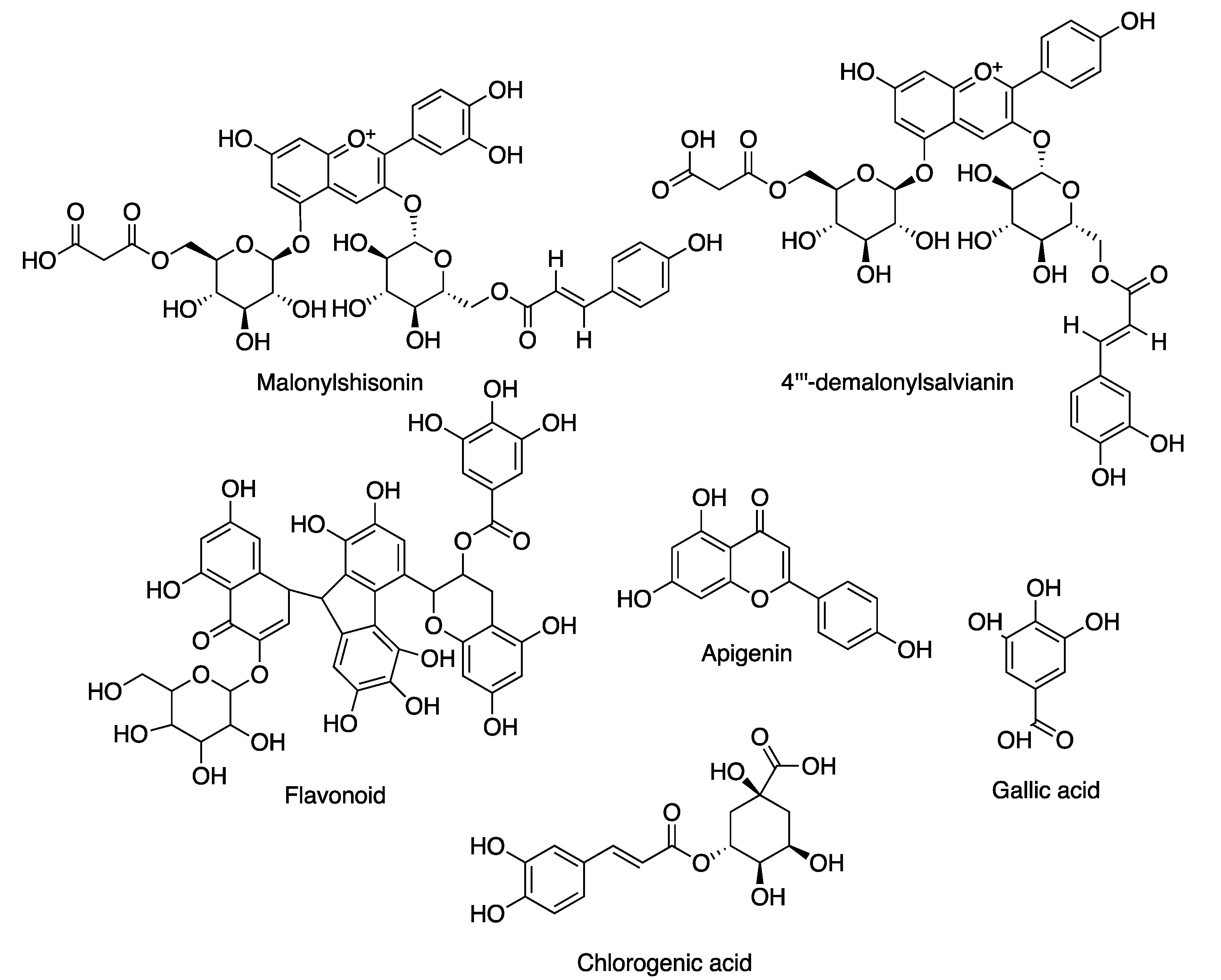
| Jania rubens | Malonylshisonin; 4′′′-demalonylsalvianin | Antiaging | [97] |
| Flavonoid | Antiwrinkle | [97] | |
| Halymenia durvillei | Sulphate polysaccharides Methanol extract | Antiaging and antiwrinkle | [100,110,111] |
| Corallina pilulifera | Methanol extract | Antiaging | [112] |
| Porphyra columbina | Phenolic extracts | Photoprotectant, antiaging | [113] |
| Gigartina skottsbergii | Phenolic extracts | Photoprotectant, antiaging | [113,114] |
| Sarcothalia radula | Phenolic extracts | Photoprotectant, antiaging | [113] |
| Gigartina chamisoi | Extracts | Antioxidant, antiaging | [114] |
| Gigartina radula | Extracts | Antioxidant, antiaging | [114] |
| Gigartina chilensis | Extracts | Antioxidant, antiaging | [114] |
| Gracilaria changii | Polyphenol | Antioxidant | [101] |
| Gracilaria corticata | Sulfated polysaccharide (galactose) | Antioxidant | [115] |
| Polysiphonia fucoides | Phenolic from ethanolic extract | Antioxidant | [102] |
| Furcellaria lumbricalis | Aqueous extract | Antiaging | [116] |
| Gracilaria birdiae | Apigenin and gallic acid | Antioxidant | [117] |
| Gracilaria cornea | Apigenin and gallic acid | Antioxidant | [117] |
| Gracilaria birdiae | Sulfated polysaccharide | Antioxidant | [118] |
| Pyrophia haitanesis | Porphyrin | Antioxidant; antiaging | [119] |
| Gelidium sesquipedale | Extract | Restoring elasticity and softness | [109] |
| Corallina officinalis | Extract | Antioxidant; antiaging | [109] |
| Palmaria palmata, Porhyra purpurea, Chondrus crispus, Mastocarpus stellatus, Gracilaria vermiculophylla | Gallic acid, Protocatechuic acid, chlorogenic acids, gentisic acid | Antiaging | [102] |
| Rhodophyta Collection | Type of MAA | References |
|---|---|---|
| Acanthophora specifera | AS, MG, PE, PL, PR, PI | [122] |
| Ahnfeltiopsis sp. | AS, PI, SH | [123] |
| Amansia sp. | AS, PR, PI, SH | [55] |
| Amphiroa sp. | PI, SH | [124] |
| Dichotomaria marginata | AS, PI, SH | [55] |
| Eucheuma sp. | PR | [125] |
| Galaxaura sp. | PR, SH | [122] |
| Ganonema sp. | PR, SH | [122] |
| Gelidiella acerosa | AS, PR, PI, SH | [122] |
| Gelidium sp. | AS, PL, PR, PI, SH | [126] |
| Gracilaria sp. | AS, MG, PE, PL, PR, PI, SH, US, PA | [62,127,128,129] |
| Gymnogongrus sp. | APA, APB, AS, M2G, MGV, MG, MMT, PE, PI, PGA, PS, PL, PR, SH, US | [130] |
| Hypnea sp. | AS, MG, PR, PI, SH | [131] |
| Jania rubens | AS, MG, PR, PI, SH | [55] |
| Laurencia sp. | AS, MG, PR, PI, SH, PL, US | [131,132] |
| Mastocarpus stellatus | APA, AS, MAG, PL, PR, PI, SH, US | [52,73,133] |
| Palmaria palmata | AS, MG, PE, PL, PR, PI, SH, US | [63,78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. https://doi.org/10.3390/molecules27227788
Kasanah N, Ulfah M, Imania O, Hanifah AN, Marjan MID. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules. 2022; 27(22):7788. https://doi.org/10.3390/molecules27227788
Chicago/Turabian StyleKasanah, Noer, Maria Ulfah, Okmalisda Imania, Annisa Nur Hanifah, and Muhammad Idham Darussalam Marjan. 2022. "Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application" Molecules 27, no. 22: 7788. https://doi.org/10.3390/molecules27227788
APA StyleKasanah, N., Ulfah, M., Imania, O., Hanifah, A. N., & Marjan, M. I. D. (2022). Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules, 27(22), 7788. https://doi.org/10.3390/molecules27227788





