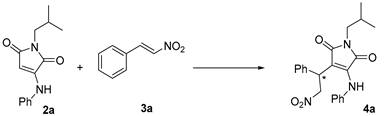
Abstract
Known as electrophiles, maleimides are often used as acceptors in Michael additions to produce succinimides. However, reactions with maleimides as nucleophiles for enantioselective functionalization are only rarely performed. In this paper, a series of bifunctional Takemoto’s catalysts were used to organocatalyze the enantioselective Michael reaction of aminomaleimides with nitroolefins. The resulting products were obtained in good yields (76–86%) with up to 94% enantiomer excess (ee). The catalyst type and the substrate scope were broadened using this methodology.
1. Introduction
Maleimide scaffolds are promising skeletons, which have been widely found in many natural alkaloids and bioactive pharmaceuticals [1,2,3,4,5,6,7,8]. Additionally, maleimide could be transformed into many important heterocyclic frameworks such as succinimides, pyrrolidines, and 2-pyrrolidones. Thus, intensive attention has been focused on the synthesis and modification of maleimide derivatives. In general, functionalization of maleimides mainly takes place on the double bond of maleimide through Michael addition, oxidative coupling, cycloaddition reaction, etc. [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Accordingly, enantioselective Michael addition of maleimides has been well established with maleimides as Michael acceptors [25,26,27]. Although maleimides are often used as electrophiles, their application as nucleophiles or Michael donors for the construction of maleimide-containing compounds is limited [28,29,30].
In 2021, Mori’s group first reported asymmetric Michael addition of α-aminomaleimides as Michael donors to β-nitroolefins using Cinchona alkaloid as the organocatalyst [31]. Furthermore, density functional theory (DFT) was employed in research to improve the enantioselectivity of the adduct and to reveal the mechanism of stereochemistry. Through DFT calculation, the author predicted that increasing the size of the N substituent of the maleimide could be favorable for stereo control. As expected, the ee value was significantly increased by 16% when N-Me was substituted with an N-iBu group of the α-aminomaleimide in this asymmetric reaction.
To the best of our knowledge, only the above one item of literature involves asymmetric Michael addition using α-aminomaleimides as nucleophiles. Therefore, it is important to develop this reaction by using new types of catalysts. Takemoto’s catalyst is the commercially available chiral organocatalyst, which was first synthesized by Takemoto in 2003 [32]. Subsequently, they were widely used in various diastereoselective and enantioselective reactions [33,34,35,36,37,38,39,40]. In this paper, we offer the first reports on the enantioselective Michael addition of α-aminomaleimides and nitroolefins by employing Takemoto-type catalysts 1a–1h bearing (thio)urea or squareamide moiety (Figure 1).

Figure 1.
(Thio)urea derivatives screened as organocatalysts (1a–1h).
2. Results and Discussion
We first applied the Takemoto-type catalysts 1a–1h in the Michael reaction of α-aminomaleimide (2a) and β-nitrostyrene (3a) to screen the optimal catalyst. According to the optimized condition reported [31], the reaction was carried out with Et2O as a solvent in the presence of 10 mol% of catalysts at r.t. (Table 1). All catalysts proceeded the reaction smoothly to give the desired product 4a in 69–78% yields with moderate to high enantioselectivities. Among of them, (R,R)-thiourea catalyst 1c was optimal in terms of the yield and enantioselectivity (entry 3). When the thiourea moiety in catalysts 1c was substituted with squareamide moiety in catalyst 1d, the similar stereoselectivity was afforded (entry 4 vs. entry 3). Additionally, N,N-dimethyl tertiary amine of 1c was changed to the steric bulk moieties in catalysts 1e–1h, led to reduction both in the yields and ees (entries 5–8 vs. entry 3). Therefore, N,N-dimethyl tertiary amine proved to be crucial. Although the optical rotation values of the products were not reported in the literature, it indicated that quinine catalyzed the reaction to obtain S configuration of the major product [31]. Therefore, we repeated a quinine-catalyzed Michael reaction of α-aminomaleimide 2a and β-nitrostyrene 3a to obtain the S-adduct 4a (entry 9). Then, the optical rotation data of two products from quinine- or 1c-induced reaction were determined with MeOH as solvent to afford two negative values (entries 3 and 9). Accordingly, the configuration of the major product catalyzed by 1c was proved to be S.

Table 1.
Asymmetric Michael reaction of α-aminomaleimide with β-nitrostyrene catalyzed by 1a–h a.
To further improve the enantioselectivity of the transformation, a screened catalyst 1c was applied to the Michael reaction of α-aminomaleimide 2a and β-nitrostyrene 3a under the different conditions (Table 2).

Table 2.
Screening of reaction condition for the asymmetric Michael reaction catalyzed by 1c a.
First, a variety of solvents were investigated. All the reactions proceeded smoothly in the screened solvent. It is noteworthy that aprotic solvents were suitable for the reaction to give 85–93% ees (entries 1–8), while protic MeOH was unfavorable for asymmetric induction (entry 9). Among them, toluene was optimal in terms of the yield and enantioselectivity (entry 7). When the reaction temperature was lowered from r.t. to 0 °C, an improved ee of 93% was afforded (entry 10 vs. entry 7). With further temperature decreases to −10 °C and −20 °C, both enantioselectivities were increased by 1%, but showed significantly lower yields (entries 11, 12 vs. entry 10). Therefore, 0 °C was regarded to be the most suitable reaction temperature. When reducing the catalyst loading to 5 mol%, the enantioselectivity was maintained at an excellent level, but with a relatively low yield (entry 13 vs. entry 10), and 20 mol% loading offered no improvement in the asymmetric induction, albeit with a slightly improved yield (entry 14 vs. entry 10). Furthermore, diluting the reaction concentration by half was detrimental for yield and enantiocontrol (entry 15 vs. entry 10). Adding 4 Å molecular sieves (MS) led to a slightly higher ee value of 94% and increased yield (entry 16 vs. entry 10). Based on these experiments, the optimized conditions were determined to be toluene as the solvent with a 10 mol% loading of catalyst 1c in the presence of 4Å MS (200 mg) at 0 °C.
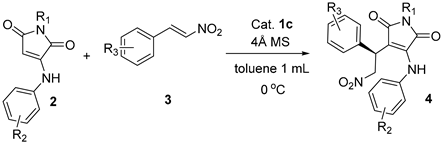
With the optimized conditions in hand, we explored the scope and general applicability of the protocol. A wide range of substituted α-aminomaleimides and β-nitroolefins were evaluated as shown in Table 3.

Table 3.
Generality of the enantioselective Michael reaction of α-aminomaleimides with β-nitroolefins a.
All of the substrates reacted smoothly to give the corresponding products in high yields (76–86%) with good ees (81–94%). Therefore, the stereoselectivities were barely affected by the type and position of the substituents on α-aminomaleimide. However, the substituent and position on the β-nitrostyrene was found to have influence on the enantioselectivity. It can be seen that the 2-Br substituent on phenyl of nitrostyrene led to slightly decreased ee values (entries 4, 18). Compared with the data of the reported literature listed in parentheses (Table 3) [31], our screened catalyst system showed similar enantioselectivities in most reactions. Exceptionally, in the reaction with N-Bn substituted maleimide as Michael donor, a markedly increased ee value was obtained (entry 17), while in the reaction with β-nitro-1-naphthalene ethylene as Michael acceptor, 10% reduction in enantioselectivity was observed (entry 12) in this paper. To extend the type of Michael acceptor in the reaction of α-aminomaleimide as donor, we tried unsaturated carbonyl compounds such as methyl cinnamate 5 and chalcone 6 to substitute the β-nitroolefin. Surprisingly, neither reaction occurred under the screened condition (Scheme 1).
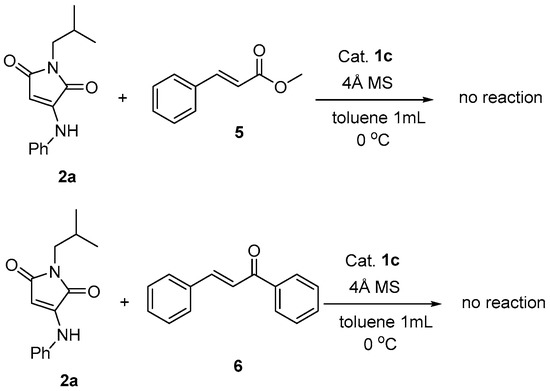
Scheme 1.
Michael reaction of α-aminomaleimide 2a with unsaturated carbonyl compounds.
Based on the obtained absolute configuration described above and the previously reported enantioselective Michael addition of α-aminomaleimide 2a and β-nitrostyrene 3a [31], a proposed transition-state model is depicted in Scheme 2. β-nitrostyrene 3a is oriented and activated by the thiourea moiety through hydrogen bonding and the NH group in 2a is deprotoned and oriented by the tertiary amine of catalyst 1c through another hydrogen bonding. Then, the reaction proceeds with a Se-face addition of α-aminomaleimide to β-nitrostyrene, affording the desired product S-4a.
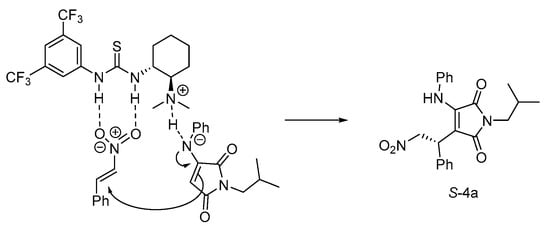
Scheme 2.
Proposed stereochemical model.
3. Experimental
3.1. Chemistry
The 1H NMR spectra were recorded on a 500 MHz for 1 H and at 125 MHz for 13 C NMR, using CDCl3 as a solvent. The chemical shifts were reported in ppm, and the residual nondeuterated solvent (CHCl3) as internal standard (7.26 and 77.0 ppm, respectively). The splitting patterns of the signals were reported as s, singlet; d, doublet; t, triplet; q, quartet; dd, doublet of doublets; m, multiplet. High-resolution mass spectra (HRMS) were measured on a triple TOF 5600+ mass spectrometer equipped with an electrospray ionization (ESI) source in the negative-ion mode. The enantiomeric excess (ee) values of the products were determined through chiral HPLC, using Daicel Chiralpak IA columns (4.6 mm × 250 mm). The optical rotation values were determined using an automatic polarimeter. The reactions were monitored by thin layer chromatography (TLC). Purifications by column chromatography were conducted over silica gel (200–300 mesh). The organocatalysts 1a–1h were purchased from Daicel Chiral Technologies (China) Co.
3.2. General Procedure for the Enantioselective Michael Reaction of α-Aminomaleimides and β-Nitrostyrenes
To a mixture of nitrostyrenes (0.1 mmol), maleimides (0.1 mmol) and organocatalyst 1c (0.01 mmol), toluene (1.0 mL) was added. The resulting mixture was stirred at 0 °C for 24 h (TLC). After the reaction was finished, it was directly poured into a column chromatography on silica gel with hexane/EtOAc (5:1) as eluent to afford the products 4a–t. Among them, 4b, 4c, 4f–h, 4o, 4p, and 4r–t were the new compounds. Experimental data can be found in the Supplementary Materials.
3.2.1. (S)-1-Isobutyl-3-(2-nitro-1-phenylethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4a)
1H NMR (500 MHz, CDCl3) δ 7.44–7.39 (m, 2H), 7.38–7.34 (m, 1H), 7.21–7.18 (m, 3H), 7.15 (d, J = 7.5 Hz, 2H), 6.97 (s, 1H), 6.91–6.80 (m, 2H), 5.47 (dd, J = 12.5, 10.0 Hz, 1H), 4.60 (dd, J = 12.5, 6.0 Hz, 1H), 4.29 (dd, J = 10.0, 6.0 Hz, 1H), 3.34 (d, J = 7.5 Hz, 2H), 2.04 (hept, J = 7.0 Hz, 1H), 0.91 (d, J = 7.0 Hz, 6H); [α]D25 = −6.70 (c 0.52, MeOH) (94% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 10.2 min (minor), 20.0 min (major).
3.2.2. (S)-3-(1-(2-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4b)
1H NMR (500 MHz, CDCl3) δ 7.35–7.27 (m, 3H), 7.25–7.17 (m, 2H), 7.09–6.98 (m, 4H), 6.94–6.88 (m, 1H), 5.39 (dd, J = 14.0, 11.5 Hz, 1H), 4.65–4.52 (m, 2H), 3.35 (d, J = 7.5 Hz, 2H), 2.05 (hept, 7.0 Hz, 1H), 0.93 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.2, 167.5, 141.1, 136.6, 129.5, 127.2, 124.5, 124.3 (d, J = 14.1 Hz), 115.5, 115.3, 95.7, 75.3, 45.4, 32.9, 27.9, 20.1. HRMS (ESI) m/z: [M + Na]+ calcd for C22H22FN3O4Na 434.1492; found 434.1497; [α]D25 = 6.15 (c 0.72, MeOH)(92% ee); HPLC (Chiralpak IA, hexane:iPrOH = 97:3, 0.5 mL/min, 254 nm), tR = 48.6 min (minor), 51.4 min (major).
3.2.3. (S)-3-(1-(2-Chlorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4c)
1H NMR (500 MHz, CDCl3) δ 7.35 (dd, J = 7.0, 2.0 Hz, 1H), 7.27–7.25 (m, 3H), 7.25–7.18 (m, 3H), 7.02 (s, 1H), 6.99–6.91 (m, 2H), 5.39 (dd, J = 13.0, 10.5 Hz, 1H), 4.69 (dd, J = 10.5, 4.5 Hz, 1H), 4.57 (dd, J = 13.0, 4.5 Hz, 1H), 3.38 (d, J = 7.5 Hz, 2H), 2.07 (hept, J = 7.0, 1H), 0.94 (dd, J = 6.5, 3.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.5, 167.4, 141.4, 136.6, 134.3, 133.6, 129.9, 129.7, 129.6, 129.1, 127.4, 127.0, 124.1, 95.6, 74.5, 45.4, 36.8, 27.9, 20.0; HRMS (ESI) m/z: [M + Na]+ calcd for C22H22ClN3O4Na 450.1197; found 450.1194; [α]D25 = 7.41 (c 0.58, MeOH) (87% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 9.8 min (minor), 11.3 min (major).
3.2.4. (S)-3-(1-(2-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5-dione(4d)
1H NMR (500 MHz, CDCl3) δ 7.44 (dd, J = 8.0, 1.0 Hz, 1H), 7.38 (dd, J = 8.0, 1.5 Hz, 1H), 7.28 (dd, J = 7.5, 1.0 Hz, 1H), 7.25 (d, J = 3.5 Hz, 3H), 7.12 (td, J = 7.5, 1.6 Hz, 1H), 7.00 (s, 1H), 6.97–6.91 (m, 2H), 5.40 (dd, J = 13.0, 10.5 Hz, 1H), 4.65 (dd, J = 10.5, 4.5 Hz, 1H), 4.59 (dd, J = 13.0, 4.5 Hz, 1H), 3.39 (d, J = 7.5 Hz, 2H), 2.08 (hept, J = 13.9, 7.0 Hz, 1H), 0.95 (dd, J = 6.5, 4.5 Hz, 6H); [α]D25 = 5.36 (c 0.60, MeOH) (86% ee); HPLC (Chiralpak IA, hexane:iPrOH = 95:5, 0.8 mL/min, 254 nm), tR = 19.1 min (minor), 24.0 min (major).
3.2.5. (S)-3-(1-(3-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4e)
1H NMR (500 MHz, CDCl3) δ 7.50–7.38 (m, 3H), 7.33 (ddd, J = 8.0, 2.0, 1.0 Hz, 1H), 7.17 (d, J = 7.5 Hz, 2H), 7.08 (t, J = 8.0 Hz, 1H), 7.00 (s, 1H), 6.89 (d, J = 8.0 Hz, 1H), 6.82 (t, J = 2.0 Hz, 1H), 5.42 (dd, J = 13.0, 10.0 Hz, 1H), 4.59 (dd, J = 13.0, 6.0 Hz, 1H), 4.22 (dd, J = 10.0, 6.0 Hz, 1H), 3.34 (d, J = 7.5 Hz, 2H), 2.03 (hept, J = 7.0 Hz, 1H), 0.92 (d, J = 6.5 Hz, 6H); [α]D25 = −24.42 (c 0.65, MeOH) (92% ee); HPLC (Chiralpak AS, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 12.6 min (minor), 15.9 min (major).
3.2.6. (S)-3-(1-(3-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4f)
1H NMR (500 MHz, CDCl3) δ 7.47–7.41 (m, 2H), 7.41–7.37 (m, 1H), 7.21–7.13 (m, 3H), 7.00 (s, 1H), 6.89 (tdd, J = 8.5, 2.5, 1.0 Hz, 1H), 6.64 (d, J = 8.0 Hz, 1H), 6.60–6.51 (m, 1H), 5.41 (dd, J = 13.0, 10.0 Hz, 1H), 4.62 (dd, J = 13.0, 6.0 Hz, 1H), 4.26 (dd, J = 10.0, 6.0 Hz, 1H), 3.34 (d, J = 7.5 Hz, 2H), 2.03 (hept, J = 7.0 Hz, 1H), 0.92 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.1, 167.5, 141.0, 140.1 (d, J = 6.9 Hz), 136.6, 130.3 (d, J = 8.1 Hz), 129.6, 127.6, 125.6, 123.5, 115.2, 115.0, 114.6 (d, J = 21.0 Hz), 96.6, 76.8, 45.4, 39.2, 27.9, 20.0. HRMS (ESI) m/z: [M + Na]+ calcd for C22H22FN3O4Na 434.1492; found 434.1497; [α]D25 = −28.98 (c 0.75, MeOH) (93% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 10.3 min (minor), 12.4 min (major).
3.2.7. (S)-3-(1-(4-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4g)
1H NMR (500 MHz, CDCl3) δ 7.45–7.41 (m, 2H), 7.40–7.36 (m, 1H), 7.16 (d, J = 7.5 Hz, 2H), 6.96 (s, 1H), 6.88 (dd, J = 12.0, 5.5 Hz, 2H), 6.86–6.80 (m, 2H), 5.39 (dd, J = 12.5, 10.0 Hz, 1H), 4.60 (dd, J = 12.5, 6.0 Hz, 1H), 4.26 (dd, J = 10.0, 6.0 Hz, 1H), 3.34 (d, J = 7.5 Hz, 2H), 2.03 (hept, J = 7.0 Hz, 1H), 0.92 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 167.6, 140.7, 136.7, 133.5, 129.7(d, J = 8.1 Hz), 129.6, 127.5, 125.5, 115.8, 115.6, 97.2, 77.1, 45.4, 38.8, 27.9, 20.0; HRMS (ESI) m/z: [M + Na]+ calcd for C22H22FN3O4Na 434.1492; found 434.1499; [α]D25 = −16.12 (c 0.55, MeOH) (93% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 10.3 min (minor), 17.2 min (major).
3.2.8. (S)-3-(1-(4-Chlorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4h)
1H NMR (500 MHz, CDCl3) δ 7.45–7.41 (m, 2H), 7.40–7.36 (m, 1H), 7.19–7.13 (m, 4H), 7.00 (s, 1H), 6.82–6.75 (m, 2H), 5.38 (dd, J = 12.5, 10.0 Hz, 1H), 4.60 (dd, J = 12.5, 6.0 Hz, 1H), 4.25 (dd, J = 10.0, 6.0 Hz, 1H), 3.33 (d, J = 7.5 Hz, 2H), 2.03 (hept, J = 7.0 Hz, 1H), 0.91 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.2, 167.5, 140.8, 136.6, 136.2, 133.5, 129.6, 129.4, 129.0, 127.6, 125.6, 96.8, 76.9, 45.4, 38.9, 31.6, 27.9, 22.6, 20.04 (d, J = 2.9 Hz), 14.1. HRMS (ESI) m/z: [M + Na]+ calcd for C22H22ClN3O4Na 450.1197; found 450.1192; [α]D25 = −58.69 (c 0.67, MeOH) (93% ee); HPLC (Chiralpak IA, hexane:iPrOH = 95:5, 0.8 mL/min, 254 nm), tR = 21.6 min (minor), 32.2min (major).
3.2.9. (S)-3-(1-(4-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4i)
1H NMR (500 MHz, CDCl3) δ 7.46–7.36 (m, 3H), 7.35–7.29 (m, 2H), 7.16 (d, J = 7.5 Hz, 2H), 6.99 (s, 1H), 6.76–6.70 (m, 2H), 5.38 (dd, J = 13.0, 9.5 Hz, 1H), 4.60 (dd, J = 13.0, 6.0 Hz, 1H), 4.23 (dd, J = 9.5, 6.0 Hz, 1H), 3.33 (d, J = 7.5 Hz, 2H), 2.02 (hept, J = 7.0 Hz,1H), 0.91 (d, J = 6.5 Hz, 6H); [α]D25 = −12.33 (c 0.49, MeOH) (90% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 11.4 min (minor), 16.6min (major)
3.2.10. (S)-1-Isobutyl-3-(2-nitro-1-(p-tolyl)ethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4j)
1H NMR (500 MHz, CDCl3) δ 7.45–7.39 (m, 2H), 7.38–7.33 (m, 1H), 7.16 (d, J = 7.5 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 6.92 (s, 1H), 6.76 (d, J = 8.0 Hz, 2H), 5.44 (dd, J = 12.5, 10.0 Hz, 1H), 4.58 (dd, J = 12.5, 6.0 Hz, 1H), 4.25 (dd, J = 10.0, 6.0 Hz, 1H), 3.33 (d, J = 7.5 Hz, 2H), 2.28 (s, 3H), 2.04 (hept, J = 7.0 Hz, 1H), 0.92 (d, J = 6.5 Hz, 6H); [α]D25 = −8.89 (c 0.51, MeOH) (93% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 9.5min (minor), 14.9min (major).
3.2.11. (S)-1-Isobutyl-3-(1-(4-methoxyphenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4k)
1H NMR (500 MHz, CDCl3) δ 7.42 (dd, J = 10.0, 5.0 Hz, 2H), 7.35 (dd, J = 8.5, 6.5 Hz, 1H), 7.15 (d, J = 7.5 Hz, 2H), 6.93 (s, 1H), 6.83–6.77 (m, 2H), 6.76–6.70 (m, 2H), 5.41 (dd, J = 12.5, 10.0 Hz, 1H), 4.57 (dd, J = 12.5, 6.0 Hz, 1H), 4.23 (dd, J = 10.0, 6.0 Hz, 1H), 3.75 (s, 3H), 3.33 (d, J = 7.5 Hz, 2H), 2.04 (hept, J = 7.0 Hz, 1H), 0.92 (d, J = 6.5 Hz, 6H); [α]D25 = −30.50 (c 0.59, MeOH) (92% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 13.2min (minor), 27.6min (major).
3.2.12. (S)-1-Isobutyl-3-(1-(naphthalen-2-yl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole- 2,5-dione (4l)
1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 6.5 Hz, 1H), 7.45 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 7.41 (t, J = 8.0 Hz, 1H), 7.28 (ddd, J = 8.5, 7.0, 1.5 Hz, 1H), 7.11 (d, J = 8.5 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 7.01–6.94 (m, 3H), 6.91 (d, J = 7.5 Hz, 2H), 5.60 (dd, J = 13.0, 11.0 Hz, 1H), 5.11 (dd, J = 11.0, 4.0 Hz, 1H), 4.56 (dd, J = 13.0, 4.0 Hz, 1H), 3.42 (d, J = 7.5 Hz, 2H), 2.11 (hept, J = 7.0 Hz, 2H), 0.97 (dd, J = 6.5, 4.5 Hz, 6H); [α]D25 = −14.13 (c 0.66, MeOH) (92% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 12.2min (minor), 28.2min (major).
3.2.13. (S)-1-Isobutyl-3-(2-nitro-1-(thiophen-2-yl)ethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4m)
1H NMR (500 MHz, CDCl3) δ 7.44 (dd, J = 10.5, 5.0 Hz, 2H), 7.37 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 7.5 Hz, 2H), 7.16 (dd, J = 5.0, 1.0 Hz, 1H), 7.00 (s, 1H), 6.87 (dd, J = 5.0, 3.5 Hz, 1H), 6.65 (d, J = 3.5 Hz, 1H), 5.33 (dd, J = 12.5, 10.0 Hz, 1H), 4.63 (dd, J = 12.5, 5.5 Hz, 1H), 4.52 (dd, J = 10.0, 5.5 Hz, 1H), 3.34 (d, J = 7.5 Hz, 2H), 2.04 (hept, J = 7.0 Hz,1H), 0.92 (d, J = 6.5 Hz, 6H); [α]D25 = −14.13 (c 0.53, MeOH) (92% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 12.2min (minor), 28.2min (major).
3.2.14. (S)-3-((4-Chlorophenyl)amino)-1-isobutyl-4-(2-nitro-1-phenylethyl)-1H-pyrrole- 2,5-dione (4n)
1H NMR (500 MHz, CDCl3) δ 7.38 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.96–6.92 (m, 3H), 5.62–5.48 (m, 1H), 4.61 (dd, J = 13.0, 5.5 Hz, 1H), 4.30 (dd, J = 10.5, 5.5 Hz, 1H), 3.36 (d, J = 7.5 Hz, 2H), 2.06 (hept, J = 7.0 Hz, 1H), 0.94 (d, J = 6.5 Hz, 6H); [α]D25 = −19.26 (c 0.65, MeOH) (92% ee); HPLC (Chiralpak IA, hexane:iPrOH = 95:5, 0.8 mL/min, 254 nm), tR = 26.7 min (minor), 49.6 min (major).
3.2.15. (S)-3-((4-Chlorophenyl)amino)-1-isobutyl-4-(1-(2-chlorophenyl)-2-nitroethyl)-1H- pyrrole-2,5-dione (4o)
1H NMR (500 MHz, CDCl3) δ 7.76–7.69 (m, 1H), 7.68–7.64 (m, 1H), 7.59 (t, J = 6.0 Hz, 4H), 7.34 (s, 1H), 7.26 (d, J = 8.5 Hz, 2H), 5.86–5.69 (m, 1H), 5.02 (dd, J = 11.0, 4.5 Hz, 1H), 4.91 (dd, J = 13.0, 4.5 Hz, 1H), 3.74 (d, J = 7.5 Hz, 2H), 2.43 (hept, J = 7.0 Hz, 1H), 1.30 (dd, J = 6.5, 4.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.4, 167.3, 141.3, 135.2, 134.1, 133.6, 132.7, 129.8, 129.2, 129.3, 127.5, 125.5, 96.4, 74.4, 45.4, 36.8, 27.9, 20.0; HRMS (ESI) m/z: [M + Na]+ calcd for C22H21Cl2N3O4Na 484.0807; found 484.0813; [α]D25 = 3.64 (c 0.50, MeOH) (89% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 10.9min (minor), 14.2min (major).
3.2.16. (S)-3-((3-Chlorophenyl)amino)-1-isobutyl-4-(2-nitro-1-phenylethyl)-1H-pyrrole- 2,5-dione (4p)
1H NMR (500 MHz, CDCl3) δ 7.35 (d, J = 7.0 Hz, 2H), 7.28 (m, 3H), 7.13 (s, 1H), 7.06 (d, J = 6.5 Hz, 1H), 6.99–6.89 (m, 3H), 5.58–5.44 (m, 1H), 4.62 (dd, J = 13.0, 5.5 Hz, 1H), 4.31 (dd, J = 9.5, 5.5 Hz, 1H), 3.36 (d, J = 7.5 Hz, 2H), 2.06 (hept, J = 7.0 Hz, 1H), 0.94 (d, J = 6.5 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 173.0, 167.5, 140.1, 138.2, 137.0, 135.2, 130.5, 129.1, 128.0, 127.8, 127.3, 125.2, 123.2, 98.9, 45.4, 39.9, 27.9, 20.0; HRMS (ESI) m/z: [M + Na]+ calcd for C22H22ClN3O4Na 450.1197; found 450.1190; [α]D25 = −30.83 (c 0.71, MeOH) (93% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 10.2min (minor), 16.0min (major).
3.2.17. (S)-1-Benzyl-3-(2-nitro-1-phenylethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4q)
1H NMR (500 MHz, CDCl3) δ 7.43–7.38 (m, 2H), 7.39–7.32 (m, 5H), 7.31–7.27 (m, 1H), 7.21–7.17 (m, 3H), 7.14 (d, J = 7.5 Hz, 2H), 6.94 (s, 1H), 6.88–6.82 (m, 2H), 5.44 (dd, J = 12.5, 9.5 Hz, 1H), 4.69 (s, 2H), 4.64 (dd, J = 12.5, 6.0 Hz, 1H), 4.28 (dd, J = 9.5, 6.0 Hz, 1H); [α]D25 = −16.15 (c 0.62, MeOH) (90% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 19.6min (minor), 24.2 min (major).
3.2.18. (S)-1-Benzyl-3-(1-(2-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4r)
1H NMR (500 MHz, CDCl3) δ 7.43 (dd, J = 8.0, 1.5 Hz, 1H), 7.40–7.29 (m, 6H), 7.26–7.23 (m, 4H), 7.11 (td, J = 7.5, 1.5 Hz, 1H), 7.00 (s, 1H), 6.95–6.90 (m, 2H), 5.40 (dd, J = 13.0, 10.5 Hz, 1H), 4.74 (dd, J = 21.0, 13.5 Hz, 2H), 4.66 (dd, J = 10.5, 4.5 Hz, 1H), 4.61 (dd, J = 13.0, 4.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 172.9, 166.9, 141.8, 136.6, 136.3, 135.8, 133.2, 130.0, 129.8, 129.3, 128.8, 128.2, 127.8, 127.1, 124.3, 124.1, 96.4, 74.5, 41.7, 39.4, 29.7; HRMS (ESI) m/z: [M + Na]+ calcd for C25H20BrN3O4Na 528.0535; found 528.0541; [α]D25 = 36.0 (c 0.69, MeOH) (84% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 18.9min (minor), 23.1min (major).
3.2.19. (S)-1-Benzyl-3-(1-(3-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4s)
1H NMR (500 MHz, CDCl3) δ 7.50–7.38 (m, 3H), 7.38–7.27 (m, 6H), 7.15 (d, J = 7.5 Hz, 2H), 7.07 (t, J = 8.0 Hz, 1H), 6.98 (s, 1H), 6.87 (d, J = 8.0 Hz, 1H), 6.81 (t, J = 2.0 Hz, 1H), 5.39 (dd, J = 13.0, 9.5 Hz, 1H), 4.69 (s, 2H), 4.63 (dd, J = 13.0, 6.0 Hz, 1H), 4.22 (dd, J = 9.5, 6.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 172.5, 166.9, 141.4, 140.0, 136.5, 136.2, 131.2, 130.8, 130.4, 129.7, 128.7, 128.2, 127.9, 127.8, 126.4, 125.9, 122.7, 97.0, 41.6, 39.2, 29.7; HRMS (ESI) m/z: [M + Na]+ calcd for C25H20BrN3O4Na 528.0535; found 528.0543; [α]D25 = −11.22 (c 0.63, MeOH) (90% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 1.0 mL/min, 254 nm), tR = 18.7min (minor), 21.6min (major).
3.2.20. (S)-1-Benzyl-3-(1-(4-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4t)
1H NMR (500 MHz, CDCl3) δ 7.40 (dd, J = 17.0, 7.0 Hz, 3H), 7.36–7.27 (m, 7H), 7.15 (d, J = 6.5 Hz, 2H), 7.02 (s, 1H), 5.34 (t, J = 11.0 Hz, 1H), 4.76–4.57 (m, 3H), 4.23 (d, J = 5.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 172.5, 167.0, 141.2, 136.7, 136.5, 136.1, 132.0, 129.7, 129.6, 128.7, 128.2, 127.8, 127.7, 125.7, 121.7, 97.1, 41.6, 39.0, 29.7; HRMS (ESI) m/z: [M + Na]+ calcd for C25H20BrN3O4Na 528.0535; found 528.0546; [α]D25 = −27.63 (c 0.72, MeOH) (90% ee); HPLC (Chiralpak IA, hexane:iPrOH = 90:10, 0.5 mL/min, 254 nm), tR = 42.9min (minor), 45.5min (major).
4. Conclusions
In summary, we described the first Takemoto-type catalyst to promote the enantioselective Michael addition of α-aminomaleimides and β-nitroolefins. The α-aminomaleimides were used as nucleophiles rather than electrophiles in this transformation to create the desired maleimide-containing adducts with high enantioselectivity (up to 94% ee). Moreover, we used our optimized conditions to expand upon the substrate scope of this reaction. Further study of α-aminomaleimides as donors in Michael additions with other acceptors is under way.
Supplementary Materials
Copies of 1H and 13C-NMR spectra and HPLC trace of products are available online at https://www.mdpi.com/article/10.3390/molecules27227787/s1. Figures S1–S30: NMR spectra of compounds 4a–t; Figures S31–S70: HPLC trace of compounds 4a–4t.
Author Contributions
H.M. and Y.J. (Yan Jin) are coauthors; they contributed equally to this work. They performed the experiments, acquired and analyzed the original data. R.Z. conducted instrumental analysis. L.W. supervised the experiment, provided funding supporting. Y.J. (Ying Jin) designed the research plan, analyzed and checked all the data, wrote and revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Natural Science Foundation of Jilin province (No. YDZJ202201ZYTS552), and the National Students’ Program for Innovation and Entrepreneurship Training (No. 202113706009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, K.J.; Choi, M.J.; Shin, J.-S.; Kim, M.J.; Choi, H.-E.; Kang, S.M.; Jin, J.H.; Lee, K.-T.; Lee, J.Y. Synthesis, biological evaluation, and docking analysis of a novel family of 1-methyl-1H-pyrrole-2,5-diones as highly potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wang, S.; Li, Y.; Zhang, F.; Song, B.; Zhao, W. Syntheses of new chlorin derivatives containing maleimide functional group and their photodynamic activity evaluation. Bioorg. Med. Chem. Lett. 2015, 25, 4078–4081. [Google Scholar] [CrossRef]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent Nematicidal Activity of Maleimide Derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]
- Eis, M.J.; Evenou, J.P.; Schuler, W.; Zenke, G.; Vangrevelinghe, E.; Wagner, J.; Matt, P. Indolyl-naphthyl-maleimides as potent and selective inhibitors of protein kinase C-α/β. Bioorg. Med. Chem. Lett. 2017, 27, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Alam, J.; Jeyaraj, D.-A.; Wang, W.; Lin, G.-R.; Ang, S.-H.; Tan, E.-S.-W.; Lee, M.-A.; Ke, Z.; Madan, B.; et al. Scaffold Hopping and Optimization of Maleimide Based Porcupine Inhibitors. J. Med. Chem. 2017, 60, 6678–6692. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Sorrell, F.J.; Berger, B.-T.; Collins, R.J.; Vasconcelos, S.N.S.; Massirer, K.B.; Knapp, S.; Bennett, J.; Fedorov, O.; Patel, H.; et al. Discovery of a Potent Dual SLK/STK10 Inhibitor Based on a Maleimide Scaffold. J. Med. Chem. 2021, 64, 13259–13278. [Google Scholar] [CrossRef] [PubMed]
- Kjærsgaard, N.L.; Hansen, R.A.; Gothelf, K.V. Preparation of Maleimide-Modified Oligonucleotides from the Corresponding Amines Using N-Methoxycarbonyl maleimide. Bioconjug. Chem. 2022, 33, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Fernandez, S.; Martinez, A.; Zeni, M.; Reyes, L.; Rey, A.; Cerecetto, H. Glycogen Synthase Kinase-3 Maleimide Inhibitors As Potential PET-Tracers for Imaging Alzheimer’s Disease: 11C-Synthesis and In Vivo Proof of Concept. J. Med. Chem. 2022, 65, 1342–1351. [Google Scholar] [CrossRef]
- Mandal, R.; Emayavaramban, B.; Sundararaju, B. Cp*Co(III)-Catalyzed C–H Alkylation with Maleimides Using Weakly Coordinating Carbonyl Directing Groups. Org. Lett. 2018, 20, 2835–2838. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Yang, H.; Liu, D.; Sun, B.; Zhang, F.-L. Assembly of Diverse Spirocyclic Pyrrolidines via Transient Directing Group Enabled Ortho-C(sp2)–H Alkylation of Benzaldehydes. Org. Lett. 2018, 1, 146–149. [Google Scholar] [CrossRef]
- Yu, J.T.; Chen, R.; Jia, H.; Pan, C. Rhodium-Catalyzed Site-Selective ortho-C–H Activation: Enone Carbonyl Directed Hydroarylation of Maleimides. J. Org. Chem. 2018, 83, 12086–12093. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, Y.; Wu, C.; Yu, J.-T. Rhodium-catalyzed C7-alkylation of indolines with maleimides. Org. Biomol. Chem. 2018, 16, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Uno, B.-E.; Dicken, R.-D.; Redfern, L.-R.; Stern, C.-M.; Krzywicki, G.-G.; Scheidt, K.-A. Calcium(II)—Catalyzed enantioselective conjugate additions of amines. Chem. Sci. 2018, 9, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Shamsianpour, M.; Issazadeh, S.; Dorrani, M.; Hazrati, H. Palladium-catalyzed direct arylation of maleimides: A simple route to bisaryl-substituted maleimides. Tetrahedron 2017, 73, 1668–1672. [Google Scholar] [CrossRef]
- Jafarpour, F.; Shamsianpour, M. Palladium-catalyzed cross-dehydrogenative coupling of maleimides with simple arenes: A fast track to highly substituted maleimides. RSC Adv. 2016, 6, 103567–103570. [Google Scholar] [CrossRef]
- Yang, Z.-H.; An, Y.-L.; Chen, Y.; Shao, Z.-Y.; Zhao, S.-Y. Copper(I) Iodide-Catalyzed Sulfenylation of Maleimides and Related 3-Indolylmaleimides with Thiols. Adv. Synth. Catal. 2016, 358, 3869–3875. [Google Scholar] [CrossRef]
- Dana, S.; Mandal, A.; Sahoo, H.; Baidya, M. Ru(II)-Catalyzed C–H Functionalization on Maleimides with Electrophiles: A Demonstration of Umpolung Strategy. Org. Lett. 2017, 19, 1902–1905. [Google Scholar] [CrossRef]
- An, Y.-L.; Zhang, H.-H.; Yang, Z.-H.; Lin, L.; Zhao, S.-Y. Cu/Ag-Cocatalyzed Aerobic Oxidative Amination and CuCl2-Mediated Aerobic Oxidative Chloroamination of Maleimides. Eur. J. Org. Chem. 2016, 2016, 5405–5414. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Tan, H.-R.; An, Y.-L.; Zhao, Y.-W.; Lin, H.-P.; Zhao, S.-Y. Three-Component Coupling Reactions of Maleimides, Thiols, and Amines: One-Step Construction of 3,4-Heteroatom-functionalized Maleimides by Copper-Catalyzed C(sp2)–H Thioamination. Adv. Synth. Catal. 2018, 360, 173–179. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Tan, H.-R.; Zhu, J.-N.; Zheng, J.; Zhao, S.-Y. Regioselective Silver-Catalyzed Carbon-Phosphorus Difunctionalization of Maleimides: One-Step Construction of Phosphonylated Indolylmaleimides and Pyrrolylmaleimides. Adv. Synth. Catal. 2018, 360, 1523–1529. [Google Scholar] [CrossRef]
- Baker, J.-R.; Tedaldi, L.-M.; Aliev, A.-E. [2 + 2] Photocycloadditions of thiomaleimides. Chem. Commun. 2012, 48, 4725–4727. [Google Scholar] [CrossRef]
- Lin, C.; Zhen, L.; Cheng, Y.; Du, H.-J.; Zhao, H.; Wen, X.; Kong, L.-Y.; Xu, Q.-L.; Sun, H. Visible-Light Induced Isoindoles Formation To Trigger Intermolecular Diels–Alder Reactions in the Presence of Air. Org. Lett. 2015, 17, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wu, X.; Jiang, L.; Zhang, Z.; Xie, X. Reduction of Benzolactams to Isoindoles via an Alkoxide-Catalyzed Hydrosilylation. Org. Lett. 2017, 19, 6048–6051. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Lee, R.; Zhu, B.; Coote, M.-L.; Zhao, X.; Jiang, Z. Highly Enantio- and Diastereoselective [4 + 2] Cycloaddition of 5H-oxazol-4-ones with N-Maleimides. J. Org. Chem. 2016, 81, 8061–8069. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Kaur, J.; Chimni, S.S. Asymmetric Organocatalytic Addition Reactions of Maleimides: A Promising Approach Towards the Synthesis of Chiral Succinimide Derivatives. Chem. Asian J. 2013, 8, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Gómez-Torres, E.; Alonso, D.A.; Gómez-Bengoa, E.; Nájera, C. Enantioselective synthesis of succinimides by Michael addition of 1,3-dicarbonyl compounds to maleimides catalyzed by a chiral bis(2-aminobenzimidazole) organocatalyst. Eur. J. Org. Chem. 2013, 2013, 1434–1440. [Google Scholar] [CrossRef]
- Yuan, F.; Duan, W.; Li, Z.; Luo, X.; Zhang, M.; Deng, H.; Song, L. One-Pot Synthesis of Trifluoromethylated Pyrazol-4-YlPyrrole-2,5-Dione Derivatives. Synthesis 2019, 51, 3345–3355. [Google Scholar] [CrossRef]
- Khan, M.M.; Shareef, S.; Khan, S.; Saigal; Sahoo, S.C. Organocatalyzed Highly Efficient Synthesis of Densely Functionalized Pyrrole-Fused 1,4-Dihydropyridine Derivatives. Synth. Commun. 2019, 49, 2884–2894. [Google Scholar] [CrossRef]
- Xie, D.-H.; Niu, C.; Du, D.-M. Enantioselective Michael/ Hemiketalization Cascade Reactions between Hydroxymaleimides and 2-Hydroxynitrostyrenes for the Construction of Chiral ChromanFused Pyrrolidinediones. Molecules 2022, 27, 5081. [Google Scholar] [CrossRef]
- Sakai, N.; Kawashima, K.; Kajitani, M.; Mori, S.; Oriyama, T. Combined Computational and Experimental Studies on the Asymmetric Michael Addition of α-Aminomaleimides to β-Nitrostyrenes Using an Organocatalyst Derived from Cinchona Alkaloid. Org. Lett. 2021, 23, 5714–5718. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Hoashi, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael Addition to α, β-unsaturated Imides Catalyzed by a Bifunctional Organocatalyst. Angew. Chem. Int. Ed. Engl. 2005, 44, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Zea, A.; Valero, G.; Alba, A.R.; Moyano, A.; Rios, R. Development of Diphenylamine-linked Bis(imidazoline) Ligands and Their Application in Asymmetric Friedel-Crafts Alkylation of Indole Derivatives with Nitroalkenes. Adv. Synth. Catal. 2010, 352, 1102–1106. [Google Scholar] [CrossRef]
- Raimondi, W.; Baslé, O.; Constantieux, T.; Bonne, D.; Rodriguez, J. Activation of 1, 2-Ketoesters with Takemoto’s Catalyst toward Michael Addition to Nitroalkenes. Adv. Synth. Catal. 2012, 354, 563–568. [Google Scholar] [CrossRef]
- Ansari, S.; Raabe, G.; Enders, D. Asymmetric Michael Addition of 1, 3-Bis(phenylthio)propan-2-one to Nitroalkenes Employing Takemoto’s Thiourea Catalyst. Monatsh. Chem. 2013, 144, 641–646. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Asymmetric Synthesis of Syn-propargylamines and Unsaturated β-amino Acids under Brønsted Base Catalysis. Nat. Commun. 2015, 6, 8544–8582. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, H.-F.; Zhao, J.-Z.; Du, Z.-H.; Da, C.-S. Organocatalytic Enantioselective Cross-aldol Reaction of o-Hydroxyarylketones and Trifluoromethyl Ketones. Org. Lett. 2017, 19, 2634–2637. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.-M.; Zhang, J.-W.; Jin, Y. Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins. Molecules 2019, 24, 3944. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, T.-Y.; Sun, Y.-H.; Wang, L.-M.; Jin, Y. Organocatalytic enantioselective aza-Friedel–Crafts alkylation of β-naphthols and isatin-derived ketimines via a Takemoto-type catalyst. New J. Chem. 2021, 45, 10481–10487. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).