Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
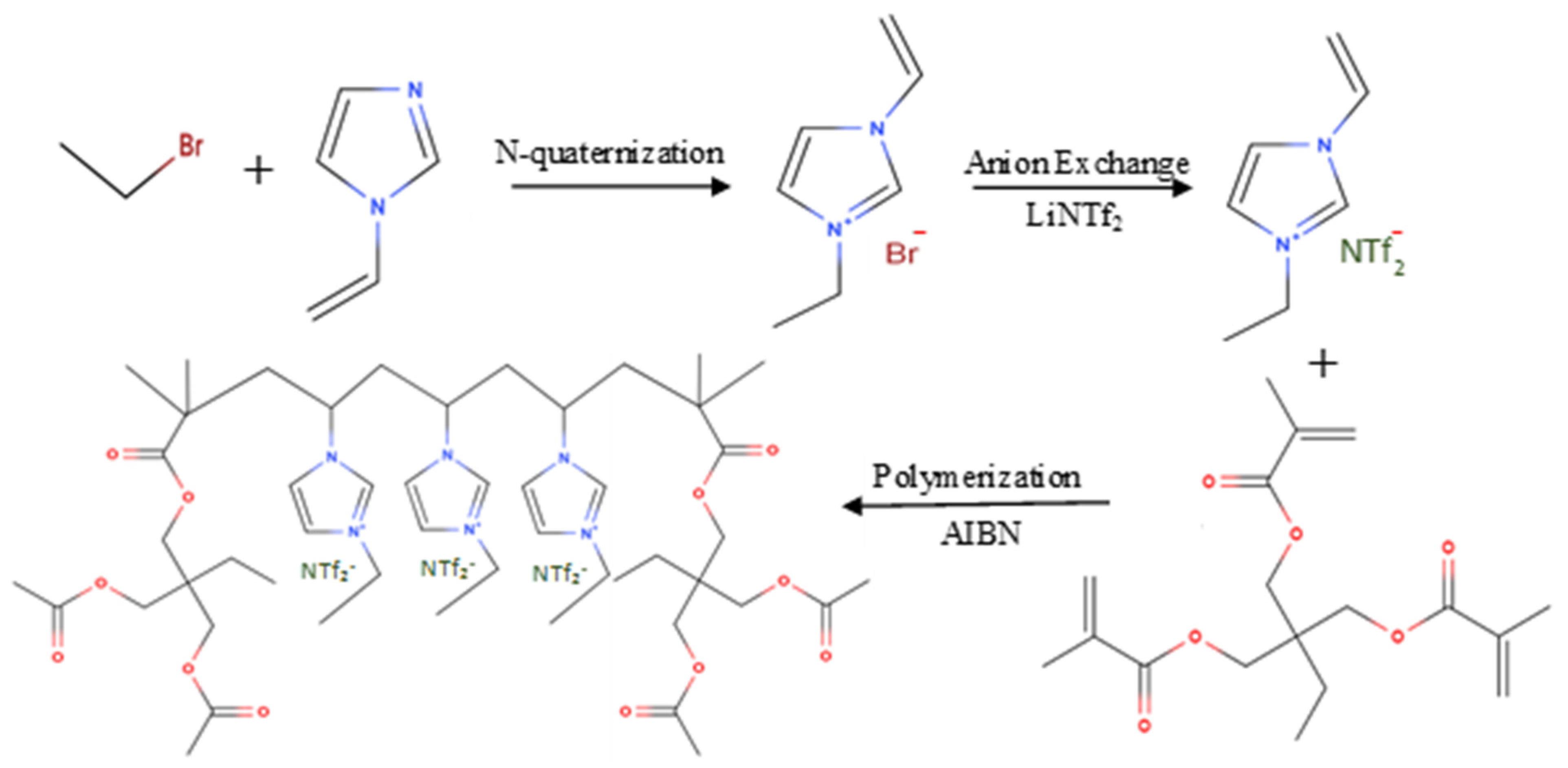
2.2. [veim][Br] and [veim][Tf2N] Monomer Synthesis
2.3. Synthesis of Cross-Linked poly[veim][Br] and poly[veim][Tf2N]
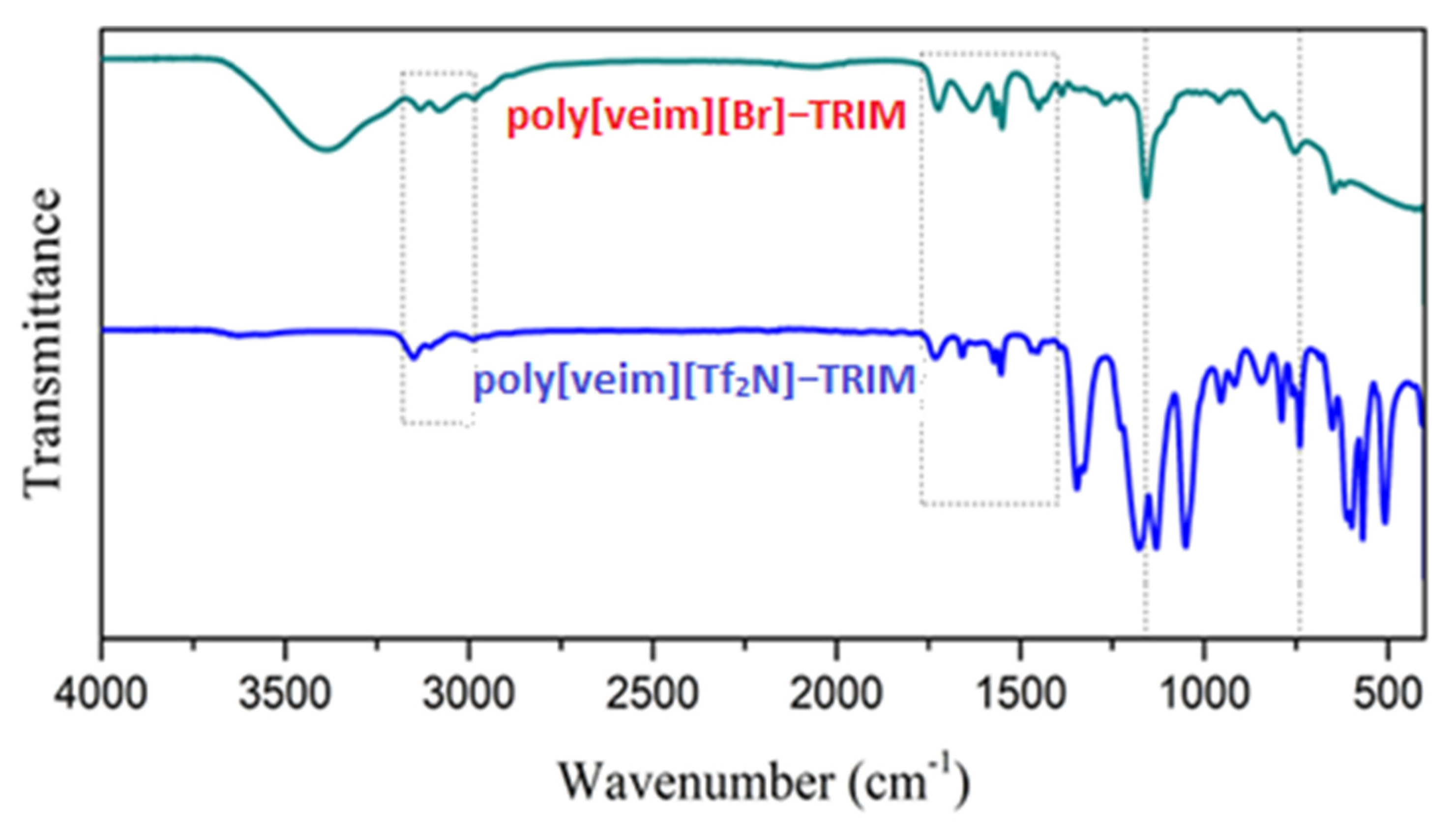
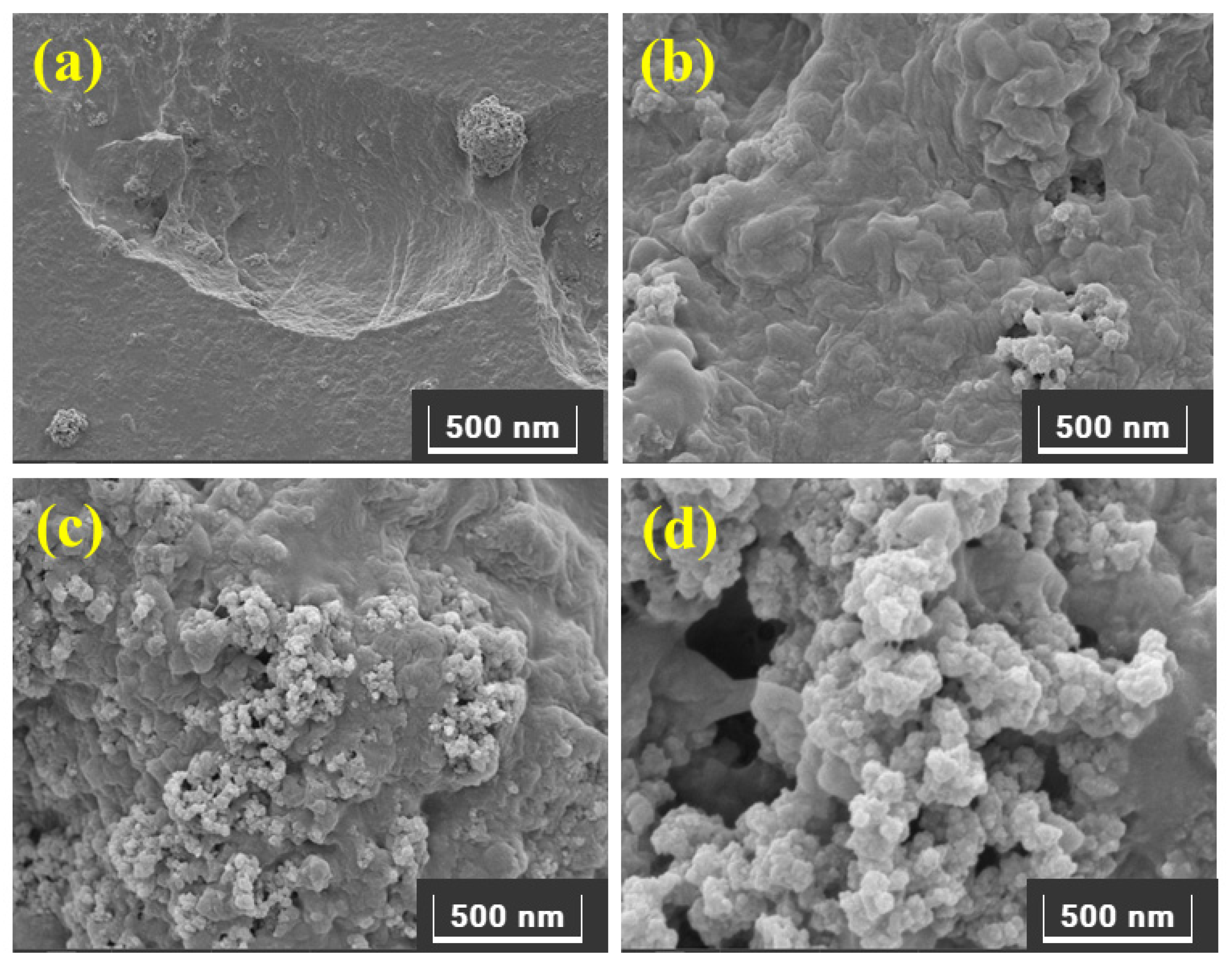
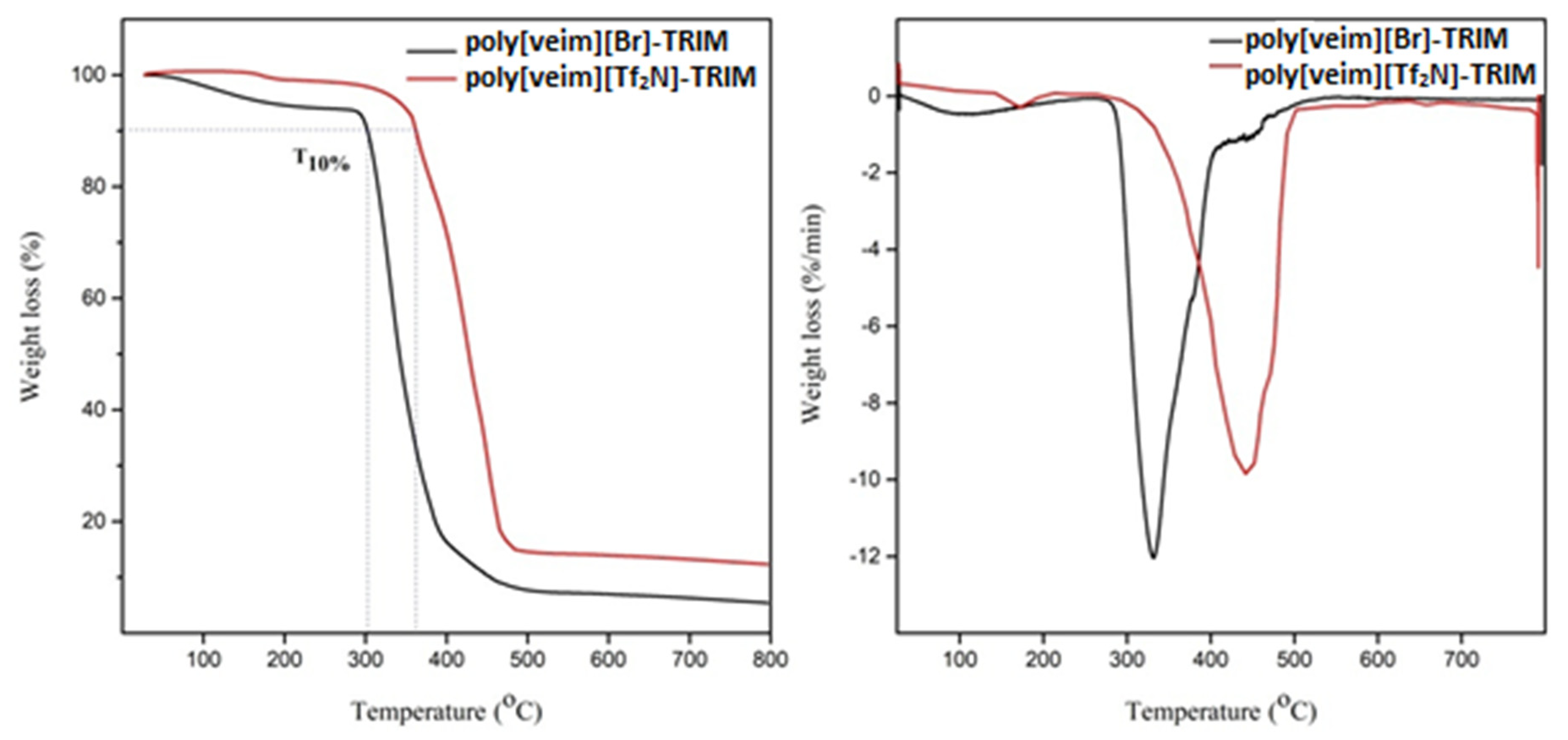
2.4. Characterization of Synthesized IL Monomer and Polymers
2.5. Dye Adsorption Experiments
2.6. Desorption
3. Results and Discussions
3.1. Synthesis and Characterization of PIL-TRIM Adsorbent
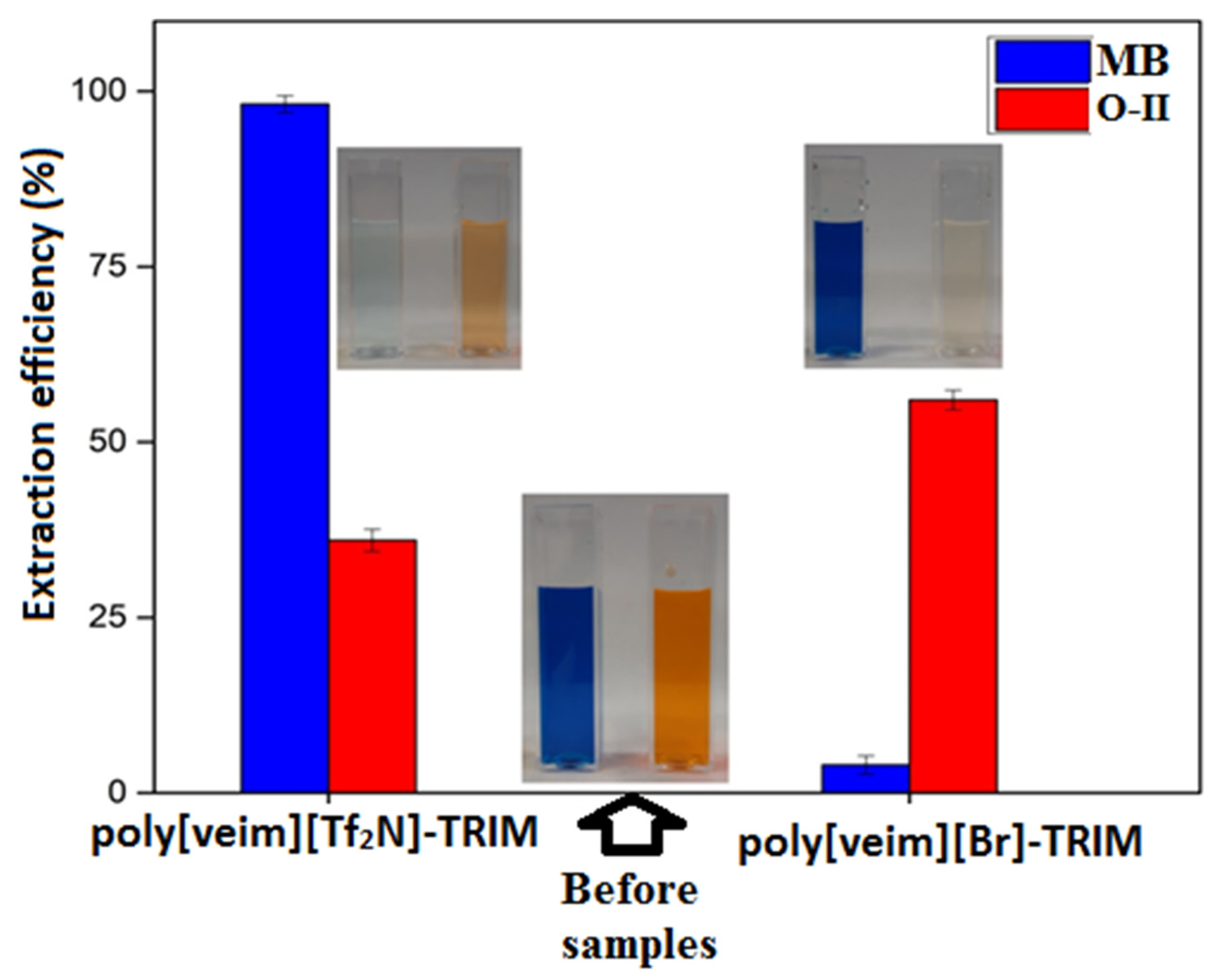
3.2. Study of Adsorption Performance
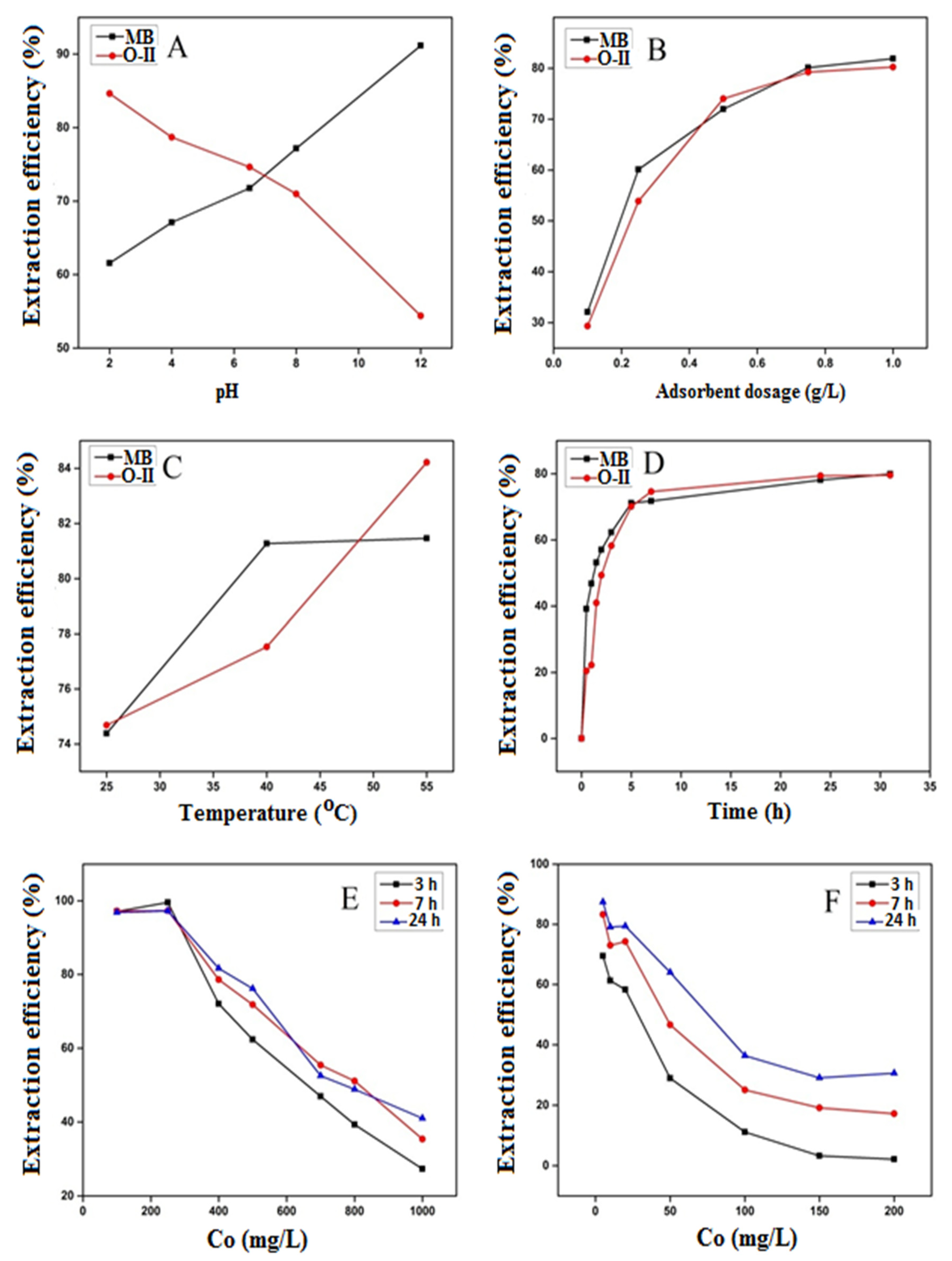
3.2.1. Effect of Solution pH
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Temperature
3.2.4. Effect of Contact Time
3.2.5. Effect of Dye Concentration
3.3. Adsorption Kinetic Study
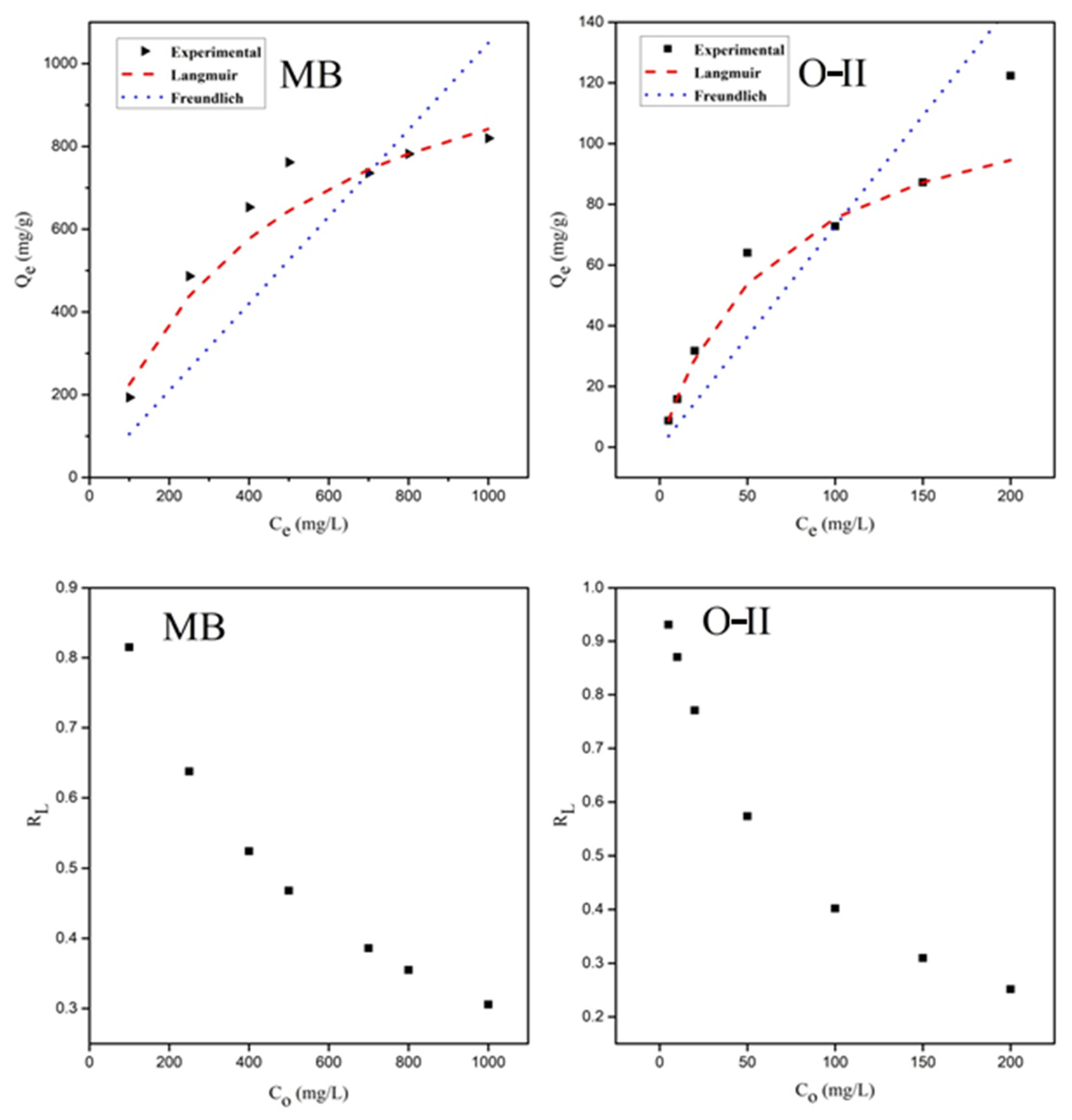
3.4. Adsorption Isotherms
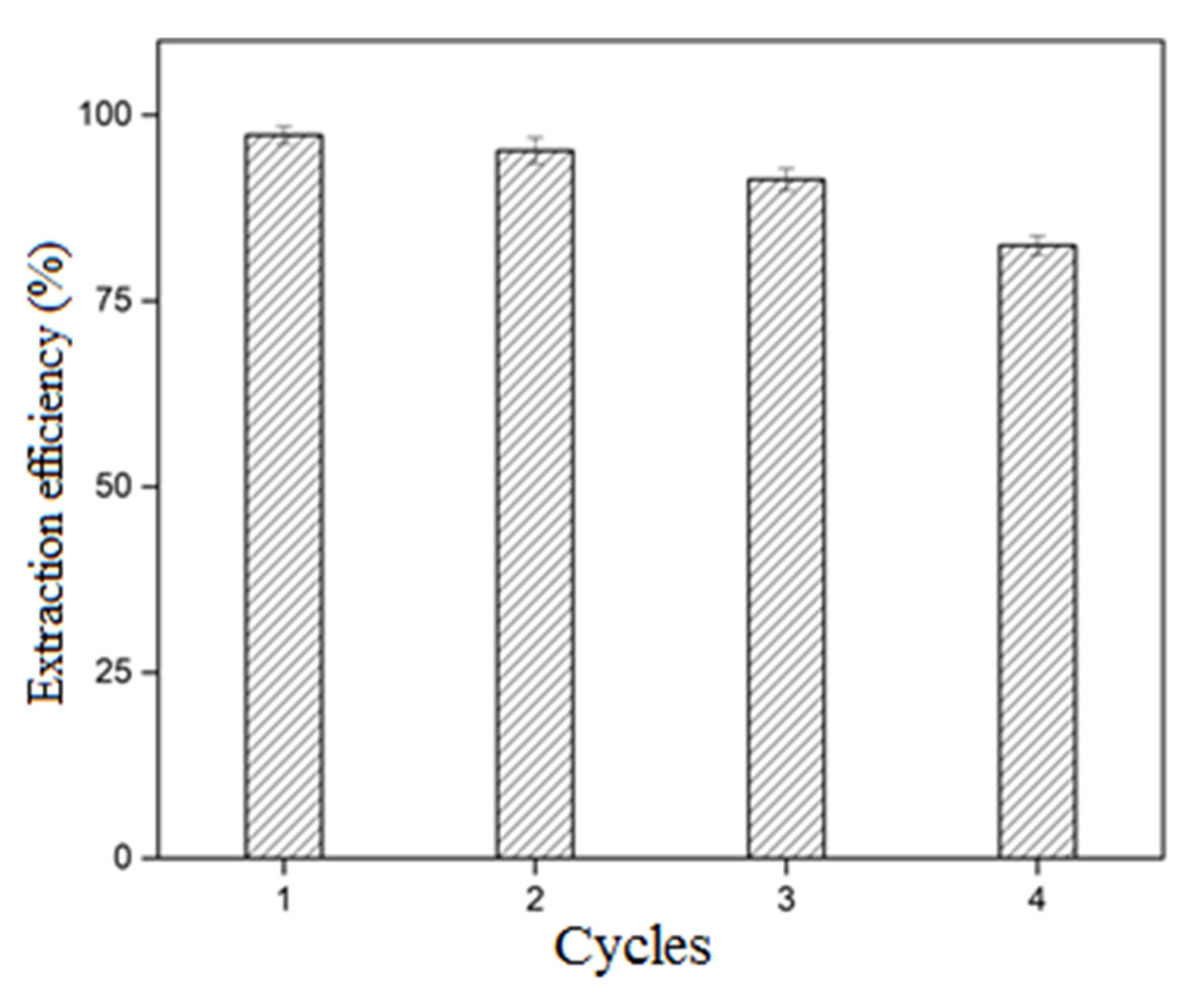
3.5. Reusability Performance
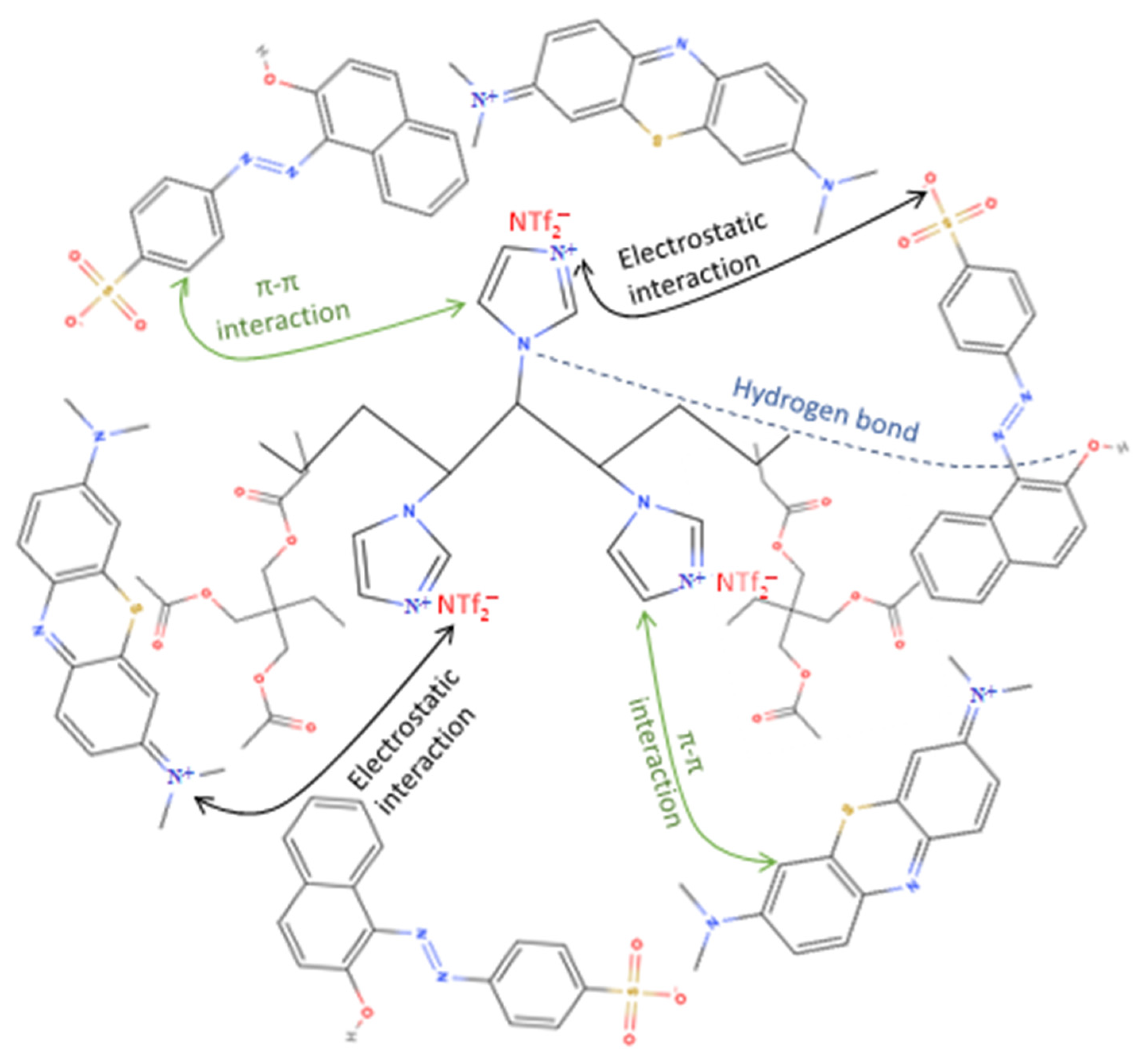
4. Adsorption Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brüschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Wever, J.; Münzner, R.; Renner, H.W. Testing of sunset yellow and orange II for genotoxicity in different laboratory animal species. Environ. Mol. Mutagen. 1989, 13, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kermanioryani, M.; Mutalib, M.I.A.; Kurnia, K.A.; Lethesh, K.C.; Krishnan, S.; Leveque, J.-M. Enhancement of π–π aromatic interactions between hydrophobic Ionic Liquids and Methylene Blue for an optimum removal efficiency and assessment of toxicity by microbiological method. J. Clean. Prod. 2016, 137, 1149–1157. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Bi, G.; Liu, J.; Wang, Z.; Xu, Q.; Xu, H.; Li, X. Mussel-inspired polydopamine biopolymer decorated with magnetic nanoparticles for multiple pollutants removal. J. Hazard. Mater. 2014, 270, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Yang, L.; Zhou, L.; You, T. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A 2015, 3, 10031–10037. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Karan, C.K.; Bhattacharjee, M. Self-Healing and Moldable Metallogels as the Recyclable Materials for Selective Dye Adsorption and Separation. ACS Appl. Mater. Interfaces 2016, 8, 5526–5535. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.-H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nat. Cell Biol. 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Meksi, N.; Moussa, A. A review of progress in the ecological application of ionic liquids in textile processes. J. Clean. Prod. 2017, 161, 105–126. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Moniruzzaman, M.; Reddy, A.V.B.; Talukder, M.R.; Yusup, S.B.; Goto, M. An environmentally benign ionic liquid based formulation for enhanced oil spill remediation: Optimization of environmental factors. J. Mol. Liq. 2020, 314, 113603. [Google Scholar] [CrossRef]
- Khan, H.W.; Reddy, A.V.B.; Nasef, M.M.E.; Bustam, M.A.; Goto, M.; Moniruzzaman, M. Screening of ionic liquids for the extraction of biologically active compounds using emulsion liquid membrane: COSMO-RS prediction and experiments. J. Mol. Liq. 2020, 309, 113122. [Google Scholar] [CrossRef]
- Kermanioryani, M.; Mutalib, M.A.; Gonfa, G.; El-Harbawi, M.; Mazlan, F.A.; Lethesh, K.C.; Leveque, J.-M. Using tunability of ionic liquids to remove methylene blue from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2327–2332. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Umar, A.; Chaudhary, G.R. Ionic liquid and surfactant functionalized ZnO nanoadsorbent for Recyclable Proficient Adsorption of toxic dyes from waste water. J. Mol. Liq. 2016, 224, 1294–1304. [Google Scholar] [CrossRef]
- Bu, R.; Chen, F.; Li, J.; Li, W.; Yang, F. Adsorption capability for anionic dyes on 2-hydroxyethylammonium acetate-intercalated layered double hydroxide. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 511, 312–319. [Google Scholar] [CrossRef]
- Shojaeipoor, F.; Elhamifar, D.; Masoumi, B.; Elhamifar, D.; Barazesh, B. Ionic liquid based nanoporous organosilica supported propylamine as highly efficient adsorbent for removal of congo red from aqueous solution. Arab. J. Chem. 2019, 12, 4171–4181. [Google Scholar] [CrossRef]
- Baharuddin, S.H.; Mustahil, N.A.; Reddy, A.V.B.; Abdullah, A.A.; Mutalib, M.I.A.; Moniruzzaman, M. Development, formulation and optimization of a novel biocompatible ionic liquids dispersant for the effective oil spill remediation. Chemosphere 2020, 249, 126125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, G.; Lu, T.; Cao, Z.; Wang, L.; Li, Q.; Fan, J. An ionic liquid functionalized polymer for simultaneous removal of four phenolic pollutants in real environmental samples. J. Hazard. Mater. 2019, 373, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, H.; Wang, W.-J.; Li, B.-G.; Zhu, S. Collectable and Recyclable Mussel-Inspired Poly(ionic liquid)-Based Sorbents for Ultrafast Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 2829–2835. [Google Scholar] [CrossRef]
- Hu, C.-C.; Gao, Q.; Liu, S.; Chang, L.-L.; Xia, K.-S.; Han, B.; Zhou, C.-G. Crosslinked poly (ionic liquid) anchored with organic probe as a new promising platform for organic solvent-free recognition, quantification, and selective removal of heavy metal ion. Chem. Eng. J. 2018, 346, 458–465. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Wang, S.; Ma, H.-L.; Peng, J.; Zhang, Y.; Chen, J.; Wang, L.; Xu, L.; Li, J.; Zhai, M. Facile synthesis of a novel polymeric ionic liquid gel and its excellent performance for hexavalent chromium removal. Dalton Trans. 2015, 44, 7618–7625. [Google Scholar] [CrossRef]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef]
- Ahmad, N.; Reddy, A.V.B.; Saha, B.B.; Nasruddin; Moniruzzaman, M. Synthesis and characterization of ionic liquid polymer composite with zeolite and its application for carbon dioxide capture. In Proceedings of the IOP Conference Series: Material Science and Engineering, Kuala Lumpur, Malaysia, 13–14 August 2018; Volume 458, p. 012009. [Google Scholar]
- Miao, L.; Duan, H.; Liu, M.; Lu, W.; Zhu, D.; Chen, T.; Li, L.; Gan, L. Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors. Chem. Eng. J. 2017, 317, 651–659. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Xu, W.; Ledin, P.A.; Shevchenko, V.V.; Tsukruk, V.V. Architecture, assembly, and emerging applications of branched functional polyelectrolytes and poly (ionic liquid) s. ACS Appl. Mater. Interfaces 2015, 7, 12570–12596. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Jung, K.-W.; Choi, J.-W.; Lee, S.-H.; Lee, Y.J.; Ahn, K.-H. Facile one-pot synthesis of imidazole-functionalized poly-(vinylbenzyl chloride) and its study on the applicability of synthetic dye removal from aqueous system. J. Clean. Prod. 2017, 168, 510–518. [Google Scholar] [CrossRef]
- Makrygianni, M.; Lada, Z.; Manousou, A.; Aggelopoulos, C.; Deimede, V. Removal of anionic dyes from aqueous solution by novel pyrrolidinium-based Polymeric Ionic Liquid (PIL) as adsorbent: Investigation of the adsorption kinetics, equilibrium isotherms and the adsorption mechanisms involved. J. Environ. Chem. Eng. 2019, 7, 103163. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Z.; Xu, L.; Lou, N.; Li, M.; Liu, L. Controllable click synthesis of poly(ionic liquid)s by surfactant-free ionic liquid microemulsions for selective dyes reduction. React. Funct. Polym. 2020, 147, 104464. [Google Scholar] [CrossRef]
- Mu, Z.; Liu, D.; Lv, J.; Chai, D.-F.; Bai, L.; Zhang, Z.; Dong, G.; Li, J.; Zhang, W. Insight into the Highly Efficient Adsorption towards Cationic Methylene Blue Dye with a Superabsorbent Polymer Modified by Esterified Starch. J. Environ. Chem. Eng. 2022, 10, 108425. [Google Scholar] [CrossRef]
- Gong, L.; Wu, W.; Lin, D.; Yang, K. A superhydrophobic and porous polymer adsorbent with large surface area. J. Mater. Chem. A 2020, 9, 254–258. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Kanmani, S.; Varthine, R.H.; Muthu, C.M.M.; Yuvaraj, D. Modelling on the Removal of Dye from Industrial Wastewater Using Surface Improved Enteromorpha intestinalis. Int. J. Environ. Res. 2019, 13, 349–366. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci. Total Environ. 2021, 812, 152456. [Google Scholar] [CrossRef]
- Saravanan, A.; Jeevanantham, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Karishma, S. Enhanced Zn(II) ion adsorption on surface modified mixed biomass—Borassus flabellifer and Aspergillus tamarii: Equilibrium, kinetics and thermodynamics study. Ind. Crops Prod. 2020, 153, 112613. [Google Scholar] [CrossRef]
- Cegłowski, M.; Kurczewska, J.; Lusina, A.; Nazim, T.; Ruszkowski, P. EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery. Polymers 2022, 14, 1027. [Google Scholar] [CrossRef]
- Dadong, L.; Xiaoshuang, W.; Jin, L.; Junbo, L.; Shanshan, T.; Bao, X.; Ruifa, J. Design, preparation and adsorption performances of norfloxacin molecularly imprinted polymers. J. Mol. Graph. Model. 2022, 114, 108197. [Google Scholar]
- Mamman, S.; Suah, F.B.M.; Raaov, M.; Mehamod, F.S.; Asman, S.; Zain, N.N.M. Removal of bisphenol A from aqueous media using a highly selective adsorbent of hybridization cyclodextrin with magnetic molecularly imprinted polymer. R. Soc. Open Sci. 2021, 8, 201604. [Google Scholar] [CrossRef] [PubMed]
- Sonia, B.; Reyhane, A.; Hashemi, S.A.; Mousavi, S.M.; Ghaedi, M. Chapter 7—Introduction to molecularly imprinted polymer, Interface Science and Technology. Elsevier 2021, 33, 511–556. [Google Scholar]
- Reddy, A.V.B.; Moniruzzaman, M.; Bustam, M.A.; Goto, M.; Saha, B.B.; Janiak, C. Ionic liquid polymer materials with tunable nanopores controlled by surfactant aggregates: A novel approach for CO2 capture. J. Mater. Chem. A 2020, 8, 15034–15041. [Google Scholar] [CrossRef]
- Sellergren, B. Polymer- and template-related factors influencing the efficiency in molecularly imprinted solid-phase extractions. TrAC Trends Anal. Chem. 1999, 18, 164–174. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.K.; Gangwar, S.; Misra, N.; Mondal, A.; Brahmachari, G. Combined experimental (FT-IR, UV–visible spectra, NMR) and theoretical studies on the molecular structure, vibrational spectra, HOMO, LUMO, MESP surfaces, reactivity descriptor and molecular docking of Phomarin. J. Mol. Struct. 2015, 1096, 94–101. [Google Scholar] [CrossRef]
- Gao, H.; Kan, T.; Zhao, S.; Qian, Y.; Cheng, X.; Wu, W.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer. J. Hazard. Mater. 2013, 261, 83–90. [Google Scholar] [CrossRef]
- Pitula, S.; Mudring, A.V. Synthesis, structure, and physico-optical properties of manganate (II)-based ionic liquids. Chem. –A Eur. J. 2010, 16, 3355–3365. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition of carboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef]
- Patinha, D.J.; Silvestre, A.J.; Marrucho, I.M. Poly(ionic liquids) in solid phase microextraction: Recent advances and perspectives. Prog. Polym. Sci. 2019, 98, 101148. [Google Scholar] [CrossRef]
- Zambare, R.; Song, X.; Bhuvana, S.; Antony Prince, J.S.; Nemade, P. Ultrafast Dye Removal Using Ionic Liquid–Graphene Oxide Sponge. ACS Sustain. Chem. Eng. 2017, 5, 6026–6035. [Google Scholar] [CrossRef]
- Min, M.; Shen, L.; Hong, G.; Zhu, M.; Zhang, Y.; Wang, X.; Chen, Y.; Hsiao, B.S. Micro-nano structure poly (ether sulfones)/poly (ethyleneimine) nanofibrous affinity membranes for adsorption of anionic dyes and heavy metal ions in aqueous solution. Chem. Eng. J. 2012, 197, 88–100. [Google Scholar] [CrossRef]
- Vimalkumar, A.; Thilagan, J.; Rajasekaran, K.; Raja, C.; Flora, M. Preparation of activated carbon from mixed peels of fruits with chemical activation (K2CO3)-application in adsorptive removal of methylene blue from aqueous solution. Int. J. Environ. Waste Manag. 2018, 22, 260–271. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. -Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Gusain, D.; Dubey, S.; Upadhyay, S.N.; Weng, C.H.; Sharma, Y.C. Studies on optimization of removal of orange G from aqueous solutions by a novel nano adsorbent, nano zirconia. J. Ind. Eng. Chem. 2016, 33, 42–50. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M. Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: Iron-benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Y.; Chen, R.; Feng, C.; Zhang, Z.; Lei, Z.; Yang, Y. A novel tablet porous material developed as adsorbent for phosphate removal and recycling. J. Colloid Interface Sci. 2013, 396, 197–204. [Google Scholar] [CrossRef]
- Fil, B.A.; Özmetin, C. Adsorption of cationic dye from aqueous solution by clay as an adsorbent: Thermodynamic and kinetic studies. J. Chem. Soc. Pak. 2012, 34, 896–906. [Google Scholar]
- Xing, X.; Chang, P.-H.; Lv, G.; Jiang, W.-T.; Jean, J.-S.; Liao, L.; Li, Z. Ionic-liquid-crafted zeolite for the removal of anionic dye methyl orange. J. Taiwan Inst. Chem. Eng. 2016, 59, 237–243. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Zheng, L. Hydroxyl-functionalized ionic liquid-based cross-linked polymer as highly efficient adsorbent for anionic azo dyes removal. Chem. Eng. J. 2013, 234, 372–379. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J. Hazard. Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Song, Y.; Xu, H.; Ren, J. Adsorption study for removal of sunset yellow by ethylenediamine-modified peanut husk. DESALINATION Water Treat. 2015, 57, 17585–17592. [Google Scholar] [CrossRef]
- Ho, Y.; Mckay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Agarwal, S.; Sharma, S. De-coloration of hazardous dye from water system using chemically modified Ficus carica adsorbent. J. Mol. Liq. 2012, 174, 86–94. [Google Scholar] [CrossRef]
- Liu, R.-L.; Liu, Y.; Zhou, X.-Y.; Zhang, Z.-Q.; Zhang, J.; Dang, F.-Q. Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour. Technol. 2014, 154, 138–147. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Vahedi, S.; Tavakoli, O.; Khoobi, M.; Ansari, A.; Faramarzi, M.A. Application of novel magnetic β -cyclodextrin-anhydride polymer nano-adsorbent in cationic dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 80, 452–463. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Guernelli, S.; Noto, R.; Riela, S. Synthesis and Characterization of Halloysite–Cyclodextrin Nanosponges for Enhanced Dyes Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 3346–3352. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Y.; Jin, Y.; Yilihan, P.; Yang, S. Dynamic adsorption of anionic dyes by apricot shell activated carbon. DESALINATION Water Treat. 2013, 53, 2990–2998. [Google Scholar] [CrossRef]
- Marçal, L.; De Faria, E.H.; Saltarelli, M.; Calefi, P.S.; Nassar, E.J.; Ciuffi, K.J.; Trujillano, R.; Vicente, M.A.; Korili, S.A.; Gil, A. Amine-Functionalized Titanosilicates Prepared by the Sol−Gel Process as Adsorbents of the Azo-Dye Orange II. Ind. Eng. Chem. Res. 2011, 50, 239–246. [Google Scholar] [CrossRef]
- Yang, H.; Bai, L.; Wei, D.; Yang, L.; Wang, W.; Chen, H.; Niu, Y.; Xue, Z. Ionic self-assembly of poly(ionic liquid)-polyoxometalate hybrids for selective adsorption of anionic dyes. Chem. Eng. J. 2018, 358, 850–859. [Google Scholar] [CrossRef]


| Name of Dye | Experimental Adsorption | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe,exp | Qe,cal | k1 | R2 | ARE | Qe,cal | k2 | R2 | ARE | |
| MB | 717.7 | 717.3 | 1.054 | 0.951 | 0.076 | 668.3 | 0.0026 | 0.981 | 0.041 |
| O-II | 29.84 | 29.12 | 0.532 | 0.990 | 0.085 | 25.12 | 0.025 | 0.979 | 0.142 |
| Dye Name | Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| Qm (mg/L) | KL | ARE | R2 | KF | n | ARE | R2 | |
| MB | 1212 | 0.002 | 0.081 | 0.968 | 10.26 | 9.77 | 0.277 | 0.870 |
| O-II | 126.32 | 0.014 | 0.078 | 0.970 | 7.97 | 10.95 | 0.362 | 0.960 |
| Name of Adsorbent | Name of Dye | Adsorption Capacity Qm (mg/g) | Reference |
|---|---|---|---|
| Poly[veim][Tf2N]−TRIM | MB | 1212 | This study |
| Graphene hydrogel | 660 | [8] | |
| Fe3O4–βCD–DCA polymer composite [Fe@CDA2] | 333 | [69] | |
| Halloysite-Cyclodextrin nano sponges | 226 | [70] | |
| PIL@PDA@Fe3O4 | 72 | [24] | |
| Poly[veim][Tf2N]−TRIM | O-II | 126 | This work |
| Apricot shell-AC | 14 | [71] | |
| Amino-functionalized titanosilicate | 189 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, A.V.B.; Rafiq, R.; Ahmad, A.; Maulud, A.S.; Moniruzzaman, M. Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms. Molecules 2022, 27, 7775. https://doi.org/10.3390/molecules27227775
Reddy AVB, Rafiq R, Ahmad A, Maulud AS, Moniruzzaman M. Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms. Molecules. 2022; 27(22):7775. https://doi.org/10.3390/molecules27227775
Chicago/Turabian StyleReddy, A. Vijaya Bhaskar, Rehan Rafiq, Aqeel Ahmad, Abdulhalim Shah Maulud, and Muhammad Moniruzzaman. 2022. "Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms" Molecules 27, no. 22: 7775. https://doi.org/10.3390/molecules27227775
APA StyleReddy, A. V. B., Rafiq, R., Ahmad, A., Maulud, A. S., & Moniruzzaman, M. (2022). Cross-Linked Ionic Liquid Polymer for the Effective Removal of Ionic Dyes from Aqueous Systems: Investigation of Kinetics and Adsorption Isotherms. Molecules, 27(22), 7775. https://doi.org/10.3390/molecules27227775








