Cytotoxic and Pro-Apoptotic Effects of Leaves Extract of Antiaris africana Engler (Moraceae)
Abstract
1. Introduction
2. Results
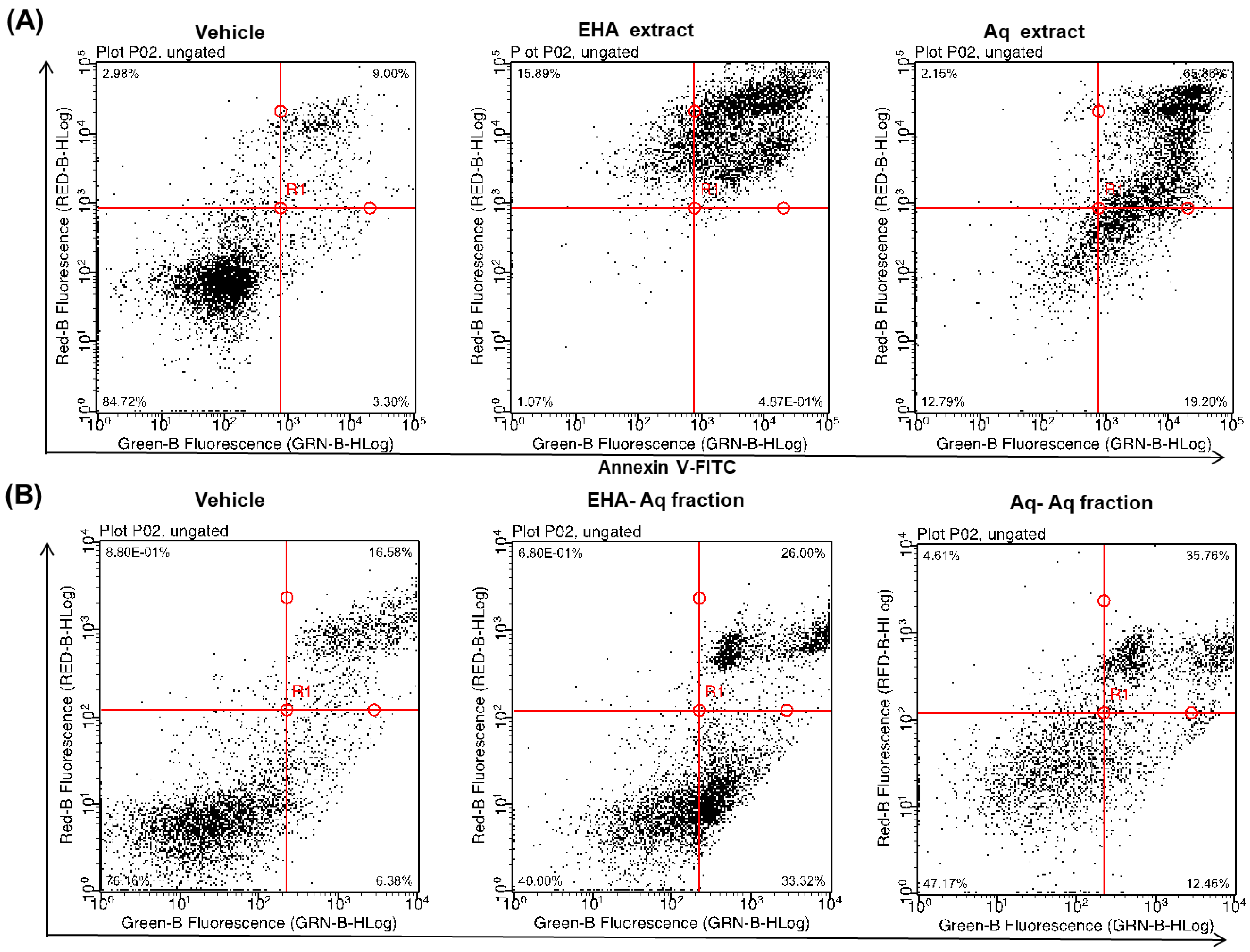
2.1. Pro-Apoptotic Effect of Antiaris Africana Engler Leaves Extracts on MCF-7 Cell Line
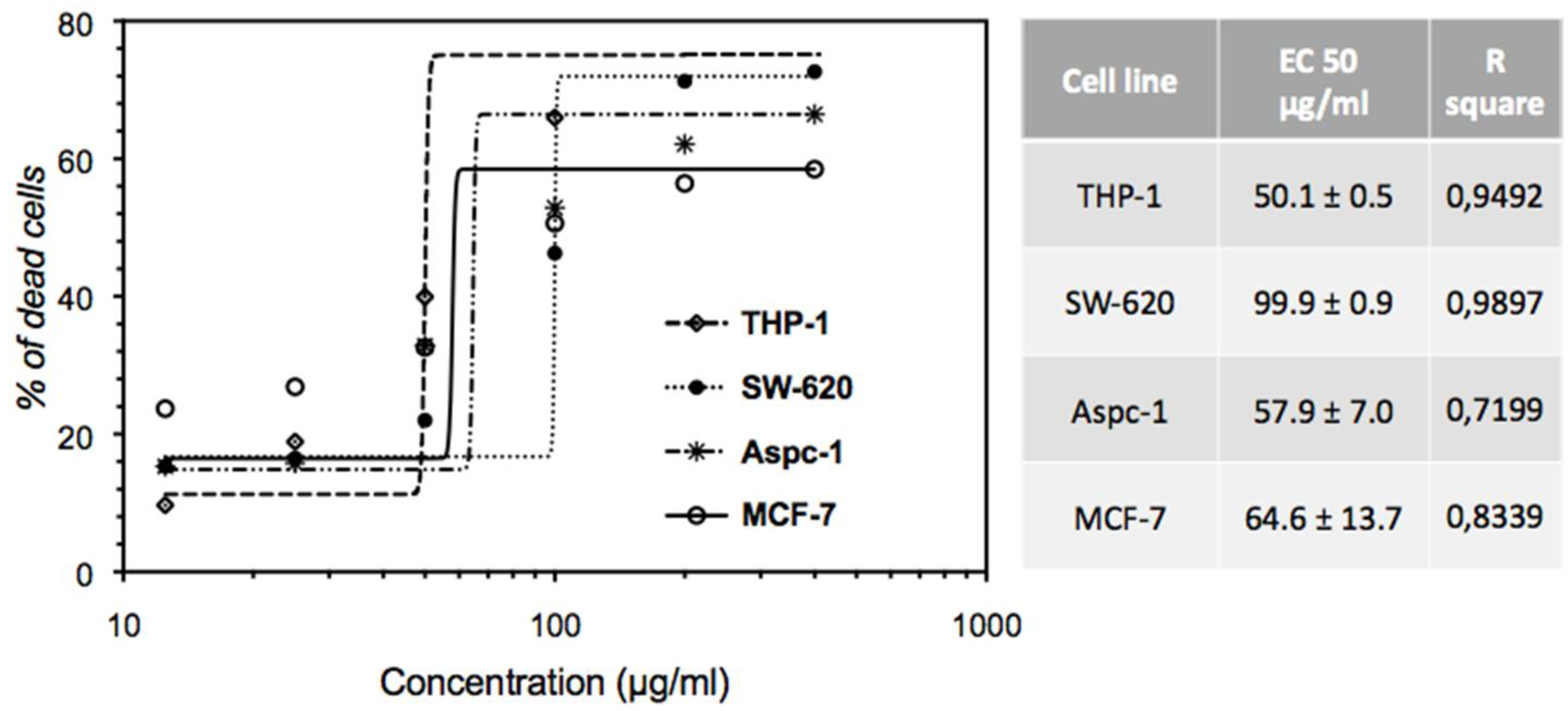
2.2. Anti-Proliferative Effect of EHA-Aq on MCF-7 Cell Line
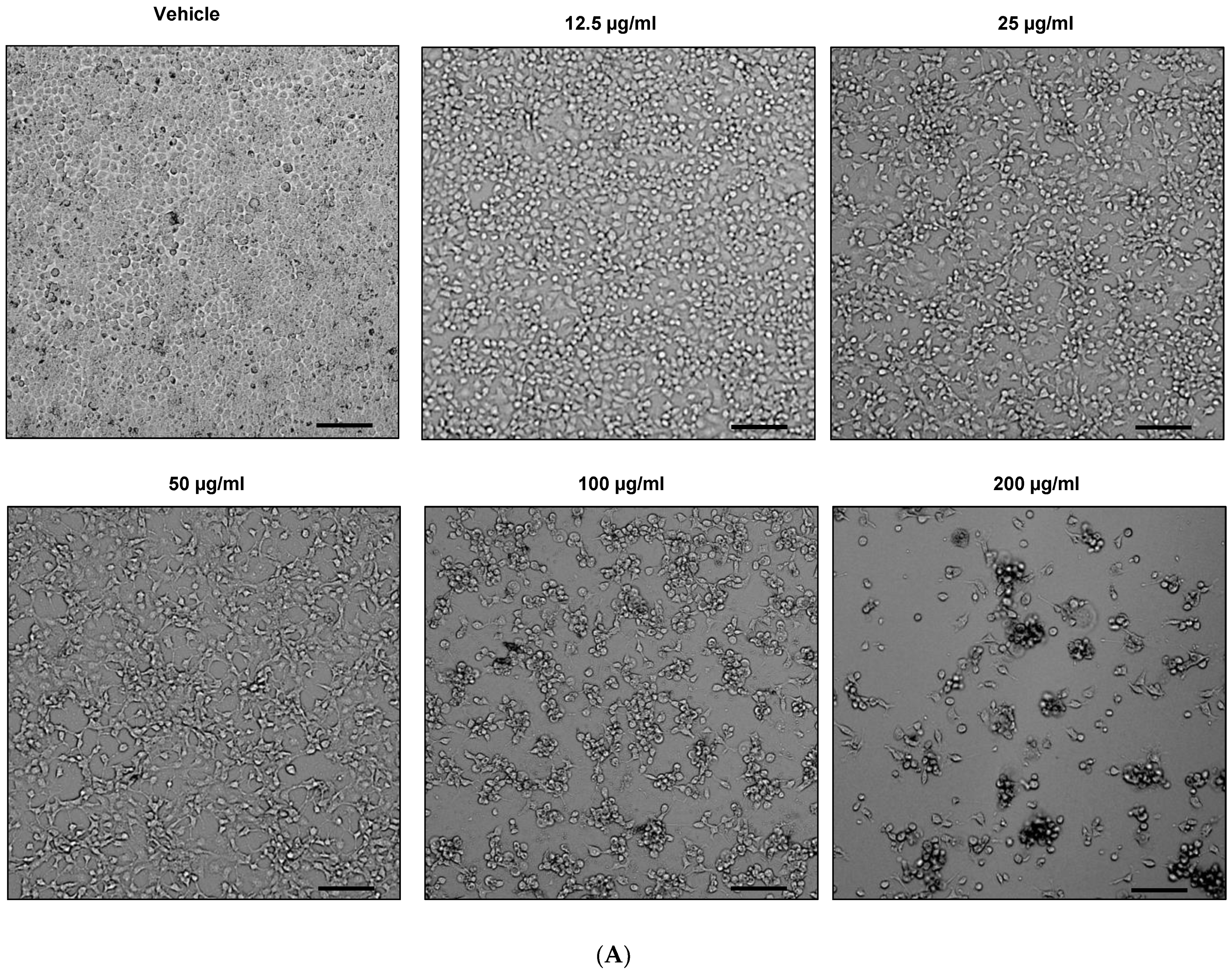
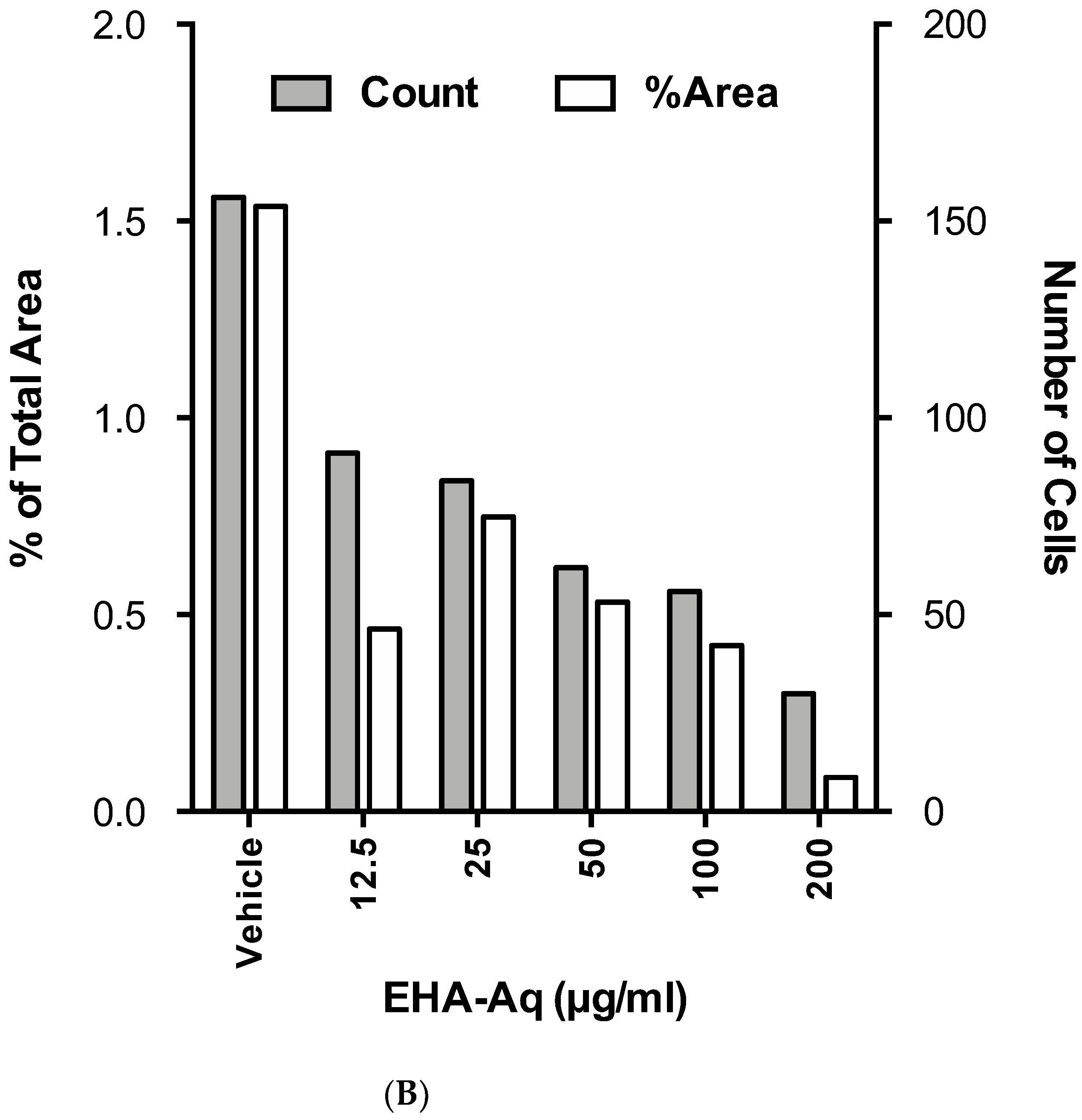
2.3. Effect of EHA-Aq Fraction on Cell Viability
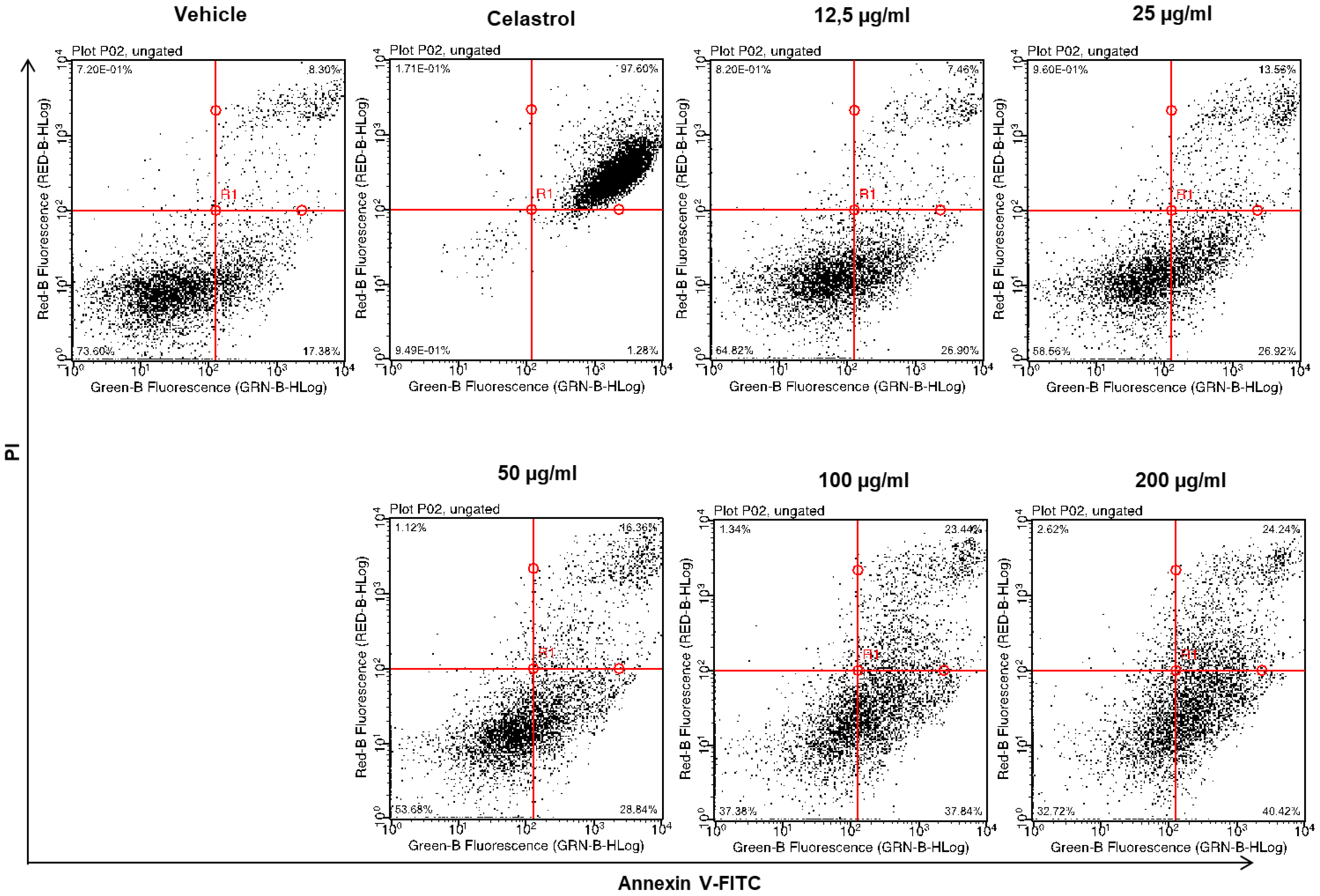
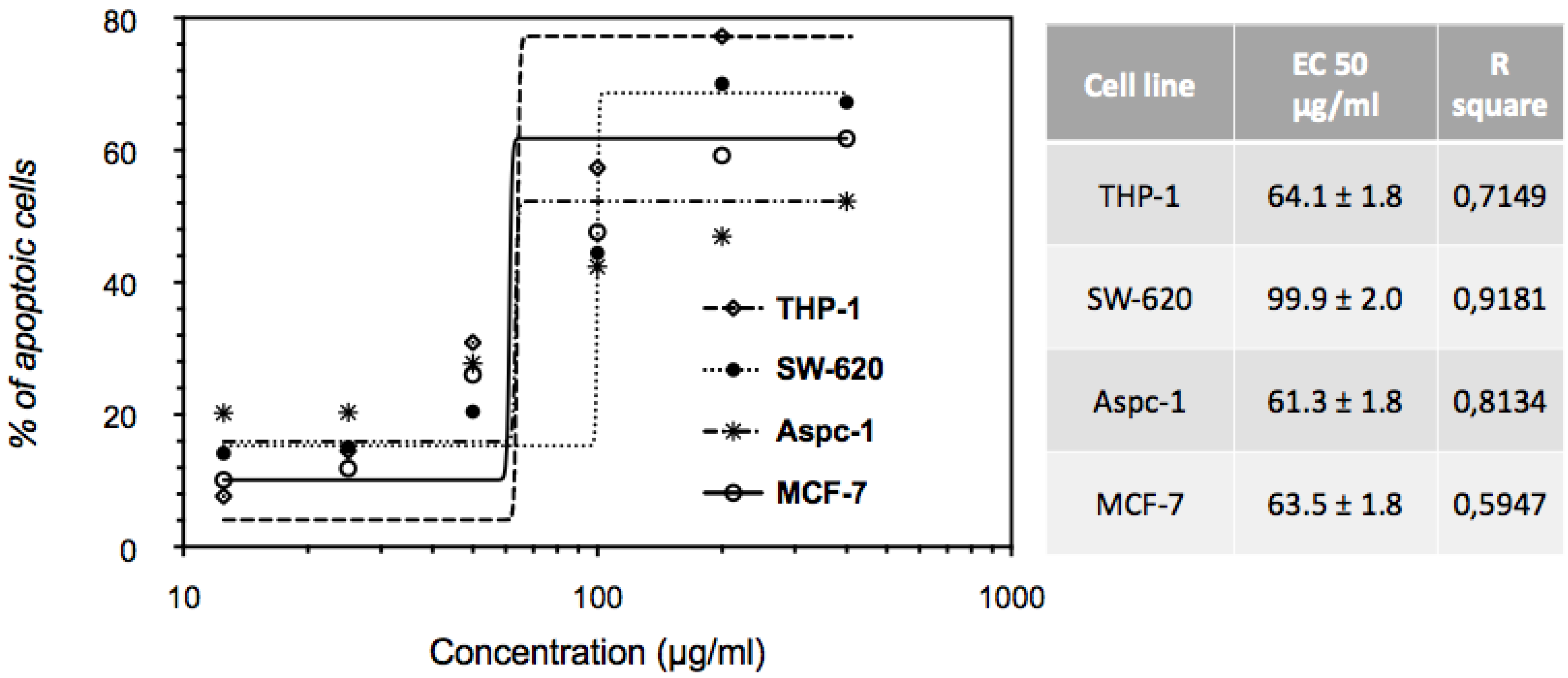
2.4. Dose-Dependent Induction of Apoptosis by EHA-Aq Fraction
2.5. UPLC-DAD-QTOF-MS/MS Analysis of EHA-Aq
3. Discussion
4. Materials and Methods
4.1. Plant Names
4.2. Materials and Reagents
4.3. Sample Preparation
4.3.1. Ultrasonic Extraction
4.3.2. Maceration Extraction
4.4. Cell Culture
4.5. Cytomic Analysis
4.5.1. Cytotoxicity Assay by Microcapillary Flow Cytometry
4.5.2. Cell Growth Assay by Image Cytometry
4.6. Phytochemical Analysis by UPLC-DAD-QTOF-MS/MS
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile |
| ASPC-1 | human pancreatic adenocarcinoma |
| ATCC | American-type culture collection |
| BPC | base peak chromatogram |
| n-BuOH | n-butanol |
| CAKI-1 | human renal cancer cell |
| CGS | cardiac glycosides |
| CO2 | carbon dioxide |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | dimethyl sulfoxide |
| EtOAc | ethyl acetate |
| EtOH | ethanol |
| FSC | forward scatter |
| HCT-9 | human adenocarcinoma colorectal |
| HL-60 | acute promyelocytic leukemia |
| KB | nasopharyngeal epidermoid carcinoma |
| MCF-7 | human breast cancer cell |
| MRC-5 | human diploid embryonic lung cell |
| MS | mass spectrometry |
| NIH-H460 | human lung carcinoma |
| PANC-1 | human epithelioid carcinoma |
| PC-3 | human Caucasian prostate adenocarcinoma |
| PBS | phosphate buffer solution |
| PS | phosphatidylserine |
| PI | propidium iodide |
| RPMI | Roswell Park Memorial Institute |
| SGC-7901 | human gastric cancer cell line |
| SMMC-7721 | human hepatoma cancer cell line |
| SW-620 | human adenocarcinoma colon |
| THP-1 | acute monocytic leukemia |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, A.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Vouffo, B.; Mbaveng, A.T.; Vouffo, E.Y.; Siagat, R.M.; Dongo, E. Evaluation of Antiaris Africana Methanol Extract and Compounds for Antioxidant and Antitumor Activities. Pharm. Biol. 2009, 47, 1042–1049. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera Indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer (Dove Med. Press) 2021, 13, 471–503. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.M.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Rani, N.N.I.M.; Seow, L.J.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Lum, P.T. Hesperidin and Its Aglycone Hesperetin in Breast Cancer Therapy: A Review of Recent Developments and Future Prospects. Saudi J. Biol. Sci. 2021, 28, 6730–6747. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Kouamé, J.; Gnoula, C.; Palé, E.; Bassolé, H.; Guissou, I.P. Etude des propriétés cytotoxiques et anti-radicalaires d’extraits de feuilles et de galles de Guiera senegalensis J. F. Gmel (Combretacae). Sci. St. 2009, 32, 15. [Google Scholar]
- Banso, A.; Mann, A. Evalution of Antibacterial Propeties of Flavonoid Fraction from Antiaris africana (Engl). J. Appl. Biosci. 2008, 12, 665–670. [Google Scholar]
- Vouffo, B.; Dongo, E.; Facey, P.; Thorn, A.; Sheldrick, G.; Maier, A.; Fiebig, H.; Laatsch, H. Antiarol Cinnamate and Africanoside, a Cinnamoyl Triterpene and a Hydroperoxy-Cardenolide from the Stem Bark of Antiaris africana. Planta Med. 2010, 76, 1717–1723. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-Oxidant and pro-Apoptotic Activity of Polyphenol Extract from Annurca Apple and Its Underlying Mechanisms in Human Breast Cancer Cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharm. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-H.; Mei, W.-L.; Zhao, Y.-X.; Zeng, Y.-B.; Zuo, W.-J.; Wang, H.; Li, X.-N.; Dai, H.-F. Cytotoxic Cardenolide Glycosides from the Seeds of Antiaris toxicaria. Planta Med. 2011, 77, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, J.-S.; Hu, M.-J.; Liu, J.; Chen, H.-F.; Gao, H.; Wang, G.-H.; Li, S.-L.; Hao, X.-J.; Zhang, X.-K.; et al. Antiproliferative Cardiac Glycosides from the Latex of Antiaris toxicaria. J. Nat. Prod. 2013, 76, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Hu, M.-J.; Liu, J.; Liu, Q.; Huang, Z.-X.; Li, S.-L.; Hao, X.-J.; Zhang, X.-K.; Yao, X.-S.; Tang, J.-S. Cardiac Glycosides from the Bark of Antiaris toxicaria. Fitoterapia 2014, 97, 71–77. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.; Baehrecke, E.; Blagosklonny, M.; El-Deiry, W.; Golstein, P.; Green, D.; et al. Classification of Cell Death. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Find-Me and Eat-Me Signals in Apoptotic Cell Clearance: Progress and Conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef]
- Dai, H.-F.; Gan, Y.-J.; Que, D.-M.; Wu, J.; Wen, Z.-C.; Mei, W.-L. Two New Cytotoxic Cardenolides from the Latex of Antiaris toxicaria. J. Asian Nat. Prod. Res. 2009, 11, 832–837. [Google Scholar] [CrossRef]
- Dai, H.-F.; Gan, Y.-J.; Que, D.-M.; Wu, J.; Wen, Z.-C.; Mei, W.-L. A New Cytotoxic 19-nor-Cardenolide from the Latex of Antiaris toxicaria. Molecules 2009, 14, 3694–3699. [Google Scholar] [CrossRef]
- Shalaby, A.S.G.; Ragab, T.I.M.; Mehany, A.B.M.; Helal, M.M.I.; Helmy, W.A. Antitumor and Prebiotic Activities of Novel Sulfated Acidic Polysaccharide from Ginseng. Biocatal. Agric. Biotechnol. 2018, 14, 402–409. [Google Scholar] [CrossRef]
- Niepel, M.; Hafner, M.; Duan, Q.; Wang, Z.; Paull, E.O.; Chung, M.; Lu, X.; Stuart, J.M.; Golub, T.R.; Subramanian, A.; et al. Common and Cell-Type Specific Responses to Anti-Cancer Drugs Revealed by High Throughput Transcript Profiling. Nat. Commun. 2017, 8, 1186. [Google Scholar] [CrossRef]
- Heiser, L.M.; Sadanandam, A.; Kuo, W.-L.; Benz, S.C.; Goldstein, T.C.; Ng, S.; Gibb, W.J.; Wang, N.J.; Ziyad, S.; Tong, F.; et al. Subtype and Pathway Specific Responses to Anticancer Compounds in Breast Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, O.B.; Olaleye, T.M.; Akinmoladun, A.C.; Alawode, T.T. HPLC Quantification of Phenolic Content and Assessment of Methanolic Extract of Antiaris africana for Toxicological Study. AJB 2016, 15, 320–330. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of Medicinal Plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, C.; Yu, Z.; Li, X.; Mu, Q.; Liao, G.; Yu, B. Natural Products as LSD1 Inhibitors for Cancer Therapy. Acta Pharm. Sin. B 2020, 11, 621–631. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Quercetin- and Rutin-Based Nano-Formulations for Cancer Treatment: A Systematic Review of Improved Efficacy and Molecular Mechanisms. Phytomedicine 2022, 97, 153909. [Google Scholar] [CrossRef]
- Ilesanmi, O.B.; Akinmoladun, A.C.; Elusiyan, C.A.; Ogungbe, I.V.; Olugbade, T.A.; Olaleye, M.T. Neuroprotective Flavonoids of the Leaf of Antiaris africana Englea against Cyanide Toxicity. J. Ethnopharmacol. 2022, 282, 114592. [Google Scholar] [CrossRef]
- Alía, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of Quercetin and Rutin on Growth and Antioxidant Defense System of a Human Hepatoma Cell Line (HepG2). Eur. J. Nutr. 2006, 45, 19–28. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular Mechanisms of Anticancer Effect of Rutin. Phytother. Res. 2020, 35, 2500–2513. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.-E.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from Chemical Structure and Pharmacological Utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-S.; Kuo, S.-C.; Sun, H.-D.; Shi, L.S.; Kuo, S.C.; Sun, H.D.; Morris-Natschke, S.L.; Lee, K.H.; Wu, T.S. Cytotoxic Cardiac Glycosides and Coumarins from Antiaris toxicaria. Bioorg. Med. Chem. 2014, 22, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.-J.; Dong, W.-H.; Jing-Chen; Zhao, Y.-X.; Chen, H.-Q.; Mei, W.-L.; Dai, H.-F. Two New Strophanthidol Cardenolides from the Seeds of Antiaris toxicaria. Phytochem. Lett. 2013, 6, 1–4. [Google Scholar] [CrossRef]
- Lindholm, H.; Ejeskär, K.; Szekeres, F. Digitoxin Affects Metabolism, ROS Production and Proliferation in Pancreatic Cancer Cells Differently Depending on the Cell Phenotype. Int. J. Mol. Sci. 2022, 23, 8237. [Google Scholar] [CrossRef]
- Hou, J.; Kang, N.; Liu, N.-N.; Tan, D.; Zhang, S.; Liu, J.; Xie, Y. Proscillaridin A Induces Mitochondrial Damage and Autophagy in Pancreatic Cancer and Reduces the Stability of SMAD4 in Panc-1 Cells. Ann. Transl. Med. 2022, 10, 820. [Google Scholar] [CrossRef]
- Francischini, C.R.D.; Mendonça, C.R.; Barcelos, K.A.; Silva, M.A.M.; Botelho, A.F.M. Antitumor Effects of Oleandrin in Different Types of Cancers: Systematic Review. Toxicon 2022, 216, 15–27. [Google Scholar] [CrossRef]
- Eklu-Natey, R.; Balet, A. Pharmacopée africaine. Dictionnaire et monographies mutilingues du potentiel médicinal des plantes africaines (Afrique de l’Ouest). J. Des Afr. 2012, 2, 202–205. [Google Scholar]
- Gill, L.S. Ethnomedical Uses of Plants in Nigeria; Uniben Press: Benin City, Nigeria, 1992; ISBN 978-978-2027-20-7. [Google Scholar]
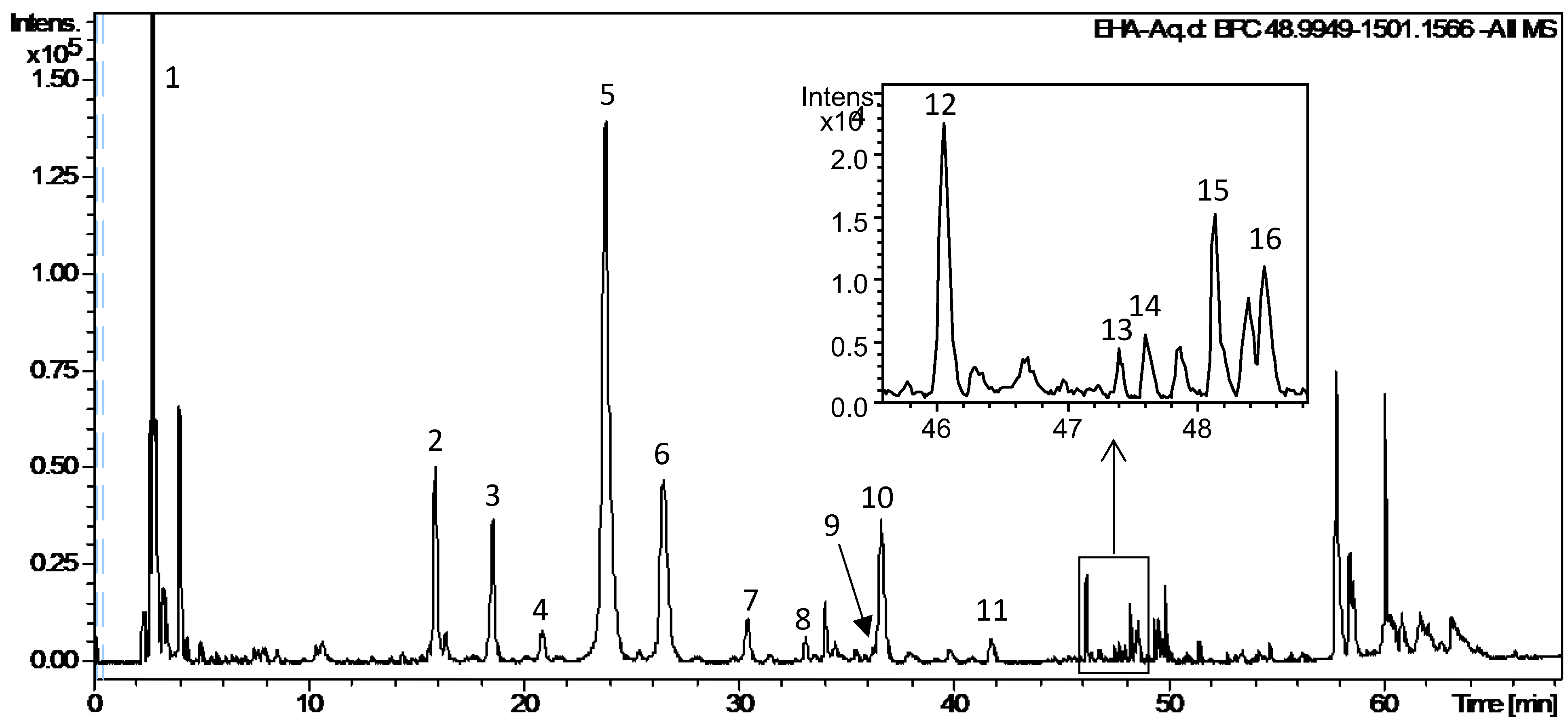
| Peak N° | Rt (min) | UV (nm) | Formula (m/z) [M-H]− | Theo. (m/z) | Exp. (m/z) | Error (ppm) | Fragmentation (m/z) | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.75 | - | C7H11O6 | 191.0561 | 191.0556 | 2.7 | - | Quinic acid |
| 2 | 15.83 | 325, 300sh | C16H17O9 | 353.0878 | 353.0867 | 3.2 | 191.0556; 179.0344; 135.0432 | Chlorogenic acid isomer |
| 3 | 18.50 | 342, 312sh, 290sh, 252 | C32H33O18 | 705.1672 | 705.1651 | 3.0 | 513.1023; 339.0496; 321.0418; 229.0129; 191.0553 | Caffeoylquinic acid derivative (biphenyl type) a |
| 4 | 20.80 | 340, 321, 285sh, 252sh | C32H33O18 | 705.1672 | 705.1662 | 1.5 | 513.1012; 339.0493; 321.0418; 191.0541 | Caffeoylquinic acid derivative (biphenyl type) a |
| 5 | 23.73 | 327, 300sh | C16H17O9 | 353.0878 | 353.0876 | 0.5 | 191.0557 | Chlorogenic acid |
| 6 | 26.44 | 325, 300sh | C16H17O9 | 353.0878 | 353.0868 | 2.8 | 191.0558; 179.0333; 173.0450, 135.0442 | Chlorogenic acid isomer |
| 7 | 30.30 | 320 | C16H17O9 | 353.0878 | 353.0870 | 2.3 | 191.0557 | Chlorogenic acid isomer |
| 8 | 33.03 | 312, 290sh | C16H17O8 | 337.0929 | 337.0922 | 2.0 | 191.0566; 163.0395 | Coumaroylquinic acid isomer |
| 9 | 36.20 | 310sh, 280sh | C16H17O8 | 337.0929 | 337.0923 | 1.6 | 191.0555 | Coumaroylquinic acid isomer |
| 10 | 36.56 | 325, 300sh | C17H19O9 | 367.1035 | 367.1019 | 4.3 | 191.0561 | Feruoylquinic acid |
| 11 | 41.65 | - | C30H45O13[M+HCOO]− | 613.2866 | 613.2828 | 6.1 | 567.2789; 405.2231 | Toxicarioside K or Toxicarioside O |
| 12 | 46.08 | 342 | C27H29O16 | 609.1461 | 609.1433 | 4.6 | 301.0328; 300.0266 | Rutin isomer b |
| 13 | 47.40 | - | C30H45O12 [M+HCOO]− | 597.2917 | 597.2889 | 4.5 | 551.2824; 389.2338 | Antiaritoxioside G |
| 14 | 47.60 | - | C36H53O16[M+HCOO]− | 741.3339 | 741.3299 | 5.4 | 695.3246 ; 533.2776 ; 385.1990 ; 161.0431 | Antiaroside ZC |
| 15 | 48.16 | 325, 290sh | C25H23O12 | 515.1195 | 515.1169 | 5.1 | 353.0841; 191.0564; 179.0339 | Caffeoylquinic acid derivative c |
| 16 | 48.44 | 327, 290sh | C25H23O12 | 515.1195 | 515.1167 | 5.5 | 353.0846; 191.0549; 179.0335 | Caffeoylquinic acid derivative c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiam, K.; Zhao, M.; Marchioni, E.; Muller, C.D.; Diop, Y.M.; Julien-David, D.; Emhemmed, F. Cytotoxic and Pro-Apoptotic Effects of Leaves Extract of Antiaris africana Engler (Moraceae). Molecules 2022, 27, 7723. https://doi.org/10.3390/molecules27227723
Thiam K, Zhao M, Marchioni E, Muller CD, Diop YM, Julien-David D, Emhemmed F. Cytotoxic and Pro-Apoptotic Effects of Leaves Extract of Antiaris africana Engler (Moraceae). Molecules. 2022; 27(22):7723. https://doi.org/10.3390/molecules27227723
Chicago/Turabian StyleThiam, Khadidiatou, Minjie Zhao, Eric Marchioni, Christian D. Muller, Yerim M. Diop, Diane Julien-David, and Fathi Emhemmed. 2022. "Cytotoxic and Pro-Apoptotic Effects of Leaves Extract of Antiaris africana Engler (Moraceae)" Molecules 27, no. 22: 7723. https://doi.org/10.3390/molecules27227723
APA StyleThiam, K., Zhao, M., Marchioni, E., Muller, C. D., Diop, Y. M., Julien-David, D., & Emhemmed, F. (2022). Cytotoxic and Pro-Apoptotic Effects of Leaves Extract of Antiaris africana Engler (Moraceae). Molecules, 27(22), 7723. https://doi.org/10.3390/molecules27227723








