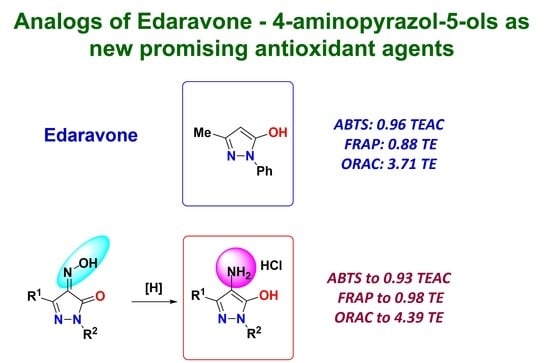
Synthesis of 4-Aminopyrazol-5-ols as Edaravone Analogs and Their Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
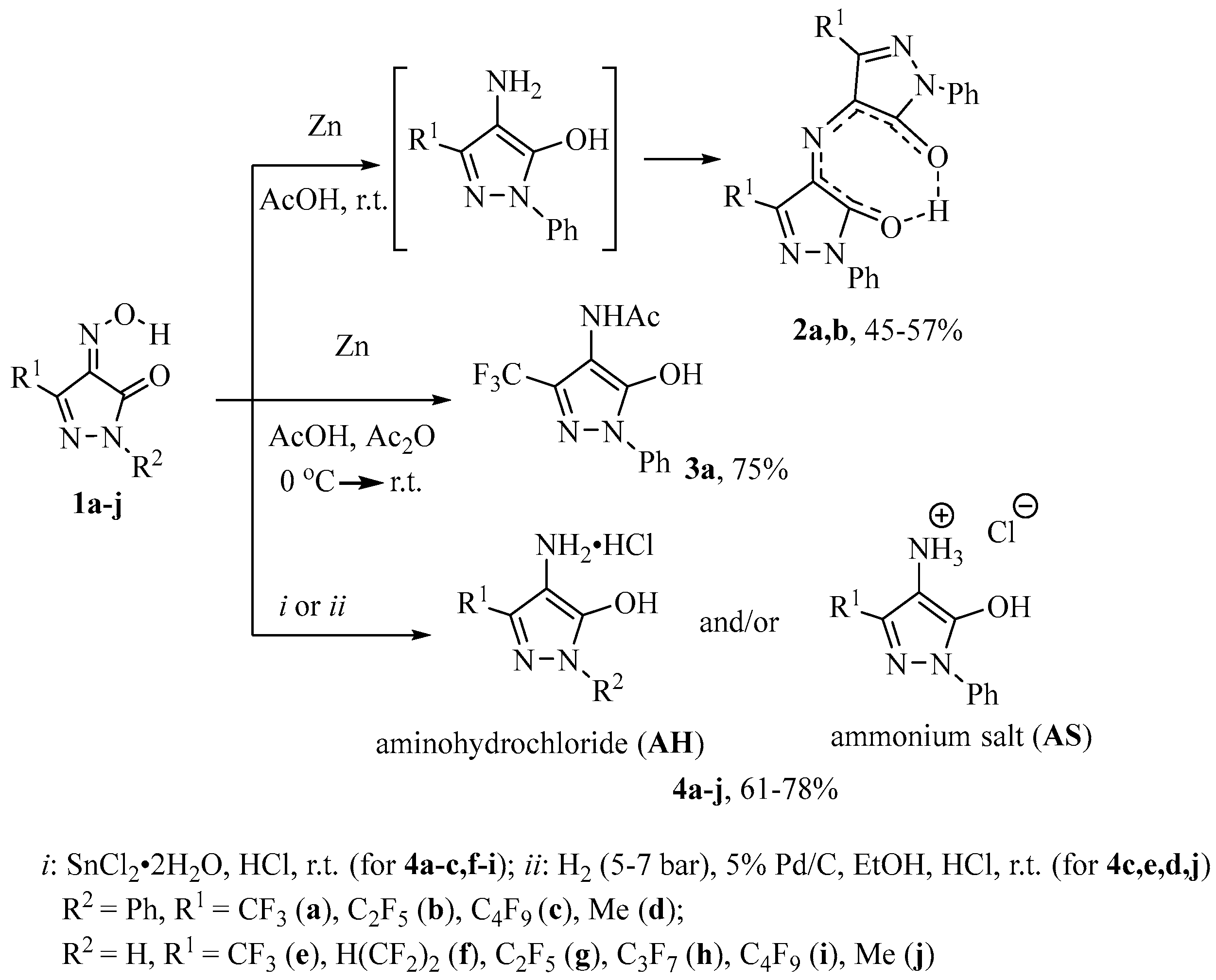
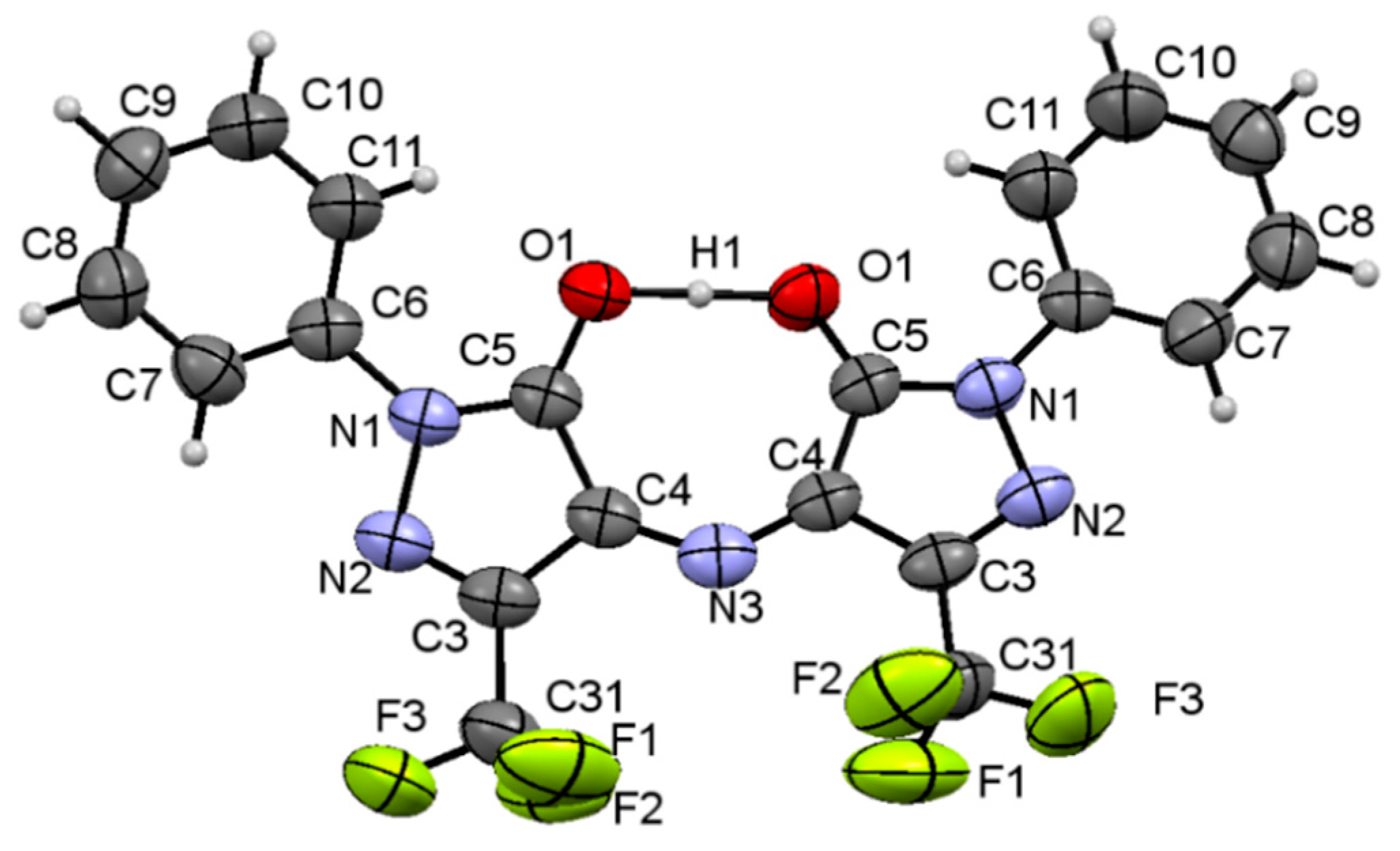
2.1. Chemistry
2.2. Evaluation of Antioxidant Potential of Pyrazoles 1 and 4
2.2.1. ABTS Assay
2.2.2. FRAP Assay
2.2.3. ORAC Assay
2.3. Assessment of Esterase Profile of Pyrazoles 1a–j and 4a–j
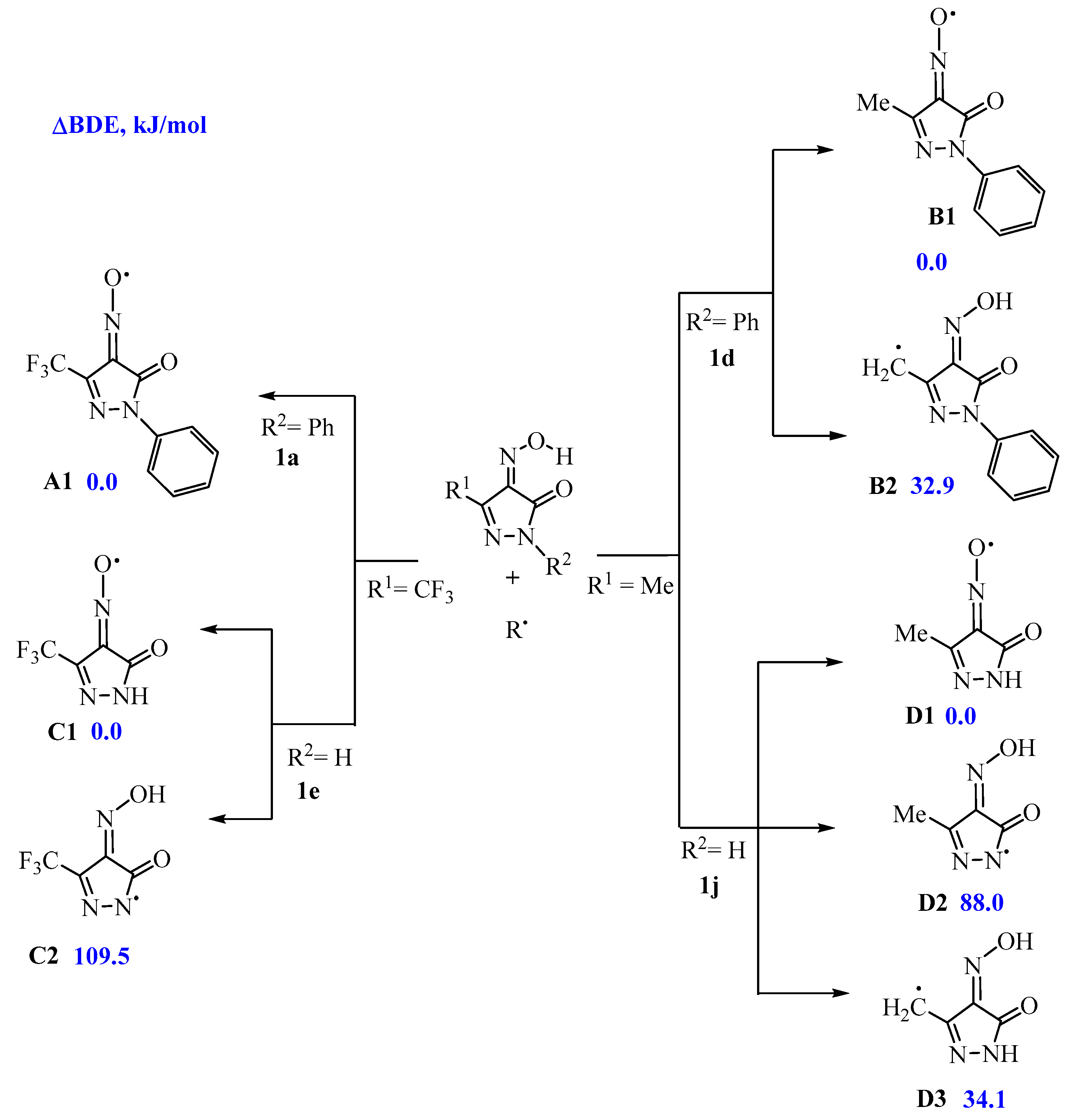
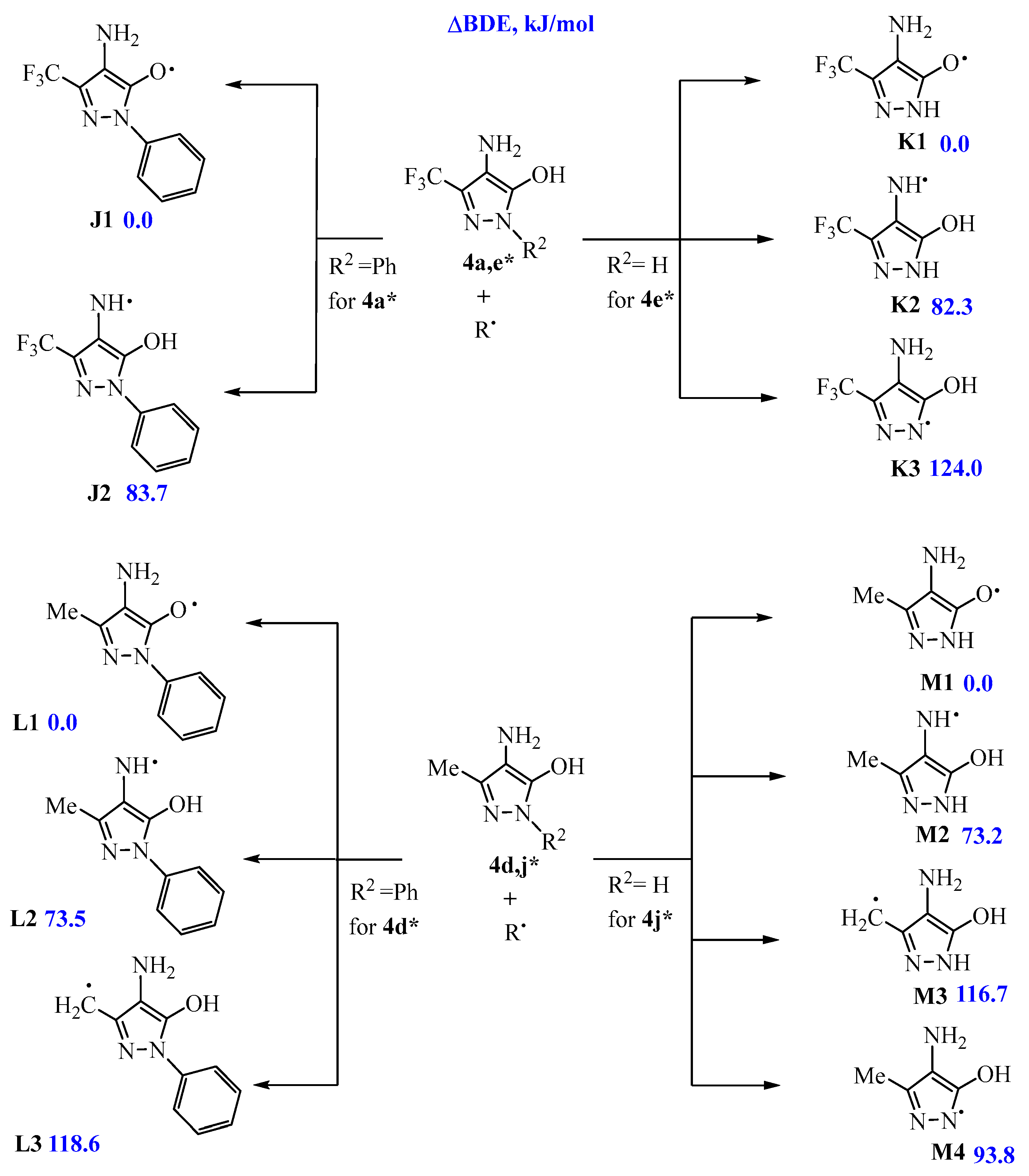
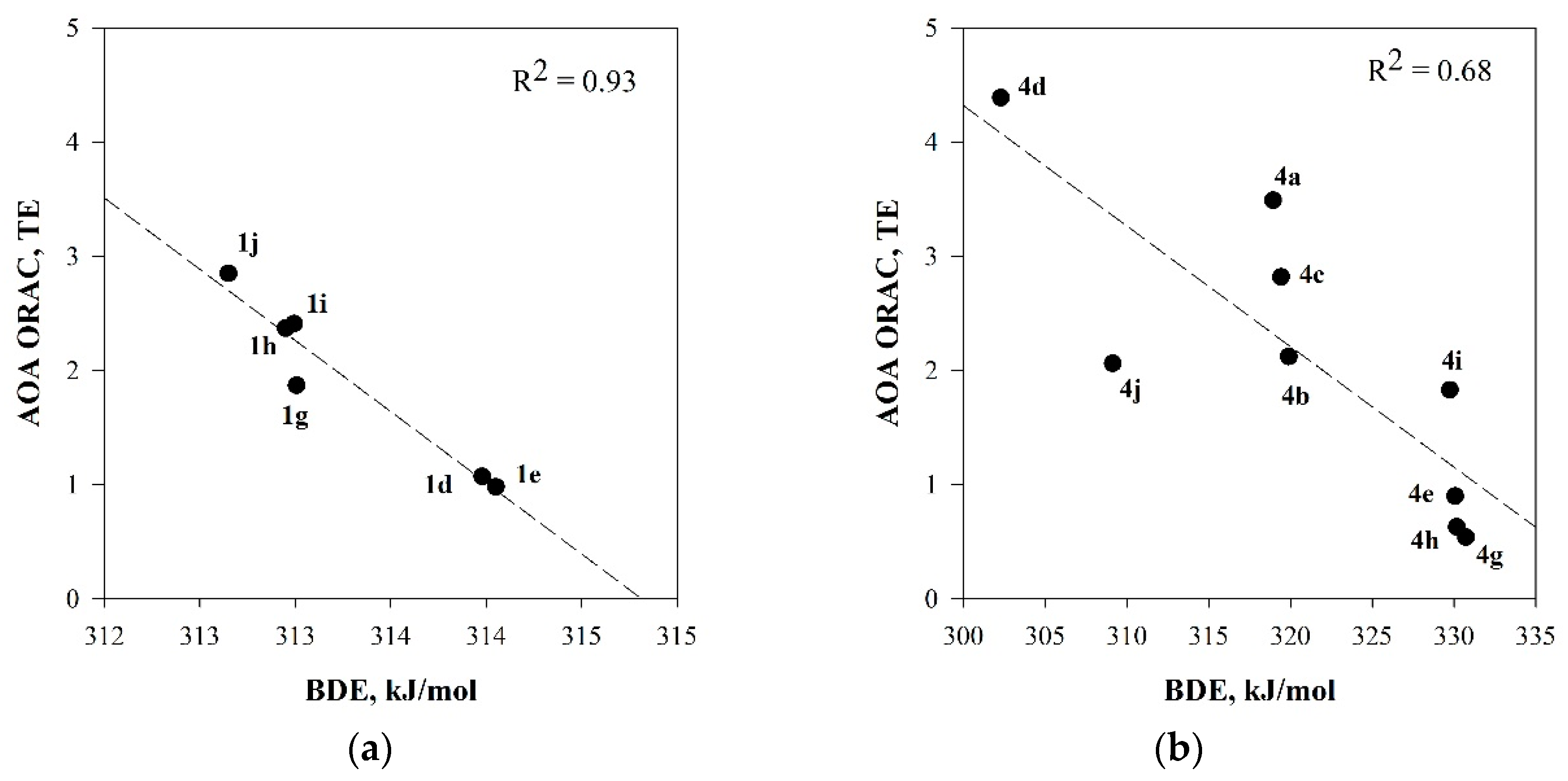
2.4. Quantum-Chemical Calculations of Antioxidant Activity
2.5. Cytotoxicity Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Rubazonic Acids 2a,b (General Procedure)
3.1.2. Synthesis of 4-Aminopyrazoles 4a–j (General Procedure)
3.2. Biochemical Methods
3.2.1. ABTS Radical Cation Scavenging Activity Assay
3.2.2. The FRAP (Ferric Reducing Antioxidant Power) Assay
3.2.3. ORAC Assay
3.2.4. Inhibition In Vitro of Human Erythrocyte AChE, Equine Serum BChE, and Porcine Liver CES
3.2.5. Cytotoxicity Studies
3.3. Quantum-Chemical Calculations
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q. Bridging free radical chemistry with drug discovery: A promising way for finding novel drugs efficiently. Eur. J. Med. Chem. 2020, 189, 112020. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L.M.; Elguero, J.; Silva, A.M.S. Current progress on antioxidants incorporating the pyrazole core. Eur. J. Med. Chem. 2018, 156, 394–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Martins, C.C.; Santos, K.L.B.; Carvalho, E.S.; Owiti, A.O.; Oliveira, K.R.M.; Herculano, A.M.; da Silva, A.B.F.; Borges, R.S. Experimental and theoretical study on structure-tautomerism among edaravone, isoxazolone, and their heterocycles derivatives as antioxidants. Saudi Pharm. J. 2020, 28, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yuki, S.; Kogure, K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988, 19, 480–485. [Google Scholar] [CrossRef]
- Miyaji, Y.; Yoshimura, S.; Sakai, N.; Yamagami, H.; Egashira, Y.; Shirakawa, M.; Uchida, K.; Kageyama, H.; Tomogane, Y. Effect of Edaravone on Favorable Outcome in Patients with Acute Cerebral Large Vessel Occlusion: Subanalysis of RESCUE-Japan Registry. Neurol. Med. Chir. 2015, 55, 241–247. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Q.; Gao, G.; Zhao, M.; Sun, C. Clinical observation in edaravone treatment for acute cerebral infarction. Niger. J. Clin. Pract. 2019, 22, 1324–1327. [Google Scholar] [CrossRef]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Two Decades-Long Journey from Riluzole to Edaravone: Revisiting the Clinical Pharmacokinetics of the Only Two Amyotrophic Lateral Sclerosis Therapeutics. Clin. Pharmacokinet. 2018, 57, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Song, Z.; Li, X.; Huiwang; Zeng, Y.; Qinwang; Meiqi; He, J. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis—A systematic review and meta-analysis. Neurol. Sci. 2018, 40, 235–241. [Google Scholar] [CrossRef]
- Shefner, J.; Heiman-Patterson, T.; Pioro, E.P.; Wiedau-Pazos, M.; Liu, S.; Zhang, J.; Agnese, W.; Apple, S. Long-term edaravone efficacy in amyotrophic lateral sclerosis: Post-hoc analyses of Study 19 (MCI186-19). Muscle Nerve 2019, 61, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers. Int. Immunopharmacol. 2019, 77, 105967. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Bai, F.; Wang, J.; Zheng, R.-H.; Yang, L.-W.; James, E.; Zhao, Z.-Q. Edaravone inhibits pressure overload-induced cardiac fibrosis and dysfunction by reducing expression of angiotensin II AT1 receptor. Drug Des. Dev. Ther. 2017, 11, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Q.; Pang, X.-F.; Zhang, L.-H.; Bai, F.; Wang, N.-P.; McKallip, R.; Garner, R. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Dev. Ther. 2015, 9, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Takeshige, N.; Miura, N.; Morimoto, Y.; Ito, T.; Tancharoen, S.; Miyata, K.E.I.; Kikuchi, C.; Iida, N.; Uchikado, H.; et al. Beyond free radical scavenging: Beneficial effects of edaravone (Radicut) in various diseases (Review). Exp. Ther. Med. 2012, 3, 3–8. [Google Scholar] [CrossRef]
- Raymer, B.; Bhattacharya, S.K. Lead-like Drugs: A Perspective. J. Med. Chem. 2018, 61, 10375–10384. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Jitsuiki, D.; Chayama, K.; Yoshizumi, M. Edaravone (3-Methyl-1-Phenyl-2-Pyrazolin-5-one), A Novel Free Radical Scavenger, for Treatment of Cardiovascular Diseases. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wei, B.; Song, X.; An, N.; Zhou, Y.; Jin, X.; Zhang, Y. Edaravone’s free radical scavenging mechanisms of neuroprotection against cerebral ischemia: Review of the literature. Int. J. Neurosci. 2014, 125, 555–565. [Google Scholar] [CrossRef]
- Kamogawa, E.; Sueishi, Y. A multiple free-radical scavenging (MULTIS) study on the antioxidant capacity of a neuroprotective drug, edaravone as compared with uric acid, glutathione, and trolox. Bioorg. Med. Chem. Lett. 2014, 24, 1376–1379. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kuwahara, T.; Watanabe, K.; Watanabe, K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 2016, 2, 333–338. [Google Scholar] [CrossRef]
- Homma, T.; Kobayashi, S.; Sato, H.; Fujii, J. Edaravone, a free radical scavenger, protects against ferroptotic cell death in vitro. Exp. Cell Res. 2019, 384, 111592. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Hecquet, P.-E.; Kouach, M.; Thuru, X.; Goossens, J.-F. Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone. Bioorg. Med. Chem. 2020, 28, 115463. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Galano, A. OH Radical Scavenging Activity of Edaravone: Mechanism and Kinetics. J. Phys. Chem. B 2010, 115, 1306–1314. [Google Scholar] [CrossRef]
- Yamamoto, Y. Plasma marker of tissue oxidative damage and edaravone as a scavenger drug against peroxyl radicals and peroxynitrite. J. Clin. Biochem. Nutr. 2017, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ohyama, R.; Kimata, A.; Suzuki, T.; Miyata, N. Hydroxyl radical scavenging by edaravone derivatives: Efficient scavenging by 3-methyl-1-(pyridin-2-yl)-5-pyrazolone with an intramolecular base. Bioorg. Med. Chem. Lett. 2006, 16, 5939–5942. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Cena, C.; Giorgis, M.; Rolando, B.; Tosco, P.; Bertinaria, M.; Fruttero, R.; Carrupt, P.-A.; Gasco, A. Edaravone Derivatives Containing NO-Donor Functions. J. Med. Chem. 2008, 52, 574–578. [Google Scholar] [CrossRef]
- Rolando, B.; Filieri, A.; Chegaev, K.; Lazzarato, L.; Giorgis, M.; De Nardi, C.; Fruttero, R.; Martel, S.; Carrupt, P.-A.; Gasco, A. Synthesis physicochemical profile and PAMPA study of new NO-donor edaravone co-drugs. Bioorg. Med. Chem. 2012, 20, 841–850. [Google Scholar] [CrossRef]
- Gaffer, H.E.; Abdel-Fattah, S.; Etman, H.A.; Abdel-Latif, E. Synthesis and Antioxidant Activity of Some New Thiazolyl-Pyrazolone Derivatives. J. Heterocycl. Chem. 2017, 54, 331–340. [Google Scholar] [CrossRef]
- Polkam, N.; Ramaswamy, V.R.; Rayam, P.; Allaka, T.R.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Gandamalla, D.; Yellu, N.R.; Balasubramanian, S.; et al. Synthesis, molecular properties prediction and anticancer, antioxidant evaluation of new edaravone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 2562–2568. [Google Scholar] [CrossRef]
- Qiang, X.; Li, Y.; Yang, X.; Luo, L.; Xu, R.; Zheng, Y.; Cao, Z.; Tan, Z.; Deng, Y. DL-3-n-butylphthalide-Edaravone hybrids as novel dual inhibitors of amyloid- β aggregation and monoamine oxidases with high antioxidant potency for Alzheimer’s therapy. Bioorg. Med. Chem. Lett. 2017, 27, 718–722. [Google Scholar] [CrossRef]
- Purohit, M.K.; Chakka, S.K.; Scovell, I.; Neschadim, A.; Bello, A.M.; Salum, N.; Katsman, Y.; Bareau, M.C.; Branch, D.R.; Kotra, L.P. Structure–activity relationships of pyrazole derivatives as potential therapeutics for immune thrombocytopenias. Bioorg. Med. Chem. 2014, 22, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Ather, M.; Khan, M.U.A.; Saeed, A.; Saleem, R.; Shabir, G.; Channar, P.A. Synthesis and characterization of chromium (III), iron (II), copper (II) complexes of 4-amino-1-(p-sulphophenyl)-3-methyl-5-pyrazolone based acid dyes and their applications on leather. Dyes Pigment. 2016, 130, 90–98. [Google Scholar] [CrossRef]
- Uchida, T.; Kawanishi, T.; Arita, E.; Sato, Y.; Aoki, S.; Ogawa, M.; Nakano, T.; Hasegawa, Y. Preparation of Oxidation Coloring Compounds, 1-(Sulfonated phenyl)-4-amino-5-pyrazolones. Patent JP 2012214419, 31 October 2011. Available online: https://patents.google.com/patent/JP2012214419A/en (accessed on 1 October 2022).
- Isaad, J. Highly water soluble dyes based on pyrazolone derivatives of carbohydrates. Tetrahedron 2013, 69, 2239–2250. [Google Scholar] [CrossRef]
- Drummond, J.; Johnson, G.; Nickell, D.G.; Ortwine, D.F.; Bruns, R.F.; Welbaum, B. Evaluation and synthesis of amino-hydroxy isoxazoles and pyrazoles as potential glycine agonists. J. Med. Chem. 1998, 32, 2116–2128. [Google Scholar] [CrossRef]
- Kawase, M.; Koiwai, H.; Yamano, A.; Miyamae, H. Regioselective reaction of mesoionic 4-trifluoroacetyl-1,3-oxazolium-5-olates and phenylhydrazine: Synthesis of trifluoromethyl substituted pyrazole and 1,2,4-triazine derivatives. Tetrahedron Lett. 1998, 39, 663–666. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Gupta, G.K. Trifluoromethylpyrazoles as anti-inflammatory and antibacterial agents: A review. J. Fluor. Chem. 2015, 178, 306–326. [Google Scholar] [CrossRef]
- Sloop, J.C.; Holder, C.; Henary, M. Selective Incorporation of Fluorine in Pyrazoles. Eur. J. Org. Chem. 2015, 2015, 3405–3422. [Google Scholar] [CrossRef]
- O’Hagan, D. Fluorine in health care: Organofluorine containing blockbuster drugs. J. Fluorine Chem. 2010, 131, 1071–1081. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2020, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Kamble, O.; Dandela, R.; Shinde, S. Recent Innovations of Organo-fluorine Synthesis and Pharmacokinetics. Curr. Org. Chem. 2021, 25, 2650–2665. [Google Scholar] [CrossRef]
- Politanskaya, L.V.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’shin, P.V.; Vinogradov, A.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; et al. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- Jeffries, B.; Wang, Z.; Graton, J.; Holland, S.D.; Brind, T.; Greenwood, R.D.R.; Le Questel, J.-Y.; Scott, J.S.; Chiarparin, E.; Linclau, B. Reducing the Lipophilicity of Perfluoroalkyl Groups by CF2–F/CF2–Me or CF3/CH3 Exchange. J. Med. Chem. 2018, 61, 10602–10618. [Google Scholar] [CrossRef]
- Mohd Faudzi, S.M.; Leong, S.W.; Auwal, F.A.; Abas, F.; Wai, L.K.; Ahmad, S.; Tham, C.L.; Shaari, K.; Lajis, N.H.; Yamin, B.M. In silico studies, nitric oxide, and cholinesterases inhibition activities of pyrazole and pyrazoline analogs of diarylpentanoids. Arch. Pharm. 2020, 354, e2000161. [Google Scholar] [CrossRef]
- Gutti, G.; Kumar, D.; Paliwal, P.; Ganeshpurkar, A.; Lahre, K.; Kumar, A.; Krishnamurthy, S.; Singh, S.K. Development of pyrazole and spiropyrazoline analogs as multifunctional agents for treatment of Alzheimer’s disease. Bioorg. Chem. 2019, 90, 103080. [Google Scholar] [CrossRef]
- Min, L.-J.; Zhai, Z.-W.; Shi, Y.-X.; Han, L.; Tan, C.-X.; Weng, J.-Q.; Li, B.-J.; Zhang, Y.-G.; Liu, X.-H. Synthesis and biological activity of acyl thiourea containing difluoromethyl pyrazole motif. Phosphorus Sulfur Silicon Relat. Elem. 2019, 195, 22–28. [Google Scholar] [CrossRef]
- Tok, F.; Koçyiğit-Kaymakçıoğlu, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new pyrazolone Schiff bases as monoamine oxidase and cholinesterase inhibitors. Bioorg. Chem. 2019, 84, 41–50. [Google Scholar] [CrossRef]
- Turkan, F.; Cetin, A.; Taslimi, P.; Gulçin, İ. Some pyrazoles derivatives: Potent carbonic anhydrase, α-glycosidase, and cholinesterase enzymes inhibitors. Arch. Pharm. 2018, 351, e1800200. [Google Scholar] [CrossRef]
- Bao, X.; Wei, S.; Qian, X.; Qu, J.; Wang, B.; Zou, L.; Ge, G. Asymmetric Construction of a Multi-Pharmacophore-Containing Dispirotriheterocyclic Scaffold and Identification of a Human Carboxylesterase 1 Inhibitor. Org. Lett. 2018, 20, 3394–3398. [Google Scholar] [CrossRef]
- Burgart, Y.V.; Agafonova, N.A.; Shchegolkov, E.V.; Maslova, V.V.; Triandafilova, G.A.; Solodnikov, S.Y.; Krasnykh, O.P.; Saloutin, V.I. New one-pot synthesis of 4-hydroxyimino-5-polyfluoroalkylpyrazol-3-ones, their structure and biological activity. Chem. Heterocycl. Compd. 2019, 55, 52–59. [Google Scholar] [CrossRef]
- Burgart, Y.V.; Agafonova, N.A.; Shchegolkov, E.V.; Krasnykh, O.P.; Kushch, S.O.; Evstigneeva, N.P.; Gerasimova, N.A.; Maslova, V.V.; Triandafilova, G.A.; Solodnikov, S.Y.; et al. Multiple biological active 4-aminopyrazoles containing trifluoromethyl and their 4-nitroso-precursors: Synthesis and evaluation. Eur. J. Med. Chem. 2020, 208, 112768. [Google Scholar] [CrossRef] [PubMed]
- Hänsel, W. 4-(5-Hydroxy-4-pyrazolylimino)-2-pyrazolin-5-one und ihre Metallchelate, I Synthese von 4-(5-Hydroxy-4-pyrazolylimino)-2-pyrazolin-5-onen (Rubazonsäuren) und strukturanalogen Verbindungen. Justus Liebigs Ann. Der Chem. 1976, 1976, 1380–1394. [Google Scholar] [CrossRef]
- Ueda, T.; Oda, N.; Ito, I. Studies on synthetic methods for 5-amino-4(3H)-pyrimidones. I. A novel ring expansion reaction of 4-aminoantipyrines to 5-amino-4(3H)-pyrimidones. Chem. Pharm. Bull. 1980, 28, 2144–2147. [Google Scholar] [CrossRef][Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Elkina, N.A.; Shchegolkov, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Rudakova, E.V.; Kovaleva, N.V.; Radchenko, E.V.; Palyulin, V.A.; et al. Synthesis, molecular docking, and biological evaluation of 3-oxo-2-tolylhydrazinylidene-4,4,4-trifluorobutanoates bearing higher and natural alcohol moieties as new selective carboxylesterase inhibitors. Bioorg. Chem. 2019, 91, 103097. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Kotelnikova, R.A.; Serkov, I.V.; et al. New Multifunctional Agents Based on Conjugates of 4-Amino-2,3-polymethylenequinoline and Butylated Hydroxytoluene for Alzheimer’s Disease Treatment. Molecules 2020, 25, 5891. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food. Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Gotsbacher, M.P.; Telfer, T.J.; Witting, P.K.; Double, K.L.; Finkelstein, D.I.; Codd, R. Analogues of desferrioxamine B designed to attenuate iron-mediated neurodegeneration: Synthesis, characterisation and activity in the MPTP-mouse model of Parkinson’s disease. Metallomics 2017, 9, 852–864. [Google Scholar] [CrossRef]
- Kraus, R.L.; Pasieczny, R.; Lariosa-Willingham, K.; Turner, M.S.; Jiang, A.; Trauger, J.W. Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J. Neurochem. 2005, 94, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Rudakova, E.V.; Serebryakova, O.G.; Aksinenko, A.Y.; Lushchekina, S.V.; Bachurin, S.O.; Richardson, R.J. Esterase profiles of organophosphorus compounds in vitro predict their behavior in vivo. Chem. Biol. Interact. 2016, 259, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Radchenko, E.V.; Palyulin, V.A.; Rudakova, E.V.; Aksinenko, A.Y.; Sokolov, V.B.; Zefirov, N.S.; Richardson, R.J. Organophosphorus compound esterase profiles as predictors of therapeutic and toxic effects. Chem. Biol. Interact. 2013, 203, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Serebryakova, O.G.; Kovaleva, N.V.; Rudakova, E.V.; Elkina, N.A.; Shchegolkov, E.V.; Burgart, Y.V.; Stupina, T.S.; et al. Novel potent bifunctional carboxylesterase inhibitors based on a polyfluoroalkyl-2-imino-1,3-dione scaffold. Eur. J. Med. Chem. 2021, 218, 113385. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Bachurin, S.O.; Richardson, R.J. Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg. Med. Chem. 2016, 24, 1050–1062. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food. Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Jiménez, A.; Selga, A.; Torres, J.L.; Julià, L. Reducing Activity of Polyphenols with Stable Radicals of the TTM Series. Electron Transfer versus H-Abstraction Reactions in Flavan-3-ols. Org. Lett. 2004, 6, 4583–4586. [Google Scholar] [CrossRef]
- Lespade, L.; Bercion, S. Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids. Free Radic. Res. 2012, 46, 346–358. [Google Scholar] [CrossRef]
- Mohajeri, A.; Asemani, S.S. Theoretical investigation on antioxidant activity of vitamins and phenolic acids for designing a novel antioxidant. J. Mol. Struct. 2009, 930, 15–20. [Google Scholar] [CrossRef]
- Amić, D.; Lučić, B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg. Med. Chem. 2010, 18, 28–35. [Google Scholar] [CrossRef]
- Marković, Z.; Milenković, D.; Đorović, J.; Dimitrić Marković, J.M.; Stepanić, V.; Lučić, B.; Amić, D. PM6 and DFT study of free radical scavenging activity of morin. Food Chem. 2012, 134, 1754–1760. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Chu, L.; Wei, Y.; Wang, D.; Cai, S.; Zhou, F.; Ji, B. Relationship Between the Structures of Flavonoids and Oxygen Radical Absorbance Capacity Values: A Quantum Chemical Analysis. J. Phys. Chem. A 2013, 117, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Davidson, E.R. Comment on “Comment on Dunning’s correlation-consistent basis sets”. Chem. Phys. Lett. 1996, 260, 514–518. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Horton, W.; Peerannawar, S.; Török, B.; Török, M. Theoretical and experimental analysis of the antioxidant features of substituted phenol and aniline model compounds. Struct. Chem. 2018, 30, 23–35. [Google Scholar] [CrossRef]
- Klein, E.; Lukeš, V.; Cibulková, Z.; Polovková, J. Study of N–H, O–H, and S–H bond dissociation enthalpies and ionization potentials of substituted anilines, phenols, and thiophenols. J. Mol. Struct. 2006, 758, 149–159. [Google Scholar] [CrossRef]
- Saqib, M.; Mahmood, A.; Akram, R.; Khalid, B.; Afzal, S.; Kamal, G.M. Density functional theory for exploring the structural characteristics and their effects on the antioxidant properties. J. Pharm. Appl. Chem. 2015, 1, 65–71. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history ofSHELX. Acta Crystallogr. Sec. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, T. Study on Chemotherapeutics. III. Yakugaku Zasshi 1957, 77, 800–802. [Google Scholar] [CrossRef]
- Jo, S.-H.; Ka, E.-H.; Lee, H.-S.; Apostolidis, E.; Jang, H.-D.; Kwon, Y.-I. Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int. J. Appl. Res. Nat. Prod. 2009, 2, 52–60. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sterri, S.H.; Johnsen, B.A.; Fonnum, F. A radiochemical assay method for carboxylesterase, and comparison of enzyme activity towards the substrates methyl [1-14C] butyrate and 4-nitrophenyl butyrate. Biochem. Pharmacol. 1985, 34, 2779–2785. [Google Scholar] [CrossRef]
- Pearson, R.G. Hardness of Closed Systems. In Chemical Reactivity Theory: A Density Functional View; Chattaraj, P.K., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 155–162. [Google Scholar]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; John Wiley & Sons: Chichester, UK, 2004; pp. 330–331. [Google Scholar]

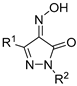
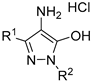
| No. | Compound | Antioxidant Activity (Mean ± SEM) | |||
|---|---|---|---|---|---|
| R1 | R2 | ABTS (n = 3; 1 h) | FRAP (n = 3; 1 h) | ORAC-FL (n = 3; 2 h) | |
| TEAC * (IC50, μM) ** | TE * | TE * (IC50, μM) ** | |||
 | |||||
| 1a | CF3 | Ph | 0.035 ± 0.002 (n.d.) | n.a. | n.a. |
| 1b | C2F5 | Ph | 0.03 ± 0.002 (n.d.) | 0.06 ± 0.01 | n.a. |
| 1c | C4F9 | Ph | 0.04 ± 0.004 (n.d.) | 0.06 ± 0.01 | n.a. |
| 1d | Me | Ph | 0.16 ± 0.02 (n.d.) | n.a. | 1.07 ± 0.17 |
| 1e | CF3 | H | 0.23 ± 0.01 (n.d.) | 0.07 ± 0.01 | 0.98 ± 0.03 |
| 1f | H(CF2)2 | H | 0.15 ± 0.007 (n.d.) | n.a. | n.d. |
| 1g | C2F5 | H | 0.13 ± 0.04 (n.d.) | 0.13 ± 0.01 | 1.87 ± 0.04 |
| 1h | C3F7 | H | 0.19 ± 0.008 (n.d.) | n.a. | 2.37 ± 0.09 |
| 1i | C4F9 | H | 0.2 ± 0.01 (n.d.) | n.a. | 2.41 ± 0.08 |
| 1j | Me | H | 0.2 ± 0.03 (n.d.) | 0.09 ± 0.01 | 2.85 ± 0.28 |
 | |||||
| 4a | CF3 | Ph | 0.75 ± 0.04 (27.2 ± 2.0) | 0.89 ± 0.05 | 3.49 ± 0.16 (8.8 ± 0.1) |
| 4b | C2F5 | Ph | 0.6 ± 0.03 (34.1 ± 2.0) | 0.75 ± 0.07 | 2.12 ± 0.17 |
| 4c | C4F9 | Ph | 0.85 ± 0.04 (23.4 ± 2.1) | 0.98 ±0.05 | 2.82 ± 0.2 |
| 4d | Me | Ph | 0.93 ± 0.03 (23.3 ± 1.9) | 0.98 ± 0.08 | 4.39 ± 0.04 (6.85 ± 0.7) |
| 4e | CF3 | H | 0.92 ± 0.04 (21.2 ± 1.8) | 1.02 ± 0.02 | 0.90 ± 0.19 |
| 4f | H(CF2)2 | H | 0.50 ± 0.02 (33.2 ± 2.3) | 0.62 ± 0.01 | n.d. |
| 4g | C2F5 | H | 0.73 ± 0.03 (25.7 ± 2.1) | 0.88 ± 0.04 | 0.54 ± 0.03 |
| 4h | C3F7 | H | 0.78 ± 0.04 (24.8 ± 1.6) | 1.03 ± 0.04 | 0.63 ± 0.04 |
| 4i | C4F9 | H | 0.45 ± 0.02 (40.8 ± 1.1) | 0.75 ± 0.05 | 1.83 ± 0.02 |
| 4j | Me | H | 0.95 ± 0.04 (20.1 ± 1.8) | 1.04 ± 0.04 | 2.06 ± 0.16 |
| CF3-EDA |  | 0.95 ± 0.04 (21.4 ± 0.8) | 0.63 ± 0.03 | 3.89 ± 0.04 (6.3 ± 0.2) | |
| EDA | 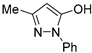 | 0.96 ± 0.04 (21.4 ± 1.1) | 0.80 ± 0.01 | 3.71 ± 0.06 (5.6 ± 0.1) | |
| Trolox | 1.0 (20.1 ± 1.2) | 1.0 | 1.0 (23.6 ± 4.4) | ||
| Quercetin | 1.20 ± 0.11 (13.8 ± 0.7) | 4.99 ± 0.02 | 5.41 ± 0.08 | ||
| No. | Compound | Inhibitory Activity against AChE, BChE, and CES % Inhibition at 20 μM 1 or IC50, μM 2 | |||
|---|---|---|---|---|---|
| R1 | R2 | AChE | BChE | CES | |
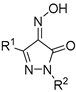 | |||||
| 1a | CF3 | Ph | n.a.3 | n.a. | n.a. |
| 1b | C2F5 | Ph | 11.9 ± 0.9% | 16.1 ± 1.4% | 8.2 ± 1.3% |
| 1c | C4F9 | Ph | 11.5 ± 1.8% | 5.3 ± 1.0% | 7.8 ± 1.4% |
| 1d | Me | Ph | 4.9 ± 1.3% | 6.6 ± 1.3% | n.a. |
| 1e | CF3 | H | n.a. | n.a. | 5.9 ± 1.1% |
| 1f | H(CF2)2 | H | 6.8 ± 1.2% | 4.7 ± 1.0% | 3.5 ± 0.9% |
| 1g | C2F5 | H | n.a. | n.a. | 7.8 ± 1.4% |
| 1h | C3F7 | H | 5.3 ± 1.3% | n.a. | 6.6 ± 1.4% |
| 1i | C4F9 | H | n.a. | n.a. | n.a. |
| 1j | Me | H | n.a. | 3.8 ± 1.3% | n.a. |
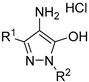 | |||||
| 4a | CF3 | Ph | 7.9 ± 1.5% | 6.9 ± 1.1% | 44.9 ± 4.0 |
| 4b | C2F5 | Ph | 4.4 ± 1.1% | 12.8 ± 1.5% | 16.6 ± 1.4% |
| 4c | C4F9 | Ph | 19.2 ± 1.7% | 10.3 ± 1.2% | 10.2 ± 0.8 |
| 4d | Me | Ph | 6.5 ± 1.2% | 5.1 ± 1.3% | 98.7 ± 8.8 |
| 4e | CF3 | H | 9.7 ± 1.5% | 5.6 ± 1.5% | n.a. |
| 4f | H(CF2)2 | H | 17.3 ± 1.5% | n.a. | 17.5 ± 1.4% |
| 4g | C2F5 | H | 17.3 ± 1.7% | 8.9 ± 1.4% | 6.7 ± 1.2% |
| 4h | C3F7 | H | n.a. | 3.4 ± 0.9% | 81.8 ± 7.3 |
| 4i | C4F9 | H | 5.1 ± 0.9% | n.a. | 21.8 ± 1.7 |
| 4j | Me | H | n.a. | 13.9 ± 1.6% | 4.3 ± 0.9% |
| CF3-EDA | 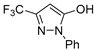 | n.a. | n.a. | 7.6 ± 1.2% | |
| EDA | 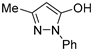 | n.a. | 4.6 ± 0.2% | 8.4 ± 0.7% | |
| R1 | CF3 | C2F5 | C4F9 | Me | CF3 | (CF2)2H | C2F5 | C3F7 | C4F9 | Me |
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Ph | Ph | Ph | Ph | H | H | H | H | H | H |
| Compounds | 4-Hydroxyiminopyrazolones | |||||||||
| 1a | 1b | 1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | |
| IP, eV | 8.45 | 8.44 | 8.44 | 7.98 | 9.84 | 9.61 | 9.78 | 9.76 | 9.77 | 9.10 |
| EA, eV | 1.49 | 1.55 | 1.60 | 0.97 | 1.21 | 1.11 | 1.28 | 1.31 | 1.34 | 0.58 |
| |HOMO-LUMO| (gap), eV | 6.96 | 6.89 | 6.84 | 7.01 | 8.63 | 8.5 | 8.5 | 8.45 | 8.43 | 8.52 |
| η, eV | 3.48 | 3.45 | 3.42 | 3.50 | 4.32 | 4.25 | 4.25 | 4.22 | 4.21 | 4.26 |
| BDE (OH), kJ/mol | 315.4 | 314.3 | 314.3 | 314.0 | 314.0 | 320.7 | 313.0 | 312.9 | 313.0 | 312.7 |
| Compounds | 4-Aminopyrazolols | |||||||||
| 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i | 4j | |
| IP, eV | 6.81 | 6.85 | 6.84 | 6.41 | 7.06 | 7.02 | 7.09 | 7.09 | 7.11 | 6.56 |
| EA, eV | 1.33 | 1.34 | 1.40 | 1.05 | 0.48 | 0.46 | 0.59 | 0.43 | 0.53 | 0.12 |
| |HOMO-LUMO| (gap), eV | −5.47 | −5.51 | −5.44 | −5.36 | −6.58 | −6.56 | −6.50 | −6.67 | −6.58 | −6.43 |
| η, eV | 2.74 | 2.75 | 2.72 | 2.68 | 3.29 | 3.28 | 3.25 | 3.33 | 3.29 | 3.22 |
| BDE (OH), kJ/mol | 318.9 | 319.9 | 319.4 | 302.3 | 330.1 | 328.0 | 330.7 | 330.2 | 329.8 | 309.2 |
| EDA Inhibitory Capability (IC, %) at Increasing Concentrations | ||
|---|---|---|
| 1 µM | 10 µM | 100 µM |
| 3.74 ± 0.05 | 19.9 ± 0.8 | 32.8 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgart, Y.V.; Makhaeva, G.F.; Krasnykh, O.P.; Borisevich, S.S.; Agafonova, N.A.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Shchegolkov, E.V.; Triandafilova, G.A.; et al. Synthesis of 4-Aminopyrazol-5-ols as Edaravone Analogs and Their Antioxidant Activity. Molecules 2022, 27, 7722. https://doi.org/10.3390/molecules27227722
Burgart YV, Makhaeva GF, Krasnykh OP, Borisevich SS, Agafonova NA, Kovaleva NV, Boltneva NP, Rudakova EV, Shchegolkov EV, Triandafilova GA, et al. Synthesis of 4-Aminopyrazol-5-ols as Edaravone Analogs and Their Antioxidant Activity. Molecules. 2022; 27(22):7722. https://doi.org/10.3390/molecules27227722
Chicago/Turabian StyleBurgart, Yanina V., Galina F. Makhaeva, Olga P. Krasnykh, Sophia S. Borisevich, Natalia A. Agafonova, Nadezhda V. Kovaleva, Natalia P. Boltneva, Elena V. Rudakova, Evgeny V. Shchegolkov, Galina A. Triandafilova, and et al. 2022. "Synthesis of 4-Aminopyrazol-5-ols as Edaravone Analogs and Their Antioxidant Activity" Molecules 27, no. 22: 7722. https://doi.org/10.3390/molecules27227722
APA StyleBurgart, Y. V., Makhaeva, G. F., Krasnykh, O. P., Borisevich, S. S., Agafonova, N. A., Kovaleva, N. V., Boltneva, N. P., Rudakova, E. V., Shchegolkov, E. V., Triandafilova, G. A., Gazizov, D. A., Serebryakova, O. G., Ulitko, M. V., Khursan, S. L., Saloutin, V. I., & Richardson, R. J. (2022). Synthesis of 4-Aminopyrazol-5-ols as Edaravone Analogs and Their Antioxidant Activity. Molecules, 27(22), 7722. https://doi.org/10.3390/molecules27227722