Abstract
Human carbonic anhydrase (CA, EC 4.2.1.1) (hCA) isoforms I, II, IX, and XII were investigated for their inhibitory activity with a series of new Schiff’s bases based on quinazoline scaffold 4–27. The hCA I isoform was efficiently inhibited by Schiff’s bases 4–6, 10–19, 22–27 and had an inhibition constant (Ki) value of 52.8–991.7 nM compared with AAZ (Ki, 250 nM). Amongst the quinazoline derivatives, the compounds 2, 3, 4, 10, 11, 16, 18, 24, 26, and 27 were proven to be effective hCA II inhibitors, with Ki values of 10.8–52.6 nM, measuring up to AAZ (Ki, 12 nM). Compounds 2–27 revealed compelling hCA IX inhibitory interest with Ki values of 10.5–99.6 nM, rivaling AAZ (Ki, 25.0 nM). Quinazoline derivatives 3, 10, 11, 13, 15–19, and 24 possessed potent hCA XII inhibitory activities with KI values of 5.4–25.5 nM vs. 5.7 nM of AAZ. Schiff’s bases 7, 8, 9, and 21 represented attractive antitumor hCA IX carbonic anhydrase inhibitors (CAIs) with KI rates (22.0, 34.8, 49.2, and 45.3 nM, respectively). Compounds 5, 7, 8, 9, 14, 18, 19, and 21 showed hCA I inhibitors on hCA IX with a selectivity index of 22.46–107, while derivatives 12, 14, and 18 showed selective hCA I inhibitors on hCA XII with a selectivity profile of 45.04–58.58, in contrast to AAZ (SI, 10.0 and 43.86). Compounds 2, 5, 7–14, 19–23, and 25 showed a selectivity profile for hCA II inhibitors over hCA IX with a selectivity index of 2.02–19.67, whereas derivatives 5, 7, 8, 13, 14, 15, 17, 20, 21, and 22 showed selective hCA II inhibitors on hCA XII with a selectivity profile of 4.84–26.60 balanced to AAZ (SI, 0.48 and 2.10).
1. Introduction
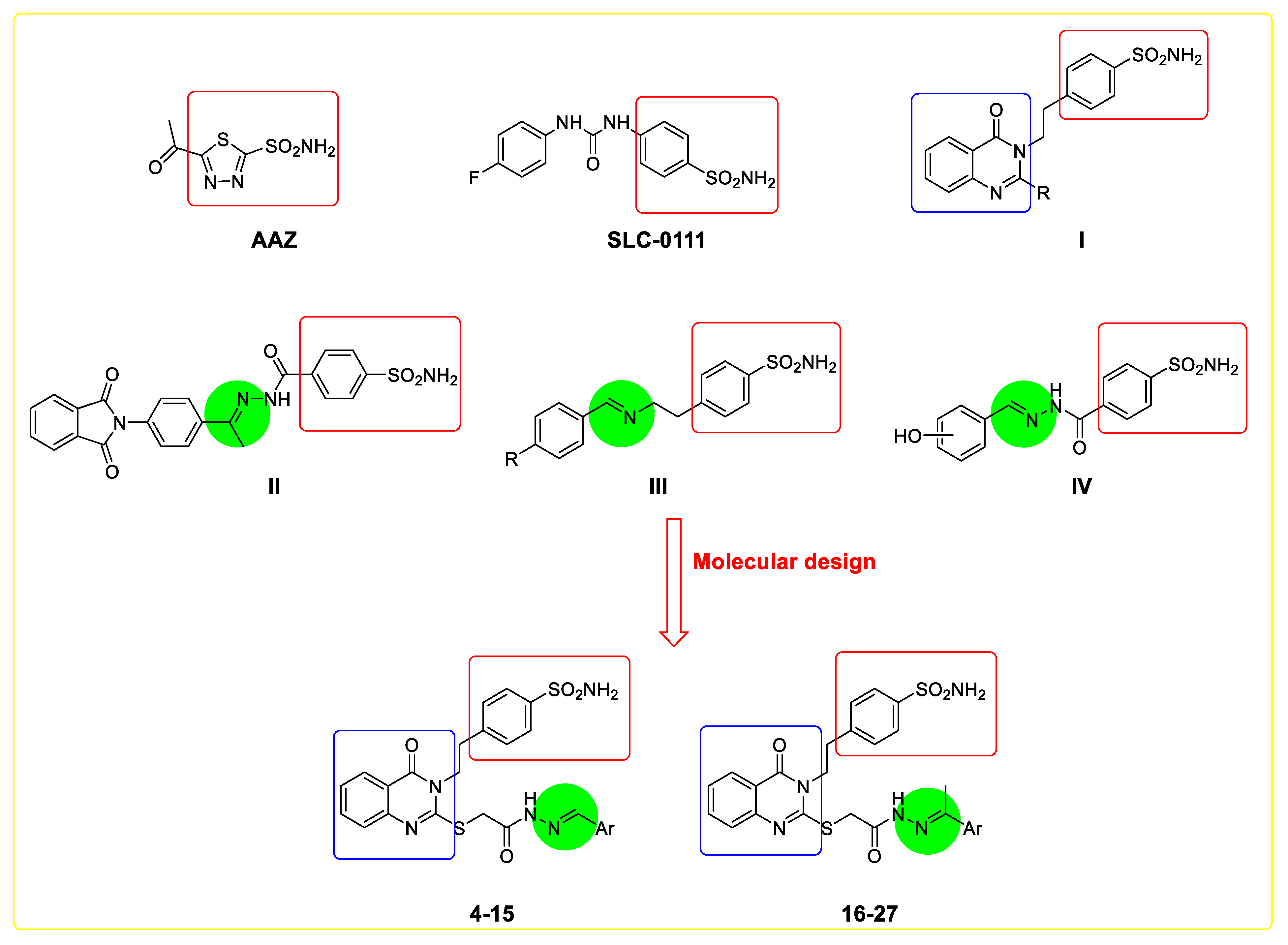
Carbonic anhydrase is a zinc-metalloenzyme (CA, EC 4.2.1.1). In vertebrates, the α-carbonic anhydrases are present in different tissues and differ in function and kinetic patterns [1,2]. Functional impairment of different CA isoforms may lead to several human diseases [1]. CA I and CA II control the acid-base equilibrium throughout the physiological pathways used for cerebral edema, glaucoma, and epilepsy treatment [3]. CA IX and CA XII have been verified in plain tissues. Furthermore, CA IX and CA XII isotypes boost several cancer cells, such as breast, urinary bladder, and lung [4]. Metamorphosis of CO2 to HCO3− and a proton is achieved via CA IX and CA XII. It simplifies the diffusion of the protons inside tumor cells, directing a decrease in extracellular pH and accelerating matrix decomposition, invasion, and drug resistance [1,5]. Significant architectural similarities in CA isoforms necessitate the production of more small compounds with high CA isoform selectivity to treat certain diseases without side effects, which is critical in medicinal chemistry [6]. Sulfonamide derivatives are a preferred class of COX-2 inhibitors and antitumor agents [7,8,9,10,11]. In addition, compounds incorporating sulfonamide fragments are highly used as CA inhibitors [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Furthermore, SLC-0111 (Figure 1) is a sensitive inhibitor of CA IX/XII with a sulfonamide moiety (Phase I) for the rehabilitation of solid metastatic tumors via zinc metalloenzyme fixation [12,13,26,27,28,29]. In pharmaceutical chemistry, the quinazolinone scaffold is also commonly employed [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Interestingly, a family of mercaptoquinazolines (I) could be exploited to develop new efficacious and sensitive CAIs countering various disorders and antitumor activities [32,40,44,45,55,56]. Indeed, Schiff’s bases containing benzenesulfonamide moieties, such as compounds II–IV (Figure 1), displayed versatile inhibition against CAs [13,57,58]. According to the logic mentioned above, this work focused on using quinazolinone scaffold as hCA isoforms inhibitor (C, Figure 1), incorporating ethylbenzenesulfonamide moiety [55,59,60], for (i) the synthesis of novel ester, acid-hydrazide, and Schiff’s bases with activating and deactivating groups; (ii) evaluation results for these new molecules’ CA inhibitory action on the isoenzymes I, II, IX, and XII; (iii) the structure–activity interactions of these Schiff’s bases together with various substituents and the inhibition of CA isoforms I, II, IX, and XII.
Figure 1.
Structures of AAZ, SLC-0111, I-IV, and the designed Schiff’s bases incorporating quinazoline scaffold (4–27) as carbonic anhydrase inhibitors (CAIs).
2. Results and Discussion
2.1. Chemistry
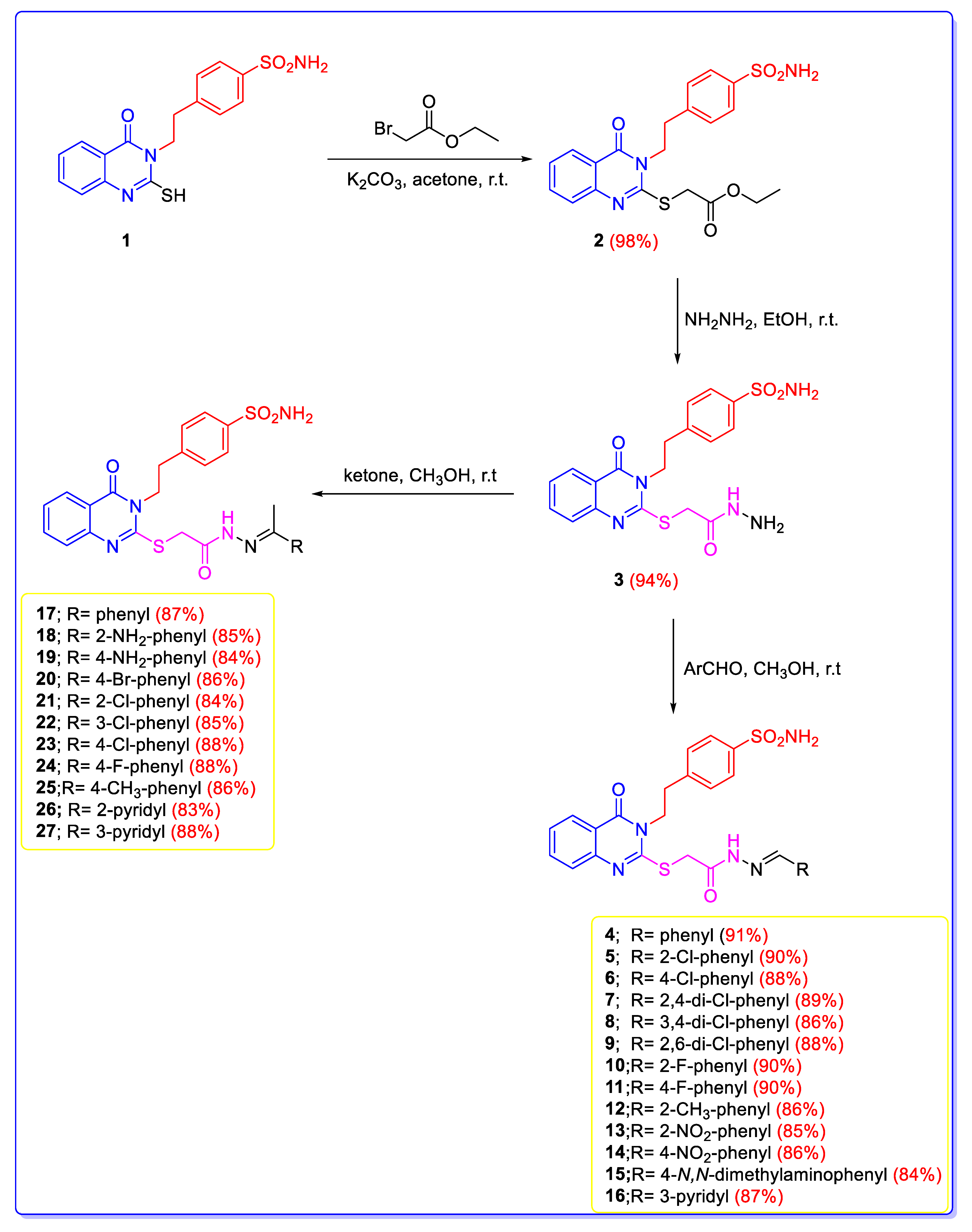
The synthetic pathway of the novel series of 2-mercapto-quinazolines-incorporating benzylidene thioacetohydrazide and phenylethylidene thioacetohydrazide derivatives is shown in Scheme 1. The stirring of 4-(2-(2-mercapto-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (1) with ethyl 2-bromoacetate in acetone and potassium carbonate afforded the corresponding 2-ethyl-thioacetate ester 2 in 98% yield. The ester 2 was assessed by thiomethyl peaks (SCH2-COOCH2CH3) at 4.16 and 33.61 ppm and ethyl ester peaks (SCH2-COOCH2CH3) at 4.28, 1.22, 61.62, and 14.62 ppm in 1H NMR and 13C NMR, respectively, as well as the carbonyl peak (SCH2-COOCH2CH3) at 168.73 ppm in 13C NMR. The acid hydrazide 3 was obtained in 94% yield by stirring 2-ethyl-thioacetate ester 2 with hydrazine hydrate in ethanol. 1H NMR and 13C NMR of compound 3 revealed thiomethyl peaks (SCH2CONHNH2) at 4.02 and 33.60 ppm, respectively. The hydrazide group of thioacetohydrazide moiety (SCH2CONHNH2) was assessed by peaks at 9.42 and 4.37 ppm in 1H NMR, together with the carbonyl peak (SCH2CONHNH2) at 166.36 ppm in 13C NMR. Derivative 3 was stirred with an aldehyde or acetophenone derivative in methanol containing acetic acid-produced Schiff’s bases 4–27 (E and Z mixtures) in 83–91% yield. Schiff’s bases 4–27 were recognized via the disappearance of the singlet peak of the NH2 group at 4.37 ppm and the presence of substituted benzylidene fragment (-SCH2CONHN = CH-R) or phenylethylidene fragment (-SCH2CONHN = C(CH3)-R) peaks. Benzylidene fragment of Schiff’s bases 4–16 was identified by a singlet mercaptomethyl peak (SCH2CONHN = CH-R) at 4.75–4.14 ppm in 1H NMR and 33.64–33.59 ppm in 13C NMR. It was further recognized by aminocarbonyl proton (SCH2CONHN = CH-R) at 12.25–11.39 ppm and benzylidene proton (SCH2CONHN = CH-R) at 9.68–7.99 in 1H NMR. 13C NMR confirmed the carbonyl and imino-carbon groups (SCH2CONHN = CH-R) at 169.60–167.78 and 156.56–156.10 ppm, respectively in 13C NMR. A singlet mercaptomethyl peak assigned phenylethylidene moiety of Schiff’s bases 17–27 (SCH2CONHN = C(CH3)-R) at 4.74–4.22 ppm in 1H NMR and 33.64–33.61 ppm in 13C NMR. Additionally, it was further identified by aminocarbonyl peaks (SCH2CONHN = C(CH3)-R) at 11.11–10.71 ppm in 1H NMR, as well as methyl peaks (SCH2CONHN = C(CH3)-R) at 2.46–2.21 ppm in 1H NMR and 18.64–12.49 in 13C NMR, respectively. 13C NMR confirmed the presence of carbonyl (CO) and imino-carbon groups of phenylethylidene moiety (SCH2CONHN = C(CH3)-R) at 170.15–169.92 and 156.48–156.11 ppm, respectively. Additionally, compounds 2–27 were characterized by three peaks at 4.64–4.12, 3.13–3.06, and 7.38–7.35 ppm for ethylbenzenesulfonamide moieties (-CH2CH2-Ph-SO2NH2) in 1H NMR, together with typical peaks at 47.05–45.47 and 45.53–35.94 ppm in 13C NMR spectra and carbonyl group (C = O) at 160.89–160.79 ppm representing quinazoline nucleus.
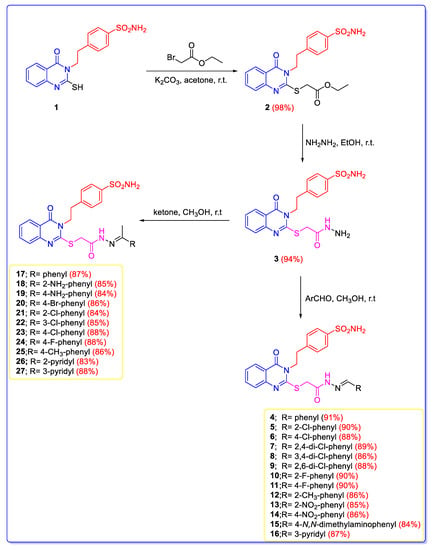
Scheme 1.
Synthesis of the designed quinazoline derivatives (2–27).
2.2. CA Inhibitory Activity
Newly prepared quinazolines 2–27 were assayed against CAI activity isoforms, such as hCA I, II, IX, and XII compared with the standard sulfonamide inhibitor acetazolamide (AAZ). The tested compounds showed a selectivity index (SI) of hCA I and hCA II/hCA IX (SI, 0.77–107 and 1.85–85.58, respectively), related to the AAZ selectivity index (SI, 10.0 and 43.86). The selectivity index of hCA I and hCA II/hCA XII of the tested compounds exhibited (SI, 0.32–19.67 and 0.31–26.60, respectively, allied to AAZ (SI, 0.48 and 2.10). Compounds 2, 5, 7, 8, 9, 10, 12, 13, 14, 18, 19, 20, 21, 22, and 25 showed selective solid hCA I inhibitory activity/hCA IX (SI, 10.16–107), while compounds 1, 9, 13, 15, and 18 displayed great selective hCA I inhibitory activity/hCA XII (SI, 40.35–85.58) associated with AAZ (SI, 10.0 and 43.86), respectively. Compounds 2 and 4–25 showed effective selective hCA II inhibitory activity/hCA IX (SI, 0.63–19.67), whereas compounds 3, 5, 7, 8, 9, 11, 13, 14, 15, 17, 19, 20, 21, 22, 23, and 25 exhibited unusual selective hCA II inhibitory activity over hCA XII (SI, 2.19–26.60), compared with AAZ (SI, 0.48 and 2.10), respectively.
2.2.1. CA I Inhibitory Activity
The CAI activity toward distinct hCA isoforms, hCA I, was efficiently inhibited by quinazolines 2, 3, 4, 6, 10, 11, 12, 14, 15, 16, 17, 19, 23, 24, 25, 26, and 27 (Ki, 52.8–635.4 nM), equated to (AAZ: Ki, 250.0 nM). Compounds 5, 13, 18, and 22 showed moderate hCA I inhibitory action (Ki, 824.3–991.7 nM). In contrast, derivatives 7, 8, 9, 20, and 21 had a feeble inhibitory action (Ki, 1145–2354 nM). Structure–activity interactions of hCA I activities with Ki values indicated the following: (i) Ester 2 and acid-hydrazide 3 showed strong hCA I activities (Ki, 106.7 and 87.6 nM, respectively); (ii) conversion of acid-hydrazide 3 into corresponding Schiff’s bases 4–27 gave different hCA I activities (Ki, 52.8–2354 nM); (iii) unsubstituted-2-benzylidene derivatives, such as 4 (Ki, 152.4 nM) are more influential than different substituted chloro-2-benzylidenes, such as hydrazones 5–9 with (Ki, 567.6–2345 nM); (iv) 4-chloro-2-benzylidene derivative 6 (Ki, 567.6 nM) is more powerful than 2-chloro-2-benzylidene derivative 5 (Ki, 940.3 nM); (v) dichloro-2-benzylidene derivatives, such as 7–9 (Ki, 1827–2354 nM) are less powerful than monochloro-2-benzylidene derivatives 5–6 (Ki, 567.6–940.3 nM); (vi) substitution of 2-chloro or 4-chloro group of compounds 5–6 (Ki, 567.6–940.3 nM) by 2-fluoro or 4-fluoro group produced fluoro-2-benzylidenehydrazineyl derivatives 10–11 with an increase in hCA I activity (Ki, 132.9–274.1 nM); (vii) substituting the 2-chloro group of hydrazone 5 (Ki, 940.3 nM) by activating a group, such as the 2-methyl group produced compound 12 with an improvement in hCA I activity (Ki, 337.8 nM), whereas substituting a 4-chloro group of hydrazone 6 (Ki, 567.6 nM) by an activating group, such as 4-N,N-dimethyl amino group produced compound 15 while maintaining the hCA I activity (Ki, 582.0 nM); (viii) substituting the chloro group of compounds 5 and 6 (Ki, 940.3 and 567.6 nM, respectively) by the NO2 group produced hydrazones 13 and 14 with a decrease in hCA I activity (Ki, 991.7 and 635.4 nM, respectively); (ix) switching phenyl nucleus of hydrazone 4 (Ki, 152.4 nM) by the 3-pyridyl moiety produced compound 16 with a mild decrease in hCA I activity (Ki, 207.9 nM); (x) 1-phenylethylidenehydrazineyl 17 (Ki, 124.3 nM) is more effective than the substituted-1-phenylethylidenehydrazineyl derivatives, such as 18–25 (Ki, 186.9–1356 nM); (xi) replacing the phenyl nucleus of hydrazone 17 (Ki, 124.3 nM) by the 2-pyridyl moiety produced compound 26 with an increase in hCA I activity (Ki, 52.8 nM), whereas replacing the phenyl nucleus of compound 17 by 3-pyridyl moiety gave compound 27 with a decrease in hCA I activity (Ki, 238.4 nM); (xii) in comparison, 4-amino or 4-chloro-phenylethylidenehydrazineyl derivatives, such as 19 and 23 (Ki, 439.5 and 563.5 nM, respectively) are more active than the corresponding 2-amino, 2-chloro-phenylethylidenes, such as 18 and 21 (Ki, 824.3 and 1145 nM, respectively); (xiii) 4-fluoro-phenylethylidene 24 (Ki, 186.9 nM) is more active than the corresponding 4-amino, 4-bromo, 4-chloro or 4-methyl-phenylethylidenes, such as 19, 20, 23, and 25 (Ki, 439.5–1356 nM); (xiv) in comparison, phenylethylidene 17 (Ki, 124.3 nM) is more forceful than the parallel benzylidene 4 (Ki, 152.4 nM), whereas benzylidenes 5, 6, and 11 (Ki, 940.3, 567.6, and 132.0 nM, respectively) are more equivalent than the comparable phenylethylidenes 21, 23, and 24 (Ki, 1145, 563.5, and 186.9 nM, respectively).
2.2.2. CA II Inhibitory Activity
Quinazolines 2, 3, 10, 11, 16, 18, 24, 26, and 27 are potent hCA II inhibitors, with Ki of 10.80–49.5 nM, superior to or nearly equally active to AAZ (Ki, 12.0 nM). Compounds 4, 12, 15, 17, and 19 showed moderate hCA II inhibitory activity with (Ki, 52.6 and 82.1 nM), whereas hydrazonoquinazolines 5, 6, 7, 8, 9, 13, 14, 20, 21, 22, 23, and 25 showed a weedy inhibitory action with Ki in the range of 126.0–698.2 nM. Structure–activity interactions of hCA II activities with Ki values designated the following: (i) Ester 2 and acid-hydrazide 3 showed intense hCA II activities (Ki, 16.9–21.3 nM), but the ester 2 (Ki, 21.3 nM) is less active than acid-hydrazide 3 (Ki, 16.9 nM); (ii) conversion of acid-hydrazide 3 into the corresponding hydrazones 4–27 gave different hCA II activities (Ki, 10.8–698.2 nM); (iii) fluoro group insertion into unsubstituted-2-benzylidene 4 (Ki, 52.6 nM) gave fluoro-2-benzylidenes 10 and 11 (Ki, 35.7–49.5 nM) with an increase in hCA II activity; (iv) substitution of fluoro group of compounds 10–11 (Ki, 35.7–49.5 nM) by chloro, nitro, methyl or dimethylamino groups produced substituted-2-benzylidenes 5–9 and 12–15 with a decrease in hCA II activity (Ki, 69.4–569.4 nM); (v) monochloro-2-benzylidenes 5 and 6 (Ki, 126.8–251.3 nM) are more powerful than dichloro-2-benzylidenes 7, 8, and 9 (Ki, 164.8–432.8 nM); (vi) in comparison, 4-chloro or 4-nitro-2-benzylidenes 6 and 14 (Ki, 126.8 and 220.7 nM, respectively) are more active than the corresponding 2-chloro or 2-nitro-2-benzylidenes 5 and 13 (Ki, 251.3 and 569.4 nM, respectively); (vii) replacing the chloro group of compounds 5 and 6 (Ki, 251.3 and 126.8 nM, respectively) by activating factions as 2-methyl or 4-N,N-dimethyl amino produced compounds 12 and 15 with an hCA II improvement in activity (Ki, 69.4 and 82.1 nM, respectively); (viii) phenyl moiety replacement of compound 4 (Ki, 52.6 nM) by the 3-pyridyl moiety produced compound 16 with a likely increase in hCA II activity (Ki, 23.8 nM); (ix) 1-phenylethylidene 17 (Ki, 67.5 nM) is more active than the substituted-1-phenylethylidene derivatives 18–25 (Ki, 126.0–698.2 nM) except for amino-1-phenylethylidenes 18, 19, and 4-fluoro-1-phenylethylidene 24 (Ki, 35.2–59.3 nM); (x) replacing the phenyl moiety of compound 17 (Ki, 67.5 nM) with the pyridyl moieties produced compounds 26 and 27 with an increase in hCA II activity (Ki, 10.8–22.8 nM); (xi) replacing the chloro group of compounds 21 and 23 (Ki, 157–324.2 nM) by activating groups, such as methyl or amino groups produced compounds 18, 19, and 25 with an hCA II improvement in activity (Ki, 35.2–126.0 nM); (xii) in comparison, 2-amino-phenylethylidene 18 (Ki, 35.2 nM) is more active than the corresponding 4-aminophenylethylidene 19 (Ki, 59.3 nM), whereas 2-chlorophenylethylidene 21 (Ki, 324.2 nM) is rarer than 4-chlorophenylethylidene 23 (Ki, 157.0 nM); (xiii) in comparison, benzylidenes 4, 5, and 6 (Ki, 52.6–251.3 nM) are forceful than the corresponding phenylethylidenes 17, 21, and 23 (Ki, 67.5–324.2 nM), whereas benzylidenes 11 and 16 (Ki, 23.8–49.5 nM) are lower in efficacy than the congruent phenylethylidenes 24 and 27 (Ki, 10.8–39.5 nM).
2.2.3. CA IX Inhibitory Activity
Hydrazonoquinazolines 2, 5, 7, 8, 9, 10, 11, 12, 14, 16, 18, 19, 21, 24, 25, and 27 displayed compelling hCA IX inhibitory effect with (Ki, 5.8–49.2 nM), being more effectual than or nearly equivalent to AAZ (Ki, 25 nM). Against hCA IX, hydrazonoquinazolines 3, 4, 6, 13, 15, 17, 20, 22, 23, and 26 exhibited moderate inhibitory vigor, with (Ki, 52.1–99.6 nM), respectively. Dialkylated hydrazones 7, 8, and 9 showed selective inhibition against tumor-associated CA IX with Ki, 22–49.2 nM. At the same time, they possessed a neglected inhibitory activity toward hCA I (Ki, 1145–2354 nM), hCA II (Ki, 164.8–432.8 nM), and hCA XII (Ki, 55.9–84.4 nM) allied to AAZ (Ki, 250.0, 12.0, and 5.7, respectively nM). Compounds 7, 8, and 9 showed the selectivity index of hCA I inhibitory activity/hCA IX (SI, 107.0, 52.5, and 45.85) matched to AAZ (SI, 10.0), and the selectivity index of hCA II inhibitory activity/hCA IX (SI, 19.67, 9.33, and 3.35) matched to AAZ (SI, 0.48).
Structure–activity interactions of hCA IX activities with Ki values focused on the following: (i) Ester 2 and acid-hydrazide 3 showed intense hCA IX activities (Ki, 10.5 and 52.1 nM); (ii) hydrazinolysis of the ester 2 (Ki, 10.5 nM) produced acid-hydrazide 3 (Ki, 52.1 nM) with depression of hCA IX inhibitory vigor; (iii) conversion of acid-hydrazide 3 into corresponding hydrazones 4–27 gave different hCA IX activities (Ki, 5.8–99.6 nM); (iv) conversion of acid-hydrazide 3 (Ki, 52.1 nM) into unsubstituted-2-benzylidene 4 (Ki, 61.7 nM) leads to a neglected decrease in hCA IX activity; (v) substituted-2-benzylidenes 5–15 (Ki, 15.4–58.5 nM) are greater in efficacy than unsubstituted-2-benzylidene 4 (Ki, 61.7 nM) except for hydrazones 6 and 15 (Ki, 88.6 and 89.1 nM, respectively); (vi) in comparison, 4-fluoro or 4-nitro-2-benzylidenes 11 and 14 (Ki, 15.4 and 26.9 nM, separately) are more active than the corresponding 2-fluoro or 2-nitro-2-benzylidenes 10 and 13 (Ki, 19.7 and 58.5 nM, respectively), whereas 2-chlor-2-benzylidene 5 (Ki, 26.4 nM) are further vigorous than 4-chlor-2-benzylidene 6 (Ki, 89.1 nM); 2,4-dichloro-2-benzylidene 7 (Ki, 22.0 nM) is more active than the congruous 3,4-dichloro and 2,6- dichlor-2-benzylidenes 8 and 9 (Ki, 34.8 and 49.2 nM, respectively); (vii) in comparison, 2-fluoro or 4-fluoro-2-benzylidenes 10 and 11 (Ki, 19.7 and 15.4 nM, respectively) are more active than the corresponding 2-chloro or 4-chloro 2-benzylidenes 5 and 6 (Ki, 26.4, 89.1 nM) and 2-nitro or 4-nitro 2-benzylidenes 13 and 14 (Ki, 58.5 and 26.9 nM, respectively); (viii) phenyl moiety replacement of compound 4 (Ki, 61.7 nM) with the 3-pyridyl moiety produced compound 16 with a likely increase in hCA IX activity (Ki, 37.8 nM); (ix) 1-phenylethylidene 17 (Ki, 55.7 nM) are low in effect than 4-fluoro-1-phenylethylidene 24 (Ki, 28.5 nM); (xi) replacing the 4-fluoro group of compound 24 (Ki, 28.5 nM) by an activating group, such as the 4-amino group produced compound 19 with an hCA IX improvement in activity (Ki, 5.8 nM), whereas replacing it with 4-bromo, 4-chloro or 4-methyl groups gave compounds 20, 23, and 25 with a decrease in hCA IX activity (Ki, 43.9–99.6 nM); (xii) in comparison, 4-amino-1-phenylethylidene 19 (Ki, 5.8 nM) is more active than the corresponding 2-amino-1-phenylethylidene 18 (Ki, 36.7 nM), whereas 4-chloro-1-phenylethylidene 23 (Ki, 65 nM) is less active than 2-chloro-1-phenylethylidene 21 (Ki, 45.3 nM); (xiii) replacing the phenyl nucleus of compound 17 (Ki, 55.7 nM) by the 2-pyridyl moiety produced hydrazone 26 with a mild decrease in hCA IX activity (Ki, 68.2 nM), whereas replacing it with 3-pyridyl grew compound 27 (Ki, 33.7 nM) with a good increase in hCA IX activity; (xiv) replacing the chloro group of compounds 21 and 23 (Ki, 45.3–65.0 nM) by activating units, such as amino or methyl groups produced compounds 18, 19, and 25 with an hCA IX improvement in activity (Ki, 5.8–43.9 nM); (xv) in comparison, benzylidenes 5 and 11 (Ki, 26.4 and 15.4 nM) are further stronger than the analogous phenylethylidenes 21 and 24 (Ki, 45.3 and 28.5 nM, respectively), whereas phenylethylidenes 17, 23, and 27 (Ki, 55.7, 65.0, and 33.7 nM, respectively) are more powerful than the equivalent benzylidenes 4, 6 and 16 (Ki, 61.7, 89.1, and 37.8 nM, respectively).
2.2.4. CA XII Inhibitory Activity
Derivatives 3, 11, 15, 17, 18, and 19 exerted intense hCA XII suppressant activities with (Ki, 5.4–18.3 nM) matched to AAZ (Ki, 5.7 nM). Compounds 2, 4, 10, 13, 14, 16, 24, 26, and 27 had moderate hCA XII inhibitory vigor with (Ki, 20.80–38.4 nM), whereas hydrazones 5, 6, 7, 8, 9, 12, 20, 21, 22, 23, and 25 showed soft hCA XII inhibitory action (Ki, 46.1–89.4 nM, Table 1). Structure–activity interactions of hCA XII activities with Ki values directed the following: (i) Ester 2 showed moderate hCA XII activity (Ki, 34.7 nM); (ii) hydrazinolysis of the ester 2 (Ki, 34.7 nM) produced acid-hydrazide 3 (Ki, 5.4 nM) with an increase in the hCA XII suppressant effect; (iii) conversion of acid-hydrazide 3 into corresponding hydrazones 4–27 gave different hCA XII activities (Ki, 5.4–89.4 nM); (iv) conversion of acid-hydrazide 3 (Ki, 5.4 nM) into unsubstituted-2-benzylidene 4 (Ki, 38.4 nM) decreased hCA XII activity; (v) insertion of fluoro or 4-N, N-dimethylamino groups into the unsubstituted-2-benzylidene 4 (Ki, 38.4 nM) gave compounds 10, 11, and 15 (Ki, 22.9, 15.8, and 6.8 nM, separately) with an increase in hCA XII activity, whereas insertion of chloro or methyl groups 5–9 and 12 (Ki, 46.1–89.4 nM) decreased the hCA XII activities; (vi) in comparison, 2-chloro or 2-nitro-2-benzylidenes 5 and 13 (Ki, 46.1 and 21.4 nM, respectively) are more active than the corresponding 4-chloro or 4-nitro-2-benzylidenes 6 and 14 (Ki, 67.8 and 30.5 nM, respectively). Meanwhile, 2-fluoro-2-benzylidene 10 (Ki, 22.9 nM) is lower in activity than 4-fluoro-2-benzylidene 11 (Ki, 15.8 nM), whereas 2,6-dichloro-2-benzylidene 9 (Ki, 55.9 nM) is more powerful than the analogous 2,4-dichloride and 3,4-dichlor-2-benzylidenes 7 and 8 (Ki, 89.4 and 63.4 nM, respectively); (vii) in comparison, fluoro-2-benzylidenes 10 and 11 (Ki, 22.9 and 15.8 nM, respectively) are more active than the corresponding chloro-2-benzylidenes 5, 6, and 4-nitro-2-benzylidene 14 (Ki, 46.1, 67.8, and 30.5 nM, respectively); (viii) phenyl moiety replacement of compound 4 (Ki, 38.4 nM) by the 3-pyridyl moiety produced compound 16 with an increasing hCA XII activity (Ki, 20.8 nM); (ix) 1-phenylethylidene 17 (Ki, 12.7 nM) is more forceful than benzylidene 4 (Ki, 38.4 nM) and insertion of an amino or fluoro group into 1-phenylethylidene 17 (Ki, 12.7 nM) gave compounds 18, 19, and 24 (Ki, 18.3, 15.9, and 25.4 nM, respectively) with a neglected moderate decrease in hCA XII activity; (x) insertion of chloro or methyl groups of 1-phenylethylidene 17 (Ki, 12.7 nM) produced compounds 21, 22, 23, and 25 (Ki, 58.0, 46.9, 61.2, and 57.5 nM, respectively) with reduction in hCA XII activity, whereas insertion of bromo group produced compound 20 (Ki, 88.8 nM) with a substantial decrease in hCA XII activity; (xi) in comparison, 4-amino-1-phenylethylidene 19 (Ki, 15.9 nM) is significantly forceful than the analogous 2-amino-1-phenylethylidene 18 (Ki, 18.3 nM), whereas 4-chloro-1-phenylethylidene 23 (Ki, 61.2 nM) is lower in effectivity than 2-chloro-1-phenylethylidene 21 (Ki, 58.0 nM); (xii) replacing the phenyl nucleus of hydrazone 17 (Ki, 12.7 nM) by the pyridyl moiety produced compounds 26 and 27 with a mild decrease in hCA XII activity (Ki, 28.5 and 34.7 nM, respectively); (xii) in comparison, benzylidenes 5, 11, and 16 (Ki, 46.1, 15.8, and 20.8 nM, respectively) are higher in power than the analogous phenylethylidenes 21, 24, and 27 (Ki, 58.0, 25.4, and 34.7 nM, respectively). Phenylethylidenes 17 and 23 (Ki, 12.7 and 61.2 nM, respectively) are extra forceful than the analogous benzylidenes 4 and 6 (Ki, 38.4 and 67.8 nM, respectively).

Table 1.
Inhibition data of human CA isoforms hCA I, II, IX, and XII for hydrazonoquinazolines 2–27 and (AAZ) standard drug.
3. Conclusions
Novel synthesized quinazolines 2–27 were evaluated for their activity against CAI isoforms (hCA I, II, IX, and XII) along with acetazolamide (AAZ). The CAI activity toward different hCA isoforms, hCA I, was powerfully inhibited by derivatives 2, 3, 4, 6, 10, 11, 12, 14, 15, 16, 17, 19, 23, 24, 25, 26, and 27 (Ki, 52.8–635.4 nM) matched to (AAZ: Ki, 250.0 nM). Compounds 2, 3, 10, 11, 16, 18, 24, 26, and 27 are efficacious hCA II inhibitors, (Ki, 10.80–49.5 nM) related to AAZ (Ki, 12.0 nM). On the other hand, Schiff’s bases 4, 12, 15, 17, and 19 showed a moderate hCA II inhibitory vigor (Ki, 52.6–82.1 nM). Quinazolines 2, 5, 7, 8, 9, 10, 11, 12, 14, 16, 18, 19, 21, 24, 25, and 27 revealed forceful hCA IX suppressant activity (Ki, 5.8–49.2 nM) equated to AAZ (Ki, 25 nM); however, quinazolines 3, 4, 6, 13, 15, 17, 20, 22, 23, and 26 demonstrated equitable hCA IX suppressant activity (Ki, 52.1–99.6 nM). Compounds 3, 11, 15, 17, 18, and 19 exerted intense hCA XII inhibitory vigor (Ki, 5.4–18.3 nM) rivaled to AAZ (Ki, 5.7 nM), while compounds 2, 4, 10, 13, 14, 16, 24, 26, and 27 had middling hCA XII suppressant activity (Ki, 20.80–38.4 nM). Quinazolines 2, 5, 7, 8, 9, 10, 12, 13, 14, 18, 19, 20, 21, 22, and 25 showed selectively fixed hCA I inhibitory activity over hCA IX (SI, 10.16–107.0) paralleled to AAZ (SI, 10.0). Derivatives 1, 9, 13, 15, and 18 displayed distinguished selective hCA I inhibitory vigor/hCA XII (SI, 40.35–85.58) matched to AAZ (SI, 43.86). Quinazolines 2 and 4–25 indicated real selective hCA II inhibitory activity/hCA IX (SI, 0.63–19.67) associated with AAZ (SI, 0.48). Quinazolines 3, 5, 7, 8, 9, 11, 13, 14, 15, 17, 19, 20, 21, 22, 23, and 25 presented notable selective hCA II suppressant activity/hCA XII (SI, 2.19–26.60) allied to AAZ (SI, 2.10). Compounds 7, 8, and 9 showed the selectivity index of hCA I inhibitory activity over hCA IX (SI, 107.0, 52.5, and 45.85) related to AAZ (SI, 10.0) and the selectivity index of hCA II inhibitory activity over hCA IX (SI, 19.67, 9.33, and 3.35) compared with AAZ (SI, 0.48).
4. Materials and Methods
4.1. Chemistry
Stirring of anthranilic acid with 4-(2-isothiocyanatoethyl)benzenesulfonamide in ethanol containing trimethylamine gave 4-(2-(2-mercapto-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (1) [55,59]. Melting points (uncorrected) were recorded on a Barnstead 9100 Electrothermal melting apparatus (APS Water Services Corporation, Van Nuys, CA, USA). In contrast, the KBr disc IR spectra are recorded on an FT-IR Perkin-Elmer spectrometer (PerkinElmer Inc., Waltham, MA, USA). The 1H NMR and 13C NMR were measured in DMSO-d6 on Bruker 700 and 176 MHz instruments, respectively (Bruker, Billerica, MA, USA). Supporting Information: 1H NMR and 13C NMR of compounds 2–27. Mass spectra were recorded on an Agilent 6320 Ion Trap mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). C, H, and N were analyzed at the Research Centre, College of Pharmacy, King Saud University, Saudi Arabia. The results were within ±0.4% of the theoretical values.
4.1.1. Ethyl 2-((4-oxo-3-(4-sulfamoylphenethyl)-3,4-dihydroquinazolin-2-yl)thio)acetate (2)
A mixture of 4-(2-(2-mercapto-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (1) (20 mmol, 7.22 gm), ethyl 2-bromoacetate (22 mmol, 3.68 gm), and anhydrous potassium carbonate (22 mmol, 3.04 gm) was stirred at room temperature in (150 mL) acetone for 24 h. The reaction mixture was filtered, dried, washed with (10 mL) of water, and dried. Mp 210–211°, 98% yield; IR (KBr, cm−1) ν: 3308, 3217 (NH), 1735, 1661 (C = O), 1334, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 8.08 (d, 1H, J = 7.24 Hz), 7.80 (d, 3H, J = 6.18 Hz), 7.49 (d, 3H, J = 7.20 Hz), 7.44 (d, 1H, J = 9.0 Hz), 7.35 (s, 2H), 4.28 (s, 2H), 4.16 (s, 4H), 3.10 (s, 2H), 1.22 (s, 3H); 13C NMR (175 MHz, DMSO-d6): δ 168.73, 160.76, 155.92, 147.00, 143.11, 142.24, 135.36, 129.65, 126.94, 126.67, 126.49, 126.23, 119.17, 61.62, 45.54, 34.56, 33.61, 14.62; Ms: [m/z, 447].
4.1.2. 4-(2-(2-((2-Hydrazineyl-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (3)
A mixture of hydrazine hydrate (32 mmol, 1.0 gm) and ethyl 2-((4-oxo-3-(4-sulfamoylphenethyl)-3,4-dihydroquinazolin-2-yl)thio)acetate (2) (15 mmol, 6.71 gm) in absolute ethanol (70 mL) was stirred for 24 h at room temperature. The reaction mixture was filtered, dried, and washed with (20 mL) of 70% ethanol. Mp 253–254°, 94% yield; IR (KBr, cm−1) ν: 3536, 3378, 3291, 3142 (NH), 1760, 1687 (C = O), 1335, 1211 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 9.42 (s, 1H), 8.08 (d, 1H, J = 7.75 Hz), 7.81 (d, 3H, J = 7.79 Hz), 7.57 (d, 1H, J = 7.84 Hz), 7.51 (d, 2H, J = 7.49 Hz), 7.47 (t, 1H, J = 15.19 Hz), 7.36 (s, 2H), 4.37 (s, 2H), 4.28 (t, 2H, J = 15.51 Hz), 4.02 (s, 2H), 3.10 (t, 2H, J = 15.51 Hz); 13C NMR (175 MHz, DMSO-d6): δ 166.63. 160.88, 156.11, 147.12, 143.07, 142.36, 135.23, 129.65, 126.86, 126.56, 126.50, 119.20, 45.46, 34.46, 33.60; Ms: [m/z, 443].
4.1.3. Synthesis of Compounds 4–27
A mixture of 4-(2-(2-((2-hydrazineyl-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (3) (1 mmol, 44 mg) and appropriate aldehyde or acetophenone derivatives (1 mmol) were stirred at room temperature in absolute methanol (5 mL) and 5 drops of acetic acid for 24 h. The reaction mixture was filtered, dried, and washed with (10 mL) of 50% methanol.
4-(2-(2-((2-(2-Benzylidenehydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (4)
Mp 296–298°, 91% yield; IR (KBr, cm−1) ν: 3388, 3300, 3143 (NH), 1759, 1687 (C = O), 1335, 1215 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 169.17, 164.02, 160.86, 160.82, 156.30, 156.19, 147.15, 147.06, 143.89, 143.08, 142.32, 135.29, 135.26, 134.58, 134.56, 130.59, 130.42, 129.67, 129.63, 129.32, 129.29, 127.57, 127.33, 126.94, 126.52, 126.50, 126.37, 126.32, 119.23, 119.18, 45.56, 45.47, 35.42, 34.27, 33.64, 33.62; 13C NMR (175 MHz, DMSO-d6): δ 11.88 (s, 0.36H), 11.73 (s, 0.64H), 8.29 (s, 0.36H), 8.10 (s, 0.64H), 8.08 (d, 1H, J = 7.49 Hz), 7.82 (d, 0.78H, J = 7.84 Hz), 7.79 (d, 1.72H, J = 7.77 Hz), 7.74 (d, 1.72H, J = 6.23 Hz), 7.71 (d, 0.78H, J = 7.00 Hz), 7.53–7.40 (m, 7H), 7.36 (s, 2H), 4.65 (s, 1.25), 4.31 (q, 2H, J = 8.01, 7.35, and 7.00 Hz), 4.17 (s, 0.75H), 3.12 (t, 2H, J = 7.0 Hz); Ms: [m/z, 521].
4-(2-(2-((2-(2-(2-Chlorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (5)
Mp 288–290°, 90% yield; IR (KBr, cm−1) ν: 3384, 3260, 3144 (NH), 1758, 1685 (C = O), 1335, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 12.12 (s, 0.35H), 11.91 (s, 0.65H), 9.69 (s, 0.35H), 8.49 (s, 0.65H), 8.08 (t, 1H, J = 7.21 Hz), 8.04 (d, 0.65H, J = 7.77 Hz), 7.94 (dd, 0.35H, J = 7.70 Hz), 7.82–7.75 (m, 3H), 7.55–7.7.49 (m, 3H), 7.45 (t, 2H, J = 14.63 Hz), 7.42–7.37 (m,2H), 7.36 (s, 2H), 4.65 (s, 1.30H), 4.31 (q, 2H, J = 7.91 Hz), 4.16 (s, 0.70H), 3.12 (t, 2H, J = 14.14 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.36, 164.29, 160.85, 160.81, 156.25, 156.15, 147.06, 147.04, 143.10, 143.09, 142.31, 139.94, 135.28, 133.62, 133.45, 132.05, 131.83, 131.82, 131.78, 130.46, 130.40, 129.67, 129.64, 128.14, 128.08, 127.33,, 127.26, 126.95, 126.63, 126.55, 126.52, 126.50, 126.37, 126.31, 119.23, 119.18, 45.57, 45.48, 35.41, 34.21, 33.64; Ms: [m/z, 555 and M + 2, 557].
4-(2-(2-((2-(2-(4-Chlorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (6)
Mp 288–289°, 88% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.94 (s, 0.40H), 11.79 (s, 0.60H), 8.28 (s, 0.38H), 8.09 (s, 0.62H), 8.07 (d, 1H, J = 7.42 Hz), 7.82–7.73 (m, 5H), 7.50 (t, 4H, J = 18.20 Hz), 7.45 (d, 1.2H, J = 7.28 Hz), 7.40 (d, 0.8H, J = 8.19 Hz), 7.36 (s, 2H), 4.64 (s, 1.2H), 4.31 (q, 2H, J = 7.70 Hz), 4.17 (s, 0.8H), 3.12 (t, 2H, J = 15.82 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.24, 164.14, 160.85, 160.82, 156.29, 156.17, 147.05, 145.84, 143.09, 142.60, 142.32, 135.27, 135.01, 134.82, 133.53, 129.65, 129.40, 129.21, 128.97, 126.94, 126.60, 126.52, 126.50, 126.38, 126.32, 119.23, 119.17, 45.56, 45.49, 35.4, 34.28, 33.64.
4-(2-(2-((2-(2-(2,4-Dichlorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (7)
Mp 304–305°, 89% yield; IR (KBr, cm−1) ν: 3386, 3267, 3142 (NH), 1758, 1684 (C = O), 1334, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 12.16 (s, 0.33H), 11.95 (s, 0.67H), 8.63 (s, 0.33H), 8.42 (s, 0.67H), 8.07 (t, 1H, J = 7.42 Hz), 8.03 (d, 0.67H, J = 8.54 Hz), 7.92 (d, 0.37H, J = 8.47 Hz), 7.80 (q, 2H, J = 8.40 Hz), 8.78–7.71 (m, 2H), 7.50 (q, 2H, J = 8.33 Hz),7.45 (t, 2H, J = 14.98 Hz), 7.39 (d, 1H, J = 8.12 Hz), 7.36 (s, 2H), 4.64 (s, 1.26H), 4.30 (q, 2H, J = 7.74 Hz), 4.16 (s, 0.74H), 3.12 (t, 2H, J = 15.86 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.38, 160.83, 160.80, 156.22, 156.10, 147.03, 143.10, 142.31, 142.07, 138.90, 135.62, 135.40, 135.27, 134.30, 134.13, 130.93, 129.90, 129.64, 128.51, 128.43, 126.93, 126.62, 126.50, 126.31, 119.16, 45.56, 45.49, 35.41, 34.26, 33.64; Ms: [m/z, 598 and M + 2, 591].
4-(2-(2-((2-(2-(3,4-Dichlorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (8)
Mp 271–272°, 86% yield; 1H NMR (700 MHz, DMSO-d6): δ 12.06 (s, 0.35H), 11.89 (s, 0.65H), 8.27 (s, 0.36H), 8.07 (t, 1.64H, J = 6.86 and 4.14 Hz), 8.00 (s, 0.63H), 7.94 (s, 0.37H), 7.80 (t, 2.3H, J = 10.57 and 8.26 Hz), 7.77 (dd, 1.4H, J = 16.12 and 8.33 Hz), 7.70 (dd, 1.3H, J = 2.31 and 8.14 Hz), 7.50 (t, 2.3H, 7.49 and 7.77 Hz), 7.45 (q, 1H, J = 8.12 Hz), 7.39 (d, 0.7H, J = 8.19 Hz), 7.36 (s, 2H), 4.65 (s, 1.18H), 4.30 (q, 2H, J = 7.65 Hz), 4.17 (s, 0.72H), 3.12 (t, 2H, J = 7.70 and 7.65 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.39, 164.35, 160.84, 160.80, 156.26, 156.14, 147.05, 147.04, 144.46, 142.31, 141.25, 135.47, 135.43, 135.25, 132.73, 132.54, 132.23, 132.14, 131.54, 131.52, 129.66, 129.63, 129.15, 128.75, 127.24, 126.94, 126.60, 126.51, 126.50, 126.38, 126.28, 119.22, 119.17, 45.56, 45.47, 35.39, 34.33, 33.65, 33.62; Ms: [m/z, 589, M + 2, 591].
4-(2-(2-((2-(2-(2,6-Dichlorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (9)
1 Mp 296–297°, 88% yield; 1H NMR (700 MHz, DMSO-d6): δ 12.17 (s, 0.25H), 11.99 (s, 0.75H), 8.49 (s, 0.25H), 8.35 (s, 0.75H), 8.07 (t, 1H, J = 7.92 Hz), 7.81 (d, 2H, J = 7.84 Hz), 7.74 (t, 1H, J = 15.06 Hz), 7.58 (d, 1.4H, J = 8.05 Hz), 7.56 (d, 0.60H, J = 8.05 Hz), 7.52 (d, 2H, J = 7.70 Hz), 7.47–7.43 (m, 2H), 7.39 (d, 1H, J = 8.12 Hz), 7.36 (s, 2H), 4.60 (s, 1.5H), 4.30 (t, 2H, J = 7.90 Hz), 4.15 (s, 0.50H), 3.12 (t, 2H, J = 15.92 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.57, 160.82, 156.28, 156.18, 147.06, 143.08, 142.54, 142.33, 142.30, 138.90, 135.20, 134.40, 131.56, 130.10, 129.92, 129.67, 129.63, 129.46, 126.93, 126.51, 126.24, 119.24, 119.17, 45.59, 45.49, 35.43, 34.60, 33.64.
4-(2-(2-((2-(2-(2-Fluorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (10)
Mp 276–277°, 90% yield; 1H NMR (700 MHz, DMSO-d6): δ 12.01 (s, 0.5H), 11.86 (s, 0.5H), 8.31–7.50 (m, 15H), 4.66 (s, 1.25H), 4.33 (s, 2.75H), 3.16 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 169.30, 164.19, 161.87, 160.85, 160.45, 156.28, 147.09, 143.10, 142.33, 136.76, 135.28, 132.36, 129.66, 126.95, 126.77, 126.52, 125.42, 122.14, 119.19, 116.52, 45.49, 40.23, 40.12, 40.01, 39.89, 39.78, 34.22, 33.65; Ms: [m/z, 539].
4-(2-(2-((2-(2-(4-Fluorobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (11)
Mp 292–293°, 90% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.89 (s, 0.38H), 11.73 (s, 0.62H), 8.29 (0.42H), 8.09 (s, 0.58H), 8.07 (s, t, 1H, J = 5.32 and 7.17 Hz), 7.82–7.74 (m, 5H), 7.50 (dd, 2.3H, J = 4.69 and 7.88 Hz), 7.45 (dd, 1H, J = 7.42 Hz), 7.40 (d, 0.7H, J = 8.12 Hz), 7.36 (s, 2H), 7.28 (a, 2H, J = 8.58 Hz), 4.60 (s, 1.22H), 4.31 (dd, 2H, J = 7.82 Hz), 4.16 (s, 0.78H), 3.12 (t, 2H, J = 15.86 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.17, 164.29, 164.16, 164.07, 162.89, 162.76, 160.87, 160.83, 156.30, 156.17, 147.07, 147.05, 146.06, 143.07, 142.78, 142.33, 135.27, 131.19, 129.79, 129.74, 129.67, 129.64, 129.52, 129.47, 126.93, 126.60, 126.51, 126.49, 126.38, 126.32, 119.22, 119.16, 116.44, 116.3, 45.55, 45.47, 35.39, 34.28, 33.638; Ms: [m/z, 539].
4-(2-(2-((2-(2-(2-Methylbenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (12)
Mp 239–240°, 86% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.86 (s, 0.39H), 11.70 (s, 0.61H), 8.25 (s, 0.35H), 8.08 (s, 0.5H), 8.07 (s, 1H), 7.82 (d, 0.75H, J = 7.93 Hz), 7.97 (d, 1.25H, J = 7.95 Hz), 7.78–7.73 (m, 1H), 7.56 (s, 0.65H), 7.53 (d, 1.5H, J = 8.40 Hz), 7.51 (s, 0.5H), 7.49 (d, 1.5 H, J = 7.92 Hz), 7.44 (q, 1H, J = 7.28 Hz), 7.40 (d, 1H, J = 8.12 Hz), 7.37 (s, 2H), 7.32 (q, 1H, J = 7.21 Hz), 7.24 (d, 1H, J = 7.42 Hz), 4.65 (1.25H), 4.30 (dd, 2H, J = 7.84 Hz), 4.16 (s, 0.75H), 3.12 (t, 2H, J = 15.84 Hz), 2.33 (d, 3H, J = 6.02 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.11, 164.00, 160.85, 160.81, 156.30, 156.17, 147.22, 147.08, 147.06, 144.04, 143.10, 143.09, 142.32, 138.57, 138.52, 135.23, 134.52, 134.51, 131.29, 131.14, 129.66, 129.62, 129.21, 129.18, 127.88, 127.65, 126.93, 126.57, 126.52, 126.50, 126.36, 126.28, 124.96, 124.70, 119.22, 119.17, 45.54, 45.45, 35.44, 34.32, 33.65, 33.62, 21.36, 21.33; Ms: [m/z, 535].
4-(2-(2-((2-(2-(2-Nitrobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (13)
Mp 288–289°, 85% yield; IR (KBr, cm−1) ν: 3392, 3287, 3142 (NH), 1755, 1682 (C = O), 1337, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 12.23 (s, 0.40H), 11.98 (s, 0.60H), 8.69 (s, 0.40H), 8.46 (s, 0.60H), 8.09 (t, 1H, J = 8.61 Hz), 8.05 (dd, 2H, J = 7.49 and 7.77 Hz), 7.81–7.7.72 (m, 4H), 7.66 (q, 1H, J = 7.37 Hz), 7.53–7.39 (m, 4H), 7.37 (s, 2H), 4.60 (s, 1.2H), 4.30 (t, 2H, J = 7.35 Hz), 4.15 (s, 0.8H), 8.09 (t, 2H, J = 8.61 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.60, 164.75, 160.89, 156.18, 156.09, 148.58, 148.48, 147.01, 142.97, 142.34, 139.55, 135.26, 134.31, 133.99, 131.22, 131.04, 129.68, 129.65, 129.10, 128.73, 128.63, 128.57, 126.90, 126.66, 126.57, 126.49, 126.42, 126.30, 125.20, 125.07, 119.15, 119.10, 45.53, 45.45, 35.32, 34.16, 33.58; Ms: [m/z, 566].
4-(2-(2-((2-(2-(4-Nitrobenzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (14)
Mp 296–297°, 86% yield; IR (KBr, cm−1) ν: 3396, 3258, 3145 (NH), 1755, 1683 (C = O), 1340, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 12.18 (s, 0.35H), 12.02 (s, 65H), 8.40 (s, 0.35H), 8.28 (t, 2H, J = 10.71 and 8.75 Hz), 8.20 (s, 0.65H), 8.07 (t, 1H, J = 5.81 and 7.07 Hz), 8.01 (dd, 2H, J = 8.40 Hz), 7.82 (dd, 2H, J = 7.88 and 7.77 Hz), 7.74 (q, 1H, J = 7.56 and 10.74 Hz), 7.50 (t, 2H, J = 8.25, 8.68 Hz), 7.45 (q, 1H, J = 7.28 Hz), 7.39 (d, 1H, J = 8.19 Hz), 7.36 (s, 2H), 4.67 (s, 1.30H), 4.31 (q, 2H, J = 8.33, 8.96, and 7.59 Hz), 4.20 (s, 0.70H), 3.12 (t, 2H, J = 15.75 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.61, 164.59, 160.83, 160.80, 156.23, 156.12, 148.31, 148.17, 147.05, 147.02, 144.68, 143.10, 143.09, 142.31, 141.55, 140.95, 140.86, 135.27, 129.67, 129.65, 128.49, 128.25, 126.93, 126.61, 126.54, 126.52, 126.49, 126.37, 126.32, 124.54, 124.51, 119.22, 119.17, 45.57, 45.50, 35.43, 34.25, 33.64; Ms: [m/z, 566].
4-(2-(2-((2-(2-(4-(Dimethylamino)benzylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (15)
Mp 280–281°, 84% yield; IR (KBr, cm−1) ν: 3398, 3260, 3141 (NH), 1755, 1682 (C = O), 1338, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 11.55 (s, 0.40H), 11.44 (s, 0.60H), 8.12 (s, 0.4H), 8.08 (d, 1H, J = 7.68 Hz), 7.95 (s, 0.60H), 7.81 (d, 1H, J = 7.98 Hz), 7.78 (dd, 2H, J = 8.12 Hz), 7.54–7.44 (m, 6H), 7.36 (d, 2H, J = 6.93 Hz), 6.73 (dd, 2H, J = 8.61 and 8.54 Hz), 4.61 (s, 1.2H), 4.30 (t, 2H, J = 8.12 and 7.28 Hz), 4.18 (s, 0.80H), 3.11 (t, 2H, J = 5.67 and 9.17 Hz), 2.96 (s, 6H); 13C NMR (175 MHz, DMSO-d6): δ 168.51, 163.24, 160.88, 160.83, 156.40, 156.25, 151.98, 151.83, 148.01, 147.10, 144.75, 143.09, 143.07, 142.34, 142.32, 135.26, 129.66, 129.63, 128.92, 128.61, 126.93, 126.57, 126.52, 126.50, 126.39, 126.36, 121.87, 121.75, 119.23, 119.20, 112.25, 112.22, 45.54, 45.44, 35.45, 34.40, 33.64, 33.61; Ms: [m/z, 564].
4-(2-(4-Oxo-2-((2-oxo-2-(2-(pyridin-3-ylmethylene)hydrazineyl)ethyl)thio)quinazolin-3(4H)-yl)ethyl)benzenesulfonamide (16)
Mp 263–264°, 87% yield; 1H NMR (700 MHz, DMSO-d6): δ 12.04 (s, 0.36H), 11.89 (s, 0.46H), 8.62 (s, 1H), 8.35 (s, 0.38H), 8.17 (d, 0.64H, J = 7.76 Hz), 8.14 (s, 0.62H), 8.11 (d, 0.36H, J = 7.73 Hz), 8.07 (t, 1H, J = 14.07 Hz), 7.81 (dd, 2.55H, J = 7.94 and 7.98 Hz), 7.74 (t,0.67 H, J = 7.59 Hz), 7.50 (t, 3H, J = 9.59 and 8.47 Hz), 7.45 (dd, 2H, J = 8.47 Hz), 7.39 (d, 0.78H, J = 8.19 Hz), 7.37 (s, 2H), 4.65 (s, 1.25H), 4.31 (q, 2H, J = 7.94 Hz), 4.18 (s, 0.75H), 3.12 (t, 2H, J = 15.96 Hz); 13C NMR (175 MHz, DMSO-d6): δ 169.35, 164.27, 160.85, 160.82, 156.27, 156.14, 151.18, 150.99, 149.24, 148.97, 147.06, 147.04, 144.48, 143.08, 142.32, 141.13, 135.29, 135.26, 133.91, 133.82, 130.56, 129.67, 129.64, 126.93, 126.60, 126.52, 126.50, 126.39, 126.30, 124.51, 119.22, 119.16, 45.57, 45.48, 35.39, 34.27, 33.64, 33.61; Ms: [m/z, 522].
4-(2-(4-Oxo-2-((2-oxo-2-(2-(1-phenylethylidene)hydrazineyl)ethyl)thio)quinazolin-3(4H)-yl)ethyl)benzenesulfonamide (17)
Mp 260–261°, 87% yield; IR (KBr, cm−1) ν: 3387, 3266, 3146 (NH), 1754, 1679 (C = O), 1339, 1156 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 10.97 (s, 0.64H), 10.83 (s, 0.36H), 8.09 (dd, 1H, J = 7.98 Hz), 7.87 (t, 1H, J = 3.50 and 3.85 Hz), 7.82–7.75 (m, 3H), 7.52–7.39 (m, 8H), 7.36 (s, 2H), 4.70 (s, 1.2H), 4.31 (t, 2.8H, J = 8.40 and 6.79 Hz), 3.12 (t, 2H, J = 7.71 and 7.84 Hz), 2.34 (s, 1H), 2.32 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.03, 164.51, 160.88, 160.83, 156.47, 156.41, 152.63, 148.47, 147.11, 147.07, 143.07, 142.33, 138.49, 138.44, 135.28, 129.82, 129.66, 129.62, 128.89, 126.93, 126.81, 126.57, 126.49, 126.29, 119.27, 119.17, 45.57. 45.42, 35.33, 34.97, 33.63, 14.72, 14.17; Ms: [m/z, 535].
4-(2-(2-((2-(2-(1-(2-Aminophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (18)
Mp 264–265°, 85% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.00 (s, 0.80H), 10.85 (s, 0.20H), 8.10 (d, 1H, J = 7.91 Hz), 7.82 (t, 3H, J = 13.86 Hz), 7.52 (t, 2.60H, J = 7.70 and 7.21 Hz), 7.47 (t, 1.4H, J = 15. 05 Hz), 7.43 (d, 1H, J = 8.05 Hz), 7.36 (s, 2.60H), 7.15 (s, 1.40H), 7.05 (tt, 1H, J = 7.63 and 7.52 Hz), 6.78 (d, 0.22H, J = 8.05 Hz), 6.70 (d, 0.78H, 8.09 Hz), 6.60 (t, 0.22H, J = 7.45 Hz), 7.53 (t, 0.78H, 7.50 Hz), 4.60 (s, 0.50H), 4.31 (t, 3.5H, J = 9.17 and 8.49 Hz), 3.12 (t, 2H, J = 8.49 and 8.40 Hz), 2.34 (ss, 3H); 13C NMR (175 MHz, DMSO-d6): δ 169.13, 164.41, 160.83, 156.41, 156.36, 155.05, 152.78, 148.41, 147.56, 147.12, 147.10, 143.11, 143.08, 142.33, 135.33, 135.25, 134.62, 132.66, 130.07, 129.74, 129.66, 129.56, 126.98, 126.61, 126.53, 126.51, 126.27, 119.28, 119.15, 117.71, 116.55, 115.72, 114.89, 45.58, 45.47, 35.57, 35.07, 33.65, 16.01, 15.03; Ms: [m/z, 550].
4-(2-(2-((2-(2-(1-(4-Aminophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (19)
Mp 308–309°, 84% yield; 1H NMR (700 MHz, DMSO-d6): δ 10.68 (s, (s, 0.63H), 10.57 (s, 0.37H), 8.09 (t, 1H, J = 9.66 and 8.54 Hz), 7.80 (ddd, 3H, J = 11.44 and 8.22 Hz), 7.56 (dd, 1.50H, J = 8.19 and 8.05 Hz), 7.50 9dd, 3H, J = 8.05 and 7.98 Hz), 7.45 (ddd, 1.50H, J = 5.18 and 8.12 Hz), 7.35 (s, 2H), 6.55 (dd, 2H, J = 8.19 and 8.40 Hz), 5.48 (d, 2H, J = 11.41 Hz), 4.66 (s, 1.22H), 4.31 (dd, 2H, J = 8.96 and 7.21 Hz), 4.25 (s, 0.78H), 3.12 (t, 2H, J = 14.84 Hz), 2.21 (s, 3H); 13C NMR (175 MHz, DMSO-d6): δ 169.44, 163.76, 160.90, 160.84, 156.52. 156.48, 153.98, 150.76, 150.57, 149.34, 147.13, 147.10, 143.09, 143.06, 142.36, 135.29, 135.25, 129.67, 129.64, 128.09, 127.78, 126.97, 126.94, 126.50, 126.31, 126.29, 125.60, 125.36, 119.27, 119.18, 113.68, 113.56, 45.56, 45.41, 35.37, 35.09, 33.64, 14.27, 13.77; Ms: [m/z, 550].
4-(2-(2-((2-(2-(1-(4-Bromophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (20)
Mp 302–303°, 86% yield; IR (KBr, cm−1) ν: 3295, 3258, 3138 (NH), 1753, 1680 (C = O), 1338, 1156 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 11.02 (s, 0.65H), 10.87 (s, 0.35H), 8.09 (dd, 1H, J = 8.33 and 7.19 Hz), 7.81–7.41 (m, 5H), 8.09 (t, 2H, J = 8.33 Hz), 7.54–7.38 (m, 4H), 7.35 (s, 2H), 4.68 (s, 1.3H), 4.31 (t, 2.7H, J = 11.41 and 7.14 Hz), 3.12 (t, 2H, J = 15 Hz), 2.33 (s, 1H), 2.31 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.04, 164.60, 160.86, 160.82, 156.40, 156.36, 151.33, 147.40, 147.10, 147.06, 143.08, 142.33, 137.68, 137.64, 135.30, 131.82, 131.76, 129.67, 129.63, 128.83, 128.59, 126.98, 126.92, 126.61, 126.51, 126.4, 126.34, 126.28, 123.30, 123.10, 119.27, 119.17, 45.58, 45.44, 35.31, 34.99, 33.63, 14.50, 14.01; Ms: [m/z, 613 and M + 2, 615].
4-(2-(2-((2-(2-(1-(2-Chlorophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (21)
Mp 273–275°, 84% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.01 (s, 0.65H), 10.88 (s, 0.35H), 7.39 (dd, 1H, J = 7.22 and 10.92 Hz), 7.18 (m, 2H), 7.55–7.7.38 (M, 7H), 7.36 (S, 2H), 7.32–7.29 (M, 1H), 4.56 (s, 1.2H), 4.30 (s, 2.8H), 3.11 (t, 2H, J = 6.43 Hz), 2.32 (s, 1H), 2.31 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.14, 164.80, 160.85, 156.39, 156.36, 156.11, 153.28, 149.20, 147.10, 147.05, 143.08, 142.34, 139.45, 139.26, 135.28, 129.91, 129.65, 129.63, 127.89, 126.92, 126.63, 126.50, 126.29, 119.28, 119.15, 45.58, 45.53, 33.62, 18.96, 18.51.
4-(2-(2-((2-(2-(1-(3-Chlorophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (22)
Mp 297–298°, 85% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.07 (s, 0.67H), 10.92 (s, 0.33H), 8.08 (dd, 1H, J = 8.12 and 7.83 Hz), 7.92 (ss, 1H), 7.82–7.74 (m, 4H), 7.51 (d, 1H, J = 8.05 Hz), 7.48 (t, 2.50H, J = 14.64 Hz), 7.46 (d, 1.5H, J = 7.98 Hz), 7.43 (d, 1H, J = 10.99 Hz), 7.36 (s, 2H), 4.71 (s, 1.40H), 4.32 (t, 2.6H, J = 7.07 Hz), 3.12 (t, 2H, J = 15.66 Hz), 2.35 (s, 1H), 2.32 (s. 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.13, 160.87, 160.82, 156.38, 147.07, 147.02, 143.08, 142.33, 140.63, 135.31, 135.25, 133.87, 133.7, 130.80, 130.74, 129.67, 129.62, 129.39, 126.98, 126.94, 126.51, 126.49, 126.34, 126.29, 126.14, 125.53, 125.32, 119.27, 119.19, 45.58, 45.43, 35.2, 35.25, 33.64, 14.61, 14.11; Ms: [m/z, 569, M + 2, 571].
4-(2-(2-((2-(2-(1-(4-Chlorophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (23)
Mp 278–280°, 88% yield; IR (KBr, cm−1) ν: 3385, 3290, 3153 (NH), 1752, 1680 (C = O), 1339, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 11.01 (s, 0.65H), 10.87 (s, 0.35H), 8.08 (t, 1H, J = 7.39 and 6Hz), 7.88 (d, 1H, J = 7.95 Hz), 7.82–7.76 (m, 4H), 7.57 (d, 1H, J = 8.12 Hz), 7.54–7.44 (m, 4H), 7.39 (d, 1H, J = 8.19 Hz), 7.36 (S, 2H), 4.69 (s, 1.2H), 4.29 (t, 2.8H, J = 8.51 Hz), 3.10 (t, 2H, J = 15. 29 Hz), 2.34 (s, 1H), 2.31 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.04, 166.63, 160.88, 160.83, 156.37, 156.10, 147.12, 147.06, 143.07, 142.36, 142.33, 137.32, 135.31, 135.23, 134.34, 129.65, 128.90, 128.84, 128.56, 128.32, 126.86, 126.56, 126.50, 119.20, 45.46, 34.46, 33.60, 14.55, 14.06; Ms: [m/z, 569 and M + 2, 571].
4-(2-(2-((2-(2-(1-(4-Fluorophenyl)ethylidene)hydrazineyl)-2-oxoethyl)thio)-4-oxoquinazolin-3(4H)-yl)ethyl)benzenesulfonamide (24)
Mp 299–300°, 88% yield; IR (KBr, cm−1) ν: 3272, 3144 (NH), 1755, 1680 (C = O), 1339, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 10.97 (s, 0.65H), 10.83 (s, 0.35H), 8.09 (dd, 1H, J = 7.78 and 7.71 Hz), 7.91 (t, 1.3H, J = 12.88 Hz), 7.84 (0.7H, J = 13.02 Hz), 7.82–7.76 (m, 3H), 7.54–7.44 (m, 3H), 7.40 (d, 1H, J = 8.12 Hz), 7.36 (s, 2H), 7.25 (t, 2H, J = 8.57 Hz), 4.69 (s, 1.3H), 4.33–4.30 (m, 2.7H), 3.12 (t, 2H, J = 12.06 Hz), 2.34 (s, 1H), 2.32 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 169.98, 164.49, 163.90, 162.50, 160.87, 160.83, 156.38, 151.67, 147.54, 147.11, 147.07, 143.08, 142.33, 135.30, 135.02, 135.00, 129.67, 129.63, 129.07, 129.02, 128.80, 128.75, 126.92, 126.48, 126.33, 119.27, 119.17, 115.82, 115.75, 115.70, 115.63, 45.57, 45.43, 35.31, 35.00, 33.63, 14.72, 14.20; Ms: [m/z, 553].
4-(2-(4-Oxo-2-((2-oxo-2-(2-(1-(p-tolyl)ethylidene)hydrazineyl)ethyl)thio)quinazolin-3(4H)-yl)ethyl)benzenesulfonamide (25)
Mp 288–290°, 86% yield; 1H NMR (700 MHz, DMSO-d6): δ 10.91 (s, 0.65H), 10.77 (s, 0.35H), 8.08 (t, 1H, J = 12.67 and 7.91), 7.81 (d, 1.3H, J = 7.56 Hz), 7.77 (t, 3H, J = 8.47 Hz), 7.69 (d, 0.7H, J = 7.77 Hz), 7.54 (d, 0. 33H, J = 8.19 Hz), 7.51 (d, 0. 67H, J = 7.70 Hz), 7.48 (dd, 2H, J = 7.70 Hz), 7.46 (d, 0.65H, J = 7.35 Hz), 7.41 (d, 0.35H, J = 8.12 Hz), 7.36 (s, 2H), 7.22 (d, 2H, J = 7.77 Hz), 4.68 (s, 1.3H), 4.31 (dd, 2.7H, J = 16.66 and 12.60 Hz), 3.11 (t, 2H, J = 7.88 and 7.56 Hz), 2.33 (s, 3H), 2.31 (s, 1H), 2.29 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 169.93, 164.36, 160.88, 160.83, 156.43, 156.41, 152.69, 148.52, 147.11, 147.08, 143.09, 143.07, 142.33, 139.45, 139.27, 135.72, 135.64, 135.29, 129.67, 129.62, 129.47, 129.38, 126.97, 126.93, 126.76, 126.50, 126.48, 126.30, 119.27, 119.18, 45.57, 45.42, 35.33, 34.97, 33.63, 21.30, 14.62, 14.10.
4-(2-(4-Oxo-2-((2-oxo-2-(2-(1-(pyridin-2-yl)ethylidene)hydrazineyl)ethyl)thio)quinazolin-3(4H)-yl)ethyl)benzenesulfonamide (26)
Mp 300–301°, 83% yield; IR (KBr, cm−1) ν: 3375, 3259, 3143 (NH), 1751, 1680 (C = O), 1340, 1157 (O = S = O); 1H NMR (700 MHz, DMSO-d6): δ 11.12 (s, 0.66H), 10.97 (s, 0.34H), 8.62 (dd, 1H, J = 4.41 and 4.13 Hz), 8.15 (d, 0.66H, J = 7.98 Hz), 8.07 (t, 1H, J = 12.32 and 7.91 Hz), 8.02 (d, 0.34H, J = 8.05 Hz), 7.84–7.75 (m, 4H), 7.53–7.39 (m, 5H), 7.36 (s, 2H), 4.73 (s, 1.3H), 4.32 (t, 2.7H, J = 8.26 and 8.04 Hz), 3.12 (t, 2H, J = 6.39 and 8.20Hz), 2.44 (s, 1H), 2.41 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.14, 164.83, 160.86, 160.83, 156.37, 156.33, 155.43, 155.39, 152.57, 149.20, 149.10, 147.09, 147.05, 143.09, 143.08, 142.32, 137.09, 135.29, 129.67, 129.63, 126.9, 126.93, 126.61, 126.52, 126.4, 126.31, 126.27, 124.59, 124.4, 120.77, 120.46, 119.26, 119.1, 45.59, 45.44, 35.31, 34.86, 33.63, 12.90, 12.48; Ms: [m/z, 536 and M + 1, 537].
4-(2-(4-Oxo-2-((2-oxo-2-(2-(1-(pyridin-3-yl)ethylidene)hydrazineyl)ethyl)thio)quinazolin-3(4H)-yl)ethyl)benzenesulfonamide (27)
Mp 297–280°, 88% yield; 1H NMR (700 MHz, DMSO-d6): δ 11.10 (s, 0.66H), 10.95 (s, 0.34H), 9.08 (s, (s, 0.66H), 8.96 (s, 0.34H), 8.60 (d, 1H, J = 3.99 Hz), 8.22 (d, 0.66H, J = 7.91 Hz), 8.15 (d, 0.34H, J = 7.91 Hz), 9.09 (d, 0.34H, J = 7.98 Hz), 8.07 (0.66H, J = 7.91 Hz), 7.81 (d, 1H, J = 7.35 Hz), 7.91 (d, 1.35H, J = 7.84 Hz), 7.75 (d, 0.65H, J = 7.70 Hz), 7.54 (d, 0.35H, J = 8.19 Hz), 7.51 (d, 0.65H, J = 7.70 Hz), 7.49 (d, 1.3H, H= 7.63 Hz), 7.45 (dd, 2H, J = 9.73 and 13.63 Hz), 7.39 (d, 0.7H, J = 8.12 Hz), 7.36 (s, 2H), 4.71 (s, 1.3H), 4.32 (d, 2.7H, J = 11.06 Hz), 3.12 (t, 2H, J = 15.75 Hz), 2.38 (s, 1H), 2.36 (s, 2H); 13C NMR (175 MHz, DMSO-d6): δ 170.16, 164.70, 160.87, 160.83, 156.39, 156.37, 150.50, 150.33, 147.98, 147.86, 147.10, 147.06, 146.42, 143.09, 143.07, 142.33, 135.32, 135.29, 134.11, 134.06, 133.91, 129.67, 129.63, 126.98, 126.93, 126.62, 126.51, 126.49, 126.29, 123.93, 123.89, 119.27, 119.17, 45.59, 45.45, 35.29, 34.97, 33.64, 33.62, 14.58, 14.02; Ms: [m/z, 536 and M + 1, 537].
4.2. CA Inhibition
The inhibition assay for the hCA I, II, IX, and XII isozymes was carried out with the SX.18MV-R stopped-flow instrument (Applied Photophysics, Oxford, UK) according to the method reported previously [58,61].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227703/s1, 1H NMR and 13C NMR of compounds 2–27.
Author Contributions
Conceptualization, A.S.E.-A. and A.A.-M.A.-A.; Data curation, A.S.E.-A. and A.A.-M.A.-A.; Formal analysis, A.S.E.-A. and A.A.-M.A.-A.; Investigation, A.S.E.-A. and H.M.A.; Methodology, A.S.E.-A., A.A.-M.A.-A., S.B., A.N. and C.T.S.; Project administration A.S.E.-A.; Supervision, C.T.S. and A.S.E.-A.; Validation, A.S.E.-A. and A.A.-M.A.-A.; Visualization, A.S.E.-A., A.A.-M.A.-A. and C.T.S.; Writing—original draft, A.S.E.-A., A.A.-M.A.-A. and H.A.G.; Writing—review and editing, A.S.E.-A., A.A.-M.A.-A., H.M.A., N.A.A., M.H.M.A.-A. and C.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this project through the Research Group Project No. RGP-163.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
It can be found in supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, R.; Vats, L.; Bua, S.; Supuran, C.T.; Sharma, P.K. Design and synthesis of novel benzenesulfonamide containing 1,2,3-triazoles as potent human carbonic anhydrase isoforms I, II, IV and IX inhibitors. Eur. J. Med. Chem. 2018, 155, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Sly, W.S. Carbonic anhydrase XII functions in health and disease. Gene 2017, 623, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Senturk, M.; Gulcin, I.; Dastan, A.; Kufrevioglu, O.I.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Mboge, M.Y.; McKenna, R.; Frost, S.C. Advances in Anti-Cancer Drug Development Targeting Carbonic Anhydrase IX and XII. Top. Anti-Cancer Res. 2015, 5, 3–42. [Google Scholar]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Carta, F.; Monti, S.M.; De Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Abou-Zeid, L.A.; ElTahir, K.E.; Abdel-Aziz, N.I.; Ayyad, R.R.; Al-Obaid, A.M. Synthesis, anti-inflammatory, analgesic and COX-1/2 inhibition activities of anilides based on 5,5-diphenylimidazolidine-2,4-dione scaffold: Molecular docking studies. Eur. J. Med. Chem. 2016, 115, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Alanazi, A.M.; Asiri, Y.A.; Al-Suwaidan, I.A.; Maarouf, A.R.; Ayyad, R.R.; Shawer, T.Z. Synthesis and potential antitumor activity of 7-(4-substituted piperazin-1-yl)-4-oxoquinolines based on ciprofloxacin and norfloxacin scaffolds: In silico studies. J. Enzyme Inhib. Med. Chem. 2016, 31, 796–809. [Google Scholar] [CrossRef]
- Al-Suwaidan, I.A.; Alanazi, A.M.; El-Azab, A.S.; Al-Obaid, A.M.; ElTahir, K.E.; Maarouf, A.R.; Abu El-Enin, M.A.; Abdel-Aziz, A.A. Molecular design, synthesis and biological evaluation of cyclic imides bearing benzenesulfonamide fragment as potential COX-2 inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2013, 23, 2601–2605. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Angeli, A.; El-Azab, A.S.; Hammouda, M.E.A.; El-Sherbeny, M.A.; Supuran, C.T. Synthesis and anti-inflammatory activity of sulfonamides and carboxylates incorporating trimellitimides: Dual cyclooxygenase/carbonic anhydrase inhibitory actions. Bioorg. Chem. 2019, 84, 260–268. [Google Scholar] [CrossRef]
- Alaa, A.-M.; El-Azab, A.S.; El-Subbagh, H.I.; Al-Obaid, A.M.; Alanazi, A.M.; Al-Omar, M.A. Design, synthesis, single-crystal and preliminary antitumor activity of novel arenesulfonylimidazolidin-2-ones. Bioorg. Med. Chem. Lett. 2012, 22, 2008–2014. [Google Scholar]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Briganti, F.; Mincione, G.; Supuran, C.T. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: Is the tail more important than the ring? J. Med. Chem. 1999, 42, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Abu El-Enin, M.A.; Almehizia, A.A.; Supuran, C.T.; Nocentini, A. Synthesis of novel isoindoline-1,3-dione-based oximes and benzenesulfonamide hydrazones as selective inhibitors of the tumor-associated carbonic anhydrase IX. Bioorg. Chem. 2018, 80, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Ekinci, D.; Senturk, M.; Supuran, C.T. Investigation of arenesulfonyl-2-imidazolidinones as potent carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2015, 30, 81–84. [Google Scholar] [CrossRef]
- Angeli, A.; Abdel-Aziz, A.A.; Nocentini, A.; El-Azab, A.S.; Gratteri, P.; Supuran, C.T. Synthesis and carbonic anhydrase inhibition of polycyclic imides incorporating N-benzenesulfonamide moieties. Bioorg. Med. Chem. 2017, 25, 5373–5379. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abdel-Aziz, A.A.; Sakr, H.M.; El-Azab, A.S.; Bua, S.; Supuran, C.T. Synthesis and human/bacterial carbonic anhydrase inhibition with a series of sulfonamides incorporating phthalimido moieties. Bioorg. Med. Chem. 2017, 25, 2524–2529. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Angeli, A.; El-Azab, A.S.; Abu El-Enin, M.A.; Supuran, C.T. Synthesis and biological evaluation of cyclic imides incorporating benzenesulfonamide moieties as carbonic anhydrase I, II, IV and IX inhibitors. Bioorg. Med. Chem. 2017, 25, 1666–1671. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Ceruso, M.; Supuran, C.T. Carbonic anhydrase inhibitory activity of sulfonamides and carboxylic acids incorporating cyclic imide scaffolds. Bioorg. Med. Chem. Lett. 2014, 24, 5185–5189. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.; Ayyad, R.R.; Ceruso, M.; Supuran, C.T. Inhibition of carbonic anhydrase isoforms I, II, IV, VII and XII with carboxylates and sulfonamides incorporating phthalimide/phthalic anhydride scaffolds. Bioorg. Med. Chem. 2016, 24, 20–25. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Ghiaty, A.H.; Gratteri, P.; Supuran, C.T.; Nocentini, A. 4-Substituted benzenesulfonamides featuring cyclic imides moieties exhibit potent and isoform-selective carbonic anhydrase II/IX inhibition. Bioorg. Chem. 2019, 83, 198–204. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Bua, S.; Nocentini, A.; Abu El-Enin, M.A.; Alanazi, M.M.; AlSaif, N.A.; Hefnawy, M.M.; Supuran, C.T. Design, synthesis, and carbonic anhydrase inhibition activity of benzenesulfonamide-linked novel pyrazoline derivatives. Bioorg. Chem. 2019, 87, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Sönmez, F.; Bilen, Ç.; Ergun, A.; Gençer, N.; Arslan, O.; Kucukislamoglu, M. Synthesis, antioxidant and carbonic anhydrase I and II inhibitory activities of novel sulphonamide-substituted coumarylthiazole derivatives. J. Enzyme Inhib. Med. Chem. 2016, 31, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Kılıcaslan, S.; Arslan, M.; Ruya, Z.; Bilen, Ç.; Ergün, A.; Gençer, N.; Arslan, O. Synthesis and evaluation of sulfonamide-bearing thiazole as carbonic anhydrase isoforms hCA I and hCA II. J. Enzyme Inhib. Med. Chem. 2016, 31, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Alaa, A.-M.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Almehizia, A.A.; Alanazi, M.M.; Hefnawy, M.M.; Supuran, C.T. New anthranilic acid-incorporating N-benzenesulfonamidophthalimides as potent inhibitors of carbonic anhydrases I, II, IX, and XII: Synthesis, in vitro testing, and in silico assessment. Eur. J. Med. Chem. 2019, 181, 111573. [Google Scholar] [CrossRef] [PubMed]
- Gökce, H.; Öztürk, N.; Sert, Y.; El-Azab, A.S.; AlSaif, N.A.; Abdel-Aziz, A.A.M. 4-[(1, 3-Dioxoisoindolin-2-yl) methyl] benzenesulfonamide: Full Structural and Spectroscopic Characterization and Molecular Docking with Carbonic Anhydrase II. ChemistrySelect 2018, 3, 10113–10124. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 1377–1378. [Google Scholar] [CrossRef]
- Borras, J.; Scozzafava, A.; Menabuoni, L.; Mincione, F.; Briganti, F.; Mincione, G.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: Is the tail more important than the ring? Bioorg. Med. Chem. 1999, 7, 2397–2406. [Google Scholar] [CrossRef]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Mincione, G.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. Bioorg. Med. Chem. Lett. 2001, 11, 575–582. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Abou-Zeid, L.A.; ElTahir, K.E.; Mohamed, M.A.; Abu El-Enin, M.A.; El-Azab, A.S. Design, synthesis of 2,3-disubstitued 4(3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents: COX-1/2 inhibitory activities and molecular docking studies. Bioorg. Med. Chem. 2016, 24, 3818–3828. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Abdel-Aziz, A.A.; Al-Suwaidan, I.A.; Abdel-Hamide, S.G.; Shawer, T.Z.; El-Azab, A.S. Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Eur. J. Med. Chem. 2014, 79, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Al-Suwaidan, I.A.; Alaa, A.-M.; Mohamed, M.A.; El_Morsy, A.M.; El-Azab, A.S. Design, synthesis and biological evaluation of some novel substituted 2-mercapto-3-phenethylquinazolines as antitumor agents. Med. Chem. Res. 2013, 22, 5566–5577. [Google Scholar] [CrossRef]
- Al-Obaid, A.M.; Abdel-Hamide, S.G.; El-Kashef, H.A.; Abdel-Aziz, A.A.; El-Azab, A.S.; Al-Khamees, H.A.; El-Subbagh, H.I. Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur. J. Med. Chem. 2009, 44, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaidan, I.A.; Abdel-Aziz, A.A.; Shawer, T.Z.; Ayyad, R.R.; Alanazi, A.M.; El-Morsy, A.M.; Mohamed, M.A.; Abdel-Aziz, N.I.; El-Sayed, M.A.; El-Azab, A.S. Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4(3H)quinazolinone analogues. J. Enzyme Inhib. Med. Chem. 2016, 31, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaidan, I.A.; Alanazi, A.M.; Abdel-Aziz, A.A.; Mohamed, M.A.; El-Azab, A.S. Design, synthesis and biological evaluation of 2-mercapto-3-phenethylquinazoline bearing anilide fragments as potential antitumor agents: Molecular docking study. Bioorg. Med. Chem. Lett. 2013, 23, 3935–3941. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Abdel-Hamide, S.G.; Sayed-Ahmed, M.M.; Hassan, G.S.; El-Hadiyah, T.M.; Al-Shabanah, O.A.; Al-Deeb, O.A.; El-Subbagh, H.I. Novel 4 (3H)-quinazolinone analogs: Synthesis and anticonvulsant activity. Med. Chem. Res. 2013, 22, 2815–2827. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Al-Omar, M.A.; Abdel-Aziz, A.A.; Abdel-Aziz, N.I.; el-Sayed, M.A.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Abdel-Hamide, S.G. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur. J. Med. Chem. 2010, 45, 4188–4198. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Eltahir, K.E. Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4(3H)-quinazoline derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.-M.; Ng, S.W.; Tiekink, E.R. 6-Methyl-3-phenyl-2-sulfanylidene-1, 2, 3, 4-tetrahydroquinazolin-4-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o862. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.; Bua, S.; Nocentini, A.; El-Gendy, M.A.; Mohamed, M.A.; Shawer, T.Z.; AlSaif, N.A.; Supuran, C.T. Synthesis of benzensulfonamides linked to quinazoline scaffolds as novel carbonic anhydrase inhibitors. Bioorg. Chem. 2019, 87, 78–90. [Google Scholar] [CrossRef]
- El-Azab, A.S.; ElTahir, K.E.; Attia, S.M. Synthesis and anticonvulsant evaluation of some novel 4 (3H)-quinazolinones. Mon. Chem.-Chem. Mon. 2011, 142, 837–848. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Ayyad, R.R.; Shawer, T.Z.; Abdel-Aziz, A.A.; El-Azab, A.S. Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur. J. Med. Chem. 2016, 112, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Abdel-Aziz, A.A.; Shawer, T.Z.; Ayyad, R.R.; Al-Obaid, A.M.; Al-Agamy, M.H.; Maarouf, A.R.; El-Azab, A.S. Synthesis, antitumor and antimicrobial activity of some new 6-methyl-3-phenyl-4(3H)-quinazolinone analogues: In silico studies. J. Enzyme Inhib. Med. Chem. 2016, 31, 721–735. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Al-Dhfyan, A.; Abdel-Aziz, A.A.; Abou-Zeid, L.A.; Alkahtani, H.M.; Al-Obaid, A.M.; Al-Gendy, M.A. Synthesis, anticancer and apoptosis-inducing activities of quinazoline-isatin conjugates: Epidermal growth factor receptor-tyrosine kinase assay and molecular docking studies. J. Enzyme Inhib. Med. Chem. 2017, 32, 935–944. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.; Ghabbour, H.A.; Al-Gendy, M.A. Synthesis, in vitro antitumour activity, and molecular docking study of novel 2-substituted mercapto-3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinone analogues. J. Enzyme Inhib. Med. Chem. 2017, 32, 1229–1239. [Google Scholar] [CrossRef]
- Al-Omary, F.A.; Abou-Zeid, L.A.; Nagi, M.N.; Habib, E.-S.E.; Alaa, A.-M.; El-Azab, A.S.; Abdel-Hamide, S.G.; Al-Omar, M.A.; Al-Obaid, A.M.; El-Subbagh, H.I. Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2010, 18, 2849–2863. [Google Scholar] [CrossRef]
- El-Azab, A.S.; ElTahir, K.E. Design and synthesis of novel 7-aminoquinazoline derivatives: Antitumor and anticonvulsant activities. Bioorg. Med. Chem. Lett. 2012, 22, 1879–1885. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Abou-Zeid, L.A.; ElTahir, K.E.H.; Ayyad, R.R.; El-Sayed, M.A.; El-Azab, A.S. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. Eur. J. Med. Chem. 2016, 121, 410–421. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.; AlSaif, N.A.; Alkahtani, H.M.; Alanazi, M.M.; Obaidullah, A.J.; Eskandrani, R.O.; Alharbi, A. Antitumor activity, multitarget mechanisms, and molecular docking studies of quinazoline derivatives based on a benzenesulfonamide scaffold: Cell cycle analysis. Bioorg. Chem. 2020, 104, 104345. [Google Scholar] [CrossRef]
- Hamdi, A.; El-Shafey, H.W.; Othman, D.I.A.; El-Azab, A.S.; AlSaif, N.A.; Abdel-Aziz, A.A. Design, synthesis, antitumor, and VEGFR-2 inhibition activities of novel 4-anilino-2-vinyl-quinazolines: Molecular modeling studies. Bioorg. Chem. 2022, 122, 105710. [Google Scholar] [CrossRef]
- Altamimi, A.S.; El-Azab, A.S.; Abdelhamid, S.G.; Alamri, M.A.; Bayoumi, A.H.; Alqahtani, S.M.; Alabbas, A.B.; Altharawi, A.I.; Alossaimi, M.A.; Mohamed, M.A. Synthesis, Anticancer Screening of Some Novel Trimethoxy Quinazolines and VEGFR2, EGFR Tyrosine Kinase Inhibitors Assay; Molecular Docking Studies. Molecules 2021, 26, 2992. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S. Synthesis of some new substituted 2-mercaptoquinazoline analogs as potential antimicrobial agents. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 333–348. [Google Scholar] [CrossRef]
- Aziza, M.; Nassar, M.; AbdelHamide, S.; ElHakim, A.; El-Azab, A. Synthesis and antimicrobial activities of some new 3-heteroaryl-quinazolin-4-ones. Indian J. Heterocycl. Chem. 1996, 6, 25–30. [Google Scholar]
- Alafeefy, A.M.; Kadi, A.A.; El-Azab, A.S.; Abdel-Hamide, S.G.; Daba, M.H.Y. Synthesis, Analgesic and Anti-Inflammatory Evaluation of Some New 3H-Quinazolin-4-one Derivatives. Arch. Pharm. 2008, 341, 377–385. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Abdel-Aziz, A.A.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Alanazi, M.M.; El-Gendy, M.A.; Ahmed, H.E.A.; Supuran, C.T. S-substituted 2-mercaptoquinazolin-4(3H)-one and 4-ethylbenzensulfonamides act as potent and selective human carbonic anhydrase IX and XII inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, H.M.; Abdalla, A.N.; Obaidullah, A.J.; Alanazi, M.M.; Almehizia, A.A.; Alanazi, M.G.; Ahmed, A.Y.; Alwassil, O.I.; Darwish, H.W.; Abdel-Aziz, A.A.; et al. Synthesis, cytotoxic evaluation, and molecular docking studies of novel quinazoline derivatives with benzenesulfonamide and anilide tails: Dual inhibitors of EGFR/HER2. Bioorg. Chem. 2020, 95, 103461. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Alaa, A.-M.; Bua, S.; Nocentini, A.; Alanazi, M.M.; AlSaif, N.A.; Al-Suwaidan, I.A.; Hefnawy, M.M.; Supuran, C.T. Synthesis and comparative carbonic anhydrase inhibition of new Schiff’s bases incorporating benzenesulfonamide, methanesulfonamide, and methylsulfonylbenzene scaffolds. Bioorg. Chem. 2019, 92, 103225. [Google Scholar] [CrossRef]
- Nocentini, A.; Bonardi, A.; Gratteri, P.; Cerra, B.; Gioiello, A.; Supuran, C.T. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J. Enzyme Inhib. Med. Chem. 2018, 33, 1453–1459. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.; Ahmed, H.E.A.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Obaidullah, A.J.; Hefnawy, M.M.; Supuran, C.T. Exploring structure-activity relationship of S-substituted 2-mercaptoquinazolin-4(3H)-one including 4-ethylbenzenesulfonamides as human carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 598–609. [Google Scholar] [CrossRef]
- Durgun, M.; Turkmen, H.; Ceruso, M.; Supuran, C.T. Synthesis of Schiff base derivatives of 4-(2-aminoethyl)-benzenesulfonamide with inhibitory activity against carbonic anhydrase isoforms I, II, IX and XII. Bioorg. Med. Chem. Lett. 2015, 25, 2377–2381. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).