Double Perovskite Mn4+-Doped La2CaSnO6/La2MgSnO6 Phosphor for Near-Ultraviolet Light Excited W-LEDs and Plant Growth
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Properties
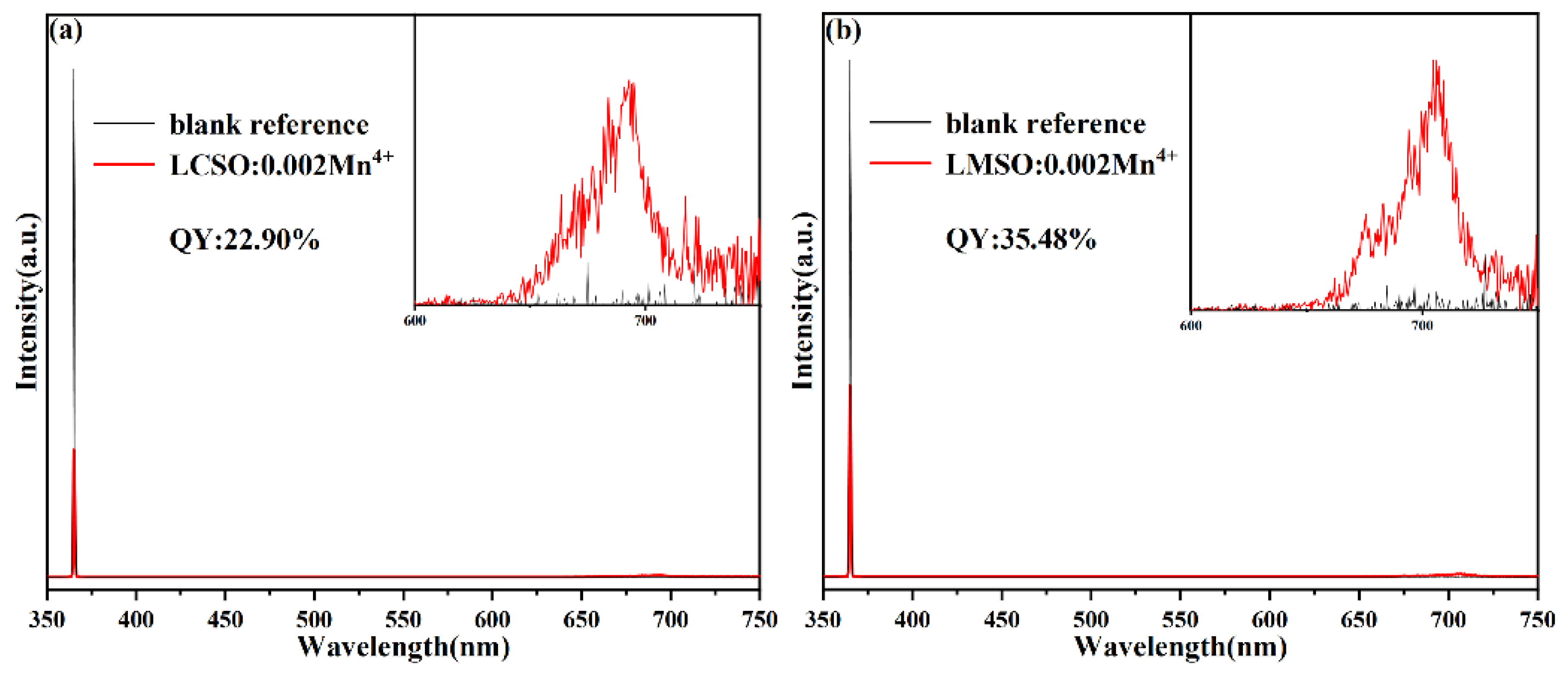
2.2. Photoluminescence Properties
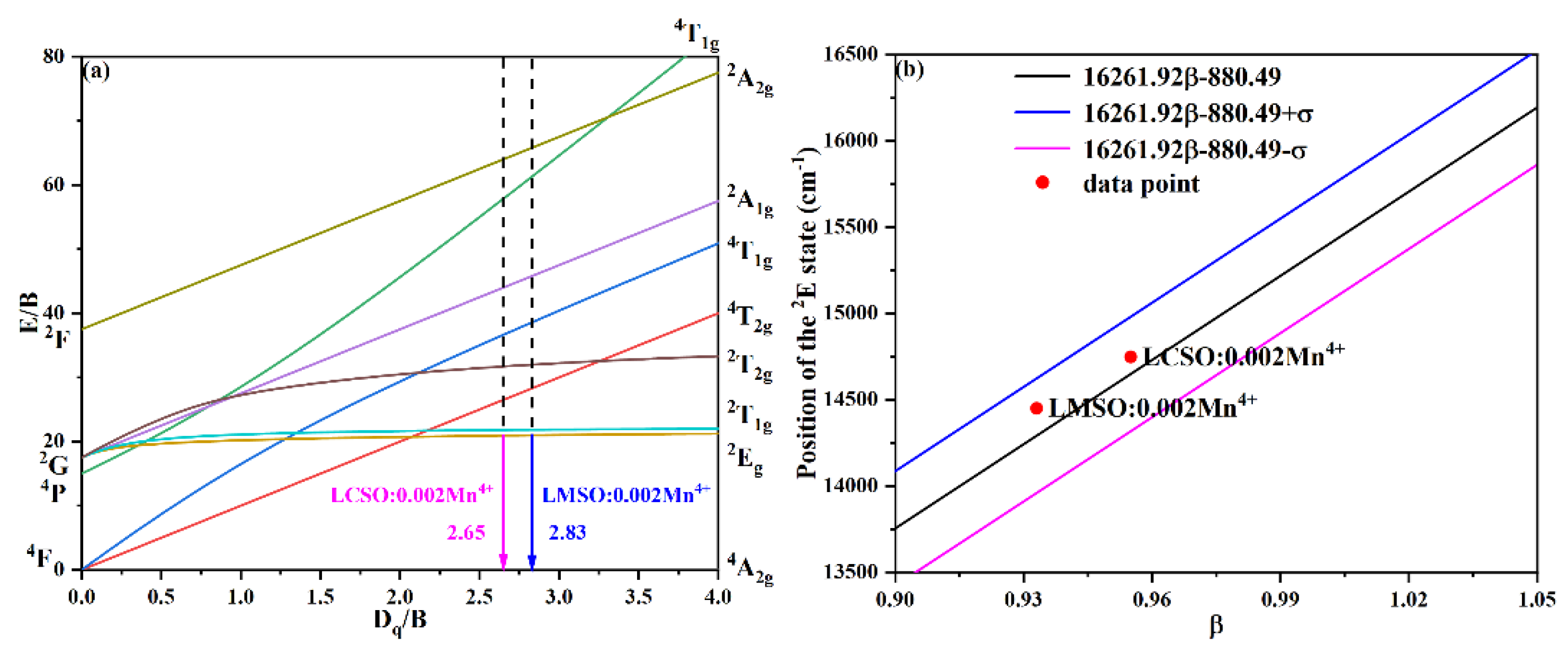
2.3. Crystal Field Analysis and Nephelauxetic Effect
2.4. Photoluminescence Thermal Stability
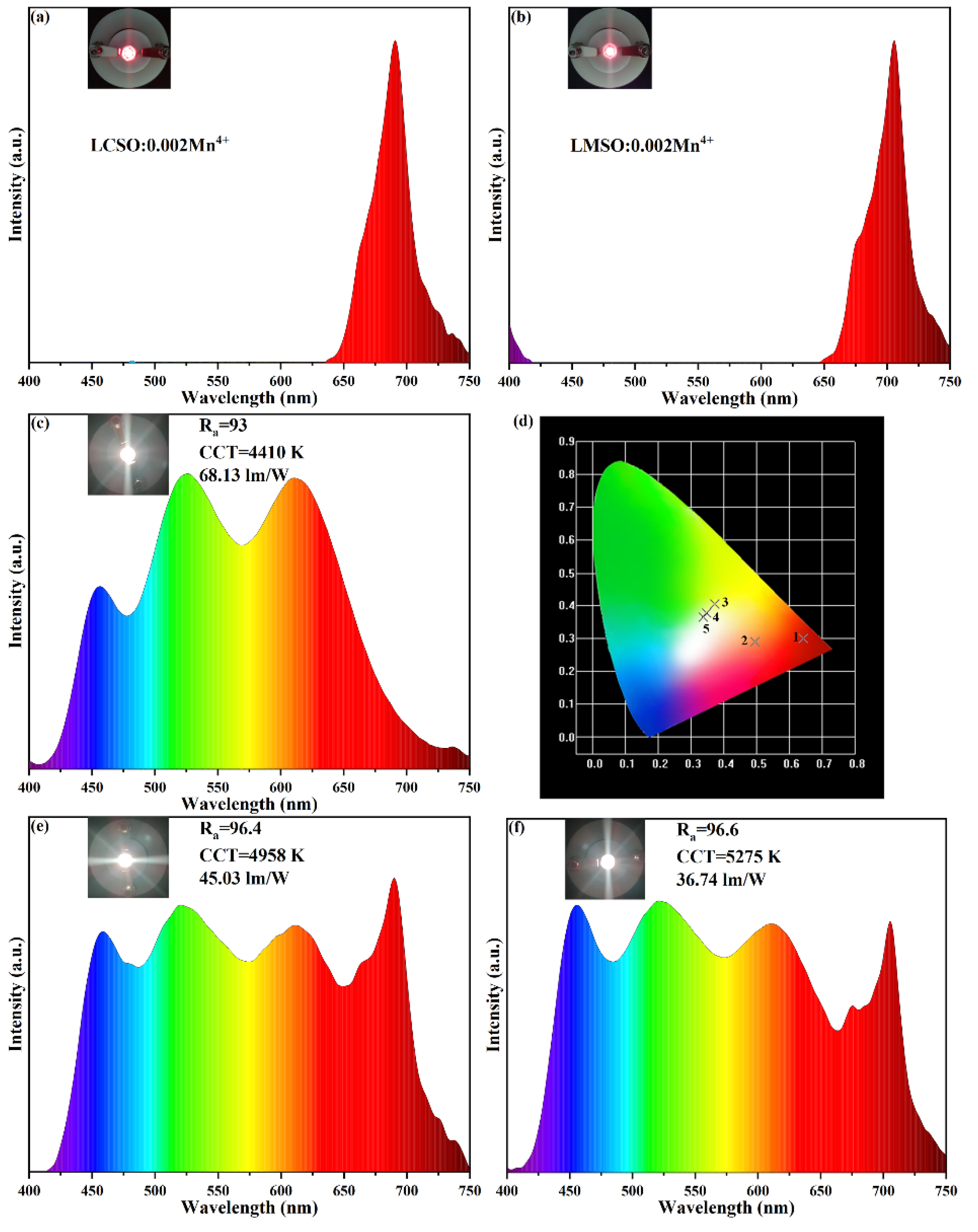
2.5. Electroluminescence Spectrum of the Fabricated LED Devices
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, D.; Li, J.; Guo, X.; Li, Q.; Wu, H.; Meng, L.; Liu, Z. Controlled Synthesis of Tb3+/Eu3+ Co-doped Gd2O3 phosphors with enhanced red emission. Molecules 2019, 24, 759. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.P.; Li, C.; Zhang, X.T.; Xu, J.; Chen, X.; Wang, X.L.; Jia, Y.; Wang, X.; Liu, Y.C. A single Eu2+-activated high-color-rendering oxychloride white-light phosphor for white-light-emitting diodes. Light Sci. Appl. 2016, 5, e16024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, M.; Fang, M.; Wu, X.; Liu, Y.; Huang, Z.; Xi, K.; Min, X. Synthesis and luminescence properties of a novel green-yellow-emitting phosphor BiOCl:Pr3+ for Blue-Light-Based w-LEDs. Molecules 2019, 24, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, C.; Wu, X.; Huang, X.; Lin, F.; Liu, Y.; Fang, M.; Min, X.; Huang, Z. Yellow emission obtained by combination of broadband emission and multi-peak emission in garnet structure Na2YMg2V3O12:Dy3+ phosphor. Molecules 2020, 25, 542. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S. Review—Tanabe−Sugano energy-level diagram and Racah parameters in Mn4+-activated red and deep red-emitting phosphors. ECS J. Solid State Sci. Technol. 2019, 8, R183–R196. [Google Scholar] [CrossRef]
- Zhou, C.; Peng, L.; Kong, Z.; Wu, M.; Molokeev, M.S.; Zhou, Z.; Wang, J.; Xia, M. A high thermal stability Cr3+-doped gallate far red phosphor for plant lighting: Structure, luminescence enhancement and application prospect. J. Mater. Chem. C 2022, 10, 5829–5839. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Yin, S.; You, H. Rare-earth-free Mn4+-doped double perovskite structure phosphor for near ultraviolet excitation of WLED and plant cultivation. J. Alloys Compd. 2022, 891, 162042. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Su, Y.; Zhang, X. Metal to metal charge transfer induced efficient yellow/far-red luminescence in Na2Ca3(Nb,Ta)2O9:Bi3+ toward the applications of white-leds and plant growth light. Adv. Opt. Mater. 2022, 10, 2200150. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, C.; Song, E.; Wang, Y.; Ming, H.; Xia, Z.; Zhang, Q. Three birds with one stone: K2SiF6:Mn4+ single crystal phosphors for high-power and laser-driven lighting. Adv. Opt. Mater. 2020, 8, 2000976. [Google Scholar] [CrossRef]
- Wu, Y.; Zhuang, Y.; Lv, Y.; Ruan, K.; Xie, R.-J. A high-performance non-rare-earth deep-red-emitting Ca14-xSrxZn6Al10O35:Mn4+ phosphor for high-power plant growth LEDs. J. Alloys Compd. 2019, 781, 702–709. [Google Scholar] [CrossRef]
- Deng, K.; Jin, Y.; Yuan, L.; Wang, B.; Wu, H.; Hu, Y. A thermal-stable Mn4+-doped far-red-emitting phosphor-converted LED for indoor plant cultivation. Mater. Today Chem. 2022, 26, 101010. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, H.; Zhang, D.; Shen, Y.; Li, Y.; Hong, R.; Tao, C.; Han, Z.; Chen, L.; Zhou, S. Deep-red emitting Mg2TiO4:Mn4+ phosphor ceramics for plant lighting. J. Adv. Ceram. 2020, 10, 88–97. [Google Scholar] [CrossRef]
- Li, L.; Tian, G.; Chang, W.; Yan, Y.; Ling, F.; Jiang, S.; Xiang, G.; Zhou, X. A novel double-perovskite LiLaMgTeO6:Mn4+ far-red phosphor for indoor plant cultivation white LEDs: Crystal and electronic structure, and photoluminescence properties. J. Alloys Compd. 2020, 832, 154905. [Google Scholar] [CrossRef]
- Gu, S.; Xia, M.; Zhou, C.; Kong, Z.; Molokeev, M.S.; Liu, L.; Wong, W.-Y.; Zhou, Z. Red shift properties, crystal field theory and nephelauxetic effect on Mn4+-doped SrMgAl10-yGayO17 red phosphor for plant growth LED light. Chem. Eng. J. 2020, 396, 125208. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Dong, L.; Yin, S.; Wu, X.; You, H. Novel SrGd2Al2O7:Mn4+, Nd3+, and Yb3+ phosphors for c-Si solar cells. Dalton Trans. 2021, 50, 7017–7025. [Google Scholar] [CrossRef]
- Qin, L.; Bi, S.; Cai, P.; Chen, C.; Wang, J.; Kim, S.I.; Huang, Y.; Seo, H.J. Preparation, characterization and luminescent properties of red-emitting phosphor: LiLa2NbO6 doped with Mn4+ ions. J. Alloys Compd. 2018, 755, 61–66. [Google Scholar] [CrossRef]
- Fu, A.; Pang, Q.; Yang, H.; Zhou, L. Ba2YNbO6:Mn4+-based red phosphor for warm white light-emitting diodes (WLEDs): Photoluminescent and thermal characteristics. Opt. Mater. 2017, 70, 144–152. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Chen, X.; Wang, K.; Li, X.; Zhu, Y.; Ji, Z. Efficient rare-earth free red-emitting Ca2YSbO6:Mn4+, M(M = Li+, Na+, K+, Mg2+) phosphors for white light-emitting diodes. Dalton Trans. 2018, 47, 6528–6537. [Google Scholar] [CrossRef]
- Cao, R.; Chen, T.; Ren, Y.; Chen, T.; Ao, H.; Li, W.; Zheng, G. Synthesis and photoluminescence properties of Ca2LaTaO6:Mn4+ phosphor for plant growth LEDs. J. Alloys Compd. 2019, 780, 749–755. [Google Scholar] [CrossRef]
- Wu, Q.; Li, P.; Ye, Z.; Huo, X.; Yang, H.; Wang, Y.; Wang, D.; Zhao, J.; Yang, Z.; Wang, Z. Near-infrared emitting phosphor LaMg0.5SnGe0.5O3:Cr3+ for plant growth applications: Crystal structure, luminescence, and thermal stability. Inorg. Chem. 2021, 60, 16593–16603. [Google Scholar] [CrossRef]
- Gai, S.; Zhu, H.; Gao, P.; Zhou, C.; Kong, Z.; Molokeev, M.S.; Qi, Z.; Zhou, Z.; Xia, M. Structure analysis, tuning photoluminescence and enhancing thermal stability on Mn4+-doped La2-xYxMgTiO6 red phosphor for agricultural lighting. Ceram. Int. 2020, 46, 20173–20182. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, H.; Zhang, H.; Xia, Z.; Liu, Y.; Molokeev, M.S.; Lei, B. Co-substitution in Ca1−xYxAl12−xMgxO19 phosphors: Local structure evolution, photoluminescence tuning and application for plant growth LEDs. J. Mater. Chem. C 2018, 6, 4217–4224. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Huang, Q.; Huang, F.; Xu, J.; Wang, B.; Lin, Z.; Zhou, J.; Wang, Y. A novel double-perovskite Gd2ZnTiO6:Mn4+ red phosphor for UV-based w-LEDs: Structure and luminescence properties. J. Mater. Chem. C 2016, 4, 2374–2381. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Lu, W.; Shao, B.; Zhao, S.; You, H. Preparation and photoluminescence of novel La8Ca2(Si4P2O22N2)O2 oxynitride phosphors containing Eu2+/Ce3+/Tb3+ ions. Dalton Trans. 2019, 48, 3028–3037. [Google Scholar] [CrossRef]
- Wu, D.; Xiao, Y.; Zhang, L.; Dong, X.; Zhao, S.; Zhou, W.; Lu, Q.; Zhang, J. Highly efficient and thermally stable luminescence of Ca3Gd2Si6O18:Ce3+,Tb3+ phosphors based on efficient energy transfer. J. Mater. Chem. C 2020, 8, 17176–17184. [Google Scholar] [CrossRef]
- Shao, B.; Huo, J.; You, H. Prevailing strategies to tune emission color of lanthanide-activated phosphors for WLED applications. Adv. Opt. Mater. 2019, 7, 1900319. [Google Scholar] [CrossRef]
- Zhu, M.; Pan, Y.; Huang, Y.; Lian, H.; Lin, J. Designed synthesis, morphology evolution and enhanced photoluminescence of a highly efficient red dodec-fluoride phosphor, Li3Na3Ga2F12:Mn4+, for warm WLEDs. J. Mater. Chem. C 2018, 6, 491–499. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, Z.; Xu, L.; Jia, H.; Sun, X.; Liu, K. La2MgTiO6:Bi3+/Mn4+ photoluminescence materials: Molten salt preparation, Bi3+ → Mn4+ energy transfer and thermostability. J. Lumin. 2020, 224, 117290. [Google Scholar] [CrossRef]
- Xing, G.; Feng, Y.; Pan, M.; Wei, Y.; Li, G.; Dang, P.; Liang, S.; Molokeev, M.S.; Cheng, Z.; Lin, J. Photoluminescence tuning in a novel Bi3+/Mn4+ co-doped La2ATiO6:(A = Mg, Zn) double perovskite structure: Phase transition and energy transfer. J. Mater. Chem. C 2018, 6, 13136–13147. [Google Scholar] [CrossRef]
- Cao, R.; Shi, Z.; Quan, G.; Chen, T.; Guo, S.; Hu, Z.; Liu, P. Preparation and luminescence properties of Li2MgZrO4:Mn4+ red phosphor for plant growth. J. Lumin. 2017, 188, 577–581. [Google Scholar] [CrossRef]
- Brik, M.G.; Camardello, S.J.; Srivastava, A.M. Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2014, 4, R39–R43. [Google Scholar] [CrossRef]
- Reisfeld, M.J.; Matwiyoff, N.A.; Asprey, L.B. The electronic spectrum of cesium hexafluoromanganese(IV). J. Mol. Spectrosc. 1971, 39, 8–20. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M. Electronic energy levels of the Mn4+ ion in the Perovskite, CaZrO3. ECS J. Solid State Sci. Technol. 2013, 2, R148–R152. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.; Shi, R.; Zhang, N.; Chen, J.; Zhang, R.; Suo, H.; Goldys, E.M.; Guo, C. Ab initio site occupancy and far-red emission of Mn4+ in cubic-phase La(MgTi)1/2O3 for plant cultivation. ACS Appl. Mater. Inter. 2017, 9, 6177–6185. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, J.; Liu, S.; Li, Y.; Qiu, J. Deep-red photoluminescence and long persistent luminescence in double perovstkite-type La2MgGeO6:Mn4+. J. Am. Ceram. Soc. 2018, 101, 1576–1584. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Wu, H.; Duan, H.; Chen, L.; Fu, Y.; Ju, G.; Mu, Z.; He, M. A deep red phosphor Li2MgTiO4:Mn4+ exhibiting abnormal emission: Potential application as color converter for warm w-LEDs. Chem. Eng. J. 2016, 288, 596–607. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Beers, W.W. Luminescence of Mn4+ in the distorted Perovskite Gd2MgTiO6. J. Electrochem. Soc. 2019, 143, L203–L205. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Huang, L.; Brik, M.G.; Si, S.; Lin, L.; Xuan, T.; Liang, H.; Qiu, J.; Wang, J. High-performance and moisture-resistant red-emitting Cs2SiF6:Mn4+ for high-brightness LED backlighting. J. Mater. Chem. C 2019, 7, 2401–2407. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Jia, Y.; Shao, B.; Lu, W.; Zhao, S.; You, H. Enhancing luminescence and controlling the Mn valence state of Gd3Ga5−x-#Alx−y+#O12:yMn phosphors by the design of the garnet structure. ACS Appl. Mater. Inter. 2020, 12, 7334–7344. [Google Scholar]
- Li, L.; Chang, W.; Chen, W.; Feng, Z.; Zhao, C.; Jiang, P.; Wang, Y.; Zhou, X.; Suchocki, A. Double perovskite LiLaMgWO6:Eu3+ novel red-emitting phosphors for solid sate lighting: Synthesis, structure and photoluminescent properties. Ceram. Int. 2017, 43, 2720–2729. [Google Scholar] [CrossRef]
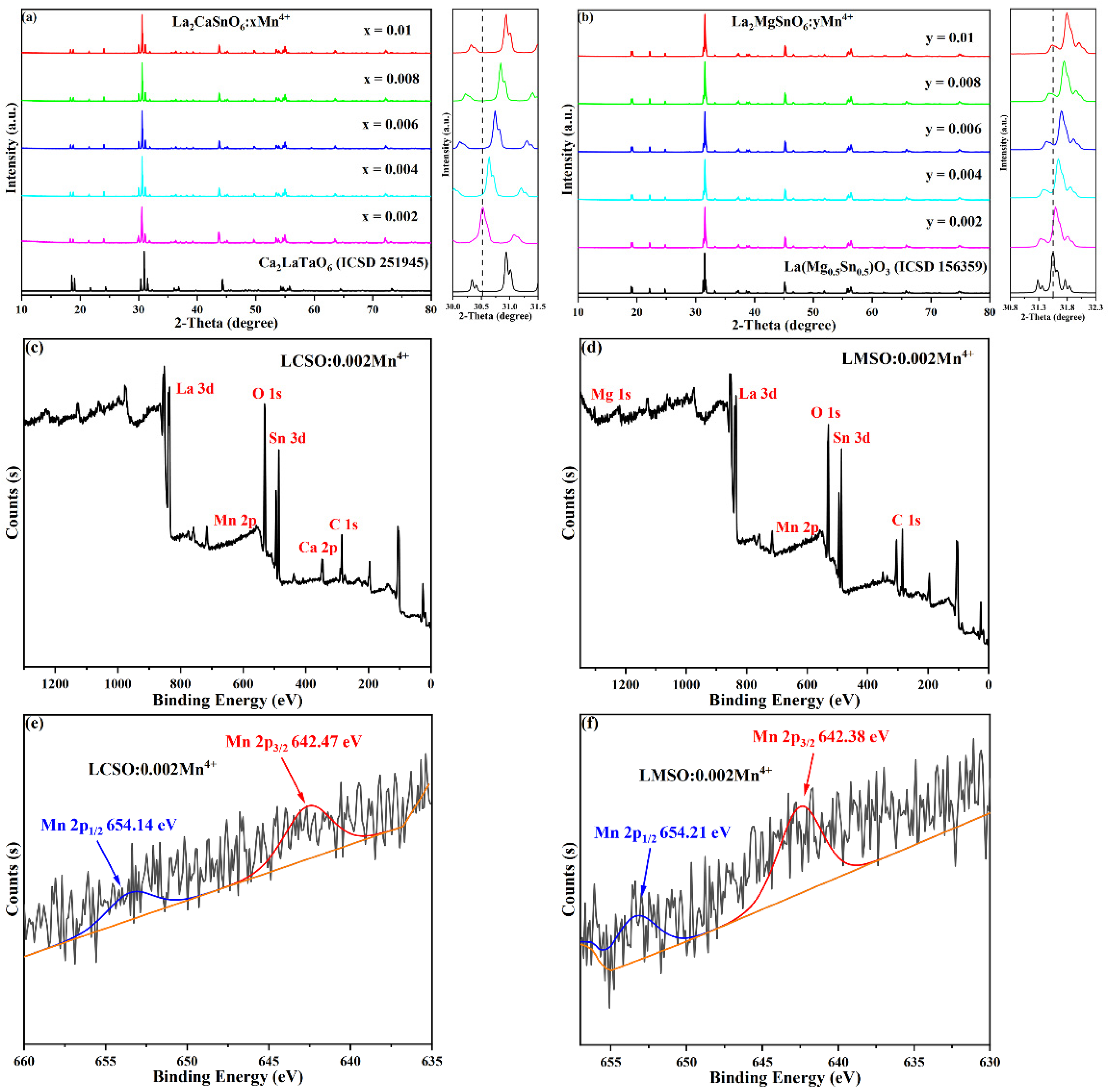

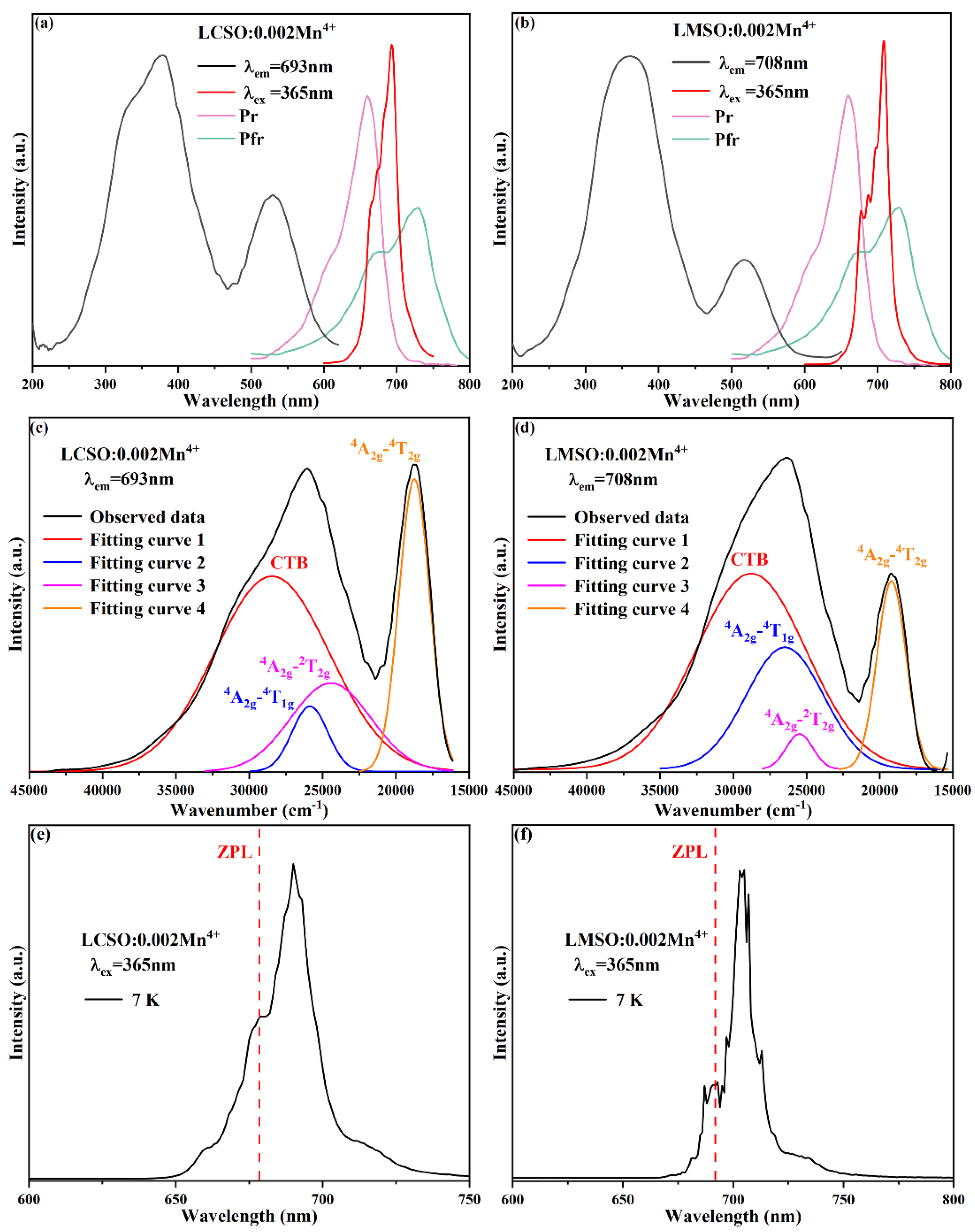
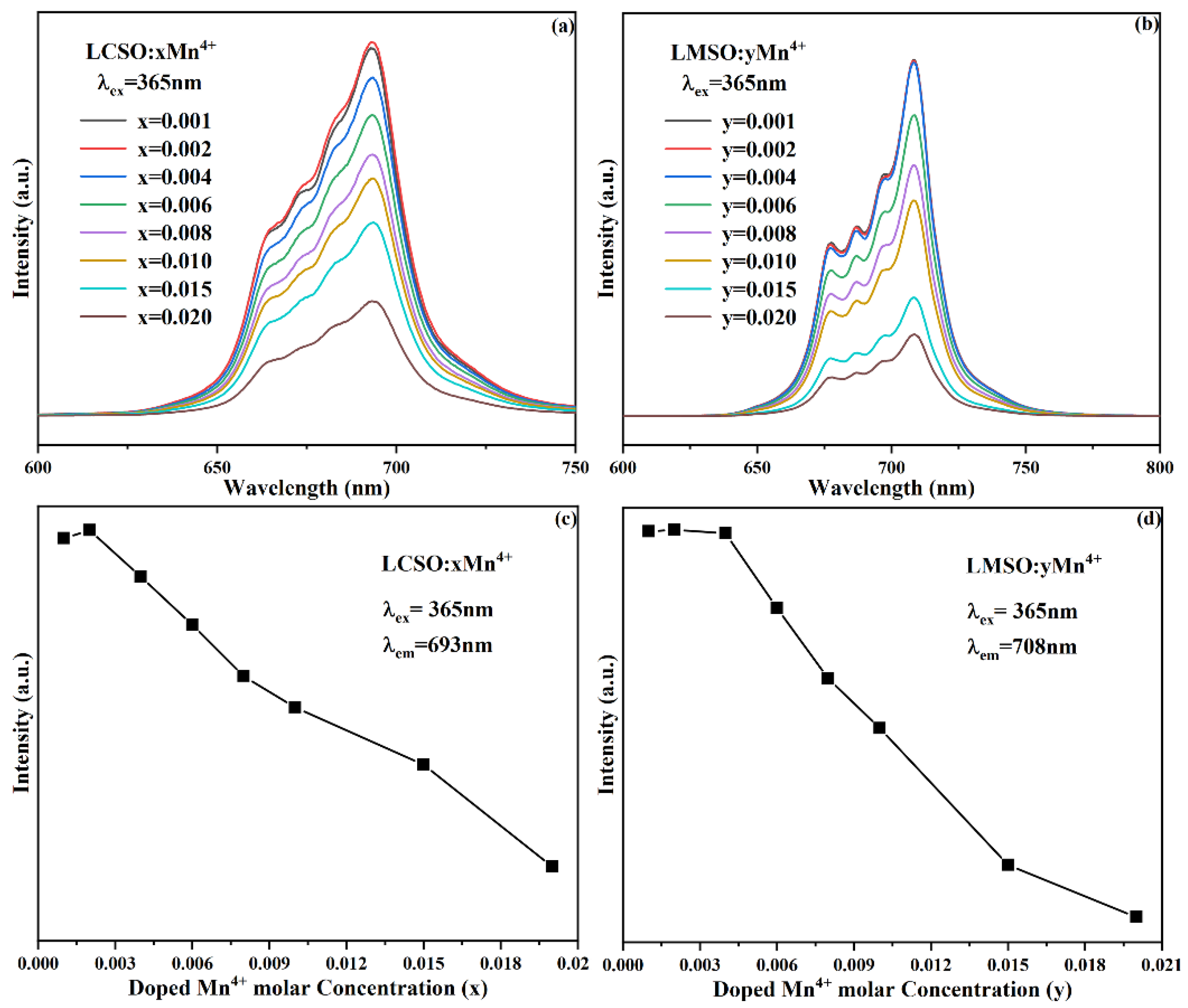
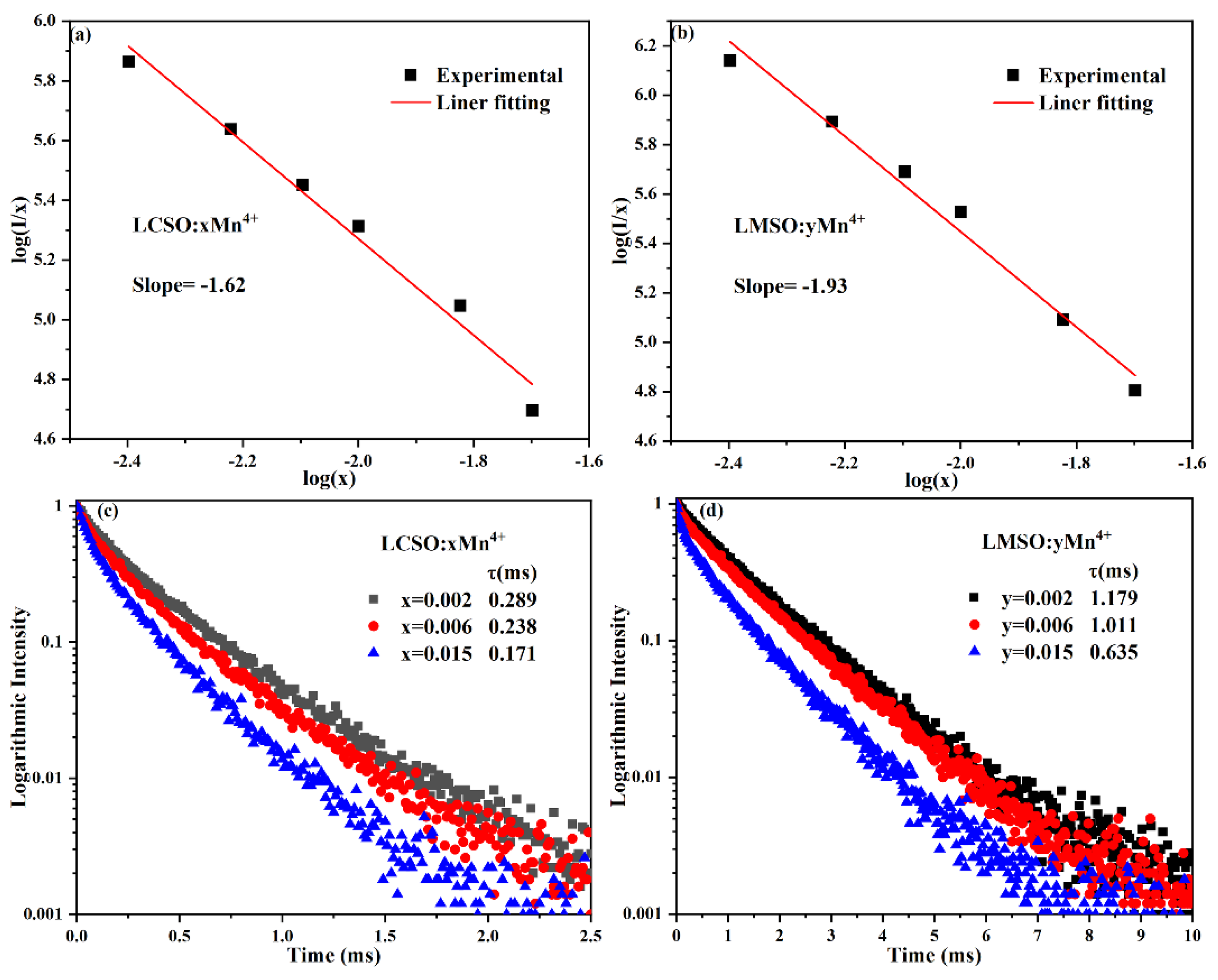


| Compound | La2CaSnO6:0.004Mn4+ | La2MgSnO6:0.010Mn4+ |
|---|---|---|
| Space group | P21/n | P21/n |
| a, Å | 5.74019 | 5.63776 |
| b, Å | 5.95969 | 5.72036 |
| c, Å | 8.25804 | 8.02015 |
| α = γ | 90° | 90° |
| β | 89.99° | 90.06° |
| V | 282.506 | 258.650 |
| Crystal system | Monoclinic | Monoclinic |
| Z | 2 | 2 |
| Rwp, % | 6.08 | 8.26 |
| Rp, % | 4.83 | 5.33 |
| χ2 | 1.008 | 2.173 |
| Phosphor Composition | λex (nm) | λem (nm) | IQY | References |
|---|---|---|---|---|
| Ba2YNbO6:Mn4+ | 360 | 695 | 29.2 | [17] |
| Ca2YSbO6:Mn4+ | 470 | 680 | 19.72 | [18] |
| SrLaGa3O7:Mn4+ | 380 | 715 | 13.83 | [21] |
| La2MgTiO6:Mn4+ | 350 | 710 | 32.5 | [29] |
| Li2MgTiO4:Mn4+ | 460 | 676 | 32 | [30] |
| Li2MgZrO4:Mn4+ | 335 | 670 | 32.3 | [31] |
| LCSO:Mn4+ | 365 | 693 | 22.90 | This work |
| LMSO:Mn4+ | 365 | 708 | 35.48 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Sun, D.; Lyu, Z.; Shen, S.; Wang, J.; Zhao, H.; Wang, L.; You, H. Double Perovskite Mn4+-Doped La2CaSnO6/La2MgSnO6 Phosphor for Near-Ultraviolet Light Excited W-LEDs and Plant Growth. Molecules 2022, 27, 7697. https://doi.org/10.3390/molecules27227697
Lu Z, Sun D, Lyu Z, Shen S, Wang J, Zhao H, Wang L, You H. Double Perovskite Mn4+-Doped La2CaSnO6/La2MgSnO6 Phosphor for Near-Ultraviolet Light Excited W-LEDs and Plant Growth. Molecules. 2022; 27(22):7697. https://doi.org/10.3390/molecules27227697
Chicago/Turabian StyleLu, Zheng, Dashuai Sun, Zeyu Lyu, Sida Shen, Jianhui Wang, Hanwei Zhao, Lixuan Wang, and Hongpeng You. 2022. "Double Perovskite Mn4+-Doped La2CaSnO6/La2MgSnO6 Phosphor for Near-Ultraviolet Light Excited W-LEDs and Plant Growth" Molecules 27, no. 22: 7697. https://doi.org/10.3390/molecules27227697
APA StyleLu, Z., Sun, D., Lyu, Z., Shen, S., Wang, J., Zhao, H., Wang, L., & You, H. (2022). Double Perovskite Mn4+-Doped La2CaSnO6/La2MgSnO6 Phosphor for Near-Ultraviolet Light Excited W-LEDs and Plant Growth. Molecules, 27(22), 7697. https://doi.org/10.3390/molecules27227697






