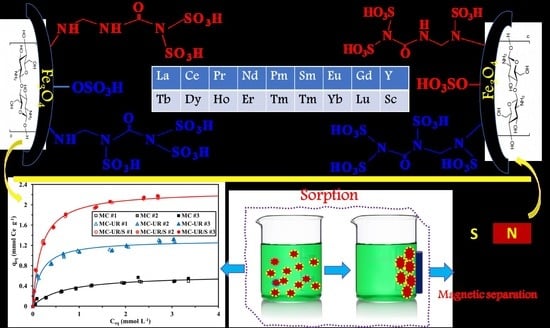
Enhancement of Cerium Sorption onto Urea-Functionalized Magnetite Chitosan Microparticles by Sorbent Sulfonation—Application to Ore Leachate
Abstract
1. Introduction
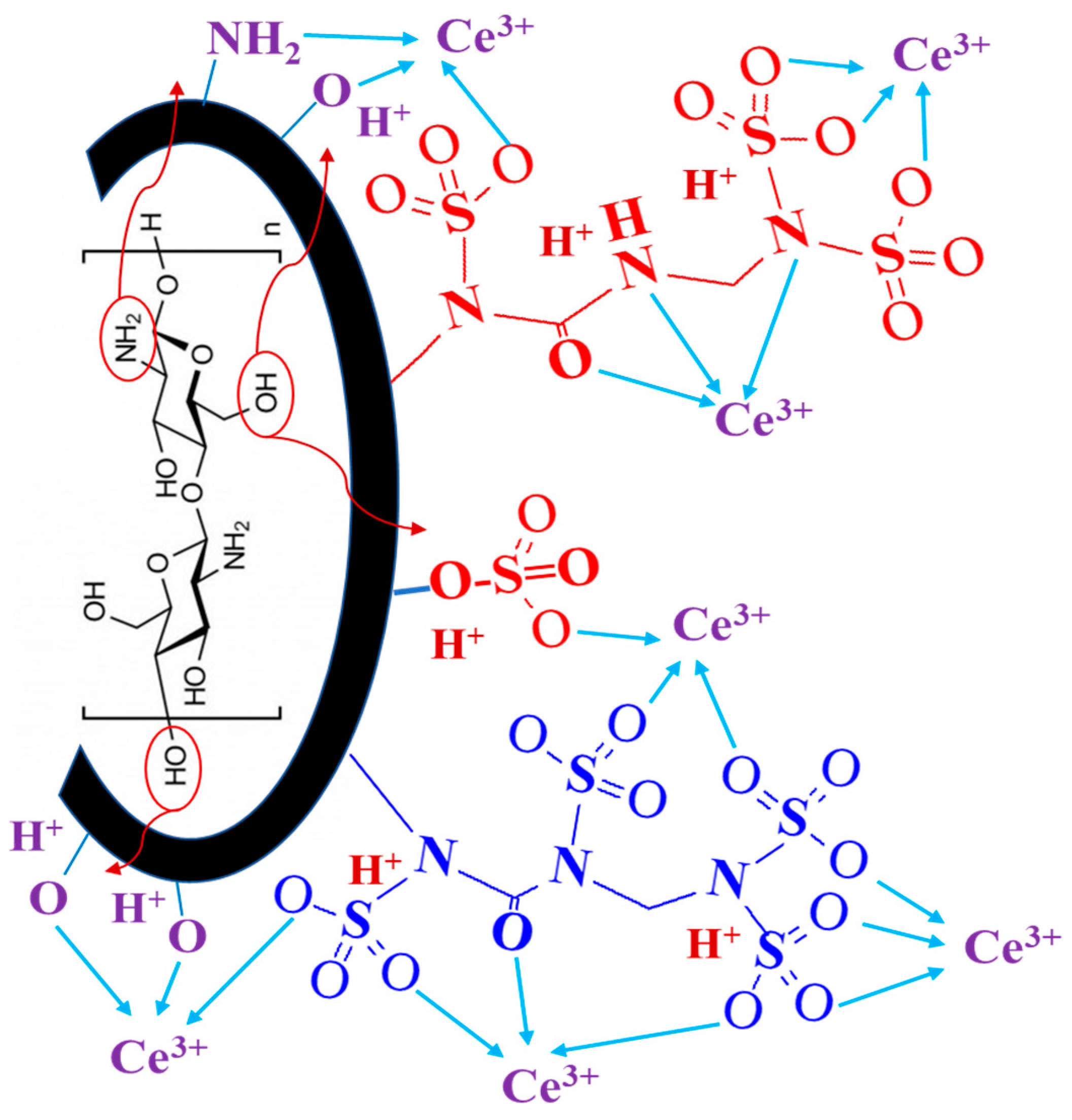
2. Selected Strategy for Sorbent Synthesis—Rationales
3. Results and Discussion
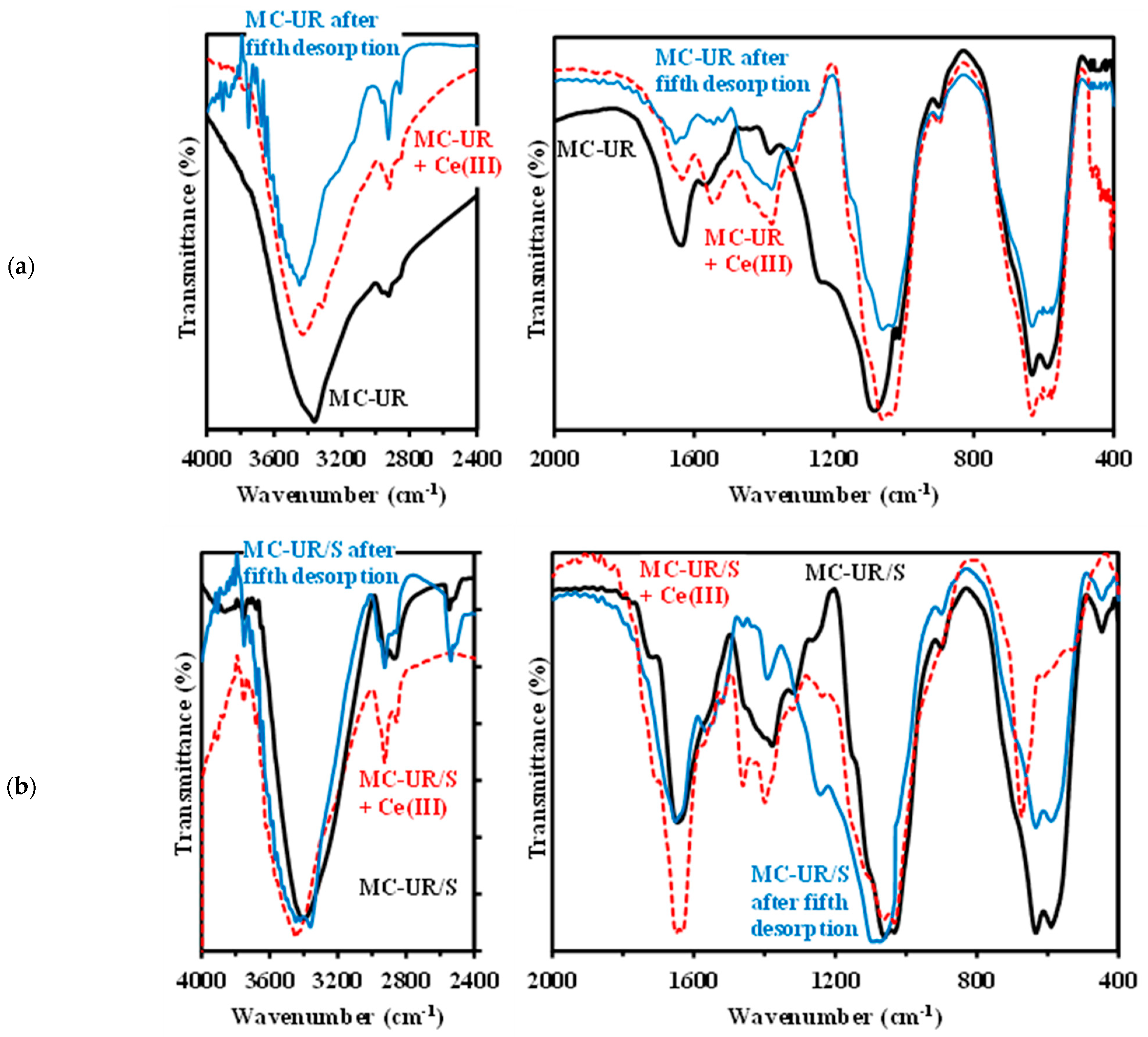
3.1. Characterization of the Sorbents
3.2. Sorption Properties
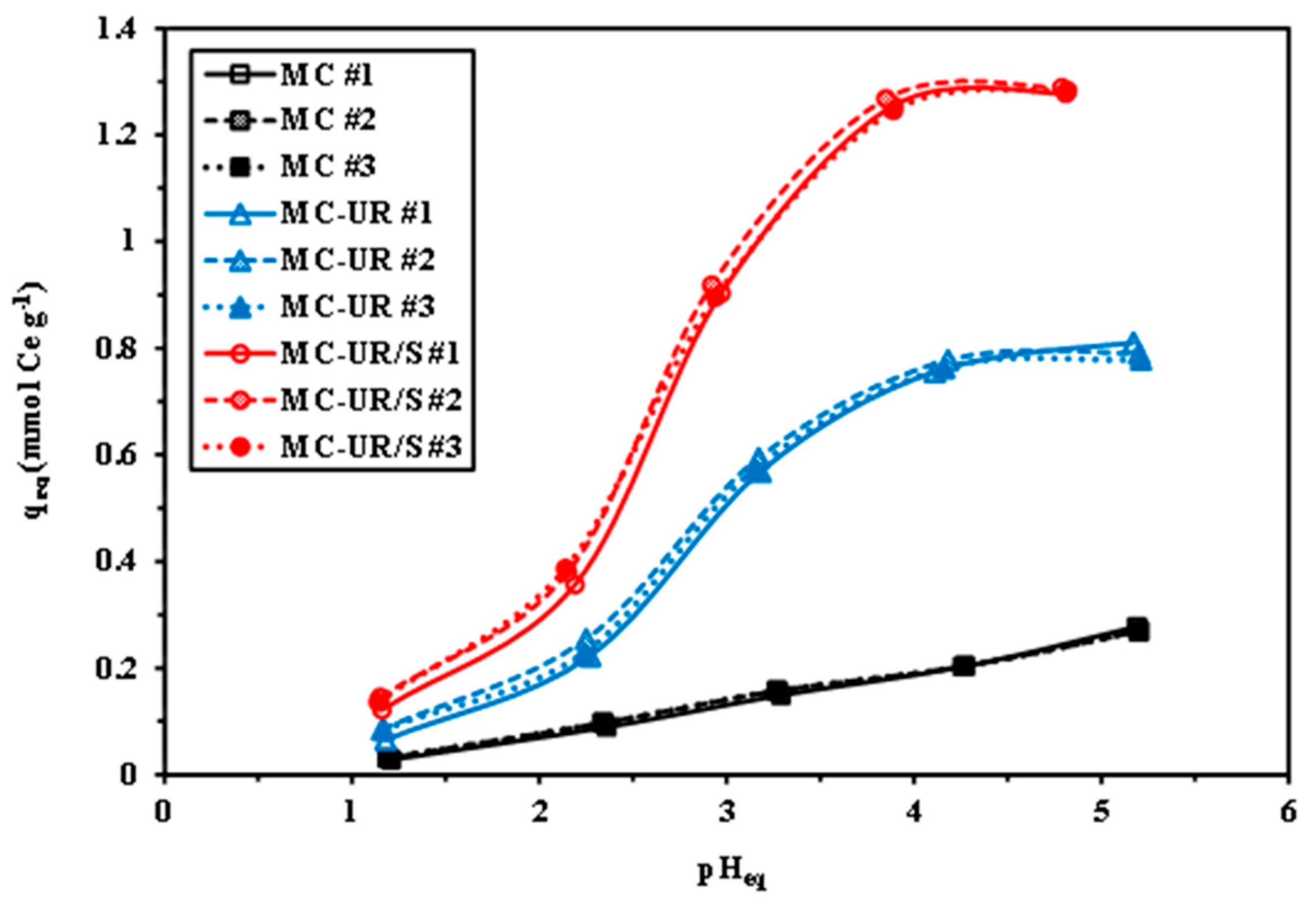
3.2.1. Effect of pH
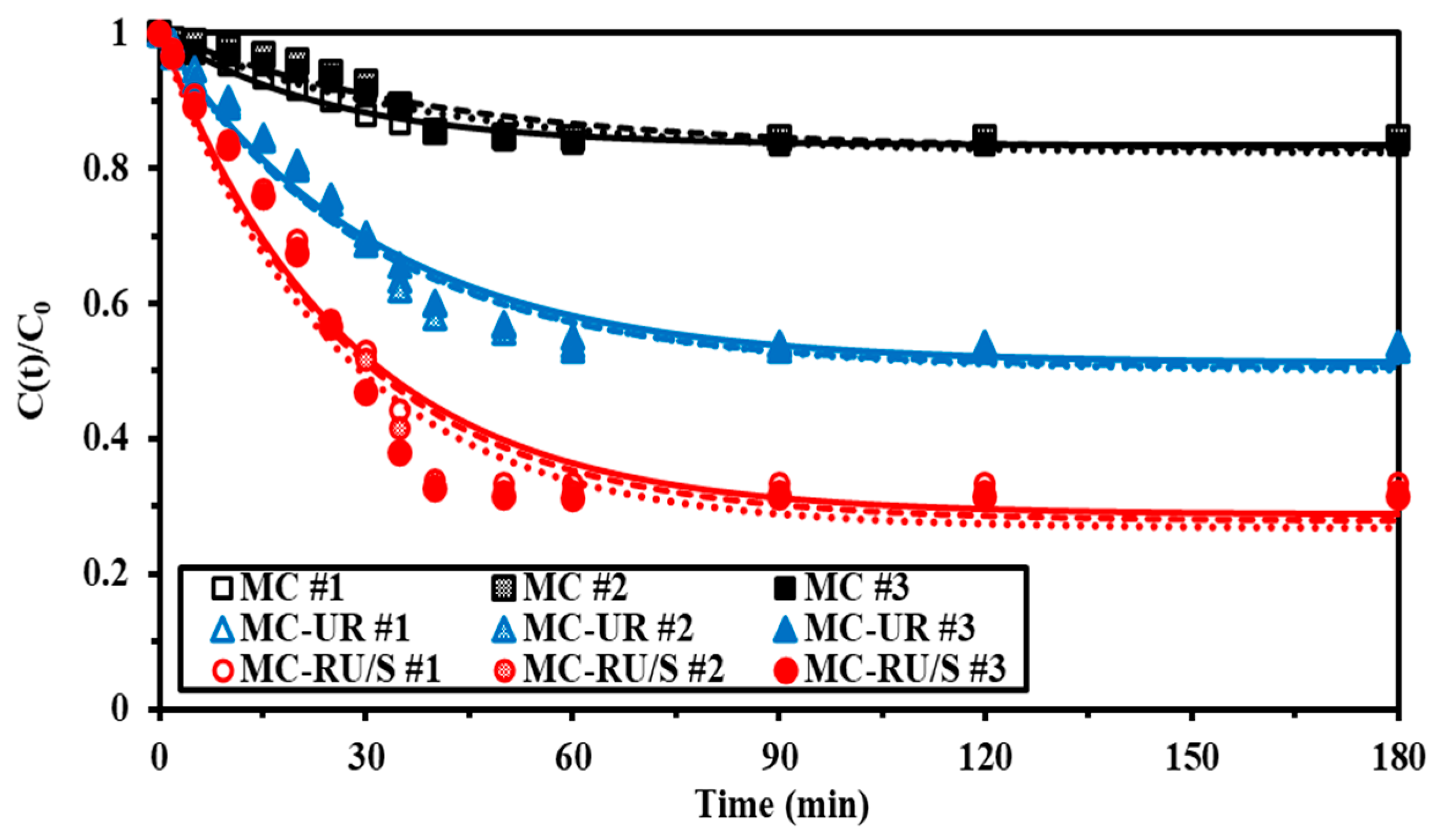
3.2.2. Uptake Kinetics
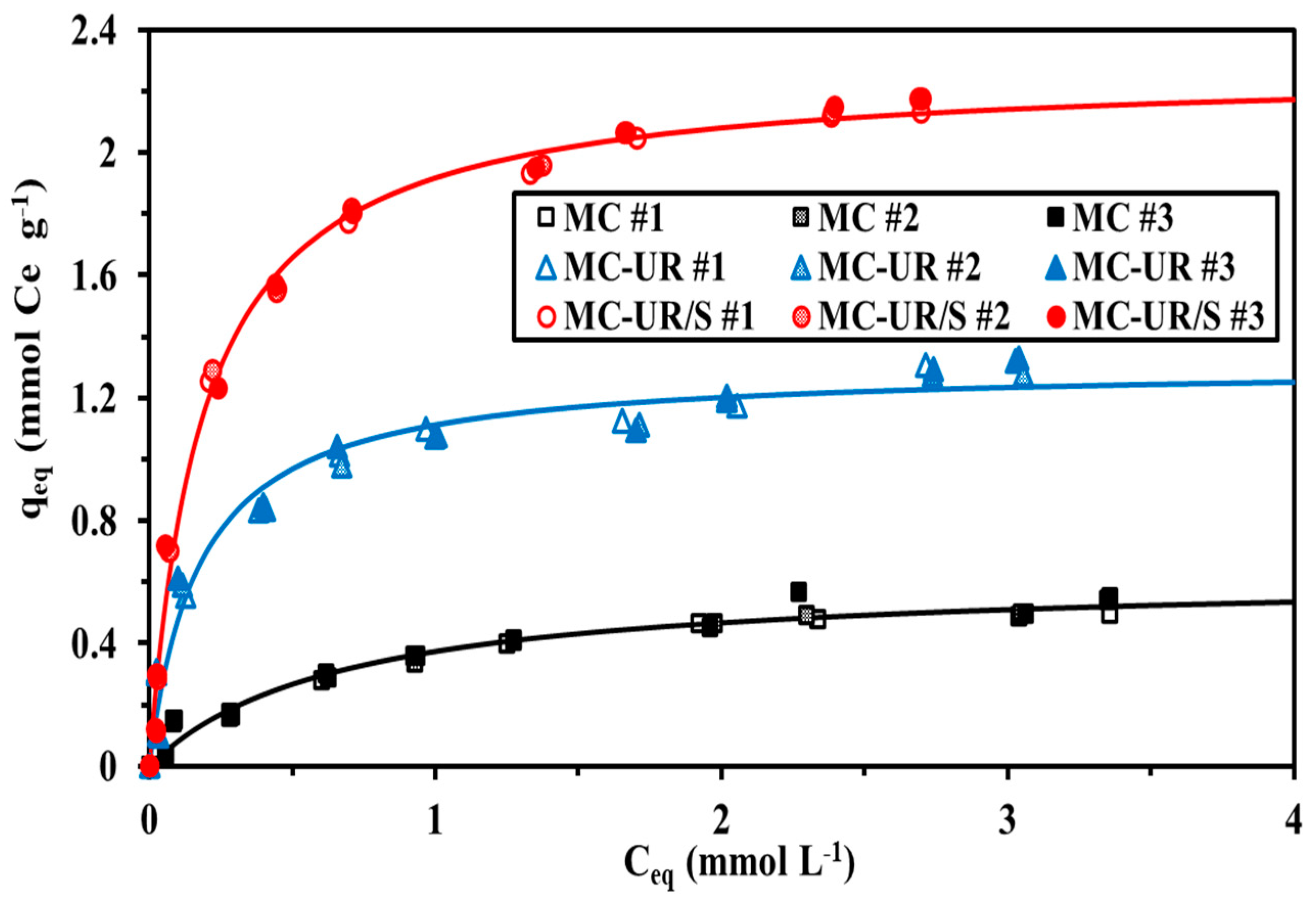
3.2.3. Sorption Isotherms
3.2.4. Sorption Selectivity
3.2.5. Metal Desorption and Sorbent Recycling
3.2.6. Application to Ore Raffinate
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Sorbents
4.3. Characterization of Sorbents
4.4. Sorption Studies
4.5. Modeling of the Sorption Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- RSC. Periodic Table. Available online: https://www.rsc.org/periodic-table/ (accessed on 5 October 2021).
- Hermassi, M.; Granados, M.; Valderrama, C.; Ayora, C.; Cortina, J.L. Recovery of rare earth elements from acidic mine waters: An unknown secondary resource. Sci. Total Environ. 2022, 810, 152258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, C.; Li, C.; Jiang, M. Separation and recovery of valuable metals from low-grade REE-Nb-Fe ore. Int. J. Miner. Process. 2016, 150, 16–23. [Google Scholar] [CrossRef]
- Ai-Thyabat, S.; Zhang, P. Extraction of rare earth elements from upgraded phosphate flotation tailings. Miner. Metall. Process. 2016, 33, 23–30. [Google Scholar]
- Prameswara, G.; Trisnawati, I.; Mulyono, P.; Prasetya, A.; Petrus, H.T.B.M. Leaching behaviour and kinetic of light and heavy rare earth elements (REE) from zircon tailings in Indonesia. JOM 2021, 73, 988–998. [Google Scholar] [CrossRef]
- Reynier, N.; Gagne-Turcotte, R.; Coudert, L.; Costis, S.; Cameron, R.; Blais, J.-F. Bioleaching of uranium tailings as secondary sources for rare earth elements production. Minerals 2021, 11, 302. [Google Scholar] [CrossRef]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.-W.; Song, M.-H.; Wei, W.; Reddy, D.H.K.; Cho, C.-W.; Yun, Y.-S. Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J. Clean. Prod. 2020, 252, 119389. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Akcil, A.; Agcasulu, I.; Swain, B. Valorization of waste LCD and recovery of critical raw material for circular economy: A review. Resour. Conserv. Recycl. 2019, 149, 622–637. [Google Scholar] [CrossRef]
- Kumari, A.; Raj, R.; Randhawa, N.S.; Sahu, S.K. Energy efficient process for recovery of rare earths from spent NdFeB magnet by chlorination roasting and water leaching. Hydrometallurgy 2021, 201, 105581. [Google Scholar] [CrossRef]
- Ahn, N.-K.; Shim, H.-W.; Kim, D.-W.; Swain, B. Valorization of waste NiMH battery through recovery of critical rare earth metal: A simple recycling process for the circular economy. Waste Manag. 2020, 104, 254–261. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J.; Zeng, X. Rare earth elements recovery from waste fluorescent lamps: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 749–776. [Google Scholar] [CrossRef]
- Lie, J.; Liu, J.-C. Selective recovery of rare earth elements (REEs) from spent NiMH batteries by two-stage acid leaching. J. Environ. Chem. Eng. 2021, 9, 106084. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.Y.; Wang, B.; Wang, K.; Wu, R.Z.; Ekberg, C.; Volinsky, A.A. Multiscale recycling rare earth elements from real waste trichromatic phosphors containing glass. J. Clean. Prod. 2019, 238, 117998. [Google Scholar] [CrossRef]
- Garcia-Balboa, C.; Garcia, P.M.-A.; Lopez-Rodas, V.; Costas, E.; Baselga-Cervera, B. Microbial biominers: Sequential bioleaching and biouptake of metals from electronic scraps. Microbiologyopen 2022, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Noble, A.; Ji, B.; Li, Q. Effects of contaminant metal ions on precipitation recovery of rare earth elements using oxalic acid. J. Rare Earths 2022, 40, 482–490. [Google Scholar] [CrossRef]
- Trinopiawan, K.; Avifa, V.N.; Susilo, Y.S.B.; Rakhma, E.; Supriyatna, Y.I.; Susanto, I.; Permana, S.; Soedarsono, J.W. Preliminary study of cerium, lanthanum, and neodymium precipitation from chloride solution using sodium carbonate in the processing of Bangka monazite. Eksplorium-Bul. Pus. Teknol. Bahan Galian Nukl. 2020, 41, 37–44. [Google Scholar]
- Hermassi, M.; Granados, M.; Valderrama, C.; Ayora, C.; Cortina, J.L. Recovery of rare earth elements from acidic mine waters by integration of a selective chelating ion-exchanger and a solvent impregnated resin. J. Environ. Chem. Eng. 2021, 9, 105906. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Teixeira, L.V.; Oliveira, É.D. Selective precipitation of high-quality rare earth oxalates or carbonates from a purified sulfuric liquor containing soluble impurities. Min. Metall. Explor. 2019, 36, 967–977. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Cui, Y.; Jin, L.; Peng, X.; Zhang, H.; Sun, G. Recovery of rare earths from nitric acid leach solutions of phosphate ores using solvent extraction with a new amide extractant (TODGA). Hydrometallurgy 2018, 180, 132–138. [Google Scholar] [CrossRef]
- Talebi, A.; Marra, A.; Cesaro, A.; Belgiorno, V.; Norli, I. The recovery of rare earth metals from WEEE leaching solution via liquid-liquid extraction. Glob. Nest J. 2018, 20, 719–724. [Google Scholar]
- Pavon, S.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Rare earths separation from fluorescent lamp wastes using ionic liquids as extractant agents. Waste Manag. 2018, 82, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Dakroury, G.A.; Maree, R.M.; El-Shazly, E.A.A.; Allan, K.F. Synthesize of poly (acrylamide-co-itaconic/TiO2) nanocomposite for Ce(III) sorption from monazite leachate. J. Polym. Environ. 2022, 30, 1942–1958. [Google Scholar] [CrossRef]
- Kusrini, E.; Alhamid, M.I.; Widiantoro, A.B.; Daud, N.Z.A.; Usman, A. Simultaneous adsorption of multi-lanthanides from aqueous silica sand solution using pectin-activated carbon composite. Arab. J. Sci. Eng. 2020, 45, 7219–7230. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Ismail, L.S.; Khalili, F.I. Biosorption of neodymium(III) and cerium(III) ions by Loquat leaves (Eriobotrya japonica) kinetics and thermodynamic studies. Desalin. Water Treat. 2021, 229, 291–301. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A.; Khosravi, A. Biosorption of lanthanum and cerium from aqueous solutions by grapefruit peel: Equilibrium, kinetic and thermodynamic studies. Res. Chem. Intermed. 2013, 41, 559–573. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Interaction of rare earth elements with a brown marine alga in multi-component solutions. Desalination 2011, 265, 54–59. [Google Scholar] [CrossRef]
- Sert, Ş.; Kütahyali, C.; İnan, S.; Talip, Z.; Çetinkaya, B.; Eral, M. Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy 2008, 90, 13–18. [Google Scholar] [CrossRef]
- de Farias, A.B.V.; da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Cerium recovery from aqueous solutions by bio/adsorption: A review in a circular economy context. J. Clean. Prod. 2021, 326, 129395. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Majdańska, M.; Fila, D. Sorption of lanthanide ions on biochar composites. J. Rare Earths 2018, 36, 1212–1220. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Baquero, E.E.; Largo, O.R.; Lopez, F.A. On the adsorption of cerium(III) using multiwalled carbon nanotubes. Metals 2020, 10, 1057. [Google Scholar] [CrossRef]
- Khalil, M.; El-Aryan, Y.F.; El Afifi, E.M. Sorption performance of light rare earth elements using zirconium titanate and polyacrylonitrile zirconium titanate ion exchangers. Part. Sci. Technol. 2018, 36, 618–627. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Demcak, S.; Humelnicu, I. Sorption of Ce(III) by silica SBA-15 and titanosilicate ETS-10 from aqueous solution. Water 2021, 13, 3263. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z. Investigation of sorption and separation of lanthanides on the ion exchangers of various types. In Ion Exchange Technologies; Kilislioglu, A., Ed.; IntechOpen: London, UK, 2012; pp. 101–154. [Google Scholar]
- Kolodynska, D.; Hubicki, Z.; Fila, D. Recovery of rare earth elements from acidic solutions using macroporous ion exchangers. Sep. Sci. Technol. 2019, 54, 2059–2076. [Google Scholar] [CrossRef]
- Ang, K.L.; Li, D.; Nikoloski, A.N. The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 1. Anionic and cationic resins. Hydrometallurgy 2017, 174, 147–155. [Google Scholar] [CrossRef]
- Jain, V.K.; Handa, A.; Pandya, R.; Shrivastav, P.; Agrawal, Y.K. Polymer supported calix[4]arene-semicarbazone derivative for separation and preconcentration of La(III), Ce(III), Th(IV) and U(VI). React. Funct. Polym. 2002, 51, 101–110. [Google Scholar] [CrossRef]
- Jain, V.K.; Pandya, R.A.; Pillai, S.G.; Agrawal, Y.K.; Kanaiya, P.H. Solid-phase extractive preconcentration and separation of lanthanum(III) and cerium(III) using a polymer-supported chelating calix 4 arene resin. J. Anal. Chem. 2007, 62, 104–112. [Google Scholar] [CrossRef]
- Nghiem Van, N.; Iizuka, A.; Shibata, E.; Nakamura, T. Study of adsorption behavior of a new synthesized resin containing glycol amic acid group for separation of scandium from aqueous solutions. Hydrometallurgy 2016, 165, 51–56. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Pinheiro, E.F.; Espinosa, D.C.R.; Tenorio, J.A.S.; Baltazar, M.D.P.G. Adsorption of lanthanum and cerium on chelating ion exchange resins: Kinetic and thermodynamic studies. Sep. Sci. Technol. 2021, 57, 60–69. [Google Scholar] [CrossRef]
- Kolodynska, D.; Fila, D.; Hubicki, Z. Recovery of lanthanum(III) and nickel(II) ions from acidic solutions by the highly effective ion exchanger. Molecules 2020, 25, 3718. [Google Scholar] [CrossRef]
- Jose, L.B.; Ladeira, A.C.Q. Recovery and separation of rare earth elements from an acid mine drainage-like solution using a strong acid resin. J. Water Process Eng. 2021, 41, 102052. [Google Scholar] [CrossRef]
- Kolodynska, D.; Fila, D.; Hubicki, Z. Evaluation of possible use of the macroporous ion exchanger in the adsorption process of rare earth elements and heavy metal ions from spent batteries solutions. Chem. Eng. Process. Process Intensif. 2020, 147, 107767. [Google Scholar] [CrossRef]
- Kolodynska, D.; Fila, D.; Hubicki, Z. Static and dynamic studies of lanthanum(III) ion adsorption/desorption from acidic solutions using chelating ion exchangers with different functionalities. Environ. Res. 2020, 191, 110171. [Google Scholar] [CrossRef]
- Araucz, K.; Aurich, A.; Kolodynska, D. Novel multifunctional ion exchangers for metal ions removal in the presence of citric acid. Chemosphere 2020, 251, 126331. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, C.; Wang, Y.; Tang, C.; Zhou, S.; Zhao, Y.; Ma, R.; Duan, P. Synthesis of activated carbon-based amino phosphonic acid chelating resin and its adsorption properties for Ce(III) removal. Environ. Technol. 2015, 36, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Hamza, M.F.; Fujita, T.; Rodríguez-Castellón, E.; Guibal, E. Synthesis of a new phosphonate-based sorbent and characterization of its interactions with lanthanum (III) and terbium (III). Polymers 2021, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Lupa, L.; Visa, A. Synthesis, characterization and rare earth elements adsorption properties of phosphonate metal organic frameworks. Appl. Surf. Sci 2019, 481, 83–91. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Diethylenetriamine-functionalized chitosan magnetic nano-based particles for the sorption of rare earth metal ions Nd(III), Dy(III) and Yb(III). Cellulose 2015, 22, 2589–2605. [Google Scholar] [CrossRef]
- Callura, J.C.; Perkins, K.M.; Baltrus, J.P.; Washburn, N.R.; Dzombak, D.A.; Karamalidis, A.K. Adsorption kinetics, thermodynamics, and isotherm studies for functionalized lanthanide-chelating resins. J. Colloid Interface Sci. 2019, 557, 465–477. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. Assess. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants-A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.H.N.; Sulong, A. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Joseph, T.; Jacob, M.; Nair, V.R.; Varkey, J.T. Removal of metal ions using Chitosan based electro spun nanofibers: A review. Nanosyst.-Phys. Chem. Math. 2021, 12, 728–748. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhao, M.W.; Cheng, Q.; Wang, C.; Li, H.J.; Han, X.G.; Fan, Z.H.; Su, G.Y.; Pan, D.; Li, Z.Y. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Puhakka, V.; Iftekhar, S.; Wojtus, A.; Repo, E.; Ben Hammouda, S.; Iakovleva, E.; Sillanpaa, M. N- and O- ligand doped mesoporous silica-chitosan hybrid beads for the efficient, sustainable and selective recovery of rare earth elements (REE) from acid mine drainage (AMD): Understanding the significance of physical modification and conditioning of the polymer. J. Hazard. Mater. 2018, 348, 84–91. [Google Scholar]
- Cui, J.; Li, W.; Song, X.; Zhang, Z.; Yu, H.; Shan, W.; Xiong, Y. Microwave-assisted one-pot rapid synthesis of mesoporous silica-chitosan composites for efficient recovery of rhenium(VII). Sep. Purif. Technol. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Ruiz, M.; Sastre, A.; Guibal, E. Pd and Pt recovery using chitosan gel beads. I. influence of the drying process on diffusion properties. Sep. Sci. Technol. 2002, 37, 2143–2166. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J. Chitosan based nano adsorbents and its types for heavy metal removal: A mini review. Mater. Lett. 2022, 312, 131670. [Google Scholar] [CrossRef]
- Briao, G.D.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Removal of toxic metals from water using chitosan-based magnetic adsorbents. A review. Environ. Chem. Lett. 2020, 18, 1145–1168. [Google Scholar] [CrossRef]
- Michailidou, G.; Koumentakou, I.; Liakos, E.V.; Lazaridou, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Adsorption of uranium, mercury, and rare earth elements from aqueous solutions onto magnetic chitosan adsorbents: A review. Polymers 2021, 13, 3137. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.B.; Cagnetta, G.; Wang, W.; Meng, P.P.; Liu, D.C.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Gao, Y.; Zhang, S.; Wu, K. Adsorption of rare earths(III) using an efficient sodium alginate hydrogel cross-linked with poly-gamma-glutamate. PLoS ONE 2015, 10, 124826. [Google Scholar]
- Sahu, B.B.; Parida, K. Cation exchange and sorption properties of crystalline α-titanium(IV) phosphate. J. Colloid Interface Sci. 2002, 248, 221–230. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar]
- Marcus, Y. Ion Properties; Marcel Dekker, Inc.: New York, NY, USA, 1997; p. 259. [Google Scholar]
- Chu, K.H. Revisiting the Temkin isotherm: Dimensional inconsistency and approximate forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar]
- Puccia, V.; Avena, M.J. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Shahnaz, T.; Vishnu Priyan, V.; Jayakumar, A.; Narayanasamy, S. Magnetic nanocellulose from Cyperus rotundas grass in the absorptive removal of rare earth element cerium (III): Toxicity studies and interpretation. Chemosphere 2022, 287, 131912. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Zheng, Y.; Wang, F.; Wang, A. Rapid enrichment of rare-earth metals by carboxymethyl cellulose-based open-cellular hydrogel adsorbent from HIPEs template. Carbohydr. Polym. 2016, 140, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, Q.; Wang, S.; Man, R. Preparation of a novel polystyrene-poly(hydroxamic acid) copolymer and its adsorption properties for rare earth metal ions. Polymers 2020, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Azhar, M.R.; Li, X.J.; Duan, X.G.; Sun, H.Q.; Wang, S.B.; Fang, X.C. Adsorption of cerium (III) by HKUST-1 metal-organic framework from aqueous solution. J. Colloid Interface Sci. 2019, 542, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sadovsky, D.; Brenner, A.; Astrachan, B.; Asaf, B.; Gonen, R. Biosorption potential of cerium ions using Spirulina biomass. J. Rare Earths 2016, 34, 644–652. [Google Scholar] [CrossRef]
- Tran, T.N.; Do, Q.C.; Kim, D.; Kim, J.; Kang, S. Urchin-like structured magnetic hydroxyapatite for the selective separation of cerium ions from aqueous solutions. J. Hazard. Mater. 2022, 430, 128488. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2020, 55, 4193–4212. [Google Scholar] [CrossRef]
- He, C.; Salih, K.A.M.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.H.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient recovery of rare earth elements (Pr(III) and Tm(III)) from mining residues using a new phosphorylated hydrogel (algal biomass/PEI). Metals 2021, 11, 294. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Hamza, M.F.; Rodriguez Castellon, E.; Guibal, E. Novel phosphonate-functionalized composite sorbent for the recovery of lanthanum(III) and terbium(III) from synthetic solutions and ore leachate. Chem. Eng. J. 2021, 424, 130500. [Google Scholar] [CrossRef]
- Han, K.N.; Kim, R. Thermodynamic analysis of precipitation characteristics of rare earth elements with sulfate in comparison with other common precipitants. Minerals 2021, 11, 670. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Sinha, S.; De, S.; Mishra, D.; Shekhar, S.; Agarwal, A.; Sahu, K.K. Phosphonomethyl iminodiacetic acid functionalized metal organic framework supported PAN composite beads for selective removal of La(III) from wastewater: Adsorptive performance and column separation studies. J. Hazard. Mater. 2022, 425, 127802. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, D.M.; Hamad, N.A.; Abdel-Rahman, A.A.H.; Fouda, A.; Wei, Y.; Guibal, E.; El-Etrawy, A.-A.S. Functionalization of magnetic chitosan microparticles for high-performance removal of chromate from aqueous solutions and tannery effluent. Chem. Eng. J. 2022, 428, 131775. [Google Scholar] [CrossRef]
- Zhang, Y.; Hamza, M.F.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Tuning the sorption properties of amidoxime-functionalized algal/polyethyleneimine beads for La(III) and Dy(III) using EDTA: Impact of metal speciation on selective separation. Chem. Eng. J. 2021, 431, 133214. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Rabie, K.; Elwakeel, K.Z.; Zayed, Y.E.; Hamza, M.F.; Guibal, E. Development of phosphoryl-functionalized algal-PEI beads for the sorption of Nd(III) and Mo(VI) from aqueous solutions—Application for rare earth recovery from acid leachates. Chem. Eng. J. 2021, 412, 127399. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Li, H. Application of titanium phosphate prepared from acidic titanium dioxide wastewater to remove cerium (III) in aqueous solution. Colloids Surf. A 2021, 630, 127613. [Google Scholar] [CrossRef]
- Lei, C.; Wen, F.; Chen, J.; Chen, W.; Huang, Y.; Wang, B. Mussel-inspired synthesis of magnetic carboxymethyl chitosan aerogel for removal cationic and anionic dyes from aqueous solution. Polymer 2021, 213, 123316. [Google Scholar] [CrossRef]
- Yang, X.; Debeli, D.K.; Shan, G.; Pan, P. Selective adsorption and high recovery of La3+ using graphene oxide/poly (N-isopropyl acrylamide-maleic acid) cryogel. Chem. Eng. J. 2020, 379, 122335. [Google Scholar] [CrossRef]
- Nkinahamira, F.; Alsbaiee, A.; Zeng, Q.; Li, Y.; Zhang, Y.; Feng, M.; Yu, C.-P.; Sun, Q. Selective and fast recovery of rare earth elements from industrial wastewater by porous beta-cyclodextrin and magnetic beta-cyclodextrin polymers. Water Res. 2020, 181, 115857. [Google Scholar] [CrossRef]
- Kegl, T.; Kosak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Li, Z.; Liu, C.; Liu, X.; Liu, Y.; Dong, G.; Lan, T.; Wei, Y. Fabrication of efficient alginate composite beads embedded with N-doped carbon dots and their application for enhanced rare earth elements adsorption from aqueous solutions. J. Colloid Interface Sci. 2020, 562, 224–234. [Google Scholar] [CrossRef]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]
- Cheraghipour, E.; Pakshir, M. Process optimization and modeling of Pb(II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 2020, 260, 127560. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, Z.; Khan, A.A.; Rehman, N.U. Terbium extraction by functionalized surface: Experimental and DFT approach. Adsorpt.-J. Int. Adsorpt. Soc. 2020, 26, 117–125. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Bian, T.; Zhang, Y.; Zhang, F.; Yan, Y. Selective extraction of gadolinium using free-standing imprinted mesoporous carboxymethyl chitosan films with high capacity. Cellulose 2019, 26, 1209–1219. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Liu, H.; Luo, Y.; Wang, W. Preparation of double carboxylic corn stalk gels and their adsorption properties towards rare earths(III). Waste Biomass Valoriz. 2018, 9, 1945–1954. [Google Scholar] [CrossRef]
- Ravi, S.; Lee, Y.-R.; Yu, K.; Ahn, J.-W.; Ahn, W.-S. Benzene triamido-tetraphosphonic acid immobilized on mesoporous silica for adsorption of Nd3+ ions in aqueous solution. Microporous Mesoporous Mater. 2018, 258, 62–71. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kolodynska, D.; Gorbyk, P.P. Gd(III) adsorption on the DTPA-functionalized chitosan/magnetite nanocomposites. Sep. Sci. Technol. 2018, 53, 1006–1016. [Google Scholar] [CrossRef]
- Ibanescu, A.; Alexandrica, M.C.; Hritcu, D.; Chiscan, O.; Popa, M.I. Magnetite/chitosan composite particles as adsorbents for Reactive Blue 19 dye. Green Mater. 2018, 6, 149–156. [Google Scholar]
- Hisada, M.; Kawase, Y. Recovery of rare-earth metal neodymium from aqueous solutions by poly-gamma-glutamic acid and its sodium salt as biosorbents: Effects of solution pH on neodymium recovery mechanisms. J. Rare Earths 2018, 36, 528–536. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum decorated magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Romero, C.; McCloy, J. Magnetic analysis of commercial hematite, magnetite, and their mixtures. AIP Adv. 2018, 8, 056807. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.L.; Chen, L.N.; Huang, Z.Y. Preparation of thiol-functionalized cellulose and its application to the removal of Hg(II) from water environment. Cellul. Chem. Technol. 2017, 51, 559–567. [Google Scholar]
- Rahman, M.L.; Biswas, T.K.; Sarkar, S.M.; Yusoff, M.M.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Adsorption of rare earth metals from water using a kenaf cellulose-based poly(hydroxamic acid) ligand. J. Mol. Liq. 2017, 243, 616–623. [Google Scholar] [CrossRef]
- Bezdorozhev, O.; Kolodiazhnyi, T.; Vasylkiv, O. Precipitation synthesis and magnetic properties of self-assembled magnetite-chitosan nanostructures. J. Magn. Magn. Mater. 2017, 428, 406–411. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpaa, M. An EDTA-beta-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Gui, W.; Yang, Y.; Zhu, X. High-efficiency recovery of rare earth ions by hydrolyzed poly(styrene-co-maleic anhydride). J. Appl. Polym. Sci. 2016, 133, 43676. [Google Scholar] [CrossRef]
- Ghamami, S.; Anari, S.K.; Bakhshi, M.; Lashgari, A.; Salgado-Morán, G.; Glossman-Mitnik, D. Preparation and characterization of Cerium (III) doped captopril nanoparticles and study of their photoluminescence properties. Open Chem. 2016, 14, 60–64. [Google Scholar] [CrossRef][Green Version]
- Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-functionalized chitosan magnetic nano-based particles for the recovery of light and heavy rare earth metals: Uptake kinetics and sorption isotherms. Nanomaterials 2015, 5, 154–179. [Google Scholar] [CrossRef]
- Dos Santos Menegucci, J.; Santos, M.-K.M.S.; Santos Dias, D.J.; Chaker, J.A.; Sousa, M.H. One-step synthesis of magnetic chitosan for controlled release of 5-hydroxytryptophan. J. Magn. Magn. Mater. 2015, 380, 117–124. [Google Scholar] [CrossRef]
- Corazzari, I.; Nistico, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Borai, E.H.; Hamed, M.G.; El-kamash, A.M.; Siyam, T.; El-Sayed, G.O. Synthesis, characterization and application of a modified acrylamide–styrene sulfonate resin and a composite for sorption of some rare earth elements. New J. Chem. 2015, 39, 7409–7420. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M. Biosorption of lanthanum and cerium from aqueous solutions using tangerine (Citrus reticulate) peel: Equilibrium, kinetic and thermodynamic studies. Chem. Ind. Chem. Eng. Q. 2013, 19, 79–88. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ, version 3.1; Royal Institute of Technology: Stockholm, Sweden, 2013. [Google Scholar]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805–4812. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, J.L. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar]
- Wang, S.; Yu, D. Adsorption of Cd(II), Pb(II), and Ag(I) in aqueous solution on hollow chitosan microspheres. J. Appl. Polym. Sci. 2010, 118, 733–739. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Biosorption of lanthanum, cerium, europium, and ytterbium by a brown marine alga, Turbinaria conoides. Ind. Eng. Chem. Res. 2010, 49, 4405–4411. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Eng. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 1–23. [Google Scholar]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, J.; Grant, J.T.; Su, S.J.; Bunning, T.J.; Cooper, T.M.; Adams, W.W. Characterization of chitosan and rare-earth-metal-ion doped chitosan films. Macromol. Chem. Phys. 1997, 198, 1561–1578. [Google Scholar] [CrossRef]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Newton, MA, USA, 1994; p. 243. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. APPENDIX 3—A Summary of Characteristic Raman and Infrared Frequencies. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 477–490. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 10—Compounds Containing –NH2, –NHR, and –NR2 Groups. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 155–178. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 9—Compounds Containing the Carbonyl Group. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 117–154. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 14—Organic Sulfur Compounds. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 225–250. [Google Scholar]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1249. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Szymanski, H.A. Introduction to Theoretical Infrared Spectroscopy. In Progress in Infrared Spectroscopy: Volume 1; Szymanski, H.A., Ed.; Springer US: Boston, MA, USA, 1962; pp. 1–6. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in lasungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]


| Cycle | MC | MC-UR | MC-UR/S | ||||
| SE (%) | DE (%) | SE (%) | DE (%) | SE (%) | DE (%) | ||
| 1 | Aver. | 16.1 | 100.6 | 45.9 | 99.9 | 71.8 | 100.4 |
| Std. Dev. | 0.6 | 0.5 | 0.5 | 1.1 | 1.5 | 0.5 | |
| 2 | Aver. | 15.6 | 99.8 | 45.2 | 100.3 | 71.7 | 100.2 |
| Std. Dev. | 0.4 | 0.7 | 0.4 | 0.1 | 1.5 | 0.9 | |
| 3 | Aver. | 15.5 | 100.3 | 44.9 | 100.6 | 70.8 | 99.7 |
| Std. Dev. | 0.6 | 0.2 | 0.7 | 0.4 | 1.1 | 0.5 | |
| 4 | Aver. | 15.1 | 100.5 | 44.6 | 100.0 | 70.2 | 100.0 |
| Std. Dev. | 0.7 | 0.5 | 1.0 | 0.9 | 0.8 | 0.5 | |
| 5 | Aver. | 14.7 | 99.9 | 44.2 | 100.0 | 69.7 | 100.3 |
| Std. Dev. | 0.7 | 0.4 | 0.8 | 0.5 | 0.8 | 0.2 | |
| Loss (5th/1st) (%) | 8.7 | - | 3.8 | - | 3.1 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Guibal, E.; Abdel-Rahman, A.A.-H.; Salem, M.; Khalafalla, M.S.; Wei, Y.; Yin, X. Enhancement of Cerium Sorption onto Urea-Functionalized Magnetite Chitosan Microparticles by Sorbent Sulfonation—Application to Ore Leachate. Molecules 2022, 27, 7562. https://doi.org/10.3390/molecules27217562
Hamza MF, Guibal E, Abdel-Rahman AA-H, Salem M, Khalafalla MS, Wei Y, Yin X. Enhancement of Cerium Sorption onto Urea-Functionalized Magnetite Chitosan Microparticles by Sorbent Sulfonation—Application to Ore Leachate. Molecules. 2022; 27(21):7562. https://doi.org/10.3390/molecules27217562
Chicago/Turabian StyleHamza, Mohammed F., Eric Guibal, Adel A.-H. Abdel-Rahman, Marwa Salem, Mahmoud S. Khalafalla, Yuezhou Wei, and Xiangbiao Yin. 2022. "Enhancement of Cerium Sorption onto Urea-Functionalized Magnetite Chitosan Microparticles by Sorbent Sulfonation—Application to Ore Leachate" Molecules 27, no. 21: 7562. https://doi.org/10.3390/molecules27217562
APA StyleHamza, M. F., Guibal, E., Abdel-Rahman, A. A.-H., Salem, M., Khalafalla, M. S., Wei, Y., & Yin, X. (2022). Enhancement of Cerium Sorption onto Urea-Functionalized Magnetite Chitosan Microparticles by Sorbent Sulfonation—Application to Ore Leachate. Molecules, 27(21), 7562. https://doi.org/10.3390/molecules27217562