Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS
Abstract
1. Introduction
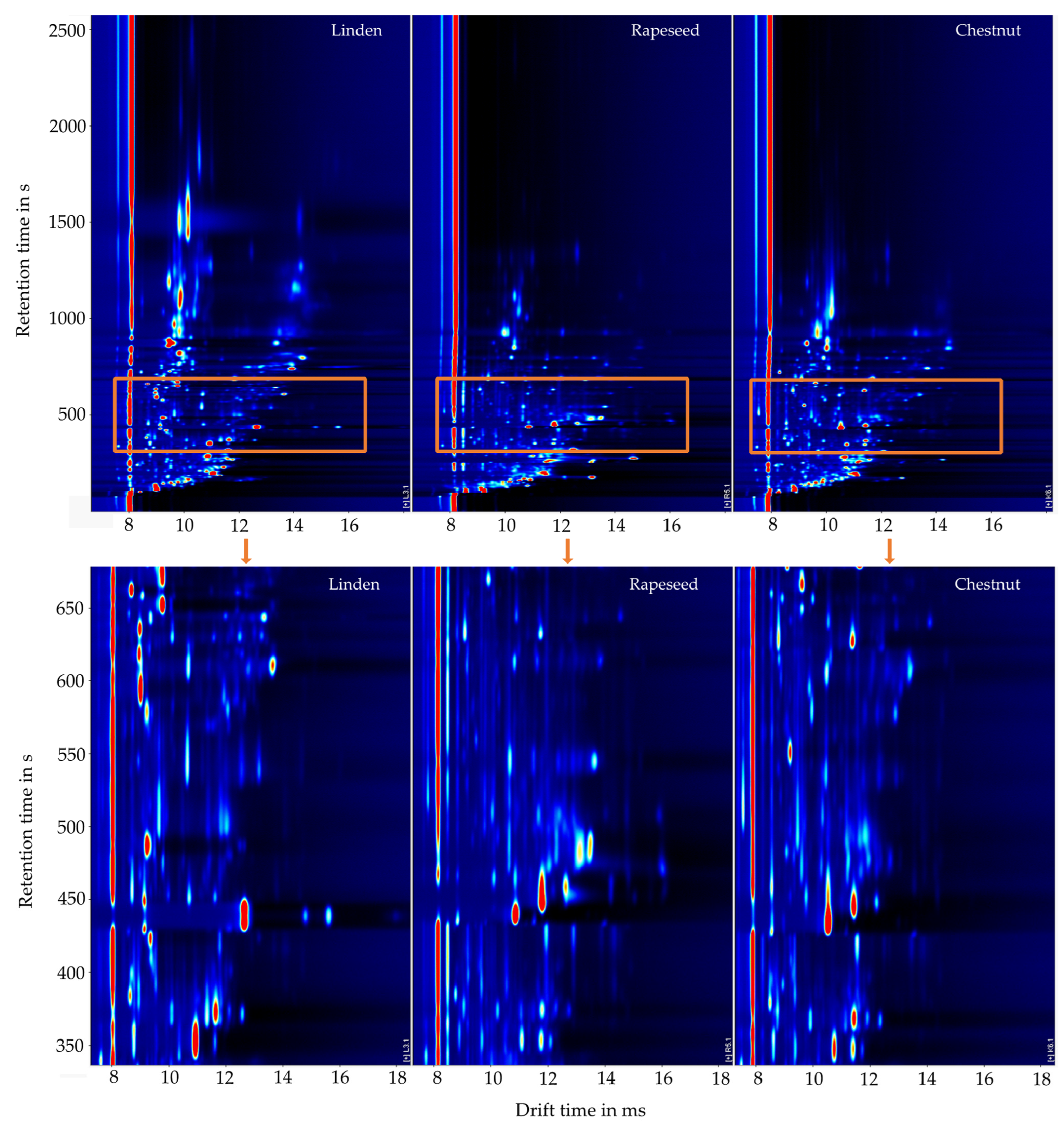
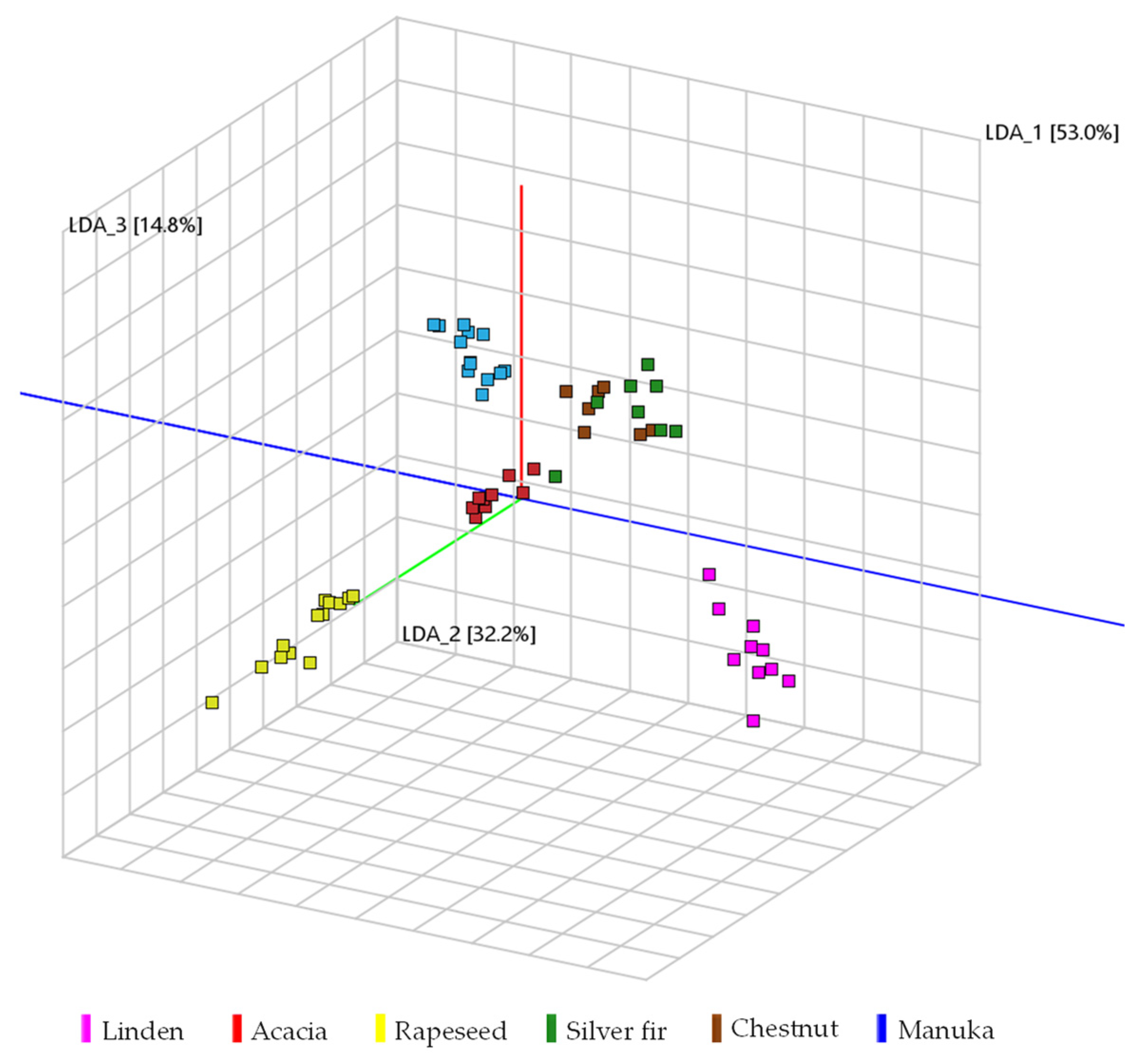
2. Results and Discussion
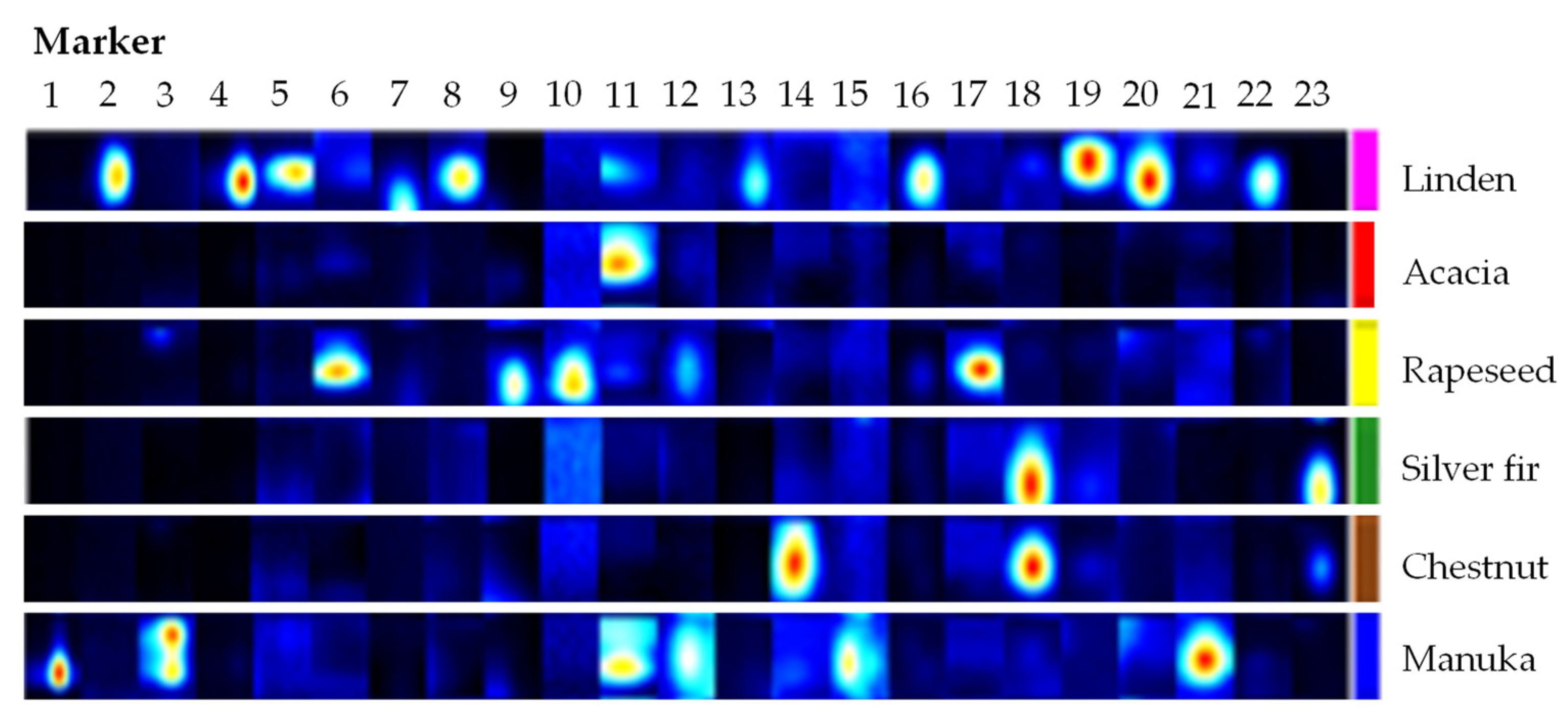
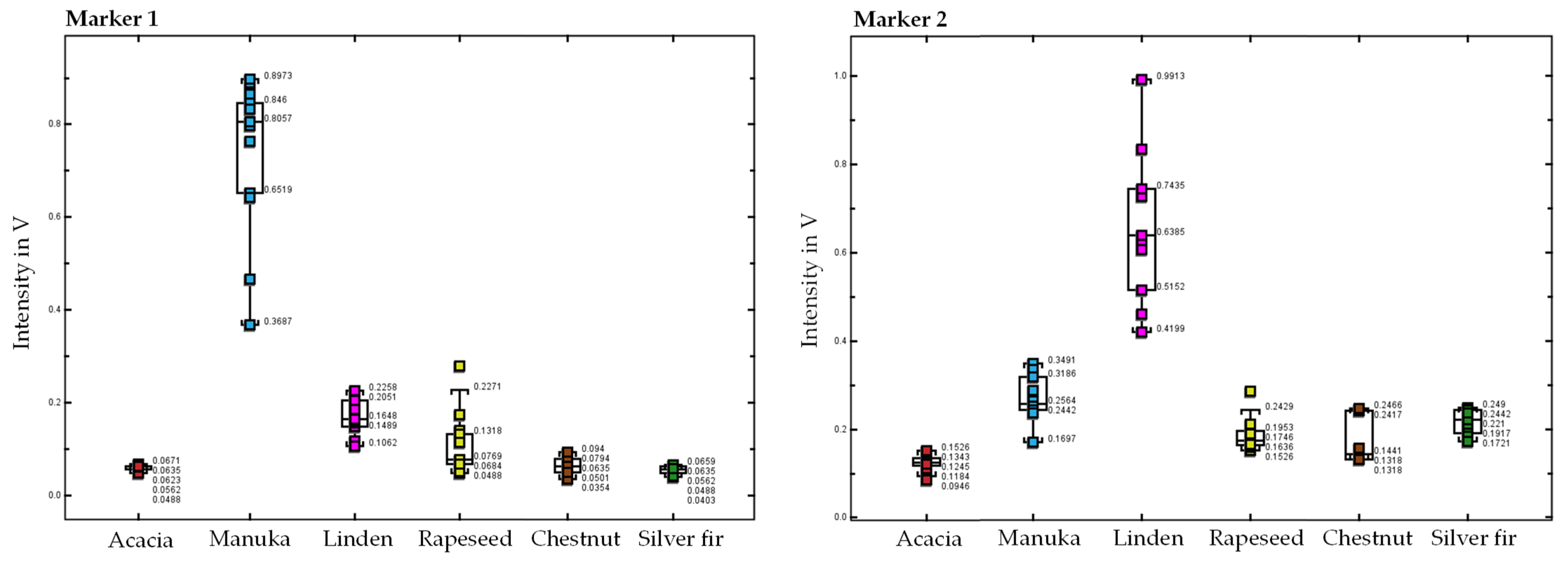
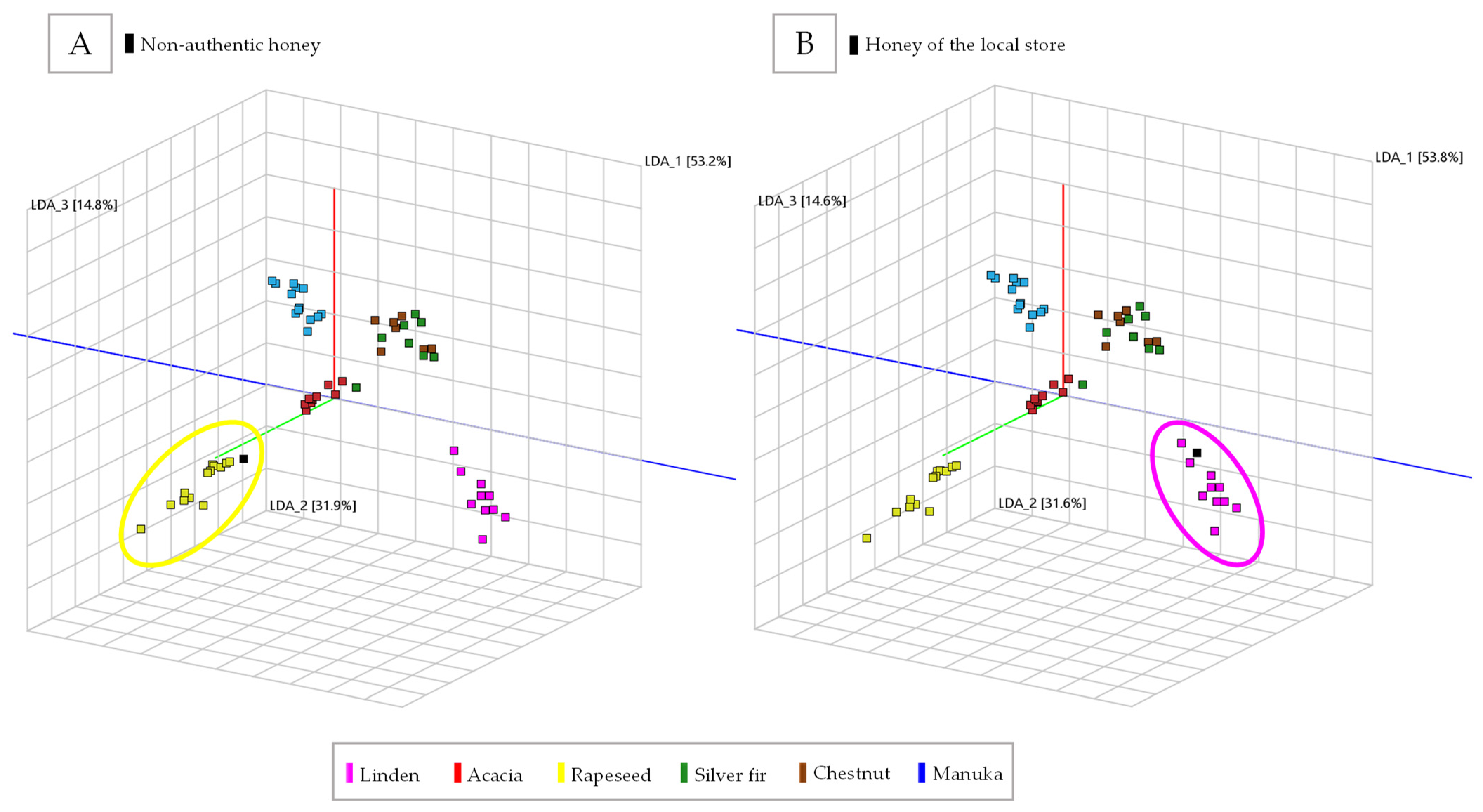
2.1. Classification of Honey Types Using the Volatile Organic Profile
2.2. Identification of Substances
3. Materials and Methods
3.1. Chemicals and Samples
3.2. Instrumentation
3.3. Data Analysis
3.4. Identification of Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruoff, K.; Bogdanov, S. Authenticity of Honey and other Bee Products. Apiacta 2004, 38, 317–327. [Google Scholar]
- Baroni, M.V.; Nores, M.L.; Del Díaz, M.P.; Chiabrando, G.A.; Fassano, J.P.; Costa, C.; Wunderlin, D.A. Determination of volatile organic compound patterns characteristic of five unifloral honey by solid-phase microextraction-gas chromatography-mass spectrometry coupled to chemometrics. J. Agric. Food Chem. 2006, 54, 7235–7241. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; Villarejo, M.; Espejo, R.; Jodral, M. Chemical and physical parameters of Andalusian honey: Classification of Citrus and Eucalyptus honeys by discriminant analysis. Food Chem. 2004, 87, 619–625. [Google Scholar] [CrossRef]
- Guyot, C.; Bouseta, A.; Scheirman, V.V.; Collin, S. Floral Origin Markers of Chestnut and Lime Tree Honeys. J. Agric. Food Chem. 1998, 46, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.S.; Miorini, M.; Giomo, A.; Bertacco, G.; Zironi, R. Evaluation of Some Fixed Components for Unifloral Honey Characterization. J. Agric. Food Chem. 1998, 46, 1844–1849. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Codex Alimentarius—Standard for Honey: International Food Standard, 2019th ed.; World Health Organization: Rome, Italy, 2019.
- Deutsche Lebensmittelbuch Kommission. Leitsätze für Honig; Bundesanzeiger, 10.05.2022.BMEL—Publikationen—Leitsätze für Honig; 2022. Available online: https://berufsimker.de/leitsaetze-fuer-honig/ (accessed on 27 September 2022).
- Karabournioti, S.; Zervalaki, P. The effect of heating on honey HMF and invertase. Apiacta 2001, 36, 177–181. [Google Scholar]
- Zhang, G.-Z.; Tian, J.; Zhang, Y.-Z.; Li, S.-S.; Zheng, H.-Q.; Hu, F.-L. Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey. Foods 2021, 10, 2882. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Chromatographic analysis of sugars applied to the characterisation of monofloral honey. Anal. Bioanal. Chem. 2004, 380, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- García, N.L. The Current Situation on the International Honey Market. Bee World 2018, 95, 89–94. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Finotello, C.; Uddin, J.; Mammi, S.; Piana, L. Objective Definition of Monofloral and Polyfloral Honeys Based on NMR Metabolomic Profiling. J. Agric. Food Chem. 2016, 64, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Boffo, E.F.; Tavares, L.A.; Tobias, A.C.T.; Ferreira, M.M.C.; Ferreira, A.G. Identification of components of Brazilian honey by 1H NMR and classification of its botanical origin by chemometric methods. LWT 2012, 49, 55–63. [Google Scholar] [CrossRef]
- Ohmenhaeuser, M.; Monakhova, Y.B.; Kuballa, T.; Lachenmeier, D.W. Qualitative and Quantitative Control of Honeys Using NMR Spectroscopy and Chemometrics. ISRN Anal. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. Recent developments in honey characterization. RSC Adv. 2015, 5, 59696–59714. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-Compound Fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a Benchtop Alternative to 1H NMR Profiling for Assessment of the Authenticity of Honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Bonaga, G.; Giumanini, A.G. The Volatile Fraction of Chestnut Honey. J. Apic. Res. 1986, 25, 113–120. [Google Scholar] [CrossRef]
- Tan, S.T.; Wilkins, A.L.; Molan, P.C.; Holland, P.T.; Reid, M. A Chemical Approach to the Determination of Floral Sources of New Zealand Honeys. J. Apic. Res. 1989, 28, 212–222. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, M.; Fagúndez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile compounds of Argentinean honeys: Correlation with floral and geographical origin. Food Chem. 2018, 246, 32–40. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Sánchez-Brunete, C.; Calvo, R.M.; Tadeo, J.L. Analysis of volatiles from Spanish honeys by solid-phase microextraction and gas chromatography-mass spectrometry. J. Agric. Food Chem. 2002, 50, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Arce, L.; Valcárcel, M. Multi-capillary column-ion mobility spectrometry: A potential screening system to differentiate virgin olive oils. Anal. Bioanal. Chem. 2012, 402, 489–498. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Zhu, W.; Benkwitz, F.; Kilmartin, P.A. Volatile-Based Prediction of Sauvignon Blanc Quality Gradings with Static Headspace-Gas Chromatography-Ion Mobility Spectrometry (SHS-GC-IMS) and Interpretable Machine Learning Techniques. J. Agric. Food Chem. 2021, 69, 3255–3265. [Google Scholar] [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Gas-phase volatilomic approaches for quality control of brewing hops based on simultaneous GC-MS-IMS and machine learning. Anal. Bioanal. Chem. 2020, 412, 7085–7097. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A Screening Method Based on Headspace-Ion Mobility Spectrometry to Identify Adulterated Honey. Sensors 2019, 19, 1621. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography—Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Augustini, A.L.R.M.; Sielemann, S.; Telgheder, U. Strategy for the identification of flavor compounds in e-liquids by correlating the analysis of GCxIMS and GC-MS. Talanta 2021, 230, 122318. [Google Scholar] [CrossRef] [PubMed]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Blank, I.; Fischer, K.-H.; Grosch, W. Intensive neutral odourants of linden honey Differences from honeys of other botanical origin. Z. Lebensm. Unters. Forsch. 1989, 189, 426–433. [Google Scholar] [CrossRef]
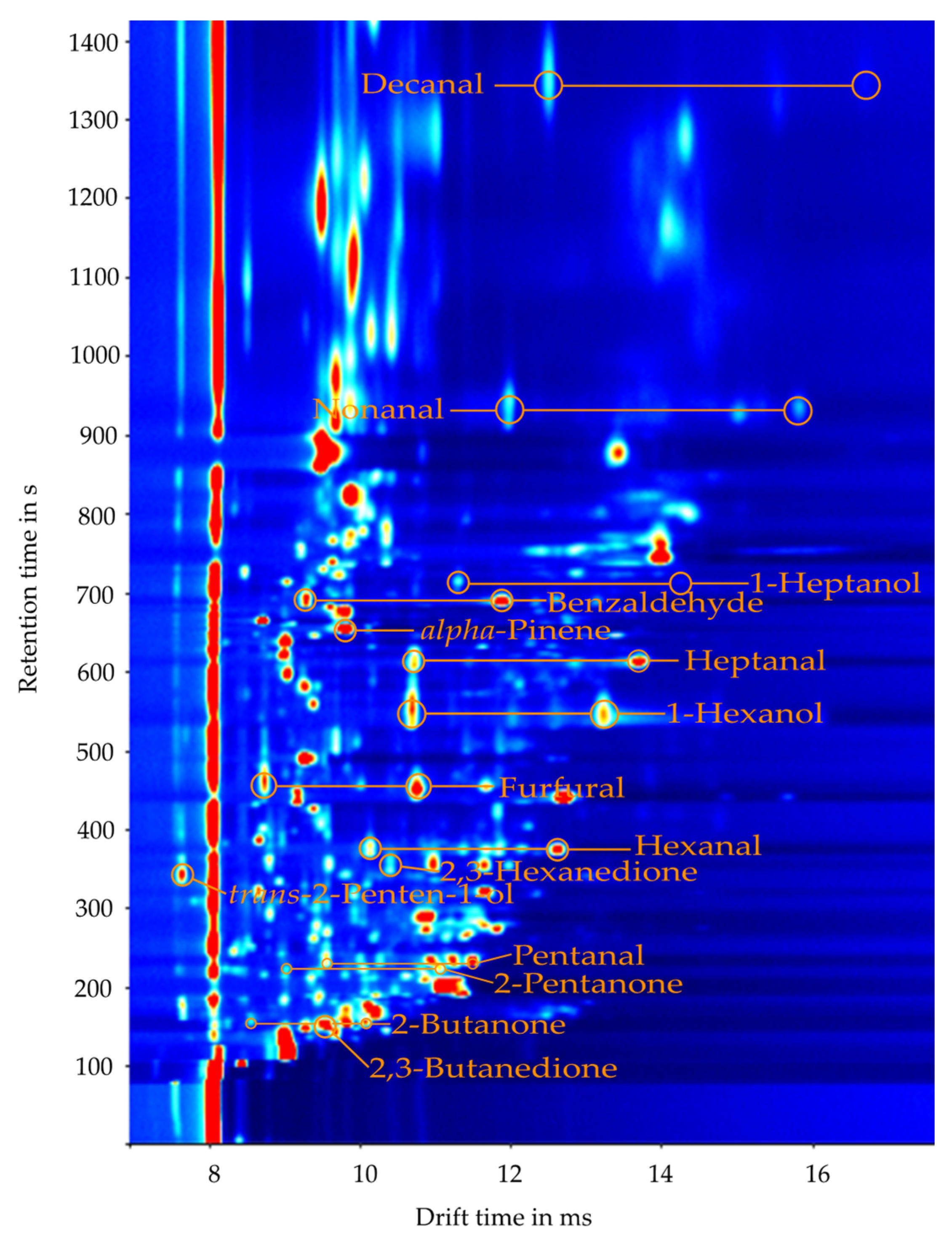
| Substance | CAS Number | Retention Time in s | K0Monomer in cm2(Vs)−1 | K0Dimer in cm2(Vs)−1 |
|---|---|---|---|---|
| 2,3-Butanedione | 431-03-8 | 151 | 1.77 | |
| 2-Butanone | 78-93-3 | 153 | 1.97 | 1.67 |
| 2-Pentanone | 107-87-9 | 220 | 1.86 | 1.52 |
| Pentanal | 110-62-3 | 232 | 1.77 | 1.46 |
| trans-2-Penten-1-ol | 1576-96-1 | 341 | 2.19 | 1.29 |
| 2,3-Hexanedione | 3848-24-6 | 355 | 1.62 | |
| Hexanal | 66-25-1 | 373 | 1.65 | 1.34 |
| Furfural | 98-01-1 | 446 | 1.91 | 1.57 |
| 1-Hexanol | 111-27-3 | 546 | 1.57 | 1.27 |
| Heptanal | 111-71-7 | 611 | 1.57 | 1.23 |
| α-Pinene | 80-56-8 | 653 | 1.71 | |
| Benzaldehyde | 100-52-7 | 691 | 1.81 | 1.42 |
| 1-Heptanol | 11-70-6 | 713 | 1.49 | 1.18 |
| Nonanal | 124-19-6 | 942 | 1.41 | 1.06 |
| Decanal | 112-31-2 | 1360 | 1.35 | 1.01 |
| Honey Type | Botanical Origin | Number of Samples | Color Coding |
|---|---|---|---|
| Acacia | Robinia pseudoacacia | 10 | Red |
| Chestnut | Castanea sativa | 7 | Brown |
| Linden | Tilia spp. | 11 | Magenta |
| Manuka | Leptospermum scoparium | 9 | Blue |
| Rapeseed | Brassica napus | 13 | Yellow |
| Silver fir | Abies alba | 8 | Green |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schanzmann, H.; Augustini, A.L.R.M.; Sanders, D.; Dahlheimer, M.; Wigger, M.; Zech, P.-M.; Sielemann, S. Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules 2022, 27, 7554. https://doi.org/10.3390/molecules27217554
Schanzmann H, Augustini ALRM, Sanders D, Dahlheimer M, Wigger M, Zech P-M, Sielemann S. Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules. 2022; 27(21):7554. https://doi.org/10.3390/molecules27217554
Chicago/Turabian StyleSchanzmann, Hannah, Alexander L. R. M. Augustini, Daniel Sanders, Moritz Dahlheimer, Modestus Wigger, Philipp-Marius Zech, and Stefanie Sielemann. 2022. "Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS" Molecules 27, no. 21: 7554. https://doi.org/10.3390/molecules27217554
APA StyleSchanzmann, H., Augustini, A. L. R. M., Sanders, D., Dahlheimer, M., Wigger, M., Zech, P.-M., & Sielemann, S. (2022). Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules, 27(21), 7554. https://doi.org/10.3390/molecules27217554






