Phytochemistry and Pharmacology of Medicinal Plants Used by the Tenggerese Society in Java Island of Indonesia
Abstract
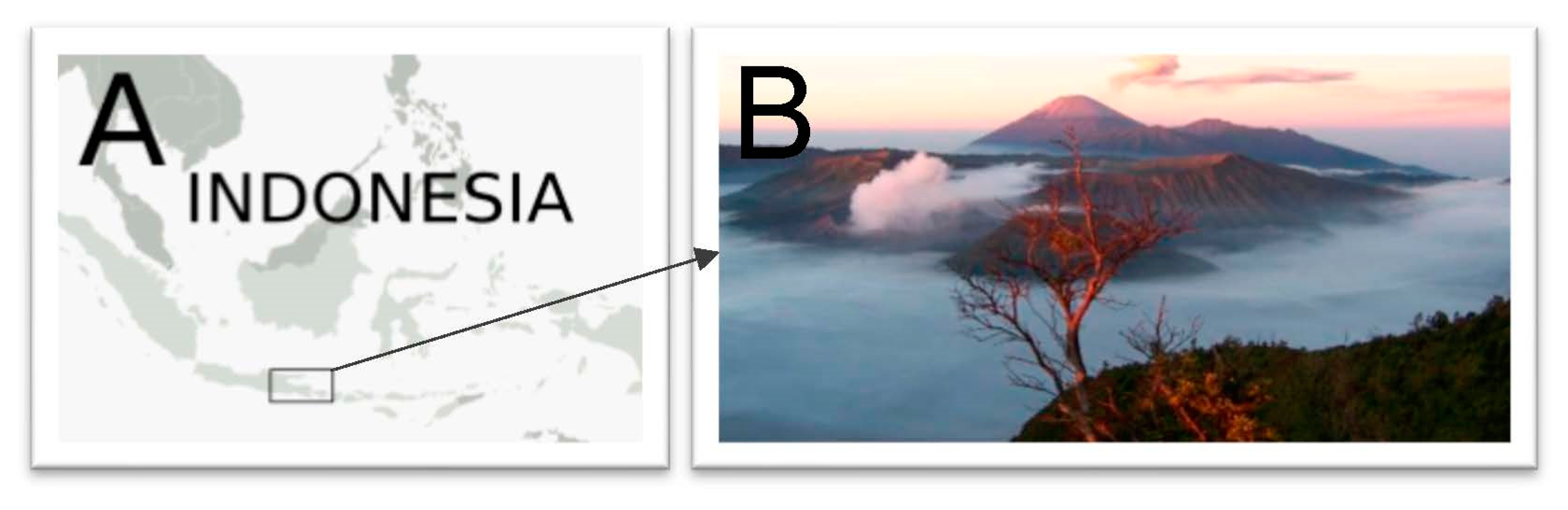
1. Introduction
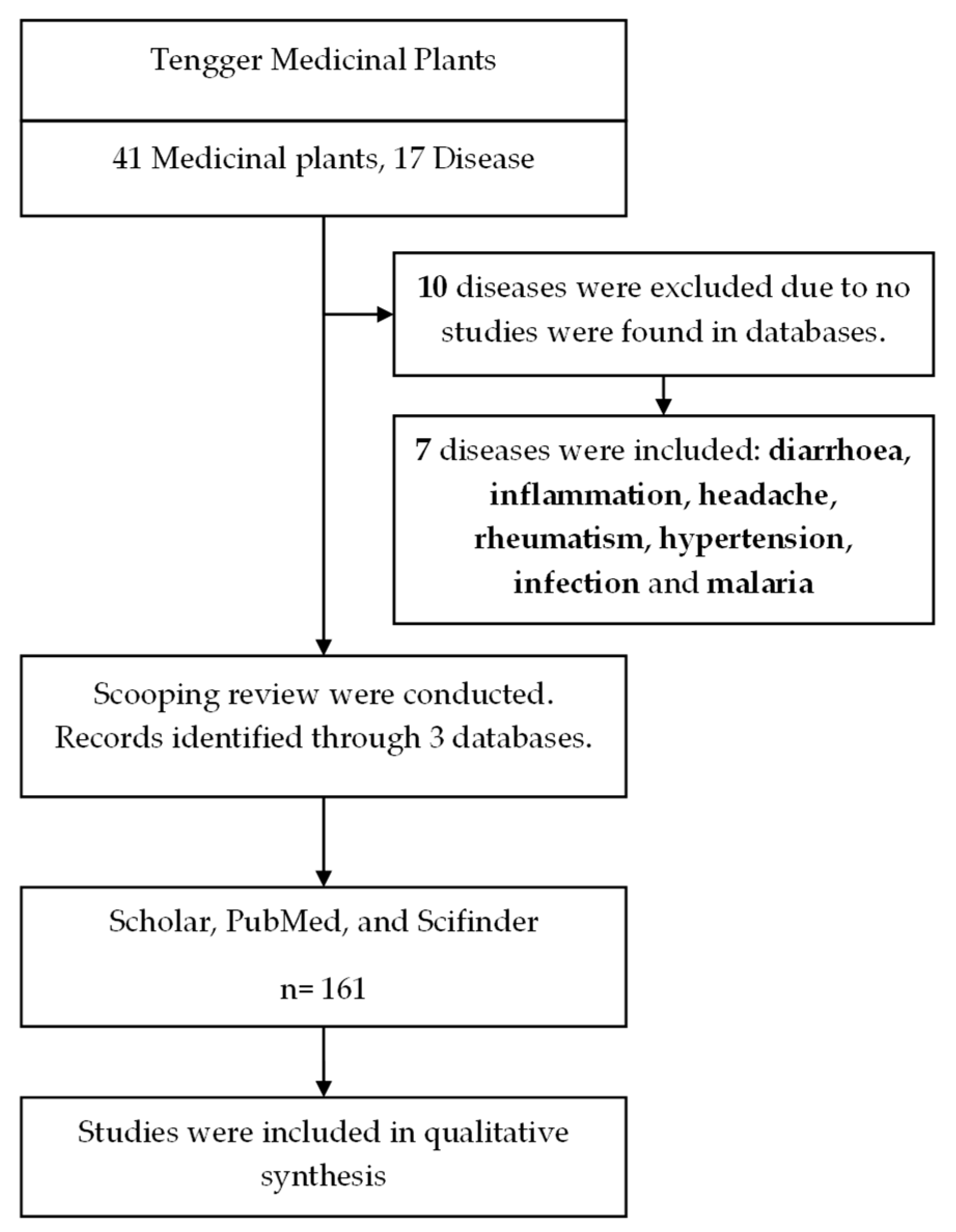
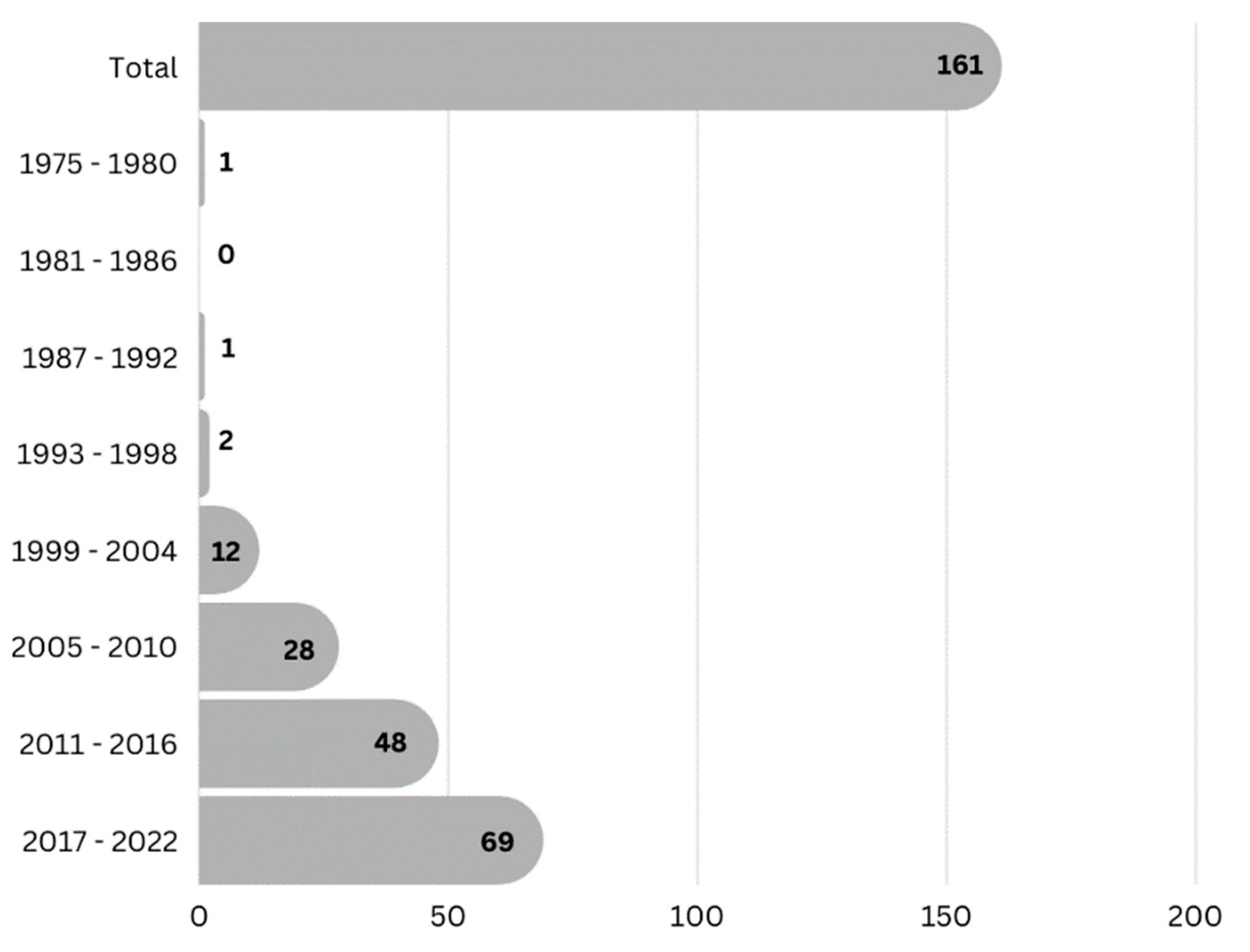
2. Phytochemistry of Tenggerese Medicinal Plants
| Species | Family | Tenggeresse Ethnopharmacological Uses of Plants | Parts Used for Chemical Isolation | Countries (Chemical Studies Reported) | Isolated Compounds |
|---|---|---|---|---|---|
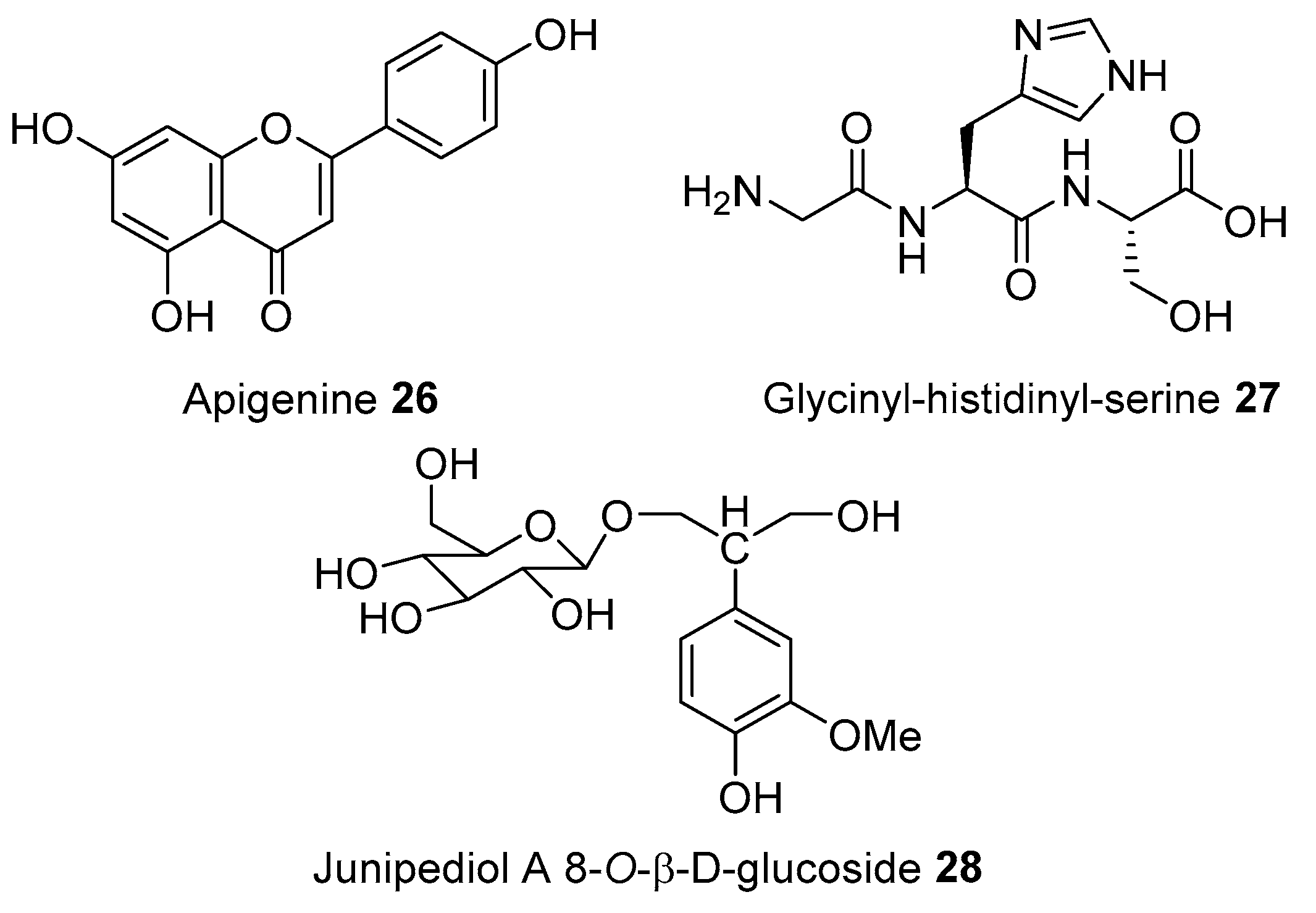
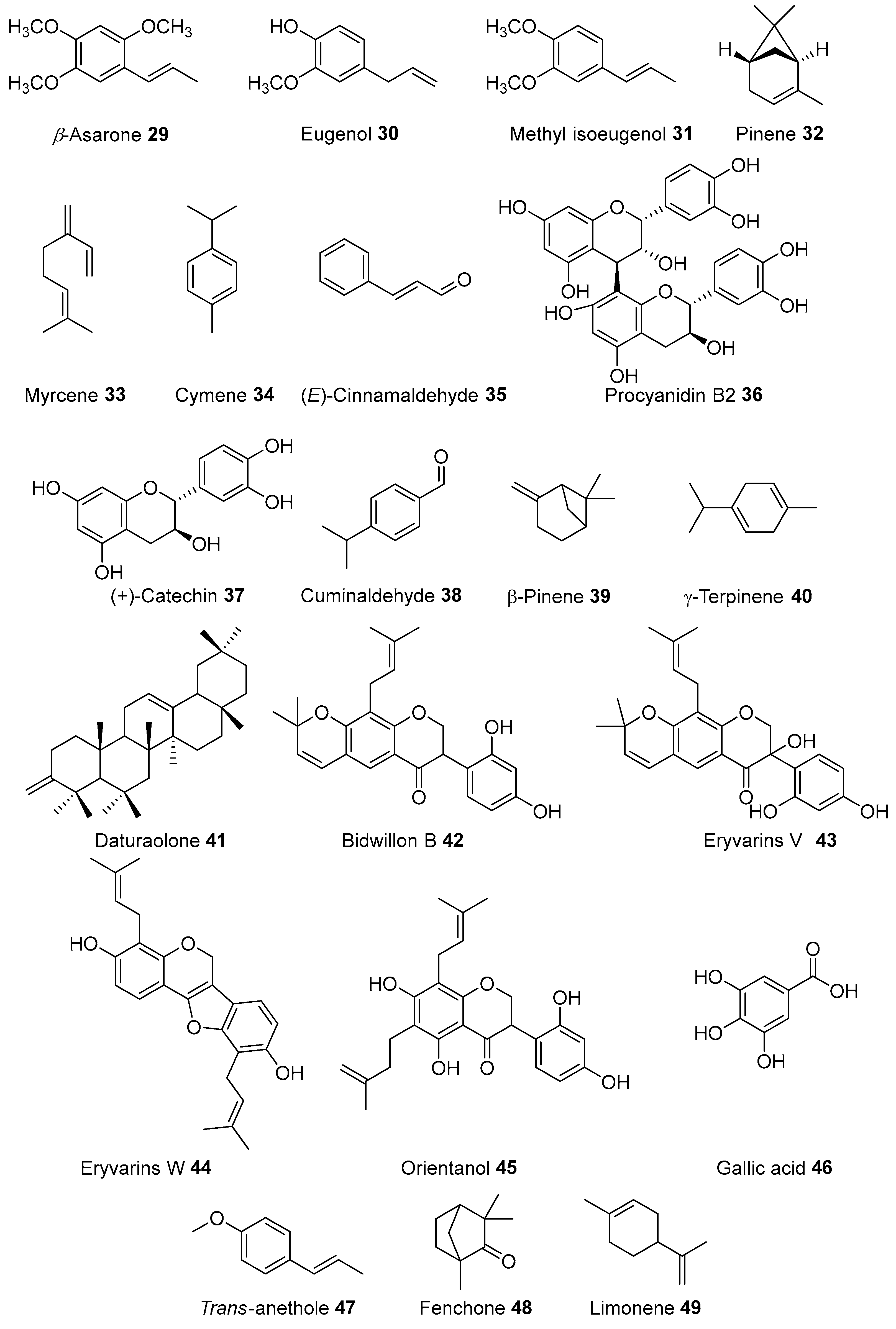
| Acorus calamus Linnaeus | Acoraceae | Fever | Leaves, rhizome, stem | India | β-Asarone, Camphene, Cymene, Calarene, α-Selinene, s-Cadinol, Isoshyobunone, β-Sesquiphellandrene, Preiso-calamendiol, Acorone [13]; (-)-4-Terpineol, Epieudesmin, Lysidine, (-)-Spathulenol, Borneol, Furyl ethyl ketone, Nonanoic acid, Bornyl acetate, Galgravin, Retusin, Butyl butanoate, Geranylacetate, Sakuranin, Acetic acid, Camphor, Isoelemicin, α-Ursolic acid, Acetophenone, Dehydroabietic acid, Isoeugenol Methylether, Apigenin, Dehydrodiisoeugenol, Linalool, Elemicin, Linolenic acid [14]; 2-Deca-4,7-dienol, Acoradin, Acoragermacrone, Acrenone, Aterpineol, β-Cadinene, Calacorene, Calamendiol, Galangin, Shyobunones, Sitosterol [15]; Calamusins A-I [16]. |
| Allium sativum Linnaeus | Alliaceae | Wound or cut | Rhizome | Iraq | E-Ajoene, Z-Ajoene, Alliin, Allicin, 2-Vinyl-4H-1,3-dithiin, Diallyl sulfide (DAS), Diallyl disulfide (DADS), Diallyl trisulfide (DATS), Allyl methyl sulfide (AMS) [17]. |
| Alyxia reinwardtii Blume | Apocynaceae | Fever, Rheumatism | Stem | Thailand | Coumarin, 3-Hydroxycoumarin, 6-Hydroxycoumarin, 8-Hydroxycoumarin, Scopoletin, (+)-Pinoresinol, Zhebeiresinol and p-Hydroxybenzoic acid [18]. |
| Anredera cordifolia (Ten.) Steenis | Basellaceae | Itchiness, Wound | Leaves | Brazil | Phytol, α-pinene, Larreagenin A, Vitexin, Isovitexin, Myricetin, Morin, Lupeol, β-Sitosterol, Ursolic acid [19]. |
| Apium graveolens Linnaeus | Apiaceae | Hypertension | Leaves | China | Apigenin, Luteolin, Chlorogenic acid [20]; Linalool, D-Limonene, 3-N-Butylphthalide (NBP) [21]. |
| Borreria laevis (Lam.) Griseb | Rubiaceae | Rheumatism | Aerial parts | Thailand | Borreline, Asperulosidic acid, 6-O-Acetylscandoside, 6α-Hydroxyadoxoside, Kaempferol 3-O-β-d-glucopyranoside, Kaempferol 3-O-rutinoside, Quercetin 3-O-β-d-galactopyranoside, Rutin [22,23]. |
| Brassica rapa Linnaeus | Brassicaceae | Fever, Hypertension, Nutrition | Leaves, stem, flower buds, roots | Portugal | Kaempferol 3-O-sophoroside-7-O-glucoside, Kaempferol 3-O (feruloyl/caffeoyl)-sophoroside7-O-glucoside, Isorhamnetin 3,7-O-diglucoside, Isorhamnetin 3-O-glucoside [24]. |
| Capsicum pubescens Dun. | Solanaceae | Tonic after hard labour | Fruit | Mexico | Carotenoids (Violaxanthin, cis-Violaxanthin, Luteoxanthin, Antheraxanthin, Lutein, Zeaxanthin, β-Carotene), Ascorbic acid and Capsaicinoids (Capsaicin, Dihydrocapsaicin) [25]. |
| Cayratia clematidea (F. Müll.) Domin | Vitaceae | Stomach disorder | NA | NA | NA |
| Cinnamomum burmannii (Nees & T. Nees) Bl. | Laruaceae | Fever | China, Indonesia | Trans-Cinnamaldehyde, Coumarin, and Trans-Cinnamic acid [26]. Styrene, Benzaldehyde, Camphene, β-Pinene, Borneol, α-Terpineol, Procyanidin B1, Procyanidin B2, Procyanidin trimer, Catechin, Procyanidin dimer, Epicatechin, Coumarin, (E)-Cinnamic acid, (E)-Cinnamaldehyde, (Z)-Cinnamaldehyde, Cinnamyl alcohol, (E)-cinnamaldehyde, eugenol, and coumarin, procyanidin trimer, (E)-cinnamaldehyde, and (Z)-cinnamaldehyde [27]. catechin, epicatechin, procyanidin B2, quercitrin, 3,4-dihydroxybenzaldehyde, protocatechuic acid, and cinnamic acid [28]. (E)-Cinnamaldehyde, Cinnamyl alcohol, Coumarin, 3,4-Dihydrocoumarin, Kaempferol, Procyanidin dimer, Procyanidin trimer, Linalool [29] | |
| Cocos nucifera Linnaeus | Aracaceae | Foetus health | Fruit | India, Indonesia, Brazil, UK | 2-Furaldehyde diethyl acetal and Palmitic acid [30]; Jezonofol, Cirrhusin A, Cassigarol G, Maackin A, Treoguiacyl glycerol-8`-vanil ether acid, Erythroguiacyl glycerol-8′-vanillic acid ether, Apigenin-7-O-β-d-glucoside, Piceatannol, p-Hydroxybenzoic acid, Protocatechuic acid, and Vanillic acid [31]; Two phenol compounds-catechin and Chlorogenic acid [32]. |
| Cuminum cyminum Linnaeus | Apiaceae | Fever | Seed | USA, Iraq | Cuminaldehyde, α-Pinene, β-Pinene, γ-Cymene, γ-Terpinene, α-Terpinen-7-al and β-Terpinen-7-al [33]; Bergapten, Methoxsalen [34]; Luteolin, Apigenin-7-O-glucoside [35]. |
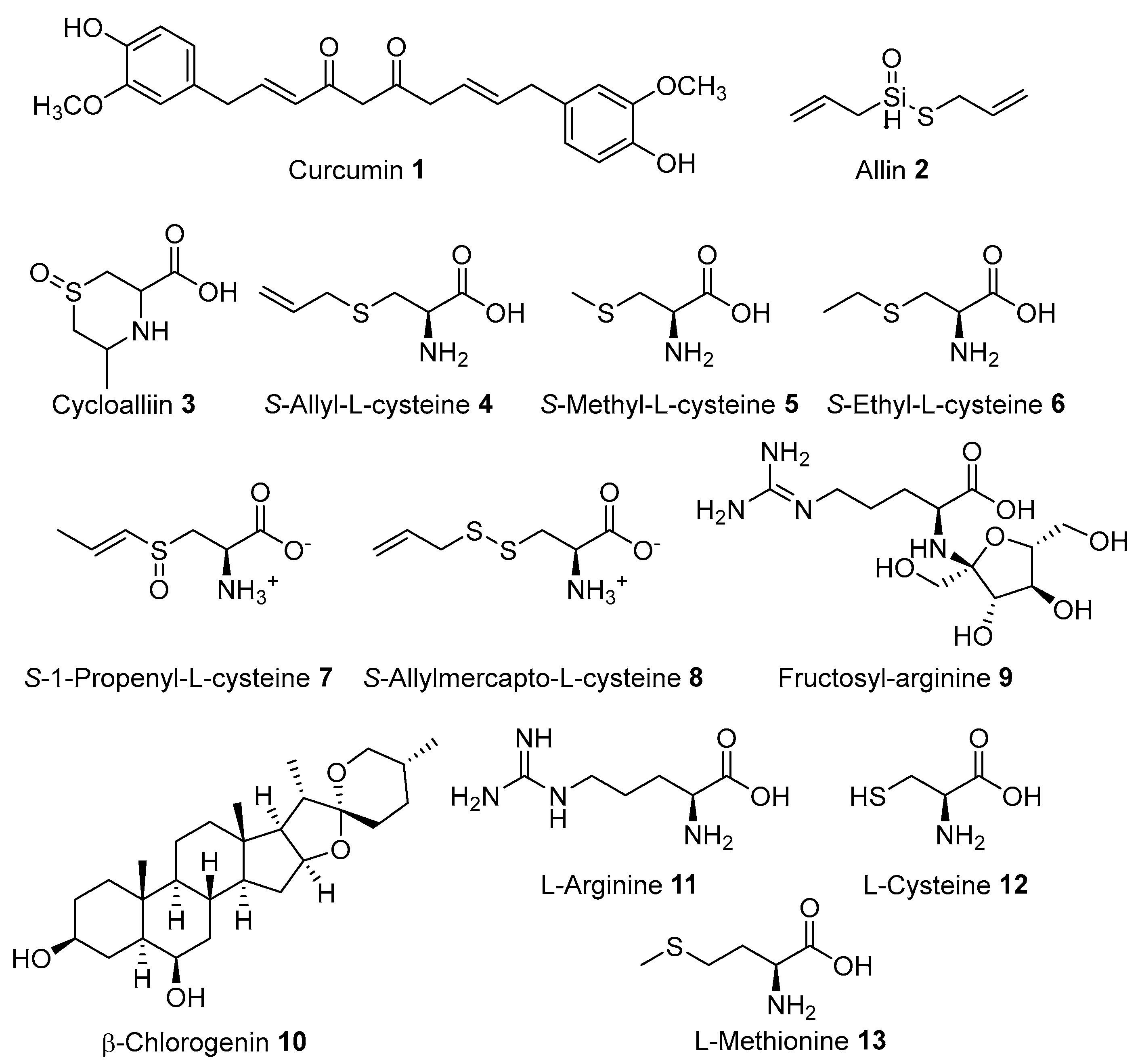
| Curcuma longa Linnaeus | Zingiberaceae | Fever, Headache, Wound | Rhizome | Thailand, China, Belgium, Vietnam, Germany | Curcuminoids, Demethoxycurcumin, Bisdemethoxycurcumin [36]; Calebin-A [37]; α-Turmerone [38]; Epicatechins [39]; Cucurbitacin B, Curcumin [40]; Bisacurone B [41]; α-Curcumene, Zingiberene, Bisabolene, Sesquiphellandrene [42]; Turmeronol B, Turmeronol A, (E)-α-Atlantone [43]; Curlone [44]. |
| Datura metel Linnaeus | Solanaceae | Fever | Leaves, Flower | China | Daturafolisides, Daturametelin [45]; Dmetelisproside A, Citroside A, Staphylionoside D [46]; Baimantuoluolines, Baimantuoluoside [47]; Cyclosieversioside F, Astragaloside II, Ginsenoside Rg1, Astrojanoside A, Celerioside E [48]; Isofraxidin, Scopatone, Daturadiol (3),1,4-Benzenediol, Arenarine D, Vanillin, N-trans-Feruloyl-tyramine, Scopoletin, G-Sitosterol and Hyoscyamilactol [49]. |
| Daucus carota | Apiaceae | Eyesight | Roots, Stems, Flower | Italy, Korea | β-carotene, carotenoids [50]; β-Phellandrene, γ-Terpinene [51]; 6-methoxymellein [52]; Camphorene, Carotol, β-Bisabolene, Isoelemicin [53]. |
| Drymocallis arguta subsp. arguta | Rosaceae | Diarrhoea Anaemia | NA | NA | NA |
| Elaeocarpus longifolius Bl. | Elaeocarpaceae | NA | NA | NA | |
| Erythrina variegata Linnaeus | Leguminoseae | Diarrhoea | Whole plant | China | Xanthoxyletin [54]; eryvarinols A and B [55]; Protocatechuic acid, Chlorogenic acid, and Caffeic acid [56]; Erythrinin B [57]. |
| Foeniculum vulgare | Apiaceae | Fever, Rheumatism | Leaves, Stem | Serbia, Italy, Tunisia Turkey, Romania, China, India, Italy, Turkey, Algeria, Italy, Spain, Turkey, and Egypt | Quercetin 3-glucuronide, Isoquercitrin, Rutin, Quercetin 3-arabinoside, Isorhammetin glycosides [58]; Dillapiol, Bergapten, Imperatorin, Psolaren [59]; Anethole, Limonene [60]; Gallic acid, Diosmin, Hesperidin, Kaempferol [61]; Carvacrol, Thymol, Anethol, p-Cymene and γ-Terpinene [62]; (E)-Anethole and p-Acetonylanisole [63]. α-Thujene, 1,8-Cineol, β-Ocimene, Linalool, Germacrene D, Anisketone, Apiol, n-Hexadecanoic acid, Cubebene, Benzene-1-methyl-4-(1-methylethyl)-p-cymene, 1,3,6-Octatriene, 3,7-dimethyl-, (E)-3-carene, 2-Heptene, 3-Methyl-butanal, β-Pinene, Camphene, Hexanal, α-Pinene, β-Phellandrene, α-Phellanrrene, β-Myrcene, 4-Carene, 2-Heptanohe, Limonene, 4-Methyl-bicyclo[3.1.0]hex-2-ene, Eucalyptol, α-Pinene, γ-Terpinene, 7-Dimethyl-1,3,7-octriene, 2,4-Dimethyl-benzenamine, 3-Carene, Cathine, 2-Heptanol, 2-Propyn-1-ol, 2,6-Dimethyl-2,4,6-octatriene, Fenchone, 1-Methyl-4-(1-methylethyl)-benzene, cis-Limonene oxide, trans-Limonene oxide, 6-Methylene-bicyclo[3.1.0]hexane, Sabinene hydrate, Fenchyl acetate, Camphor, Benzaldehyde, 1,3-Butanediol, Dicyclopropyl carbinol, Fenchol, 1-Octanol, 5-Methyl-2-heptanol, Tetradecyl-oxirane, Estragole, Trans-p-2,8-menthadien-1-ol, β-Terpinol, cis-p-2,8-Menthadien, 4-Methyl-1-(methylethyl)-3-cyclohexen, 2-Methyl-5-(1-methylethyl)-2-cyclohexen-1-one, Phenylmethyl-formic ester, 2,3-Cyclohexen-1-methanol, Epi-bicyclosesquiphellardrene, cis-p-Menth-2,8-dienol, 1,4-Dimethoxy-benzene, 1-Methoxy-4-(1-propenyl)-benzene, 1,2,4a,5,8,8a-Hexadehyde-naphthalene, 4-Methyl-bicyclo[3.1.1]hept-3-en-2-ol, trans-Anethole 73.20 73.27 66.71, Allantoic acid, 2-Methyl-5-(1-methylethyl)-phenol, Mannoheptulose, 2-Methyl-5-(1-methylethyl)-2-cyclohexen-1-ol, 1-Undecanol, Benzothiazole, E-Pinane, 2-Cyclohexen-1-ol, 2-Methyl-bezenemethanol, 4-Methoxy-benzaldehyde, 1,6-Hexanediol, 2-Methoxycyclohexanone, β-Elemenone, Mephenesin, 4φ-Methoxy-acetophenone, 2-Methyl-3-methylethyl-butanoic acid, Folic acid, 1-(Methoxyphenyl)-2-propanone, 1-Methyl-3-(1-methylethyl)-benzene, 4-Fluorohistamine, 1,2-Dimethoxy-4-(1-propenyl)-benzene, (E)-2-Hydroxy-4-cyano-stilbene, 1-(3-Methoxyphenyl)-1-propanone [12], eriodictyol-7-rutinoside, quercetin-3-rutinoside, and rosmarinic acid [64], quercetin-3-glucuronide, isoquercitrin, quercetin-3-arabinoside, kaempferol-3-glucuronide and kaempferol-3-arabinoside, and isorhamnetin glucoside [58], Quercetin-3-O-galactoside, kaempferol-3-O-rutinoside, and kaempferol-3-O-glucoside [65],Isorhamnetin 3-O-α-rhamnoside, quercetin, and kaempferol, quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, and quercetin 3-O-β-glucoside [66], quercetin, rutin, isoquercitrin [67], 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-Ocaffeoylquinic acid, 1,3-O-di-caffeoylquinic acid, 1,4-O-dicaffeoylquinic acid, and 1,5-O-di-caffeoylquinic acid [64], 3,4-dihydroxyphenethylalchohol-6-O-caffeoyl-β-Dglucopyranoside and 3′,8′-binaringenin [68]. |
| Garcinia mangostana Linnaeus | Clusiaceae | Stomach disorder | Fruit | India | α-Mangostin, β-Mangostin, γ-Mangostin, Garcinone-E, Methoxy-β-mangostin, Xanthone [69]; Mangostin, BR-Xanthone, Gartanin, 8-Desoxygartanin, Garcinone-D, Euxanthone, Xanthione [70]; Epichatechin, and Tannin [71]. |
| Jatropha gossypiifolia Linnaeus | Euphorbiaceae | Rheumatism | Whole plant, Stem, Leaves | India, Nigeria, Thailand | Gossypifan, Gossypilin, Gossypidien [72]; Gadain, Jatroiden [73]; Jatrodien [74]; Arylnaphthalene, Galic, Vanilic, Syringic, 2,5-Dihydroxy benzoic, Caffeic, Rosmarinic, and p-Coumaric [75]. |
| Kaempferia galanga Linnaeus | Zingiberaceae | Rheumatism | Rhizome | Thailand | (−)-Sandaracopimaradiene, Boesenberol, Sandaracopimaradien-1α,9α-diol, Kaempulchraol C, Kaempulchraol D [76]. |
| Malus prunifolia (Willd.) Borkh. | Rosaceae | Diarrhoea | Fruit | China | Citric acid, p-Coumaric acid, Hyperoside, Myricetin, Naringenin, Quercetin, Kaempferol, Gentiopicroside, Ursolic acid, and 8-Epiloganic acid [77]. |
| Manihot esculenta Crantz | Euphorbiaceae | Hypertension | Stem | Switzerland, China | Sporoge, Thecacorin, Longifoamide-B (Zeng Y, 2015); Yucalexin P-23, Yucalexin P-15, Protocatechuic acid, and Catalpinic acid [78]; Coniferaldehyde, Isovanillin, 6-Deoxyjacareubin, Scopoletin, Syringaldehyde, Pinoresinol, p-Coumaric acid, Ficusol, Balanophonin and Ethamivan [79]. |
| Musa paradisiaca Linnaeus | Musaceae | Diarrhoea Stomach disorder | Fruit | Brazil, India | Cycloeucalenone, 31-Norcyclolaudenone, 24-Methylene-cicloartanol [80]; α-Thujene, γ-Terpinene, α- and β-Pinene, Sabinene, β-Myrcene, Limonene, α-Capaene, Caryophyllene and (Z,E)-α Farnesene, Aceteugenol, Palmitic acid, Stearic acid, Palmitin, and Stearin [81]. |
| Oryza sativa Linnaeus | Poaceae | Vitaliser | Seed, Roots | Japan, Korea | Momilactones A and B [82]; Momilactone D, Momilactone E, Momilactone A, Sandaracopimaradien-3-one, Oryzalexin A [83]; Oryzativol C [84]; Oryzativol A [85]; ferulic acid, γ-Oryzanol, and Phytic acid [86]; Vanillin, Methyl trans-ferulate, Trans-p-Coumaric acid Methyl ester, N-Benzoyltryptamine, and N-(Trans-cinnamoyl)tryptamine [87]. |
| Persea americana Mill. | Lauraceae | Hypertension | Seed | Brazil | Quercetin and Epicatechin [88]; Avocadene, Avocadyne, Avocadenol-A [89]; γ-Lactone Perseanolide [90]. |
| Physalis lagascae Roem. & Schult. | Solanaceae | Diarrhoea Stomach disorder | NA | NA | NA |
| Piper amplum Kunth | Piperaceae | Rheumatism | NA | NA | NA |
| Piper betle Linnaeus | Piperaceae | Bleeding | Leaves | India, Myanmar, China | Estragole, Linalool, α-Copaene, Anethole, Caryophyllene, α-Terpinene, p-Cymene, 1,8-Cineole, β-Caryophyllene, α-Humulene, Allyl pyrocatechol, Allylcatechol, Methyl eugenol, Estragol (methyl chavicol), Chavibetol, Chavibetol acetate, Safrol, 4-Allyl-2-methoxy-phenolacetate, and 3-Allyl-6-methoxyphenol [91]; Pipeneolignan A, Piperneolignan B, Hydroxychavicol, p-Hydroxycinnamaldehyde, Diallylcatechol [92]; Pipercerebrosides A and B [93]; Piperolactam A [94]. |
| Rosa tomentosa Sm. | Rosaceae | Fever | NA | NA | NA |
| Rubus rosa L. H. Bailey | Rosaceae | Diarrhoea | Whole plant | USA | Elagic acid [95]. |
| Saccharum officinarum Linnaeus | Poaceae | Rheumatism | Stem | Brazil | Phenolic acid: p-Hydroxybenzoic, p-Hydroxycinnamic, Vanillic and Ferulic acid, Terpenoids: α-Tocopherol and β-Carotene, Flavonoid aglycone Tricin (5,7,4-trihydroxy-3,5-dimethoxyflavone) [96]. |
| Sechium edule | Cucurbitaceae | Fever (kindern) | Whole plant, Fruit | Mexico | Cinnamic acid, Linoleic, Palmitic, and Myristic acids [97]. |
| Sesbania grandiflora (L.)Pers. | Fabaceae | Fever | Leaves, Bark, Flowers | Indonesia | Gallic acid [98], 2-Arylbenzofuran [99]; Sesbagrandiflorains A and B [100]; Sesbagrandiflorain D and E, Spinosan A and Spinosan B [101]. |
| Solanum lycopersicum Linnaeus | Solanaceae | Nutrition | Fruit | USA, Japan, Korea, Chile | Monoterpenes, Glycoalkaloids, and Acyl sugars [102]; 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic acid (13-oxo-OTA), a linolenic acid derivative [103]; Steroidal saponins, Alkaloids, Cerebroside, Phenolic compounds, Sterols, and Nucleosides [104]; Guanosine [105]. |
| Solanum nigrum Linnaeus | Solanaceae | Hypertension, Tonic drink after hard labour | Whole plant, Fruit, Seed | China, Korea | Lignanamides [106]; Solanine A, 7α-OH Khasianine, 7α-OH Solamargine and 7α-OH Solasonine [107]; Saponins, Solanigroside A and Solanigroside B [108]; Steroidal glycosides (β2-Solamargine, Solamargine, and Degalactotigonin), Saponin (degalactotigonin) [108]; Lunasin [109]. |
| Tagetes tenuifolia Cavanille | Asteraceae | Nasal bleeding | NA | NA | NA |
| Tamarindus indica Linnaeus | Fabaceae | Nausea | Fruit | India | 9,12-Octadecadienoic acid (Z,Z)-, Cis-vaccenic acid, n-Hexadecanoic acid, Beta-Sitosterol, and Octadecanoic acid [110]; Proanthcyanidins, (+)-Catechin, Procyanidin B2, (-)-Epicatechin, Procyanidin trimer, Procyanidin tetramer, Procyanidin pentamer, Procyanidin hexamer, Taxifolin, Apigenin, Eriodictyol, Luteolin and Naringenin [111]. |
| Zingiber officinale Roscoe | Zingiberaceae | Headache | Rhizome | Japan, Thailand | Myristicin, Plumbagin, Methyl piperate, 6-Shogaol, 6-Gingerol and Piperine [112]; Geranyl 6-O-α-l-arabinopyranosyl-β-d-glucopyranoside, Geranyl 6-O-β-d-apiofuranosyl-β-d-glucopyranoside, and Geranyl 6-O-β-d-xylopyranosyl-β-d-glucopyranoside [113]. |
3. Biological Activities of Tenggerese Medicinal Plants
3.1. Diarrhoea
3.2. Wound Healing
3.3. Headache
3.4. Rheumatism and Anti-Inflammatory Agents
3.5. Hypertension
3.6. Antimicrobial Activities
3.7. Antimalarial Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Tobgay, T. Contributions of medicinal plants to the Gross National Happiness and Biodiscovery in Bhutan. J. Ethnobiol. Ethnomed. 2015, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Al Rashid, M.H.; Kundu, A.; Mandal, V.; Wangchuk, P.; Mandal, S.C. Preclinical and Clinical Trials of Indian Medicinal Plants in Disease Control. In Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and Its Value; Sen, S., Chakraborty, R., Eds.; Springer: Singapore, 2020; pp. 119–142. [Google Scholar] [CrossRef]
- Yeshi, K.; Gyal, Y.; Sabernig, K.; Phuntsho, J.; Tidwell, T.; Jamtsho, T.; Dhondup, R.; Tokar, E.; Wangchuk, P. An integrated medicine of Bhutan: Sowa Rigpa concepts, botanical identification, and the recorded phytochemical and pharmacological properties of the eastern Himalayan medicinal plants. Eur. J. Integr. Med. 2019, 29, 100927. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Keller, P.a. Revealing indigenous Indonesian traditional medicine: Anti-infective agents. Nat. Prod. Commun. 2011, 6, 1953–1966. [Google Scholar] [CrossRef]
- Roosita, K.; Kusharto, C.M.; Sekiyama, M.; Fachrurozi, Y.; Ohtsuka, R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J. Ethnopharmacol. 2008, 115, 72–81. [Google Scholar] [CrossRef]
- Gewali, M.B.; Awale, S. Aspects of Traditional Medicine in Nepal; Institute of Natural Medicine University of Toyama: Toyama, Japan, 2008. [Google Scholar]
- Smith-Hefner, N.J. A Social History of Language Change in Highland East Java. J. Asian Stud. 2011, 48, 257–271. [Google Scholar] [CrossRef]
- Azrianingsih, R.; Kusumahati, A. Perception and appreciation of tenggerese of medicinal plants in Wonokitri Village, Tosari subdistrict, Pasuruan Regency. AIP Conf. Proc. 2018, 2019, 020016. [Google Scholar] [CrossRef]
- Jadid, N.; Kurniawan, E.; Himayani, C.E.S.; Andriyani; Prasetyowati, I.; Purwani, K.I.; Muslihatin, W.; Hidayati, D.; Tjahjaningrum, I.T.D. An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS ONE 2020, 15, e0235886. [Google Scholar] [CrossRef]
- Badgujar, S.A.-O.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 32. [Google Scholar] [CrossRef]
- Paithankar, V.V.; Belsare, S.L.; Charde, R.M.; Vyas, J.V. Acorus Calamus: An Overview. Int. J. Biomed. Res. 2011, 2, 518–529. [Google Scholar] [CrossRef]
- Balakumbahan, R.; Rajamani, K.; Kumanan, K. Acorus calamus: An overview. J. Med. Plants Res. 2010, 4, 2740–2745. [Google Scholar]
- Imam, H.; Riaz, Z.; Azhar, M.; Sofi, G.; Hussain, A. Sweet flag (Acorus calamus Linn.): An incredible medicinal herb. Int. J. Green Pharm. 2013, 7, 288. [Google Scholar] [CrossRef]
- Hao, Z.-Y.; Liang, D.; Luo, H.; Liu, Y.-F.; Ni, G.; Zhang, Q.-J.; Li, L.; Si, Y.-K.; Sun, H.; Chen, R.-Y.; et al. Bioactive Sesquiterpenoids from the Rhizomes of Acorus calamus. J. Nat. Prod. 2012, 75, 1083–1089. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; GWasef, L.; Elewa, Y.H.; AAl-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; MAbd-Elhakim, Y.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Rattanapan, J.; Sichaem, J.; Tip-Pyang, S. Chemical constituents and antioxidant activity from the stems of Alyxia reinwardtii. Rec. Nat. Prod. 2012, 6, 288–291. [Google Scholar]
- Souza, L.F.; de Barros, I.B.I.; Mancini, E.; Martino, L.D.; Scandolera, E.; Feo, V.D. Chemical Composition and Biological Activities of the Essential Oil from Anredera cordifolia Grown in Brazil. Nat. Prod. Commun. 2014, 9, 1934578X1400900730. [Google Scholar] [CrossRef]
- Liu, G.; Zhuang, L.; Song, D.; Lu, C.; Xu, X. Isolation, purification, and identification of the main phenolic compounds from leaves of celery (Apium graveolens L. var. dulce Mill./Pers.). J. Sep. Sci. 2017, 40, 472–479. [Google Scholar] [CrossRef]
- Hedayati, N.; Bemani Naeini, M.; Mohammadinejad, A.; Mohajeri, S.A. Beneficial effects of celery (Apium graveolens) on metabolic syndrome: A review of the existing evidences. Phytother. Res. 2019, 33, 3040–3053. [Google Scholar] [CrossRef]
- Conserva, L.M.; Jesu Costa Ferreira, J. Borreria and Spermacoce species (Rubiaceae): A review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacogn. Rev. 2012, 6, 46. [Google Scholar] [CrossRef]
- Noiarsa, P.; Yu, Q.; Matsunami, K.; Otsuka, H.; Ruchirawat, S.; Kanchanapoom, T. (Z)-3-Hexenyl diglycosides from Spermacoce laevis Roxb. J. Nat. Med. 2007, 61, 406–409. [Google Scholar] [CrossRef]
- Fernandes, F.; Valentão, P.; Sousa, C.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem. 2007, 105, 1003–1010. [Google Scholar] [CrossRef]
- Pérez-Vázquez, M.A.K.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Mosso-González, C.; Ramírez-García, S.A.; Romero-Arenas, O.; Villa-Ruano, N. Peppermint Essential Oil and Its Major Volatiles as Protective Agents against Soft Rot Caused by Fusarium sambucinum in Cera Pepper (Capsicum pubescens). Chem. Biodivers. 2022, 19, e202100835. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Arifianti, A.E.; Sakti, A.S.; Saputri, F.C.; Abdul, M.i. Simultaneous natural deep eutectic solvent-based ultrasonic-assisted extraction of bioactive compounds of cinnamon bark and sappan wood as a dipeptidyl peptidase IV inhibitor. Molecules 2020, 25, 3832. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Sun, A.; Liu, X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci. Nutr. 2019, 7, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, D.U.; Hakim, R.A.; Mawarni, S.A.; Satriani, A.R. Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid. Cosmetics 2022, 9, 57. [Google Scholar] [CrossRef]
- Sethupathy, S.; Nithya, C.; Pandian, S.K. 2-Furaldehyde diethyl acetal from tender coconut water (Cocos nucifera) attenuates biofilm formation and quorum sensing-mediated virulence of Chromobacterium violaceum and Pseudomonas aeruginosa. Biofouling 2015, 31, 721–733. [Google Scholar] [CrossRef]
- Elsbaey, M.; Ibrahim, M.A.A.; Bar, F.A.; Elgazar, A.A. Chemical constituents from coconut waste and their in silico evaluation as potential antiviral agents against SARS-CoV-2. South Afr. J. Bot. 2021, 141, 278–289. [Google Scholar] [CrossRef]
- Lima, E.B.C.; de Sousa, C.N.S.; Vasconcelos, G.S.; Meneses, L.N.; Silva Pereira, Y.F.E.; Ximenes, N.C.; Santos Júnior, M.A.; Matos, N.C.B.; Brito, R.; Miron, D.; et al. Antidepressant, antioxidant and neurotrophic properties of the standardized extract of Cocos nucifera husk fiber in mice. J. Nat. Med. 2016, 70, 510–521. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Schlegel, V. Distillation Time as Tool for Improved Antimalarial Activity and Differential Oil Composition of Cumin Seed Oil. PLoS ONE 2015, 10, e0144120. [Google Scholar] [CrossRef] [PubMed]
- Aldulaimi, O. Screening of Fruits of Seven Plants Indicated for Medicinal Use in Iraq. Pharmacogn. Mag. 2017, 13, S189–S195. [Google Scholar] [CrossRef] [PubMed]
- Neethu, S.; Veena, S.K.; Indulekha, V.C.; Eapen, J.; Radhakrishnan, K.V. Phytoconstituents assessment and development of standardization protocol for ‘Nayopayam Kwatha’, a polyherbal Ayurvedic formulation. J. Ayurveda Integr. Med. 2021, 12, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Wuthi-udomlert, M.; Grisanapan, W.; Luanratana, O.; Caichompoo, W. Antifungal activity of Curcuma longa grown in Thailand. Southeast Asian J. Trop. Med. Public Health 2000, 31 (Suppl. 1), 178–182. [Google Scholar] [PubMed]
- Kim, D.S.; Kim, J.Y. Total synthesis of Calebin-A, preparation of its analogues, and their neuronal cell protectivity against beta-amyloid insult. Bioorganic Med. Chem. Lett. 2001, 11, 2541–2543. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Komiya, T.; Moteki, H.; Katsuzaki, H.; Imai, K.; Hibasami, H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L.) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002, 9, 481–484. [Google Scholar] [CrossRef]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Suganuma, M.; Fujiki, H. New role of (-)-epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev. Res. 2010, 3, 953–962. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhou, J.; Huang, Z.; Hu, H.; Qiao, M.; Zhao, X.; Chen, D. Synergistic effect of cucurbitacin B in combination with curcumin via enhancing apoptosis induction and reversing multidrug resistance in human hepatoma cells. Eur. J. Pharmacol. 2015, 768, 28–40. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhu, X.; Wang, D.; Li, X. Choleretic Activity of Turmeric and its Active Ingredients. J. Food Sci. 2016, 81, H1800–H1806. [Google Scholar] [CrossRef]
- Lv, G.-P.; Hu, D.-J.; Zhou, Y.-Q.; Zhang, Q.-W.; Zhao, J.; Li, S.-P. Preparation and Application of Standardized Typical Volatile Components Fraction from Turmeric (Curcuma longa L.) by Supercritical Fluid Extraction and Step Molecular Distillation. Molecules 2018, 23, 1831. [Google Scholar] [CrossRef]
- Akter, J.; Islam, M.Z.; Takara, K.; Hossain, M.A.; Sano, A. Isolation and structural elucidation of antifungal compounds from Ryudai gold (Curcuma longa) against Fusarium solani sensu lato isolated from American manatee. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2019, 219, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Beaufay, C.; Nghiem, D.; Pham, T.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules 2019, 24, 1158. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, Y.; Xu, Z.-P.; Xia, Y.-G.; Yang, B.-Y.; Kuang, H.-X. Withanolides from the leaves of Datura metel L. Phytochemistry 2018, 155, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, Y.; Pan, J.; Guan, W.; Yang, B.-Y.; Kuang, H.-X. A new sesquiterpenoid with cytotoxic and anti-inflammatory activity from the leaves of Datura metel L. Nat. Prod. Res. 2021, 35, 607–613. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Sun, Y.-P.; Wang, X.; Liu, Y.; Yang, B.-Y.; Kuang, H.-X. Immunosuppressive withanolides from the flower of Datura metel L. Fitoterapia 2020, 141, 104468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, D.-D.; Zhou, Y.-Q.; Wu, J.-T.; Qi, Z.-T.; Algradi, A.M.; Pan, J.; Guan, W.; Yang, B.-Y.; Kuang, H.-X. A new ent-kaurane diterpenoid from the pericarps of Datura metel. J. Asian Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Han, X.-L.; Wang, H.; Zhang, Z.-H.; Tan, Y.; Wang, J.-H. Study on Chemical Constituents in Seeds of Datura metel from Xinjiang. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2015, 38, 1646–1648. [Google Scholar]
- Miękus, N.; Iqbal, A.; Marszałek, K.; Puchalski, C.; Świergiel, A. Green Chemistry Extractions of Carotenoids from Daucus carota L.-Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules 2019, 24, 4339. [Google Scholar] [CrossRef]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Liu, R.; Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Yun, B.-S.; Lee, D.-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbera, M.; Aleo, A.; Lommatzsch, I.; La Mantia, T.; Settanni, L. Inhibitory Activity and Chemical Characterization of Daucus carota subsp. maximus Essential Oils. Chem. Biodivers. 2017, 14, e1600477. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Khan, M.; Yu, B.; Ma, T.; Yang, H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac. J. Cancer Prev. APJCP 2011, 12, 1219–1223. [Google Scholar] [PubMed]
- Tanaka, H.; Hirata, M.; Etoh, H.; Watanabe, N.; Shimizu, H.; Ahmad, M.; Terada, Y.; Fukai, T. Two diphenylpropan-1,2-diol syringates from the roots of Erythrina variegata. J. Nat. Prod. 2002, 65, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, J.; Liao, X.; Zhang, P.; Chen, X. One-step separation of antioxidant compounds from Erythrina variegata by high speed counter-current chromatography. J. Chromatogr. Sci. 2015, 53, 730–735. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mahmud, T.; Yoshioka, N.; Shibuya, H.; Kitagawa, I. Indonesian medicinal plants. XXI. Inhibitors of Na+/H+ exchanger from the bark of Erythrina variegata and the roots of Maclura cochinchinensis. Chem. Pharm. Bull. 1997, 45, 1615–1619. [Google Scholar] [CrossRef][Green Version]
- Kunzemann, J.; Herrmann, K. [Isolation and identification of flavon(ol)-O-glycosides in caraway (Carum carvi L.), fennel (Foeniculum vulgare Mill.), anise (Pimpinella anisum L.), and coriander (Coriandrum sativum L.), and of flavon-C-glycosides in anise. I. Phenolics of spices (author’s transl)]. Z. Fur Lebensm. Unters. Forsch. 1977, 164, 194–200. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Choi, W.G.; Kim, W.J.; cKim, W.K.; Kim, M.J.; Kang, W.H.; Kim, C.M. Antimicrobial constituents of foeniculum vulgare. Arch. Pharmacal. Res. 2002, 25, 154–157. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef]
- Váradyová, Z.; Pisarčíková, J.; Babják, M.; Hodges, A.; Mravčáková, D.; Kišidayová, S.; Königová, A.; Vadlejch, J.; Várady, M. Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus. Exp. Parasitol. 2018, 195, 71–77. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep-In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef]
- Chiboub, W.; Sassi, A.B.; Amina, C.M.H.; Souilem, F.; El Ayeb, A.; Djlassi, B.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Valorization of the Green Waste from Two Varieties of Fennel and Carrot Cultivated in Tunisia by Identification of the Phytochemical Profile and Evaluation of the Antimicrobial Activities of Their Essentials Oils. Chem. Biodivers. 2019, 16, e1800546. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum vulgare) Using Liquid Chromatography−Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.I.; Aboutabl, E.S.A.; Makled, Y.A.; El–Khrisy, E.D.A.; Osman, A.F. Secondary metabolites and pharmacology of Foeniculum vulgare Mill. Subsp. Piperitum. Rev. Latinoam. Química 2010, 38, 10. [Google Scholar]
- Cherng, J.-M.; Chiang, W.; Chiang, L.-C. Immunomodulatory activities of common vegetables and spices of Umbelliferae and its related coumarins and flavonoids. Food Chem. 2008, 106, 944–950. [Google Scholar] [CrossRef]
- Ghanem, M.T.M.; Radwan, H.M.A.; Mahdy, E.S.M.; Elkholy, Y.M.; Hassanein, H.D.; Shahat, A.A. Phenolic Compounds from Foeniculum vulgare (Subsp. Piperitum) (Apiaceae) Herb and Evaluation of Hepatoprotective Antioxidant Activity. Pharmacogn. Res. 2012, 4, 5. [Google Scholar]
- Dharmaratne, H.R.W.; Piyasena, K.G.N.P.; Tennakoon, S.B. A geranylated biphenyl derivative from Garcinia malvgostana. Nat. Prod. Res. 2005, 19, 239–243. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Banumathi, B.; Suresh, G. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J. Nat. Prod. 1997, 60, 519–524. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Opanasopi, P.; Sukma, M.; Sittisombut, C.; Kat, A.; Adachi, I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharm. Biol. 2010, 48, 55–62. [Google Scholar] [CrossRef]
- Das, B.; Anjani, G. ChemInform Abstract: Phytochemicals. Part 29. Gossypidien, a Lignan from Stems of Jatropha gossypifollia. Cheminform 2010, 30, 1. [Google Scholar] [CrossRef]
- Oduola, T.; Adeosun, G.; Oduola, T.; Avwioro, G.; Oyeniyi, M. Mechanism of Action of Jatropha Gossypifolia Stem Latex As a Haemostatic Agent. Eur. J. Gen. Med. 2004, 2, 140–143. [Google Scholar] [CrossRef]
- Saishri, R.; Ravichandran, N.; Vadivel, V.; Brindha, P. Pharmacognostic studies on leaf of Jatropha Gossypifolia L. Int. J. Pharm. Sci. Res. 2016, 7, 163–173. [Google Scholar] [CrossRef]
- Povichit, N.; Phrutivorapongkul, A.; Suttajit, M.; Chaiyasut, C.; Leelapornpisid, P. Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak. J. Pharm. Sci. 2010, 23, 403–408. [Google Scholar] [PubMed]
- Tungcharoen, P.; Chatchai, W.; Tansakul, P.; Nakamura, S.; Matsuda, H.; Tewtrakul, S. Anti-inflammatory effect of isopimarane diterpenoids from Kaempferia galanga. Phytother. Res. 2019, 34, 612–623. [Google Scholar] [CrossRef]
- Wen, C.; Wang, D.; Li, X.; Huang, T.; Huang, C.; Hu, K. Targeted isolation and identification of bioactive compounds lowering cholesterol in the crude extracts of crabapples using UPLC-DAD-MS-SPE/NMR based on pharmacology-guided PLS-DA. J. Pharm. Biomed. Anal. 2018, 150, 144–151. [Google Scholar] [CrossRef]
- Li, S.-S.; Hu, L.-F.; Zhao, Y.-X.; Zuo, W.-J.; Zeng, Y.-B.; Li, X.-N.; Mei, W.-L.; Dai, H.-F. A new diterpene from the stems of Manihot esculenta. J. Asian Nat. Prod. Res. 2011, 13, 961–964. [Google Scholar] [CrossRef]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef]
- Silva, A.A.S.; Morais, S.M.; Falcão, M.J.C.; Vieira, I.G.P.; Ribeiro, L.M.; Viana, S.M.; Teixeira, M.J.; Barreto, F.S.; Carvalho, C.A.; Cardoso, R.P.A.; et al. Activity of cycloartane-type triterpenes and sterols isolated from Musa paradisiaca fruit peel against Leishmania infantum chagasi. Phytomedicine 2014, 21, 1419–1423. [Google Scholar] [CrossRef]
- Fahim, M.; Ibrahim, M.; Zahiruddin, S.; Parveen, R.; Khan, W.; Ahmad, S.; Shrivastava, B.; Shrivastava, A.K. TLC-bioautography identification and GC-MS analysis of antimicrobial and antioxidant active compounds in Musa × paradisiaca L. fruit pulp essential oil. Phytochem. Anal. PCA 2019, 30, 332–345. [Google Scholar] [CrossRef]
- Quan, N.V.; Tran, H.-D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B Are α-Amylase and α-Glucosidase Inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef]
- Cho, J.-G.; Cha, B.-J.; Min Lee, S.; Shrestha, S.; Jeong, R.-H.; Sung Lee, D.; Kim, Y.-C.; Lee, D.-G.; Kang, H.-C.; Kim, J.; et al. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Lee, D.; Yu, J.S.; Jo, M.S.; Baek, S.C.; Shin, M.-S.; Ko, Y.-J.; Kang, K.S.; Kim, K.H. Biological Evaluation of a New Lignan from the Roots of Rice (Oryza sativa). Chem. Biodivers. 2018, 15, e1800333. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Yun, H.S.; Lee, T.K.; Lee, S.; Kim, S.-H.; Moon, E.; Park, K.-M.; Kim, K.H. Chemical Characterization of Novel Natural Products from the Roots of Asian Rice (Oryza sativa) that Control Adipocyte and Osteoblast Differentiation. J. Agric. Food Chem. 2018, 66, 2677–2684. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224. [Google Scholar] [CrossRef]
- Cho, J.-G.; Huh, J.; Jeong, R.-H.; Cha, B.-J.; Shrestha, S.; Lee, D.-G.; Kang, H.-C.; Kim, J.-Y.; Baek, N.-I. Inhibition effect of phenyl compounds from the Oryza sativa roots on melanin production in murine B16-F10 melanoma cells. Nat. Prod. Res. 2015, 29, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef]
- Louis, M.R.L.M.; Rani, V.P.; Krishnan, P.; Reegan, A.D.; Balakrishna, K.; Ignacimuthu, S.; Packiam, S.M.; Maheswaran, R.; Shirota, O. Mosquito Larvicidal Activity of Compounds from Unripe Fruit Peel of Avocado (Persea americana Mill.). Appl. Biochem. Biotechnol. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Reis, I.M.A.; Umehara, E.; Conceição, R.S.; de M. Oliveira, L.; Coelho Dos, S.M., Jr.; Costa-Silva, T.A.; Amaral, M.; Tempone, A.G.; Branco, A.; Lago, J.H.G. γ-Lactones from Persea americana and Persea fulva—In Vitro and in Silico Evaluation of Trypanosoma cruzi Activity. Chem. Biodivers. 2021, 18, e2100362. [Google Scholar] [CrossRef]
- Nayaka, N.M.D.M.W.; Sasadara, M.M.V.; Sanjaya, D.A.; Yuda, P.E.S.K.; Dewi, N.L.K.A.A.; Cahyaningsih, E.; Hartati, R. Piper betle (L): Recent Review of Antibacterial and Antifungal Properties, Safety Profiles, and Commercial Applications. Molecules 2021, 26, 2321. [Google Scholar] [CrossRef]
- San, T.T.; Wang, Y.-H.; Hu, D.-B.; Yang, J.; Zhang, D.-D.; Xia, M.-Y.; Yang, X.-F.; Yang, Y.-P. A new sesquineolignan and four new neolignans isolated from the leaves of Piper betle, a traditional medicinal plant in Myanmar. Bioorganic Med. Chem. Lett. 2021, 31, 127682. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Xiong, H.-B.; Tian, K.; Guo, J.-M.; Huang, X.-Z.; Jiang, Z.-Y. Two new sphingolipids from the leaves of Piper betle L. Molecules 2013, 18, 11241–11249. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Bhattacharya, P.; Basak, S.; Gayen, S.; Nandy, A.; Saha, A. Pharmacoinformatics study of Piperolactam A from Piper betle root as new lead for non steroidal anti fertility drug development. Comput. Biol. Chem. 2017, 67, 213–224. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.G.; Souza, A.G.; Chiavelli, L.U.R.; Ruiz, A.L.T.G.; Carvalho, J.E.; Pomini, A.M.; Silva, C.C. Phenolic compounds and anticancer activity of commercial sugarcane cultivated in Brazil. An. Acad. Bras. Cienc. 2016, 88, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Moreno, C.; Castro-Martínez, G.; Méndez-Martínez, M.; Jiménez-Ferrer, J.E.; Pedraza-Chaverri, J.; Arrellín, G.; Zamilpa, A.; Medina-Campos, O.N.; Lombardo-Earl, G.; Barrita-Cruz, G.J.; et al. Acetone fraction from Sechium edule (Jacq.) S.w. edible roots exhibits anti-endothelial dysfunction activity. J. Ethnopharmacol. 2018, 220, 75–86. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Hamamoto, H.; Sekimizu, K.; Okonogi, S. Biological activities and antibacterial biomarker of Sesbania grandiflora bark extract. Drug Discov. Ther. 2017, 11, 70–77. [Google Scholar] [CrossRef][Green Version]
- Noviany, N.; Nurhidayat, A.; Hadi, S.; Suhartati, T.; Aziz, M.; Purwitasari, N.; Subasman, I. Sesbagrandiflorain A and B: Isolation of two new 2-arylbenzofurans from the stem bark of Sesbania grandiflora. Nat. Prod. Res. 2018, 32, 2558–2564. [Google Scholar] [CrossRef]
- Noviany, N.; Samadi, A.; Carpenter, E.L.; Abugrain, M.E.; Hadi, S.; Purwitasari, N.; Indra, G.; Indra, A.; Mahmud, T. Structural revision of sesbagrandiflorains A and B, and synthesis and biological evaluation of 6-methoxy-2-arylbenzofuran derivatives. J. Nat. Med. 2020, 75, 66–75. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Tanjung, M.; Saputri, R.D.; Rahayu, D.O.; Gunawan Alfiah Nur, I.; Aldin, M.F. Two new 2-arylbenzofurans from Sesbania grandiflora L. and their cytotoxicity towards cancer cell. Nat. Prod. Res. 2020, 35, 5637–5642. [Google Scholar] [CrossRef]
- Kang, J.-H.; Shi, F.; Jones, A.D.; Marks, M.D.; Howe, G.A. Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 2009, 61, 1053–1064. [Google Scholar] [CrossRef]
- Takahashi, H.; Hara, H.; Goto, T.; Kamakari, K.; Wataru, N.; Mohri, S.; Suzuki, H.; Shibata, D.; Kawada, T. 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic Acid Activates Peroxisome Proliferator-Activated Receptor γ in Adipocytes. Lipids 2014, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Jung, H.A.; Choi, J.S. Discovery of Flazin, an Alkaloid Isolated from Cherry Tomato Juice, As a Novel Non-Enzymatic Protein Glycation Inhibitor via in Vitro and in Silico Studies. J. Agric. Food Chem. 2021, 69, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Alarcón, M.; Astudillo, L.; Valenzuela, C.; Gutiérrez, M.; Palomo, I. Protective mechanisms of guanosine from Solanum lycopersicum on agonist-induced platelet activation: Role of sCD40L. Molecules 2013, 18, 8120–8135. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Song, X.-Y.; Zhao, W.-Y.; Yao, G.-D.; Lin, B.; Huang, X.-X.; Li, L.-Z.; Song, S.-J. Characterization of enantiomeric lignanamides from Solanum nigrum L. and their neuroprotective effects against MPP(+)-induced SH-SY5Y cells injury. Phytochemistry 2019, 161, 163–171. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Shen, X.-F.; Wang, L.; Wu, Z.-W.; Li, F.; Chen, B.; Zhang, G.-L.; Wang, M.-K. Bioactive steroidal alkaloids from the fruits of Solanum nigrum. Phytochemistry 2018, 147, 125–131. [Google Scholar] [CrossRef]
- Zhou, X.-L.; He, X.-J.; Zhou, G.-X.; Ye, W.-C.; Yao, X.-S. Pregnane glycosides from Solanum nigrum. J. Asian Nat. Prod. Res. 2007, 9, 517–523. [Google Scholar] [CrossRef]
- Jeong, J.B.; De Lumen, B.O.; Jeong, H.J. Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Lett. 2010, 293, 58–64. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Olajuyigbe, O.O. Soxhlet Extraction versus Hydrodistillation Using the Clevenger Apparatus: A Comparative Study on the Extraction of a Volatile Compound from Tamarindus indica Seeds. Sci. World J. 2021, 2021, 5961586. [Google Scholar] [CrossRef]
- Sudjaroen, Y.; Haubner, R.; Würtele, G.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Changbumrung, S.; Bartsch, H.; Owen, R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2005, 43, 1673–1682. [Google Scholar] [CrossRef]
- Rattarom, R.; Sakpakdeejaroen, I.; Hansakul, P.; Itharat, A. Cytotoxic activity against small cell lung cancer cell line and chromatographic fingerprinting of six isolated compounds from the ethanolic extract of Benjakul. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2014, 97 (Suppl. 8), S70-5. [Google Scholar]
- Sekiwa, Y.; Kobayashi, A.; Kubota, K.; Takenaka, M. First isolation of geranyl disaccharides from ginger and their relations to aroma formation. Nat. Prod. Lett. 2001, 15, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, A.; Dimmito, M.P.; Zengin, G.; Pieretti, S.; Mollica, A.; Locatelli, M.; Cichelli, A.; Novellino, E.; Ak, G.; Yerlikaya, S.; et al. Exploring the Nutraceutical Potential of Dried Pepper Capsicum annuum L. on Market from Altino in Abruzzo Region. Antioxidants 2020, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T.; Nurudeen, Q.O.; Salimon, S.S.; Yakubu, M.O.; Jimoh, R.O.; Nafiu, M.O.; Akanji, M.A.; Oladiji, A.T.; Williams, F.E. Antidiarrhoeal Activity of Musa paradisiaca Sap in Wistar Rats. Evid.-Based Complement. Altern. Med. Ecam 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, Å.V.; Parsons, B.N.; Prorok-Hamon, M.; Knight, P.; Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Soluble plantain fibre blocks epithelial adhesion and M-cell translocation of intestinal pathogens. Gut 2011, 60, A96. [Google Scholar] [CrossRef][Green Version]
- Gunasekaran, D.; Chandramohan, A.; Karthikeyan, K.; Balasubramaniam, B.; Jagadeesan, P.; Soundararajan, P. Effect of Green Banana (Musa paradisiaca) on Recovery in Children With Acute Watery Diarrhea With No Dehydration: A Randomized Controlled Trial. Indian Pediatr. 2020, 57, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Acosta, T.; León, C.; Acosta-González, S.; Parra-Soto, H.; Cluet-Rodriguez, I.; Rossell, M.R.; Colina-Chourio, J.A. Beneficial Role of Green Plantain [Musa paradisiaca] in the Management of Persistent Diarrhea: A Prospective Randomized Trial. J. Am. Coll. Nutr. 2009, 28, 169–176. [Google Scholar] [CrossRef]
- Yuniarti, W.M.; Lukiswanto, B.S. Effects of herbal ointment containing the leaf extracts of Madeira vine (Anredera cordifolia (Ten.) Steenis) for burn wound healing process on albino rats. Vet. World 2017, 10, 808–813. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Pazyar, N.; Feily, A. Garlic in dermatology. Dermatol. Rep. 2011, 3, e4. [Google Scholar] [CrossRef]
- Ejaz, S.; Chekarova, I.; Cho, J.; Lee, S.; Ashraf, S.; Lim, C. Effect of aged garlic extract on wound healing: A new frontier in wound management. Drug Chem. Toxicol. 2009, 32, 191–203. [Google Scholar] [CrossRef]
- Mustafa, T.; Srivastava, K.C. Ginger (Zingiber officinale) in migraine headache. J. Ethnopharmacol. 1990, 29, 267–273. [Google Scholar] [CrossRef]
- Martins, L.B.; Rodrigues, A.M.d.S.; Monteze, N.M.; Tibaes, J.R.B.; Amaral, M.H.A.; Gomez, R.S.; Teixeira, A.L.; Ferreira, A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia 2019, 40, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.-S.; Kim, B.-Y.; Seo, C.-S.; Jeong, S.-J. Quantitative Analysis and Biological Efficacies regarding the Neuroprotective and Antineuroinflammatory Actions of the Herbal Formula Jodeungsan in HT22 Hippocampal Cells and BV-2 Microglia. Evid.-Based Complement. Altern. Med. 2017, 2017, 6360836. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R. Multimodal Care for Headaches, Lumbopelvic Pain, and Dysmenorrhea in a Woman With Endometriosis: A Case Report. J. Chiropr. Med. 2021, 20, 148–157. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Mittal, N.; Joshi, R.; Hota, D.; Chakrabarti, A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother. Res. 2009, 23, 507–512. [Google Scholar] [CrossRef]
- Young, H.-Y.; Luo, Y.-L.; Cheng, H.-Y.; Hsieh, W.-C.; Liao, J.-C.; Peng, W.-H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef]
- Choi, E.M.; Hwang, J.K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- Li, C.; Cai, Q.; Wu, X.; Tan, Z.; Yao, L.; Huang, S.; Zhang, W.; Hong, Z.; Chen, Z.; Zhang, L.A.-O. Anti-inflammatory Study on the Constituents of Angelica sinensis (Oliv.) Diels, Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav., Angelica pubescence Maxim and Foeniculum vulgare Mill. J. Essent. Oils 2022, 71, 1207–1219. [Google Scholar]
- Alazadeh, M.; Azadbakht, M.; Niksolat, F.; Asgarirad, H.; Moosazadeh, M.; Ahmadi, A.; Yousefi, S.S. Effect of sweet fennel seed extract capsule on knee pain in women with knee osteoarthritis. Complement. Ther. Clin. Pract. 2020, 40, 101219. [Google Scholar] [CrossRef]
- Rifqiyati, N.; Wahyuni, A. Fennel (Foeniculum vulgare) leaf infusion effect on mammary gland activity and kidney function of lactating rats. Nusant. Biosci. 2019, 11, 5. [Google Scholar] [CrossRef]
- Dwita, L.P.; Hikmawanti, N.P.E.; Yeni; Supandi. Extract, fractions, and ethyl-p-methoxycinnamate isolate from Kaempferia galanga Elicit anti-inflammatory activity by limiting leukotriene B4 (LTB4) production. J. Tradit. Complement. Med. 2021, 11, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Atangwho, I.J.; Yam, M.F.; Altaf, R.; Ahmed, A. Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules 2012, 17, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, P.C.; Latha, K.P.; Mudgal, J.; Nampurath, G.K. Extraction, characterization and evaluation of Kaempferia galanga L. (Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016, 194, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Ihtisham, U.; Mohd Zaini, A.; Amirin, S.; Amin Malik Shah Abdul, M.; Fouad Saleih, R.A.-S.; Loiy Elsir Ahmed, H.; Rabia, A.; Mohamed, B.K.A. Ethyl-p-methoxycinnamate isolated from kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α, and angiogenesis by blocking endothelial functions. Clinics 2014, 69, 134–144. [Google Scholar] [CrossRef]
- Xavier-Santos, J.B.; Félix-Silva, J.; Passos, J.G.R.; Gomes, J.A.S.; Fernandes, J.M.; Garcia, V.B.; de Araujo-Junior, R.F.; Zucolotto, S.M.; Silva-Junior, A.A.; Fernandes-Pedrosa, M.F. Development of an effective and safe topical anti-inflammatory gel containing Jatropha gossypiifolia leaf extract: Results from a pre-clinical trial in mice. J. Ethnopharmacol. 2018, 227, 268–278. [Google Scholar] [CrossRef]
- Ledon, N.; Casaco, A.; Remirez, D.; González, A.; Cruz, J.; Gonzalez, R.; Capote, A.; Tolón, Z.; Rojas, E.; Rodríguez, V.J.; et al. Effects of a mixture of fatty acids from sugar cane (Saccharum officinarum L.) wax oil in two models of inflammation: Zymosan-induced arthritis and mice tail test of psoriasis. Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 690–695. [Google Scholar] [CrossRef]
- Ledon, N. Anti-inflammatory and analgesic effects of a mixture of fatty acids isolated and purified from sugar cane wax oil. Planta Med. 2003, 2069, 367–369. [Google Scholar] [CrossRef]
- Alotaibi, B.; Mokhtar, F.A.-O.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A.-O. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential Against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021, 14, 7411. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Wijayanti, A.D.; Mutmainah, M.; Susilowati, R.; Rahmawati, N. Gastroprotective Effect of Combination of Hot Water Extracts of Licorice (Glycyrrhiza glabra), Pulasari Stem Bark (Alyxia reinwardtii), and Sembung Leaf (Blumea balsamifera) Against Aspirin-Induced Gastric Ulcer Model Rats. J. Evid. Based Complement. Altern. Med. 2016, 21, NP77–NP84. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; et al. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2020, 11, 155–184. [Google Scholar] [CrossRef]
- Siska, S.; Mun`im, A.; Bahtiar, A.; Suyatna, F.D. Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats. Sci. Pharm. 2018, 86, 6. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ramírez, C.; Paz, J.; Ortiz-Avila, O.; Raya-Farias, A.; González-Hernández, J.; Rodríguez-Orozco, A.; Salgado-Garciglia, R.; Saavedra-Molina, A.; Godínez-Hernández, D.; Cortés-Rojo, C. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function and mitochondrial oxidative stress in hypertensive rats. Nutrition 2018, 54, 60–67. [Google Scholar] [CrossRef]
- Sokpe, A.; Mensah, M.; Koffuor, G.; Thomford, K.P.; Arthur, R.; Jibira, Y.; Baah, M.; Adedi, B.; Agbemenyah, H. Hypotensive and Antihypertensive Properties and Safety for Use of Annona muricata and Persea americana and Their Combination Products. Evid. Based Complement. Altern. Med. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Alashi, A.; Malomo, S.; Girgih, A.; Chao, D.; Ju, X.; Aluko, R. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Glycinyl-Histidinyl-Serine (GHS), a Novel Rapeseed Protein-Derived Peptide Has Blood Pressure-Lowering Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2013, 61, 8396–8402. [Google Scholar] [CrossRef]
- Simaratanamongkol, A.; Umehara, K.; Noguchi, H.; Panichayupakaranant, P. Identification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plants. Food Chem. 2014, 165, 92–97. [Google Scholar] [CrossRef]
- Prasad, A.; Devi, A.T.; Prasad, M.N.N.; Zameer, F.; Shruthi, G.; Shivamallu, C. Phyto anti-biofilm elicitors as potential inhibitors ofHelicobacter pylori. 3 Biotech 2019, 9, 53. [Google Scholar] [CrossRef]
- Kim, W.-J.; Hwang, K.-H.; Park, D.-G.; Kim, T.-J.; Kim, D.-W.; Choi, D.-K.; Moon, W.-K.; Lee, K.-H. Major constituents and antimicrobial activity of Korean herb Acorus calamus. Nat. Prod. Res. 2011, 25, 1278–1281. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Owais, M. Evaluation of anti-methicillin-resistantStaphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S.; Al Aboody, M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Aqil, F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol. Res. 2007, 162, 264–275. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, I.; Chaudhary, P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014, 28, 1454–1466. [Google Scholar] [CrossRef]
- Yao, X.; Ling, Y.; Guo, S.; He, S.; Wu, W.; Zhang, Q.; Zou, M.; Nandakumar, K.S.; Chen, X.; Liu, S. Tatanan A from the Acorus calamus L. root inhibited dengue virus proliferation and infections. Phytomedicine 2018, 42, 258–267. [Google Scholar] [CrossRef]
- Shan, B.; Cai Yz Fau-Brooks, J.D.; Brooks Jd Fau-Corke, H.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef] [PubMed]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D.A.-O. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019, 16, 1. [Google Scholar] [CrossRef]
- Ordoñez, A.A.; Ordoñez Rm Fau-Zampini, I.C.; Zampini Ic Fau-Isla, M.I.; Isla, M.I. Design and quality control of a pharmaceutical formulation containing natural products with antibacterial, antifungal and antioxidant properties. Int. J. Pharm. 2009, 378, 51–58. [Google Scholar] [CrossRef]
- Bawazeer, S.; Rauf, A. In Vitro Antibacterial and Antifungal Potential of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus. BioMed. Res. Int. 2021, 2021, 1543574. [Google Scholar] [CrossRef]
- Zorofchian, S.; Kadir, H.; Hassandarvish, P.; Tajik, H.; Abu Bakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Tanaka, H.; Atsumi, I.; Shirota, O.; Sekita, S.; Sakai, E.; Sato, M.; Murata, J.; Murata, H.; Darnaedi, D.; Chen, I.-S. Three new constituents from the roots of Erythrina variegata and their antibacterial activity against methicillin-resistant Staphylococcus aureus. Chem. Biodivers. 2011, 8, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Atsumi, I.; Hasegawa, M.; Hirata, M.; Sakai, T.; Sato, M.; Yamaguchi, R.; Tateishi, Y.; Tanaka, T.; Fukai, T. Two New Isoflavanones from the Roots of Erythrina variegata. Nat. Prod. Commun. 2015, 10, 1934578X1501000330. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Yamaguchi, R.; Kato, K.; Etoh, H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2004, 24, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Corona, M.D.R.; Ramírez-Cabrera, M.A.; Santiago, O.G.; Garza-González, E.; Palacios, I.d.P.; Luna-Herrera, J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother. Res. PTR 2008, 22, 82–85. [Google Scholar] [CrossRef]
- Yeshi, K.; Wangchuk, P. Chapter 11—Essential oils and their bioactive molecules in healthcare. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Dayli, I.R.; Sukrisno Putri, C.P.Z.; Firli, L.N.; Widhi Pratama, A.N.; Triatmoko, B.; Untari, L.F.; Wongso, H.; Keller, P.A.; Wangchuk, P. Isolation of Antibacterial Depside Constituents from Indonesian Folious Lichen, Candelaria fibrosa. J. Biol. Act. Prod. Nat. 2022, 12, 24–32. [Google Scholar] [CrossRef]
- Maregesi, S.; Van Miert, S.; Pannecouque, C.; Feiz Haddad, M.H.; Hermans, N.; Wright, C.W.; Vlietinck, A.J.; Apers, S.; Pieters, L. Screening of Tanzanian Medicinal Plants against Plasmodium falciparum and Human Immunodeficiency Virus. Planta Med. 2010, 76, 195–201. [Google Scholar] [CrossRef]
- Nethengwe, M. Antiplasmodial/Antipyretic activity of some Zulu medicinal plants. J. Med. Plants Res. 2012, 6, 1255–1262. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Ali, A.; Sudi, S.; Sidek, H.; Embi, N.; Basir, R. The Antimalarial Effect of Curcumin Is Mediated by the Inhibition of Glycogen Synthase Kinase-3β. J. Med. Food 2016, 20, 152–161. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Rahuman, A.A.; Mohanakrishnan, D.; Bagavan, A.; Elango, G.; Zahir, A.A.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; et al. Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J. Ethnopharmacol. 2012, 141, 796–802. [Google Scholar] [CrossRef]
- Sathiyamoorthy, P.; Lugasi-Evgi, H.; Schlesinger, P.; Kedar, I.; Gopas, J.; Pollack, Y.; Golan-Goldhirsh, A. Screening for cytotoxic and antimalarial activities in desert plants of the negev and bedouin market plant products. Pharm. Biol. 1999, 37, 188–195. [Google Scholar] [CrossRef]
- Herlina, T.; Supratman, U.; Soedjanaatmadja, U.; Subarnas, A.; Sutardjo, S.; Abdullah, N.; Hayashi, H. Anti-malarial compound from the stem bark of Erythrina variegata. Indones. J. Chem. 2010, 9, 308–311. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, A.S.; Agustina, R.P.; Mirza, S.; Rani, D.M.; Winarto, N.B.; Triatmoko, B.; Pratama, A.N.W.; Keller, P.A.; Wangchuk, P. Phytochemistry and Pharmacology of Medicinal Plants Used by the Tenggerese Society in Java Island of Indonesia. Molecules 2022, 27, 7532. https://doi.org/10.3390/molecules27217532
Nugraha AS, Agustina RP, Mirza S, Rani DM, Winarto NB, Triatmoko B, Pratama ANW, Keller PA, Wangchuk P. Phytochemistry and Pharmacology of Medicinal Plants Used by the Tenggerese Society in Java Island of Indonesia. Molecules. 2022; 27(21):7532. https://doi.org/10.3390/molecules27217532
Chicago/Turabian StyleNugraha, Ari Satia, Riza Putri Agustina, Syafi Mirza, Dinar Mutia Rani, Naura Bathari Winarto, Bawon Triatmoko, Antonius Nugraha Widhi Pratama, Paul A. Keller, and Phurpa Wangchuk. 2022. "Phytochemistry and Pharmacology of Medicinal Plants Used by the Tenggerese Society in Java Island of Indonesia" Molecules 27, no. 21: 7532. https://doi.org/10.3390/molecules27217532
APA StyleNugraha, A. S., Agustina, R. P., Mirza, S., Rani, D. M., Winarto, N. B., Triatmoko, B., Pratama, A. N. W., Keller, P. A., & Wangchuk, P. (2022). Phytochemistry and Pharmacology of Medicinal Plants Used by the Tenggerese Society in Java Island of Indonesia. Molecules, 27(21), 7532. https://doi.org/10.3390/molecules27217532







