Methods for Elemental Analysis of Size-Resolved PM Samples Collected on Aluminium Foils: Results of an Intercomparison Exercise
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Checks
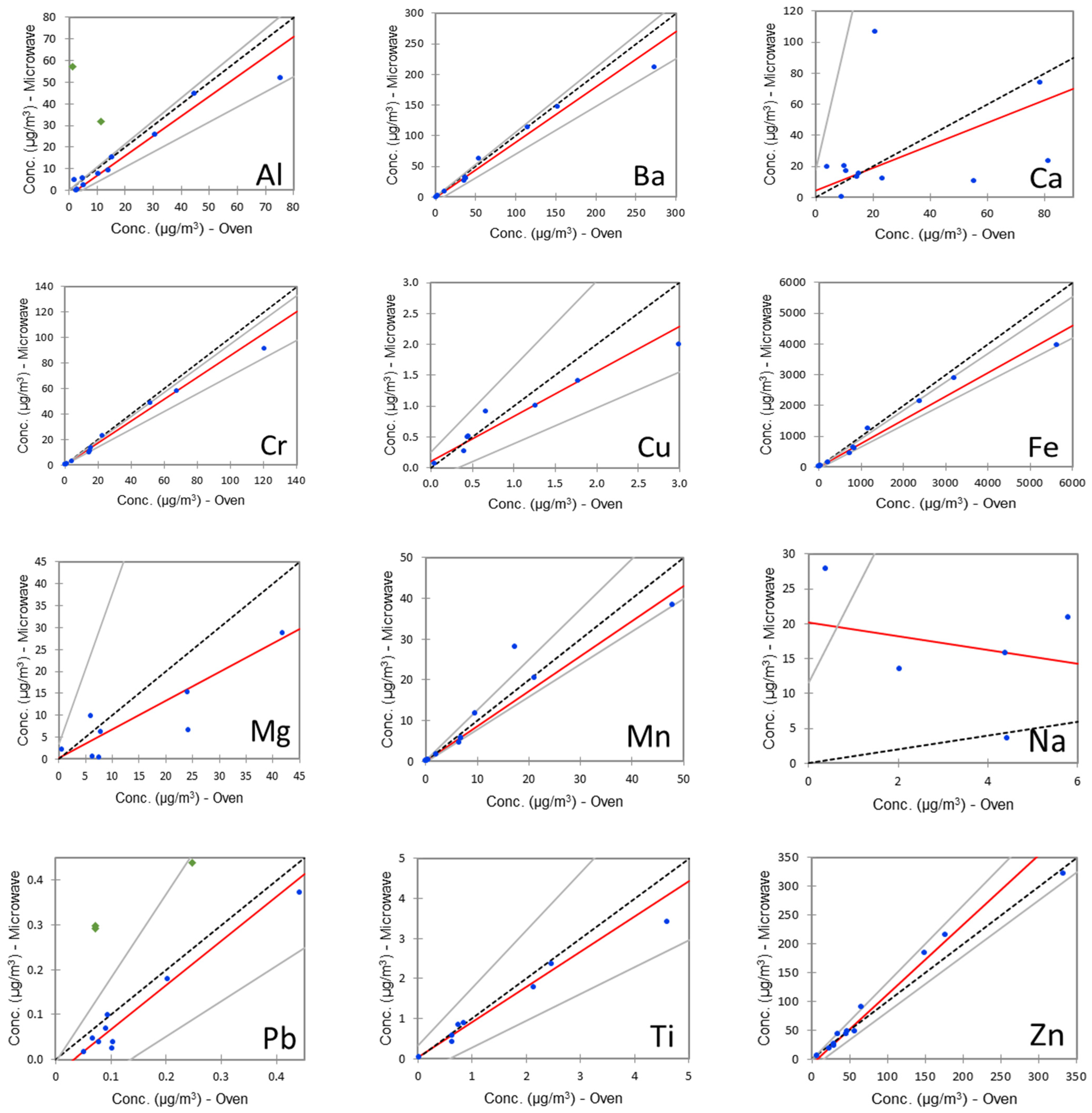
2.2. Evaluation of the Method Performances
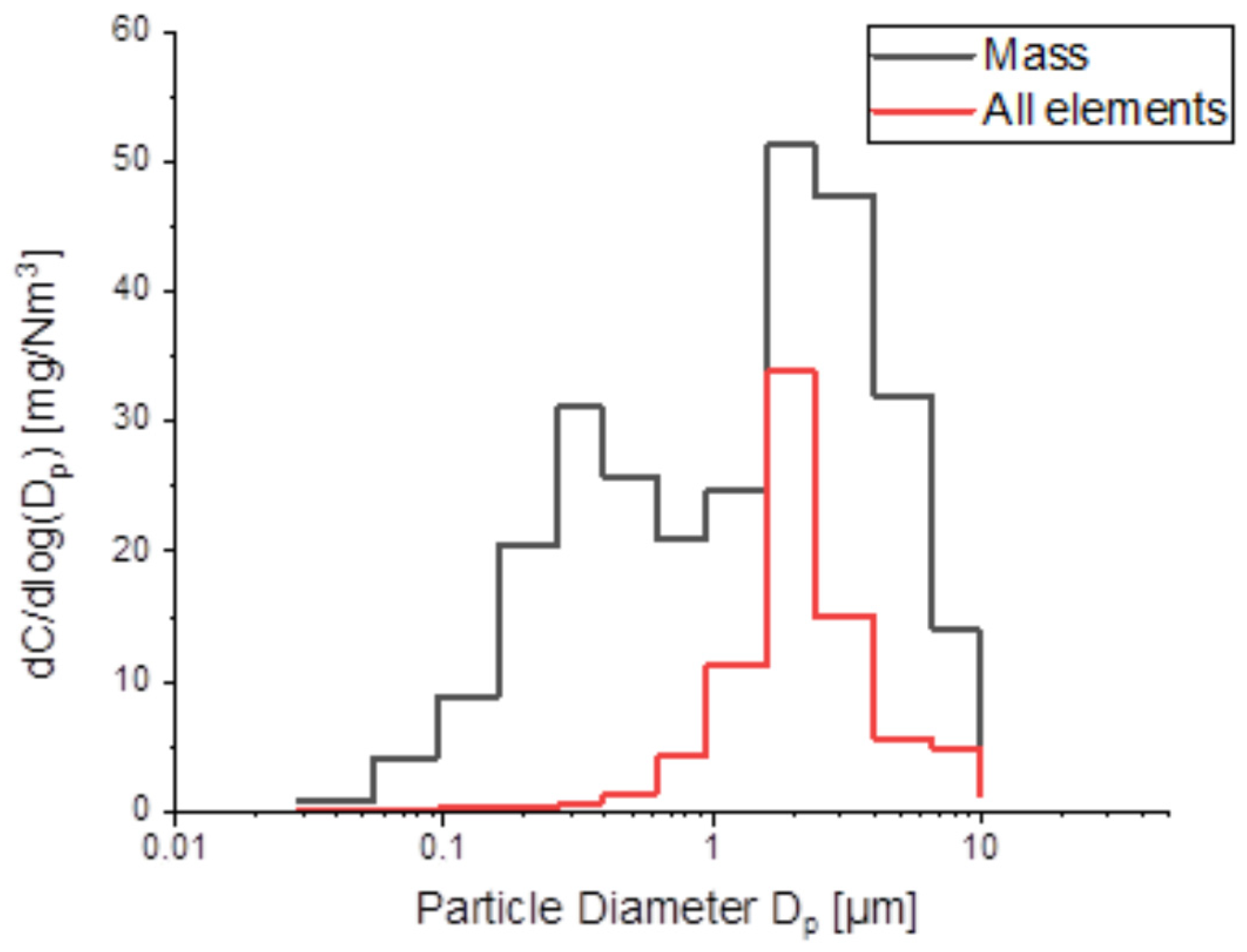
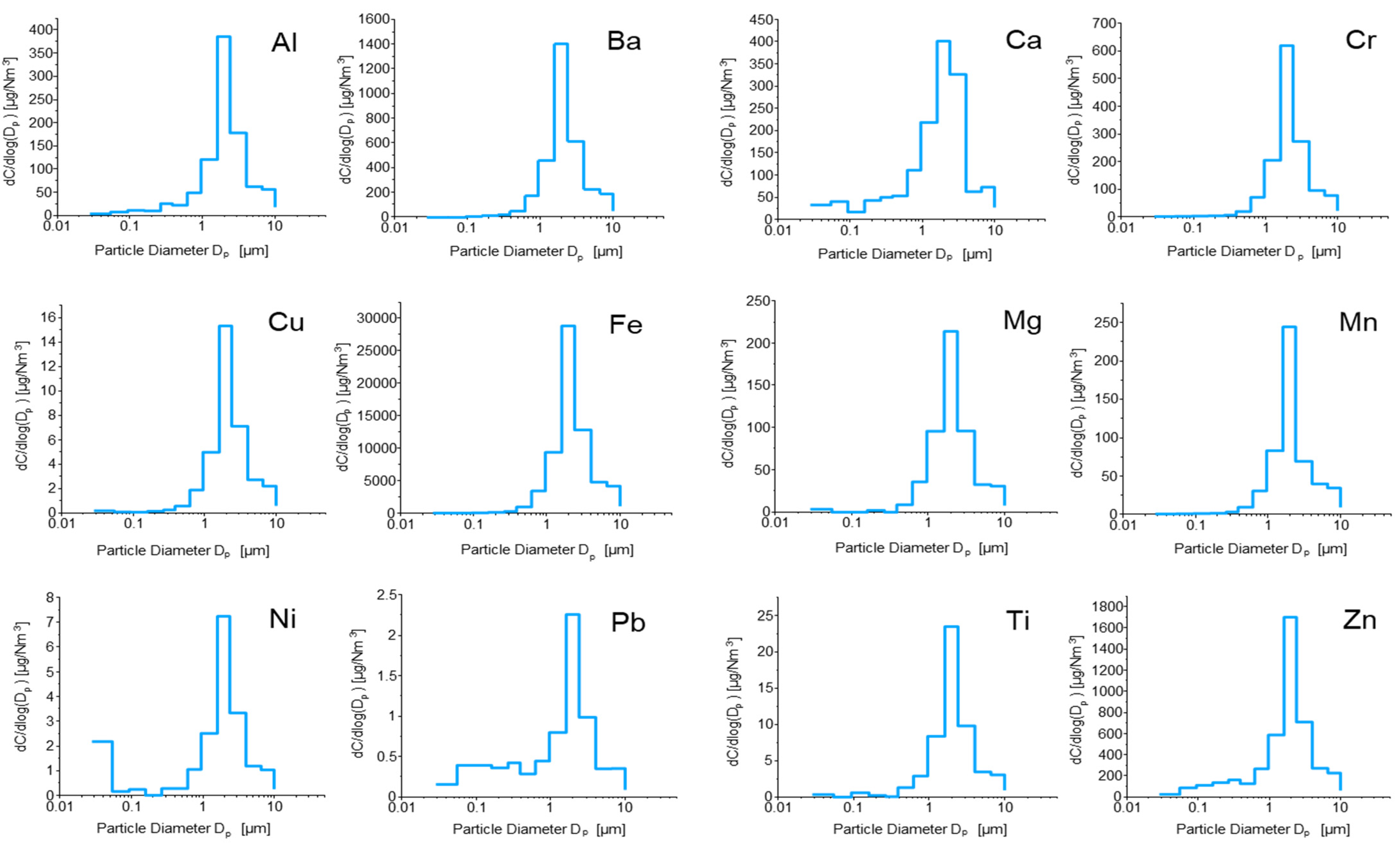
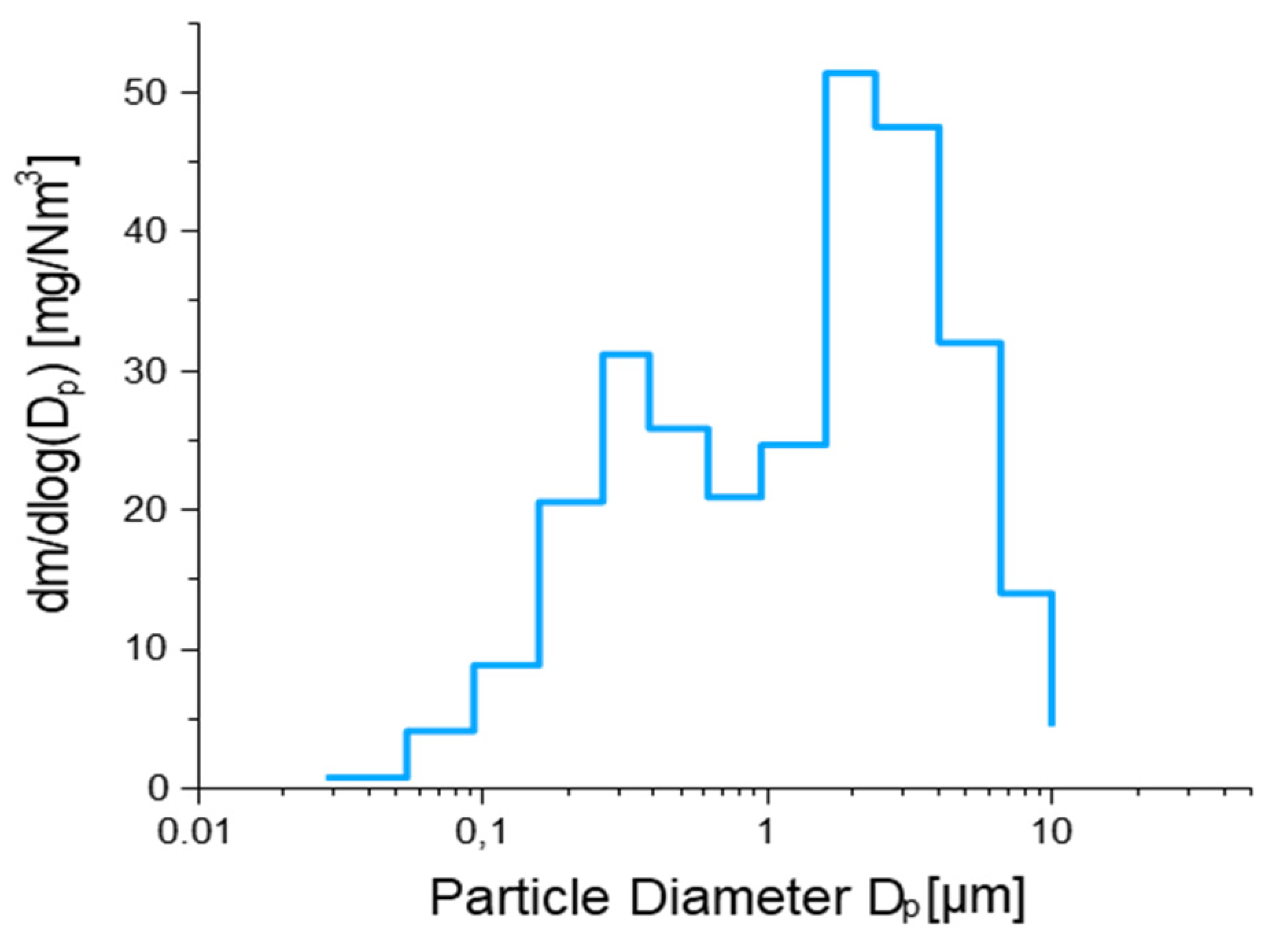
2.3. Sample Results and Data Analysis
3. Materials and Methods
3.1. Sample Collection
3.2. Apparatus and Reagents
3.3. Procedures
3.3.1. Oven Digestion
3.3.2. Microwave-Assisted Digestion
3.4. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fraction | d50 (µm) | Mass (mg/Nm3) |
|---|---|---|
| 1 | 0.0282 | 0.235 |
| 2 | 0.0549 | 1.076 |
| 3 | 0.0934 | 2.273 |
| 4 | 0.157 | 5.151 |
| 5 | 0.262 | 5.693 |
| 6 | 0.382 | 5.920 |
| 7 | 0.614 | 4.405 |
| 8 | 0.949 | 6.239 |
| 9 | 1.60 | 10.03 |
| 10 | 2.39 | 11.85 |
| 11 | 4.00 | 7.780 |
| 12 | 6.60 | 2.807 |
| 13 | 9.96 | 3.496 |
| Technique | Model | Features | Analytes | |
|---|---|---|---|---|
| Oven digestion | ICP-OES | Thermo Fisher Scientific iCAP 6500 Radial | MicroFlow PFA-ST nebuliser, cyclonic spray chamber, Échelle monochromator, CID detector for simultaneous analysis | Al, Ca, Fe, K, Mg, Na |
| ICP-MS | Thermo Fisher Scientific X-Series II | Meinhard nebuliser, Peltier-cooled conical spray chamber, quadrupole analyser, discrete dynode electron multiplier | Ba, Cd, Co, Cr, Cu, Mn, Ni, Pb, Ti, Zn | |
| Microwave digestion | ICP-OES | Perkin Elmer Optima 7000 DV | Mira Mist nebuliser, cyclonic spray chamber, dual Échelle monochromator, dual CCD detector | Al, Ba, Ca, Fe, K, Mg, Mn, Na, Ti, Zn |
| GF-AAS | Perkin Elmer 5100 | Zeeman-effect background correction, HGA 600 graphite furnace | Cd, Co, Cr, Cu, Ni, Pb |
| ICP-OES | ||||
|---|---|---|---|---|
| Analyte | Λ (nm) | Torch Position | DL (μg/L) | |
| Al | 167.0 | Radial | 30 | |
| Ca | 317.9 | Radial | 300 | |
| Fe | 259.9 | Radial | 14 | |
| K | 766.4 | Radial | 150 | |
| Mg | 279.5 | Radial | 42 | |
| Na | 589.5 | Radial | 170 | |
| Al | 396.1 | Axial | 2.7 | |
| Ba | 233.5 | Axial | 1.3 | |
| Ca | 317.9 | Radial | 7.6 | |
| Fe | 238.2 | Axial | 1.0 | |
| K | 766.5 | Radial | 1.8 | |
| Mg | 280.3 | Radial | 0.50 | |
| Mn | 257.6 | Axial | 0.79 | |
| Na | 589.6 | Radial | 30 | |
| Ti | 334.9 | Axial | 0.2 | |
| Zn | 206.2 | Axial | 3.3 | |
| ICP-MS | ||||
| Analyte | Isotope | DL (μg/L) | ||
| Ba | 138 | 0.13 | ||
| Cd | 111 | 0.054 | ||
| Co | 59 | 0.018 | ||
| Cr | 52 | 3.0 | ||
| Cu | 65 | 0.40 | ||
| Mn | 55 | 0.40 | ||
| Ni | 60 | 0.17 | ||
| Pb | 208 | 1.6 | ||
| Ti | 47 | 2.3 | ||
| Zn | 64 | 7.3 | ||
| GF-AAS | ||||
| Analyte | λ (nm) | T Roast. (°C) | T Atom. (°C) | DL (μg/L) |
| Cd | 228.8 | 500 | 1500 | 0.043 |
| Co | 240.7 | 1400 | 2400 | 0.30 |
| Cr | 357.9 | 1650 | 2500 | 0.33 |
| Cu | 324.8 | 1200 | 2000 | 0.91 |
| Ni | 232.0 | 1400 | 1800 | 1.8 |
| Pb | 283.3 | 850 | 1800 | 0.18 |
| Mann–Whitney p-Values | |||
|---|---|---|---|
| Analyte | Cotton Leaching | Al Wiping | Al Wiping |
| Oven | Oven | Microwave | |
| Al | 0.298 | 0.228 | 0.100 |
| Ba | 0.449 | 0.967 | 0.719 |
| Ca | 0.008 | 0.001 | 1.000 |
| Cr | 0.047 | 0.392 | - |
| Fe | 0.289 | 0.012 | 0.048 |
| K | 0.067 | 0.124 | - |
| Mg | 0.006 | 0.721 | 0.834 |
| Mn | 0.005 | 0.298 | 0.048 |
| Na | 0.011 | 0.197 | 0.912 |
| Ti | 0.178 | 0.222 | 0.807 |
| Zn | - | - | 0.408 |
| Analyte (conc) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
|---|---|---|---|---|---|---|---|---|
| Al | (µg/Nm3) | 1.28 ± 0.03 | 1.976 ± 0.003 | 2.829 ± 0.001 | 2.59 ± 0.05 | 4.69 ± 0.08 | 5.20 ± 0.08 | 10.45 ± 0.09 |
| Ba | (µg/Nm3) | 0.156 ± 0.002 | 0.431 ± 0.005 | 0.60 ± 0.01 | 1.239 ± 0.007 | 2.46 ± 0.04 | 10.66 ± 0.04 | 35.6 ± 0.1 |
| Ca | (µg/Nm3) | 10.0 ± 0.1 | 10.56 ± 0.04 | 4.01 ± 0.02 | 10.54 ± 0.05 | 9.07 ± 0.04 | 11.98 ± 0.02 | 23.2 ± 0.1 |
| Cd | (ng/Nm3) | <3.7 | <3.8 | <3.9 | <4.0 | <3.8 | <3.6 | <4.2 |
| Co | (ng/Nm3) | <SB | <SB | <SB | <SB | <SB | <SB | 39.4 ± 0.1 |
| Cr | (µg/Nm3) | 0.0611 ± 0.0009 | 0.138 ± 0.002 | 0.272 ± 0.005 | 0.445 ± 0.002 | 0.96 ± 0.01 | 4.24 ± 0.02 | 14.8 ± 0.1 |
| Cu | (ng/Nm3) | 53 ± 3 | 19.0 ± 0.9 | 12.2 ± 0.3 | 34.8 ± 0.5 | 43 ± 1 | 128 ± 9 | 396 ± 7 |
| Fe | (µg/Nm3) | 4.0 ± 0.1 | 9.40 ± 0.04 | 14.4 ± 0.1 | 26.4 ± 0.1 | 48.6 ± 0.4 | 221 ± 2 | 723 ± 7 |
| K | (µg/Nm3) | 8.8 ± 0.4 | <7.3 | <7.6 | <7.8 | <7.3 | <7.0 | <8.1 |
| Mg | (ng/Nm3) | 1100 ± 10 | <SB | <SB | 553.9 ± 0.4 | 18.63 ± 0.05 | 2070 ± 30 | 7560 ± 80 |
| Mn | (µg/Nm3) | 0.0224 ± 0.0006 | 0.081 ± 0.002 | 0.116 ± 0.004 | 0.240 ± 0.006 | 0.490 ± 0.006 | 2.021 ± 0.008 | 6.39 ± 0.02 |
| Na | (µg/Nm3) | 2.04 ± 0.01 | <SB | <SB | <SB | <SB | <SB | 4.4 ± 0.2 |
| Ni | (ng/Nm3) | 690 ± 10 | 41 ± 2 | 59 ± 4 | < 13 | 51 ± 2 | 62 ± 3 | 220 ± 10 |
| Pb | (ng/Nm3) | 50 ± 1 | 104 ± 3 | 102 ± 2 | 91.6 ± 0.6 | 78 ± 1 | 66.2 ± 0.1 | 94.9 ± 0.9 |
| Ti | (ng/Nm3) | 114 ± 2 | <SB | 155 ± 7 | 59 ± 3 | 15.7 ± 0.9 | 310 ± 10 | 620 ± 20 |
| Zn | (µg/Nm3) | 7.11 ± 0.05 | 22.82 ± 0.04 | 28.6 ± 0.4 | 34.08 ± 0.09 | 29.5 ± 0.3 | 29.08 ± 0.07 | 56.1 ± 0.4 |
| Analyte (conc) | F8 | F9 | F10 | F11 | F12 | F13 | ||
| Al | (µg/Nm3) | 30.7 ± 0.7 | 75.4 ± 0.8 | 44.5 ± 0.6 | 15.2 ± 0.3 | 11.34 ± 0.06 | 14.02 ± 0.07 | |
| Ba | (µg/Nm3) | 115 ± 1 | 274 ± 1 | 152 ± 1 | 53.5 ± 0.4 | 37.39 ± 0.08 | 37.2 ± 0.1 | |
| Ca | (µg/Nm3) | 55.2 ± 0.3 | 78.326 ± 0.001 | 81.4 ± 0.1 | 15.04 ± 0.03 | 14.48 ± 0.06 | 20.9 ± 0.3 | |
| Cd | (ng/Nm3) | <3.9 | <3.6 | <3.8 | <3.5 | <4.0 | <3.1 | |
| Co | (ng/Nm3) | 161 ± 4 | 384 ± 7 | 218 ± 9 | 73 ± 3 | 42 ± 4 | 45.3 ± 0.4 | |
| Cr | (µg/Nm3) | 51.5 ± 0.6 | 120.6 ± 0.7 | 67.8 ± 0.7 | 22.8 ± 0.2 | 15.4 ± 0.1 | 15.64 ± 0.08 | |
| Cu | (ng/Nm3) | 1260 ± 20 | 2990 ± 20 | 1770 ± 20 | 660 ± 30 | 440 ± 20 | 451 ± 4 | |
| Fe | (µg/Nm3) | 2375 ± 2 | 5630 ± 20 | 3200 ± 10 | 1169 ± 1 | 840 ± 9 | 823 ± 4 | |
| K | (µg/Nm3) | <7.6 | <7.1 | <7.4 | <6.9 | <7.8 | <6.0 | |
| Mg | (ng/Nm3) | 24270 ± 50 | 41800 ± 20 | 24000 ± 200 | 7897 ± 5 | 6234 ± 2 | 5990 ± 20 | |
| Mn | (µg/Nm3) | 21.0 ± 0.2 | 47.9 ± 0.3 | 17.2 ± 0.3 | 9.6 ± 0.2 | 6.86 ± 0.05 | 6.84 ± 0.06 | |
| Na | (µg/Nm3) | 9.6 ± 0.2 | 5.79 ± 0.02 | 4.42 ± 0.03 | <SB | <SB | 0.383 ± 0.001 | |
| Ni | (ng/Nm3) | 640 ± 10 | 1410 ± 50 | 830 ± 20 | 287 ± 5 | 207 ± 2 | 190 ± 20 | |
| Pb | (ng/Mm3) | 203 ± 3 | 441 ± 4 | 247 ± 2 | 86.7 ± 0.3 | 72 ± 1 | 71.8 ± 0.4 | |
| Ti | (ng/Nm3) | 2130 ± 80 | 4600 ± 30 | 2460 ± 30 | 850 ± 30 | 620 ± 20 | 750 ± 10 | |
| Zn | (µg/Nm3) | 148.9 ± 0.8 | 332.5 ± 0.3 | 177 ± 2 | 65.5 ± 0.6 | 45.4 ± 0.2 | 46.6 ± 0.2 | |
References
- Mukhtar, A.; Limbeck, A. Recent developments in assessment of bio-accessible trace metal fractions in airborne particulate matter: A review. Anal. Chim. Acta 2013, 774, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Pelfrene, A.; Cave, M.R.; Wragg, J.; Douay, F. In Vitro Investigations of Human Bioaccessibility from Reference Materials Using Simulated Lung Fluids. Int. J. Environ. Res. Public Health 2017, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Che, H.; Zhang, X.; Ma, Y.; Wang, Y.; Wang, H.; Wang, Y. Characteristics of visibility and particulate matter (PM) in an urban area of Northeast China. Atmos. Pollut. Res. 2013, 4, 427–434. [Google Scholar] [CrossRef]
- Zou, J.; Liu, Z.; Hu, B.; Huang, X.; Wen, T.; Ji, D.; Liu, J.; Yang, Y.; Yao, Q.; Wang, Y. Aerosol chemical compositions in the North China Plain and the impact on the visibility in Beijing and Tianjin. Atmos. Res. 2018, 201, 235–246. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chapter 14: Global Tropospheric Chemistry and Climate Change. In Climate Change, Chemistry of the Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 1999; pp. 762–843. [Google Scholar]
- Giardi, F.; Becagli, S.; Traversi, R.; Frosini, D.; Severi, M.; Caiazzo, L.; Ancillotti, C.; Cappelletti, D.; Moroni, B.; Grotti, M.; et al. Size distribution and ion composition of aerosol collected at Ny-Ålesund in the spring–summer field campaign 2013. Rend. Lincei-Sci. Fis. 2016, 27, 47–58. [Google Scholar] [CrossRef]
- Asgharian, B.; Hofmann, W.; Bergmann, R. Particle Deposition in a Multiple-Path Model of the Human Lung. Aerosol Sci. Technol. 2001, 34, 332–339. [Google Scholar] [CrossRef]
- Sullivan, R.C.; Prather, K.A. Recent Advances in Our Understanding of Atmospheric Chemistry and Climate Made Possible by On-Line Aerosol Analysis Instrumentation. Anal. Chem. 2005, 77, 3861–3886. [Google Scholar] [CrossRef]
- Romanazzi, V.; Casazza, M.; Malandrino, M.; Maurino, V.; Piano, A.; Schilirò, T.; Gilli, G. PM10 size distribution of metals and environmental-sanitary risk analysis in the city of Torino. Chemosphere 2014, 112, 210–216. [Google Scholar] [CrossRef]
- Elmes, M.; Gasparon, M. Sampling and single particle analysis for the chemical characterisation of fine atmospheric particulates: A review. J. Environ. Manag. 2017, 202, 137–150. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chapter 11: Analytical Methods and Typical Atmospheric Concentrations for Gases and Particles. In Climate change, Chemistry of the Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 1999; pp. 547–656. [Google Scholar]
- Dekati Ltd. Substrates and Filters for Dekati® Impactors (Vers 6.3), Dekati® Accessory; Dekati Ltd.: Kangasala, Finland, 2016. [Google Scholar]
- Kleeman, M.J.; Schauer, J.J.; Cass, G.R. Size and Composition Distribution of Fine Particulate Matter Emitted from Wood Burning, Meat Charbroiling, and Cigarettes. Environ. Sci. Technol. 1999, 33, 3516–3523. [Google Scholar] [CrossRef]
- Noel, A.; L’Esperance, G.; Cloutier, Y.; Plamondon, P.; Boucher, J.; Philippe, S.; Dion, C.; Truchon, G.; Zayed, J. Assessment of the contribution of electron microscopy to nanoparticle characterization sampled with two cascade impactors. J. Occup. Environ. Hyg. 2013, 10, 155–172. [Google Scholar] [CrossRef]
- Pakkanen, T.A.; Kerminen, V.M.; Loukkola, K.; Hillamo, R.E.; Aarnio, P.; Koskentalo, T.; Maenhaut, W. Size distributions of mass and chemical components in street-level and rooftop PM1 particles in Helsinki. Atmos. Environ. 2003, 37, 1673–1690. [Google Scholar] [CrossRef]
- Scheinhardt, S.; Spindler, G.; Leise, S.; Müller, K.; Iinuma, Y.; Zimmermann, F.; Matschullat, J.; Herrmann, H. Comprehensive chemical characterisation of size-segregated PM10 in Dresden and estimation of changes due to global warming. Atmos. Environ. 2013, 75, 365–373. [Google Scholar] [CrossRef]
- Carabali, G.; Castro, T.; De La Cruz, W.; Peralta, O.; Varela, A.; Amelines, O.; Rivera, M.; Ruiz-Suarez, G.; Torres-Jardón, R.; Martines-Quiroz, E.; et al. Morphological and chemical characterization of soot emitted during flaming combustion stage of native-wood species used for cooking process in western Mexico. J. Aerosol Sci. 2016, 95, 1–14. [Google Scholar] [CrossRef]
- Gligorovski, S.; Van Elteren, J.T.; Grgic, I. A multi-element mapping approach for size-segregated atmospheric particles using laser ablation ICP-MS combined with image analysis. Sci. Total Environ. 2008, 407, 594–602. [Google Scholar] [CrossRef]
- Xue, J.; Li, Y.; Xie, X.; Xiong, C.; Liu, H.; Chen, S.; Nie, Z.; Chen, C.; Zhao, J. Characterization of organic aerosol in Beijing by laser desorption ionization coupled with Fourier Transform Ion Cyclotron Resonance Mass spectrometry. Atmos. Environ. 2017, 159, 55–65. [Google Scholar] [CrossRef]
- Gälli Purghart, B.C.; Nyffeler, U.P.; Schindler, P.W. Metals in airborne particulate matter in rural Switzerland. Atmos. Environ. 1990, 24A, 2191–2206. [Google Scholar] [CrossRef]
- Hsieh, Y.K.; Chen, L.K.; Hsieh, H.F.; Huang, C.H.; Wang, C.F. Elemental analysis of airborne particulate matter using an electrical low-pressure impactor and laser ablation/inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2011, 26, 1502–1508. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; Zhen, N.; He, K.; Hao, J. Chemical characteristics and source of size-fractionated atmospheric particle in haze episode in Beijing. Atmos. Res. 2016, 167, 24–33. [Google Scholar] [CrossRef]
- van Gulijk, C.; Marijnissen, J.C.M.; Makkee, M.; Moulijn, J.A. Oil-soaked sintered impactors for the ELPI in diesel particulate measurements. J. Aerosol Sci. 2003, 34, 635–640. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Rodriguez, S.; Plana, F.; Mantilla, E.; Ruiz, C.R. Monitoring of PM10 and PM2.5 around primary par-ticulate anthropogenic emission sources. Atmos. Environ. 2001, 35, 845–858. [Google Scholar] [CrossRef]
- Vicente, E.D.; Duarte, M.A.; Tarelho, L.A.C.; Nunes, T.F.; Amato, F.; Querol, X.; Colombi, C.; Gianelle, V.; Alves, C.A. Particulate and gaseous emissions from the combustion of different biofuels in a pellet stove. Atmos. Environ. 2015, 120, 15–27. [Google Scholar] [CrossRef]
- Minguillón, M.C.; Campos, A.A.; Cárdenas, B.; Blanco, S.; Molina, L.T.; Querol, X. Mass concentration, composition and sources of fine and coarse particulate matter in Tijuana, Mexico, during Cal-Mex campaign. Atmos. Environ. 2014, 88, 320–329. [Google Scholar] [CrossRef]
- Pay, M.T.; Jiménez-Guerrero, P.; Jorba, O.; Basart, S.; Querol, X.; Pandolfi, M.; Baldasano, J.M. Spatio-temporal variability of concentrations and speciation of particulate matter across Spain in the CALIOPE modeling system. Atmos. Environ. 2012, 46, 376–396. [Google Scholar] [CrossRef]
- Aldabe, J.; Santamaría, C.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Application of microwave digestion and ICP-MS to simultaneous analysis of major and trace elements in aerosol samples collected on quartz filters. Anal. Methods 2013, 5, 554–559. [Google Scholar] [CrossRef]
- Wilson, M.A.; Burt, R.; Lee, C.W. Improved Elemental Recoveries in Soils with Heating Boric Acid Following Microwave Total Digestion. Commun. Soil Sci. Plan. 2006, 37, 513–524. [Google Scholar] [CrossRef]
- Harrison, R.M.; Allan, J.; Carruthers, D.; Heal, M.R.; Lewis, A.C.; Marner, B.; Murrells, T.; Williams, A. Non-exhaust vehicle emissions of particulate matter and VOC from road traffic: A review. Atmos. Environ. 2021, 262, 118592. [Google Scholar] [CrossRef]
- Sanders, P.G.; Xu, N.; Dalka, T.M.; Maricq, M.M. Airborne brake wear debris: Size distributions, composition, and a comparison of dynamometer and vehicle tests. Environ. Sci. Technol. 2003, 37, 4060–4069. [Google Scholar] [CrossRef]
- Garg, B.D.; Cadle, S.H.; Mulawa, P.A.; Groblicki, P.J.; Laroo, C.; Parr, G.A. Brake wear particulate matter emissions. Environ. Sci. Technol. 2000, 34, 4463–4469. [Google Scholar] [CrossRef]
- Wahlstrom, J.; Söderberg, A.; Olander, L.; Jansson, A.; Olofsson, U. A pin-on-disc simulation of airborne wear particles from disc brakes. Wear 2010, 268, 763–769. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Development of copper-free eco-friendly brake-friction material using novel ingredients. Wear 2016, 352–353, 79–91. [Google Scholar] [CrossRef]
- J2522_201409; Dynamometer Global Brake Effectiveness, Surface Vehicle Recommended Practice. SAE International: Warrendale, PA, USA, 2014; pp. 1–20.
- Malandrino, M.; Casazza, M.; Abollino, O.; Minero, C.; Maurino, V. Size resolved metal distribution in the PM matter of the city of Turin (Italy). Chemosphere 2016, 147, 477–489. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Sheppard, P.R.; Helsel, D.R.; Speakman, R.J.; Ridenour, G.; Witten, M.L. Additional analysis of dendrochemical data of Fallon, Nevada. Chem-Biol. Interact. 2012, 196, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part 1. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar] [CrossRef]
- Malegori, C.; Nascimento Marques, E.J.; de Freitas, S.T.; Pimentel, M.F.; Pasquini, C.; Casiraghi, E. Comparing the analytical performances of Micro-NIR and FT-NIR spectrometers in the evaluation of acerola fruit quality, using PLS and SVM regression algorithms. Talanta 2017, 165, 112–116. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Comparing the analytical performance of near and mid infrared spectrometers for evaluating pomegranate juice quality. LWT 2018, 91, 180–190. [Google Scholar] [CrossRef]
| Relative Percentage Errors (%) | |||||
|---|---|---|---|---|---|
| Analyte | Mixture A | Mixture B | Mixture C | Mixture D | Mixture E |
| Ba | −12 | −3 | −46 | −1 | −28 |
| Ca | −3 | 9 | −70 | 9 | −61 |
| Cd | −14 | −19 | −22 | −21 | −18 |
| Co | −53 | 1 | −8 | −8 | −14 |
| Cr | −63 | −53 | −62 | −70 | −72 |
| Cu | −7 | 3 | −4 | −3 | 3 |
| Fe | −19 | 4 | −29 | 1 | 1 |
| K | −19 | −1 | −6 | −4 | 3 |
| Mg | −43 | −14 | −88 | −6 | −80 |
| Mn | −23 | −12 | −23 | −9 | 13 |
| Na | −11 | −10 | −16 | −5 | −13 |
| Ni | −37 | −9 | −5 | −12 | −6 |
| Pb | −2 | 2 | −5 | −3 | 2 |
| Ti | −67 | −4 | −5 | −4 | 5 |
| Sample Blank Concentration (μg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Analyte | Aluminium Foil | Cotton | Leached Cotton | Leached Cotton on Al | Aluminium Foil | Cotton | Cotton on Al |
| Oven | Oven | Oven | Oven | Microwave | Microwave | Microwave | |
| Al | 5 × 105 ± 1 × 105 | 34 ± 1 | 34 ± 9 | 40 ± 10 | 6 × 104 ± 1 × 104 | 40 ± 10 | 60 ± 10 |
| Ba | <SB | 0.52 ± 0.02 | 0.52 ± 0.05 | 0.46 ± 0.06 | n.a. | 3.1 ± 0.8 | 3.1 ± 0.5 |
| Ca | <SB | 310 ± 20 | 160 ± 30 | 300 ± 50 | <7.6 | 560 ± 70 | 360 ± 70 |
| Cd | <0.054 | <0.054 | <0.054 | <0.054 | <0.043 | <0.043 | <0.043 |
| Co | 2.0 ± 0.4 | <0.018 | <0.018 | <0.018 | 0.7 ± 0.2 | <0.30 | <0.30 |
| Cr | 6.4 ± 0.7 | 0.92 ± 0.09 | 0.5 ± 0.2 | 0.5 ± 0.2 | 3 ± 2 | <0.33 | <0.33 |
| Cu | 120 ± 10 | <SB | <SB | 0.09 ± 0.02 | 24 ± 3 | <0.91 | <0.91 |
| Fe | 3400 ± 300 | 14 ± 2 | 13 ± 4 | 20 ± 3 | 3000 ± 700 | 31 ± 8 | 53 ± 9 |
| K | 20 ± 10 | 38 ± 4 | 20 ± 5 | 26 ± 4 | 27 ± 8 | 44 ± 9 | 110 ± 10 |
| Mg | <SB | 80 ± 20 | 46 ± 7 | 44 ± 8 | <0.50 | 160 ± 20 | 140 ± 40 |
| Mn | 110 ± 10 | 1.07 ± 0.03 | 0.8 ± 0.2 | 0.9 ± 0.1 | 560 ± 70 | 0.8 ± 0.5 | 1.59 ± 0.08 |
| Na | 30 ± 20 | 290 ± 20 | 21 ± 5 | 30 ± 10 | <30 | 370 ± 20 | 330 ± 60 |
| Ni | 22 ± 2 | 0.5 ± 0.1 | <0.17 | <0.17 | 13 ± 2 | <1.8 | <1.8 |
| Pb | 6 ± 4 | 0.17 ± 0.04 | <SB | 0.02 ± 0.01 | 4 ± 1 | <0.18 | <0.18 |
| Ti | 90 ± 20 | 0.9 ± 0.3 | 0.6 ± 0.4 | 0.8 ± 0.3 | 113 ± 7 | 3 ± 1 | 2.3 ± 0.7 |
| Zn | 120 ± 50 | 1.3 ± 0.7 | <SB | 2.1 ± 0.6 | 70 ± 10 | 4 ± 2 | 4 ± 2 |
| Relative Percentage Errors (%) | ||||
|---|---|---|---|---|
| Analyte | Aluminium Foil | Aluminium Foil | Leached Cotton | Cotton |
| Oven | Microwave | Oven | Microwave | |
| Al | −600 | 92 | 2 | −9 |
| Ba | −27 | n.a. | −10 | n.a. |
| Ca | −37 | −78 | 6 | −1 |
| Cd | −1 | −5 | −22 | −1 |
| Co | −11 | −32 | −8 | −31 |
| Cr | −50 | −62 | −44 | −38 |
| Cu | −13 | −14 | −12 | −12 |
| Fe | −15 | −26 | −8 | −9 |
| K | −11 | −29 | 3 | −1 |
| Mg | −12 | −91 | −2 | −11 |
| Mn | −25 | −18 | −3 | −9 |
| Na | −4 | −30 | 5 | −6 |
| Ni | −8 | 9 | −3 | −2 |
| Pb | −10 | −20 | 4 | −10 |
| Ti | −15 | −10 | −47 | −10 |
| Zn | −31 | −23 | −3 | −15 |
| Analyte | Value | Lower Bound | Upper Bound | |
|---|---|---|---|---|
| Al | Intercept | −2.070 | −2.971 | 0.309 |
| Slope | 0.913 | 0.696 | 1.067 | |
| Ba | Intercept | −0.324 | −7.572 | 1.012 |
| Slope | 0.902 | 0.777 | 1.055 | |
| Ca | Intercept | 4.363 | −101.804 | 18.038 |
| Slope | 0.732 | −0.081 | 7.941 | |
| Cr | Intercept | 0.104 | −0.077 | 0.258 |
| Slope | 0.859 | 0.699 | 0.949 | |
| Cu | Intercept | 0.103 | −0.196 | 0.255 |
| Slope | 0.729 | 0.583 | 1.385 | |
| Fe | Intercept | 3.438 | −42.951 | 13.079 |
| Slope | 0.766 | 0.705 | 0.921 | |
| Mg | Intercept | 0.305 | −23.327 | 3.420 |
| Slope | 0.653 | 0.187 | 3.440 | |
| Mn | Intercept | 0.055 | −0.107 | 0.180 |
| Slope | 0.861 | 0.799 | 1.238 | |
| Na | Intercept | 20.223 | −39.626 | 11.560 |
| Slope | −1.000 | 0.971 | 12.617 | |
| Pb | Intercept | −0.033 | −0.107 | −0.006 |
| Slope | 0.993 | 0.792 | 1.878 | |
| Ti | Intercept | 0.032 | −0.398 | 0.327 |
| Slope | 0.880 | 0.674 | 1.438 | |
| Zn | Intercept | −8.514 | −15.368 | −1.238 |
| Slope | 1.210 | 0.972 | 1.344 |
| Analyte | Cleaned Blank Supports (µg/kg) | Cleaned Sample Supports (µg/kg) | Mann-Whitney p-Values |
|---|---|---|---|
| Al | 5000 ± 2000 | 6000 ± 3000 | 0.278 |
| Ba | 0.4 ± 0.1 | 4 ± 4 | 0.005 |
| Ca | 130 ± 10 | 130 ± 20 | 0.558 |
| Cd | <0.054 | <0.054 | - |
| Co | <0.018 | <0.018 | - |
| Cr | 0.7 ± 0.3 | 1.3 ± 0.7 | 0.127 |
| Cu | 0.7 ± 0.4 | <SB | - |
| Fe | 11 ± 2 | 130 ± 80 | 0.018 |
| K | 4 ± 3 | <SB | - |
| Mg | 50 ± 9 | 40 ± 6 | 0.980 |
| Mn | 1.5 ± 0.6 | 3 ± 1 | 0.070 |
| Na | 60 ± 40 | 30 ± 20 | 0.821 |
| Ni | <0.17 | <0.17 | - |
| Pb | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.278 |
| Ti | 0.3 ± 0.1 | 1.3 ± 0.4 | 0.022 |
| Zn | 2 ± 2 | 5 ± 2 | 0.232 |
| Mixture | Acids | H3BO3 |
|---|---|---|
| A | 4 mL HNO3 + 1 mL H2O2 | No |
| B | 4 mL HNO3 + 2 mL HF | 0.7 g |
| C | 4 mL HNO3 + 2 mL HF | No |
| D | 4 mL HNO3 + 1 mL HF + 1 mL HCl | 0.7 g |
| E | 4 mL HNO3 + 1 mL HF + 1 mL HCl | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conca, E.; Malandrino, M.; Diana, A.; Abollino, O.; Giacomino, A.; Bartrolí, R.; Moreno, T.; Querol, X.; Amato, F. Methods for Elemental Analysis of Size-Resolved PM Samples Collected on Aluminium Foils: Results of an Intercomparison Exercise. Molecules 2022, 27, 7442. https://doi.org/10.3390/molecules27217442
Conca E, Malandrino M, Diana A, Abollino O, Giacomino A, Bartrolí R, Moreno T, Querol X, Amato F. Methods for Elemental Analysis of Size-Resolved PM Samples Collected on Aluminium Foils: Results of an Intercomparison Exercise. Molecules. 2022; 27(21):7442. https://doi.org/10.3390/molecules27217442
Chicago/Turabian StyleConca, Eleonora, Mery Malandrino, Aleandro Diana, Ornella Abollino, Agnese Giacomino, Rafael Bartrolí, Teresa Moreno, Xavier Querol, and Fulvio Amato. 2022. "Methods for Elemental Analysis of Size-Resolved PM Samples Collected on Aluminium Foils: Results of an Intercomparison Exercise" Molecules 27, no. 21: 7442. https://doi.org/10.3390/molecules27217442
APA StyleConca, E., Malandrino, M., Diana, A., Abollino, O., Giacomino, A., Bartrolí, R., Moreno, T., Querol, X., & Amato, F. (2022). Methods for Elemental Analysis of Size-Resolved PM Samples Collected on Aluminium Foils: Results of an Intercomparison Exercise. Molecules, 27(21), 7442. https://doi.org/10.3390/molecules27217442










