The Design of Rapid Self-Healing Alginate Hydrogel with Dendritic Crosslinking Network
Abstract
1. Introduction
2. Results and Discussion
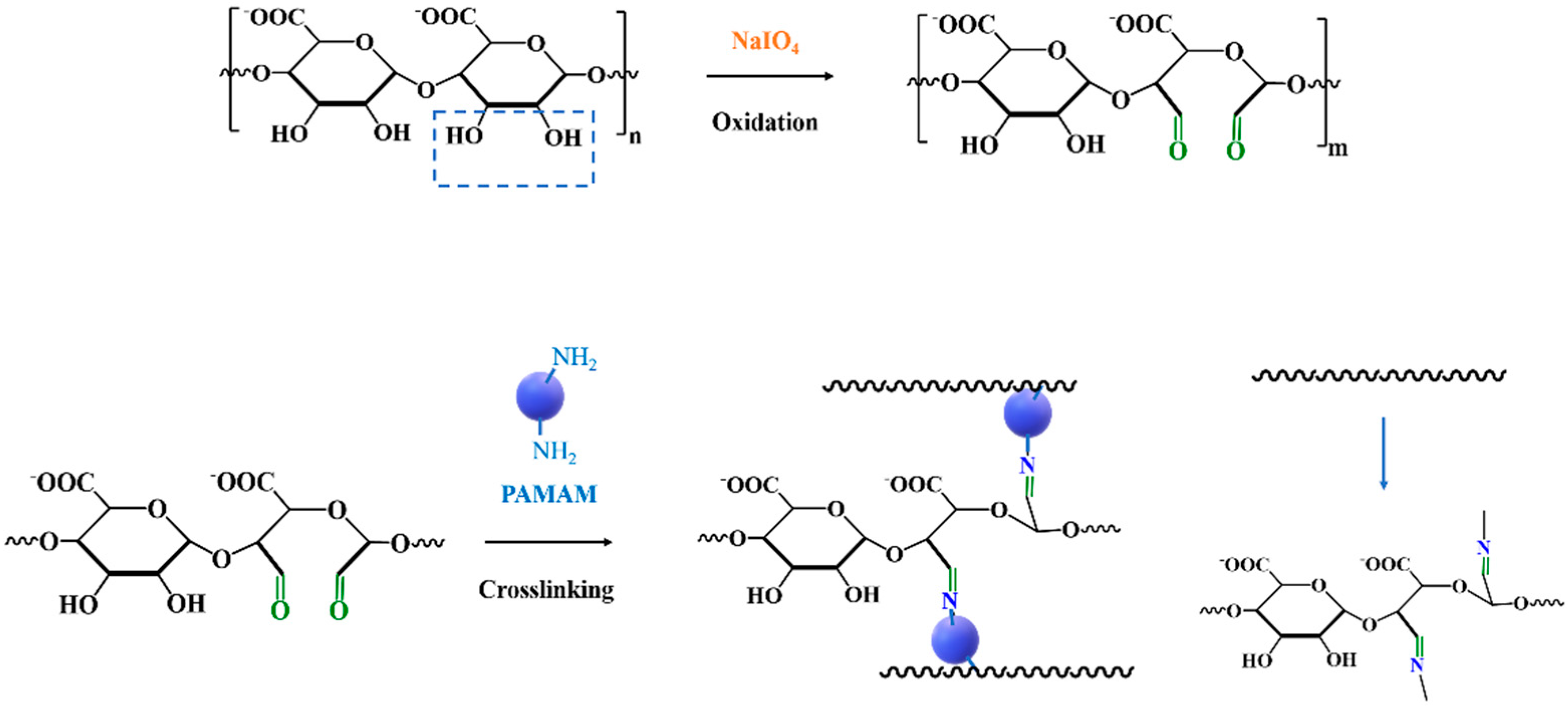
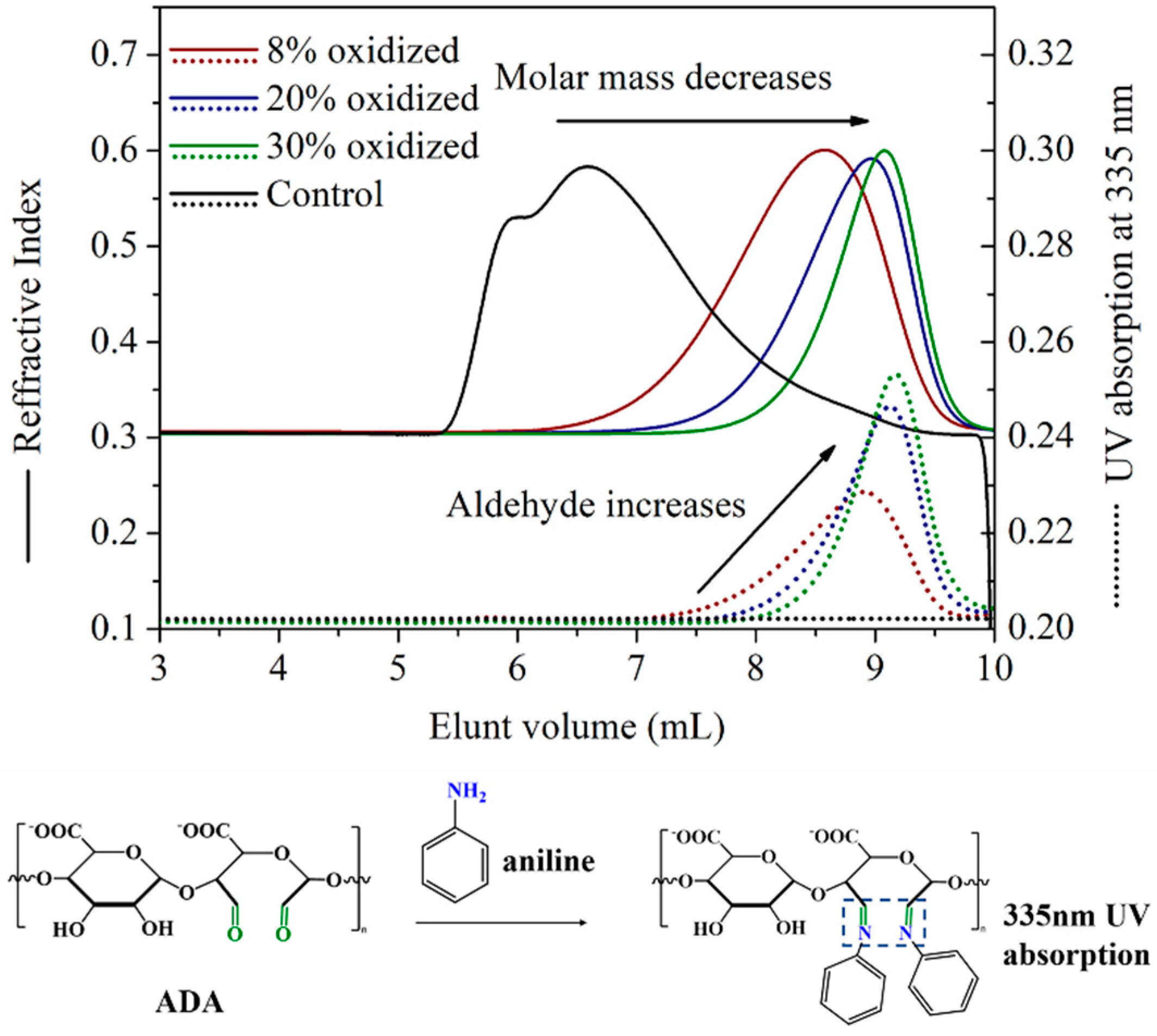
2.1. Preparation of Alginate Dialdehyde with Different Oxidation Degree
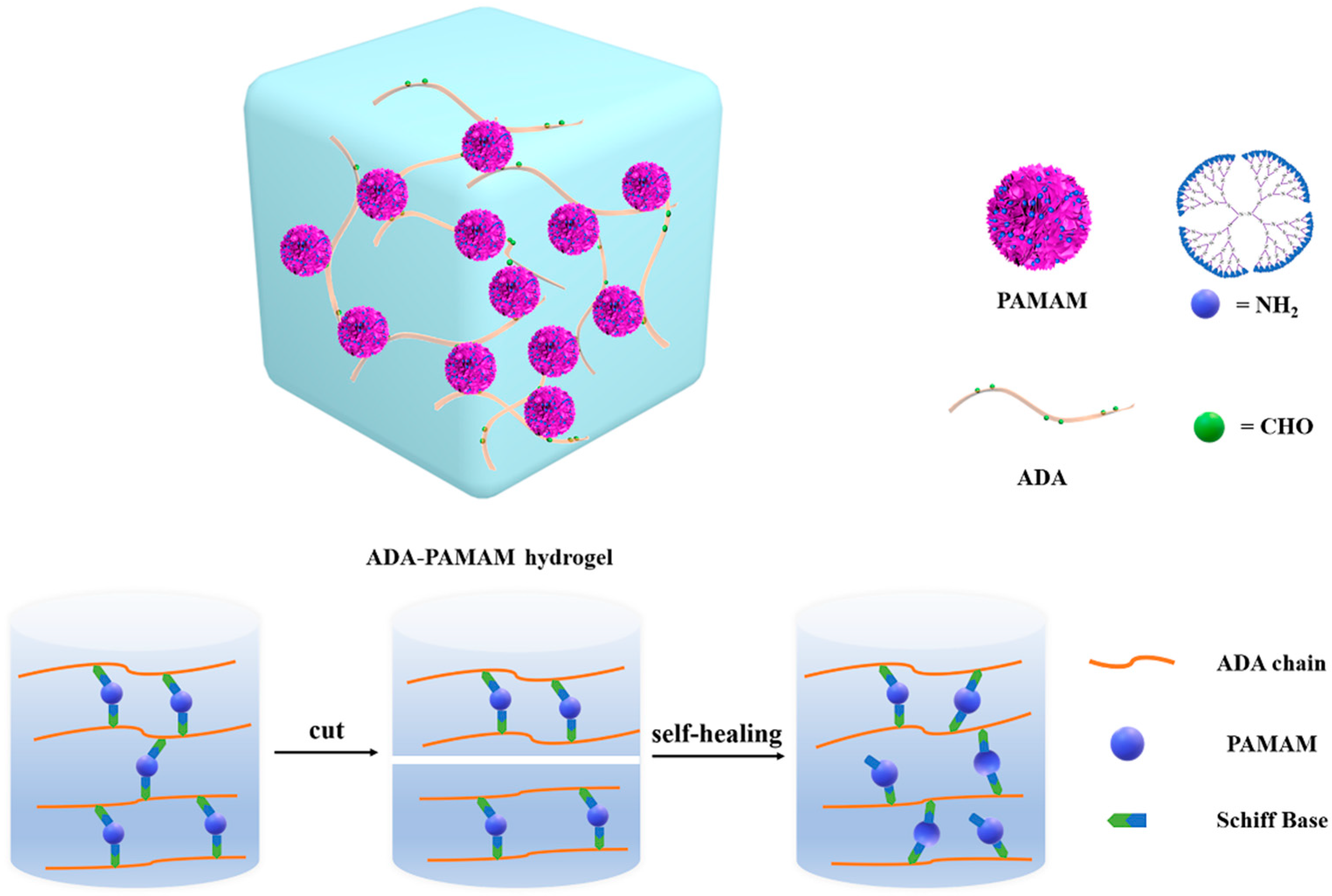
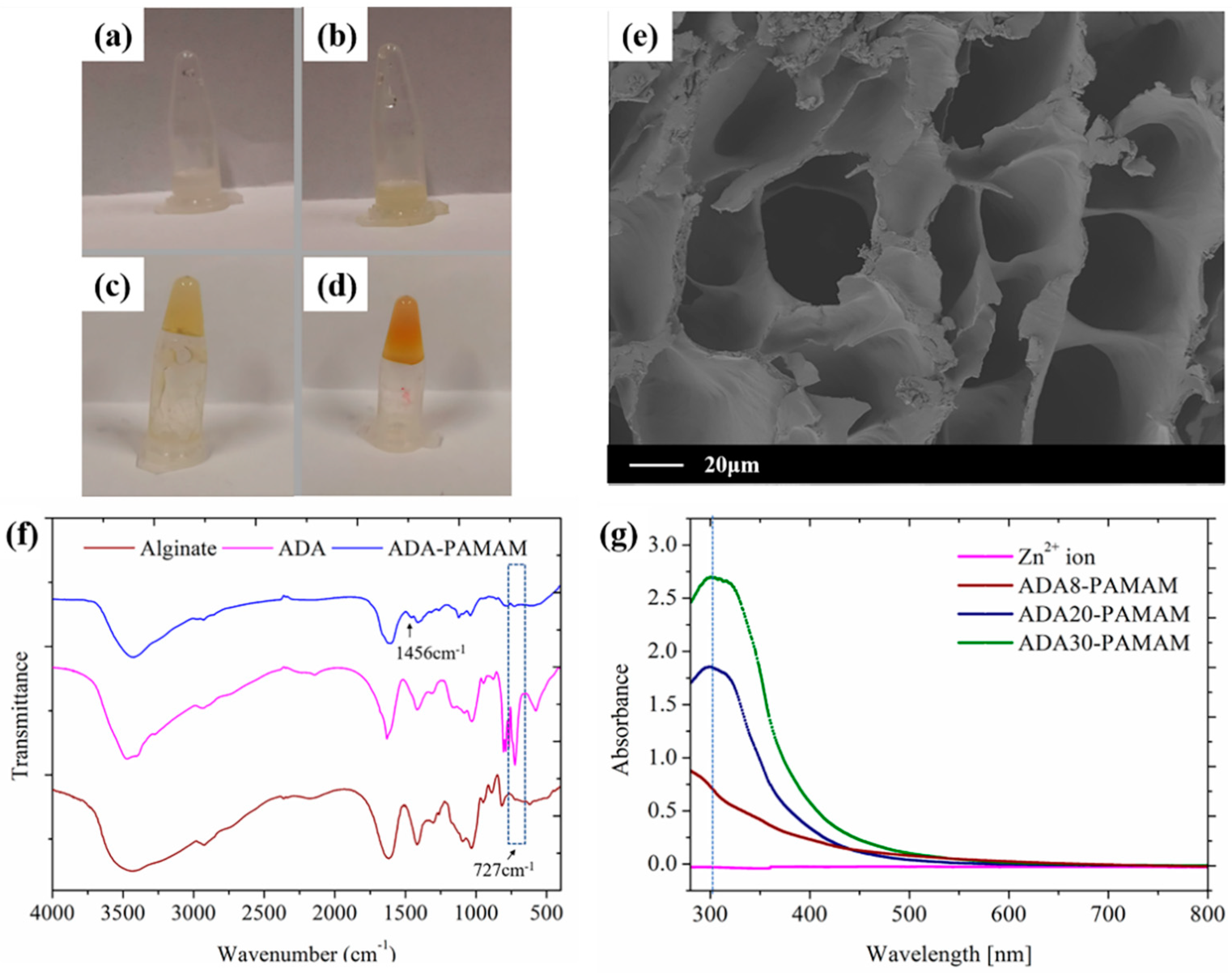
2.2. Synthesis and Characterization of ADA-PAMAM through Schiff Base Reaction
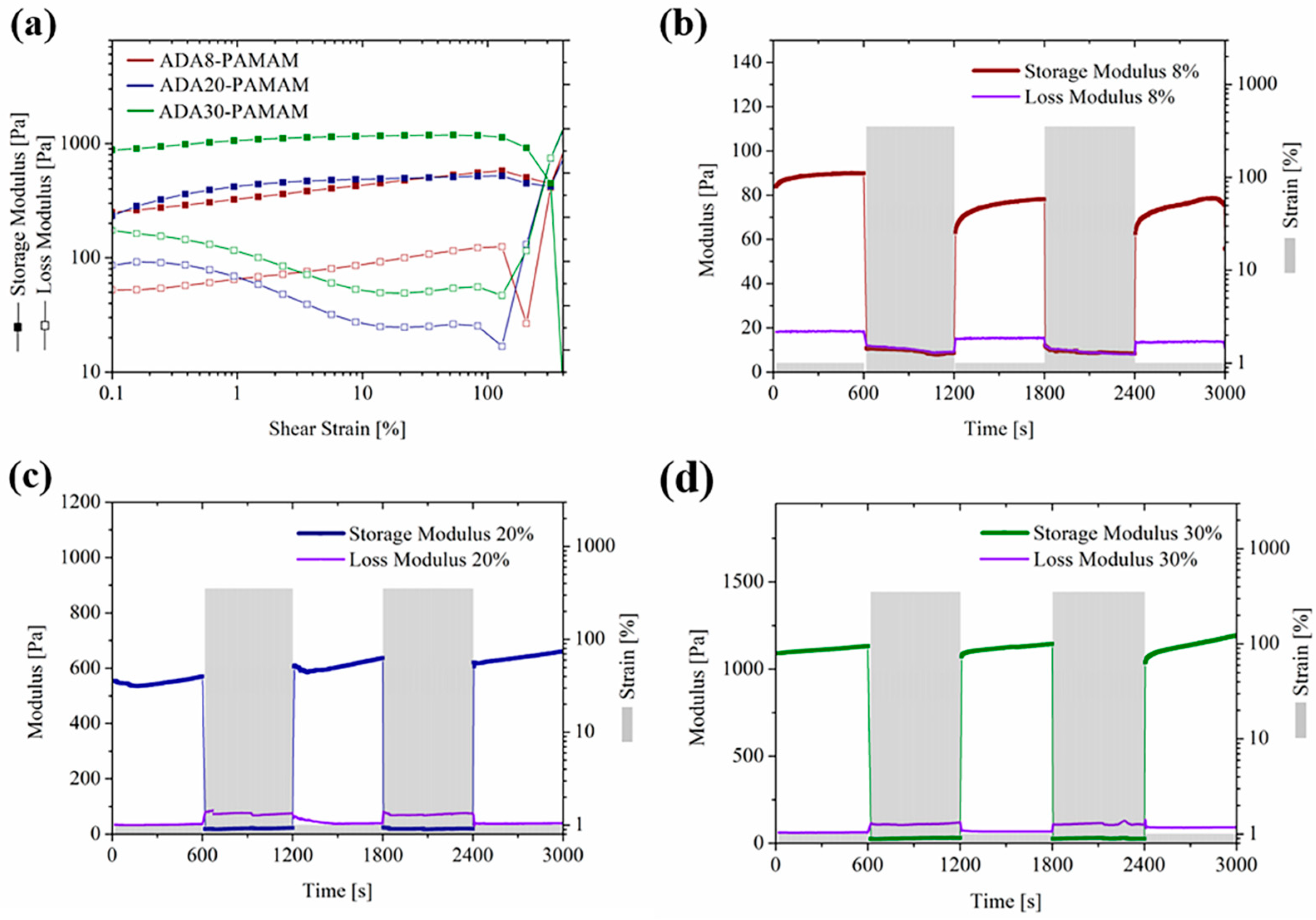
2.3. Rheological Properties of ADA-PAMAM
2.4. Self-Healing Ability of ADA-PAMAM
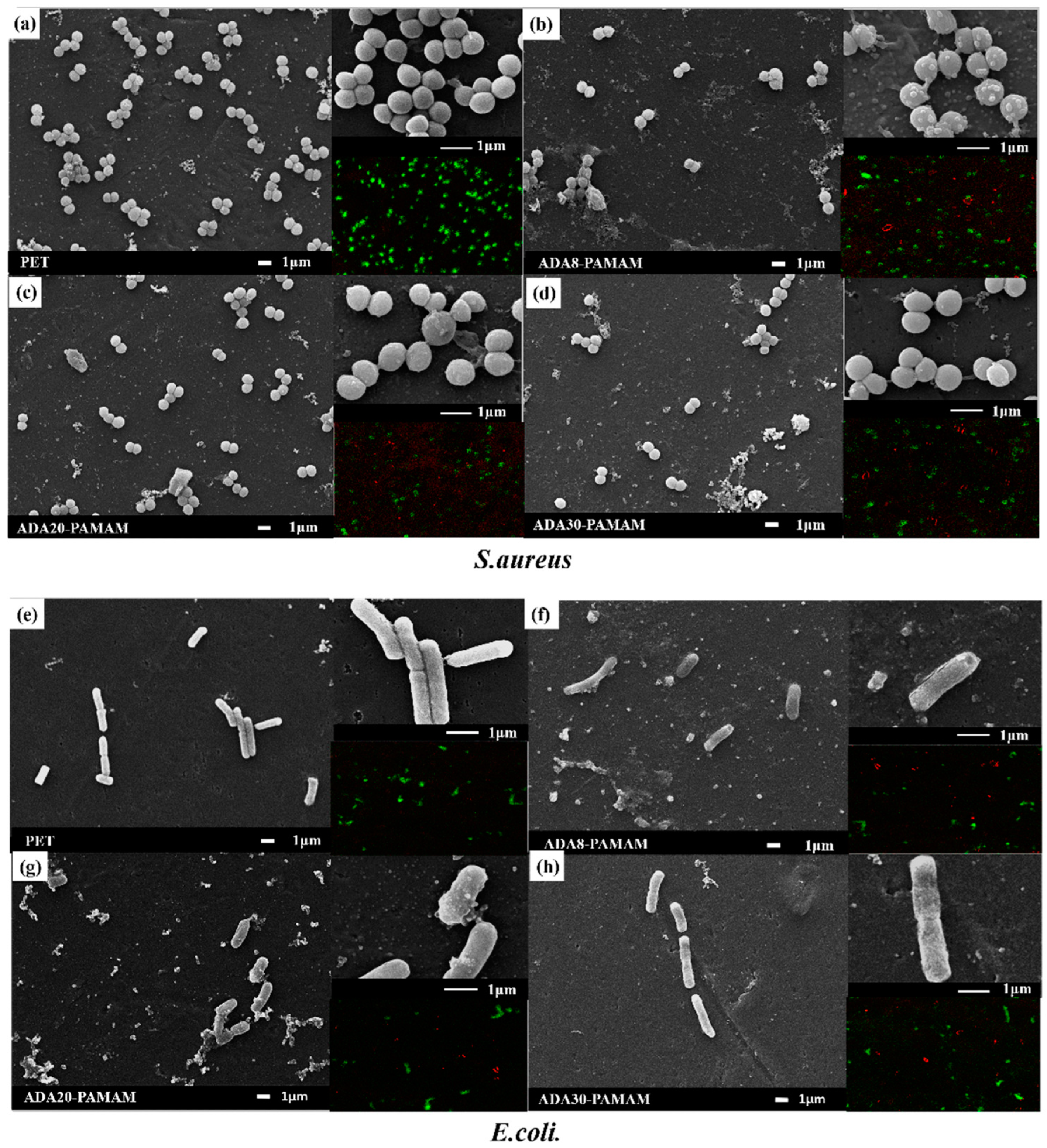
2.5. Antibiotic Ability of ADA-PAMAM
3. Materials and Methods
3.1. Materials
3.2. Preparation of Alginate Dialdehyde (ADA)
3.3. Preparation of PAMAM Crosslinked Alginate Hydrogel (ADA-PAMAM)
3.4. Characterization
3.5. Rheology Test
3.6. Self-Healing Characterization
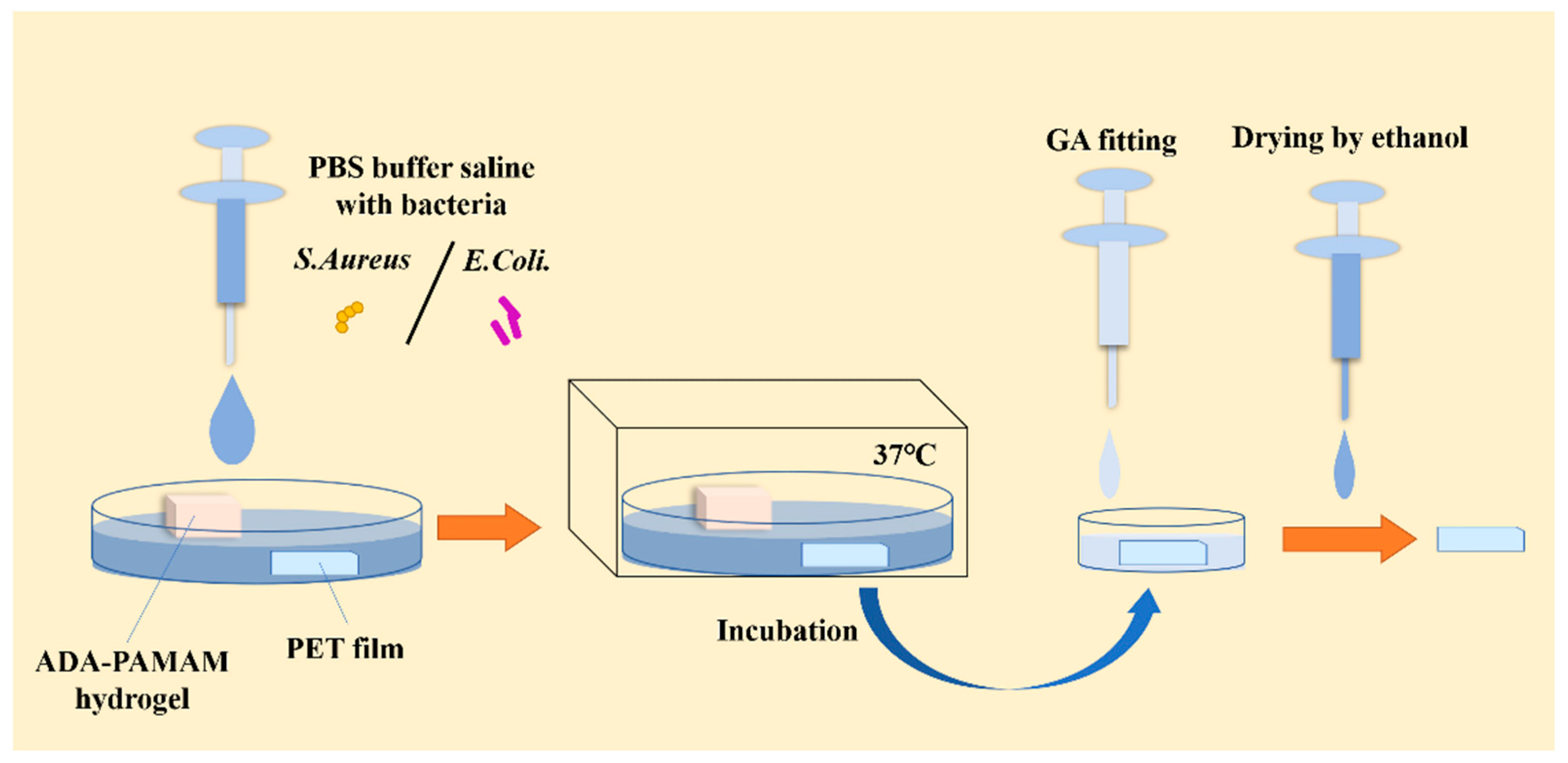
3.7. Bactericidal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAMAM | poly(amidoamine) |
| ADA | alginate dialdehyde |
| ADA-PAMAM | alginate dialdehyde hydrogel crosslinked by poly(amidoamine) |
| ADA(Xa)-PAMAM | (X)% oxidated alginate dialdehyde hydrogel crosslinked by poly(amidoamine) |
| G(X) PAMAM | Generation (X) poly(amidoamine) |
| FTIR | Fourier transform infrared spectroscopy |
| GPC | gel permeation chromatograph |
| GPC-MALLS-UV | GPC aligned with multi-angle laser light scattering and UV-absorption |
| SEM | Scanning electron microscopy |
| CLSM | laser scanning confocal microscope |
| NHS | N-hydroxysuccinimide |
| EDC | N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride |
| OD | oxidation degree |
| AD | aldehyde degree |
| RI | Refractive Index |
| DI water | deionized water |
| PET | polyethylene glycol terephthalate |
| GA | Glutaraldehyde |
References
- Gao, C.; Liu, M.; Chen, S.; Jin, S.; Chen, J. Preparation of oxidized sodium alginate-graft-poly((2-dimethylamino) ethyl methacrylate) gel beads and in vitro controlled release behavior of BSA. Int. J. Pharm. 2009, 371, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Roquero, D.M.; Bollella, P.; Katz, E.; Melman, A. Controlling Porosity of Calcium Alginate Hydrogels by Interpenetrating Polyvinyl Alcohol-Diboronate Polymer Network. ACS Appl. Polym. Mater. 2021, 3, 1499–1507. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F. Thermosensitive alginate-gelatin-nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 2021, 11, 18423–18431. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodríguez-fernández, J.; Ferrer, G.G.; Groth, T. Synthesis and characterization of oxidized polysaccharides for in situ forming hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, C.; Li, Y.; Wang, K.; Wang, X.; Wei, Y.; Tao, L. Synthesis of an injectable, self-healable and dual responsive hydrogel for drug delivery and 3D cell cultivation. Polym. Chem. 2017, 8, 537–544. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, L.; Diaz-Dussan, D.; Zhang, J.; Yang, W.; Gong, L.; Chen, J.; Narain, R.; Zeng, H. Dynamic Flexible Hydrogel Network with Biological Tissue-like Self-Protective Functions. Chem. Mater. 2020, 32, 10545–10555. [Google Scholar] [CrossRef]
- Fan, Z.; Deng, J.; Li, P.Y.; Chery, D.R.; Su, Y.; Zhu, P.; Kambayashi, T.; Blankenhorn, E.P.; Han, L.; Cheng, H. A new class of biological materials: Cell membrane-derived hydrogel scaffolds. Biomaterials 2019, 197, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 2100025. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohydr. Polym. 2020, 234, 115902. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with pH-Regulated Recovery of self-healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, L.; Shi, X. Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv. 2019, 9, 36858–36866. [Google Scholar] [CrossRef]
- Magalhães, T.M.; Guerra, R.C.; San Gil, R.A.D.S.; Valente, A.P.; Simão, R.A.; Soares, B.G.; de Carvalho Mendes, T.; dos Santos Pyrrho, A.; de Sousa, V.P.; Rodrigues-Furtado, V.L. PAMAM dendrimer hydrogel film—Biocompatible material to an efficient dermal delivery of drugs. J. Nanoparticle Res. 2017, 19, 277. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- De Araújo, R.V.; Santos, S.D.; Ferreira, E.I.; Giarolla, J. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef]
- Gao, F.; Djordjevic, I.; Pokholenko, O.; Zhang, H.; Zhang, J.; Steele, T.W.J. On-Demand Bioadhesive Dendrimers with Reduced Cytotoxicity. Molecules 2018, 23, 796. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Gopinath, P. Chemically Cross-Linked Hybrid Nanogels of Alginate and PAMAM Dendrimers as Efficient Anticancer Drug Delivery Vehicles. ACS Biomater. Sci. Eng. 2016, 2, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lecaros, R.L.G.; Ho, S.-Y.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Ionically cross-linked sodium alginate and polyamidoamine dendrimers for ethanol/water separation through pervaporation. Sep. Purif. Technol. 2021, 275, 119125. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Xiao, C.; Chen, X. Enhancing the Stability of Hydrogels by Doubling the Schiff Base Linkages. Macromol. Chem. Phys. 2019, 220, 1800484. [Google Scholar] [CrossRef]
- Hosseini, M.; Vaezi, Z.; Ganjali, M.R.; Faridbod, F.; Abkenar, S.D.; Alizadeh, K.; Salavati-Niasari, M. Fluorescence “turn-on” chemosensor for the selective detection of zinc ion based on Schiff-base derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, Y.; Zhong, X.; Hu, J.; Shi, F.; Mi, H. Preparation and catalytic performance of alginate-based Schiff Base. Carbohydr. Polym. 2019, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, W.; Wang, Q.; Xu, C.; Tang, Q.; Yang, H. An injectable, dual responsive, and self-healing hydrogel based on oxidized sodium alginate and hydrazide-modified poly (ethyleneglycol). Molecules 2018, 23, 546. [Google Scholar] [CrossRef]
- Holmes, A.M.; Heylings, J.R.; Wan, K.W.; Moss, G.P. Antimicrobial efficacy and mechanism of action of poly(amidoamine) (PAMAM) dendrimers against opportunistic pathogens. Int. J. Antimicrob. Agents 2019, 53, 500–507. [Google Scholar] [CrossRef]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. BioSyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Lesieur, S.; Labarre, D.; Jayakrishnan, A. Periodate oxidation of sodium alginate in water and in ethanol-water mixture: A comparative study. Carbohydr. Res. 2005, 340, 1425–1429. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.A.; Steele, T.W.; Bhuthalingam, R.; Li, M.; Boujday, S.; Prawirasatya, M.; Neoh, K.G.; Boey, F.Y.C.; Venkatraman, S.S. Quantification of aldehyde terminated heparin by SEC-MALLS–UV for the surface functionalization of polycaprolactone biomaterials. Colloids Surfaces B Biointerfaces 2015, 132, 253–263. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, Y.; Zhang, H.; Ren, Z.; Fan, K.; Cheng, J.; Zhang, J.; Gao, F. The Design of Rapid Self-Healing Alginate Hydrogel with Dendritic Crosslinking Network. Molecules 2022, 27, 7367. https://doi.org/10.3390/molecules27217367
Wang D, Li Y, Zhang H, Ren Z, Fan K, Cheng J, Zhang J, Gao F. The Design of Rapid Self-Healing Alginate Hydrogel with Dendritic Crosslinking Network. Molecules. 2022; 27(21):7367. https://doi.org/10.3390/molecules27217367
Chicago/Turabian StyleWang, Dingxuan, Yuhan Li, Haobo Zhang, Zhaorong Ren, Kefan Fan, Jue Cheng, Junying Zhang, and Feng Gao. 2022. "The Design of Rapid Self-Healing Alginate Hydrogel with Dendritic Crosslinking Network" Molecules 27, no. 21: 7367. https://doi.org/10.3390/molecules27217367
APA StyleWang, D., Li, Y., Zhang, H., Ren, Z., Fan, K., Cheng, J., Zhang, J., & Gao, F. (2022). The Design of Rapid Self-Healing Alginate Hydrogel with Dendritic Crosslinking Network. Molecules, 27(21), 7367. https://doi.org/10.3390/molecules27217367





