Abstract
The synthesis of cyclotetrapeptides analogues of the natural products tentoxin and versicotide D was achieved in good yield by solid phase peptide synthesis (SPPS) of their linear precursors and solution phase cyclization. All the cyclopeptides and several open precursors were evaluated as herbicides. Five cyclopeptides and five lineal peptides showed a significant inhibition (>70%) of Ryegrass seed’s radicle growth at 67 μg/mL. The evaluation at lower concentrations (4–11 μM) indicates two cyclopeptides analogs of tentoxin, which present one (N-Methyl-d-Phe), and two N-MeAA (N-Methyl-Ala and N-Methyl-Phe), respectively, as the most active of them, showing remarkable phytotoxic activity. In two cases, the open precursors are as active as their corresponding cyclopeptide. However, many linear peptides are inactive and their cyclization derivatives showed herbicidal activity. In addition, two cyclopeptide analogues of versicotide D showed more improved activity than the natural product. The results indicate that the peptide sequence, the amino acid stereochemistry and the presence of N-methyl group have important influence on the phytotoxic activity. Moreover, several compounds could be considered as lead candidates in the development of bioherbicides.
1. Introduction
Agrochemicals play a key role in agriculture as their use has dramatically increased productivity. Weeds represent one of the most important pests that need to be controlled. In fact, about 50% of the commercial pesticides used worldwide are herbicides [1]. However, many factors are causing an urgent need for the development of novel herbicides. In the first place, the toxicity and long-term impact on human health and the environment of extensively used agrochemicals, such as glyphosate, have been deeply studied during the last decades [2,3,4]. As a result, many agrochemicals have been banned by governmental agencies in many countries [5]. Furthermore, agrochemicals have caused the eutrophication of water bodies, with environmental consequences, such as the increase in cyanobacteria blooms [6], which produce harmful toxins [7], affecting animal and human health. On the other hand, many herbicides have become ineffective by the development of weed resistance [8,9,10], and the discovery of new ones during the recent decades have been scarce [11,12,13].
Natural products have long been a source of inspiration in the discovery of bioactive molecules. Furthermore, natural products could provide an environmentally friendlier approach to weed management [14,15,16]. In the search for herbicides with novel modes of action and safer for both human health and the environment, plants, fungi extracts and metabolites have been investigated as bioherbicides [17].
Naturally occurring cyclic peptides and synthetic cyclic peptides inspired in natural products have found application in a large variety of fields, such as drug discovery [18], imaging [19] and materials chemistry [20]. Relevant features that justify this fact are their great binding affinity, low toxicity, and the capability of targeting traditionally “undruggable” protein surfaces [21]. In addition, cyclopeptides exhibit increased metabolic stability in comparison with their linear counterparts.
In particular, a relatively large number of cyclic tetrapeptides reported in the literature have shown interesting bioactivities. However, due to their size, their synthesis can be challenging. As the ring size decreases, peptide cyclization becomes more difficult due to energy constraints [22]. The presence of turn-inducing motifs, such as D-amino acids or N-methyl amino acids (N-MeAA), are relevant in promoting a preorganized conformation for cyclization. In addition, cyclopeptides containing N-MeAA could produce an impact on bioactivity as cell permeability and chemical stability are increased [23].
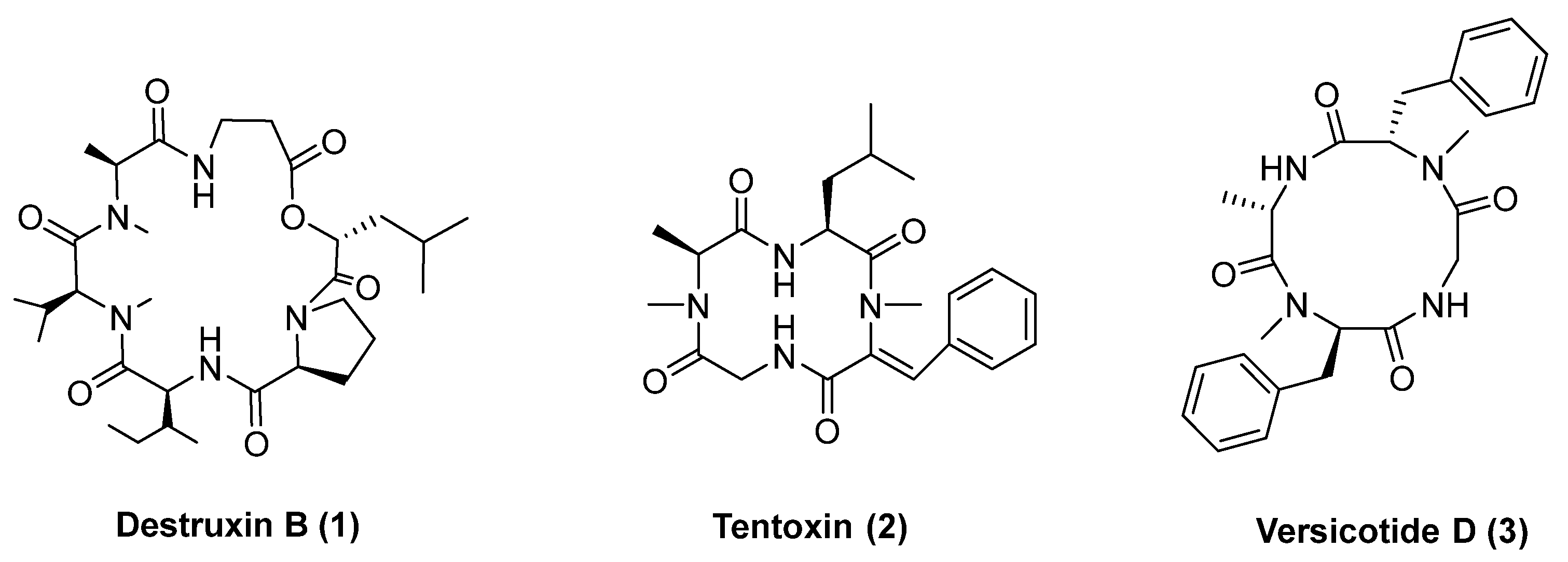
Relevant examples of bioactive cyclopeptides with phytotoxic activity are destruxin B (1), Figure 1, and tentoxin (2). Tentoxin, a cyclic tetrapeptide produced by the fungus Alternaria tenuis, causes seedling chlorosis [24]. Although tentoxin has an interesting herbicide activity, studies suggest it can be hepatotoxic [25], and its synthesis is very challenging. In fact, several synthetic routes for obtaining tentoxin were described [26,27,28,29,30], and many of them show low yields.
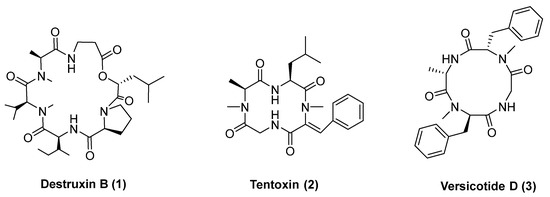
Figure 1.
Cyclopeptide natural products with herbicidal activity: destruxin, tentoxin and versicotide D.
Our group has recently reported the phytotoxic evaluation of natural products versicotides, among which versicotide D (3, Figure 1) shows low cytotoxicity on HepG2 cells [31], high phytotoxicity (74% radicle growth inhibition at 67 μg/mL) and the ability to inhibit cyanobacteria population with a substantial depletion of the concentration of microcystins in the media [32]. Here, we report the synthesis of a library of cyclic tetrapeptides inspired on tentoxin and versicotide D and the evaluation of their phytotoxicity against Ryegrass (Lolium multiflorum) seeds. The design of the compounds was focused on small modifications of the peptide backbone, exploring the influence on the bioactivity of L- or D-amino acids and N-MeAA.
2. Results and Discussion
2.1. Synthesis
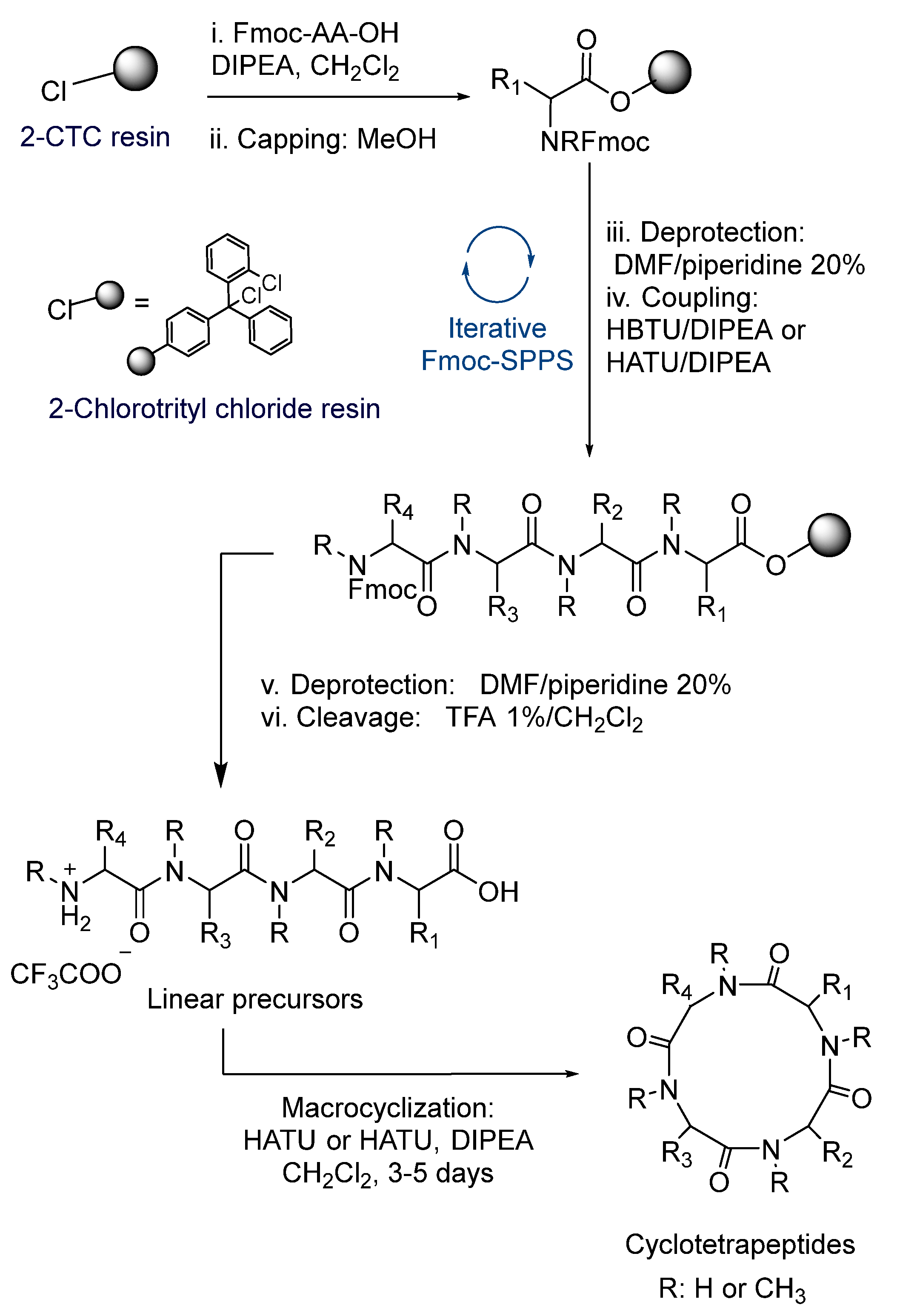
The synthesis of the linear tetrapeptides, 4–15, as shown in Table 1, and the corresponding cyclopeptides 16–27, as shown in Figure 2, were carried out following the procedure shown in Scheme 1. The methodology was based on the Fmoc-strategy of SPPS (solid phase peptide synthesis), employing the 2-chlorotrityl chloride resin (2-CTC resin). To avoid racemization, and thereby the formation of diastereomers during ring closure, we started most peptide sequences with Glycine at the C-terminus, which also minimizes steric hindrance during the macrocyclization process. The yields and purities of the obtained tetrapeptides are shown in Table 1. HBTU was used for coupling to primary amine and HATUwhen coupling to an N-MeAA. (Characterization Data of Products Can be founded in Supplementary Material)

Table 1.
Linear peptides obtained by Fmoc- SPPS.
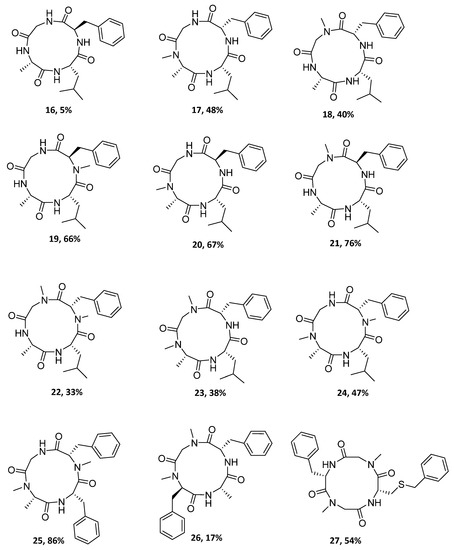
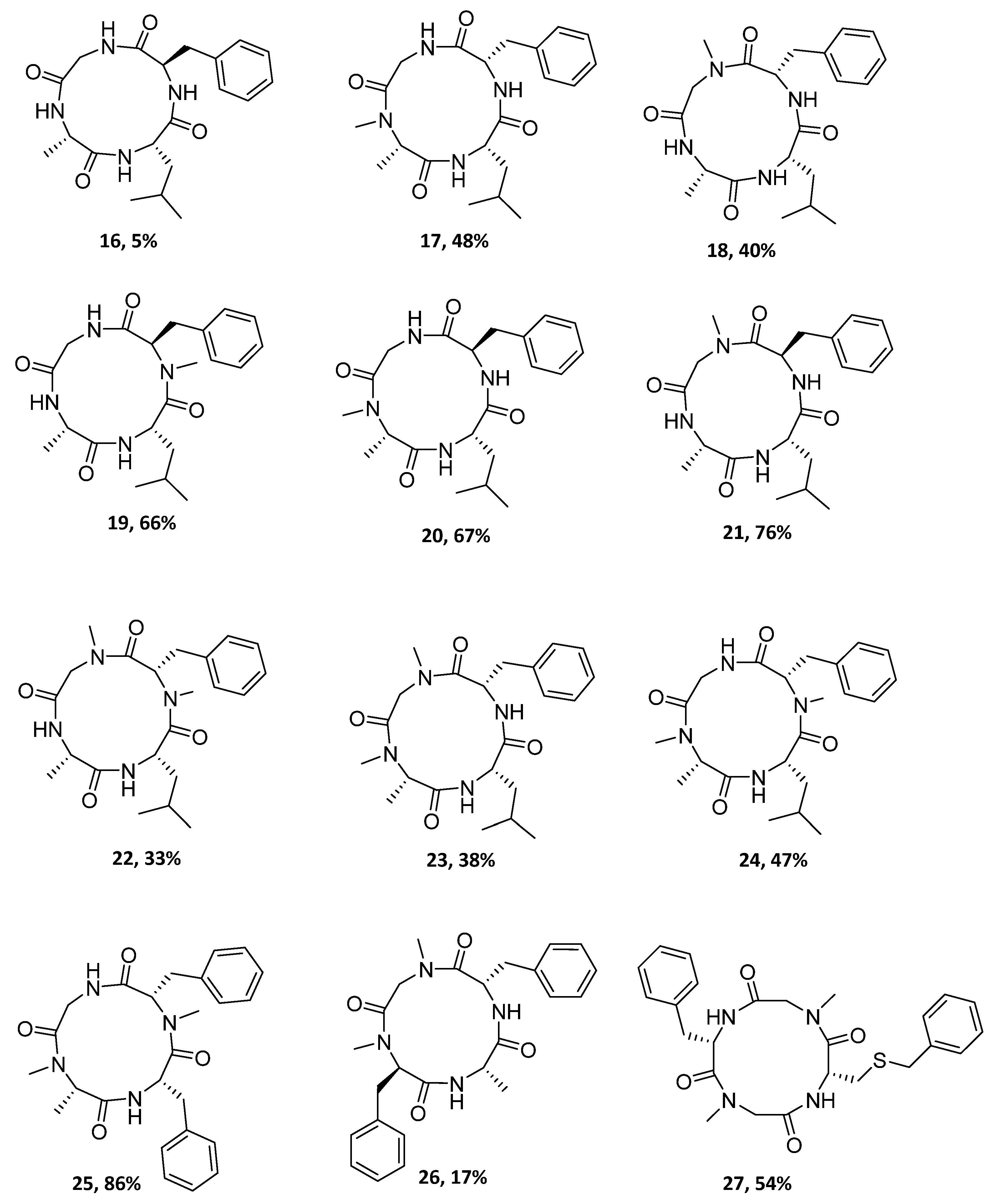
Figure 2.
Structures and yields of the synthesized cyclopeptides.
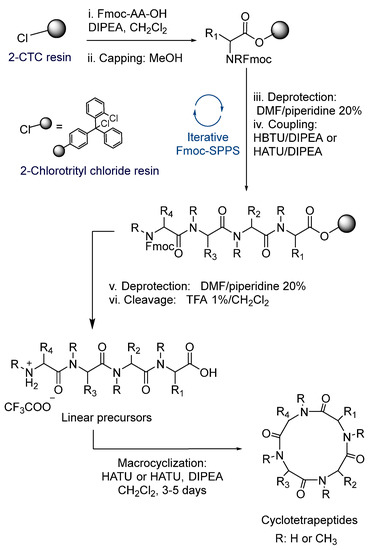
Scheme 1.
General procedure of the SPPS Fmoc-strategy and solution macrocyclization.
In order to maximize the expected outcome of the cyclization reaction, the linear sequences of the peptide precursors were carefully chosen. Gly (compounds 4–8, 11–13) or N-MeGly (compounds 9–11 and 14–15) were selected as C-terminals to avoid epimerization of this residue and minimize steric hindrance during the cyclization process.
The anchorage of Fmoc-Gly-OH or Fmoc-N-MeGly-OH to the resin was achieved with excess of DIPEA in CH2Cl2. For the elongation of the peptide chain, we carried out successive steps of deprotection employing piperidine and amide bond formation, using the corresponding Fmoc-AA-OH and HBTU (N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate) for coupling to primary amines and HATU for secondary amines ((1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate). The elongation of the peptide chain was monitored by HPLC; in some cases, additional steps of deprotection or coupling were needed. Once the desired linear tetrapeptides were reached, cleavage with a solution of TFA 1% in CH2Cl2 rendered them as trifluoroacetate salts in very good yields and excellent purities.
Diketopiperazine formation is an important side reaction that can hinder peptide synthesis on solid phase. This usually occurs during the coupling between the second and third amino acid in the sequence. It is well known that the use of 2-CTC resin decreases the formation of diketopiperazine due to the presence of the bulky trityl group [33]. Nevertheless, when the second amino acid is an N-MeAA, the formation of diketopiperazine can be promoted. In order to avoid this, short times during Fmoc deprotection of N-MeAA were used. This procedure allowed us to obtain compounds 7, 12 and 13 in very good yields. However, low yields were obtained for compounds 6, 9 and 11, possibly due to their particular peptide sequences.
Once the linear precursors were obtained as trifluoroacetate salts, we proceeded with the head-to-tail lactamization in solution phase, obtaining the corresponding cyclotetrapetides. It is well known that macrocyclization reaction is a low yielding process. The E-geometry of the amide bond prevents the peptides from adopting a ring-like conformation conducive to cyclization [34,35]. In addition, the cyclization yield is very dependent on the peptide sequence and on the ring size. In general, the cyclization of peptides with less than seven amino acids is a difficult process, even though small cyclic peptides containing a β-turn such as a d-amino acid, an N-MeAA or proline, a thiazole or oxazole ring, etc., can be prepared in higher yields.
To obtain the desired cyclotetrapeptides, high dilution conditions were used, and cyclization reactions were performed for 1–5 days (Scheme 1). Figure 2 and Table 2 show the twelve cyclotetrapeptides analogues to tentoxin (cyclopeptides 16–24) and versicotide D (25–27) that were prepared using HBTU/DIPEA in the cases where the N-terminal residue is a primary amine and HATU/DIPEA or Oxyma/DIC when the N-terminal residue was a secondary amine. To obtain compound 17, we also tried the combination of Oxyma/DIC, instead of HATU or HBTU, but the best results were obtained with HATU. For the cyclization of compound 15, Oxyma/DIC was used and 27 was obtained in 54% yield.

Table 2.
Peptide precursors, cyclotetrapeptides and macrocyclization yield.
It is important to highlight that in the case of 17 vs. 20, and 18 vs. 21, the change of Phe by D-Phe, respectively, leads to a higher cyclization yield. However, 16 containing a D-Phe, was obtained in the lowest yield (5%). Nevertheless, this yield was not optimized and could be improved by the use of others coupling reagents. Taking into account the number of N-MeAA in the peptide precursor, most of the higher yields were for compounds with one N-MeAA (17–21). An interesting result is the case of the compound 25, which contains two N-MeAA and was obtained in very good yield. In fact, this was the highest yield for the cyclopeptides synthesized in this work. In contrast, when Phe was substituted by Leu (25 vs. 24, respectively), the yield decreased.
2.2. Herbicidal Activity
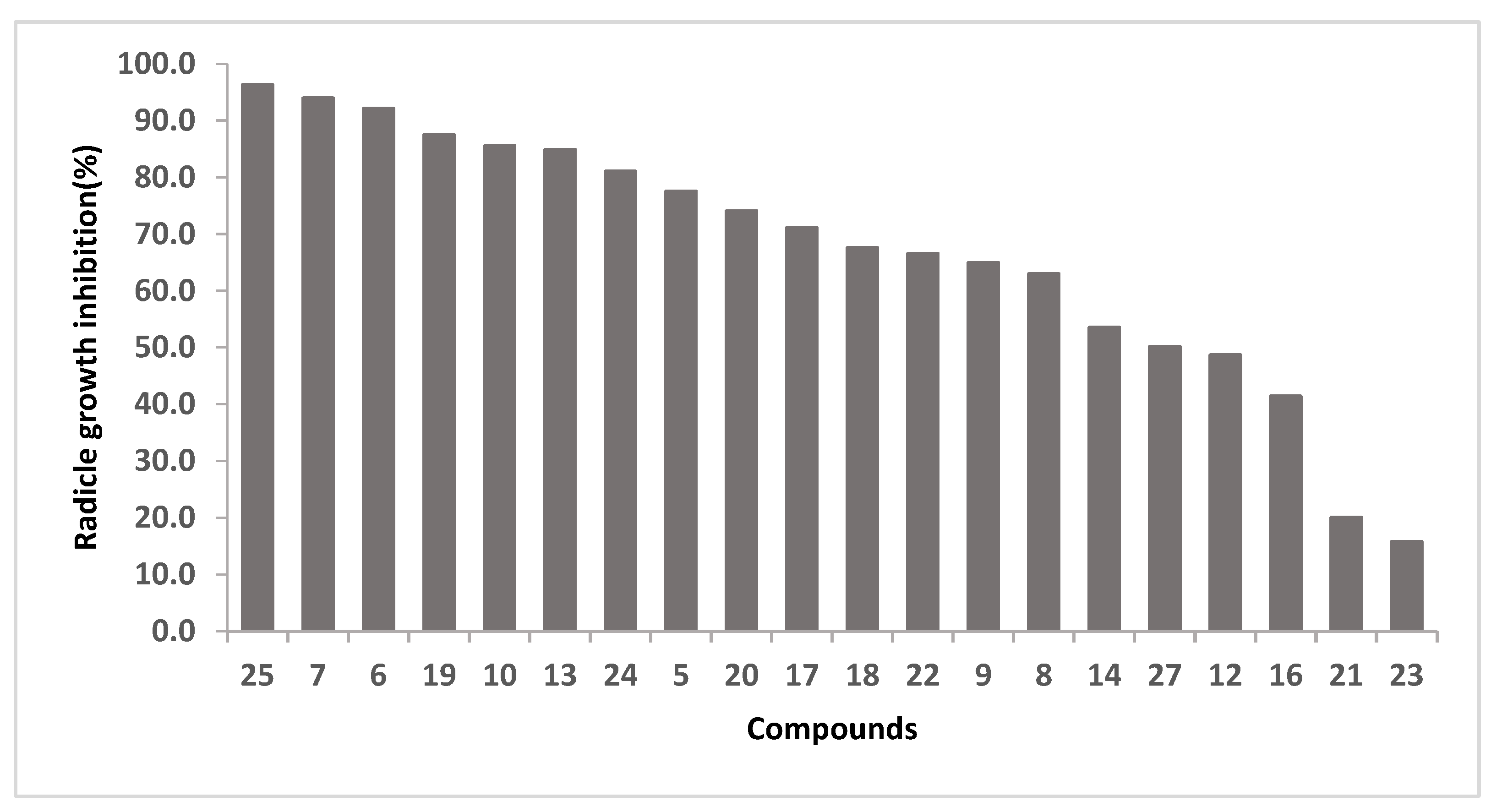
The herbicidal activity of cyclotetrapeptides 16–25, 27 and the linear precursors 4–8, 10, 12–15 were evaluated for their influence on germination, leaf development and radicle length growth of ryegrass seeds at 67 μg/mL using germination in agar methodology [32]. DMSO was used as negative control and the herbicide S-metolachlor as positive control. In this trial, the results showed that root length was the only variable for which significant differences were found after a statistical analysis. The results were expressed as % of radicle growth inhibition, calculated as the percentage between the treatment with each compound and radicle length growth for the negative control (DMSO), as shown in Figure 3. Cyclopeptide 26 was tested at 45 μg/mL and showed 67% inhibition.
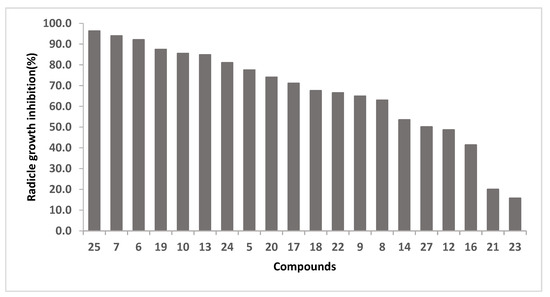
Figure 3.
Radicle growth inhibition (%) of compounds at 67 µg/mL, positive control: S-metolachlor, 100% inhibition radicle growth at 2.1 µg/mL.
The most active compounds are the cyclotetrapeptide 25, and the tetrapeptides 7 and 6, for which an inhibition of 96%, 94% and 92% at 67 µg/mL was observed, respectively. At this concentration, the cyclization of 13 to obtain 25 leads to an increase in activity. On the contrary, the cyclization of 6 and 10 to render 18 and 22, respectively, leads to a decrease in herbicidal activity.
By comparison of the herbicidal activity of 16 and 19, we concluded that the presence of N-methyl group is relevant for the bioactivity. Moreover, cyclopeptides 20 and 19, which differ in the position of N-methyl group, present root growth inhibition of 74% and 87%, respectively. On the other hand, 17, which presents a Phe instead of a D-Phe, seems to be as active as 20. However, the substitution of Phe in 6 (NH2-Ala-Leu-Phe-N-MeGly-OH) by D-Phe (9) leads to a decrease on the herbicidal activity.
The activity results for cyclotetrapeptides analogues of tentoxin, which contain two N-MeAA (22–24), allowed us to conclude that the position of this type of amino acid is relevant for the bioactivity, whereas 23 is inactive (inhibition 16%), 22 and 24 present 66% and 81% of inhibition, respectively. It is important to note that unlike 24, 22 and 23 present two contiguous N-MeAA.
In the case of cyclotetrapeptide 24, the change of Leu for Phe (compound 25) leads to an increase on the activity, and for their corresponding linear precursors (12 and 13), this fact becomes more noticeable.
The most active compounds, which exhibited inhibition greater than 85% at 67 μg/mL (compounds 6, 7, 10, 13, 19, 25, Figure 3) and cyclopeptides 17, 20, 24 and 26, were selected to assay them at a lower concentration in order to obtain more experimental results for a deep discussion about chemical structure requirements, as shown in Table 3. The linear peptide 6, did not show inhibition at 23 µM and 10 presented only 15% of root grow inhibition at 11 µM. All the other compounds (7, 13, 17, 19, 20, 24, 25 and 26) showed considerable inhibition at concentrations between 4 and 11 µM. The open precursors of the cyclopeptides 19 and 25, the peptides 7 and 13, presented similar activity, suggesting that cyclization does not have influence on the bioactivity. However, 26 is a very active compound and its open precursor (14) showed lower activity at 67 µM (53%).

Table 3.
Root growth inhibition (%) of the most active compounds at lowest concentration.
At lower concentration (6 μM), cyclopeptides 20 and 17, which differ on the stereochemistry of Phe, present different bioactivity, 32% and 10% inhibition, respectively.
Compound 24 containing two NMe-AA in the (1, 3) relative position in the cycle, presents substantial activity, 62% inhibition at 10 μM. In addition, the open peptide precursor, 12, is one of the less active of this series (Figure 3).
3. Materials and Methods
3.1. Synthesis
All reactions were carried out under nitrogen atmosphere with dry, freshly distilled solvents, under anhydrous conditions, unless otherwise stated. All solvents were purified following procedures described in the literature [36]. The cyclopeptides were analyzed using HPLC-DAD-ESI-MS/MS Shimadzu LCMS 8040 equipped with LC-20 AD pump, a DGU solvent degasser solvent, a SPD-M20AD detector, a CTO.20A oven, a Sil-20A injector. The mass spectrometer is connecting by a split 4:1 of flow. The data were processed by the Labsolutions LCMS software. Chromatographic analysis were developed using a Kinetex EVO C18 (100 mm × 4.6 mm, 5 μm solid core particle), using a flow of 1.25 mL/min and 40 °C. Analysis were developed by a gradient solvent system using 0.1% formic acid (mobile phase A) and acetonitrile (mobile phase B). The gradient program was: t = 0’8% B, t = 18’90% B.
Analysis of LC-DAD-MS was recorded by UV absorbance in a 220–360 nm range, and by full scan ESI + ions with a range of 200–1000 uma.
1H and 13C-NMR spectra were recorded at 25 °C on a Bruker Neo 400 using a BBO z-gradient probe, operating at 400.13 and 100.62 MHz for 1H and 13C, respectively.
Solid Phase Peptide Synthesis of linear peptides (4–15):
- (I)
- Resin loading: 500 mg of 2-Chlorotrityl chloride resin (2-CTC) were added to a plastic syringe. The resin was swelled in CH2Cl2 (3 × 5 min). A solution of first protected amino acid Fmoc-AA-OH (1 eq. for 0.8 mmol/g loading) and DIPEA (3 eq.) in CH2Cl2 was added and shaken for 10 min. Then, an extra 7.0 eq. of DIPEA were added, and shaking was continued for 50 min. MeOH (0.8 mL/g of resin) was added to the previous mixture and shaken for 10 min. After filtering, the resin was washed with CH2Cl2 (×3), MeOH (×3), CH2Cl2 (×3), DMF (×3).
- (II)
- Removal of NHFmoc group: The resin was shaken with piperidine-DMF solution (1:4) (1 × 1 min and 2 × 5 min). In exceptional cases, deprotection step was accomplished by a single treatment with piperidine-DMF solution for 5 min in order to prevent side reactions.
- (III)
- Coupling of subsequent N-Fmoc protected amino acids to primary or secondary amines: After removal of NHFmoc-protecting group, as previously described, the resin was washed with DMF (×3), CH2Cl2 (×3) and DMF (×3). Then, a solution of Fmoc-AA-OH (3 eq.) and DIPEA (6 eq.) in DMF was added to the resin, followed by a solution of HBTU, for coupling to primary amines, or HATU (2.9 eq.) in DMF, in case of coupling to an N-methylamino acid. The mixture was stirred for 60 min. After the coupling was completed, the resin was washed with DMF (×3) and CH2Cl2 (×3). Successive deprotection and coupling cycles were made with the appropriate amino acids to obtain the desired compound. The coupling was monitored by colorimetric assays; Kaiser test in case of primary amines and Chloranil test for secondary amines. Coupling procedure was repeated in case of positive results.
- (IV)
- Cleavage: The peptide was cleaved from the resin by treatment with 1% TFA in CH2Cl2 for 2–3 min at room temperature, followed by filtration and collection of the filtrate in MeOH. The treatment was repeated three times and then the resin was washed with CH2Cl2 (×5) and MeOH (×3). Solvents were removed under vacuum to obtain the crude peptide. LC-MS was used to identify the desired product
- (V)
- General procedure for macrocyclization in solution phase to obtain (16–27):Method I: Macrocyclization reaction was performed in diluted conditions (1–5 mM) using HBTU or HATU (1.5 eq.), DIPEA (3 eq.), 4-DMAP (catalytic) in dried CH2Cl2 at room temperature during 1–5 days. The reaction mixture was washed with HCl 5%, later with saturated aqueous NaHCO3, dried over MgSO4, filtered and concentrated in vacuo. The crude was purified by flash chromatography to obtain the pure macrocycle.Method II: The trifluoroacetate salt of the corresponding linear peptide was dissolved in dried CH2Cl2 and diluted to a concentration of 1–5 mM. DIPEA (1 eq.) was added to enable dissolution. EDCI (1.2 eq) and oxyma (1.2 eq.) were added at 0 °C and the reaction mixture was stirred for 10 min. Then, the reaction mixture is allowed to reach room temperature and stirred for 48 h. The reaction mixture was washed with HCl 5%, later with saturated aqueous NaHCO3, dried over MgSO4, filtered and concentrated in vacuo. The crude was purified by flash chromatography to obtain the pure macrocycle.
Cyclo-[Ala-Leu-d-Phe-Gly] (16): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 5 days), starting from the trifluoroacetate salt of 4: NH2-Ala-Leu-d-Phe-Gly-OH (300 mg, 0.58 mmol); HBTU were used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (1:0.1), rendered the desired macrocycle 16. White solid (5%). Rf = 0.3 (AcOEt). 1H NMR (400 MHz, 0.1% MeOD-d4 in CDCl3) δ (ppm): 8.01 (d, J = 7.2 Hz, 1H), 7.77 (d, J = 5.6 Hz, 1H), 7.39–6.99 (m, 5H), 4.48–4.34 (m, 1H), 4.22–3.82 (m, 4H), 3.32 (dd, J = 13.9, 3.7 Hz, 1H), 2.96 (dd, J = 14.0, 11.2 Hz, 1H), 1.74–1.49 (m, 2H), 1.46 (d, J = 7.1 Hz, 3H), 0.86 (d, J = 6.5 Hz, 3H), 0.82 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, 0.1% MeOD-d4 in CDCl3) multiple conformers were observed, δ (ppm): 175.2, 172.9, 172.4, 172.3, 171.6, 171.5, 137.0, 129.2, 129.0, 128.7, 128.6, 127.0, 56.8, 56.7, 51.8, 50.9, 42.5, 37.0, 36.7, 24.8, 23.0, 21.7, 16.8. ESI-MS m/z calc. for C40H56N8NaO8+: 799.41 ([2M + Na+]+), found 799.50. The purity (90.1%) was determined by HPLC, linear gradient, t = 0’ 30% B, t = 15’ 90% B, 25 °C, 220 nm, (rt = 6.79 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[N-MeAla-Leu-Phe-Gly] (17): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 84 h), starting from the trifluoroacetate salt of 5: NH-MeAla-Leu-Phe-Gly-OH (200 mg, 0.37 mmol). HATU was used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (3:0.2), rendered the desired macrocycle 17. White solid (48%). Rf = 0.5 (AcOEt:MeOH, 3:0.2). 1H NMR (400 MHz, DMSO-d6), multiple conformers were observed, δ (ppm): 8.16–7.71 (m, 2H), 7.65–7.54 (m, 1H), 7.30–7.13 (m, 5H), 5.00–4.92 (m, 0.2H), 4.84–4.76 (m, 0.1H), 4.67 (q, J = 7.4 Hz, 0.5H), 4.62–4.38 (m, 0.8H), 4.41–4.20 (m, 1.4H), 4.20–4.06 (m, 0.8H), 4.05–3.90 (m, 0.6H), 3.92–3.82 (m, 0.3H), 3.74 (dd, J = 16.8, 3.6 Hz, 0.5H), 3.14–2.97 (m, 1.4H), 2.93 (s, 1.4H), 2.88–2.70 (m, 1.6H), 2.7–2.6 (m, 0.6H), 1.59–1.32 (m, 3H), 1.33–1.12 (m, 3H), 0.80 (d, J = 6.1 Hz, 3H), 0.76 (d, J = 5.7 Hz, 3H). 13C NMR (100 MHz, DMSO-d6), multiple conformers were observed, δ (ppm): 172.2, 171.6, 170.7, 169.9, 138.6, 129.7, 129.6, 129.5, 128.6, 128.5, 127.7, 126.7, 55.8, 55.5, 52.7, 41.7, 37.1, 32.5, 25.4, 25.0, 24.7, 24.6, 23.5, 23.2, 22.0, 21.9, 14.1. ESI-MS m/z calc. for C42H60N8NaO8: 827.44 (2M + Na+]+), found 827.55. The purity (90.7%) was determined by HPLC, linear gradient 1.25 mL/min, t = 0′ 30% B, t = 15′ 90% B, 25 °C, 220 nm, (rt = 7.41 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[Ala-Leu-Phe-N-MeGly] (18): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 5 days), starting from the trifluoroacetate salt of 6: NH2-Ala-Leu-Phe-NMeGly-OH (207 mg, 0.39 mmol); HBTU was used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (1:0.1), rendered the desired macrocycle 18. White solid (40%). Rf = 0.3 (AcOEt). 1H NMR (400 MHz, MeOD-d4), multiple conformers were observed, δ (ppm): 7.43–7.03 (m, 5H), 5.29–5.05 (m, 0.2H), 5.01–4.81 (m, 0.5H), 4.66–4.56 (m, 0.5H), 4.54–4.27 (m, 1.4H), 4.18–4.05 (m, 0.5H), 4.04–3.94 (m, 0.6 H), 3.77–3.66 (m, 0.3 H), 3.35–2.93 (m, 3H), 2.72 (s, 3H), 1.82–1.19 (m, 3H), 1.50 (d, J = 7.3 Hz, 3H), 1.03–0.67 (m, 6H). 13C NMR (100 MHz, CDCl3), δ (ppm)), multiple conformers were observed: 175.1, 174.2, 173.2, 168.5, 135.3, 129.4, 129.1, 128.9, 128.7, 128.5, 127.5, 127.2, 54.0, 52.0, 50.5, 39.2, 37.5, 37.0, 36.7, 29.7, 24.7, 24.6, 23.0, 22.9, 22.7, 21.9, 21.4, 16.8, 14.1. ESI-MS m/z calc. for C21H31N4O4+: 403.23 ([M + H]+), found 403.05. The purity (97.6%) was determined by HPLC, linear gradient, t = 0′ 30% B, t = 10′ 90% B, 25 °C, 220 nm, (rt = 7.53 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[Ala-Leu-N-Me-d-Phe-Gly] (19): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 5 days), starting from the trifluoroacetate salt of 7: NH2-Ala-Leu-N-Me-d-Phe-Gly-OH (300 mg, 0.56 mmol); HBTU was used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (1:0.1) to AcOEt:MeOH (1:0.5), rendered the desired macrocycle 19. White solid (66%). Rf = 0.4 (AcOEt). 1H NMR (400 MHz, DMSO-d6), multiple conformers were observed, δ (ppm): 8.10–8.01 (m, 1H), 8.01–7.84 (m, 2H), 7.44 (d, J = 7.51 Hz., 1H), 7.27–7.09 (m, 5H), 5.51–5.28 (m, 1H), 4.62–4.40 (m, 1H), 4.37–4.18 (m, 1H), 3.98–3.55 (m, 2H), 3.29–3.18 (m, 1H), 2.96–2.80 (m, 4H), 1.25–1.09 (m, 3H), 1.11–0.76 (m, 3H), 0.76–0.57 (m, 6H). 13C NMR (100 MHz, DMSO-d6), multiple conformers were observed, δ (ppm): 173.4, 173.1, 173.0, 172.3, 172.2, 172.1, 170.7, 170.4, 170.2, 168.8, 168.6, 168.6, 138.2, 138.2, 138.1, 129.7, 129.3, 129.3, 128.5, 126.7, 58.0, 57.7, 52.2, 48.3, 48.1, 47.7, 47.7, 42.8, 42.6, 41.0, 33.6, 31.9, 31.2, 24.1, 24.1, 23.3, 23.2, 22.5, 22.2, 22.2, 19.1, 18.8, 18.8. ESI-MS m/z calc. for C42H60N8NaO8+: 827.44 ([2M + Na]+) found 827.60. The purity (98.7%) was determined by HPLC, isocratic flow 1 mL/min, MeOH: H2O (70:30), 30 °C, 220 nm, (rt = 9.97 min).
Cyclo-[N-MeAla-Leu-d-Phe-Gly] (20): solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 5 days), starting from the trifluoroacetate salt of 8: NH-MeAla-Leu-d-Phe-Gly-OH (300 mg, 0.56 mmol); HATU was used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (1:0.1) to AcOEt:MeOH (1:0.5), rendered the desired macrocycle 20. White solid (67%). Rf = 0.4 (EtOAc). 1H NMR (400 MHz, CDCl3, 0.1% MeOD-d4), multiple conformers were present, δ (ppm): 8.91–8.46 (m, 1H), 8.16–7.83 (m, 1H), 7.60–7.34 (m, 1H), 7.35–7.05 (m, 5H), 7.03–6.71 (m, 1H), 5.28–4.05 (m, 3H), 3.80–3.60 (m, 1H), 3.60–3.36 (m, 1H), 3.29–2.65 (m, 3H), 2.34–2.62 (m, 1H), 1.81–1.11 (m, 6H), 1.08–0.56 (m, 6H). 13C NMR (100 MHz, 0.1% MeOD-d4 in CDCl3), multiple conformers were present, δ (ppm): 172.7, 172.3, 172.2, 170.6, 170.3, 137.5, 129.7, 129.2, 129.1, 128.5, 128.3, 128.2, 126.8, 126.7, 72.0, 71.2, 61.6, 55.2, 52.6, 52.4, 52.3, 41.2, 39.5, 36.2, 31.7, 29.7, 29.4, 24.7, 22.7, 22.3, 22.2, 22.1, 19.3, 18.5, 14.7, 14.6, 14.1, 13.9, 13.6. ESI-MS m/z calc. for C42H60N8NaO8+: 827.44 ([2M + Na]+), found 827.50. The purity (91.7%) was determined by HPLC, linear gradient flow 1.25 mL/min, t = 0′ 30% B, t = 15′ 90% B, 25 °C, 220 nm, (rt = 7.41 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo [Ala-Leu-d-Phe-N-MeGly] (21): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 5 days), starting from the trifluoroacetate salt of 9: NH2-Ala-Leu-d-Phe-N-MeGly-OH (200 mg, 0.37 mmol); HBTU was used as coupling reagent. Purification by flash chromatography, AcOEt, rendered the desired macrocycle 21. White solid (76%). Rf = 0.3 (AcOEt:MeOH 1:0.1). 1H NMR (400 MHz, CDCl3), two conformers were observed a:b (1:0.3), δ (ppm): 8.08 (d, J = 7.7 Hz, 1Ha), 7.96 (bs, 1Hb), 7.78–7.69 (m, 1Ha,b), 7.37–7.13 (m, 5Ha,b), 5.48 (d, J = 17.2 Hz, 1Ha), 5.04 (d, J = 17.2 Hz, 1Hb), 4.85 (td, J = 9.9, 3.5 Hz, 1Ha), 4.75 (d, J = 10.1, 2.5 Hz, Hb), 4.60–4.52 (m, 1Ha), 4.52–4.45 (m, 1Hb), 4.17–4.09 (m, 1Ha,b), 3.39–3.31 (m, 1Hb), 3.21 (d, J = 17.2 Hz, 1Ha), 3.17–3.04 (m, 2Ha,b), 2.97–2.82 (m, 1Ha,b), 2.57 (s, 3Ha), 2.46 (s, 3Hb), 1.91–1.74 (m, 1Ha), 1.55 (d, J = 7.3 Hz, 3Ha,b), 1.45–1.19 (m, 2Ha,b), 0.93–0.80 (m, 6Ha,b). 13C NMR (100 MHz, CDCl3), two conformers were observed a:b (1:0.3), δ (ppm): 174.0 (Ca,b), 173.7 (Ca,b), 172.0 (Ca,b), 169.8 (Ca,b), 135.0 (Ca), 134.6 (Cb), 129.0 (Cb), 128.2 (Ca), 127.8 (Ca), 127.4 (Cb), 126.5 (Cb), 126.4 (Ca), 52.4 (Ca), 52.1 (Cb), 51.7 (Cb), 50.8 (Ca), 48.8 (Cb), 47.9 (Ca), 38.8 (Cb), 38.4 (Ca), 36.1 (Cb), 35.6 (Ca), 35.1 (Cb), 34.6 (Ca), 28.7 (Cb), 23.9 (Ca), 22.5 (Cb), 22.3 (Ca), 20.5 (Ca), 20.2 (Cb), 16.1 (Cb), 16.0 (Ca). ESI-MS m/z calc. for C21H31N4O4+: 403.23 ([M + H+]+), found 403.00. The purity (95.2%) was determined by HPLC, linear gradient, t = 0′ 30% B, t = 9′ 90% B, 25 °C, 220 nm, (rt = 7.26 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[Ala-Leu-N-MePhe-N-MeGly] (22): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 3 days), starting from the trifluoroacetate salt of 10: NH2-Ala-Leu-N-MePhe-N-MeGly-OH (200 mg, 0.36 mmol); HBTU was used as coupling reagent. Purification by flash chromatography, AcOEt:MeOH (3:0.1), rendered the desired macrocycle 22. White solid (33%). Rf = 0.6 (AcOEt:MeOH 3:0.1). 1H NMR (400 MHz, CDCl3), δ (ppm): 8.51 (bs, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.35–7.14 (m, 5H), 4.66 (dq, J = 13.4, 6.7 Hz, 1H), 3.92–3.88 (m, 1H), 3.84 (d, J = 18.5 Hz, 1H), 3.70 (d, J = 18.5 Hz, 1H), 3.64–3.53 (m, 1H), 3.48–3.34 (m, 2H), 3.06 (s, 3H), 2.40 (s, 3H), 2.00–1.87 (m, 1H), 1.78–1.60 (m, 2H), 1.25 (d, J = 6.5 Hz, 3H), 1.05 (d, J = 6.7 Hz, 3H), 0.86 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3), δ (ppm): 174.0, 172.2, 170.1, 167.2, 138.5, 129.7, 128.7, 127.0, 66.71, 54.7, 51.7, 45.7, 40.3, 38.6, 37.8, 37.6, 35.1, 24.5, 23.5, 21.0, 16.5. ESI-MS m/z calc. for C22H33N4O4+: 417.24 ([M + H+]+) found 417.65. The purity (92.8%) was determined by HPLC, isocratic flow 1mL/min, MeOH:H2O (70:30), 30 °C, 220 nm, (rt = 3.01 min).
Cyclo-[N-MeAla-Leu-Phe-N-MeGly] (23): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 3 days), starting from the trifluoroacetate salt of 11: N-MeAla-Leu-Phe-N-MeGly-OH (168 mg, 0.31 mmol); HBTU was used as coupling reagent. Purification by flash chromatography, AcOEt, rendered the desired macrocycle 23. White solid (38%). Rf = 0.5 (AcOEt). 1H NMR (400 MHz, CDCl3), multiple conformers were observed, δ (ppm): 7.94 (d, J = 9.9 Hz, 0.1 H), 7.66 (d, J = 7.7 Hz, 0.1 H), 7.49 (d, J = 8.9 Hz, 0.2 H), 7.36–7.12 (m, 5H), 7.04–6.96 (m, 0.5H), 6.89–6.81 (m, 0.5H), 6.29 (d, J = 9.1 Hz, 0.1 H), 6.04–5.92 (m, 0.2 H), 5.23–5.13 (m, 0.5 H), 5.12–5.06 (m, 0.2 H), 5.05–4.98 (m, 0.4H), 4.87 (q, J = 6.4 Hz, 0.2 H), 4.66–4.41 (m, 1H), 4.40–4.28 (m, 0.5 H), 3.36–2.72 (m, 9H), 2.07–1.97 (m, 0.25H), 1.87–1.80 (m, 0.5H), 1.74–1.67 (m, 0.5H), 1.55–1.47 (m, 1H), 1.46 (d, J = 7.2Hz, 1.2 H), 1.37 (d, J = 7.2 Hz, 1.8H), 1.32–1.2 (m, 1H), 1.03–0.76 (m, 6H). 13C NMR (100 MHz, CDCl3), multiple conformers were observed, δ (ppm): 172.7, 172.6, 172.3, 171.8, 171.8, 171.3, 171.3, 170.7, 170.2, 170.0, 168.9, 168.3, 137.5, 137.3, 136.7, 129.6, 129.5, 129.5, 129.1, 128.5, 128.4, 128.3, 126.8, 126.7, 126.5, 56.4, 54.4, 53.8, 53.0, 51.6, 51.3, 51.1, 50.4, 50.4, 49.6, 41.2, 40.1, 39.0, 38.8, 38.7, 38.6, 29.7, 28.9, 25.3, 25.0, 23.3, 23.0, 21.1, 14.8, 14.1, 13.2. ESI-MS m/z calc. for C22H33N4O4+: 417.24 ([M + H+]+), found 417.55. The purity (95.5%) was determined by HPLC, linear gradient, t = 0′ 30% B, t = 15′90% B, 220nm, (rt = 10.98 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[N-MeAla-Leu-N-MePhe-Gly] (24): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 84 h), starting from the trifluoroacetate salt of 12: N-MeAla-Leu-N-MePhe-Gly-OH (300 mg, 0.51 mmol); HATU was used as coupling reagent. Purification by flash chromatography, AcOEt, rendered the desired macrocycle 24. Yellow oil (47%). Rf = 0.5 (EtOAc). 1H NMR (400 MHz, CDCl3) δ (ppm): 7.81 (d, J = 9.3 Hz, 1H), 7.40–7.17 (m, 5H), 4.96 (dd, J = 15.2, 10.0 Hz, 1H), 4.62 (dd, J = 11.3, 3.5 Hz, 2H), 4.27 (q, J = 7.0 Hz, 1H), 3.70 (dd, J = 13.7, 1.5 Hz, 1H), 3.51 (d, J = 15.1 Hz, 1H), 2.96–2.85 (m, 1H), 2.83 (s, 3H), 2.77 (s, 3H), 1.73–1.63 (m, 1H), 1.56 (d, J = 7.1 Hz, 3H), 1.45–1.33 (m, 1H), 1.32–1.19 (m, 1H), 0.83 (d, J = 6.5 Hz, 3H), 0.77 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 172.3, 171.8, 170.6, 170.1, 137.1, 129.0, 128.2, 127.1, 63.0, 57.2, 48.4, 44.5, 41.0, 34.3, 30.6, 30.1, 24.6, 22.6, 22.4, 15.6. ESI-MS m/z calc. for C22H33N4O4: 417.25 ([M + H]+), found 417.30. The purity (95.8%) was determined by HPLC, linear gradient 1.25 mL/min, t = 0′ 30% B, t = 15′ 90% B, 30 °C, 220 nm, (rt = 5.34 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[N-MeAla-Phe-N-MePhe-Gly] (25): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 72 h), starting from the trifluoroacetate salt of 13: N-MeAla-Phe-N-MePhe-Gly-OH (220 mg, 0.38 mmol); HATU was used as coupling reagent. Purification by flash chromatography rendered the desired macrocycle 25. White solid (86%). Rf = 0.35 (AcOEt). 1H NMR (400 MHz, CDCl3) δ 1.20 (d, J = 7.0 Hz, 3H), 2,50 (s, 3H), 2.77 (s, 3H), 3.12 (d, J = 7.6 Hz, 2H), 3.34–3.42 (m, 1H), 3.46 (dd, J = 16.5, 1.9 Hz, 1H), 3.56 (dd, J = 14.0, 4.5 Hz, 1H), 3.77 (dd, J = 10.6, 4.1 Hz, 1H), 4.47 (dd, J = 16.6, 6.8 Hz, 1H), 5.13 (q, J = 7.68 Hz, 1H), 5.46 (q, J = 6.9 Hz, 1H), 6.88 (d, J = 6.8 Hz, 1H), 7.00- 7.11 (m, 2H), 7.12–7.24 (m, 3H), 7.28–7.32 (m, 2H), 7.32–7.43 (m, 3H), 7.58 (d, J = 9.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 13.6, 29.1, 33.8, 37.9, 39.2, 41.0, 50.6, 51.4, 68.6, 126.7, 126.8, 128.6, 128.7, 129.2, 129.9, 136.8, 138.7, 169.6, 169.6, 170.2, 171.4. ESI-MS m/z calc. for C25H31N4O4 450.23 ([M + H]+), found 450.85. The purity (95.6%) was determined by HPLC, linear gradient 1.25 mL/min, t = 0′30% B, t = 15′ 90% B, 30 °C, 220 nm, (rt = 11.25 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[N-Me-d-Phe-Ala-Phe-N-MeGly] (26): Solution phase macrocyclization reaction was carried out following general procedure Method I (dilution 5 mM, 24 h), starting from the trifluoroacetate salt of 14: N-Me-d-Phe-Ala-Phe-N-MeGly-OH (40 mg, 0.07 mmol); HATU was used as coupling reagent. Purification by flash chromatography rendered the desired macrocycle 26. White solid (17%). Rf = 0.4 (AcOEt:EP, 3:2). 1H NMR (400 MHz, CDCl3) δ 1.14 (d, J = 5.8 Hz, 3H), 2,98 (s, 3H), 2.95–3.08 (m, 3H), 3.10 (s, 3H), 3.23–3.37 (m, 2H), 4.39–4.60 (m, 3H), 5.41 (d, J = 14.5 Hz, 1H), 7.16–7.25 (m, 5H), 7.27–7.31 (m, 1H), 7.36–7.42 (m, 4H), 8.12 (s, 1H), 9.21 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 16.6, 29.7, 30.8, 34.3, 36.1, 38.5, 45.3, 51.2, 54.7, 62.0, 127.0, 127.6, 128.32, 128.8, 128.9, 129.0, 129.1, 129.8, 136.1, 137.2, 169.3, 169.5, 172.0, 173.9. ESI-MS m/z calc. for 451.23 C25H31N4O4 ([M + H]+) found 451.24. The purity (91.9%) was determined by HPLC, linear gradient 1.25 mL/min, t = 0′ 30% B, t = 15′ 90% B, 30 °C, 220 nm, (rt = 9.03 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
Cyclo-[Phe-N-MeGly-Cys(Bn)-N-MeGly] (27): Solution phase macrocyclization reaction was carried out following general procedure Method II (dilution 5 mM, 48 horas), starting from the trifluoroacetate salt of 15: NH2-Phe-N-MeGly-Cys(Bn)-N-MeGly-OH (196 mg, 0.31mmol). Oxyma/EDCI were used as coupling reagents. Purification by flash chromatography rendered the desired macrocycle 27. White solid (54%). Rf = 0.35 (AcOEt). 1H NMR (400 MHz, CDCl3) δ 2.64 (dd, J = 13.6, 6.5 Hz, 1H), 2.72–3.28 (m, 9H), 3.46 (d, J = 15.2 Hz, 3H), 3.64–3.82 (m, 3H), 4.20 (d, J = 14.9 Hz, 1H), 4.46 (d, J = 15.1 Hz, 1H), 4.87–5.00 (m, 1H), 5.02–5.19 (m, 1H), 6.97 (d, J = 8.2 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 7.14–7.24 (m, 4H), 7.27–7.40 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 32.8, 35.8, 36.1, 36.2, 37.9, 48.0, 50.0, 51.4, 52.1, 126.7, 126.8, 128.1, 128.2, 128.4, 128.5, 128.6, 129.0, 131.6, 131.8, 135.7, 137.5, 167.3, 167.4, 170.6, 171.6. ESI-MS m/z calc. for C50H60N8O8S2Na 987.4 ([2M + Na]+), found 987.5. The purity (94.3%) was determined by HPLC, linear gradient 1.25 mL/min, t0′—30% B, t15′—90% B, 30 °C, 220 nm, (rt = 10.18 min). A: H2O, 0.1% formic acid, B: MeCN, 0.1% formic acid.
3.2. Herbicidal Activity
The experiments to determine the herbicidal activity of the cyclopeptide compounds were carried out on Lolium multiflorum (Raygrass) plants. Germination, root length and leaf development were evaluated, compared to a control without herbicide, a negative control (100 μL DMSO in 15 mL agar, used as solvent) and an herbicide control (1/8 of the commercial dose of the herbicide S-metolachlor).
Serial experiments were conducted using the Agar germination methodology, where the tested compounds and the respective controls were placed in glass Petri dishes (6 cm diameter) in three replicates per treatment. Ten ryegrass seeds were germinated in a growth chamber (20 °C, day/night temperature). The seeds were previously sterilized by immersing them in 70% alcohol for 10 s. When distributed in the Petri dish on the agar, the seeds were placed in such a way as to ensure that they remained submerged in the solution.
Agar–water solution was prepared at 0.3%, 3 g of agar was placed in 1 liter of deionized water, and the solution was autoclaved at 100 °C for 45 min. Once the agar medium had cooled to approximately 60 °C, the solutions were prepared.
The negative control–DMSO control was prepared by adding 100 μL of DMSO per plate in 15 mL of agar and then the seeds were distributed, as mentioned above. A control without DMSO was also carried out to check that the product was not altering the correct development of the ryegrass seeds. For this test, 15 ml of agar were placed in each Petri dish and, before it solidified, the seeds of the species evaluated were placed on top. The herbicide treatment, positive control–Control S-Metolachlor (960 g/L), was carried out for a conversion of 1/8 of the dose of 1 L/ha of commercial product. For this purpose, a stock solution of S-Metolachlor was prepared by placing 0.28 mL of the herbicide in a volumetric flask and topping up to 1000 mL. A total of 25 mL of this stock solution were taken, placed in a volumetric flask and brought to 200 mL, thus, generating the 1/8x solution of S-Metolachlor. A volume of 3 mL of 1/8x herbicide solution was mixed with 45 mL of the agar solution to bring 16 mL into each Petri dish. This ensured that there was 1 mL of 1/8x S-Metolachlor solution per plate: 0.28 μL of herbicide solution. Seeds were arranged in the same way. Using the same method, the agar media corresponding to each plate (15 mL) was mixed with the cyclopeptides dissolved in 100 μL of DMSO.
Germination, root length and leaf development were evaluated 12 days after preparation. The variables of germinated plants and plants with developed leaves of the total number of plants placed to germinate were analyzed by fitting a generalized linear model, since they presented a binomial distribution.
Glinmix procedure of the SAS statistical package. Based on the model and for the comparison of the treatments with the different controls, the contrasts of interest were carried out. The effect of the treatments on the root length variable was studied by comparing means using a Tukey test (p-value < 0.05) in INFOSTAT.
4. Conclusions
In summary, cyclopeptides and their open precursors, analogues of tentoxin and versicotide D were successfully synthesized by SPPS of their linear precursor and solution-phase macrolactamization. After evaluating the herbicidal activity of cyclopeptides analogues of tentoxin, we can conclude that 19 and 24 are the most active of them showing remarkable phytotoxic activity. Cyclotetrapeptides 19 and 24 present one (N-Methyl- D-Phe) and two N-MeAA (N-Methyl-Ala and N-Methyl-Phe), respectively. The cyclopeptide without N-MeAA (16) is inactive. In two cases, the open precursors (7 and 13) are as active as their corresponding cyclopeptide (19 and 25). However, many linear peptides are inactive and their cyclization derivatives showed herbicidal activity. An example of these observed results is 24 and its open precursor 12, which are the most active and one of the less active compounds, respectively. Moreover, the cyclopeptide analogues of versicotide D, 25 and 26, showed improved activity over the natural product.
All these findings allowed us to conclude that the conformation adopted by the peptides and cyclopeptides would have great influence on the herbicidal activity. As a consequence, the peptide sequence, the amino acid stereochemistry and the presence of N-methyl group would play an important role on the phytotoxic activity of this type of compound.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217350/s1, 1. General Experimental information; 2. Solid Phase Peptide Synthesis and Solution Phase Macrocyclization; 3. Characterization Data of Products; 4. NMR Spectra, ESI-MS and chromatographic data; 5. Procedure for evaluation of phytotoxic activity.
Author Contributions
Data curation, C.I., L.R., L.S. and D.D.; Formal analysis, L.S. and D.D.; Funding acquisition, G.S.; Investigation, C.I., L.P. and G.S.; Methodology, C.I., L.P., L.R. and J.V.; Project administration, G.S.; Supervision, J.V. and G.S.; Writing—review & editing, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCE-ANII grant number 2019_155516, CSIC Grupos-UdelaR grant number 250725 and PEDECIBA. And The APC was funded by Universidad de la República and PEDECIBA.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge a postgraduate fellowship from ANII (Camila Irabuena).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Lopes, F.M.; Sandrini, J.Z.; Souza, M.M. Toxicity Induced by Glyphosate and Glyphosate-Based Herbicides in the Zebrafish Hepatocyte Cell Line (ZF-L). Ecotoxicol. Environ. Saf. 2018, 162, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Peillex, C.; Pelletier, M. The Impact and Toxicity of Glyphosate and Glyphosate-Based Herbicides on Health and Immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Asaduzzaman, M.; Parven, A.; Megharaj, M. Controversies over Human Health and Ecological Impacts of Glyphosate: Is It to Be Banned in Modern Agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef]
- Wheeler, W.B. Role of Research and Regulation in 50 Years of Pest Management in Agriculture Prepared for the 50th Anniversary of the Journal of Agricultural and Food Chemistry. J. Agric. Food Chem. 2002, 50, 4151–4155. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paer, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Heap, I. Global Perspective of Herbicide-Resistant Weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The Challenge of Herbicide Resistance around the World: A Current Summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Green, J.M.; Owen, M.D.K. Herbicide-Resistant Crops: Utilities and Limitations for Herbicide-Resistant Weed Management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef]
- Davis, A.S.; Frisvold, G.B. Are Herbicides a Once in a Century Method of Weed Control? Pest Manag. Sci. 2017, 73, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Why Have No New Herbicide Modes of Action Appeared in Recent Years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Strek, H.J. Herbicide Discovery in Light of Rapidly Spreading Resistance and Ever-Increasing Regulatory Hurdles. Pest Manag. Sci. 2018, 74, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Boari, A. Natural Compounds for Novel Strategies of Parasitic Plant Management. In Natural Products for Pest Management; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; Volume 927, pp. 76–87. [Google Scholar] [CrossRef]
- Evidente, A. Chemical and Biological Characterization of Toxins Produced by Weed Pathogenic Fungi as Potential Natural Herbicides. In Natural Products for Pest Management; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; Volume 927, pp. 62–75. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Yin, Y.; Suga, H. Macrocyclic Peptides as Drug Candidates: Recent Progress and Remaining Challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181. [Google Scholar] [CrossRef]
- Staderini, M.; Megia-Fernandez, A.; Dhaliwal, K.; Bradley, M. Peptides for Optical Medical Imaging and Steps towards Therapy. Bioorg. Med. Chem. 2018, 26, 2816–2826. [Google Scholar] [CrossRef]
- Brea, R.J.; Reiriz, C.; Granja, J.R. Towards Functional Bionanomaterials Based on Self-Assembling Cyclic Peptide Nanotubes. Chem. Soc. Rev. 2010, 39, 1448–1456. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding Cell Penetration of Cyclic Peptides. Chem. Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef]
- Sarojini, V.; Cameron, A.J.; Varnava, K.G.; Denny, W.A.; Sanjayan, G. Cyclic Tetrapeptides from Nature and Design: A Review of Synthetic Methodologies, Structure, and Function. Chem. Rev. 2019, 119, 10318–10359. [Google Scholar] [CrossRef]
- Martì-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem. Rev. 2015, 115, 8736–8834. [Google Scholar] [CrossRef] [PubMed]
- Lax, A.R.; Shepherd, H.S.; Edwards, J.V. Tentoxin, A Chlorosis-Inducing Toxin from Alternaria as a Potential Herbicide. Weed Technol. 1988, 2, 540–544. [Google Scholar] [CrossRef]
- Hessel-Pras, S.; Kieshauer, J.; Roenn, G.; Luckert, C.; Braeuning, A.; Lampen, A. In Vitro Characterization of Hepatic Toxicity of Alternaria Toxins. Mycotoxin Res. 2019, 35, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Rich, D.H.; Mathiaparanam, P. Synthesis of the Cyclic Tetrapeptide Tentoxin. Effect of an N-Methyldehydrophenylalanyl Residue on Conformation of Linear Tetrapeptides. Tetrahedron Lett 1974, 15, 4037–4040. [Google Scholar] [CrossRef]
- Jiménez, J.C.; Chavarría, B.; López-Macià, À.; Royo, M.; Giralt, E.; Albericio, F. Tentoxin as a Scaffold for Drug Discovery. Total Solid-Phase Synthesis of Tentoxin and a Library of Analogues. Org. Lett. 2003, 5, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, N.; Cavelier, F.; Noel, J.-P.; Gomis, J.-M. High Yield Synthesis of Tentoxin, a Cyclic Tetrapeptide. J. Pept. Sci. 2002, 8, 335–346. [Google Scholar] [CrossRef]
- Cavelier, F.; Verducci, J. New Synthesis of the Cyclic Tetrapeptide Tentoxin Employing an Azlactone as Key Intermediate. Tetrahedron Lett. 1995, 36, 4425–4428. [Google Scholar] [CrossRef]
- Edwards, J.V.; Lax, A.R.; Lillehoj, E.B.; Boudreaux, G.J. New Synthesis and Biological Activity of the Cyclic Tetrapeptides Tentoxin and [Pro 1] Tentoxin. Int. J. Pept. Protein Res 1986, 28, 603–612. [Google Scholar] [CrossRef]
- Posada, L.; Serra, G. First Total Synthesis of Versicotide D and Analogs. Tetrahedron Lett. 2019, 60, 151281. [Google Scholar] [CrossRef]
- Posada, L.; Rey, L.; Villalba, J.; Colombo, S.; Aubriot, L.; Badagian, N.; Brena, B.; Serra, G. Cyclopeptides Natural Products as Herbicides and Inhibitors of Cyanobacteria: Synthesis of Versicotides E and F. Chem. Sel. 2022, 7, e202201956. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D.; Schäfer, W. Synthesis of prothymosin a (ProTα)—A protein consisting of 109 amino acid residues. Angew. Chem. Int. Ed. Engl. 1991, 30, 590. [Google Scholar] [CrossRef]
- Schmidt, U.; Langner, J. Cyclotetrapeptides and cyclopentapeptides: Occurrence and synthesis. J. Pep. Res. 1997, 49, 67. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Yudin, A. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).