Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f.
Abstract
1. Introduction
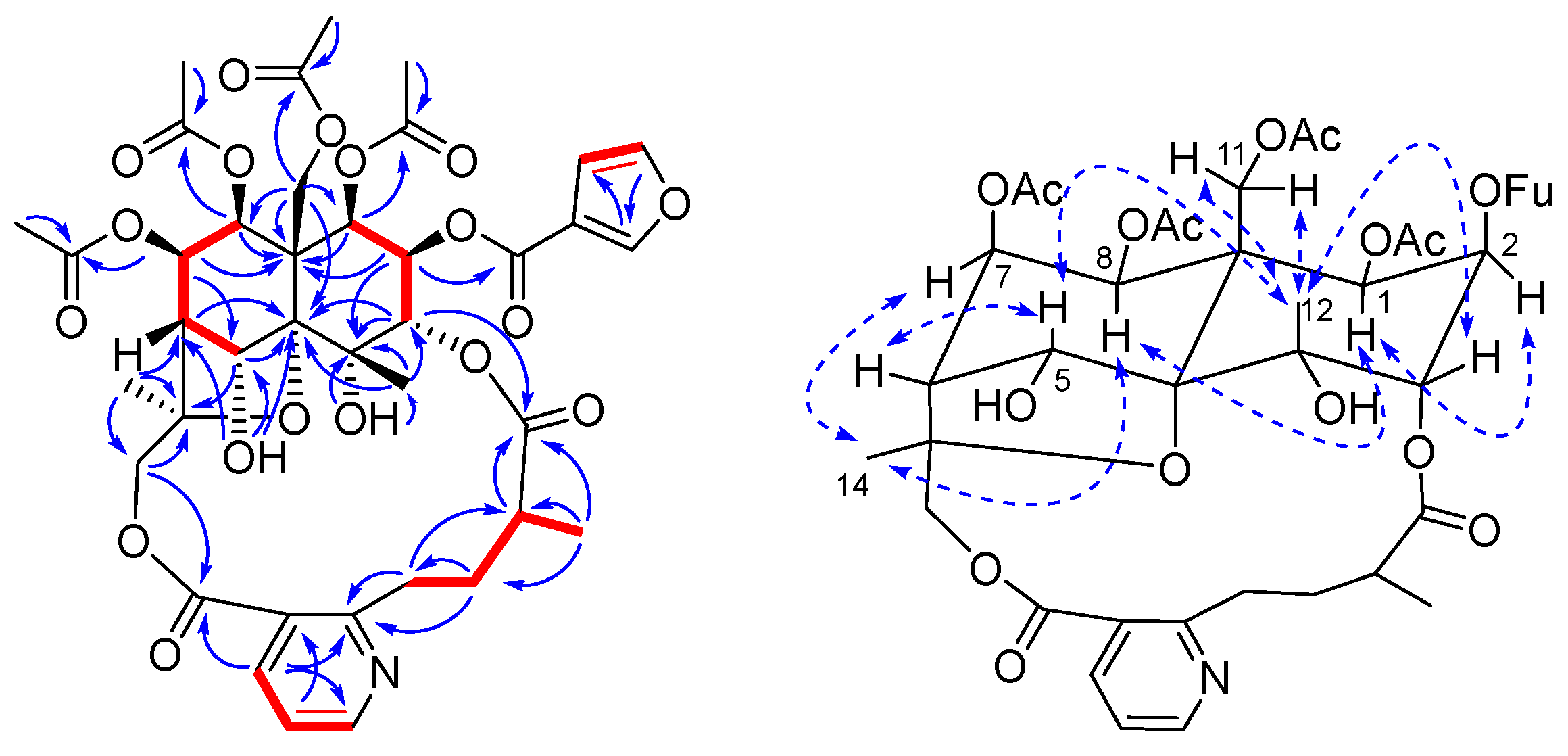
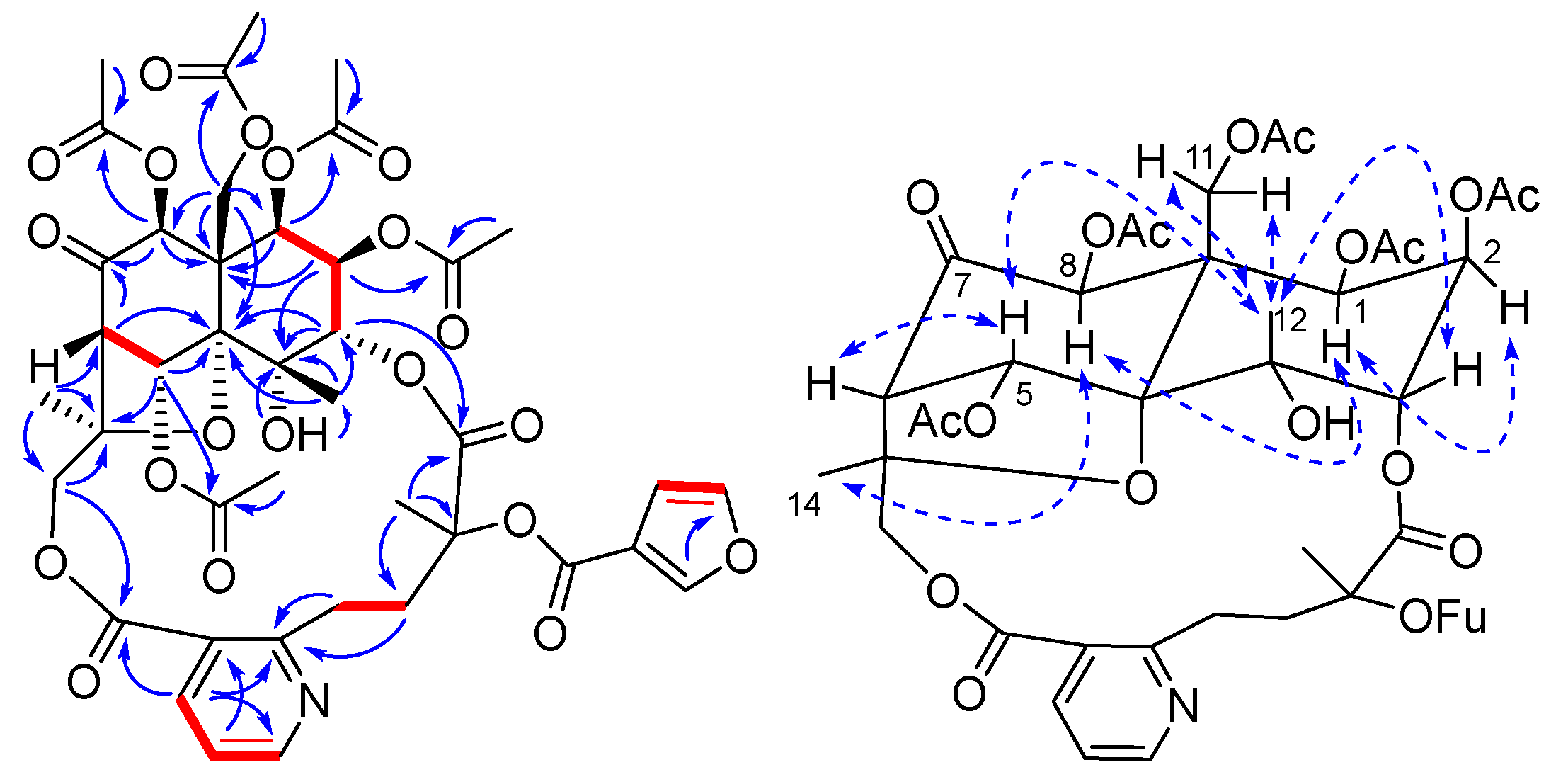
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–10
3.5. Cell Viability Assay
3.6. Immunosuppressive Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Luo, D.; Zuo, Z.; Zhao, H.; Tan, Y.; Xiao, C. Immunoregulatory effects of Tripterygium wilfordii Hook F and its extracts in clinical practice. Front. Med. 2019, 13, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Y.; Xu, Y.G.; Lu, Y.Q. Use of Tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ. Sci. B 2020, 21, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, X.X.; Bai, M.; Wu, J.; Li, J.Y.; Liu, Q.B.; Li, L.Z.; Song, S.J. Anti-inflammatory sesquiterpene pyridine alkaloids from Tripterygium wilfordii. Fitoterapia 2015, 105, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pu, X.; Luo, G.; Zhou, M.; Ye, Q.; Liu, Y.; Gu, J.; Qi, H.; Li, G.; Zhang, G. Nitrogen-containing dihydro-β-agarofuran derivatives from Tripterygium wilfordii. J. Nat. Prod. 2014, 77, 1650–1657. [Google Scholar] [CrossRef]
- Ye, H.L.; Liu, Y.; Pan, J.; Guan, W.; Liu, Y.; Li, X.M.; Wang, S.Y.; Algradi, A.M.; Yang, Y.; Kuang, H.X. Three new sesquiterpenoid alkaloids from the roots of Tripterygium wilfordii and its cytotoxicity. Nat. Prod. Res. 2022, 36, 3979–3987. [Google Scholar] [CrossRef]
- Gao, C.; Lou, L.L.; Wang, D.; Zhang, Y.; Huang, X.X.; Song, S.J. Chemical constituents from the roots of Tripterygium wilfordii and their cytotoxic activity. J. Asian Nat. Prod. Res. 2017, 19, 725–731. [Google Scholar] [CrossRef]
- Horiuch, M.; Murakami, C.; Fukamiya, N.; Yu, D.; Chen, T.H.; Bastow, K.F.; Zhang, D.C.; Takaishi, Y.; Imakura, Y.; Lee, K.H. Tripfordines A-C, sesquiterpene pyridine alkaloids from Tripterygium wilfordii, and structure anti-HIV activity relationships of Tripterygium alkaloids. J. Nat. Prod. 2006, 69, 1271–1274. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Imakura, Y.; Jia, Y.; Li, D.; Cosentino, L.M.; Lee, K.H. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: A new class of potent anti-HIV agents. J. Nat. Prod. 2000, 63, 357–361. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Wu, W.; Ji, Z.; Hu, Z. Insecticidal sesquiterpene pyridine alkaloids from Euonymus species. Phytochemistry 2002, 61, 699–704. [Google Scholar]
- Gao, J.M.; Wu, W.J.; Zhang, J.W.; Konishi, Y. The dihydro-beta-agarofuran sesquiterpenoids. Nat. Prod. Rep. 2007, 24, 1153–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Li, H.; Zhang, X.; Xia, Y.; Chu, K.; Chen, L. Therapeutic effects of total alkaloids of Tripterygium wilfordii Hook f. on collagen-induced arthritis in rats. J. Ethnopharm. 2013, 145, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Xia, Z.; Xu, R.; Chen, J. The structures of triptotetraolide and wilforjine. Acta Bot. Sin. 1992, 34, 618–621. [Google Scholar]
- Sugiura, K.; Yamada, K.; Hirata, Y. Isoevonine, a novel alkaloid from Enonymus europaea containing wilfordic acid. Tetrahedron Lett. 1973, 14, 113–116. [Google Scholar] [CrossRef]
- Morota, T.; Yang, C.X.; Ikeya, Y.; Qin, W.Z.; Nishimura, H.; Xu, L.H.; Ando, M.; Miao, K.L.; Maruno, M.; Yang, B.H. Chemical studies on the root bark of Tripterigium wilfordii 3. Sesquiterpene alkaloids from Tripterigium-wilfordii. Phytochemistry 1995, 39, 1219–1222. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Momota, H.; Ohmoto, Y.; Taki, T.; Jia, Y.; Li, D. Immunosuppressive sesquiterpene alkaloids from Tripterygium wilfordii. J. Nat. Prod. 2001, 64, 582–587. [Google Scholar] [CrossRef]
- Ye, H.; Ignatova, S.; Luo, H.; Li, Y.; Peng, A.; Chen, L.; Sutherland, I. Preparative separation of a terpenoid and alkaloids from Tripterygium wilfordii Hook. f. using high-performance counter-current chromatography. Comparison of various elution and operating strategies. J. Chromatogr. A 2008, 1213, 145–153. [Google Scholar] [CrossRef]
- He, Z.S.; Li, Y.; Fang, S.D.; Hong, S.H. Structures of wilforgine, wilforzine and wilformine from Tripterygium wilfordii. Acta Chim. Sin. 1987, 45, 510–513. [Google Scholar]
- Nunez, M.J.; Guadano, A.; Jimenez, I.A.; Ravelo, A.G.; Gonzalez-Coloma, A.; Bazzocchi, I.L. Insecticidal sesquiterpene pyridine alkaloids from Maytenus chiapensis. J. Nat. Prod. 2004, 67, 14–18. [Google Scholar] [CrossRef]
- Wu, C.M.; Zhou, L.M.; Chai, Y.F.; Wu, Y.T.; Fan, G.R. Three new sesquiterpene alkaloids from the root of Tripterygium wilfordii. Chin. Chem. Lett. 2010, 21, 830–833. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Zheng, X. Isolation of wilforgine and wilforine and the determination of their structures. Acta Sci. Nat. Univ. Pek. 1988, 24, 392–397. [Google Scholar]
- Duan, H.; Takaishi, Y.; Bando, M.; Kido, M.; Imakura, Y.; Lee, K.H. Novel sesquiterpene esters with alkaloid and monoterpene and related compounds from Tripterygium hypoglaucum: A new class of potent anti-HIV agents. Tetrahedron Lett. 1999, 40, 2969–2972. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Liu, X.; Wu, X.; Huang, L.; Gao, W. Triptolide: Pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 2021, 11, 7199–7221. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic compounds isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef]
- Chang, S.F.; Yang, L.M.; Lo, C.H.; Liaw, J.H.; Wang, L.H.; Lin, S.J. Microbial transformation of isosteviol and bioactivities against the glucocorticoid/androgen response elements. J. Nat. Prod. 2008, 71, 87–92. [Google Scholar] [CrossRef]
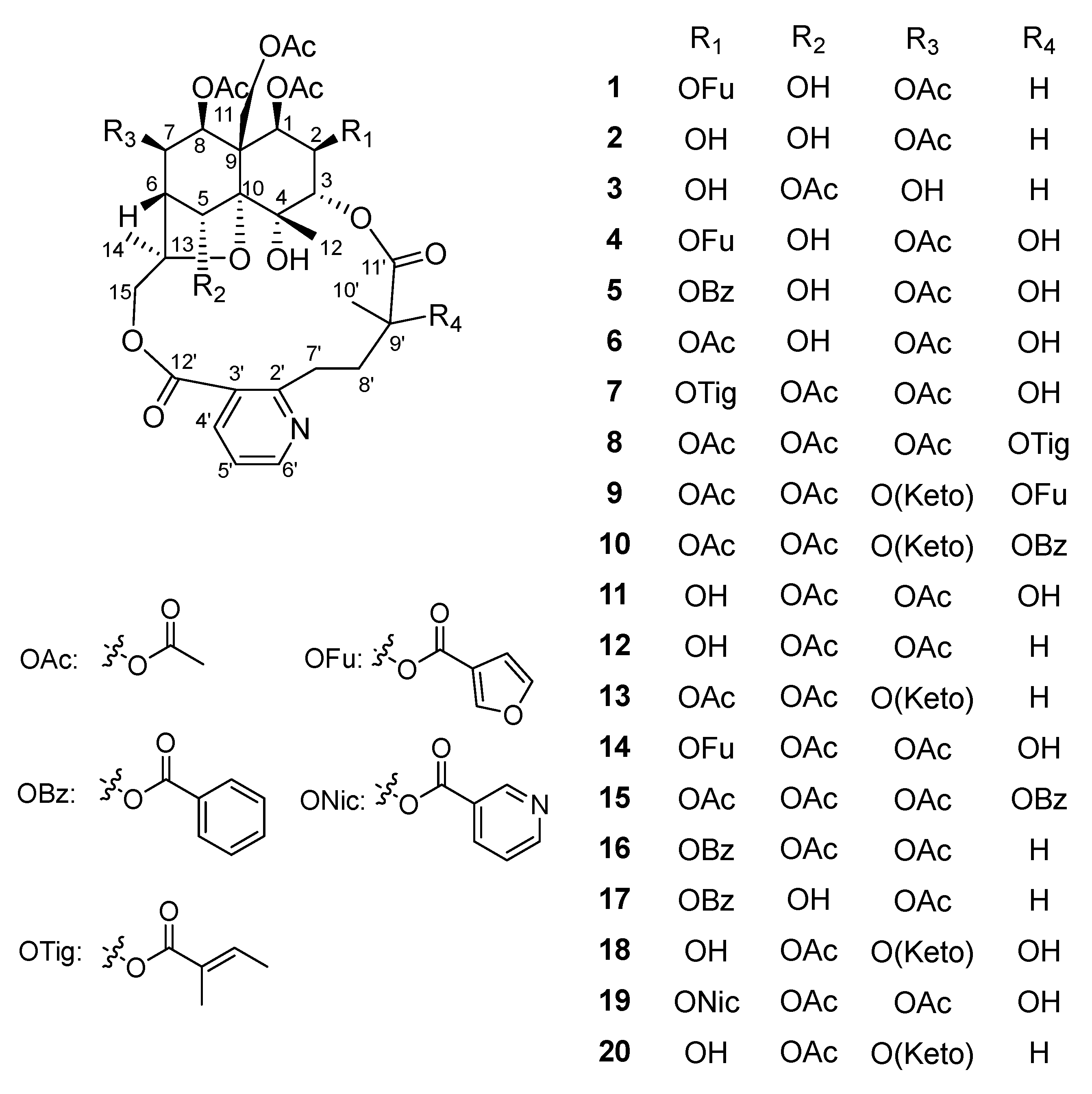
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1 | 5.71, d (3.6) | 5.58, d (3.6) | 5.57, d (3.6) | 5.70, d (3.6) | 5.76, d (3.6) | 5.57, d (3.0) | 5.63, d (3.6) | 5.56, d (4.2) | 5.65, d (3.0) | 5.76, d (3.6) |
| 2 | 5.37, t (3.0) | 3.97, q (2.4) | 3.97, t (3.0) | 5.35, t (3.6) | 5.47, t (3.0) | 5.13, t (3.0) | 5.24, t (3.0) | 5.11, t (2.4) | 5.17, t (3.0) | 5.47, t (3.0) |
| 3 | 5.08, d (2.4) | 5.10, d (3.0) | 5.01, d (3.0) | 5.10, d (3.0) | 5.16, d (3.0) | 5.00, d (2.4) | 4.97, d (3.0) | 4.93, d (2.4) | 4.95, d (2.4) | 4.98, d (3.0) |
| 5 | 5.38, d (2.4) | 5.29, d (1.8) | 6.70, s | 5.33, d (3.6) | 5.26, d (3.0) | 5.35, s | 6.95, s | 6.89, s | 6.66, s | 6.63, s |
| 6 | 2.44, d (4.2) | 2.42, d (3.6) | 2.36, d (3.6) | 2.46, d (3.6) | 2.47, d (4.2) | 2.46, d (3.6) | 2.36, d (4.2) | 2.32, d (4.2) | 2.96, s | 2.96, s |
| 7 | 5.52, dd (5.4, 4.2) | 5.52, dd (5.4, 4.2) | 4.38, d (4.2) | 5.50, dd (5.4, 4.2) | 5.51, t (3.0) | 5.48, d (3.6) | 5.52, dd (5.4, 4.2) | 5.55, dd (6.0, 3.6) | ||
| 8 | 5.39, d (6.0) | 5.34, d (6.0) | 5.31, d (6.0) | 5.36, d (5.4) | 5.35, d (6.0) | 5.32, d (6.0) | 5.37, d (6.0) | 5.28, d (6.0) | 5.47, s | 5.41, s |
| 11a | 5.43, d (13.2) | 5.34, d (12.6) | 5.37, d (13.2) | 5.46, d (13.2) | 5.47, d (13.2) | 5.23, d (13.2) | 5.33, d (13.2) | 5.19, d (13.2) | 4.82, d (13.2) | 4.80, d (13.2) |
| 11b | 4.37, d (13.2) | 4.61, d (12.6) | 4.45, d (13.2) | 4.38, d (13.2) | 4.48, d (13.2) | 4.52, d (13.2) | 4.37, d (13.2) | 4.46, d (13.2) | 4.43, d (13.2) | 4.43, d (13.2) |
| 12 | 1.89, s | 1.90, d (0.6) | 1.57, s | 1.94, d (1.2) | 2.04, s | 1.89, s | 1.61, s | 1.56, s | 1.63, s | 1.64, s |
| 14 | 1.67, s | 1.64, s | 1.59, s | 1.64, s | 1.62, s | 1.61, s | 1.64, s | 1.57, s | 1.36, s | 1.22, s |
| 15a | 5.87, d (12.6) | 5.86, d (12.0) | 5.68, d (12.0) | 5.90, d (12.6) | 5.90, d (12.6) | 5.96, d (12.6) | 5.86, d (12.0) | 5.49, d (12.0) | 5.80, d (12.0) | 5.82, d (12.6) |
| 15b | 3.73, d (12.6) | 3.72, d (12.0) | 3.86, d (12.0) | 3.70, d (12.6) | 3.71, d (12.6) | 3.73, d (12.6) | 3.71, d (12.0) | 3.92, d (12.0) | 3.78, d (12.0) | 3.72, d (12.6) |
| 4′ | 8.37, d (7.8) | 8.34, dd (7.8, 1.8) | 8.30, dd (7.8, 1.8) | 8.15, dd (7.8, 1.8) | 8.16, d (7.8) | 8.47, d (7.8) | 8.20, d (7.8) | 8.13, dd (7.8, 1.8) | 8.17, dd (7.8, 1.8) | 8.14, dd (7.8, 1.8) |
| 5′ | 7.29, dd (7.8, 4.8) | 7.28, dd (7.8, 4.8) | 7.27, dd (7.8, 4.8) | 7.22, dd (7.8, 4.8) | 7.22, dd (7.8, 4.8) | 7.52, dd (7.8, 4.8) | 7.28, dd (7.8, 4.8) | 7.28, dd (7.8, 4.8) | 7.34, dd (7.8, 4.8) | 7.35, dd (7.8, 4.8) |
| 6′ | 8.76, d (4.8) | 8.75, dd (4.8, 1.8) | 8.74, dd (4.8, 1.8) | 8.69, dd (4.8, 1.8) | 8.70, d (4.8) | 8.92, d (4.8) | 8.75, d (4.8) | 8.72, dd (4.8, 1.8) | 8.77, dd (4.8, 1.8) | 8.79, dd (4.8, 1.8) |
| 7′a | 4.09, m | 4.05, m | 3.85, m | 4.09, m | 4.09, m | 4.22, m | 4.09, m | 3.61, m | 3.82, m | 3.83, m |
| 7′b | 2.87, m | 2.87, m | 2.99, m | 2.84, m | 2.85, m | 3.10, m | 2.92, m | 2.95, m | 2.98, m | 3.00, m |
| 8′a | 2.38, m | 2.33, m | 2.22, m | 2.45, m | 2.45, m | 2.69, m | 2.55, m | 2.65, m | 2.67, m | 2.73, m |
| 8′b | 1.85, m | 1.87, m | 2.03, m | 2.16, m | 2.19, m | 2.10, m | 2.16, m | 2.20, m | 2.28, m | 2.36, m |
| 9′ | 2.33, m | 2.28, m | 2.38, m | |||||||
| 10′ | 1.18, d (6.6) | 1.12, d (6.6) | 1.16, d (7.2) | 1.48, s | 1.51, s | 1.45, s | 1.45, s | 1.73, s | 1.81, s | 1.84, s |
| 1-OAc | 1.85, s | 1.95, s | 2.00, s | 1.87, s | 1.87, s | 1.87, s | 1.86, s | 1.82, s | 1.80, s | 2.17, s |
| 2-OAc | 2.15, s | 2.15, s | 2.16, s | 1.65, s | ||||||
| 5-OAc | 2.16, s | 2.19, s | 2.16, s | 2.20, s | 2.20, s | |||||
| 7-OAc | 2.15, s | 2.16, s | 2.17, s | 2.17, s | 2.17, s | 2.17, s | 2.15, s | |||
| 8-OAc | 1.93, s | 2.17, s | 2.32, s | 1.93, s | 1.88, s | 1.95, s | 2.18, s | 1.96, s | 2.03, s | 1.99, s |
| 11-OAc | 2.07, s | 1.96, s | 2.13, s | 2.02, s | 1.76, s | 2.16, s | 1.98, s | 2.26, s | 2.00, s | 1.98, s |
| 2-OFu-2 | 8.20, s | 8.17, br s | ||||||||
| 2-OFu-4 | 6.80, t (0.6) | 6.78, br s | ||||||||
| 2-OFu-5 | 7.49, s | 7.50, t (1.8) | ||||||||
| 2-OBz-2, 6 | 8.01, d (8.4) | |||||||||
| 2-OBz-3, 5 | 7.50, d (7.8) | |||||||||
| 2-OBz-4 | 7.63, t (7.8) | |||||||||
| 2-OTig-1 | 1.88, s | |||||||||
| 2-OTig-3 | 6.96, q (7.2) | |||||||||
| 2-OTig-4 | 1.87, d (7.8) | |||||||||
| 9′-OTig-1 | 1.75, s | |||||||||
| 9′-OTig-3 | 6.71, q (6.6) | |||||||||
| 9′-OTig-4 | 1.74, d (6.6) | |||||||||
| 9′-OFu-2 | 7.82, s | |||||||||
| 9′-OFu-4 | 6.59, d (1.2) | |||||||||
| 9′-OFu-5 | 7.32, t (1.8) | |||||||||
| 9′-OBz-2, 6 | 7.81, dd (8.4, 1.2) | |||||||||
| 9′-OBz-3, 5 | 7.35, t (7.8) | |||||||||
| 9′-OBz-4 | 7.49, t (7.8) | |||||||||
| 4-OH | 6.37, s | 6.19, d (1.2) | 4.69, d (1.2) | 6.30, d (1.2) | 6.31, s | 6.07, s | 5.03, s | 4.15, s | 4.71, s | 4.74, s |
| 5-OH | 6.01, s | 6.02, d (3.6) | 5.82, d (3.6) | 5.81, d (3.6) |
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 1 | 73.5, CH | 75.7, CH | 75.0, CH | 73.1, CH | 73.2, CH | 73.2, CH | 73.2, CH | 72.3, CH | 70.3, CH | 70.3, CH |
| 2 | 69.3, CH | 70.6, CH | 70.4, CH | 68.9, CH | 69.4, CH | 68.7, CH | 68.6, CH | 69.6, CH | 69.5, CH | 69.5, CH |
| 3 | 75.0, CH | 76.6, CH | 77.6, CH | 76.0, CH | 76.1, CH | 76.1, CH | 77.0, CH | 78.2, CH | 77.8, CH | 77.8, CH |
| 4 | 71.7, C | 71.8, C | 69.5, C | 71.6, C | 71.6, C | 71.8, C | 69.8, C | 69.7, C | 69.9, C | 69.9, C |
| 5 | 74.2, CH | 74.1, CH | 72.7, CH | 74.1, CH | 74.1, CH | 74.1, CH | 73.6, CH | 73.9, CH | 73.8, CH | 73.8, CH |
| 6 | 52.5, CH | 52.3, CH | 53.7, CH | 52.4, CH | 52.3, CH | 52.0, CH | 51.1, CH | 51.1, CH | 62.2, CH | 62.2, CH |
| 7 | 69.1, CH | 69.2, CH | 67.8, CH | 69.0, CH | 69.2, CH | 69.0, CH | 68.9, CH | 68.8, CH | 195.5, C | 195.7, C |
| 8 | 71.0, CH | 71.5, CH | 73.9, CH | 70.9, CH | 71.2, CH | 70.9, CH | 70.7, CH | 71.6, CH | 79.4, CH | 79.4, CH |
| 9 | 50.8, C | 51.1, C | 53.4, C | 50.7, C | 50.5, C | 50.8, C | 52.0, C | 52.2, C | 52.5, C | 52.5, C |
| 10 | 92.6, C | 93.0, C | 93.8, C | 93.0, C | 93.0, C | 93.5, C | 94.3, C | 93.4, C | 94.9, C | 94.9, C |
| 11 | 60.9, CH2 | 61.1, CH2 | 61.3, CH2 | 60.9, CH2 | 61.1, CH2 | 60.5, CH2 | 60.4, CH2 | 60.3, CH2 | 60.2, CH2 | 60.2, CH2 |
| 12 | 23.4, CH3 | 23.7, CH3 | 23.1, CH3 | 23.4, CH3 | 23.9, CH3 | 23.1, CH3 | 22.5, CH3 | 23.2, CH3 | 24.1, CH3 | 24.1, CH3 |
| 13 | 85.0, C | 84.9, C | 84.2, C | 85.3, C | 85.4, C | 85.1, C | 84.7, C | 84.2, C | 86.4, C | 86.4, C |
| 14 | 18.2, CH3 | 18.2, CH3 | 17.8, CH3 | 18.0, CH3 | 18.0, CH3 | 18.4, CH3 | 17.9, CH3 | 17.8, CH3 | 18.2, CH3 | 18.1, CH3 |
| 15 | 71.1, CH2 | 71.1, CH2 | 70.4, CH2 | 70.7, CH2 | 70.7, CH2 | 71.5, CH2 | 70.0, CH2 | 70.2, CH2 | 70.3, CH2 | 70.2, CH2 |
| 2′ | 165.2, C | 164.9, C | 163.5, C | 165.4, C | 165.3, C | 164.8, C | 164.9, C | 161.5, C | 162.4, C | 162.2, C |
| 3′ | 123.8, C | 124.1, C | 124.8, C | 125.1, C | 124.1, C | 127.6, C | 123.9, C | 125.0, C | 125.0, C | 125.1, C |
| 4′ | 138.7, CH | 138.6, CH | 138.8, CH | 137.9, CH | 137.9, CH | 141.5, CH | 138.5, CH | 138.6, CH | 138.5, CH | 138.2, CH |
| 5′ | 121.2, CH | 121.2, CH | 121.2, CH | 120.7, CH | 120.7, CH | 122.3, CH | 120.9, CH | 121.3, CH | 121.4, CH | 121.3, CH |
| 6′ | 153.6, CH | 153.4, CH | 152.9, CH | 152.5, CH | 152.5, CH | 148.2, CH | 152.5, CH | 152.4, CH | 152.4, CH | 152.6, CH |
| 7′ | 33.0, CH2 | 32.9, CH2 | 33.5, CH2 | 31.5, CH2 | 31.6, CH2 | 28.7, CH2 | 38.2, CH2 | 38.1, CH2 | 37.9, CH2 | 38.1, CH2 |
| 8′ | 33.5, CH2 | 33.5, CH2 | 33.2, CH2 | 39.0, CH2 | 39.1, CH2 | 38.4, CH2 | 33.5, CH2 | 33.2, CH2 | 39.0, CH2 | 39.1, CH2 |
| 9′ | 38.1, CH | 38.3, CH | 38.7, CH | 78.0, C | 77.9, C | 78.2, C | 78.0, C | 80.5, C | 81.5, C | 81.8, C |
| 10′ | 19.0, CH3 | 18.8, CH3 | 18.5, CH3 | 27.5, CH3 | 27.3, CH3 | 28.5, CH3 | 28.4, CH3 | 21.9, CH3 | 22.1, CH3 | 22.1, CH3 |
| 11′ | 175.3, C | 175.7, C | 175.6, C | 172.6, C | 172.6, C | 172.0, C | 172.3, C | 171.6, C | 171.2, C | 171.3, C |
| 12′ | 167.1, C | 167.1, C | 167.2, C | 168.4, C | 168.3, C | 166.7, C | 167.5, C | 167.7, C | 167.4, C | 167.5, C |
| 1-OAc | 20.5, CH3 /169.5, C | 20.5, CH3 /169.5, C | 20.9, CH3 /169.5, C | 20.5, CH3 /169.9, C | 20.3, CH3 /170.2, C | 20.5, CH3 /169.9, C | 20.5, CH3 /169.6, C | 20.3, CH3 /168.1, C | 20.0, CH3 /168.0, C | 20.0, CH3 /168.0, C |
| 2-OAc | 21.3, CH3 /168.6, C | 21.0, CH3 /168.3, C | 21.0, CH3 /168.1, C | 21.0, CH3 /167.8, C | ||||||
| 5-OAc | 21.6, CH3 /169.9, C | 21.6, CH3 /169.8, C | 21.3, CH3 /169.8, C | 21.4, CH3 /169.3, C | 21.4, CH3 /169.3, C | |||||
| 7-OAc | 21.0, CH3 /170.0, C | 21.0, CH3 /170.0, C | 21.0, CH3 /170.0, C | 20.9, CH3 /170.0, C | 21.0, CH3 /170.0, C | 21.0, CH3 /170.1, C | 21.1, CH3 /170.0, C | |||
| 8-OAc | 20.4, CH3 /169.0, C | 21.5, CH3 /169.4, C | 21.1, CH3 /169.3, C | 20.4, CH3 /169.0, C | 20.6, CH3 /169.0, C | 20.4, CH3 /169.0, C | 20.5, CH3 /169.0, C | 20.4, CH3 /168.8, C | 20.2, CH3 /168.8, C | 20.2, CH3 /168.8, C |
| 11-OAc | 21.1, CH3 /170.1, C | 20.8, CH3 /169.1, C | 20.9, CH3 /169.2, C | 21.0, CH3 /170.0, C | 20.7, CH3 /169.7, C | 21.0, CH3 /169.6, C | 21.2, CH3 /170.3, C | 21.3, CH3 /170.2, C | 20.4, CH3 /169.6, C | 20.4, CH3 /169.6, C |
| 2-OFu-2 | 148.5, CH | 148.5, CH | ||||||||
| 2-OFu-3 | 118.4, C | 118.3, C | ||||||||
| 2-OFu-4 | 109.7, CH | 109.6, CH | ||||||||
| 2-OFu-5 | 144.3, CH | 144.4, CH | ||||||||
| 2-OFu-6 | 161.0, C | 160.9, C | ||||||||
| 2-OBz-1 | 128.8, C | |||||||||
| 2-OBz-2, 6 | 129.7, CH | |||||||||
| 2-OBz-3, 5 | 128.8, CH | |||||||||
| 2-OBz-4 | 133.9, CH | |||||||||
| 2-OBz-7 | 164.9, C | |||||||||
| 2-OTig-1 | 12.2, CH3 | |||||||||
| 2-OTig-2 | 127.4, CH | |||||||||
| 2-OTig-3 | 140.0, C | |||||||||
| 2-OTig-4 | 14.7, CH3 | |||||||||
| 2-OTig-5 | 166.0, C | |||||||||
| 9′-OTig-1 | 11.7, CH3 | |||||||||
| 9′-OTig-2 | 128.4, CH | |||||||||
| 9′-OTig-3 | 138.9, C | |||||||||
| 9′-OTig-4 | 14.5, CH3 | |||||||||
| 9′-OTig-5 | 167.4, C | |||||||||
| 9′-OFu-2 | 149.0, CH | |||||||||
| 9′-OFu-3 | 118.7, C | |||||||||
| 9′-OFu-4 | 110.2, CH | |||||||||
| 9′-OFu-5 | 143.3, CH | |||||||||
| 9′-OFu-6 | 162.1, C | |||||||||
| 9′-OBz-1 | 129.5, C | |||||||||
| 9′-OBz-2, 6 | 130.2, CH | |||||||||
| 9′-OBz-3, 5 | 128.2, CH | |||||||||
| 9′-OBz-4 | 133.1, CH | |||||||||
| 9′-OBz-7 | 165.8, C |
| Compounds | Cell Viability a (%) (N = 3) | NF-κB Inhibitory Rates (%) (N = 3) | IC50 (μM) |
|---|---|---|---|
| TA | 95.59 ± 6.56 | 64.22 ± 4.53 | 7.25 b |
| 4 | 100.94 ± 3.58 | 7.27 ± 2.28 | |
| 5 | 93.85 ± 2.20 | 65.17 ± 6.12 | 8.75 |
| 9 | 99.51 ± 5.71 | 37.43 ± 1.99 | |
| 10 | 102.85 ± 0.89 | 21.73 ± 4.42 | |
| 11 | 101.60 ± 3.66 | 64.61 ± 5.15 | 0.74 |
| 12 | 93.50 ± 2.76 | 23.04 ± 3.43 | |
| 13 | 100.13 ± 6.40 | 26.49 ± 5.99 | |
| 14 | 93.76 ± 1.82 | 40.20 ± 3.92 | |
| 15 | 110.06 ± 5.83 | 8.73 ± 2.07 | |
| 16 | 102.76 ± 2.21 | 69.07 ± 4.36 | 15.66 |
| Blank control | 100.00 ± 3.33 | ||
| JSH23 c | 74.56 ± 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, J.; Zhang, Z.; Chen, M.; Wu, X.; Ma, S. Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. Molecules 2022, 27, 7274. https://doi.org/10.3390/molecules27217274
Wang Y, Yan J, Zhang Z, Chen M, Wu X, Ma S. Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. Molecules. 2022; 27(21):7274. https://doi.org/10.3390/molecules27217274
Chicago/Turabian StyleWang, Yadan, Jiangong Yan, Zhongmou Zhang, Minghui Chen, Xianfu Wu, and Shuangcheng Ma. 2022. "Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f." Molecules 27, no. 21: 7274. https://doi.org/10.3390/molecules27217274
APA StyleWang, Y., Yan, J., Zhang, Z., Chen, M., Wu, X., & Ma, S. (2022). Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. Molecules, 27(21), 7274. https://doi.org/10.3390/molecules27217274






