Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Chemicals
2.3. Preparation of Protein Hydrolysate from Grass Carp Waste
2.4. Preparation of Cat Food Attractor by Maillard Reaction
2.5. Antioxidant Activity Analysis
2.5.1. DPPH Free Radical Scavenging Ability
2.5.2. Hydroxyl Radical Scavenging Ability
2.5.3. Fe2+ Chelation Ability
2.6. Volatile Compounds Analysis
2.7. Evaluation of Application Effect of Cat Food Attractants
2.8. Statistical Analysis
3. Results and Discussion
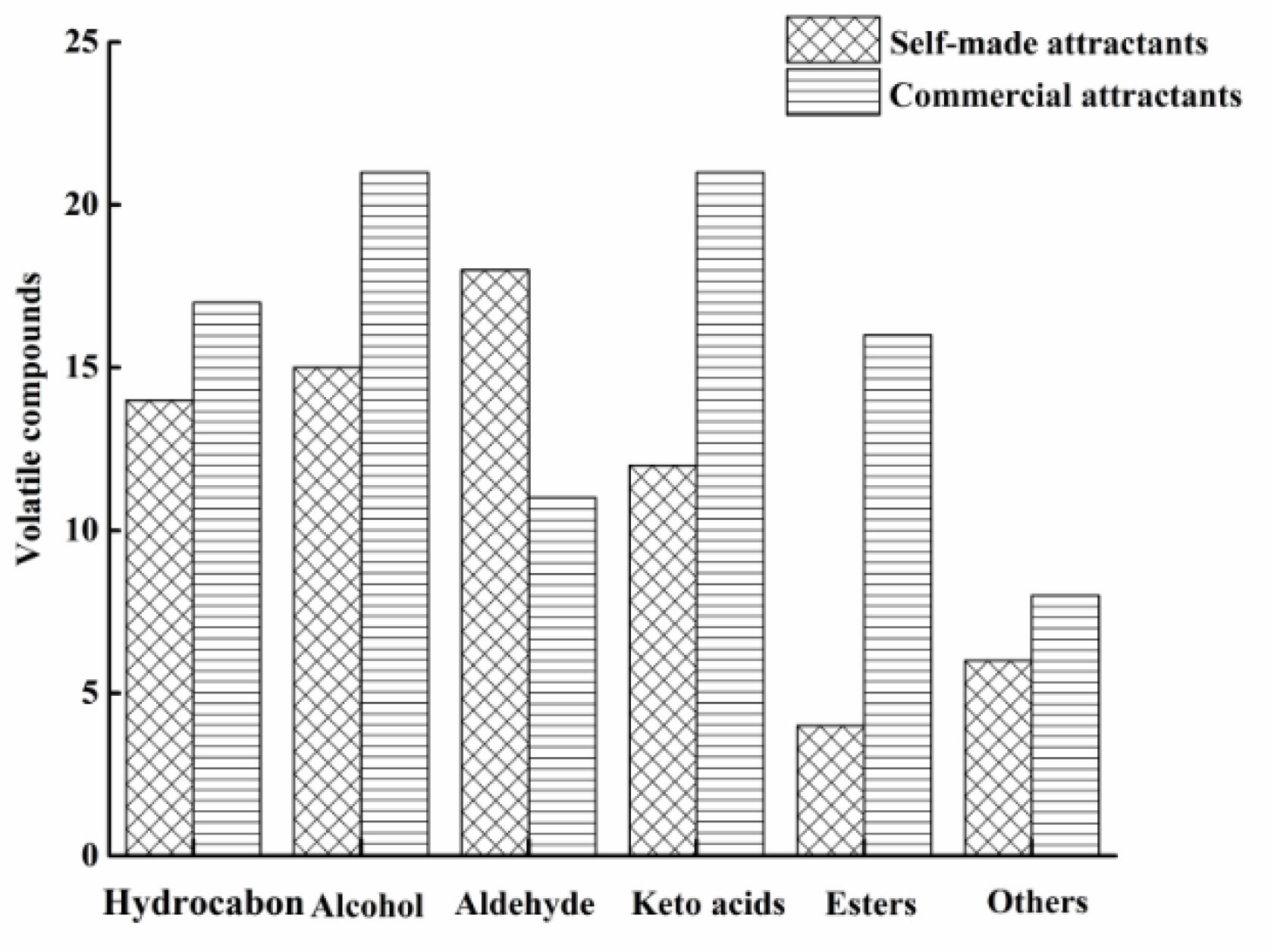
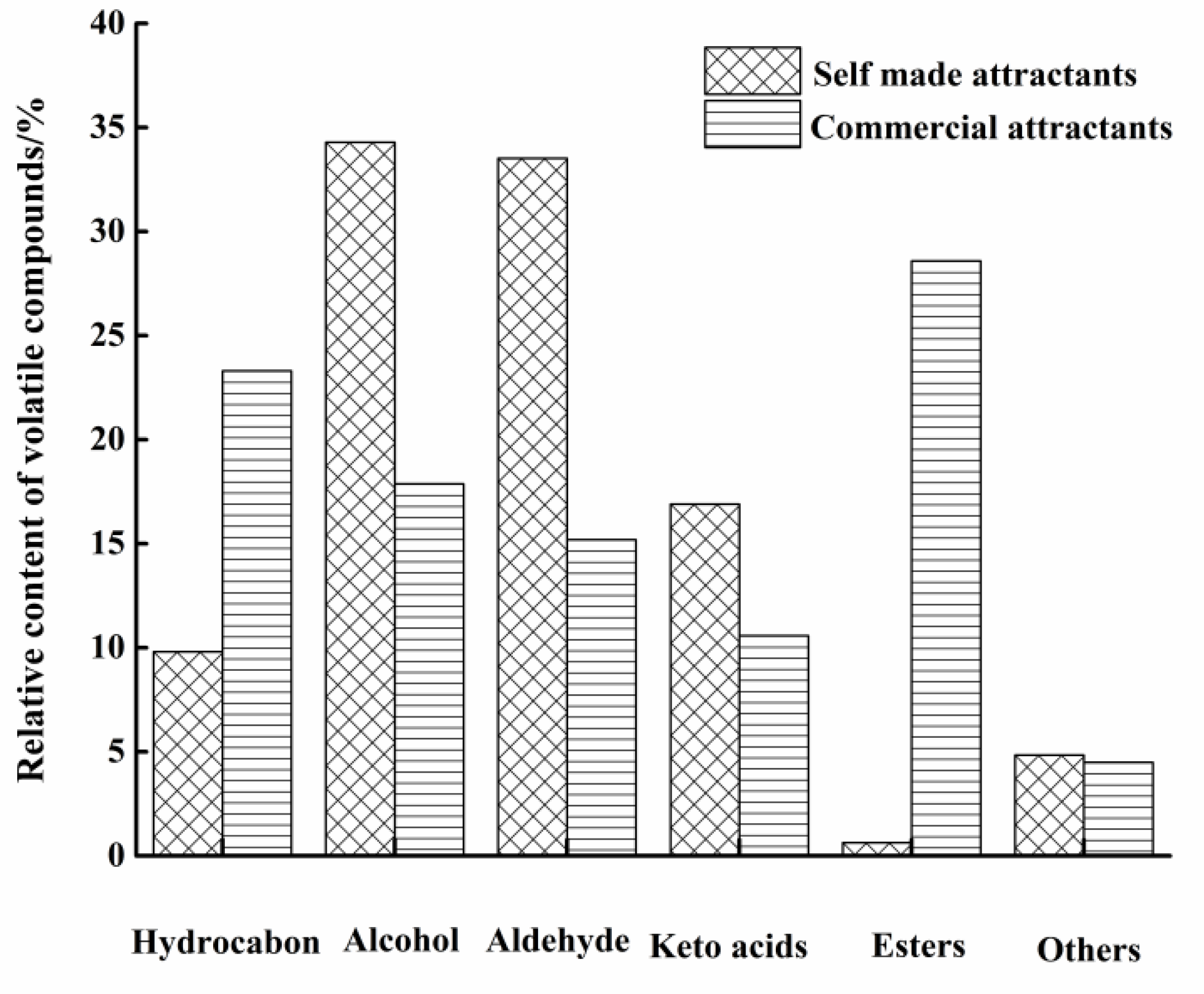
3.1. Effect of Maillard Reaction on Volatile Compounds
3.1.1. Hydrocarbon
3.1.2. Alcohol
3.1.3. Aldehyde
3.1.4. Keto Acids
3.1.5. Esters and Others
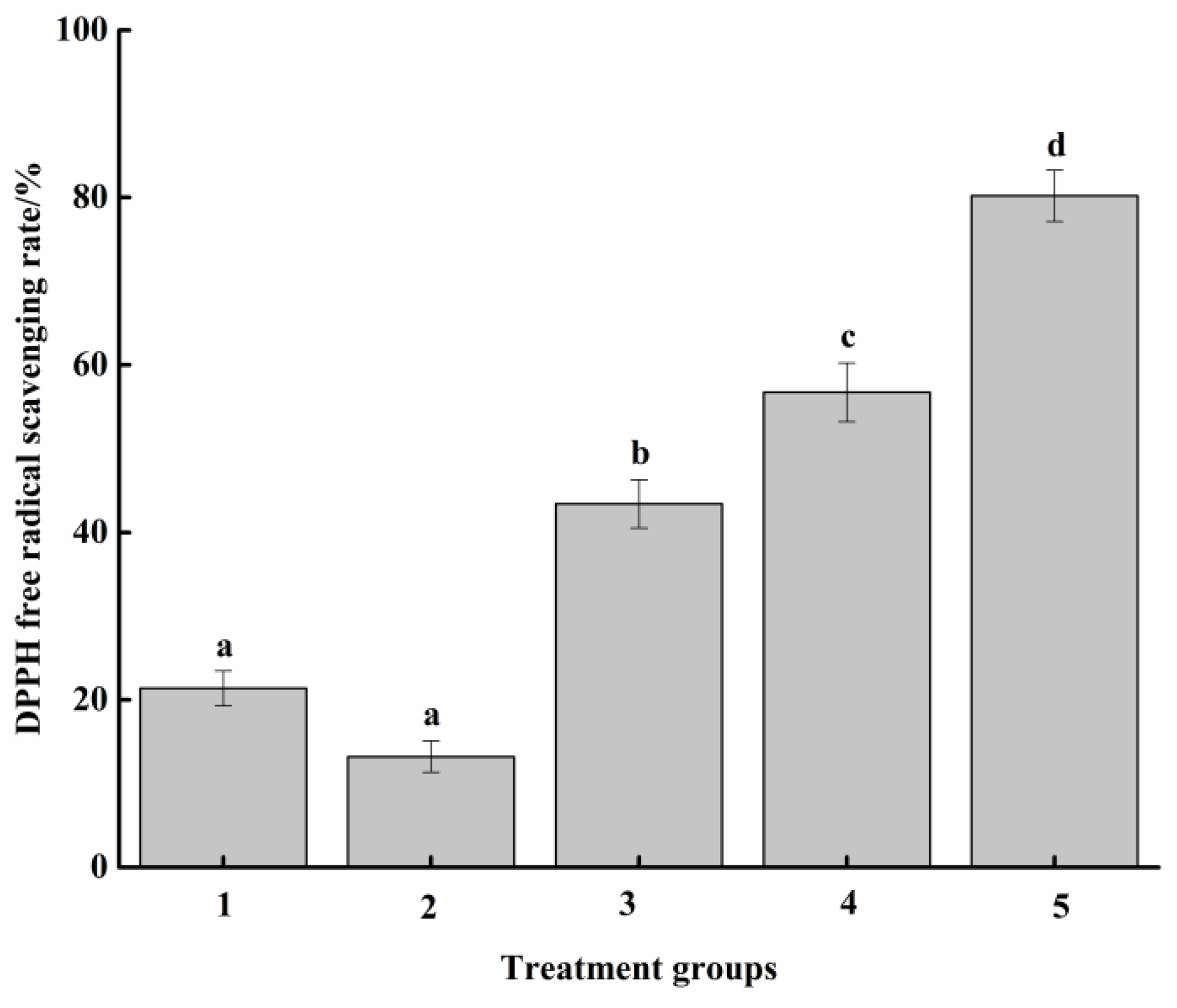
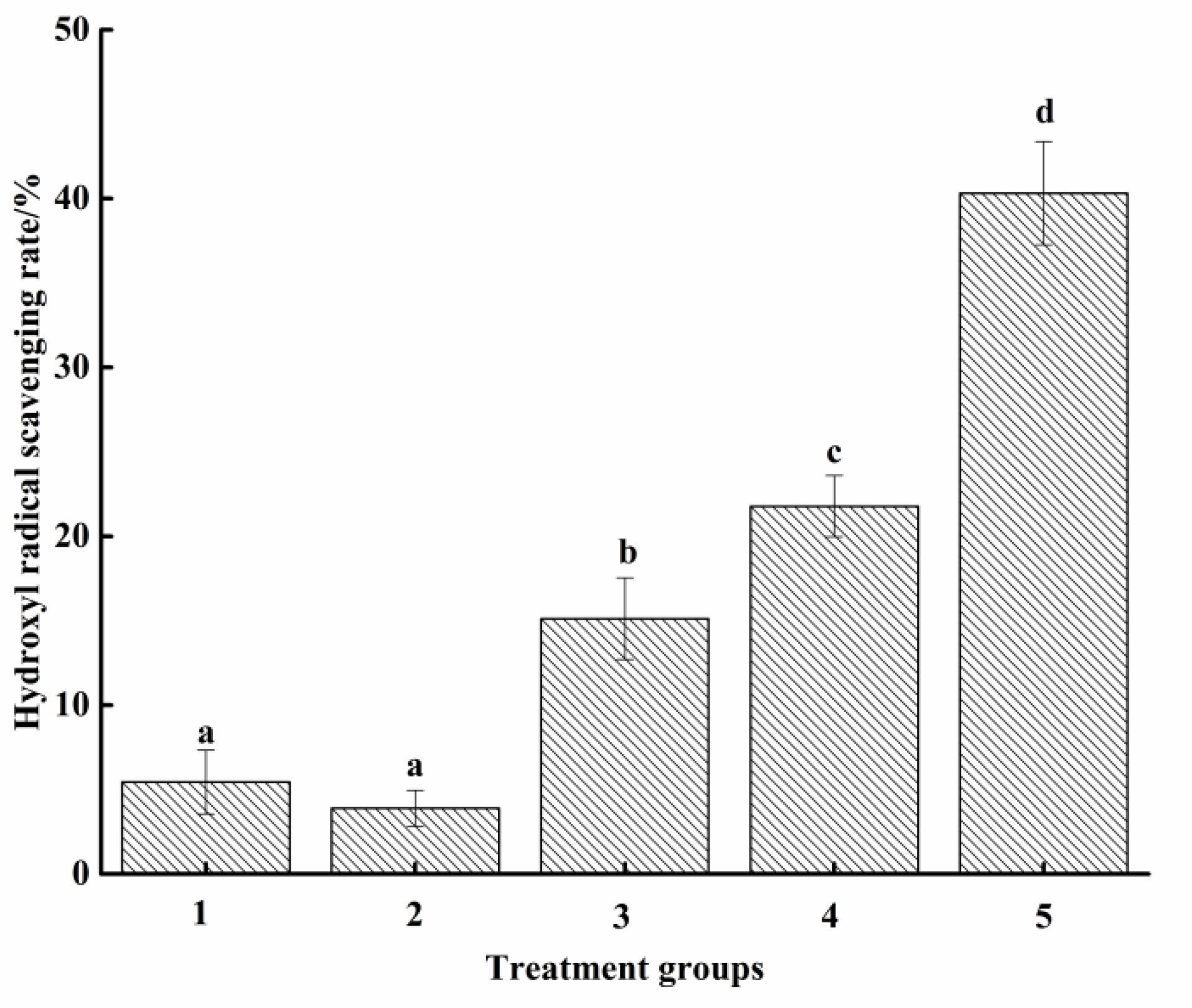
3.2. Antioxidant Activity Analysis of Food Attractants
3.2.1. DPPH Free Radical Scavenging Ability
3.2.2. Hydroxyl Radical Scavenging Ability
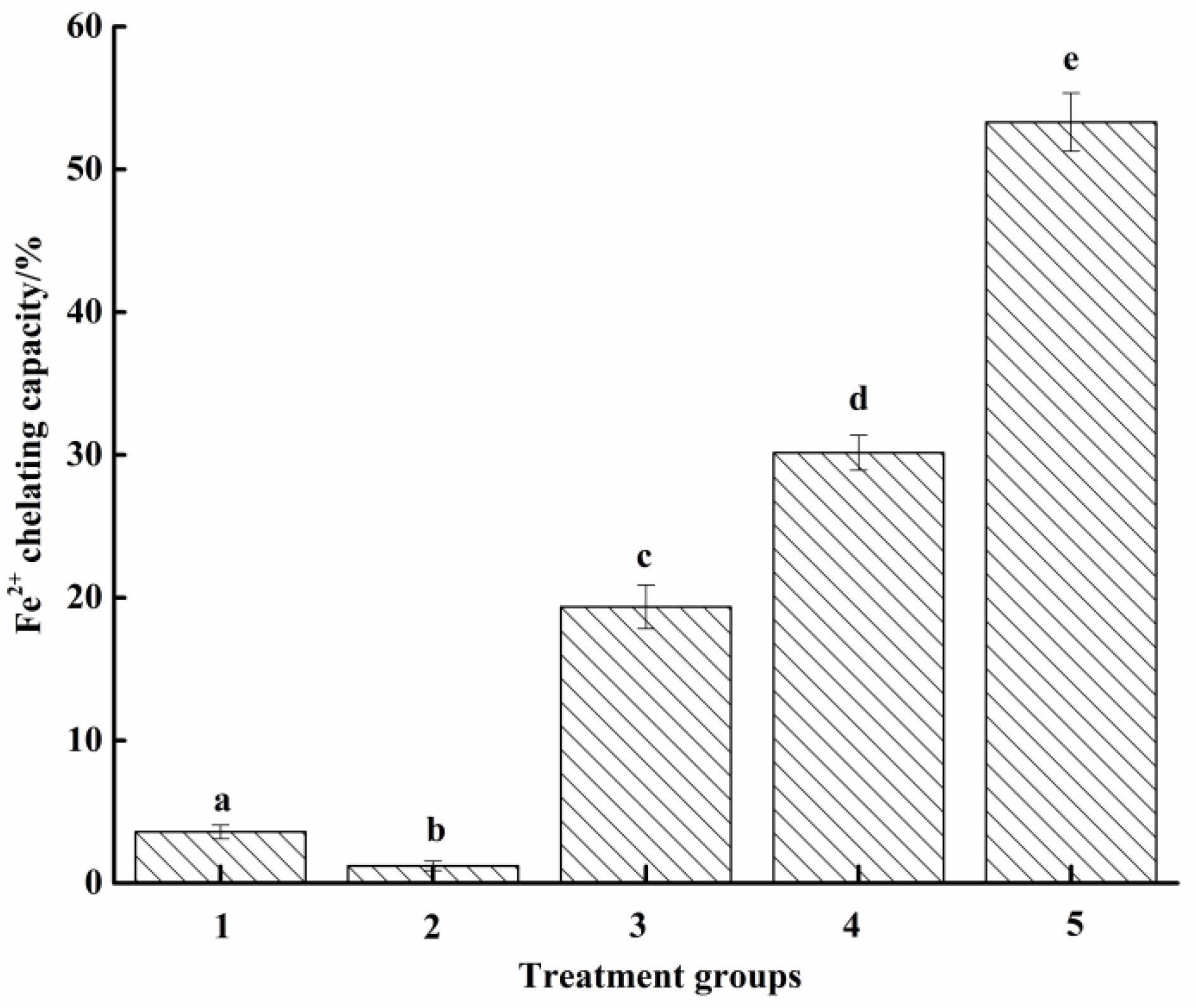
3.2.3. Fe2+ Chelation Ability
3.3. Application Effect of Self-made Cat Food Attractant
3.3.1. Analysis of Acceptability of Cat Food Attractant
3.3.2. Palatability Analysis of Cat Food Attractant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, J.; Li, Y.; Yang, X.; Liu, X.; Hong, H.; Luo, Y. Effects of oregano essential oil and nisin on the shelf life of modified atmosphere packed grass carp (Ctenopharyngodon idellus). LWT Food Sci. Technol. 2021, 147, 111609. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef] [PubMed]
- Morimura, S.; Nagata, H.; Uemura, Y.; Fahmi, A.; Shigematsu, T.; Kida, K. Development of an effective process for utilization of collagen from livestock and fish waste. Process Biochem. 2002, 37, 1403–1412. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments—Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Zent, J.B. Maillard Browning of Common Amino Acids and Sugars. J. Food Sci. 1984, 49, 1206–1207. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2001, 11, 364–373. [Google Scholar] [CrossRef]
- Scaman, C.; Nakai, S.; Aminlari, M. Effect of pH, temperature and sodium bisulfite or cysteine on the level of Maillard-based conjugation of lysozyme with dextran, galactomannan and mannan. Food Chem. 2006, 99, 368–380. [Google Scholar] [CrossRef]
- Chen, S.L.; Jin, S.Y.; Chen, C.S. Relative reactivites of glucose and galactose in browning and pyruvaldehyde formation in sugar/glycine model systems. Food Chem. 2005, 92, 597–605. [Google Scholar] [CrossRef]
- Osada, Y.; Shibamoto, T. Antioxidative activity of volatile extracts from Maillard model systems. Food Chem. 2006, 98, 522–528. [Google Scholar] [CrossRef]
- Hodge, J.E.; Rist, C.E. The Amadori Rearrangement under New Conditions and its Significance for Non-enzymatic Browning Reactions2. J. Am. Chem. Soc. 1953, 75, 316–322. [Google Scholar] [CrossRef]
- Affes, S.; Nasri, R.; Li, S.; Thami, T.; Van Der Lee, A.; Nasri, M.; Maalej, H. Effect of glucose-induced Maillard reaction on physical, structural and antioxidant properties of chitosan derivatives-based films. Carbohydr. Polym. 2021, 255, 117341. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Mu, T.-H.; Zhang, M. Structural, antioxidant, aroma, and sensory characteristics of Maillard reaction products from sweet potato protein hydrolysates as influenced by different ultrasound-assisted enzymatic treatments. Food Chem. 2021, 361, 130090. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, Q.; Hong, H.; Feng, L.; Liu, J.; Luo, Y. Physicochemical and functional properties of Maillard reaction products derived from cod (Gadus morhua L.) skin collagen peptides and xylose. Food Chem. 2020, 333, 127489. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, X.; Nsor-Atindana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Optimization of key aroma compounds for dog food attractant. Anim. Feed Sci. Technol. 2017, 225, 173–181. [Google Scholar] [CrossRef]
- Koppel, K.; Gibson, M.; Alavi, S.; Aldrich, G. The Effects of Cooking Process and Meat Inclusion on Pet Food Flavor and Texture Characteristics. Animals 2014, 4, 254–271. [Google Scholar] [CrossRef]
- Parker, J.K.; Balagiannis, D.P.; Desforges, N.; Mottram, D.S. Flavor Development in a Meat-Based Petfood Containing Added Glucose and Glycine. ACS Symp. Ser. 2010, 1042, 85–93. [Google Scholar]
- Xie, G.J.; Yan, J.F.; Yan, Z.Y.; Wang, Y.F. Enzymatic hydrolysis process research of grass carp offal. Sci. Technol. Food Ind. 2018, 39, 209–214. [Google Scholar]
- Saraswati Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. In-vitro anti-inflammatory activity, free radical (DPPH) scavenging, and ferric reducing ability (FRAP) of Sargassum cristaefolium lipid-soluble fraction and putative identification of bioactive compounds using UHPLC-ESI-ORBITRAP-MS/MS. Food Res. Int. 2020, 137, 109702. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef]
- Oliveira, G.; Marques, C.; de Oliveira, A.; Santos, A.D.A.D.; Amaral, W.D.; Ineu, R.P.; Leimann, F.V.; Peron, A.P.; Igarashi-Mafra, L.; Mafra, M.R. Extraction of bioactive compounds from Curcuma longa L. using deep eutectic solvents: In vitro and in vivo biological activities. Innov. Food Sci. Emerg. Technol. 2021, 70, 102697. [Google Scholar] [CrossRef]
- Luo, J.; Nasiru, M.M.; Zhuang, H.; Zhou, G.; Zhang, J. Effects of partial NaCl substitution with high-temperature ripening on proteolysis and volatile compounds during process of Chinese dry-cured lamb ham. Food Res. Int. 2021, 140, 110001. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Studzinski, C.M.; Larson, B.T.; Milgram, N.W. Comparison of the cognitive palatability assessment protocol and the two-pan test for use in assessing palatability of two similar foods in dogs. Am. J. Vet. Res. 2004, 65, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Koppel, K.; Adhikari, K.; Di Donfrancesco, B. Volatile Compounds in Dry Dog Foods and Their Influence on Sensory Aromatic Profile. Molecules 2013, 18, 2646–2662. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A. Specialty Ingredients: Considerations and Use. In Petfood Technology, 1st ed.; Kvamme, J.L., Phillips, T.D., Eds.; Watt Publishing Co.: Mt. Morris, IL, USA, 2003; pp. 85–100. [Google Scholar]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Kosowska, M.; MAJCHER, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Zhao, J.-L.; Tian, W.; Liu, Y.-X.; Li, M.-Y.; Zhao, G.-M. Contribution of Histidine and Lysine to the Generation of Volatile Compounds in Jinhua Ham Exposed to Ripening Conditions Via Maillard Reaction. J. Food Sci. 2018, 83, 46–52. [Google Scholar] [CrossRef]
- Bermudez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of type of muscle on volatile compounds throughout the manufacture of Celta dry-cured ham. Food Sci. Technol. Int. 2015, 21, 581–592. [Google Scholar] [CrossRef]
- Marusic, N.; Vidacek, S.; Janci, T.; Petrak, T.; Medic, H. Determination of volatile compounds and quality parameters of traditional Istrian dry-cured ham. Meat Sci. 2014, 96, 1409–1416. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Song, H.; Meng, Q. Characterization of key aroma compounds in traditional Chinese soy sauce through the molecular sensory science technique. LWT Food Sci. Technol. 2020, 128, 109413. [Google Scholar] [CrossRef]
- Petričević, S.; Marusic Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Moretti, V.M.; Vasconi, M.; Caprino, F.; Bellagamba, F. Fatty Acid Profiles and Volatile Compounds Formation During Processing and Ripening of a Traditional Salted Dry Fish Product. J. Food Process. Pres. 2017, 41, e13133. [Google Scholar] [CrossRef]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Pickering, G.J. Optimizing the sensory characteristics and acceptance of canned cat food: Use of a human taste panel. J. Anim. Physiol. Anim. Nutr. 2009, 93, 52–60. [Google Scholar] [CrossRef]
- Ying, W.; Ya-Ting, J.; Jin-Xuan, C.; Yin-Ji, C.; Yang-Ying, S.; Xiao-Qun, Z.; Dao-Dong, P.; Chang-Rong, O.; Ning, G. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Arrigoni, O.; Tullio, M. Ascorbic acid: Much more than just an antioxidant. Biochim Biophys Acta 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009, 113, 196–201. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Wyk, J.V. Antioxidant activity of Maillard reaction products (MRPs) derived from fructose-lysine and ribose-lysine model systems. Food Chem. 2013, 137, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, G.C.; Koppel, K. Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Animals 2015, 5, 43–55. [Google Scholar] [CrossRef]
- Thombre, A.G. Oral delivery of medications to companion animals: Palatability considerations. Adv. Drug Deliv. Rev. 2004, 56, 1399–1413. [Google Scholar] [CrossRef]
| Factors | Reducing Sugar (%) | Cysteine Hydrochloride (%) | Thiamine (%) | Na2HPO4 (%) | Temperature (°C) | Time (min) | pH |
|---|---|---|---|---|---|---|---|
| Parameter | 4 | 1 | 0.5 | 1 | 115 | 45 | 7.0 |
| Hydrocarbons | Self-Made Attractants | Commercial Attractants | ||
|---|---|---|---|---|
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | Tetradecane | 0.041 ± 0.01 e | Tetradecane | 2.199 ± 0.31 b |
| 2 | Nonadecane | 0.902 ± 0.02 b | Nonadecane | 0.991 ± 0.04 c |
| 3 | 1-Limonene | 0.397 ± 0.01 c | 1-Limonene | 2.281 ± 0.32 b |
| 4 | Octane | 0.870 ± 0.02 b | 2,2,4,6,6-Pentamethylheptane | 8.456 ± 0.45 a |
| 5 | Trichloromethane | 0.342 ± 0.00 c | Decane | 1.999 ± 0.21 b |
| 6 | Ethylene oxide | 0.436 ± 0.03 c | 2,2,4,4-Tetramethyloctane | 1.922 ± 0.26 b |
| 7 | Hexadecane | 0.424 ± 0.05 c | 6-Methyl-tridecane | 0.799 ± 0.05 c |
| 8 | Octodecane | 0.132 ± 0.02 d | 2,5,9-Trimethyl-decane | 0.187 ± 0.03 e |
| 9 | 3-(propyl-2-enoyloxy) dodecane | 0.104 ± 0.05 d | Undecane | 2.181 ± 0.28 b |
| 10 | 1,3-Butadiene | 0.177 ± 0.01 a | Dodecyl-cyclopropyl siloxane | 0.114 ± 0.17 e |
| 11 | 2,4-heptadiene | 1.301 ± 0.07 b | 2,3-Dihydroxybutane | 0.682 ± 0.02 c |
| 12 | (Z) 2-Octene | 0.456 ± 0.03 c | 3-Methyl-tridecane | 0.312 ± 0.02 d |
| 13 | 1,6,10-Hexadecatene | 0.653 ± 0.05 c | Ethylbenzene | 0.259 ± 0.01 d e |
| 14 | (E)-2-decene | 3.574 ± 0.75 a | Xylene | 0.083 ± 0.00 e |
| 15 | 2-Ethyl-1-decene | 0.301 ± 0.01 d | ||
| 16 | 2,2-Dimethyl-4-decene | 0.124 ± 0.01 e | ||
| 17 | 1-Tetradecene | 0.408 ± 0.05 d | ||
| Total | 9.812 ± 1.01 | 23.299 ± 6.33 | ||
| Alcohols | Self-Made Attractants | Commercial Attractants | ||
|---|---|---|---|---|
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | 1-Pentene-3 alcohol | 0.124 ± 0.02 e | 1-Pentene-3 alcohol | 0.138 ± 0.02 d |
| 2 | N -pentanol | 2.673 ± 0.15 b | N -pentanol | 0.830 ± 0.07 b |
| 3 | 1,5-Octandiene-3-ol | 0.855 ± 0.09 c | 1,5-Octandiene-3-ol | 0.295 ± 0.03 d |
| 4 | 1-Octanol | 4.679 ± 0.34 b | 1-Octanol | 0.316 ± 0.05 c d |
| 5 | 2,4-Dimethyl-cyclohexanol | 0.403 ± 0.05 d | 2,4-Dimethyl-cyclohexanol | 4.057 ± 0.56 a |
| 6 | N -heptanol | 2.845 ± 0.30 b | N -heptanol | 0.065 ± 0.00 e |
| 7 | Hexyl alcohol | 3.726 ± 0.46 b | Hexyl alcohol | 0.529 ± 0.06 c |
| 8 | 1-Octene-3-ol | 9.360 ± 0.81 a | 1-Octene-3-ol | 3.133 ± 0.66 a |
| 9 | 2-Octene alcohol | 2.633 ± 0.17 b | 2-Octene alcohol | 0.624 ± 0.05 b c |
| 10 | Benzyl alcohol | 0.479 ± 0.05 d | Benzyl alcohol | 0.061 ± 0.00 e |
| 11 | 1-Tridecane-1-ol | 0.550 ± 0.06 d | 1-Heptene-1-ol | 0.232 ± 0.01 d |
| 12 | 3-Heptene-1-ol | 2.947 ± 0.25 b | Heptanol | 0.453 ± 0.05 c |
| 13 | 2,7-Octandiene-1-ol | 0.328 ± 0.05 d | 2-Hexadecanol | 0.613 ± 0.07 b c |
| 14 | 3-Nonene-1-ol | 1.102 ± 0.08 c | 2-Tetradecanol | 0.161 ± 0.02 d |
| 15 | 4-Ethyl cyclohexanol | 1.587 ± 0.65 c | N -caprylic alcohol | 0.871 ± 0.08 b |
| 16 | (+)-5-Methyl-2-hexanol | 0.426 ± 0.05 c | ||
| 17 | 2-Cyclopropyl 1-propanol | 0.208 ± 0.03 d | ||
| 18 | Furfuryl alcohol | 1.302 ± 0.35 b | ||
| 19 | 2,4-Dimethyl-cyclohexanol | 0.138 ± 0.02 d | ||
| 20 | 2-Phenethyl alcohol | 3.269 ± 0.29 a | ||
| 21 | 3,7-Dimethyl-1-octanol | 0.149 ± 0.02 d | ||
| Total | 34.292 ± 3.05 | 17.868 ± 2.07 | ||
| Aldehydes | Self-Made Attractants | Commercial Attractants | ||
|---|---|---|---|---|
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | Hexanal | 3.758 ± 0.66 b | Hexanal | 3.912 ± 0.38 a |
| 2 | Heptanal | 1.222 ± 0.31 c | Heptanal | 0.544 ± 0.04 c |
| 3 | Octanal | 2.373 ± 0.25 b,c | Octanal | 0.449 ± 0.05 c |
| 4 | Nonanal | 6.699 ± 0.75 a | Nonanal | 0.971 ± 0.09 b |
| 5 | 2-Octene aldehyde | 0.144 ± 0.02 e | 2-Octene aldehyde | 0.524 ± 0.05 c |
| 6 | Benzaldehyde | 0.145 ± 0.03 e | Benzaldehyde | 1.497 ± 0.20 b |
| 7 | E -2-heptene aldehyde | 4.687 ± 0.55 a,b | 2-Methylpropyl aldehyde | 1.124 ± 0.18 b |
| 8 | 2,4-Heptanedienal | 0.296 ± 0.03 d | Isovaleraldehyde | 5.721 ± 0.65 a |
| 9 | 2-Octene aldehyde | 4.015 ± 0.46 a | 2-Pentyl-2-nonenal | 0.119 ± 0.25 d |
| 10 | Decanal | 0.258 ± 0.02 d | α-Ethylene-phenylacetaldehyde | 0.102 ± 0.02 d |
| 11 | Benzaldehyde | 1.181 ± 0.20 c | 5-Methyl-2-(1-methylethyl)-2-hexenal | 0.226 ± 0.09 d |
| 12 | (Z)-6-Nonene aldehyde | 2.312 ± 0.25 b,c | ||
| 13 | Dimethyl-silane dialdehyde | 0.692 ± 0.07 d | ||
| 14 | 4-Ethyl benzaldehyde | 0.252 ± 0.03 a | ||
| 15 | Trans-undecane-2-enal | 1.313 ± 0.05 c | ||
| 16 | (E,E)-2,4-decanodienal | 0.963 ± 0.08 c | ||
| 17 | 2,4-Decanodienal | 2.845 ± 0.36 b,c | ||
| 18 | Furfuraldehyde | 0.362 ± 0.05 d | ||
| Total | 33.515 ± 3.25 | 15.189 ± 1.85 | ||
| Keto Acids | Self-Made Attractants | Commercial Attractants | ||
|---|---|---|---|---|
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | 2-Octanone | 0.168 ± 0.02 e | 2-Octanone | 0.160 ± 0.02 c |
| 2 | 1-Octene-3-ketone | 2.620 ± 0.31 b,c | 1-Octene-3-ketone | 0.517 ± 0.05 b |
| 3 | N -caprylic acid | 6.834 ± 0.75 a | N -caprylic acid | 2.667 ± 0.31 a |
| 4 | Nonanoic acid | 0.198 ± 0.25 e | Nonanoic acid | 0.082 ± 0.00 d |
| 5 | Heptylic acid | 1.690 ± 0.15 c | Heptylic acid | 0.169 ± 0.02 c |
| 6 | 3-Hexene-2-ketone | 0.655 ± 0.77 d | 2,3-Pentarone | 0.251 ± 0.01 c |
| 7 | 2-Heptanone | 0.589 ± 0.60 d | 2-Hydroxy-acetone | 0.387 ± 0.03 b,c |
| 8 | 3-Octene-2-ketone | 0.283 ± 0.03 e | Hydroxy acetone | 0.679 ± 0.00 b |
| 9 | 3-Nonene-2 ketone | 0.240 ± 0.02 e | 2,2,6-Trimethyl-cyclohexanone | 0.242 ± 0.02 c |
| 10 | Linolenic acid | 0.369 ± 0.05 e | 2,3-Diketone | 1.603 ± 0.08 a |
| 11 | Hexanoic acid | 1.841 ± 0.21 c | 6-Methyl-5-heptene-2-ketone | 0.498 ± 0.05 b |
| 12 | Acetic acid | 1.413 ± 0.17 c | 2-Nonyl ketone | 0.098 ± 0.01 c,d |
| 13 | 3,6-Dimethyl-oct-2-ketone | 0.050 ± 0.00 d | ||
| 14 | Geranylacetone | 0.041 ± 0.00 d | ||
| 15 | 2-Pyrrolidone | 0.063 ± 0.01 d | ||
| 16 | 2-Methyl-2-ethyl-1-propyl propionic acid | 0.329 ± 0.05 b,c | ||
| 17 | 2-Isobutyric acid | 0.192 ± 0.02 c | ||
| 18 | N-decanoic acid | 0.981 ± 0.05 a | ||
| 19 | 9-Decanoic acid | 0.103 ± 0.02 c | ||
| 20 | Pentanoic acid | 1.011 ± 0.04 a | ||
| 21 | 4-Methylvaleric acid | 0.450 ± 0.05 b | ||
| Total | 16.900 ± 1.33 | 10.574 ± 1.45 | ||
| Esters | Self-Made Attractants | Commercial Attractants | ||
|---|---|---|---|---|
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | N -pentylbutyllactone | 0.071 ± 0.01 b | N -pentylbutyllactone | 0.069 ± 0.00 f |
| 2 | Methyl 2-hydroxyisobutyrate | 0.052 ± 0.01 b | Propyl 2-hydroxypropionate | 0.214 ± 0.03 e |
| 3 | P -caproic acid-p-nitrophenyl ester | 0.266 ± 0.03 a | Butyl acetate | 0.306 ± 0.05 e |
| 4 | 2-Hexene-4-lactone | 0.249 ± 0.03 a | Buty propionate | 0.265 ± 0.02 e |
| 5 | Decyl-butyl phthalate | 0.637 ± 0.05 e | ||
| 6 | Butyl acrylate | 1.652 ± 0.20 d | ||
| 7 | Butyl butyrate | 1.127 ± 0.15 d | ||
| 8 | Ethyl caprylate | 6.710 ± 0.55 b | ||
| 9 | Ethyl caprate | 11.037 ± 0.97 a | ||
| 10 | Ethyl 9-decenoate | 1.126 ± 0.16 d | ||
| 11 | Octanoic acid-3-Methyl butyl ester | 0.419 ± 0.45 a | ||
| 12 | Ethyl laurate | 3.265 ± 0.35 c | ||
| 13 | 3-Methylbutyrate | 0.718 ± 0.07 d | ||
| 14 | E -11-hexadecanoenoic acid ethyl ester | 0.875 ± 0.08 d | ||
| 15 | Ethyl palmitate | 0.166 ± 0.02 e | ||
| Total | 0.638 ± 0.05 | 28.585 ± 2.11 | ||
| Others | Self-Made Attractants | Commercial Attractants | ||
| Volatile Compounds | Relative Content (%) | Volatile Compounds | Relative Content (%) | |
| 1 | 2-N-pentylfuran | 2.459 ± 0.25 a | 2-N-pentylfuran | 2.045 ± 0.21 a |
| 2 | 2-Acetylfuran | 1.619 ± 0.17 a | 2-Acetylfuran | 0.633 ± 0.05 b |
| 3 | 2-Heptyl furan | 0.246 ± 0.02 b | 2-Acetyl pyrrole | 0.321 ± 0.03 b |
| 4 | 2-Ethyl furan | 0.114 ± 0.15 b | 2-Methylpyrazine | 0.635 ± 0.06 b |
| 5 | 4-Methyl-5-hydroxyethyl thiazole | 0.270 ± 0.02 b | 2, 6-Dimethylpiperazine | 0.276 ± 0.02 b |
| 6 | 5-Pentyl-2-(5H) furan | 0.136 ± 0.02 b | 2-Ethyl-6-methyl-pyrazine | 0.166 ± 0.01 b |
| 7 | 2-ethyl-5-methyl-tetrahydrofuran | 0.113 ± 0.01 b | ||
| 8 | Nucleoside chrysanthemum ring | 0.299 ± 0.04 b | ||
| Total | 4.843 ± 0.35 | 4.487 ± 0.38 | ||
| Treatment Groups | A | B | CK |
|---|---|---|---|
| Feeding rate/% | 75.37 ± 7.32 a | 80.07 ± 8.33 a | 37.63 ± 10.37 b |
| Treatment Groups | First Sniffing | First Bite | Feeding Rate/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | CK | A | B | CK | A | B | CK | |
| First day | 5 | 25 | 0 | 3 | 27 | 0 | 27.85 ± 9.31 b | 69.52 ± 7.49 a | 0.00 ± 0.00 c |
| Second day | 6 | 24 | 0 | 4 | 26 | 0 | 22.68 ± 6.77 b | 75.51 ± 8.52 a | 0.00 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Dai, Z.; Hong, W.; Zhao, J.; Zhao, H.; Luo, J.; Xie, G. Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant. Molecules 2022, 27, 7239. https://doi.org/10.3390/molecules27217239
Sun K, Dai Z, Hong W, Zhao J, Zhao H, Luo J, Xie G. Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant. Molecules. 2022; 27(21):7239. https://doi.org/10.3390/molecules27217239
Chicago/Turabian StyleSun, Kekui, Zhaoqi Dai, Wenlong Hong, Jianying Zhao, Hang Zhao, Ji Luo, and Guangjie Xie. 2022. "Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant" Molecules 27, no. 21: 7239. https://doi.org/10.3390/molecules27217239
APA StyleSun, K., Dai, Z., Hong, W., Zhao, J., Zhao, H., Luo, J., & Xie, G. (2022). Effects of Maillard Reaction on Volatile Compounds and Antioxidant Capacity of Cat Food Attractant. Molecules, 27(21), 7239. https://doi.org/10.3390/molecules27217239





