Pharmacodynamic Interactions between Puerarin and Metformin in Type-2 Diabetic Rats
Abstract
1. Introduction
2. Results
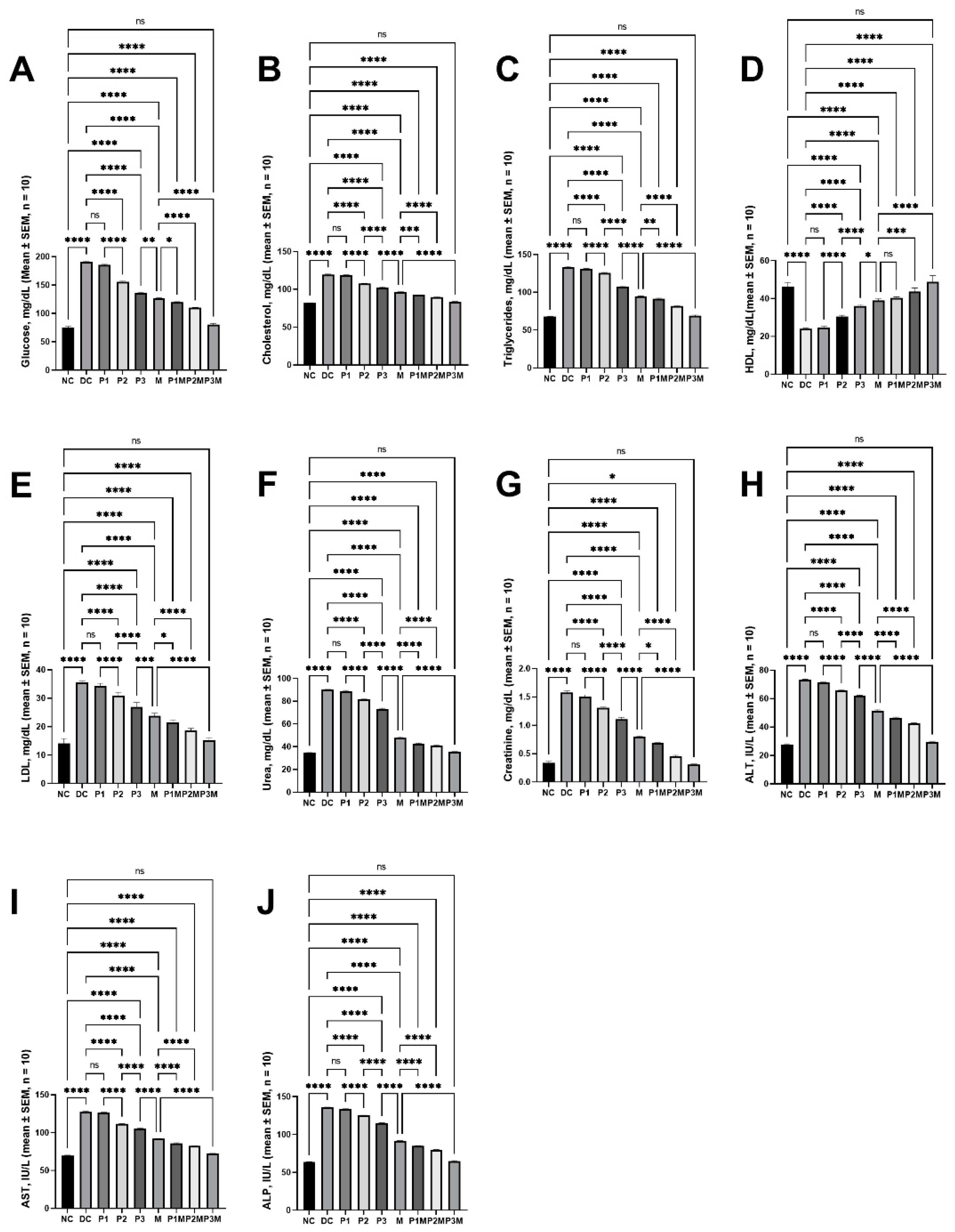
2.1. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Hyperglycaemia
2.2. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Dysregulated Lipidaemia
2.3. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Kidney Function
2.4. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Liver Function
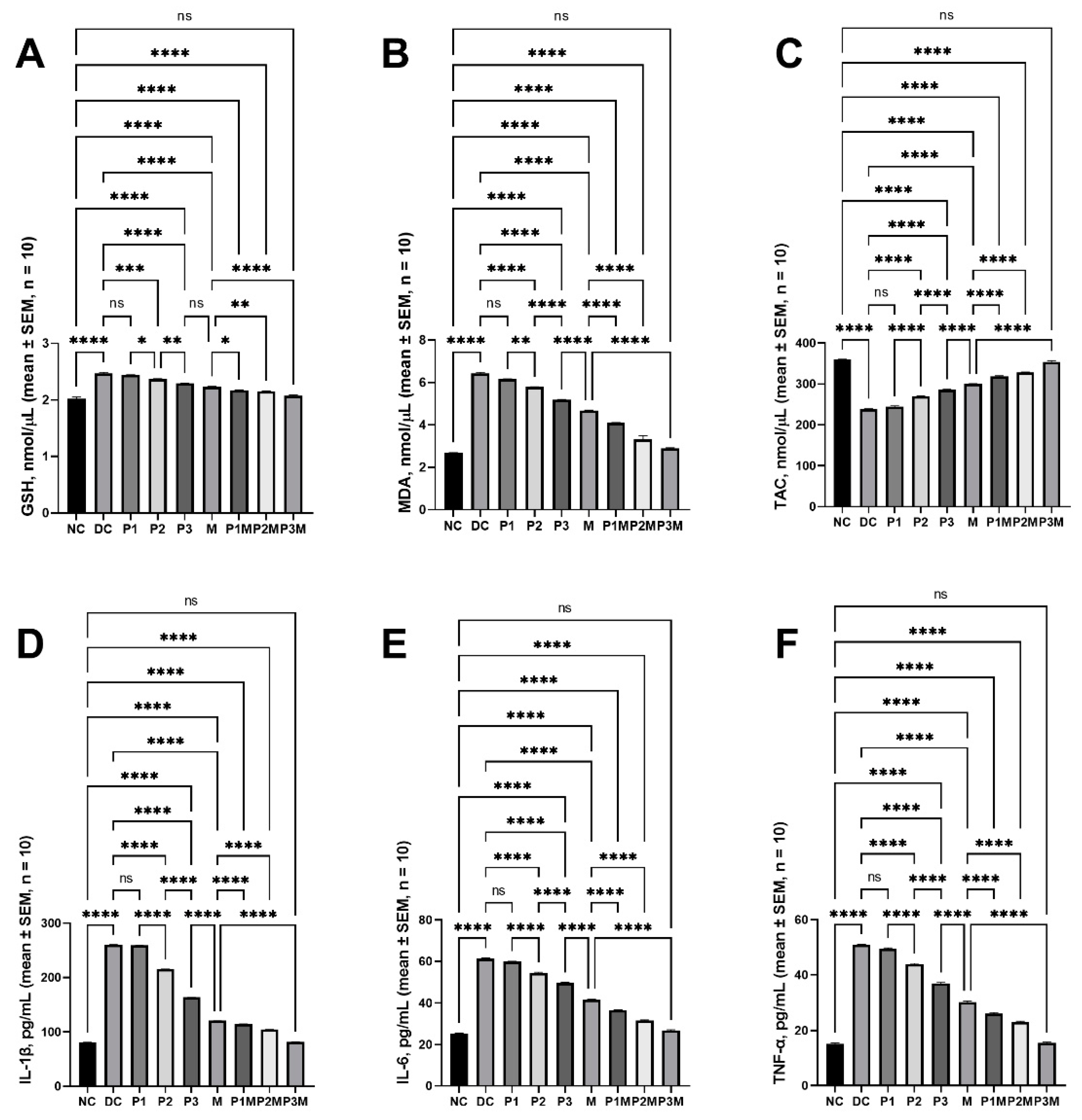
2.5. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Oxidative Stress
2.6. Effects of Pharmacodynamic Interactions between Puerarin and Metformin on Inflammation
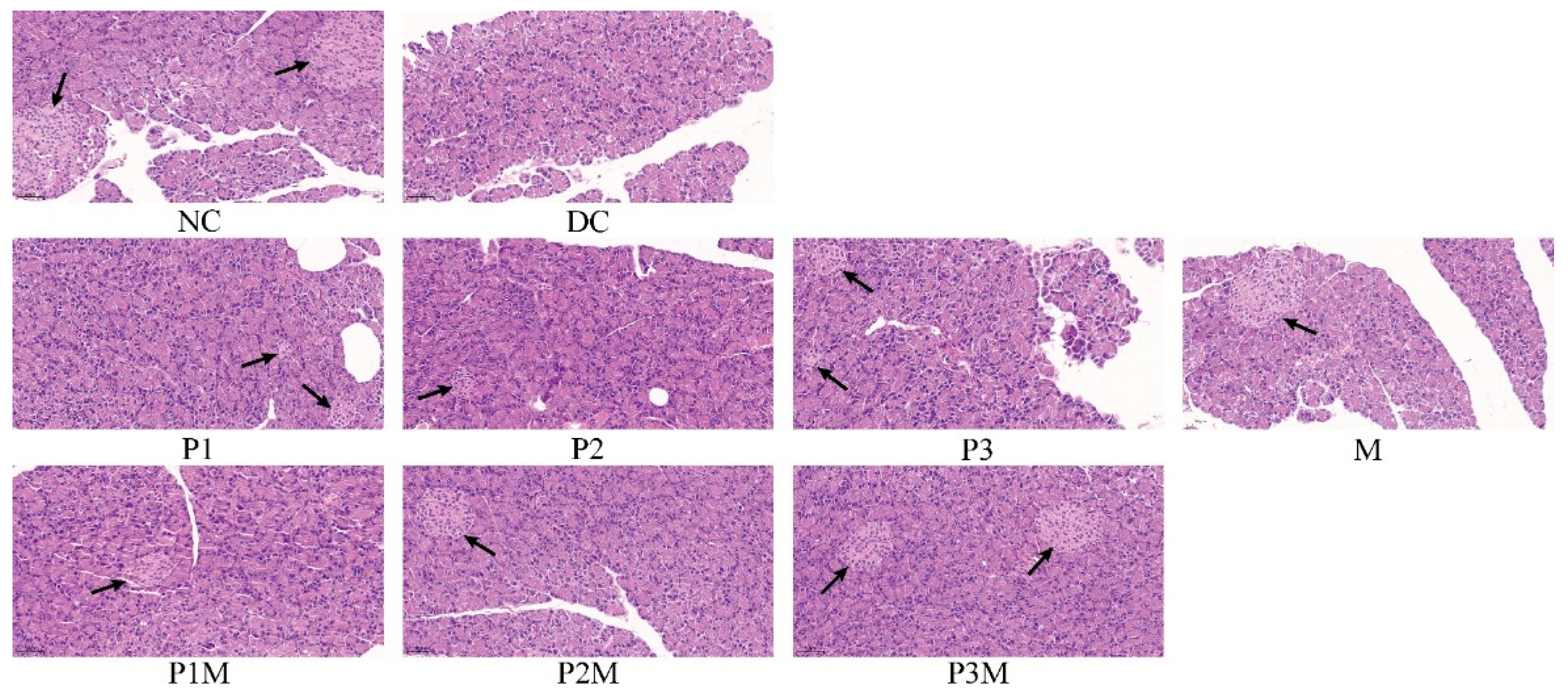
2.7. Histology Studies
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Biochemical Experiments
4.3. Histology Studies
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C. Drug interactions of medications commonly used in diabetes. Diabetes Spectr. 2006, 19, 202–211. [Google Scholar] [CrossRef]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liang, T.; Duan, X.; Xu, L.; Zhang, K.; Li, R. Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem. Toxicol. 2013, 60, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jain, V.; Singh, D.P. Effect of Pueraria tuberosa DC. (Indian Kudzu) on blood pressure, fibrinolysis and oxidative stress in patients with stage 1 hypertension. Pak. J. Biol. Sci. PJBS 2012, 15, 742–747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yusaku, G.; Putalun, W.; Udomsin, O.; Juengwatanatraku, T.; Chaichantipyuth, C. Comparative analysis of the chemical constituents of two varieties of Pueraria candollei. Fitoterapia 2011, 82, 203–207. [Google Scholar] [CrossRef]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.-T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef]
- Lynch, T.N.; Marois, J.J.; Wright, D.L.; Harmon, P.F.; Harmon, C.L.; Miles, M.R.; Hartman, G.L. First Report of Soybean Rust Caused by Phakopsora pachyrhizi on Phaseolus spp. in the United States. Plant Dis. 2006, 90, 970. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Yang, C.-C. Multi-target strategy and experimental studies of traditional Chinese medicine for Alzheimer’s disease therapy. Curr. Top. Med. Chem. 2016, 16, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; He, C. An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis. J. Ethnopharmacol. 2016, 193, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, Z.; Cao, F.; Wang, L.; Liao, Y.; Liu, X.; Pan, R.; Chang, Q. Brain Pharmacokinetics and the Pharmacological Effects on Striatal Neurotransmitter Levels of Pueraria lobata Isoflavonoids in Rat. Front. Pharmacol. 2017, 8, 599. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (–)-1-epi-Adenophorine and a Homoimindosugar. Eur. J. Org. Chem. 2018, 2018, 6574–6581. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me3SiN3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Yang, L.F.; Shimadate, Y.; Kato, A.; Li, Y.X.; Jia, Y.M.; Fleet, G.W.J.; Yu, C.Y. Synthesis and glycosidase inhibition of N-substituted derivatives of 1,4-dideoxy-1,4-imino-D-mannitol (DIM). Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef]
- Prasain, J.K.; Peng, N.; Rajbhandari, R.; Wyss, J.M. The Chinese Pueraria root extract (Pueraria lobata) ameliorates impaired glucose and lipid metabolism in obese mice. Phytomedicine 2012, 20, 17–23. [Google Scholar] [CrossRef]
- Tanaka, T.; Yokota, Y.; Tang, H.; Zaima, N.; Moriyama, T.; Kawamura, Y. Anti-Hyperglycemic Effect of a Kudzu (Pueraria lobata) Vine Extract in Ovariectomized Mice. J. Nutr. Sci. Vitaminol. 2016, 62, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae Lobatae Radix and Puerariae Thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Han, L.L.; Qian, J.H.; Wang, H.Z. Molecular Mechanism of Puerarin Against Diabetes and its Complications. Front. Pharmacol. 2021, 12, 780419. [Google Scholar] [CrossRef]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar] [CrossRef]
- Hu, X.; Duan, T.; Wu, Z.; Tang, C.; Cao, Z. Puerarin Inhibits the PERK-eIF2 [Formula: See text]-ATF4-CHOP Pathway through Inactivating JAK2/STAT3 Signal in Pancreatic beta-Cells. Am. J. Chin. Med. 2021, 49, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Wang, L.; Wu, Y.-Z.; Song, S.-Y.; Min, H.-Y.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8, 1. [Google Scholar] [CrossRef]
- Yin, L.; Chen, X.; Li, N.; Jia, W.; Wang, N.; Hou, B.; Yang, H.; Zhang, L.; Qiang, G.; Yang, X.; et al. Puerarin ameliorates skeletal muscle wasting and fiber type transformation in STZ-induced type 1 diabetic rats. Biomed. Pharmacother. 2021, 133, 110977. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y. Puerarin improve insulin resistance of adipocyte through activating Cb1 binding protein path. Chin. J. Integr. Med. 2012, 18, 293–298. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Liu, I.-M.; Kuo, D.-H.; Chen, W.-C.; Su, H.-C.; Cheng, J.-T. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J. Nat. Prod. 2003, 66, 788–792. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef]
- Hou, B.; Zhao, Y.; Qiang, G.; Yang, X.; Xu, C.; Chen, X.; Liu, C.; Wang, X.; Zhang, L.; Du, G. Puerarin Mitigates Diabetic Hepatic Steatosis and Fibrosis by Inhibiting TGF-β Signaling Pathway Activation in Type 2 Diabetic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 4545321. [Google Scholar] [CrossRef] [PubMed]
- Shiming, Z.; Mak, K.K.; Balijepalli, M.K.; Chakravarthi, S.; Pichika, M.R. Swietenine potentiates the antihyperglycemic and antioxidant activity of Metformin in Streptozotocin induced diabetic rats. Biomed. Pharmacother. 2021, 139, 111576. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Han, E.J.; Sung, J.H.; Chung, S.H. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol. Pharm. Bull. 2007, 30, 2196–2200. [Google Scholar] [CrossRef] [PubMed]
- Tongia, A.; Tongia, S.K.; Dave, M. Phytochemical determination and extraction of Momordica charantia fruit and its hypoglycemic potentiation of oral hypoglycemic drugs in diabetes mellitus (NIDDM). Indian J. Physiol. Pharmacol. 2004, 48, 241–244. [Google Scholar] [PubMed]
- Sobieraj, D.M.; Freyer, C.W. Probable hypoglycemic adverse drug reaction associated with prickly pear cactus, glipizide, and metformin in a patient with type 2 diabetes mellitus. Ann. Pharmacother. 2010, 44, 1334–1337. [Google Scholar] [CrossRef]
- Neha, S.; Anand, K.; Sunanda, P. Administration of Fenugreek Seed Extract Produces Better Effects in Glibenclamide-Induced Inhibition in Hepatic Lipid Peroxidation: An in vitro Study. Chin. J. Integr. Med. 2019, 25, 278–284. [Google Scholar] [CrossRef]
- Ashraf, R.; Khan, R.A.; Ashraf, I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak. J. Pharm. Sci. 2011, 24, 565–570. [Google Scholar]
- El-Dahiyat, F.; Rashrash, M.; Abuhamdah, S.; Abu Farha, R.; Babar, Z.U. Herbal medicines: A cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharm. Policy Pract. 2020, 13, 2. [Google Scholar] [CrossRef]
- Liu, X.C. xiao Overview on development of ASEAN traditional and herbal medicines. Chin. Herb. Med. 2021, 13, 441–450. [Google Scholar] [CrossRef]
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal Medicines for Diabetes Management and its Secondary Complications. Curr. Diabetes Rev. 2021, 17, 437–456. [Google Scholar] [CrossRef]
- Xue, Z.; Li, Y.; Zhou, M.; Liu, Z.; Fan, G.; Wang, X.; Zhu, Y.; Yang, J. Traditional Herbal Medicine Discovery for the Treatment and Prevention of Pulmonary Arterial Hypertension. Front. Pharmacol. 2021, 12, 720873. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phyther. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Boadi, E.O.; Li, J.; Pang, X.; He, J.; Chang, X.Y. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef] [PubMed]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement Altern Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Moradi, B.; Abbaszadeh, S.; Shahsavari, S.; Alizadeh, M.; Beyranvand, F. The most useful medicinal herbs to treat diabetes. Biomed. Res. Ther. 2018, 5, 2538–2551. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Willcox, M.L.; Elugbaju, C.; Al-Anbaki, M.; Lown, M.; Graz, B. Effectiveness of Medicinal Plants for Glycaemic Control in Type 2 Diabetes: An Overview of Meta-Analyses of Clinical Trials. Front. Pharmacol. 2021, 12, 777561. [Google Scholar] [CrossRef]
- Valli, G.; Giardina, E.G.V. Benefits, adverse effects and drug interactions of herbal therapies with cardiovascular effects. J. Am. Coll. Cardiol. 2002, 39, 1083–1095. [Google Scholar] [CrossRef]
- Gerber, W.; Steyn, J.D.; Kotzé, A.F.; Hamman, J.H. Beneficial Pharmacokinetic Drug Interactions: A Tool to Improve the Bioavailability of Poorly Permeable Drugs. Pharmaceutics 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.; Hamman, J.H. What are the dangers of drug interactions with herbal medicines? Expert Opin. Drug Metab. Toxicol. 2020, 16, 165–167. [Google Scholar] [CrossRef]
- Levy, I.; Attias, S.; Ben-Arye, E.; Goldstein, L.; Schiff, E. Adverse events associated with interactions with dietary and herbal supplements among inpatients. Br. J. Clin. Pharmacol. 2017, 83, 836–845. [Google Scholar] [CrossRef]
- Tan, M.H.; Alquraini, H.; Mizokami-Stout, K.; MacEachern, M. Metformin: From Research to Clinical Practice. Endocrinol. Metab. Clin. N. Am. 2016, 45, 819–843. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, B.; Jiang, B.; Zeng, L.; Tang, Z.-R.; Fan, L.; Zhou, H.-H. The effects of puerarin on CYP2D6 and CYP1A2 activities in vivo. Arch. Pharm. Res. 2010, 33, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Liang, D.L.; Xu, Z.S.; Ye, Q. In vivo inhibitory effects of puerarin on selected rat cytochrome P450 isoenzymes. Pharmazie 2014, 69, 367–370. [Google Scholar] [PubMed]
- Kim, S.B.; Yoon, I.S.; Kim, K.S.; Cho, S.J.; Kim, Y.S.; Cho, H.J.; Chung, S.J.; Chong, S.; Kim, D.D. In vitro and in vivo evaluation of the effect of puerarin on hepatic cytochrome P450-mediated drug metabolism. Planta Med. 2014, 80, 561–567. [Google Scholar] [CrossRef]
- Liu, A.C.; Zhao, L.X.; Yu, S.W.; Lou, H.X. Pre-treatment with puerarin affects pharmacokinetics of warfarin, but not clopidogrel, in experimental rats. Chin. J. Nat. Med. 2015, 13, 257–263. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Dai, H.; Liu, Y.; Wang, L. Effects of puerarin on the pharmacokinetics of astragaloside IV in rats and its potential mechanism. Pharm. Biol. 2020, 58, 328–332. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, X.; Dong, G. Effects of verapamil on the pharmacokinetics of puerarin in rats. Xenobiotica 2019, 49, 1178–1182. [Google Scholar] [CrossRef]
- Liao, Z.G.; Liang, X.L.; Zhu, J.Y.; Zhao, G.W.; Guan, Y.M.; Cao, Y.C.; Zhao, L.J. Transport properties of puerarin and effect of extract of Radix Angelicae dahuricae on puerarin intestinal absorption using in situ and in vitro models. Phytother. Res. 2014, 28, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Wang, H.; Feng, L. Effects of glycyrrhizin on the pharmacokinetics of puerarin in rats. Xenobiotica 2018, 48, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhong, L.; Yang, L.; Bai, J.; Lu, Y.; Du, S. Coexisting flavonoids and administration route effect on pharmacokinetics of Puerarin in MCAO rats. Open Life Sci. 2020, 15, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lee, M.G. Effects of enzyme inducers and inhibitors on the pharmacokinetics of metformin in rats: Involvement of CYP2C11, 2D1 and 3A1/2 for the metabolism of metformin. Br. J. Pharmacol. 2006, 149, 424. [Google Scholar] [CrossRef]
- Abbasi, M.M.; Valizadeh, H.; Hamishehkar, H.; Zakeri-Milani, P. Inhibition of P-glycoprotein expression and function by anti-diabetic drugs gliclazide, metformin, and pioglitazone in vitro and in situ. Res. Pharm. Sci. 2016, 11, 177. [Google Scholar]
- Kim, H.G.; Hien, T.T.; Han, E.H.; Hwang, Y.P.; Choi, J.H.; Kang, K.W.; Kwon, K.I.; Kim, B.H.; Kim, S.K.; Song, G.Y.; et al. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharmacol. 2011, 162, 1096–1108. [Google Scholar] [CrossRef]
- Hien, T.T.; Kim, H.G.; Han, E.H.; Kang, K.W.; Jeong, H.G. Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol. Nutr. Food Res. 2010, 54, 918–928. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Wu, Q.; Lu, Y.; Shi, J.; Chen, X. Puerarin Improves Diabetic Aorta Injury by Inhibiting NADPH Oxidase-Derived Oxidative Stress in STZ-Induced Diabetic Rats. J. Diabetes Res. 2016, 2016, 8541520. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M. Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO-1 signaling. Mol. Med. Rep. 2019, 20, 1017. [Google Scholar] [CrossRef]
- Li, N.H.; Wang, M.; Jun-Ling, M.; Yang, T. Puerarin decreases apoptosis of retinal pigment epithelial cells in diabetic rats by reducing peroxynitrite level and iNOS expression - PubMed. Sheng Li Xue Bao 2012, 64, 199–206. [Google Scholar]
- Hao, L.N.; Zhang, Y.Q.; Shen, Y.H.; Wang, Z.Y.; Wang, Y.H.; Zhang, H.F.; He, S.Z. Effect of puerarin on retinal pigment epithelial cells apoptosis induced partly by peroxynitrite via Fas/FasL pathway. Int. J. Ophthalmol. 2010, 3, 283. [Google Scholar] [PubMed]
- Li, Z.; Shangguan, Z.; Liu, Y.; Wang, J.; Li, X.; Yang, S.; Liu, S. Puerarin protects pancreatic β-cell survival via PI3K/Akt signaling pathway. J. Mol. Endocrinol. 2014, 53, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Xiufang, C.; Kangfu, L.; Min, D. Effect of puerarin on pancreatic β-cell injury in type 2 diabetic rats. J. Wezhou Med. Univ. 2017, 47, 859–863. [Google Scholar]
- Chen, X.; Wang, L.; Fan, S.; Song, S.; Min, H.; Wu, Y.; He, X.; Liang, Q.; Wang, Y.; Yi, L.; et al. Puerarin acts on the skeletal muscle to improve insulin sensitivity in diabetic rats involving μ-opioid receptor. Eur. J. Pharmacol. 2018, 818, 115–123. [Google Scholar] [CrossRef]
- Song, C.; Bi, H. Effects of puerarin on plasma membrane GLUT4 content in skeletal muscle from insulin-resistant Sprague-Dawley rats under insulin stimulation. Zhongguo Zhong Yao Za Zhi 2004, 29, 172–175. [Google Scholar]
- Juanjuan, L.I.; Huimin, B.I. Effects of Puerarin on GLUT4 in Adipocyte of Rats with Insulin Resistance. Chin. J. Clin. Pharmacol. Ther. 2004, 9, 885–888. [Google Scholar]
- Sun, R.; Deng, X.; Zhang, D.; Xie, F.; Wang, D.; Wang, J.; Tavallaie, M.S.; Jiang, F.; Fu, L. Anti-diabetic potential of Pueraria lobata root extract through promoting insulin signaling by PTP1B inhibition. Bioorg. Chem. 2019, 87, 12–15. [Google Scholar] [CrossRef]
- Shen, J.G.; Yao, M.F.; Chen, X.C.; Feng, Y.F.; Ye, Y.H.; Tong, Z.H. Effects of puerarin on receptor for advanced glycation end products in nephridial tissue of streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2009, 36, 2229–2233. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yu, L.; Chen, J. The Puerarin improves renal function in STZ-induced diabetic rats by attenuating eNOS expression. Ren. Fail. 2015, 37, 699–703. [Google Scholar] [CrossRef]
- Del Prete, E.; Lutz, T.A.; Scharrer, E. Acute Increase in Food Intake After Intraperitoneal Injection of Metformin in Rats. Physiol. Behav. 1999, 67, 685–689. [Google Scholar] [CrossRef]
- Kisfalvi, K.; Moro, A.; Sinnett-Smith, J.; Eibl, G.; Rozengurt, E. Metformin Inhibits the Growth of Human Pancreatic Cancer Xenografts. Pancreas 2013, 42, 781. [Google Scholar] [CrossRef] [PubMed]
| Group | Number of Rats | Intervention | Dose, Administration Route |
|---|---|---|---|
| NC | 10 | PBS | 2 mL/kg, IP |
| DC | 10 | PBS | 2 mL/kg, IP |
| P1 | 10 | Puerarin | 80 mg/kg, IP |
| P2 | 10 | Puerarin | 120 mg/kg, IP |
| P3 | 10 | Puerarin | 160 mg/kg, IP |
| M | 10 | Metformin | 100 mg/kg, IP |
| P1M | 10 | Puerarin + Metformin | 80 mg/kg, IP + 100 mg/kg IP |
| P2M | 10 | Puerarin + Metformin | 120 mg/kg, IP + 100 mg/kg IP |
| P3M | 10 | Puerarin + Metformin | 160 mg/kg, IP + 100 mg/kg IP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, S.; Wang, X.; Gao, P.; Zhang, S.; Mo, Y.; Zhao, D.; Dai, L. Pharmacodynamic Interactions between Puerarin and Metformin in Type-2 Diabetic Rats. Molecules 2022, 27, 7197. https://doi.org/10.3390/molecules27217197
Li Z, Wang S, Wang X, Gao P, Zhang S, Mo Y, Zhao D, Dai L. Pharmacodynamic Interactions between Puerarin and Metformin in Type-2 Diabetic Rats. Molecules. 2022; 27(21):7197. https://doi.org/10.3390/molecules27217197
Chicago/Turabian StyleLi, Zhen, Shengguang Wang, Xinyu Wang, Peng Gao, Shiming Zhang, Yingning Mo, Dongsheng Zhao, and Long Dai. 2022. "Pharmacodynamic Interactions between Puerarin and Metformin in Type-2 Diabetic Rats" Molecules 27, no. 21: 7197. https://doi.org/10.3390/molecules27217197
APA StyleLi, Z., Wang, S., Wang, X., Gao, P., Zhang, S., Mo, Y., Zhao, D., & Dai, L. (2022). Pharmacodynamic Interactions between Puerarin and Metformin in Type-2 Diabetic Rats. Molecules, 27(21), 7197. https://doi.org/10.3390/molecules27217197





