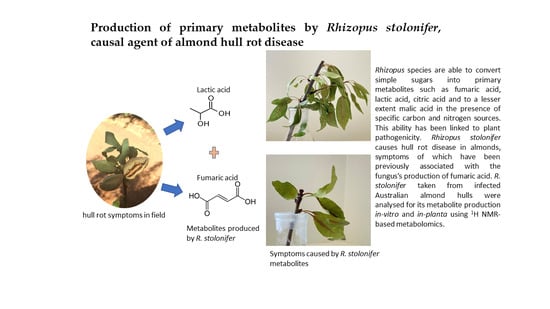
Production of Primary Metabolites by Rhizopus stolonifer, Causal Agent of Almond Hull Rot Disease
Abstract
1. Introduction
2. Results
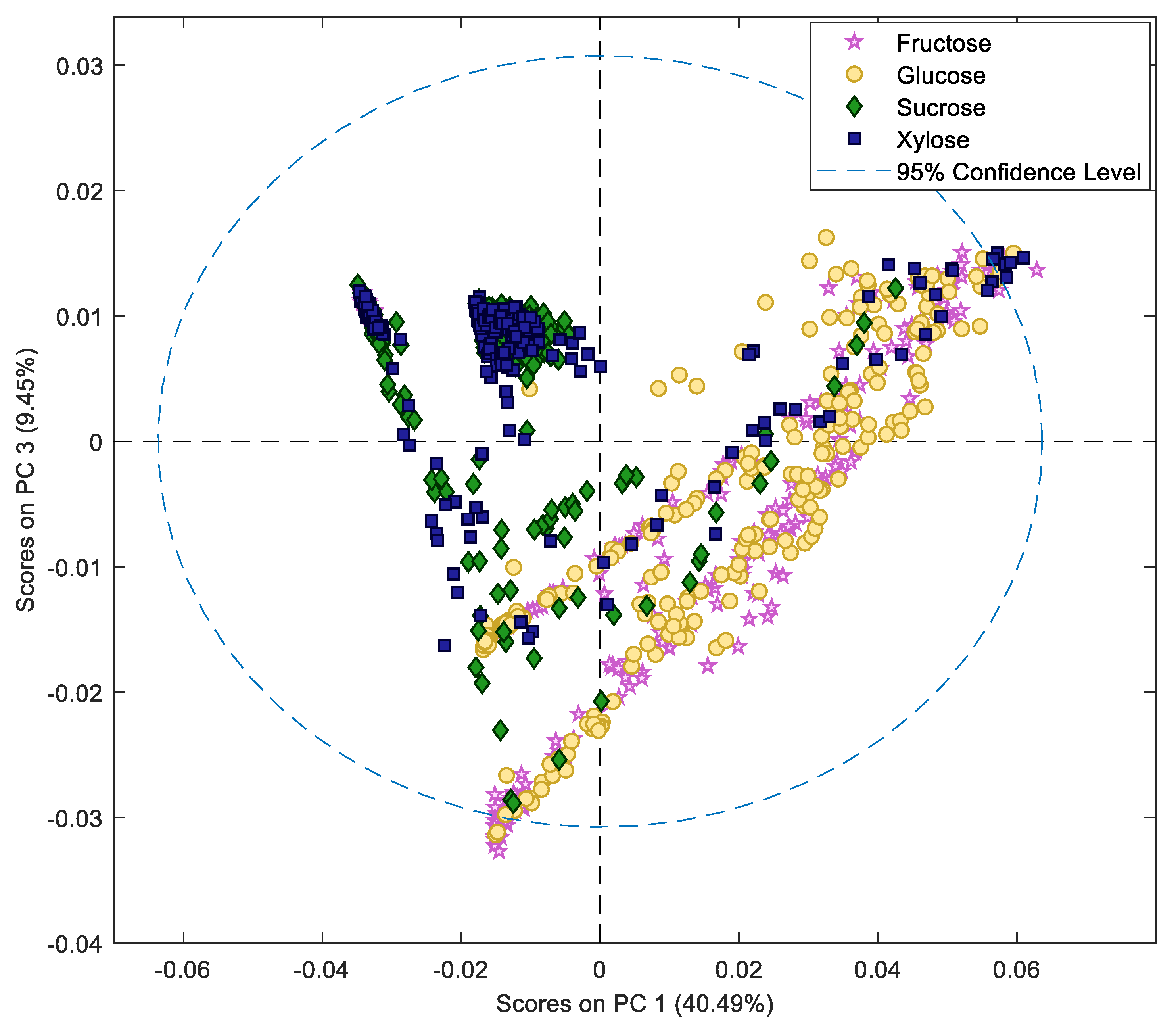
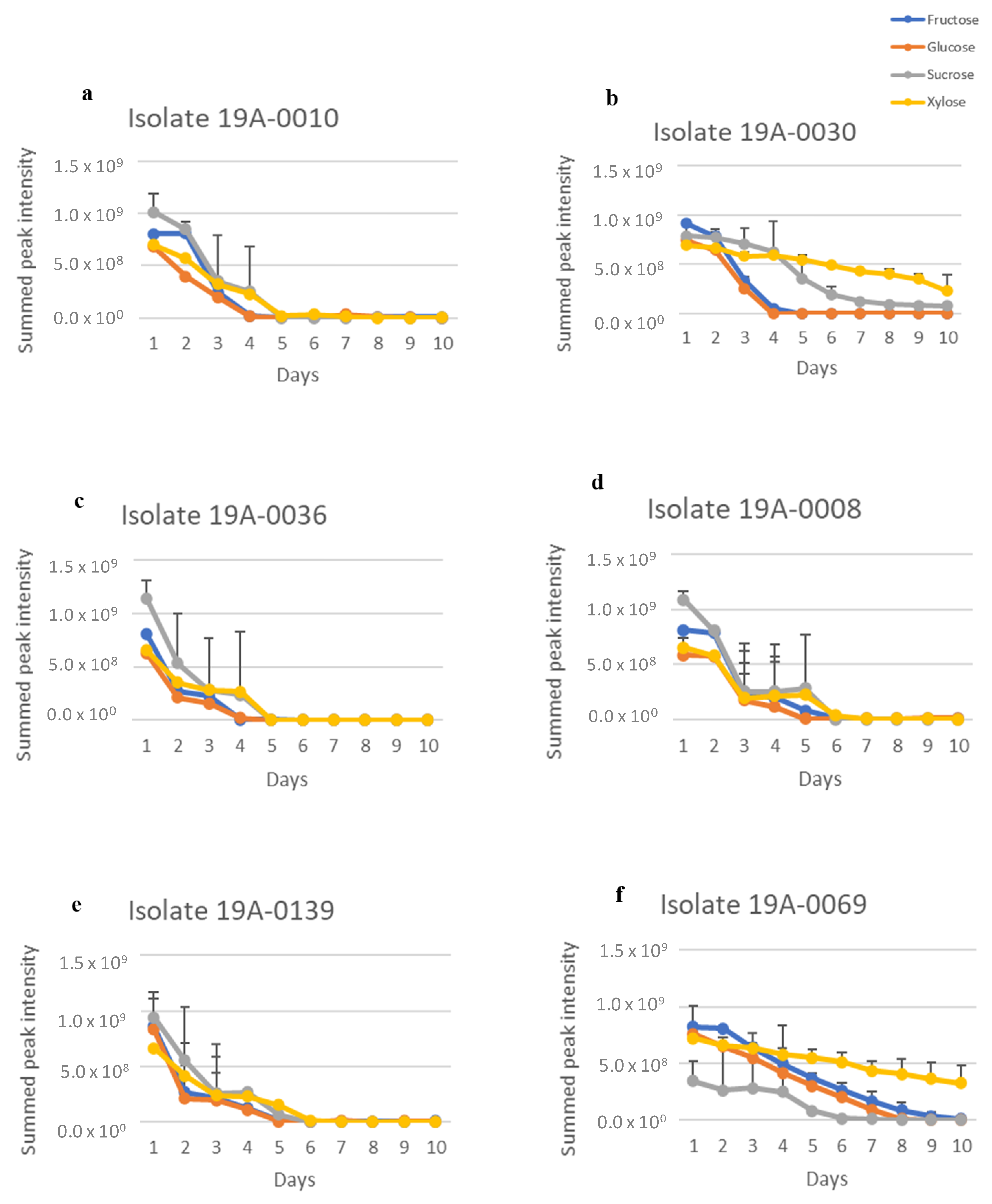
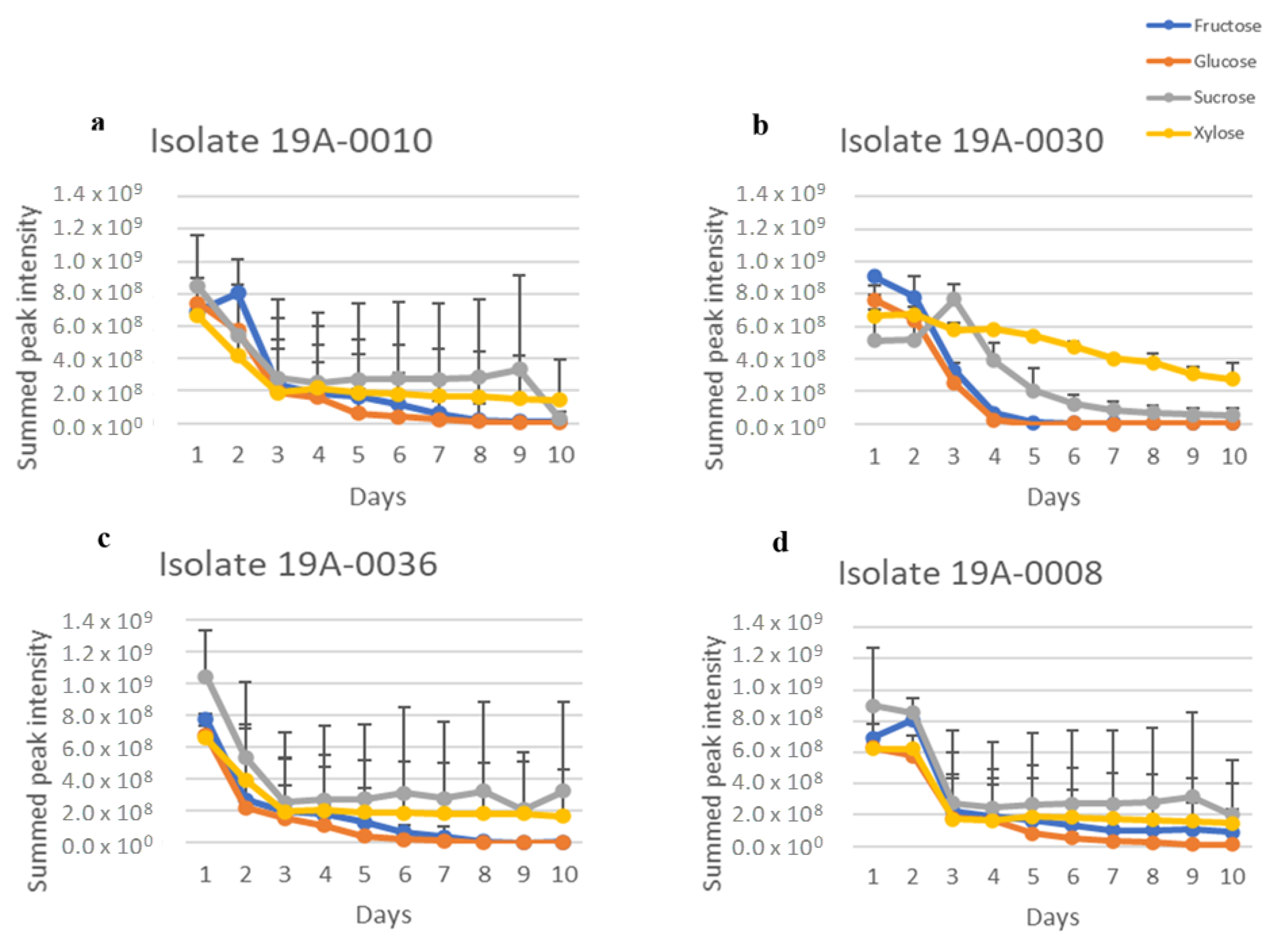
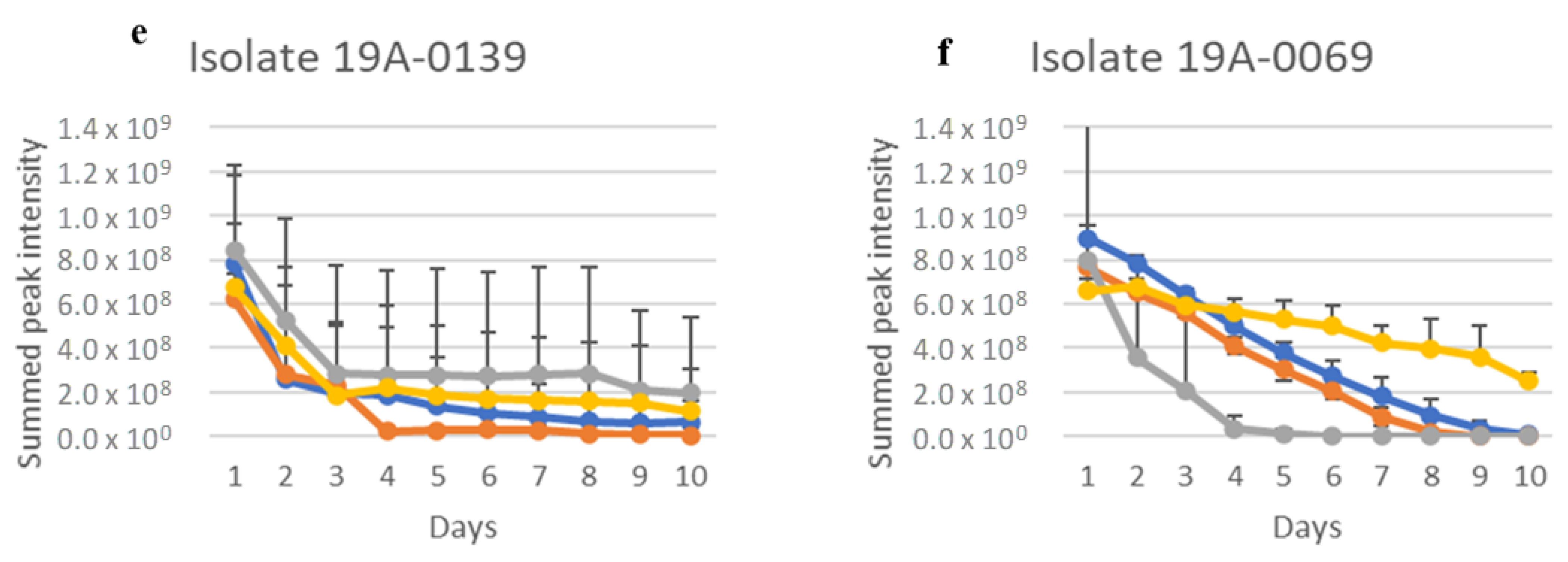
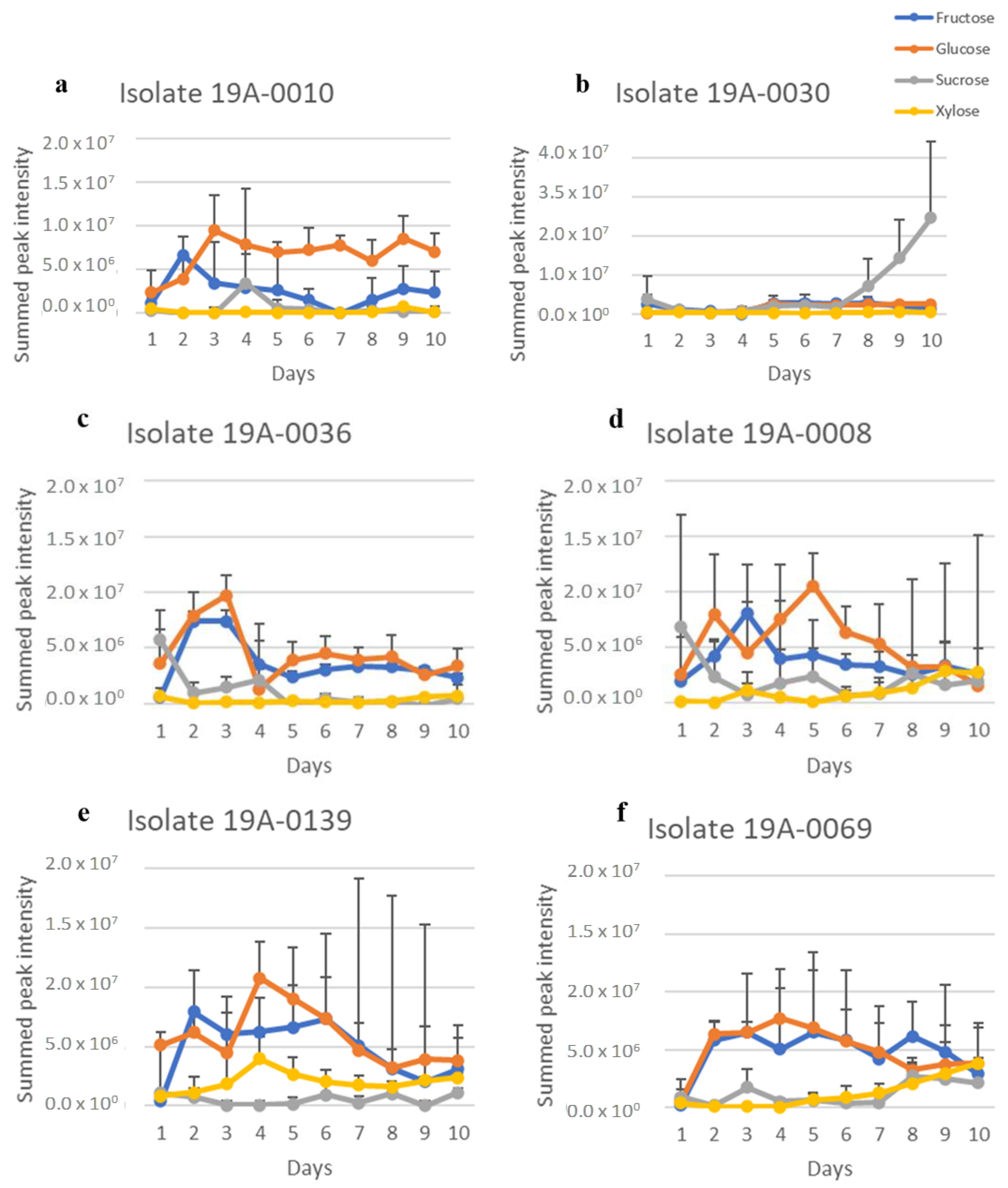
2.1. In Vitro Sugar and Nitrogen Metabolization by Rhizopus stolonifer
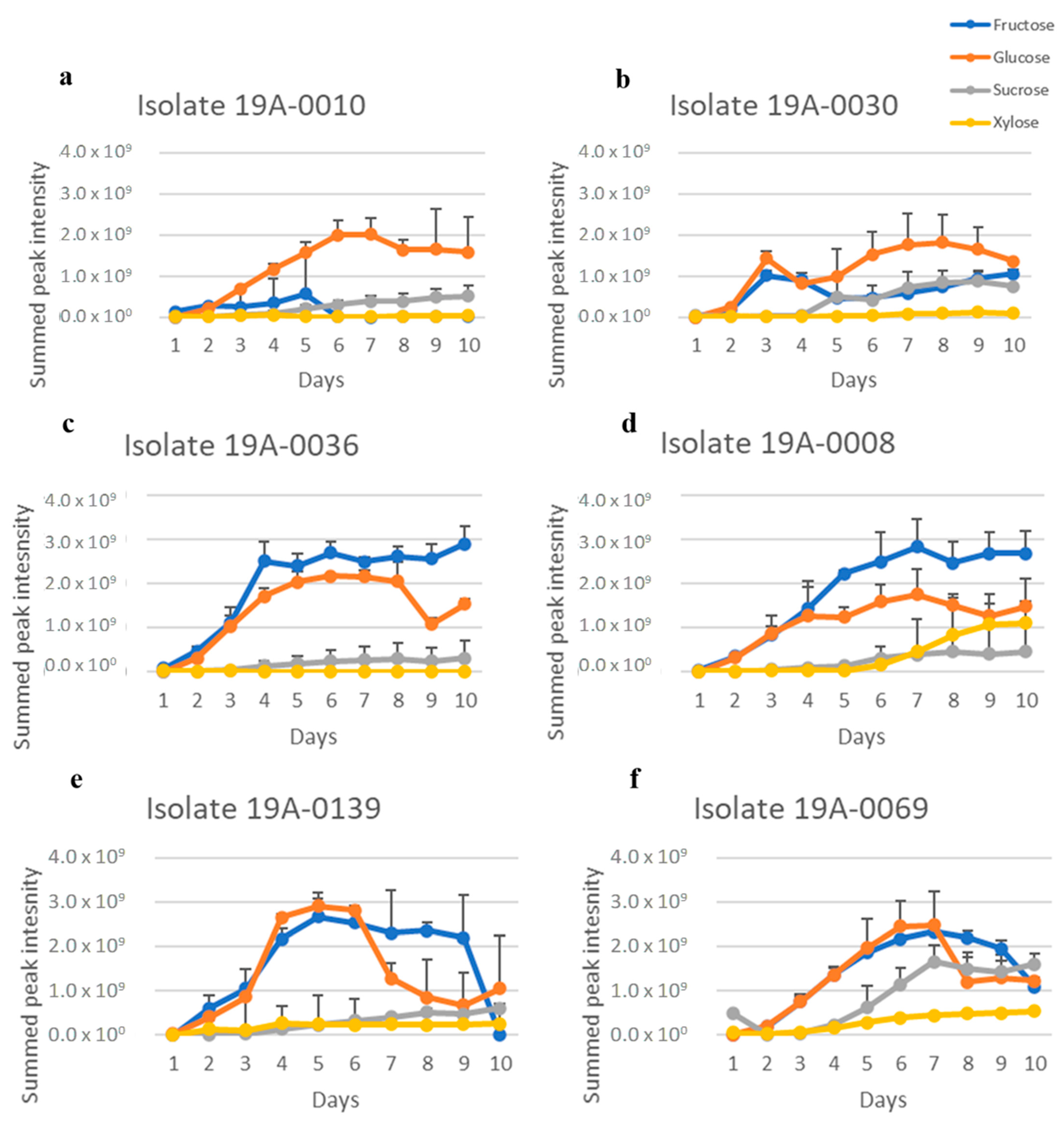
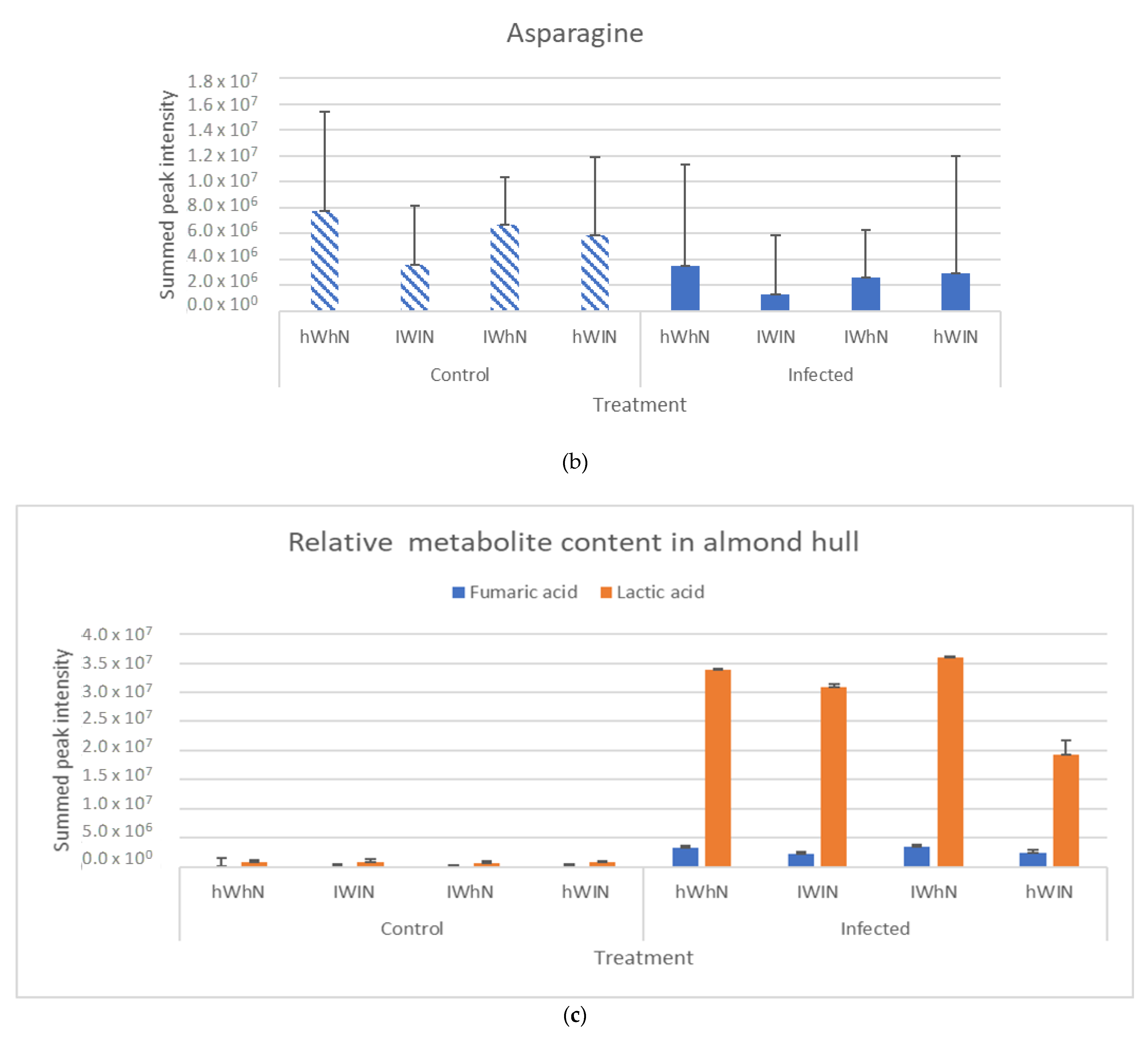
2.2. Production of Primary Metabolites
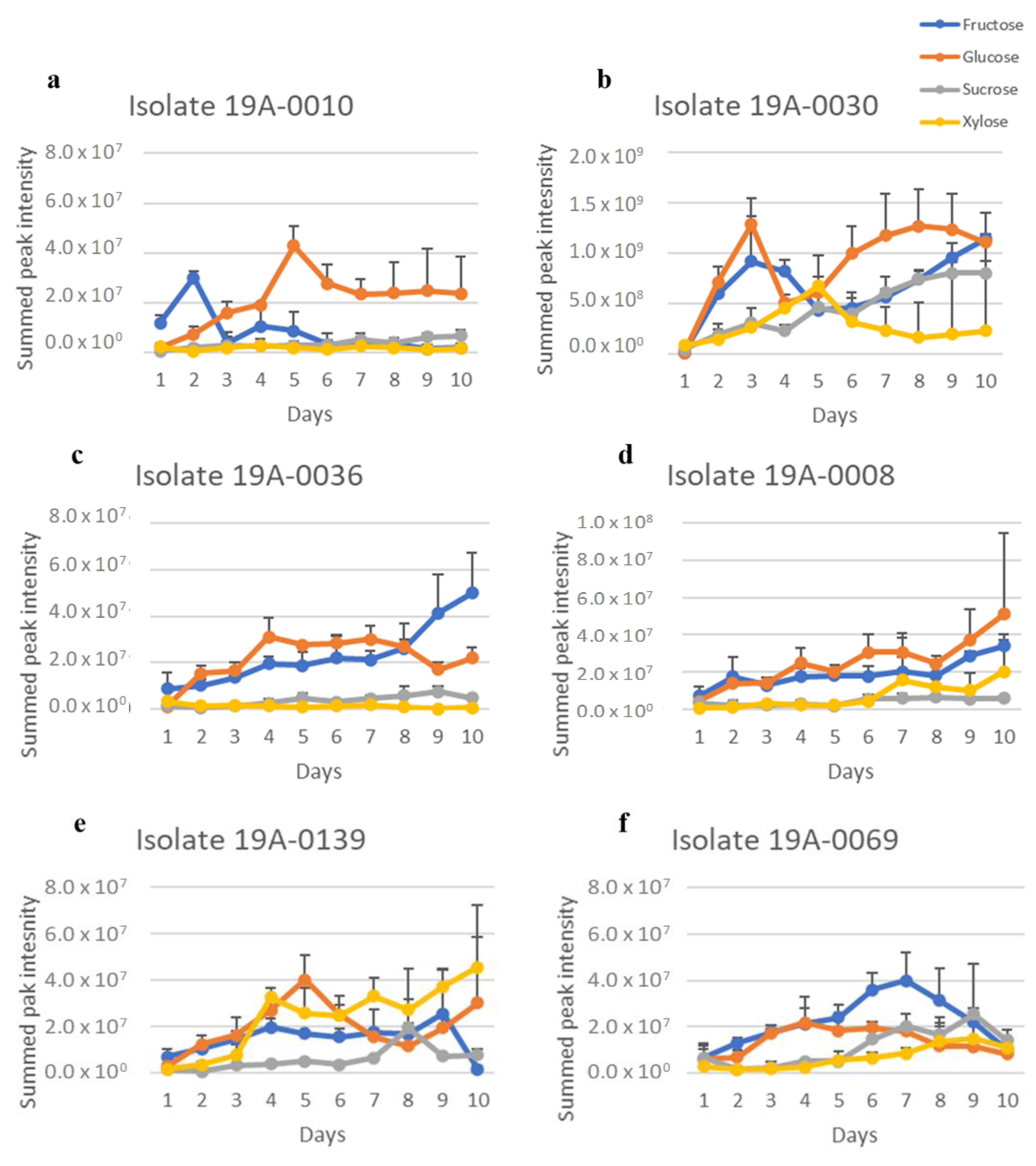
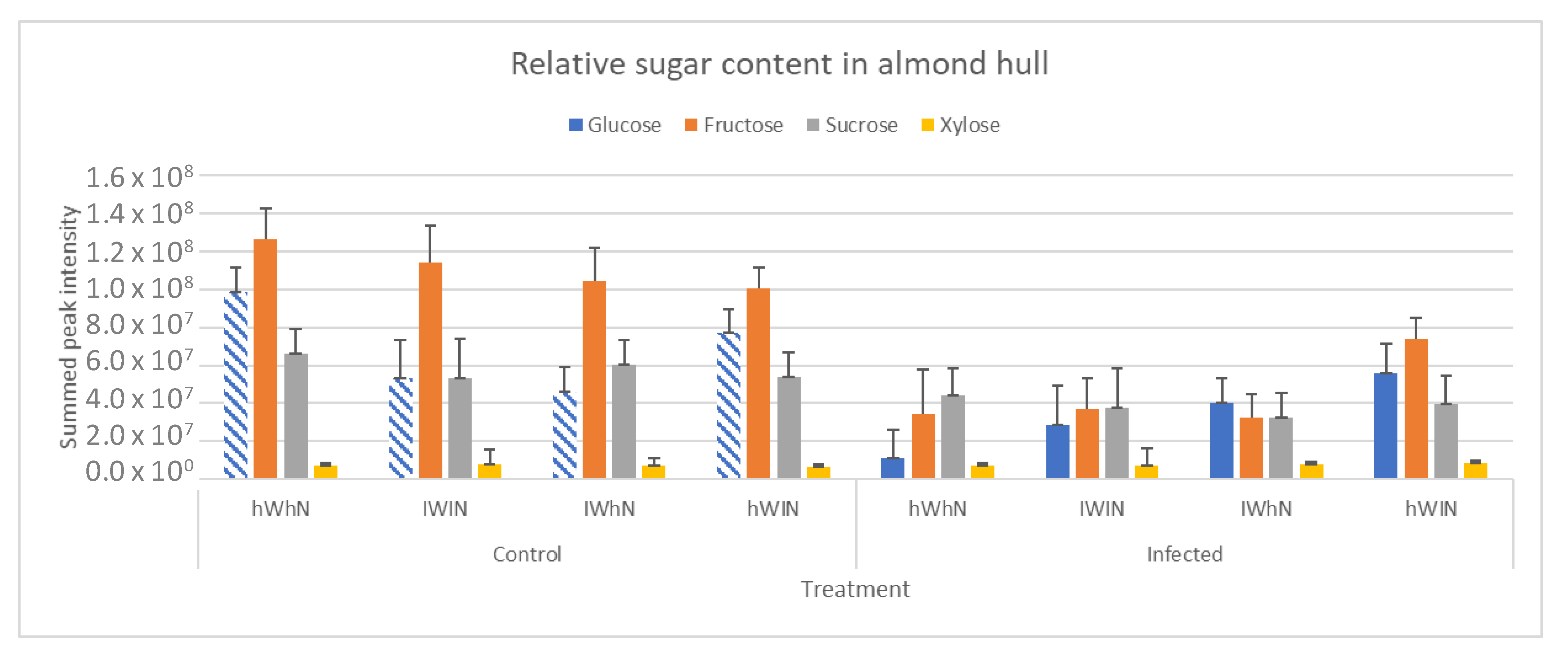
2.3. Sugar and Nitrogen Metabolisation of Rhizopus stolonifer in Infected Hull Samples
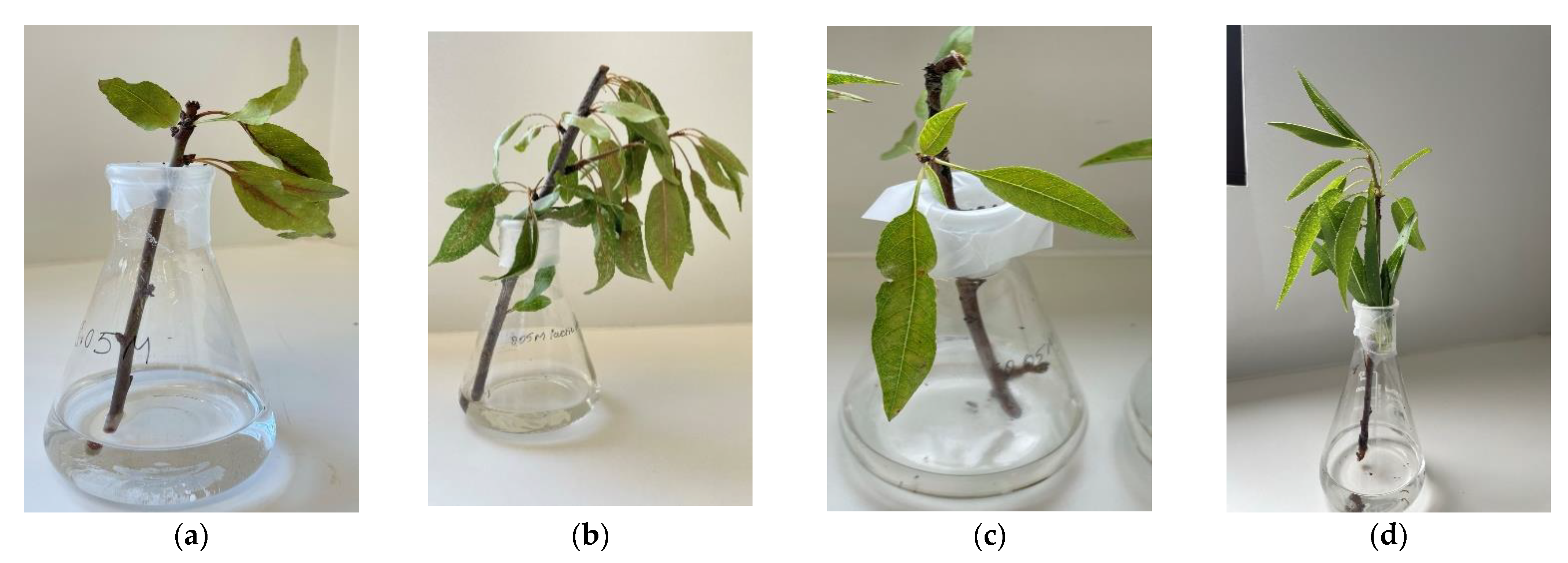
2.4. Toxicity of Fumaric Acid, Lactic Acid, and Ethanol to Almond Shoots
3. Discussion
4. Materials and Methods
4.1. Site Descriptions of Fungal Isolates and Culture Conditions of In Vitro Metabolite Analysis
4.1.1. Culture Medium
4.1.2. Culture Conditions
4.1.3. NMR Sample Preparation
4.2. Site Description, Fungal Isolates, and Culture Conditions of In Vitro Metabolite Analysis
4.3. Inoculum Preparation
4.4. Inoculation
4.5. Sample Collection
4.6. Infected Almond Hull Sample Preparation for NMR
4.7. 1H NMR Data Acquisition and Preprocessing of Fungal Metabolites and Hull Composition
4.8. Statistical Analysis
4.9. Toxicity Analysis of Metabolites Produced by R. stolonifer
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meussen, B.; Graaff, L.; Sanders, J.; Weusthuis, R. Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl. Microbiol. Biotechnol. 2012, 94, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, P.R.; Taherzadeh, M.J.; Edebo, L. RHIZOPUS. In Encyclopedia of Food Microbiology (Second Edition); Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 284–290. [Google Scholar]
- Jin, B.; Huang, L.P.; Lant, P. Rhizopus arrhizus—A producer for simultaneous saccharification and fermentation of starch waste materials to l(+)–lactic acid. Biotechnol. Lett. 2003, 25, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Marták, J.; Schlosser, Š.; Sabolová, E.; Krištofıková, L.; Rosenberg, M. Fermentation of lactic acid with Rhizopus arrhizus in a stirred tank reactor with a periodical bleed and feed operation. Process Biochem. 2003, 38, 1573–1583. [Google Scholar] [CrossRef]
- Bulut, S.; Elibol, M.; Ozer, D. Effect of different carbon sources on l(+)–lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2004, 21, 33–37. [Google Scholar] [CrossRef]
- Moon, S.K.; Wee, Y.J.; Yun, J.S.; Ryu, H.W. Production of fumaric acid using rice bran and subsequent conversion to succinic acid through a two–step process. Appl. Biochem. Biotechnol. 2004, 113–116, 843–855. [Google Scholar] [CrossRef]
- Maas, R.H.W.; Bakker, R.R.; Eggink, G.; Weusthuis, R.A. Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2006, 72, 861–868. [Google Scholar] [CrossRef]
- Kowalczyk, S.; Komoń–Janczara, E.; Glibowska, A.; Kuzdraliński, A.; Czernecki, T.; Targoński, Z. A co–utilization strategy to consume glycerol and monosaccharides by Rhizopus strains for fumaric acid production. AMB Express 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Cao, N.; Du, J.; Gong, C.S.; Tsao, G.T. Simultaneous Production and Recovery of Fumaric Acid from Immobilized Rhizopus oryzae with a Rotary Biofilm Contactor and an Adsorption Column. Appl. Environ. Microbiol. 1996, 62, 2926–2931. [Google Scholar] [CrossRef]
- Zhou, Y.; Domínguez, J.M.; Cao, N.; Du, J.; Tsao, G.T. Optimization of L–Lactic acid production from glucose by Rhizopus oryzae ATCC 52311. Appl. Biochem. Biotechnol. 1999, 78, 401–407. [Google Scholar] [CrossRef]
- Ward, G.E.; Lockwood, L.B.; May, O.E.; Herrick, H.T. Biochemical studies in the genus Rhizopus. I. The production of dextro–lactic acid1. J. Am. Chem. Soc. 1936, 58, 1286–1288. [Google Scholar] [CrossRef]
- Roa Engel, C.; Straathof, A.; Zijlmans, T.; Gulik, W.; Wielen, L. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Fumaric Acid: Production and Application Aspects. In Platform Chemical Biorefinery; Kaur Brar, S., Jyoti Sarma, S., Pakshirajan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–157. [Google Scholar]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; Machado de Castro, A.; Papanikolaou, S.; Koutinas, A. Biotechnological production of fumaric acid: The effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef]
- Thoni, H. Wachstum Kinetische Untersuchungen an einer Mucor Idee (Rhizopus oryzae W. et Pr. G.). Der Einfluss von Glycin auf das Wachstum. Growth kinetic studies on a Mucor idea (Rhizopus oryzae W. et Pr. G.). The influence of glycine on growth. Arch. Mikrobiol 1963, 46, 338–368. [Google Scholar]
- Sorenson, W.G.; Hesseltine, C.W. Carbon and Nitrogen Utilization by Rhizopus oligosporus. Mycologia 1966, 58, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.V.; Rombouts, F.M.; Nout, M.J.R. Effect of individual amino acids and glucose on activation and germination of Rhizopus oligosporus sporangiospores in tempe starter. J. Appl. Microbiol. 2005, 99, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.G. Microbial Kinetics during Batch, Countinuous and Fed–Batch Processes. In Bioprocess Engineering: An Introductory engineering and Life Science Approach; Elsevier Science & Technology: Jordon Hill, UK, 2013. [Google Scholar]
- Cessna, S.G.; Sears, V.E.; Dickman, M.B.; Low, P.S. Oxalic Acid, a Pathogenicity Factor for Sclerotinia sclerotiorum, Suppresses the Oxidative Burst of the Host Plant. Plant Cell 2000, 12, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant. Microbe Interact. 2001, 14, 1105–1113. [Google Scholar] [CrossRef]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef]
- Ruijter, G.J.G.; van de Vondervoort, P.J.I.; Visser, J. Oxalic acid production by Aspergillus niger: An oxalate–non–producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology 1999, 145, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship Between Host Acidification and Virulence of Penicillium spp. on Apple and Citrus Fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef]
- Prusky, D.B.; Bi, F.; Moral, J.; Barad, S. How Does Host Carbon Concentration Modulate the Lifestyle of Postharvest Pathogens during Colonization? Front. Plant Sci. 2016, 7, 1306. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.T.; Lawrence, D.P.; Yaghmour, M.A.; Michailides, T.J.; Trouillas, F.P. Neoscytalidium dimidiatum causing canker, shoot blight and fruit rot of almond in California. Plant Dis. 2018, 102, 1638–1647. [Google Scholar] [CrossRef]
- Michailides, T.J.; Trouillas, F.P.; Yaghmour, M.; Gordon, P. New foes of almonds at hull split stage. West Coast Nut. 2021, 38–43. [Google Scholar]
- Mirocha, C.J.; DeVay, J.E.; Wilson, E.E. Role of fumaric acid in the hull rot disease of almond. Phytopathology 1961, 51, 851–860. [Google Scholar]
- Mirocha, C.J.; Wilson, E.E. Hull rot disease of almonds. Phytopathology 1961, 51, 843–847. [Google Scholar]
- Cline, H. Hull rot is the "gout" of almond diseases. West. Farm Press 2008, 30, 8. [Google Scholar]
- Teviotdale, B.L.; Michailides, T.J.; Goldhamer, D.A.; Viveros, M.; Schmidt, L.; Hines, V. Cutting off irrigation early may reduce almond hull rot. Calif. Agric. 1994, 48, 33–36. [Google Scholar] [CrossRef]
- Adaskaveg, J.E. Epidemiology & management of almond brown rot, scab, and other diseases in California. In Proceedings of the 2010:2011 Annual Research Report, Almond Board of California, Riverside, CA, USA; 2011. [Google Scholar]
- Peach–Fine, E. Fertilization of Irrigation Effects on the disease Dynamics of Hull Rot: The “Good Growers Disease”. Master Dissertation, Univeristy of California, Davis, CA, USA, 2013. [Google Scholar]
- Saa, S.; Peach–Fine, E.; Brown, P.; Michailides, T.; Castro, S.; Bostock, R.; Laca, E. Nitrogen increases hull rot and interferes with the hull split phenology in almond (Prunus dulcis). Sci. Hortic. 2016, 199, 41–48. [Google Scholar] [CrossRef]
- Teviotdale, B.L. Effect of four level of applied nitrogen on three fungal diseases of almond trees. In Proceedings of the 22nd Almond Research Conference: Almond Board of California, Riverside, CA, USA; 1994. [Google Scholar]
- Teviotdale, B.L.; Michailides, T.J.; Goldhamer, D.A.; Viveros, M. Reduction of almond hull rot disease caused by Rhizopus stolonifer by early termination of preharvest irrigation. Plant Dis. 1995, 79, 402–405. [Google Scholar] [CrossRef]
- Teviotdale, B.L.; Goldhamer, D.A.; Viveros, M. Effects of deficit irrigation on hull rot disease of almond trees caused by Monilinia fructicola and Rhizopus stolonifer. Plant Dis. 2001, 85, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Goldhamer, D.A.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Bi, F.; Barad, S.; Ment, D.; Luria, N.; Dubey, A.; Casado, V.; Glam, N.; Mínguez, J.D.; Espeso, E.A.; Fluhr, R.; et al. Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi. Mol. Plant Pathol. 2016, 17, 1178–1195. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, R.M.; Lew, R.B. Carbohydrate composition of almond hulls. J. Agric. Food Chem. 1970, 18, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Calixto, F.S.; Cañellas, J. Chemical composition of hulls of the sweet almond (Prunus amygdalus). J. Sci. Food Agric. 1982, 33, 336–339. [Google Scholar] [CrossRef]
- DePeters, E.J.; Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M. Nutritional composition of almond hulls. Prof. Anim. Sci. 2020, 36, 761–770. [Google Scholar] [CrossRef]
- Zaveri, A.; Edwards, J.; Rochfort, S. Restricted nitrogen and water applications in the orchard modify the carbohydrate and amino acid composition of Nonpareil and Carmel almond hulls. Metabolites 2021, 11, 674. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, Q.; Jiang, Z.; Teng, C.; Wang, X. Efficient production of lactic acid from sucrose and corncob hydrolysate by a newly isolated Rhizopus oryzae GY18. J. Ind. Microbiol. Biotechnol. 2010, 37, 1137–1143. [Google Scholar] [CrossRef]
- Nwokoro, O. Studies on the production of citric acid by Rhizopus stolonifer. Chem. Sci. Int. J. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Stocks, P.K.; Ward, B.Q. Utilization of amino acids by Rhizopus nigricans. Can. J. Microbiol. 1962, 8, 761–767. [Google Scholar] [CrossRef]
- Luke, T.D.W.; Pryce, J.E.; Wales, W.J.; Rochfort, S.J. A tale of two biomarkers: Untargeted 1H NMR metabolomic fingerprinting of BHBA and NEFA in early lactation dairy cows. Metabolites 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R. Improved methods for the acquisition and interpretation of NMR metabolomic data. Biochem. Biophys. Res. Commun. 2003, 310, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.–J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA–simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
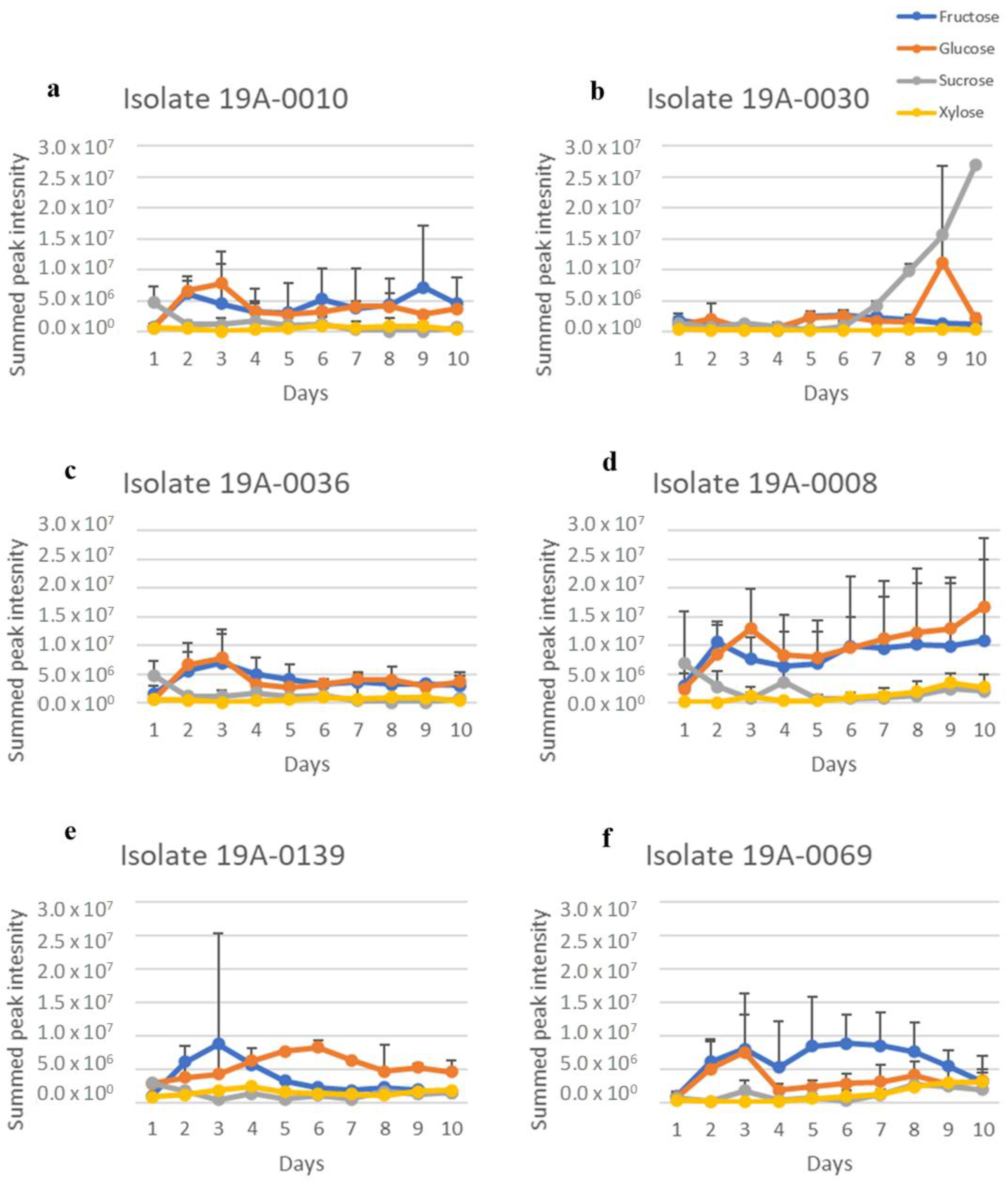
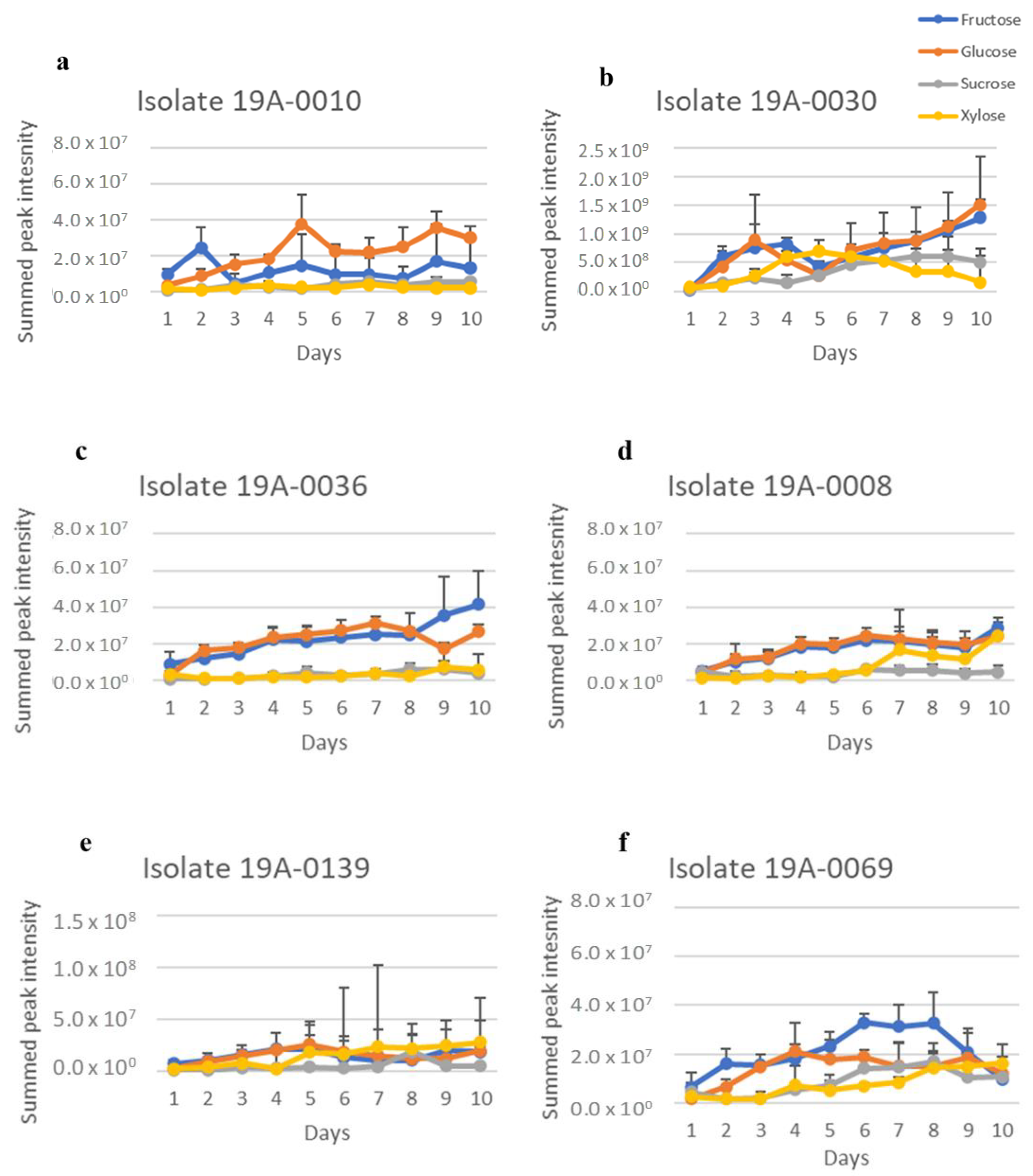
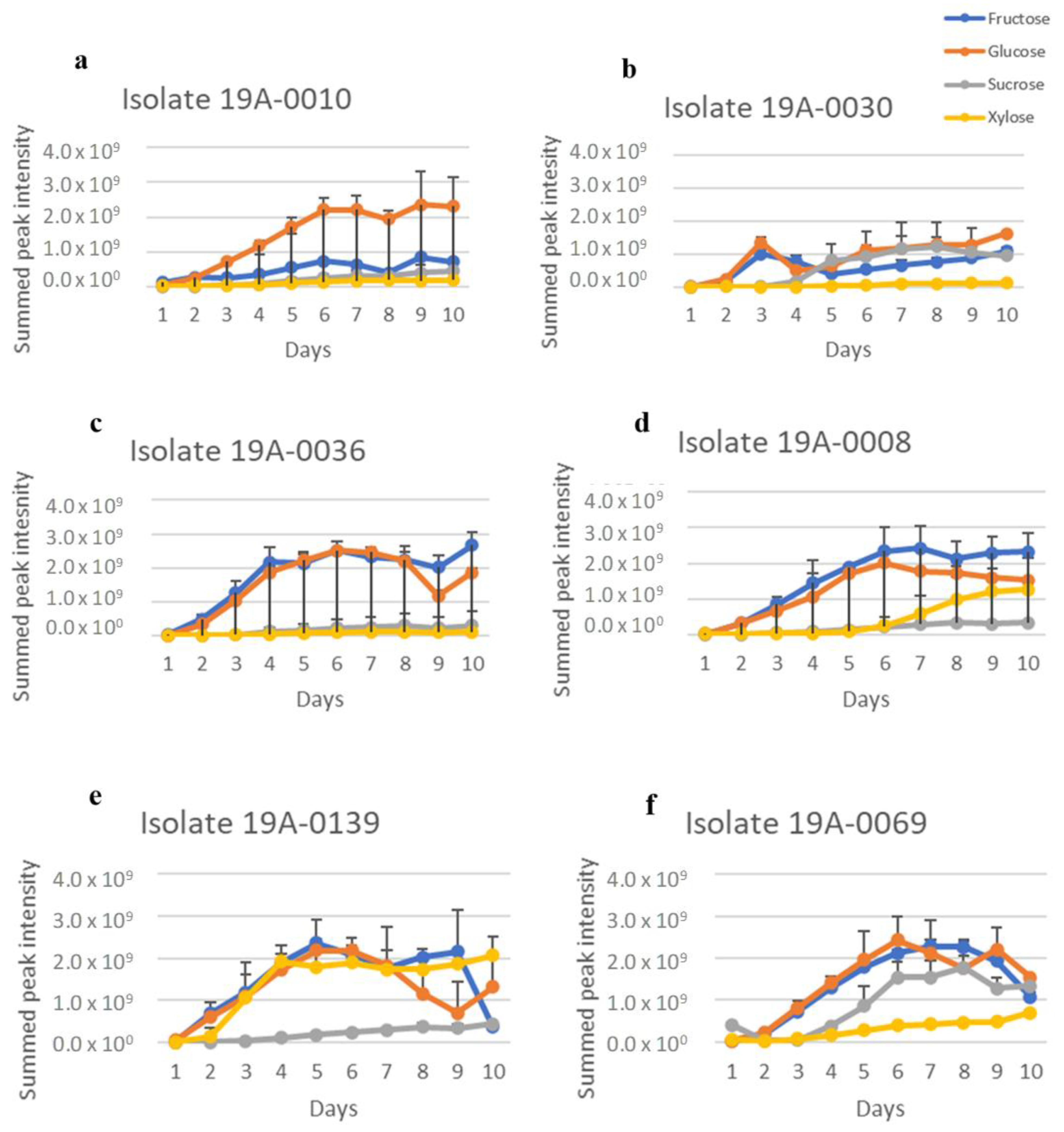
| Isolate | Location | Variety * | Strike or without Strike |
|---|---|---|---|
| 19A–0010 | Merbein, VIC | NP | Yes |
| 19A–0030 | Cullullera, VIC | NP | Yes |
| 19A–0036 | Mildura, VIC | NP | Yes |
| 19A–0008 | Nangiloc, VIC | NP | No |
| 19A–0069 | Coleambally, NSW | P | No |
| 19A–00139 | Bennerton, VIC | C | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaveri, A.; Edwards, J.; Rochfort, S. Production of Primary Metabolites by Rhizopus stolonifer, Causal Agent of Almond Hull Rot Disease. Molecules 2022, 27, 7199. https://doi.org/10.3390/molecules27217199
Zaveri A, Edwards J, Rochfort S. Production of Primary Metabolites by Rhizopus stolonifer, Causal Agent of Almond Hull Rot Disease. Molecules. 2022; 27(21):7199. https://doi.org/10.3390/molecules27217199
Chicago/Turabian StyleZaveri, Anjali, Jacqueline Edwards, and Simone Rochfort. 2022. "Production of Primary Metabolites by Rhizopus stolonifer, Causal Agent of Almond Hull Rot Disease" Molecules 27, no. 21: 7199. https://doi.org/10.3390/molecules27217199
APA StyleZaveri, A., Edwards, J., & Rochfort, S. (2022). Production of Primary Metabolites by Rhizopus stolonifer, Causal Agent of Almond Hull Rot Disease. Molecules, 27(21), 7199. https://doi.org/10.3390/molecules27217199