Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications
Abstract
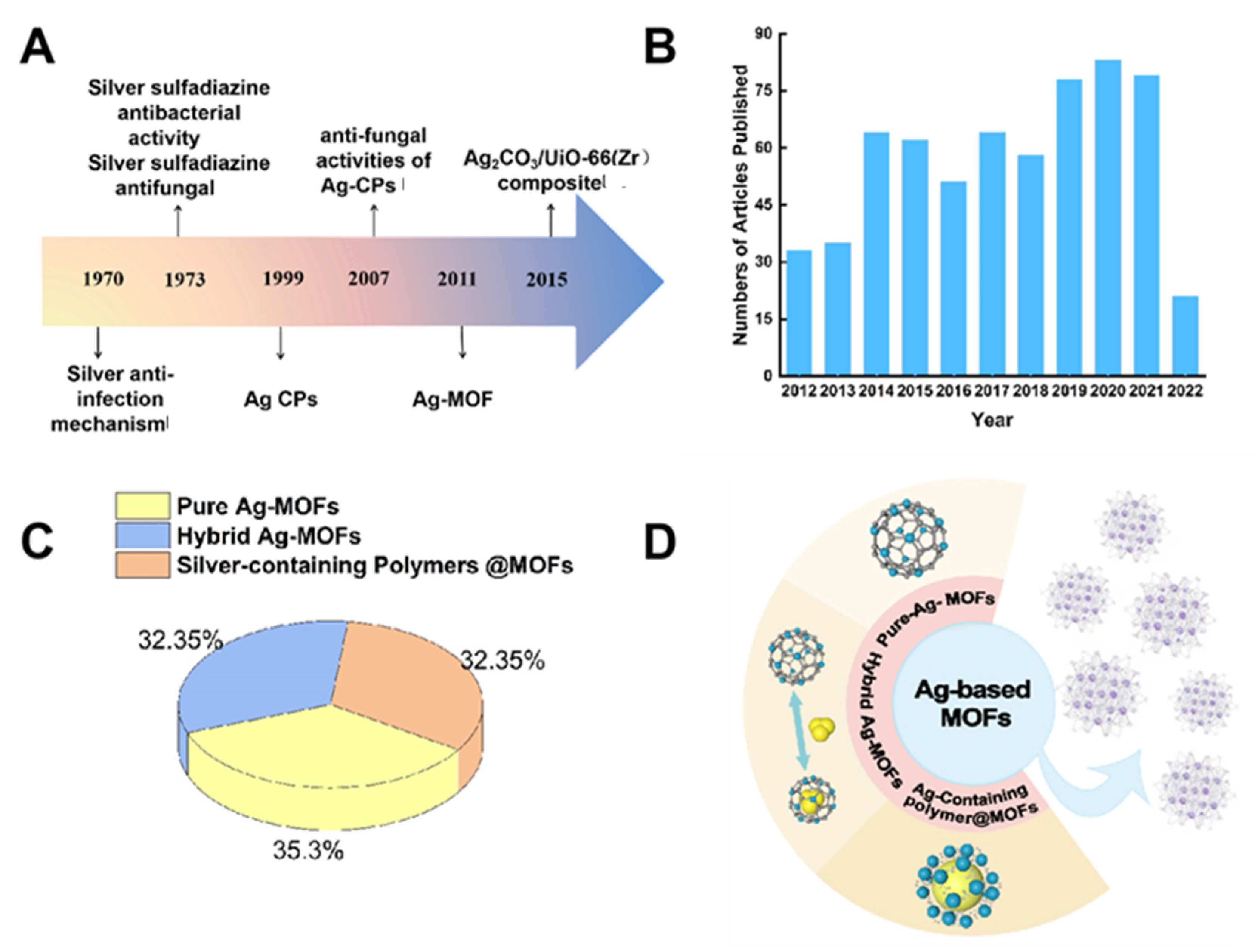
1. Introduction
2. Silver-Based MOFs
2.1. Pure Ag-MOFs
2.2. Hybrid Ag-MOFs
| No. | Composition | Organic Ligands | Bacterial Strain | Test Value | Antibacterial Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Pure Ag-MOFs | ||||||
| 1 | Ag6(m-O3PC6H4CO2)2 | m-Phosphonobenzoic acid | S. aureus P. aeruginosa | MBC = 50–70 µM MBC = 20–30 µM | The consequent release of silver ions. | [98] |
| 2 | [Ag2(Cedcp)]n | N-(carboxyethyl)-(3,5-dicarboxyl)-Pyridinium bromide | E. coli S. aureus | MIC >37.84 µM MIC = 37.84 µM | 1: The synergistic effect of the aromatic ring and pyridine. 2:The release of Ag+. | [99] |
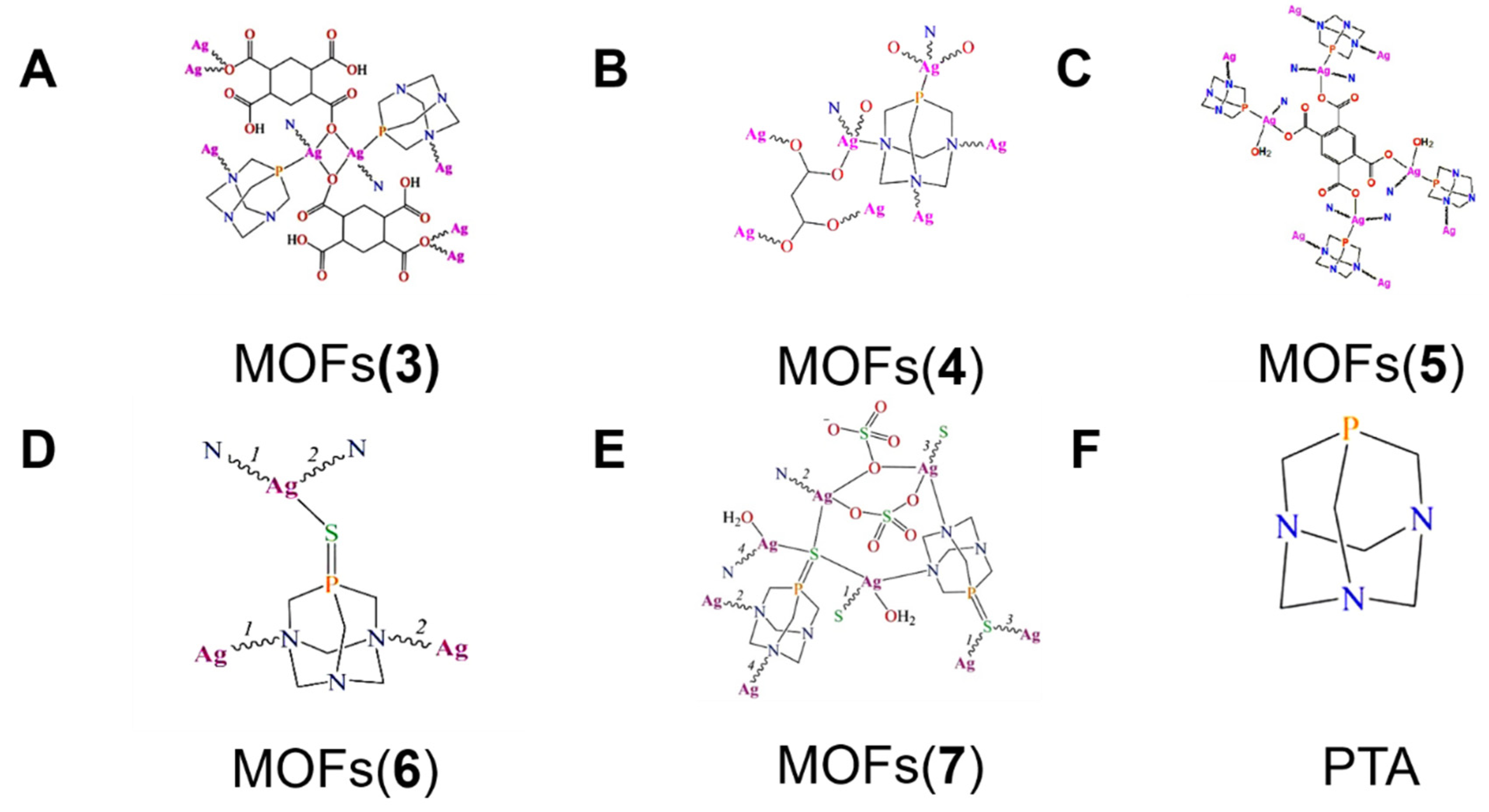
| 3 | [Ag2(μ3-PTA)2(μ2-chdc)]n·5nH2O | 1,3,5-triaza-7-Phosphaadamantane | S. aureus E. coli P. aeruginosa | MIC = 10 µg mL−1 MIC = 7 µg mL−1 MIC = 6 µg mL−1 | The release of Ag+. | [100] |
| 4 | [Ag2(μ4-PTA)(μ4-mal)]n | 1,3,5-triaza-7-Phosphaadamantane | E. coli P. aeruginosa S. aureus | MIC = 7 µg mL−1 MIC = 6 µg mL−1 MIC = 8 µg mL−1 | The weak binding tendency of O and N donor atoms toward the center helps the slow release of Ag(I). | [101] |
| 5 | [Ag4(µ-PTA)2(µ3-PTA)2(µ4-pma)(H2O)2]n·6nH2O | 1,3,5-triaza-7-Phosphaadamantane | E. coli P. aeruginosa S. aureus | MIC = 5 µg mL−1 MIC = 5 µg mL−1 MIC = 8 µg mL−1 | Bond strengthens between Ag(I) and the ligand donor atoms and the Ag+ release. | [102] |
| 6 | [Ag(u3-PTA=S)] n(NO3) n·nH2O | 1,3,5-triaza-7-Phosphaadamantane-7-sulfide | E. coli P. aeruginosa | MIC = 4 µg mL−1 MIC = 5 µg mL−1 | Presence of silver nodes. | [103] |
| 7 | [Ag4(u4-PTA=S)(u5-PTA=S)(u2-SO4)2(H2O)2]n·2nH2O | 1,3,5-triaza-7-Phosphaadamantane-7-sulfide | E. coli S. aureus | MIC = 20 µg mL−1 MIC = 40 µg mL−1 | Presence of silver nodes. | [103] |
| 8 | Ag5(PYDC)2(OH) | Pyridine-3, 5-dicarboxylic acid | E. coli S. aureus | MIC = 10–15 ppm MIC = 15–20 ppm | 1: The Ag+ interacts with bacteria. 2: The damage to the cell membrane. | [104] |
| 9 | [Ag2(O-IPA)(H2O)·(H 3O)] | 5-Hydroxyisophthalic acid | E. coli | MIC = 5 µg mL−1 ZOI = 11.12 mm | Fastest Ag+ release rate and highest equilibrium concentration. | [104] |
| 10 | [AgL]n·nH2O | 4-Cyanobenzoate | S. mutans F. nucleatum P. gingivalis | GIB = 5.29 ppm GIB = 5.29 ppm GIB = 5.29 ppm | Sustained-release of Ag+. | [105] |
| 11 | Ag (NDI-1)0.5(H2O) | Naphthalenediimide | E. coli S. aureus | IR = 100% IR = 99.52% | The synergistic reaction of the organic radical and the silver cation. | [106] |
| 12 | Ag7(NDI-2)1.5(CH3S)4(DMSO)3(DMSO) | Naphthalenediimide | E. coli S. aureus | IR = 99.96% IR = 100% | The synergistic reaction of the organic radical and the silver cation. | [106] |
| Hybrid Ag-MOFs | ||||||
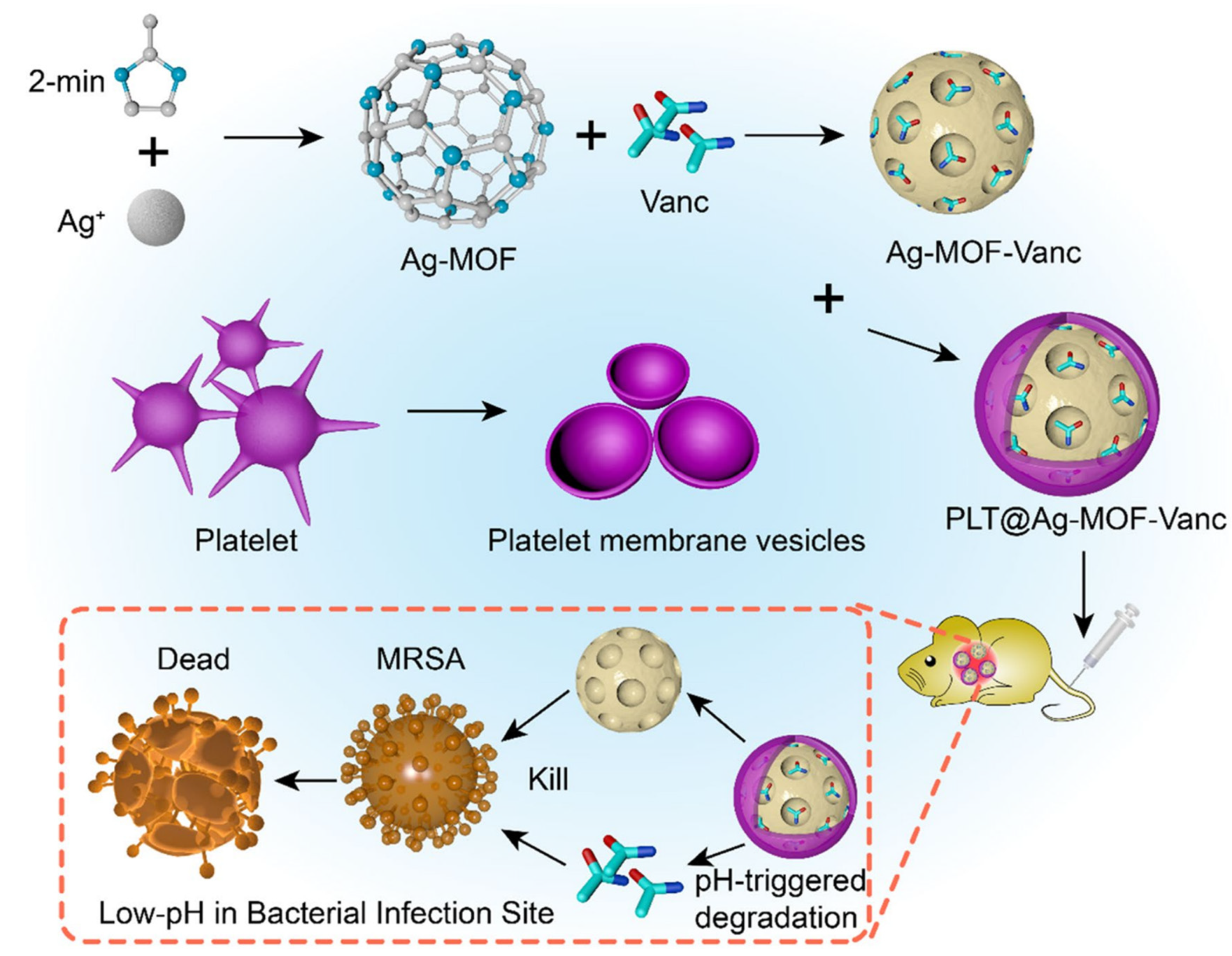
| 13 | PLT@ Ag-MOF-Vanc | 2-Methylimidazole | MRSA | MIC = 0.5 µg mL−1 | 1: Interfering with the intracellular metabolism of bacteria. 2: Catalytic production of the ROS. 3: Damage to the cell membrane integrity. | [111] |
| 14 | Ag-MOF @TFN | 2-Aminoterephthalic acid | E. coli | MR = 90–96% | The release of Ag+. | [112] |
| 15 | Ag-MOF/TFC | 2-Aminoterephthalic acid | P. aeruginosa | MR ~100% | The release of Ag+. | [113] |
| 16 | PVA/Ag-MOF @CS | S. aureus E. coli | ZOI = 12.1 mm ZOI = 9.7 mm | The release of Ag+. | [114] | |
| 17 | CQDs @Ag-MOF | 1,3,5-Benzenetricarboxylic acid | E. coli | MIC= 4 µg mL−1 | 1: Nanocomposite interactions with the cell membrane. 2: Degradation of the composite material. 3: The release of Ag+. | [115] |
| 18 | {[Ag6(μ3-HMNA)4(μ3-MNA)2]2−·[(Et3NH)+]2·(DMSO)2·(H2O)} | 2-Thio-nicotinic acid | P. aeruginosa S. epidermidis S. aureus | ZOI = 14.0 ± 1.1 mm ZOI = 11.3 ± 1.3 mm ZOI = 11.8 ± 1.8 mm | [116] | |
| 19 | GO−Ag-MOF TFN | 1,3,5-Benzenetricarboxylic | E. coli | ER: 95% | The synergistic effect of the release of Ag + and the GO. | [117] |
| 20 | GO-Ag-MOF | 1,3,5-Benzenetricarboxylic | E. coli B. subtilis | MIC = 50 ppm MIC = 50 ppm | The ROS of the GO damages the bacteria. The release of Ag+. | [117] |
| 21 | CS/SS/Ag- MOF–GO | 1,3,5-Benzenetricarboxylic | S. aureus E. coli | Synergistic effect of the composite GO and the continuously released Ag. | [121] | |
| 22 | P-CS @Ag-MOF | Pyridine-3, 5-dicarboxylic acid | S. aureus E. Coli | ZOI = 7.82 mm ZOI = 4.32 mm | 1: The disruption of cells. 2: Ag(I)interaction with thiol proteins. 3: The combination between the bacterial cell cations and the organic linkers. 4: The release of the ROS. | [122] |
| 23 | P-CS @Ag- MOF | Pyridine-3, 5-dicarboxylic acid | S. aureus E. Coli | ZOI = 4.45 mm ZOI = 3.76 mm | 1: The disruption of cells. 2: Ag(I) interaction with thiol proteins. 3: The combination between the bacterial cell cations and the organic linkers. 4: The release of the ROS. | [122] |
| Silver-containing polymer @MOFs | ||||||
| 24 | SD@Ag@CD-MOF | Cyclodextrin | E. coli S. aureus | MIC = 4 µg mL−1 MIC = 4 µg mL−1 | The synergistic activity of the releasing Ag+ ions and SD. | [124] |
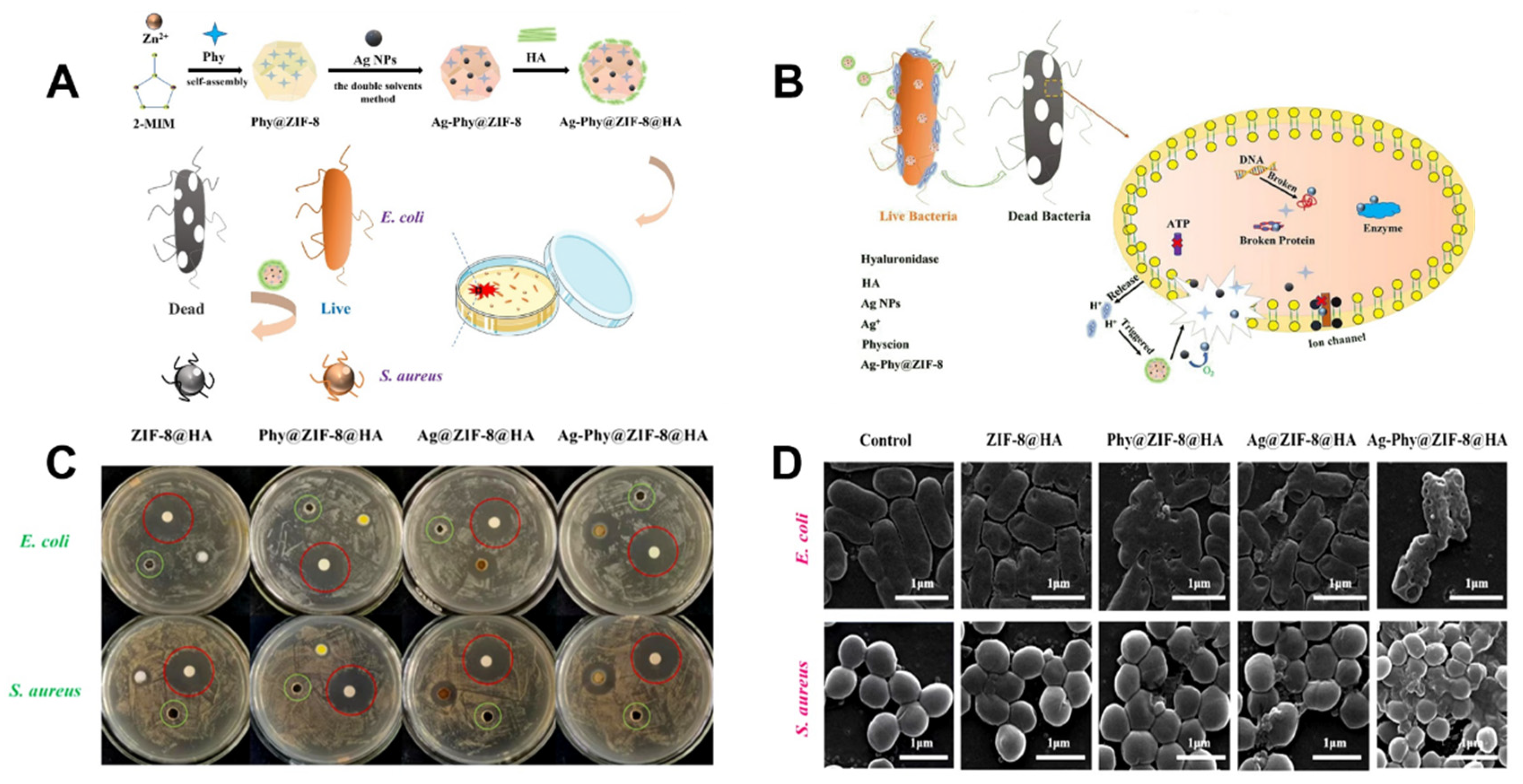
| 25 | Ag-Phy@ZIF-8@HA | 2-Methylimidazole | S. aureus E. Coli | MIC = 0.13 µg mL−1 MIC = 0.25 µg mL−1 | The synergistic activity of ZIF-8, Ag +, and Phy. | [125] |
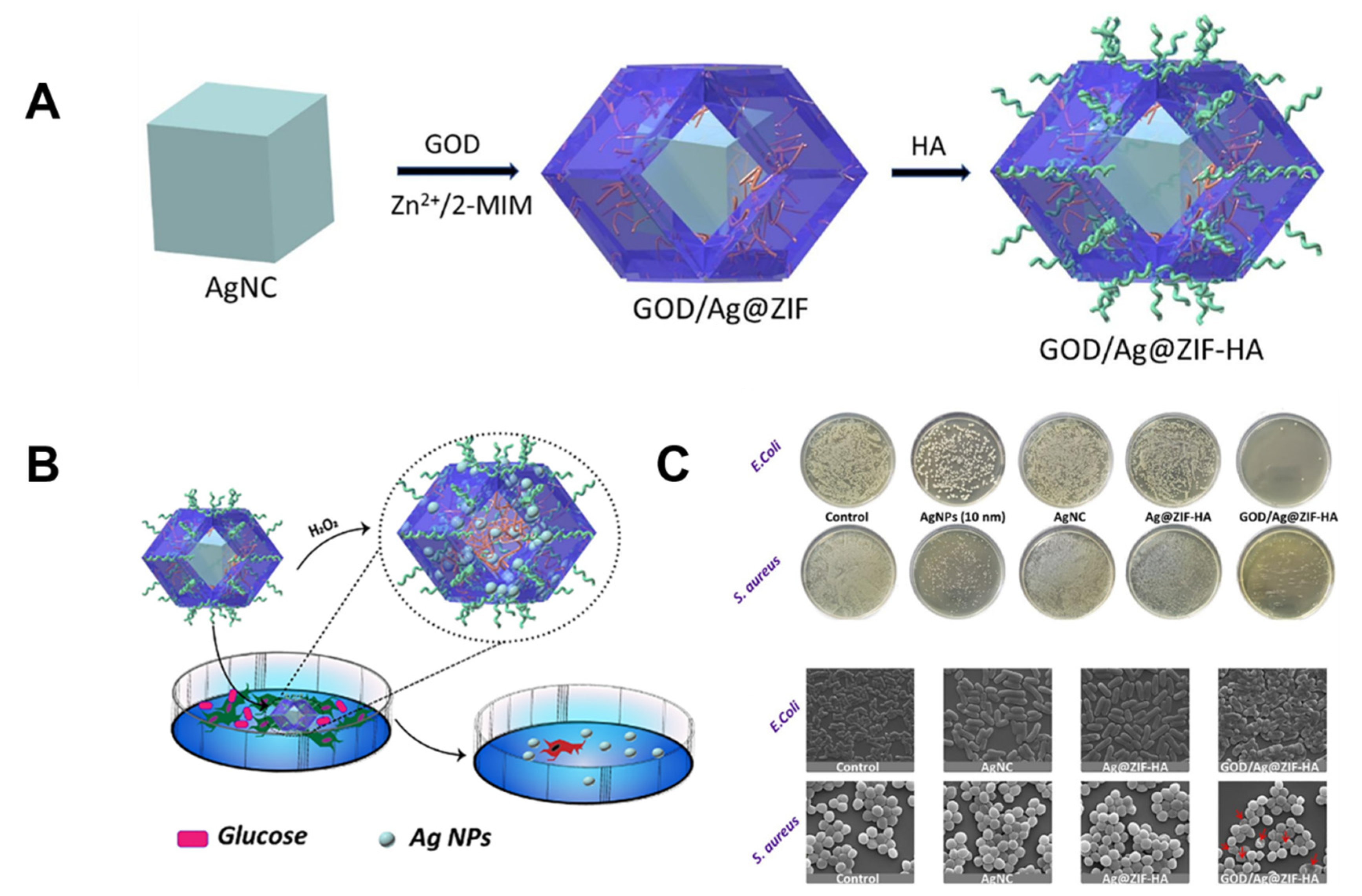
| 26 | Ag-GOD@ ZIF-HA | 2-Methylimidazole | E. Coli S. aureus | MIC = 39.7 µg mL−1 MIC = 79.3 µg mL−1 | Synergetic effect of the release of Ag+ and GOD. | [126] |
| 27 | Ag-NPs@Ni-MOFs | Di-topic carboxylate | E. Coli P. aeruginosa | MIC = 0.025 ìg/mL MIC = 0.025 ìg/mL | Synergetic effect of the release of Ag+ and Ni-MOF. | [81] |
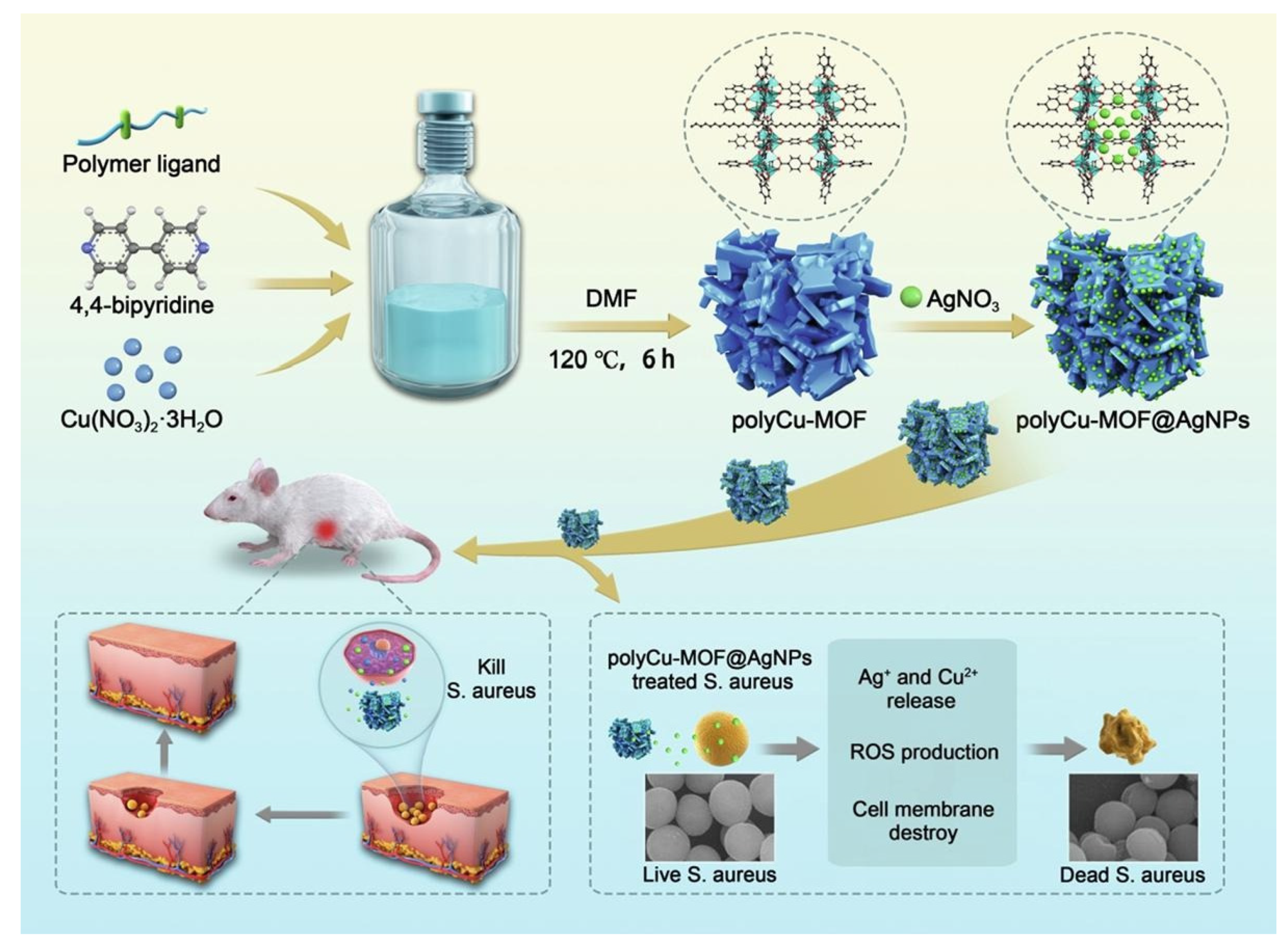
| 28 | Poly Cu-MOF@ Ag | Poly(terephthalic acid) | E. coli S. aureus | MIC = 2–5 µg mL−1 MIC = 10 µg mL−1 | 1: Release of Ag+ and Cu2+. 2: Generation of the ROS. | [127] |
| 29 | Ag-MIL-101(Cr) | Ditopic terephthalic acid | E. coli P. aeruginosaand S. aureus | MIC = 1ug mL−1 MIC = 1ug mL−1 MIC = 1ug mL−1 | The release of smaller-sized Ag+ ions. | [128] |
| 30 | Ag@MOF-5 | 1,4-Benzenedicar-boxylic acid | E. Coli S. aureus | ZOI = 16.05 mm ZOI = 14.62 mm | 1: Silver ions hinder the bacterial DNA replication. 2: Nano-silver destroys the cell membrane. 3: Produces the ROS of | [129] |
| 31 | Ag@Mg-MOF-PVDF | Sebacic acid | S. aureus | ZOI = 10 mm | the release of Ag+. | [130] |
| 32 | Ag/Zn-MOF | 2-Aminoterephthalic acid | E. coli S. aureus | ZOI= 11 mm ZOI= 12 mm | 1: Slow release of the silver ions. 2: Ag+ interacts with the S, O, and N atoms. | [131] |
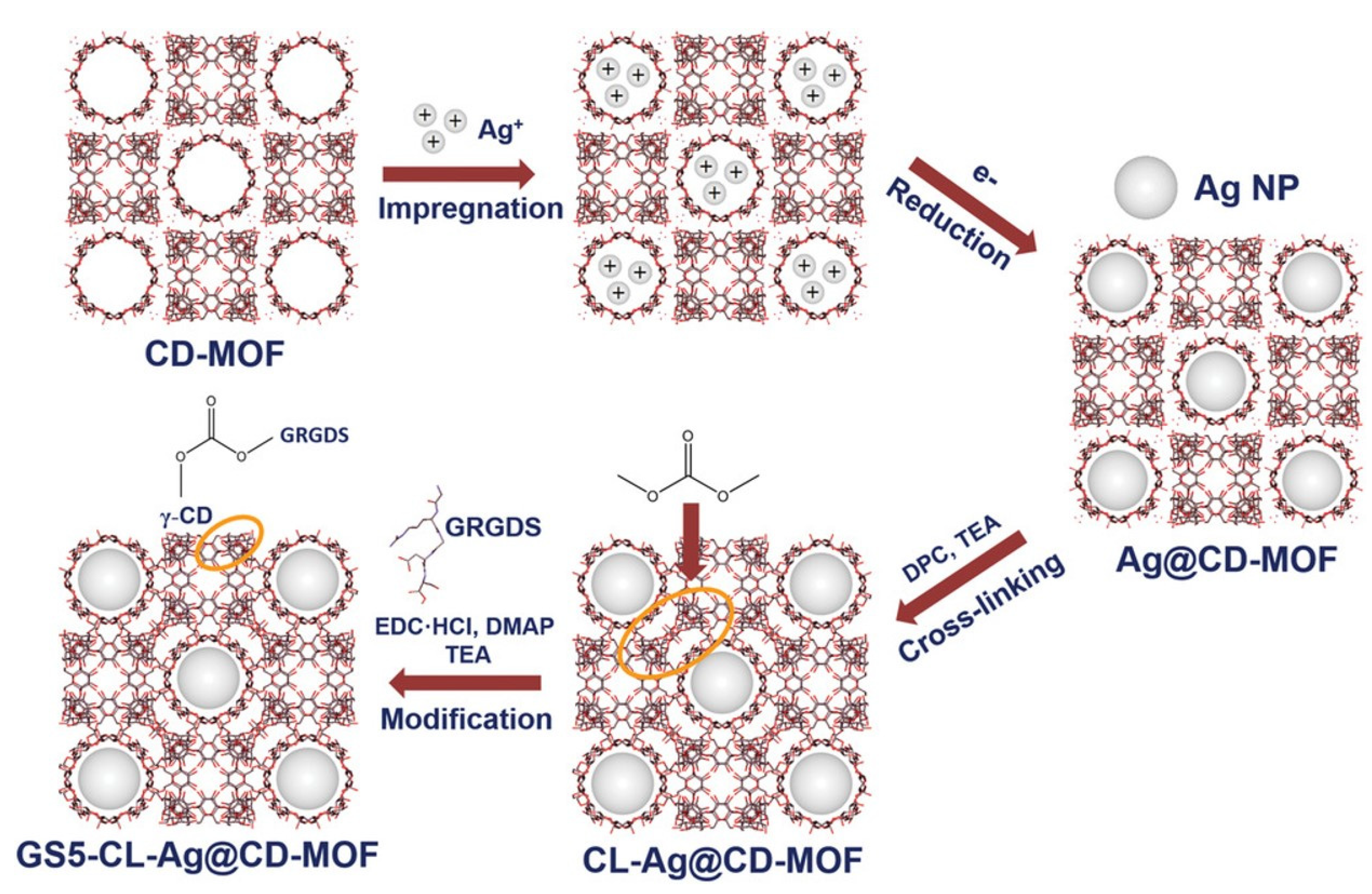
| 33 | GS5-CL-Ag@CD-MOF | Cyclodextrin | E. Coli S. aureus | MIC = 16 µg mL−1 MIC = 64 µg mL−1 | The Ag NPs released. | [127] |
| 34 | MN-MOF-GO-Ag | Gallic acid | S. aureus E. coli P. aeruginosa | The synergistic reaction of the GO and Ag. | [128] | |
2.3. Silver-Containing Polymer @ MOF
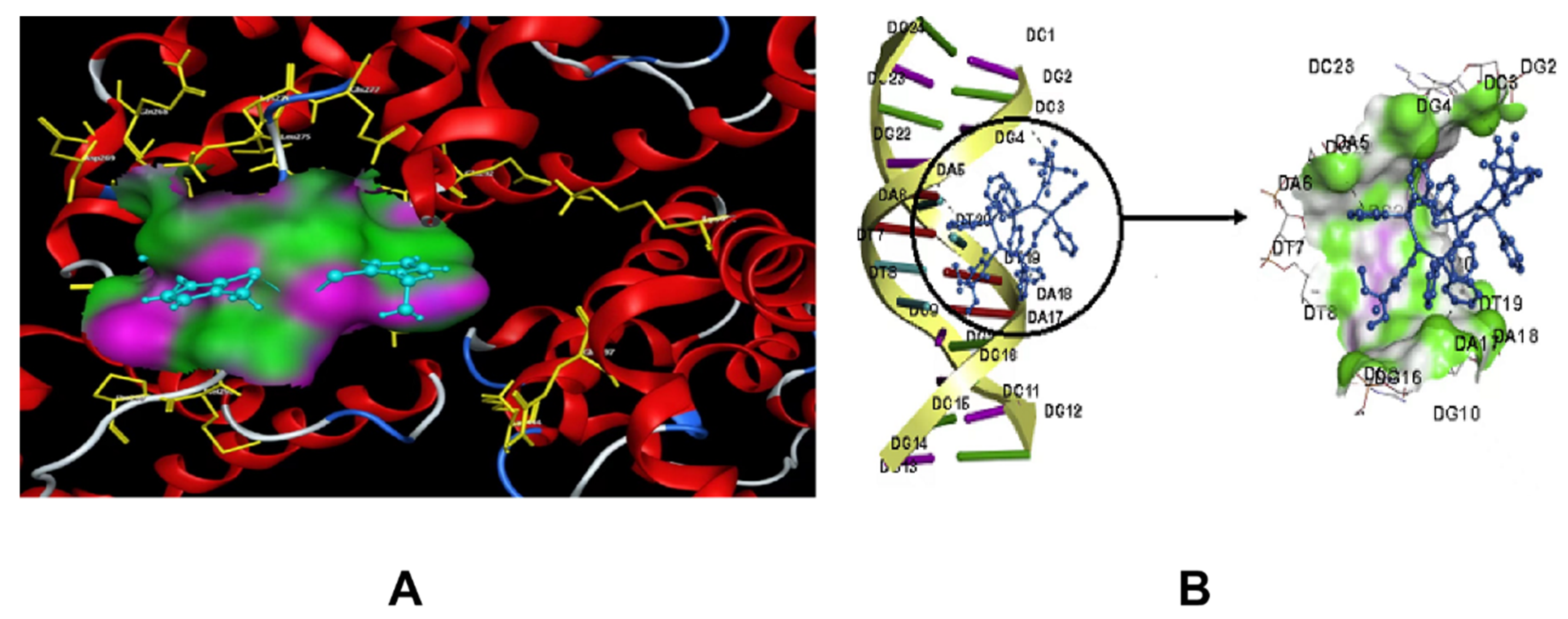
3. Molecular Docking
4. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MOF | Metal-Organic Frameworks |
| ROS | Reactive Oxygen Species |
| ZOI | Zone Of Inhibition |
| CP | Coordination Polymers |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| GIB | Generation Inhibition Rate |
| SR | Survive Rate |
| MR | Mortality Rate |
| ER | Extirpation Rates |
| IH | Inhibition Halo |
| IR | Inhibition Rate |
| GI | Generation Inhibition |
| HO-H2IPA | 5-Hydroxyisophthalic Acid |
| H2PYDC | Pyridine-3, 5-Dicarboxylic Acid |
| L | 4-Cyanobenzoate |
| PTA | 1,3,5-Triaza-7-Phosphaadamantane |
| PTA=S | 1,3,5-Triaza-7-Phosphaadamantane-7-Sulfide |
| TFC | Thin-Film Composite |
| GO | Graphene Oxide |
| TFN | Thin-Film Nanocomposite |
| SD | Solubilized Sulfadiazine |
| CD-MOF | Cyclodextrin Metal-Organic Frameworks |
| GOD | Glucose Oxidase |
| MIL | Materials Of Institute Lavoisier |
| CQD | Carbon Quantum Dots |
| CS/SS | Chitosan/Silk Sericin |
| PVA | Polyvinyl Alcohol |
| P-CS | P-Coumaric Acid Modified Chitosan |
| PLT | Platelets |
| HEMA | Hydroxyethyl-Methacrylate |
| H2 MNA | 2-Thio-Nicotinic Acid |
| Et3 N | 3-Ethylene-Amine |
| DMSO | Dimethylsulfoxide |
| Phy | Physcion |
| NDI | Naphthalenediimide |
| chdc | 1,4-Cyclohexanedicarboxylic |
| mal | Malonic |
References
- Lázaro, I.A.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Dong, Y.; Wang, R.; Pan, Y.; Zhuang, S.Z.; Liu, D.; Liu, J.Q. Recent developments on MOF-based platforms for antibacterial therapy. RSC Med. Chem. 2021, 12, 915–928. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.Y.; Weichselbaum, R.R.; Lin, W.B. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Feng, X.; Li, R.; Wang, L.; Qin, G.Z.; Ma, L.F. A series of homonuclear lanthanide coordination polymers based on a fluorescent conjugated ligand: Syntheses, luminescence and sensor for pollutant chromate anion. Cryst. Eng. Comm. 2015, 17, 7878–7887. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.Q.; Kumar, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.Q.; Kumar, A. Syntheses, design strategies, and photocatalytic charge dynamics of metal-organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.M.; Liu, C.Q.; Wang, Z.Z.; Kang, L.H.; Huang, Y.Y.; Dong, K.; Ren, J.S.; Qu, X.G. Nanozyme decorated metal–organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.Y.; Li, A.J.; Yang, P.; Zhang, C.Y.; Tang, B. H2S-activable MOF nanoparticle photosensitizer for effective photodynamic therapy against cancer with controllable singlet-oxygen release. Angew. Chem. 2017, 129, 13940–13944. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B. 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.Q.; Chen, J.J.; Wang, L.Y. Reticular three-dimensional 3d-4f frameworks constructed through substituted imidazole-dicarboxylate: Syntheses, luminescence and magnetic properties study. Dalton Trans. 2015, 44, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, N.; Chen, H.P.; Wang, H.L.; Yue, L.Y.; Chen, X.; Ng, S.W.; Liu, X.F.; Ma, L.F.; Wang, L.Y. A series of anionic host coordination polymers based on azoxybenzene carboxylate: Structures, luminescence and magnetic properties. Dalton Trans. 2017, 46, 14192–14200. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, J.L.; Bai, R.F.; Wang, L.Y.; Wei, J.T.; Chen, X.X. Two unique cobalt-organic frameworks based on substituted imidazole-dicarboxylate and dipyridyl-type ancillary ligands: Crystal structures and magnetic properties. Inorg. Chem. Commun. 2016, 66, 41–46. [Google Scholar] [CrossRef]
- Lian, X.; Chen, Y.P.; Liu, T.F.; Zhou, H.C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 2016, 7, 6969–6973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K.; Am, J. Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: Protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Qin, J.H.; Qin, W.J.; Xiao, Z.; Yang, J.K.; Wang, H.R.; Yang, X.G.; Li, D.S.; Ma, L.F. Efficient energy-transfer-induced high photoelectric conversion in a dye-encapsulated ionic pyrene-based metal-organic framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, D.; Liu, S.; Qiu, Y.Z.; Liu, Y.F.; Zhang, W.F.; Dai, Z.; Kumar, A.; Pan, Y.; Liu, J.Q. Recent advances on bimetallic metal-organic frameworks (BMOFs): Syntheses, applications and challenges. New J. Chem. 2022, 46, 13818–13837. [Google Scholar]
- He, H.; Hashemi, L.; Hu, M.L.; Morsali, A. The role of the counter-ion in metal-organic frameworks’ chemistry and applications. Coord. Chem. Rev. 2018, 376, 319–347. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Singh, A.K.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Chen, C.; Liu, S.; Lu, C.Y.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalton Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, Y.; Xiao, W.; Xu, H.; Liu, D.; Ren, F.; Peng, X.; Liu, J. Recent developments on zinc (ii) metal-organic framework nanocarriers for physiological pH-responsive drug delivery. Med. Chem. Comm. 2019, 10, 2038–2051. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, N.; Razavi, S.A.A.; Morsali, A.; Hu, M.L. High-capacity Hg (II) and Pb (II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, C.; Wang, Z.Q.; Tang, S.; Fu, W.; Ji, B.; Wang, L.Y. Aerobic oxidation of alcohols and the synthesis of benzoxazoles catalyzed by a cuprocupric coordination polymer (Cu+-CP) assisted by tempo. Inorg. Chem. 2015, 54, 2088–2090. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.; Guo, N.; Sun, Y.L.; Zhang, T.; Ma, L.F.; Wang, L.Y. Series d-f heteronuclear metal-organic frameworks: Color tunability and luminescent probe with switchable properties. Inorg. Chem. 2017, 56, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS. Nano. 2017, 11, 4315–4327. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS. Appl. Mater. Interfaces. 2017, 9, 3455–3462. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, J.; Chen, Q.; Liu, Z. Nanoscale metal-organic frameworks and coordination polymers as theranostic platforms for cancer treatment. Coord. Chem. Rev. 2019, 398, 113009. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Luo, Z.; Li, B.; Yuan, D. Microporous metal-organic framework based on ligand-truncation strategy with high performance for gas adsorption and separation. Inorg. Chem. 2017, 56, 10215–10219. [Google Scholar] [CrossRef]
- Ye, G.M.; Chen, C.; Lin, J.Z.; Peng, X.S.; Kumar, A.; Liu, D.; Liu, J.Q. Alkali/alkaline earth-based metal-organic frameworks for biomedical applications. Dalton Trans. 2021, 50, 17438–17454. [Google Scholar] [CrossRef]
- Zheng, R.Q.; Guo, J.R.; Cai, X.Y.; Bin, L.J.; Lu, C.Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J.Q. Manganese complexes and manganese-based metal-organic frameworks as contrast agents in MRI and chemotherapeutics agents: Applications and prospects. Colloids Surf. B Biointerfaces. 2022, 213, 112432. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, Y.; Shi, C.; Xiao, J.; Dai, W.; Liu, D.; Chen, H.; Li, B.; Liu, J. Applications of ROS-InducedZr-MOFs platform in multimodal synergistic therapy. Mini Rev. Med. Chem. 2021, 21, 1718–1733. [Google Scholar] [CrossRef]
- Liu, K.G.; Rouhani, F.; Gao, X.M.; Abbasi-Azad, M.; Li, J.Z.; Hu, X.D.; Wang, W.; Hu, M.L.; Morsali, A. Bilateral photocatalytic mechanism of dye degradation by a designed ferrocene-functionalized cluster under natural sunlight. Catal. Sci. Technol. 2020, 10, 757–767. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, W.; Liu, R.; Zhang, J.; Zhang, D.; Li, Z.; Luan, Y. Rational design of metal organic framework nanocarrier-based codelivery system of doxorubicin hydrochloride/verapamil hydrochloride for overcoming multidrug resistance with efficient targeted cancer therapy. ACS Appl. Mater. Interfaces. 2017, 9, 19687–19697. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xiao, X.; Bai, L.; Liu, B.; Yuan, M.; Ma, P.A.; Lin, J. Conferring Ti-Based MOFs with Defects for Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2021, 33, 2100333. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, Y.; Wang, H.; Gu, Z.; Duan, C. Synthesis of Au@ ZIF-8 single-or multi-core-shell structures for photocatalysis. Chem. Commun. 2014, 50, 8651–8654. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.Y.; Zhu, J.; Liu, J.Q.; Zhu, J.F. One-Step Construction of a Hollow Au@ Bimetal-Organic Framework Core-Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces. 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd (II) MOF as a potent photocatalyst for oxytetracycline antibiotic. Cryst. Eng. Comm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, W.; Rao, C.; Li, B.; Wang, X.; Liu, D.; Pan, Y.; Liu, J. Recent Advances in Fe-MOF Compositions for Biomedical Applications. Curr. Med. Chem. 2021, 28, 6179–6198. [Google Scholar] [CrossRef]
- Liu, K.G.; Bigdeli, F.; Li, H.J.; Li, J.Z.; Yan, X.W.; Hu, M.L. Hexavalent octahedral template: A neutral high-nucleus silver alkynyl nanocluster emitting infrared light. Inorg. Chem. 2020, 59, 6684–6688. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, L.; Morsali, A.; Hu, M.L.; Tehrani, A.A.; Carlucci, L.; Mercandelli, P.; Proserpio, D. Size-selective urea-containing metal-organic frameworks as receptors for anions. Inorg. Chem. 2020, 59, 16421–16429. [Google Scholar] [CrossRef]
- Rao, C.Y.; Zhou, L.Y.; Pan, Y.; Lu, C.Y.; Qin, X.Y.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Xu, C.; Xu, L.; Sun, Y.; Shen, Q.; Hu, Q. Fabrication of metal-organic framework Mn-PBC for theranostic application. J. Solid State Chem. 2022, 313, 123349. [Google Scholar] [CrossRef]
- Qin, J.H.; Zhang, J.R.; Xiao, Z.; Wu, Y.P.; Xu, H.M.; Yang, X.G.; Ma, L.F.; Li, D.S. Topology-and guest-dependent photoelectric conversion of 2D anionic pyrene-based metal-organic framework. Cryst. Growth Des. 2022, 22, 4018–4024. [Google Scholar] [CrossRef]
- Seri, M.A.A.; Hamzah, E.; Ahdash, A. Anticorrosion performance of self-healing polymeric coatings on low carbon steel substrates. J. Teknol. 2017, 79, 12268. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.; Ma, L.; Du, Y.; Peng, N.; Ma, L.; Zhang, Q.; et al. Intercalation-Activated Layered MoO3 Nanobelts as Biodegradable Nanozymes for Tumor-Specific Photo-Enhanced Catalytic Therapy. Angew. Chem. 2022, 134, e202115939. [Google Scholar]
- He, W.M.; Zhou, Z.; Han, Z.; Li, S.; Zhou, Z.; Ma, L.; Zang, S. Ultrafast Size Expansion and Turn-On Luminescence of Atomically Precise Silver Clusters by Hydrogen Sulfide. Angew. Chem. Int. Ed. 2021, 60, 8505–8509. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Hou, A.; Cheng, C.; Wang, X.; Yue, Y.; Wu, Z.; Wu, H.; Liu, B.; Li, H.; et al. Growth of Cu2O Nanoparticles on Two-Dimensional Zr-Ferrocene-Metal-Organic Framework Nanosheets for Photothermally Enhanced Chemodynamic Antibacterial Therapy. Inorg. Chem. 2022, 61, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Yao, W.B.; Wang, J.L.; Lou, Y.P.; Wu, H.J.; Qi, X.X.; Yang, J.G.; Zhong, A.G. Chemoselective hydroborative reduction of nitro motifs using a transition-metal-free catalyst. Org. Chem. Front. 2021, 8, 4554–4559. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Zhong, A.; Li, J.S.; Yang, J.G. Combined KOH/BEt3 catalyst for selective deaminative hydroboration of aromatic carboxamides for construction of luminophores. Org. Lett. 2020, 22, 8086–8090. [Google Scholar] [CrossRef]
- Yao, W.B.; He, L.; Han, D.; Zhong, A.G. Sodium triethylborohydride-catalyzed controlled reduction of unactivated amides to secondary or tertiary amines. J. Org. Chem. 2019, 84, 14627–14635. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, T.; Zhou, S.; Wang, J.; Lv, H.; Han, M.L.; Singh, D.P.; Kumar, A.; Jin, J.C. New highly luminescent 3D Tb (III)-MOF as selective sensor for antibiotics. Inorg. Chem. Commun. 2021, 130, 108756. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, L.; Zhang, H.; Zhang, C.; Jiang, K.; Cao, X. Hierarchical MOF-on-MOF Architecture for pH/GSH-Controlled Drug Delivery and Fe-Based Chemodynamic Therapy. Inorg. Chem. 2022, 61, 3281–3287. [Google Scholar] [CrossRef]
- Su, J.; Jing, P.; Jiang, K.; Du, J. Recent advances in porous MOFs and their hybrids for photothermal cancer therapy. Dalton Trans. 2022, 51, 8938–8944. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Leong, K.W.; Kim, H.W. Progress in nanotheranostics based on mesoporous silica nanomaterial platforms. ACS Appl. Mater. Interfaces. 2017, 9, 10309–10337. [Google Scholar] [CrossRef]
- Perez, R.A.; Singh, R.K.; Kim, T.H.; Kim, H.W. Silica-based multifunctional nanodelivery systems toward regenerative medicine. Mater. Horiz. 2017, 4, 772–799. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A multimodal Metal-Organic framework based on unsaturated metal site for enhancing antitumor cytotoxicity through Chemo-Photodynamic therapy. J. Colloid Interface Sci. 2022, 621, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Jiang, X.D.; Liu, Y.W.; Liu, D.; Chen, C.; Lu, C.Y.; Zhuang, S.Z.; Kumar, A.; Liu, J.Q. Recent advances in Cu (II)/Cu (I)-MOFs based nano-platforms for developing new nano-medicines. J. Inorg. Biochem. 2021, 225, 111599. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Z.; Tan, G.J.; Fang, Y.Q.; Liu, S.; Zhou, Y.B.; Kumar, A.; Trivedi, M.; Liu, D.; Liu, J.Q. Biomedical applications of metal-organic framework (MOF) based nano-enzymes. New J. Chem. 2021, 45, 20987–21000. [Google Scholar] [CrossRef]
- Roy, A.S.; Mondal, J.; Banerjee, B.; Mondal, P.; Bhaumik, A. Pd-grafted porous metal-organic framework material as an efficient and reusable heterogeneous catalyst for C-C coupling reactions in water. Appl. Catal. A Gen. 2014, 469, 320–327. [Google Scholar] [CrossRef]
- Lee, J.Y.; Farha, O.K.; Roberts, J.K.; Scheidt, A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Cho, S.H.; Ma, B.; Nguyen, S.B.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A metal-organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem. Commun. 2006, 24, 2563–2565. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- He, C.; Lu, K.; Liu, D.; Lin, W.B. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- Baek, S.; Singh, R.K.; Khanal, D.; Patel, K.D.; Lee, E.J.; Leong, K.W.; Chrzanowski, W.; Kim, H.W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015, 7, 14191–14216. [Google Scholar] [CrossRef]
- Liu, W.C.; Pan, Y.; Zhong, Y.Y.; Li, B.H.; Ding, Q.J.; Xu, H.J.; Qiu, Y.Z.; Ren, F.; Li, B.M. A multifunctional aminated UiO-67 metal-organic framework for enhancing antitumor cytotoxicity through bimodal drug delivery. Chem. Eng. J. 2021, 412, 127899. [Google Scholar] [CrossRef]
- Liu, W.C.; Yan, Q.W.; Xia, C.; Wang, X.X.; Kumar, A.; Wang, Y.; Liu, Y.W.; Pan, Y.; Liu, J.Q. Recent advances in cell membrane coated metal-organic frameworks (MOFs) for tumor therapy. J. Mater. Chem. B 2021, 9, 4459–4474. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Wang, J.P.; Liu, W.C.; Zhuang, X.Y.; Liu, J.Q.; Fan, G.L.; Li, B.H.; Lin, W.N.; Man, J.-H. A new (4, 8)-connected topological MOF as potential drug delivery. Inorg. Chem. Commun. 2015, 55, 8–10. [Google Scholar] [CrossRef]
- Liu, J.Q.; Li, X.F.; Gu, C.Y.; da Silva, J.C.; Barros, A.L.; Alves-Jr, S.; Li, B.H.; Ren, F.; Batten, S.R.; Soares, T.A. A combined experimental and computational study of novel nanocage-based metal-organic frameworks for drug delivery. Dalton Trans. 2015, 44, 19370–19382. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Ju, E.; Liu, Z.; Chen, Z.W.; Ren, J.S.; Qu, X.G. Metal-organic-framework-based vaccine platforms for enhanced systemic immune and memory response. Adv. Funct. Mater. 2016, 26, 6454–6461. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, H.C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef]
- Sava Gallis, D.F.; Butler, K.S.; Agola, J.O.; Pearce, C.J.; McBride, A.A. Antibacterial countermeasures via metal-organic framework-supported sustained therapeutic release. ACS Appl. Mater. Interfaces 2019, 11, 7782–7791. [Google Scholar] [CrossRef]
- Singbumrung, K.; Motina, K.; Pisitsak, P.; Chitichotpanya, P.; Wongkasemjit, S.; Inprasit, T. Preparation of Cu-BTC/PVA fibers with antibacterial applications. Fibers Polym. 2018, 19, 1373–1378. [Google Scholar] [CrossRef]
- Wang, X.S.; Chen, C.H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B Environ. 2019, 253, 323–330. [Google Scholar] [CrossRef]
- Abd, E.S.H.M.; Nassar, H.N.; Khidr, A.S.A.; Zaki, K.T. Antimicrobial activities of green synthesized Ag nanoparticles@ Ni-MOF nanosheets. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2791–2798. [Google Scholar]
- Alavijeh, R.K.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of reasons for metal-organic framework’s antibacterial activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of silver nanoparticles and their biomedical applications-a comprehensive review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS. Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, Y.; Wang, Y.; Du, T.; Li, C.; Wang, X.; Jiang, H. Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord. Chem. Rev. 2021, 449, 214218. [Google Scholar] [CrossRef]
- Kim, Y.K.; Han, S.W.; Min, D.H. Graphene oxide sheath on Ag nanoparticle/graphene hybrid films as an antioxidative coating and enhancer of surface-enhanced Raman scattering. ACS Appl. Mater. Interfaces 2012, 4, 6545–6551. [Google Scholar] [CrossRef]
- Fromm, K.M. Silver coordination compounds with antimicrobial properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Mourad, R.; Helaly, F.; Darwesh, O.; Sawy, S.E. Antimicrobial and physicomechanical natures of silver nanoparticles incorporated into silicone-hydrogel films. Contact Lens Anterior Eye 2019, 42, 325–333. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Ricketts, C.R.; Lowbury, E.J.L.; Lawrence, J.C.; Hall, M.; Wilkins, M.D. Mechanism of prophylaxis by silver compounds against infection of burns. Br. Med. J. 1970, 2, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Withersby, M.A.; Blake, A.J.; Champness, N.R.; Cooke, P.A.; Hubberstey, P.; Li, W.S.; Schröder, M. Silver (I)-3, 6-bis (pyridin-3-yl)-1, 2, 4, 5-tetrazine coordination polymers: A diversity of chain motifs. Cryst. Eng. 1999, 2, 123–136. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, Y.; Du, M. Synthesis, crystal structures and in vitro anti-fungal activities of two silver (I) coordination polymers with fluconazole. Inorg. Chim. Acta 2007, 360, 3182–3188. [Google Scholar] [CrossRef]
- Berchel, M.; Le Gall, T.; Denis, C.; Hir, S.L.; Quentel, F.; Elléouet, C.; Montier, T.; Rueff, J.M.; Salaün, J.Y.; Haelters, J.P.; et al. A silver-based metal-organic framework material as a ‘reservoir’of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Sha, Z.; Chan, H.S.; Wu, J. Ag2CO3/UiO-66(Zr) composite with enhanced visible-light promoted photocatalytic activity for dye degradation. J Hazard Mater. 2015, 299, 132–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, H.; Zhang, B.; Liu, J. Potent antibacterial activity of a novel silver nanoparticle-halloysite nanotube nanocomposite powder. J. Inorg. Biochem. 2013, 118, 59–64. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Florek, M.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Aliphatic dicarboxylate directed assembly of silver (I) 1, 3, 5-triaza-7-phosphaadamantane coordination networks: Topological versatility and antimicrobial activity. Cryst. Growth Des. 2014, 14, 5408–5417. [Google Scholar]
- Jaros, S.W.; Król, J.; Bażanów, B.; Poradowski, D.; Chrószcz, A.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Antiviral, antibacterial, antifungal, and cytotoxic silver (I) BioMOF assembled from 1, 3, 5-triaza-7-phoshaadamantane and pyromellitic acid. Molecules 2020, 25, 2119. [Google Scholar] [CrossRef]
- Jaros, S.W.; Smoleński, P.; da Silva, M.F.C.G.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver BioMOFs driven by 1, 3, 5-triaza-7-phosphaadamantane-7-sulfide (PTA [double bond, length as m-dash] S): Synthesis, topological analysis and antimicrobial activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Bandosz, T.J. Carbon quantum dot surface-chemistry-dependent Ag release governs the high antibacterial activity of Ag-metal-organic framework composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Rossos, A.K.; Banti, C.N.; Kalampounias, A.G.; Papachristodoulou, C.; Kordatos, K.; Zoumpoulakis, P.; Mavromoustakos, T.; Kourkoumelis, N.; Hadjikakou, S.K. pHEMA@ AGMNA-1: A novel material for the development of antibacterial contact lens. Mater. Sci. Eng. C 2020, 111, 110770. [Google Scholar] [CrossRef] [PubMed]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedpour, S.F.; Gh, M.S.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, V.T.; Icsel, C.; Batur, J.; Aydinlik, S.; Sahinturk, P.; Aygun, M. Structures and biochemical evaluation of silver (I) 5, 5-diethylbarbiturate complexes with bis (diphenylphosphino) alkanes as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2017, 139, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Handke, M.; Weber, H.; Lange, M.; Möllmer, J.; Lincke, J.; Gläser, R.; Staudt, R.; Krautscheid, H. Network flexibility: Control of gate opening in an isostructural series of Ag-MOFs by linker substitution. Inorg. Chem. 2014, 53, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.P.; Chai, J.W.; Fan, C.; Ouyang, J.H.; Duan, W.J.; Sun, B.; Chen, J.; Yuan, L.X.; Xu, X.Q.; Chen, J.X. Water-Stable Silver-Based Metal-Organic Frameworks of Quaternized Carboxylates and Their Antimicrobial Activity. ACS Appl. Bio Mater. 2020, 3, 8525–8531. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Król, J.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive silver-organic networks assembled from 1, 3, 5-triaza-7-phosphaadamantane and flexible cyclohexanecarboxylate blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef]
- Cao, P.; Wu, X.; Zhang, W.; Zhao, L.; Sun, W.; Tang, Z. Killing oral bacteria using metal-organic frameworks. Ind. Eng. Chem. Res. 2020, 59, 1559–1567. [Google Scholar] [CrossRef]
- Hu, F.; Xia, S.; He, Y.; Huang, Z.; Ke, H.; Liao, J. Reactive organic radical-doped Ag (I)-based coordination compounds for highly efficient antibacterial wound therapy. Colloid Surf. B 2022, 213, 112425. [Google Scholar] [CrossRef]
- Altaf, M.; Stoeckli-Evans, H.; Cuin, A.; Sato, D.N.; Pavan, F.R.; Leite, C.Q.F.; Ahmad, S.; Bouakka, M.; Mimouni, M.; Khardli, F.Z.; et al. Synthesis, crystal structures, antimicrobial, antifungal and antituberculosis activities of mixed ligand silver (I) complexes. Polyhedron 2013, 62, 138–147. [Google Scholar] [CrossRef]
- Takayama, A.; Yoshikawa, R.; Iyoku, S.; Kasuga, N.C.; Nomiya, K. Synthesis, structure and antimicrobial activity of L-argininesilver (1+) nitrate. Polyhedron 2013, 52, 844–847. [Google Scholar] [CrossRef]
- Ke, H.; Hu, F.; Meng, L.; Chen, Q.H.; Lai, Q.S.; Li, Z.C.; Huang, Z.L.; Liao, J.Z.; Qiu, J.D.; Lu, C.Z. Ultrastable radical-doped coordination compounds with antimicrobial activity against antibiotic-resistant bacteria. Chem. Commun. 2020, 56, 14353–14356. [Google Scholar] [CrossRef] [PubMed]
- Medina Cruz, D.; Mi, G.; Webster, T.J. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J. Biomed. Mater. Res. A 2018, 106, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cai, G.Q.; Li, J.; Li, X.; Li, H.; Shang, X.; Zhou, J.D.; Nie, X.; Gui, R. Platelet membrane-camouflaged silver metal-organic framework drug system against infections caused by methicillin-resistant Staphylococcus aureus. J. Nanobiotechnol. 2021, 19, 229. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Dadashi Firouzjaei, M.; Rahimpour, A.; Zolghadr, E.; Shamsabadi, A.A.; Das, P.; Afkhami, F.A.; Sadrzadeh, M.; Tiraferri, A.; Elliott, M. Toward sustainable tackling of biofouling implications and improved performance of TFC FO membranes modified by Ag-MOF nanorods. ACS Appl. Mater. Interfaces 2020, 12, 38285–38298. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Najafpour, G. Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: A novel approach toward long-lasting biofouling mitigation. J. Membr. Sci. 2019, 573, 257–269. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Yadav, P.; Bhaduri, A. Synthesis and Structural Characterization of ZnO-GrapheneNanocomposite by Chemical Co-Precipitation Method. Int. J. Innov. Res. Phys. 2020, 1, 19–24. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired design of sericin/chitosan/Ag@ MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef]
- Remelli, M.; Nurchi, V.M.; Lachowicz, J.I.; Medici, S.; Zoroddu, M.A.; Peana, M. Competition between Cd (II) and other divalent transition metal ions during complex formation with amino acids, peptides, and chelating agents. Coord. Chem. Rev. 2016, 327, 55–69. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microb. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, H.; Wang, R.; Duan, M. Fabricating Ag@ MOF-5 nanoplates by the template of MOF-5 and evaluating its antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127093. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, J.; Xu, J.; Deng, J.; Guo, J. TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J. Nanosci. Nanotechnol. 2010, 10, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Du, M. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2022, 435, 134915. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Salahshoor, M. Matrices based on meso antibacterial framework. J. Chin. Chem. Soc. 2020, 67, 1579–1590. [Google Scholar] [CrossRef]
- Shakya, S.; He, Y.; Ren, X.; Guo, T.; Maharjan, A.; Luo, T.; Wang, T.; Dhakhwa, R.; Regmi, B.; Li, H.; et al. Ultrafine Silver Nanoparticles: Ultrafine Silver Nanoparticles Embedded in Cyclodextrin Metal-Organic Frameworks with GRGDS Functionalization to Promote Antibacterial and Wound Healing Application (Small 27/2019). Small 2019, 15, 1970145. [Google Scholar] [CrossRef]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef]
- Aghaee, M.; Mohammadi, K.; Hayati, P.; Sharafi-Badr, P.; Yazdian, F.; Alonso, A.G.; Eshghi, F. A novel 3D Ag (I) metal-organic coordination polymer (Ag-MOCP): Crystallography, Hirshfeld surface analysis, antibacterial effect and molecular docking studies. J. Solid State Chem. 2022, 310, 123013. [Google Scholar] [CrossRef]
- Xu, W.; Zhuang, H.; Xu, Z.; Huang, M.; Gao, S.; Li, Q.; Zhang, G. Design and construction of Ag@ MOFs immobilized PVDF ultrafiltration membranes with anti-bacterial and antifouling properties. Adv. Polym. Technol. 2020, 2020, 5456707. [Google Scholar] [CrossRef]
- Sacourbaravi, R.; Ansari-Asl, Z.; Kooti, M.; Nobakht, V.; Darabpour, E. Fabrication of Ag NPs/Zn-MOF nanocomposites and their application as antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4615–4621. [Google Scholar] [CrossRef]
- Tan, L.; Yuan, G.; Wang, P.; Feng, S.; Tong, Y.; Wang, C. pH-responsive Ag-Phy@ ZIF-8 nanoparticles modified by hyaluronate for efficient synergistic bacteria disinfection. Int. J. Biol. Macromol. 2022, 206, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Z.; Zhang, Y.; Chen, F.; An, P.; Wu, H.; You, C.; Sun, B. MOF-shielded and glucose-responsive ultrasmall silver nano-factory for highly-efficient anticancer and antibacterial therapy. Chem. Eng. J. 2021, 416, 127610. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Beheshti, A.; Babadi, S.S.; Nozarian, K.; Heidarizadeh, F.; Ghamari, N.; Mayer, P.; Motamedi, H. Crystal structure, microbiological activity and theoretical studies of Ag (I) and Cu (I) coordination polymers with 1, 1′-(butane-1, 4-diyl) bis (3-methylimidazoline-2-thione) ligand. Polyhedron 2016, 110, 261–273. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. https://doi.org/10.3390/molecules27217166
Zhang W, Ye G, Liao D, Chen X, Lu C, Nezamzadeh-Ejhieh A, Khan MS, Liu J, Pan Y, Dai Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules. 2022; 27(21):7166. https://doi.org/10.3390/molecules27217166
Chicago/Turabian StyleZhang, Wenfeng, Gaomin Ye, Donghui Liao, Xuelin Chen, Chengyu Lu, Alireza Nezamzadeh-Ejhieh, M. Shahnawaz Khan, Jianqiang Liu, Ying Pan, and Zhong Dai. 2022. "Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications" Molecules 27, no. 21: 7166. https://doi.org/10.3390/molecules27217166
APA StyleZhang, W., Ye, G., Liao, D., Chen, X., Lu, C., Nezamzadeh-Ejhieh, A., Khan, M. S., Liu, J., Pan, Y., & Dai, Z. (2022). Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules, 27(21), 7166. https://doi.org/10.3390/molecules27217166