Abstract
A unique series of sulphonamide derivatives was attempted to be synthesized in this study using a new and effective method. All of the synthesized compounds were verified using several spectroscopic methods, including FTIR, 1H-NMR, 13C-NMR, and HREI-MS, and their binding interactions were studied using molecular docking. The enzymes urease and α-glucosidase were evaluated against each derivative (1–15). When compared to their respective standard drug such as acarbose and thiourea, almost all compounds were shown to have excellent activity. Among the screened series, analogs 5 (IC50 = 3.20 ± 0.40 and 2.10 ± 0.10 µM) and 6 (IC50 = 2.50 ± 0.40 and 5.30 ± 0.20 µM), emerged as potent molecules when compared to the standard drugs acarbose (IC50 = 8.24 ± 0.08 µM) and urease (IC50 = 7.80 ± 0.30). Moreover, an anti-microbial study also demonstrated that analogs 5 and 6 were found with minimum inhibitory concentrations (MICs) in the presence of standard drugs streptomycin and terinafine.
1. Introduction
Diabetes mellitus (DM) is one of the oldest known diseases worldwide. In the past, the number of patients experiencing this disease has increased every year; therefore, it is considered a lifestyle-related disease. In addition, about 366 million people have experienced this disease by 2011 reports, and the number will increase by up to 522 million by 2030 [1]. Type-II diabetes is the major type in developed countries, often caused by impaired insulin secretion as well as a reduction in the sensitivity of insulin [2,3,4,5,6]. Two enzymes are responsible for this infection: α-Amylase and α-Glucosidase. The hydrolyzing function of α-Amylase is to convert glycogen and starch to maltose and the process further carried out by α-Glucosidase which is present in the small intestine [7]. Conversions from glycogen to glucose increase the blood glucose level, hence the term post-prandial glycemia (PPHG), which is the initial indicator for type-II diabetes [8] and causes many complications in the body, such as neuropathy, nephropathy, retinopathy and cardiovascular diseases. It also damages various organs which are significant in the human body [9,10]. To control this, researchers have attempted to use different strategies to inhibit such infections, and various marketed drugs have been approved, including acarbose, voglibose and miglitol, but they have serious complications such as diarrhea, meteorism and abdominal pain [11,12].
Sulfonamide-containing drugs exhibit potent profiles due to their basic skeletons; they are also called sulpha drugs [13,14]. In a previous comparison, sulphonamide-bearing derivatives emerged as potent inhibitors with better inhibitory potential. One of the excellent inhibitors containing sulphonamide groups used for the treatment of Alzheimer’s disease, which is associated with butyrylcholinesterase (BuChE) inhibitory activity, was synthesized from previously marketed drugs containing sulphonamide derivatives and displayed excellent biological profiles at very low concentrations [15,16]. In the last few decades, sulphonamide derivatives have been extensively used as potent inhibitors against various diseases such as diabetes [17,18], psychosis [19], central nervous system (CNS) disorders [20,21], tumors [22,23,24,25,26] and different cancer treatments [27,28].Therefore, different heterocyclic moieties have been used by researchers in order to achieve fruitful results; in this regard, we synthesized and evaluated sulphonamide-containing aliphatic moieties and tested against α-Glucosidase, anti-urease, anti-bacterial and anti-fungal properties, which were found with excellent potential.
2. Material and Methods
General Procedure of Aliphatic Hydrazide-Based Benzene Sulphonamide Derivatives (1–15)
All the required chemicals and their reagents were purchased from Sigma Aldrich (Baden-Württemberg, Germany), and the purity of the solvents were determined using distillation processes. The reaction was carried out in a fume hood, and proper investigations were maintained by checking the reaction progress after every 30 min by using thin-layer chromatography (TLC).
3. Results and Discussion
Chemistry
The synthetic route was adopted for the synthesis of new aliphatic hydrazide-based benzene sulphonamide derivatives via a single-step reaction. Initially, a different substituted hydrazide moiety (I) was mixed in ethanol along with varied substituted benzene sulphonamides (II) followed by the addition of a few drops of acetic acid under refluxed conditions for about 4h, which afforded targeted moieties (1–15). All the synthesized derivatives were washed with n-hexane in order to remove the impurities, and fine powder was collected. Furthermore, reaction completions were confirmed by using thin-layer chromatography (TLC), and their basic skeletons were obtained through different spectroscopic techniques such as 1HNMR, 13CNMR, and HREI-MS (Scheme 1).
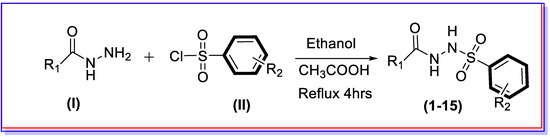
Scheme 1.
Synthesis of benzene sulphonamide derivatives (1–15).
4. Spectral Analysis
The spectral analysis of the synthesized compounds, as shown (Figures S1–S6), and their interpretation values are incorporated in the Supplementary Materials.
5. Biological Profile
5.1. α-Glucosidase Inhibitory Activity
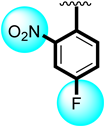
The biological profiles exhibit the inhibitory potential of the tested scaffolds against enzymes such as α-Glucosidase, in which different substituted scaffolds showed varied ranges of inhibitory profiles. The same substituted scaffolds attached to different positions of aromatic rings (Figure 1) and produced varying degrees of inhibitions, which might be the nature of substituents. Electron-donating groups activate the ring with further interactive properties with active sites of enzymes, while deactivating groups (electron with drawing) decrease the inhibitory profile of the molecules. In the present study, better interactions were found in electron-donating groups, e.g., tri-flouro and flouro moieties showed greater interactions through strong hydrogen bonds.
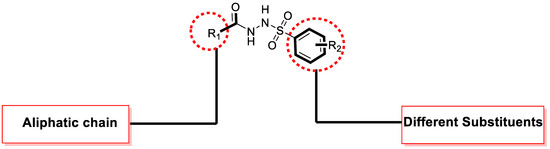
Figure 1.
General representation of molecules.
The comparison criteria were set for same substituted scaffolds attached at different positions of aromatic rings in the case of methoxy substituted scaffolds 1 (IC50 = 8.80 ± 0.20 µM), 2 (IC50 = 9.20 ± 0.30 µM) and 3 (IC50 = 8.60 ± 0.20 µM). The difference in activity profile might have been due to the position of the methoxy group. Para and ortho-substituted scaffolds (1 and 3) had somewhat better activity than the scaffold with the meta-methoxy group (2), which reduced the chances of interactions.
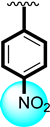
Likewise, nitro substituted scaffolds 5 (IC50 = 3.20 ± 0.40 µM), 7 (IC50 = 4.50 ± 0.80 µM) and 8 (IC50 = 6.70 ± 0.80 µM). Among the nitro substituted scaffolds, better activity was shown by analog 5; it might have been the presence of the nitro group at the ortho-position which strongly affected the enzymatic activity. When the position of the nitro group changed to para (7) and meta (8), the potentials of molecules were observed to be lower, which might have been due to the position of the substituent and the presence of the flouro group, which increased the biological activity; therefore, analog (5) showed a better activity profile than analog 7 and 8 and showed a few-fold better activity than the standard drug acarbose (IC50 = 8.24 ± 0.08).
Similarly, Chloro substituted scaffold 4 (IC50 = 5.70 ± 0.70 µM), 9 (IC50 = 7.40 ± 0.10 µM) and 14 (IC50 = 6.50 ± 0.70 µM). These scaffolds were found with minimum inhibitory concentrations (MICs). Among the chloro substituted analogs, better potential was found in the case of 4, while analog 9 and 14 had comparable activity to the standard drug acarbose.
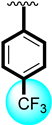
In the comparison criteria, the most attractive activity was shown by analog 6 (IC50 = 2.50 ± 0.40 µM) among the tested series. The better potential of the molecule might have been due to the presence of the tri-flouro group at the para-position, showing an excellent biological profile when compared with analog 15 (IC50 = 7.90 ± 0.80 µM)bearing the tri-flouro group at the ortho-position, while the methyl moiety was at the para-position of the ring. The change in the activity may have been due the steric hindrance of the methyl moiety; therefore, the overall effect of the molecule was found to have lower potential. Analog 6 was considered as the most potent scaffold of the tested series. The general representation of the molecule is shown in Figure 1.
The abovementioned general structure represents the different positions of molecule with varied substitutions such as the alkyl group (R = 1) and aromatic ring (R = 2). The inhibitory profile of the synthesized derivatives is based on the attached substituents on both sides of the molecule. The electron-withdrawing group (EWG) decreased the nucleophilic character of the molecule, showing a lower inhibitory profile, while the electron-donating group (EDG) increased the negative charge by donating electrons to the ring, which dominantly enhanced the biological function of the molecules. On the other hand, the alkyl group was also involved in the inhibitory profile of the molecule, which interacted through different positions of the alkyl chain. During this study, the alkyl chain with a lower number of carbons was found to have significant activity, as in the case of derivatives 5 and 6, while the other substituted molecules with the longest alkyl chains were found to have good to moderate activity, as shown in Table 1.

Table 1.
Different substituted sulphonamide derivatives with inhibitory profile (1–15).
5.2. Anti-Urease Inhibitory Activity
Similarly, the tested compound’s anti-urease profile was seen in the presence of the common standard drug thiourea (IC50 = 7.80 ± 0.30). The majority of the analogs had good to better activity profiles, and some had potential that was several folds greater than that of the reference drug, but in this case, analogs 5 and 6 demonstrated the most notable activity. Analog 5 (IC50 = 2.10 ± 0.10) bore the nitro group at the ortho-position and a flouro moiety at the para-position which created a strong ability to inhibit the enzymatic activity. Analog 5 was ranked first in the tested series because the fluoro moiety was electron withdrawing and had the ability to activate the ring system for subsequent interactions, which might boost the interaction through hydrogen bonds. Likewise, analog 6 (IC50 = 5.30 ± 0.20) containing a tri-flouro group at the para-position of the ring also showed an excellent inhibitory profile against urease enzyme and was ranked second in the series. The presence of the tri-flouro group, which similarly interacted with the active sites of enzymes by a strong hydrogen bond, may be the better interaction also measured in this analog. The fluoro group dominated in analogs 5 and 6, and the interaction had an inhibitory effect on urease. The remaining derivatives with different substitutions on both the aliphatic and aromatic side (R1 and R2, respectively) were found to have good to moderate inhibitory profiles. The lower activity profile might have been due to the presence of the electron-withdrawing group, the bulky group as well as the largest aliphatic chain; all of these were responsible for not involving the functionality toward active sites of targeted enzymes; therefore, these moieties showed less inhibitory profiles. Moreover, the involvement of the nitro/flouro and tri-flouro group with active site of enzymes through hydrogen bonds are the key factors for the excellent activity of analogs 5 and 6, whileothersubstituted derivatives having largest alkyl chain might be the reason for poor activity as compares to the standard drug thiourea.
5.3. Anti-Microbial Activity
Anti-bacterial activity has attracted substantial attention from researchers in the last few decades due to the harmful effects of bacteria [29]. The present study shows the minimum inhibitory concentration (MIC) values for the anti-microbial (anti-bacterial and anti-fungal) activity of tested compounds, which were also found to have good to moderate inhibitory profiles. Most of the scaffolds in the tested series were found to have higher inhibitory profiles in the presence of streptomycin (44% inhibition), a standard drug. The inhibitory profile of scaffolds depends on the nature and number of substituents which increase or decrease the biological potential of molecules. In addition, a few of them were found to have comparable activity with standard drugs, but in this regard, excellent activity was shown by analogs 5 and 6. These two scaffolds reveal that the functional group located on the para and ortho-position of the aromatic ring is the critical factor for the observations of antibacterial activity. Finally, it is crucial to note that the analogs showed outstanding antibacterial selectivity against E. coli, depending on the substituents. The nature of substituents attached to aromatic rings are responsible for better or poor interaction; therefore, analogs 5 with flouro and nitro moieties and analog 6 containing a tri-flouro group were regarded as the most potent analogs in the tested series. Analog 6 showed somewhat higher inhibition (43.16%) as compared to analog 5 (43.1%);this might have been due to the presence of the tri-flouro group at the para-position which produced strong inhibition against E.coli, as shown in Figure 2.
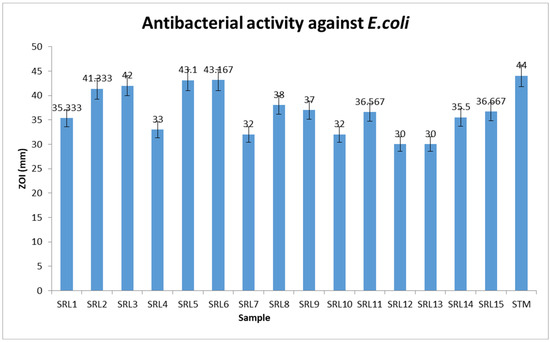
Figure 2.
The anti-bacterial activity of scaffolds 1–15.
On the other hand, anti-fungal activity was observed for the synthesized compounds in the presence of the standard drug Terinafine. The majority of the compounds bearing varied substituents were found to have poor rates of inhibitions against Alternaria alternate (Fungus specie). However, a few of them were observed with somewhat comparable activity. Analogs 2, 3, 5 and 6 exhibited anti-fungal profiles. Here, in this study, it was found that a functional group is also necessary for a better biological profile; therefore, analogs 5 and 6 showed significant profiles compared to other substituted scaffolds, as shown in Figure 3.
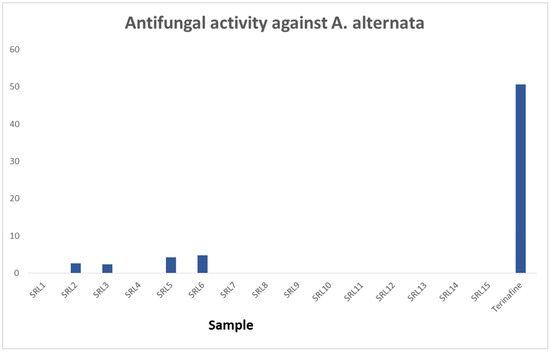
Figure 3.
The anti-fungal activity of scaffolds 1–15.
5.4. Molecular Docking Studies
Molecular docking studies were performed in order to explore the binding interactions of ligands with active sites of enzymes. Due to better interactions, all molecules were found with somewhat comparable properties, but analogs 5 and 6 were found with strong interactions due to varied substituents (Table 2).

Table 2.
The interactions of scaffolds 5 and 6 against urease and α-Glucosidase enzymes, respectively.
In the present study, scaffolds (5 and 6) bearing flouro and tri-flouro moieties, respectively, were electron-withdrawing groups, but properties to make strong hydrogen bonds were found with better interactions, and their superposed surface complex structure against urease (docking score = −9.80) and α-Glucosidase enzymes (docking score = −10.70) are illustrated in Figure 4 and Figure 5, respectively. Both scaffold 5 and 6 were found to have excellent potency in vitro and better potential in silico when compared to the standard drug acarbose (docking score = −9.30), illustrated in Figure 6.
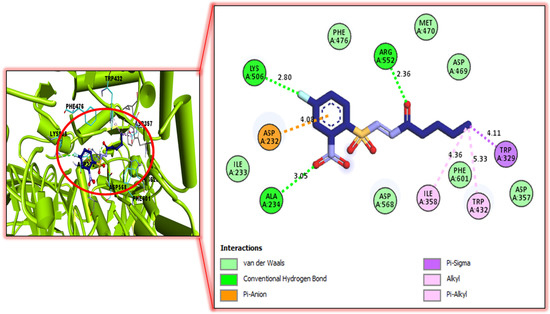
Figure 4.
Protein–ligand interaction profile for active analog against urease represents the surface of the corresponding enzyme. The PLI profile for compound 5.
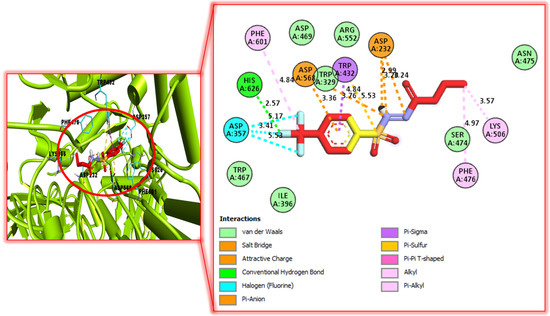
Figure 5.
Protein–ligand interaction profiles for active analog against α-Glucosidase represent the surface of the corresponding enzyme. The PLI profile for compound 6.
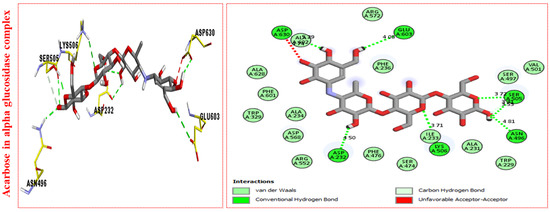
Figure 6.
Protein–ligand interaction profiles against α-Glucosidase represent the surface of the corresponding enzyme. The PLI profile for acarbose.
The better interactions of these ligands might have been due to the presence of attached substituents. In case of analog 5 and 6, interactions were found such as vander Waals, conventional hydrogen bond, Pi-anion, Pi-Sigma, alkyl, Pi-alkyl, salt bridge, halogen, Pi-sulphur, etc. The further detail of docking study were summarized as in our previous articles [30,31,32,33,34,35] and the assay protocols of both the activities have been added in Supplementary file [36,37].
6. Conclusions
The present study was carried out for the synthesis of aliphatic hydrazide-based benzene sulphonamide derivatives. Fifteen compounds were synthesized through a single-step reaction in which most of the compounds were obtained in good yields. These scaffolds were characterized through different spectroscopic techniques such as FTIR, 1HNMR, 13CNMR and HREI-MS to confirm the basic skeletons of the synthesized compounds. Furthermore, all the synthesized scaffolds were tested against α-glucosidase and urease enzymes, and their inhibitory potentials were also checked against strains of bacteria and fungi. Alpha glucosidase and urease studies were completed in the presence of standard drugs such as acarbose (IC50 = 8.24 ± 0.08) and thiourea (IC50 = 7.80 0.30), and their biological potentials against alpha glucosidase and urease enzymes were found with good to moderate activity in this regard. For analogs 5 (IC50 = 3.20 ± 0.40 µM and 2.10 ± 0.10 µM, respectively) and 6 (IC50 = 2.50 ± 0.40 µM and 5.30 ± 0.20 µM, respectively), the anti-microbial study of these analogs were found with maximum inhibitions like 5 (43.1%) and 6 (43.16%) in the presence of streptomycin (44% inhibition) Moreover, the effective nature of these analogs was also monitored through molecular docking studies. Scaffolds 5 and 6 showed different interactions with varied ranges, and their attached substituents were found prior to all the tested analogs of the series.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27207129/s1, Figures S1–S6: Spectral analysis of synthesized compounds.
Author Contributions
Conceptualization, writing-original draft Preparation, S.K., S.I. and M.S.; critical revision and response to the reviewers’ questions, writing review and editing, W.R. and R.H.; software, writing review and editing, funding, L.R. and H.A.; validation, project administration, critical revision, A.A.D., M.I.A. and R.A.P.; writing review and editing, resources, funding, E.A. and A.-E.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR58). The authors extend their apprecia-tion to the Deanship of Scientific Research at King Khalid University for funding this work under grant number (GRP/242/43).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR58). The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work under grant number (GRP/242/43).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Accili, D.; Imai, Y. Insulin resistance or insulin deficiency: Which is the primary cause of NIDDM? Diabetes 1994, 43, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Porte, D., Jr. β-cells in typeII diabetes mellitus. Diabetes 1991, 40, 166–180. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Tang, P.C.; Lin, Z.G.; Wang, Y.; Yang, F.L.; Wang, Q.; Fu, J.H.; Zhang, L.; Gong, A.S.; Luo, J.J.; Dai, J.; et al. Design and synthesis of DPP-4 inhibitor for the treatment of type 2 diabetes. Chin. Chem. Lett. 2010, 21, 253–256. [Google Scholar] [CrossRef]
- Wu, Y.H. Synthesis of (S)‐2‐Ethoxy‐3‐Phenylpropanoic Acid Derivatives and Their Insulin‐Sensitizing Activity. Chin. J. Chem. 2007, 25, 265–267. [Google Scholar] [CrossRef]
- Salar, U.; Khan, K.M.; Chigurupati, S.; Taha, M.; Wadood, A.; Vijayabalan, S.; Ghufran, M.; Perveen, S. New hybrid hydrazinyl thiazole substituted chromones: As potential α-amylase inhibitors and radical (DPPH & ABTS) scavengers. Sci. Rep. 2017, 7, 16980. [Google Scholar]
- Sun, H.; Wang, D.; Song, X.; Zhang, Y.; Ding, W.; Peng, X.; Zhang, X.; Li, Y.; Ma, Y.; Wang, R.; et al. Natural prenylchalconaringenins and prenylnaringenins as antidiabetic agents: α-glucosidase and α-amylase inhibition and in vivo antihyperglycemic and antihyperlipidemic effects. J. Agric. Food Chem. 2017, 65, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Campos, C. Chronic hyperglycemia and glucose toxicity: Pathology and clinical sequelae. Postgrad. Med. 2012, 124, 90–97. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. CellMetab. 2013, 17, 20–33. [Google Scholar]
- Miller, B.R.; Nguyen, H.; Hu, C.J.H.; Lin, C.; Nguyen, Q.T. New and emerging drugs and targets for type 2 diabetes: Reviewing the evidence. Am. Health Drug Benefits 2014, 7, 452. [Google Scholar]
- Raghu, C.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar]
- Henry, R.J. The mode of action of sulfonamides. Bacteriol. Rev. 1943, 7, 175–262. [Google Scholar] [CrossRef] [PubMed]
- Wegst-Uhrich, S.R.; Navarro, D.A.; Zimmerman, L.; Aga, D.S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Kosak, U.; Brus, B.; Knez, D.; Zakelj, S.; Trontelj, J.; Pislar, A.; Sink, R.; Jukic, M.; Zivin, M.; Podkowa, A.; et al. The magic of crystal structure-based inhibitor optimization: Development of a butyrylcholinesterase inhibitor with picomolar affinity and in vivo activity. J. Med. Chem. 2018, 61, 119–139. [Google Scholar] [PubMed]
- Košak, U.; Brus, B.; Knez, D.; Šink, R.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šlenc, J.; Gobec, M.; Živin, M.; et al. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Khan, I.U.; Bajda, M.; Ashraf, M.; Shaukat, A.; Rehman, T.U.; Mutahir, S.; Hussain, S.; Mustafa, G.; Yar, M. Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer’s disease: In vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg. Chem. 2015, 63, 64–71. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Broncel, M.; Sikora, J. Sulfenamide and sulfonamide Derivatives of Metformin—A New option to Improve endothelial Function and plasma Haemostasis. Sci. Rep. 2019, 9, 6573. [Google Scholar] [CrossRef]
- Zajdel, P.; Partyka, A.; Marciniec, K.; Bojarski, A.J.; Pawlowski, M.; Wesolowska, A. Quinoline-and isoquinoline-sulfonamide analogs of aripiprazole: Novel antipsychotic agents? Future Med. Chem. 2014, 6, 57–75. [Google Scholar] [CrossRef]
- Mutahir, S.; Jończyk, J.; Bajda, M.; Khan, I.U.; Khan, M.A.; Ullah, N.; Ashraf, M.; Riaz, S.; Hussain, S.; Yar, M. Novel biphenyl bis-sulfonamides as acetyl and butyrylcholinesterase inhibitors: Synthesis, biological evaluation and mo-lecular modeling studies. Bioorg. Chem. 2016, 64, 13–20. [Google Scholar] [CrossRef]
- Apiraksattayakul, S.; Pingaew, R.; Prachayasittikul, V.; Ruankham, W.; Jongwachirachai, P.; Songtawee, N.; Phopin, K. Neuroprotective Properties of Bis-Sulfonamide Derivatives Against 6-OHDA-Induced Parkinson's Model via Sirtuin 1 Ac-tivity and in silico Pharmacokinetic Properties. Front. Mol. Neurosci. 2022, 15, 890838. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Knudsen, G.M.; Maeda, S.; Trinidad, J.C.; Ioanoviciu, A.; Burlingame, A.L.; Mucke, L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 2015, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, Z.R.; Li, Y.; Qu, J.; Ling, Y.H.; Jiang, J.D.; Boykin, D.W. Synthesis and structure—Activity relationships of carbazole sulfonamides as a novel class of antimitotic agents against solid tumors. J. Med. Chem. 2006, 49, 6273–6282. [Google Scholar] [CrossRef] [PubMed]
- ShoaibAhmadShah, S.; Rivera, G.; Ashfaq, M. Recent advances in medicinal chemistry of sulfonamides: Rational design as anti-tumoral, anti-bacterial and an-ti-inflammatory agents. MiniRev. Med. Chem. 2013, 13, 70–86. [Google Scholar]
- Takagi, M.; Honmura, T.; Watanabe, S.; Yamaguchi, R.; Nogawa, M.; Nishimura, I.; Katoh, F.; Matsuda, M.; Hidaka, H. In vivo antitumor activity of a novel sulfonamide, HMN-214, against human tumor xenografts in mice and the spectrum of cytotoxicity of its active metabolite, HMN-176. Investig. New Drugs 2003, 21, 387–399. [Google Scholar]
- Owa, T.; Yoshino, H.; Okauchi, T.; Yoshimatsu, K.; Ozawa, Y.; Sugi, N.H.; Nagasu, T.; Koyanagi, N.; Kitoh, K. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J. Med. Chem. 1999, 42, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yamali, C.; Sakagami, H.; Angeli, A.; Leitans, J.; Kazaks, A.; Tars, K.; Ozgun, D.O.; Supuran, C.T. New anticancer drug candidate’s sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg. Chem. 2018, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Singh, H.; Singh, A.; Agrawal, D.K.; Arora, A.; Chundawat, T.S. Trifluoromethylated Quinolone-Hydantoin Hybrids: Synthesis and Antibacterial Evaluation. Science 2022, 4, 30. [Google Scholar] [CrossRef]
- Kharb, M.; Jat, R.K.; Parjapati, G.; Gupta, A. Introduction to molecular docking software technique in medicinal chemistry. Int. J. Drug Res. Tech. 2012, 2, 189–197. [Google Scholar]
- Li, Z.; Gu, J.; Zhuang, H.; Kang, L.; Zhao, X.; Guo, Q. Adaptive molecular docking method based on information entropy genetic algorithm. Appl. Soft Comput. 2015, 26, 299–302. [Google Scholar]
- Rao, C.M.M.P.; Naidu, N.; Priya, J.; Rao, K.P.C.; Ranjith, K.; Shobha, S.; Siddiraju, S. Molecular docking and dynamic simulations of benzimidazoles with beta-tubulins. Bioinformation 2021, 17, 404. [Google Scholar] [PubMed]
- Khan, S.; Ullah, H.; Rahim, F.; Nawaz, M.; Hussain, R.; Rasheed, L. Synthesis, in vitro α-amylase, α-glucosidase activities and molecular docking study of new benzimidazole bearing thiazolidinone derivatives. J. Mol. Struct. 2022, 1269, 133812. [Google Scholar] [CrossRef]
- Khan, Y.; Iqbal, S.; Shah, M.; Maalik, A.; Hussain, R.; Khan, S.; Khan, I.; Pashameah, R.A.; Alzahrani, E.; Farouk, A.E. New Quinoline-based triazole hybrid analogues as effective inhibitors of α-amylase and αglucosidase: Synthesis, in vitro evaluation and molecular docking along with in silico study. Front. Chem. 2022, 10, 1099. [Google Scholar]
- Khan, Y.; Rehman, W.; Hussain, R.; Khan, S.; Malik, A.; Khan, M.; Liaqat, A.; Rasheed, L.; Begum, F.; Fazil, S.; et al. New biologically potent benzimidazole‐based‐triazole derivatives as acetylcholinesterase and butyrylcholinesterase inhib-itors along with molecular docking study. J. Hetercyc. Chem. 2022, in press. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Srinivasa, M.G.; Aggarwal, N.N.; Gatpoh, B.F.D.; Shankar, M.K.; Byadarahalli Ravindranath, K.; Gurubasavaraj Veeranna, P.; Bistuvalli Chandrashekarappa, R. Identification of benzothiazole‐rhodanine derivatives as α-amylase and α-glucosidase inhibitors: Design, synthesis, in silico, and in vitro analysis. J. Mol. Recognit. 2022, 35, 2959. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).