Tannin-Derived Hard Carbon for Stable Lithium-Ion Anode
Abstract
1. Introduction
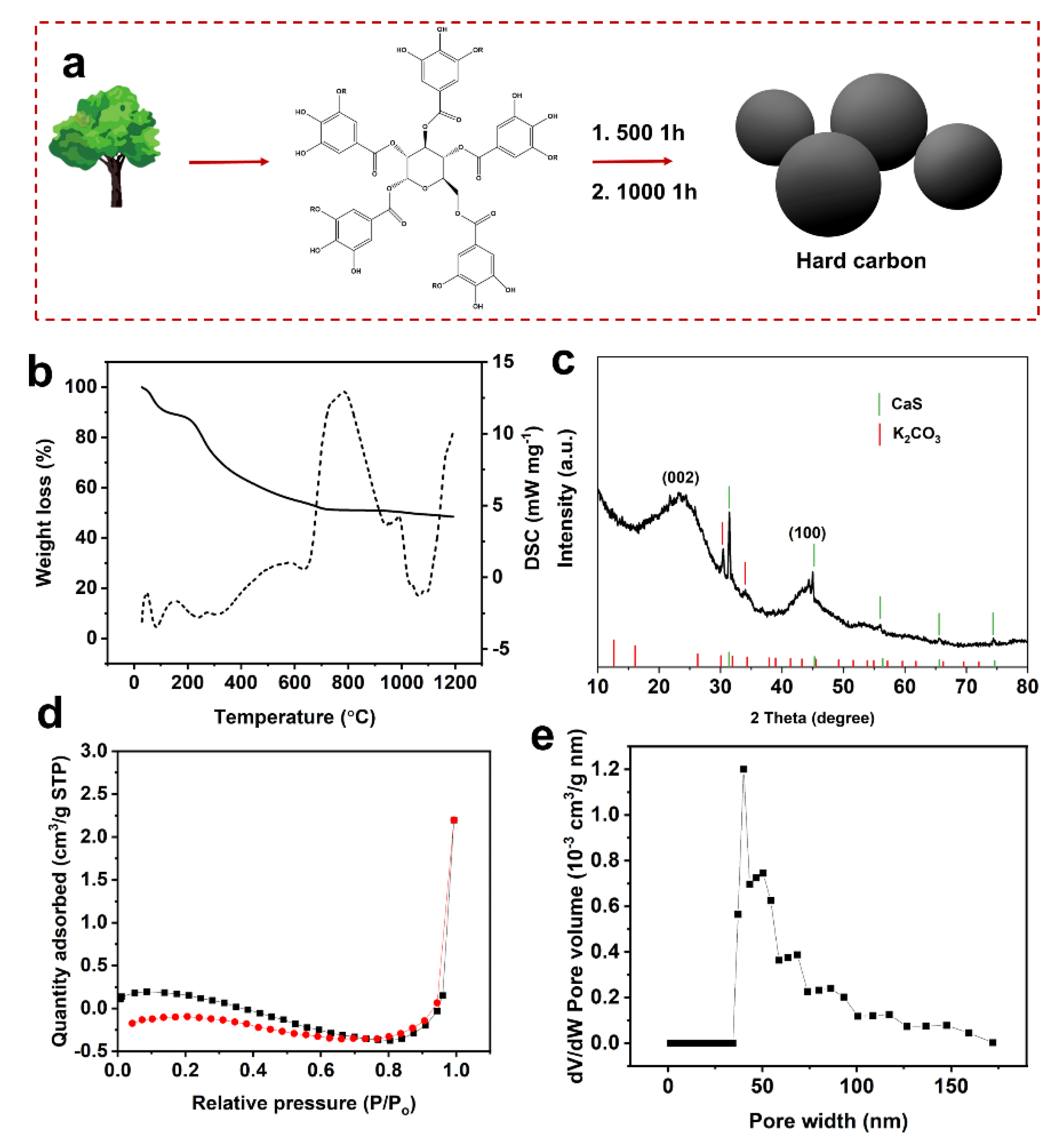
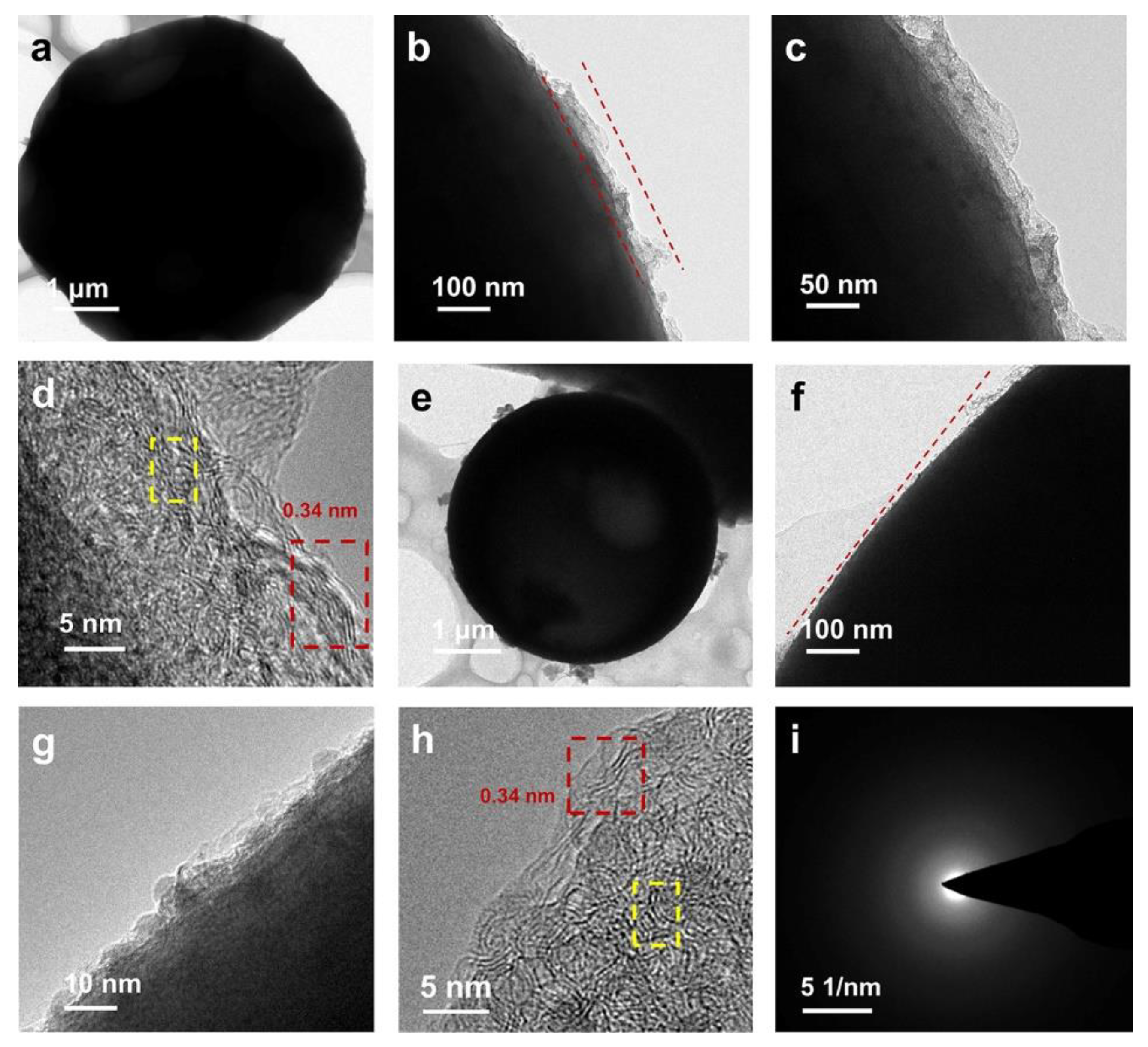
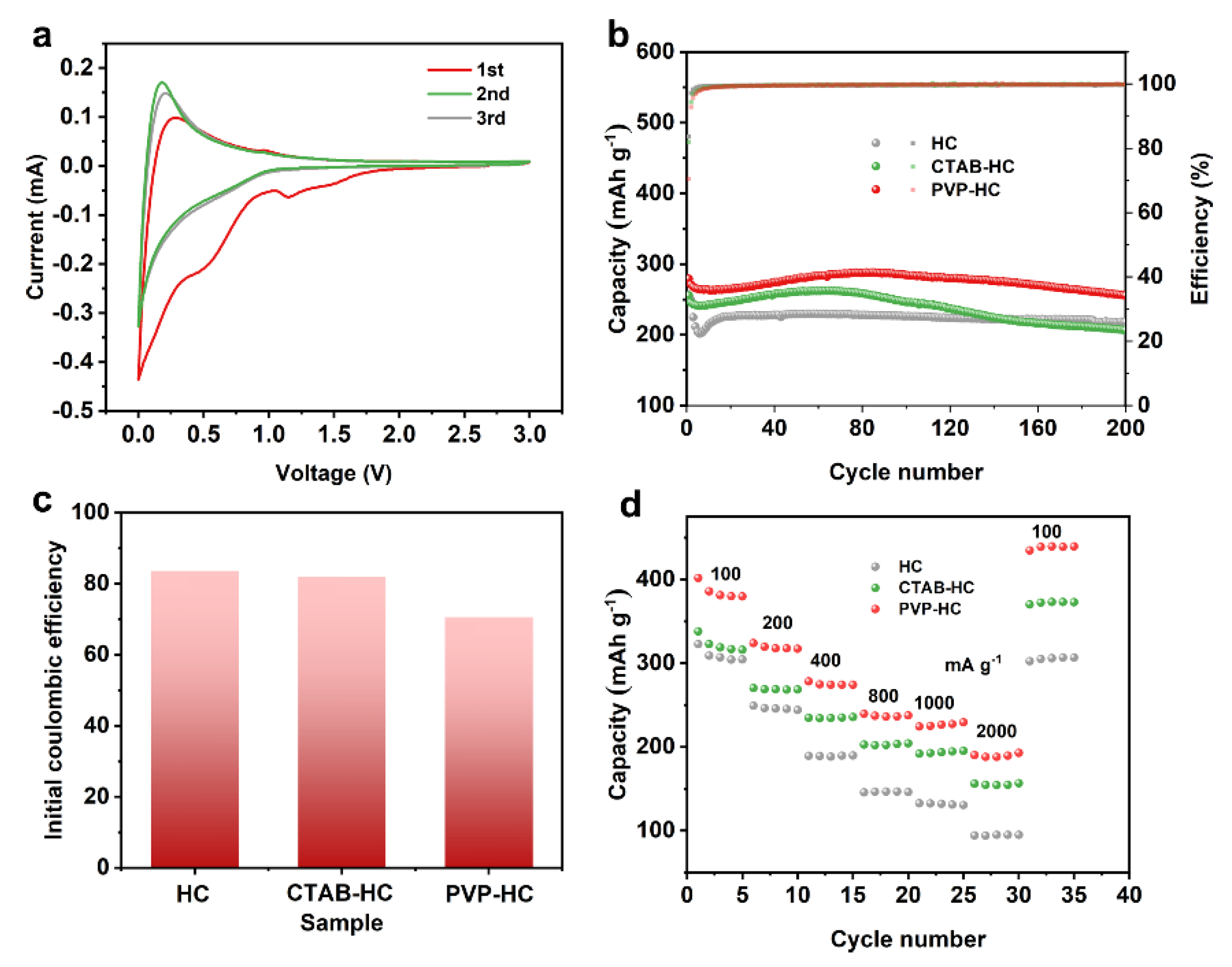
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.-Y. Progress of Nanostructured Metal Oxides Derived from Metal–Organic Frameworks as Anode Materials for Lithium–Ion Batteries. Coord. Chem. Rev. 2020, 420, 213434. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Reddy, R.C.K.; Lin, X.; Zeb, A.; Su, C.-Y. Metal–Organic Frameworks and Their Derivatives as Cathodes for Lithium-Ion Battery Applications: A Review. Electrochem. Energy Rev. 2022, 5, 312–347. [Google Scholar]
- Xu, L.; Li, J.; Deng, W.; Shuai, H.; Li, S.; Xu, Z.; Li, J.; Hou, H.; Peng, H.; Zou, G. Garnet Solid Electrolyte for Advanced All-Solid-State Li Batteries. Adv. Energy Mater. 2021, 11, 2000648. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Shuai, H.; Luo, Z.; Wang, B.; Fang, S.; Zou, G.; Hou, H.; Peng, H.; Ji, X. Recent Advances of Composite Electrolytes for Solid-State Li Batteries. J. Energy Chem. 2022, 67, 524–548. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-Safety Separators for Lithium-Ion Batteries and Sodium-Ion Batteries: Advances and Perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar]
- Xu, L.; Li, J.; Xiang, Y.; Tian, Y.; Momen, R.; Liu, H.; Zhu, F.; Tu, H.; Luo, Z.; Fang, S.; et al. Few-Layer Bismuthene Enabled Solid-State Li Batteries. Energy Storage Mater. 2022, 52, 655–663. [Google Scholar] [CrossRef]
- Xu, L.; Tu, H.; Zhu, F.; Xiang, Y.; Luo, Z.; Fang, S.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Carbon Dots for Ultrastable Solid-State Batteries. SmartMat 2022, 3, 286–297. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation Chemistry of Graphite: Alkali Metal Ions and Beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, C.; He, J.; Tu, X.; Sun, W.; Pan, H.; Zhou, Y.; Rui, X.; Zhang, B.; Huang, K. Structural Engineering in Graphite-Based Metal-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2107277. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Yang, H.; Zhong, F.; Ai, X. Chemically Prelithiated Hard-Carbon Anode for High Power and High Capacity Li-Ion Batteries. Small 2020, 16, 1907602. [Google Scholar] [CrossRef]
- Wan, H.; Shen, X.; Jiang, H.; Zhang, C.; Jiang, K.; Chen, T.; Shi, L.; Dong, L.; He, C.; Xu, Y. Biomass-Derived N/S Dual-Doped Porous Hard-Carbon as High-Capacity Anodes for Lithium/Sodium Ions Batteries. Energy 2021, 231, 121102. [Google Scholar] [CrossRef]
- Alvin, S.; Cahyadi, H.S.; Hwang, J.; Chang, W.; Kwak, S.K.; Kim, J. Revealing the Intercalation Mechanisms of Lithium, Sodium, and Potassium in Hard Carbon. Adv. Energy Mater. 2020, 10, 2000283. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, H.; Ji, W.; Zheng, D.; Ding, T.; Abegglen, C.; Qiu, D.; Qu, D. Fast and Controllable Prelithiation of Hard Carbon Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 11589–11599. [Google Scholar] [CrossRef]
- Chen, D.; Luo, K.; Yang, Z.; Zhong, Y.; Wu, Z.; Song, Y.; Chen, G.; Wang, G.; Zhong, B.; Guo, X. Direct Conversion of Ester Bond-Rich Waste Plastics into Hard Carbon for High-Performance Sodium Storage. Carbon 2021, 173, 253–261. [Google Scholar] [CrossRef]
- Hong, K.-l.; Qie, L.; Zeng, R.; Yi, Z.-q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.-j.; Zhang, W.-x. Biomass Derived Hard Carbon Used as a High Performance Anode Material for Sodium Ion Batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Rios, C.d.M.S.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from Various Biomass Types as Precursors for Hard Carbon Anodes in Sodium-Ion Batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, J.; Liu, X.; Xuan, M.; Bi, K.; Zhang, X.L.; Hu, J.; Fan, J.; Chen, S.; Shao, G. Pinecone Biomass-Derived Hard Carbon Anodes for High-Performance Sodium-Ion Batteries. RSC Adv. 2017, 7, 41504–41511. [Google Scholar] [CrossRef]
- Kamiyama, A.; Kubota, K.; Nakano, T.; Fujimura, S.; Shiraishi, S.; Tsukada, H.; Komaba, S. High-Capacity Hard Carbon Synthesized from Macroporous Phenolic Resin for Sodium-Ion and Potassium-Ion Battery. ACS Appl. Energy Mater. 2019, 3, 135–140. [Google Scholar] [CrossRef]
- Nita, C.; Zhang, B.; Dentzer, J.; Ghimbeu, C.M. Hard Carbon Derived from Coconut Shells, Walnut Shells, and Corn Silk Biomass Waste Exhibiting High Capacity for Na-Ion Batteries. J. Energy Chem. 2021, 58, 207–218. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hua, S.; Wang, C.; Hu, B. Insight into the Performance and Mechanism of Persimmon Tannin Functionalized Waste Paper for U (VI) and Cr (VI) Removal. Chemosphere 2022, 287, 132199. [Google Scholar] [CrossRef] [PubMed]
- Beda, A.; Escamilla-Pérez, A.M.; Simonin, L.; Matei Ghimbeu, C.l. Vegetal-Extracted Polyphenols as a Natural Hard Carbon Anode Source for Na-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4774–4787. [Google Scholar] [CrossRef]
- Phuriragpitikhon, J.; Ghimire, P.; Jaroniec, M. Tannin-Derived Micro-Mesoporous Carbons Prepared by One-Step Activation with Potassium Oxalate and CO2. J. Colloid Interface Sci. 2020, 558, 55–67. [Google Scholar] [CrossRef]
- Kubota, K.; Shimadzu, S.; Yabuuchi, N.; Tominaka, S.; Shiraishi, S.; Abreu-Sepulveda, M.; Manivannan, A.; Gotoh, K.; Fukunishi, M.; Dahbi, M. Structural Analysis of Sucrose-Derived Hard Carbon and Correlation with the Electrochemical Properties for Lithium, Sodium, and Potassium Insertion. Chem. Mater. 2020, 32, 2961–2977. [Google Scholar] [CrossRef]
- Liang, H.-J.; Hou, B.-H.; Li, W.-H.; Ning, Q.-L.; Yang, X.; Gu, Z.-Y.; Nie, X.-J.; Wang, G.; Wu, X.-L. Staging Na/K-Ion De-/Intercalation of Graphite Retrieved from Spent Li-Ion Batteries: In Operando X-ray Diffraction Studies and an Advanced Anode material for Na/K-Ion Batteries. Energy Environ. Sci. 2019, 12, 3575–3584. [Google Scholar] [CrossRef]
- Liu, J.; Yin, T.; Tian, B.; Zhang, B.; Qian, C.; Wang, Z.; Zhang, L.; Liang, P.; Chen, Z.; Yan, J. Unraveling the Potassium Storage Mechanism in Graphite Foam. Adv. Energy Mater. 2019, 9, 1900579. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zhu, Y.; Ji, K.; Wang, C.; Ruan, D.; Xia, Y. Engineering Hard Carbon with High Initial Coulomb Efficiency for Practical Sodium-Ion Batteries. J. Power Sources 2021, 492, 229656. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and Improving the Initial Coulombic Efficiency of High-Capacity Anode Materials for Practical Sodium Ion Batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B. Understanding and Applying Coulombic Efficiency in Lithium Metal Batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, W.; Luo, K.; Song, Y.; Zhong, Y.; Liu, Y.; Wang, G.; Zhong, B.; Wu, Z.; Guo, X. Hard Carbon for Sodium Storage: Mechanism and Optimization Strategies Toward Commercialization. Energy Environ. Sci. 2021, 14, 2244–2262. [Google Scholar] [CrossRef]
- Cheng, D.; Zhou, X.; Hu, H.; Li, Z.; Chen, J.; Miao, L.; Ye, X.; Zhang, H. Electrochemical Storage Mechanism of Sodium in Carbon Materials: A Study from Soft Carbon to Hard Carbon. Carbon 2021, 182, 758–769. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Bai, Y.; Yang, H.; Dong, R.; Wang, X.; Xu, H.; Wang, Q.; Li, H.; Gao, H. Probing the Energy Storage Mechanism of Quasi-Metallic Na in Hard Carbon for Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003854. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Helen, M.; Groß, A.; Fichtner, M.; Euchner, H. Insight into Sodium Insertion and the Storage Mechanism in Hard Carbon. ACS Energy Lett. 2018, 3, 2851–2857. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.-L.; Wang, Y.-G.; Wang, C.-X.; Xia, Y.-Y. Pitch Modified Hard Carbons as Negative Materials for Lithium-Ion Batteries. Electrochim. Acta 2012, 74, 1–7. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Liang, H.-J.; Gu, Z.-Y.; Meng, Y.-F.; Yang, M.; Yu, M.-X.; Zhao, C.-D.; Wu, X.-L. Waste-to-Wealth: Low-Cost Hard Carbon Anode Derived from Unburned Charcoal with High Capacity and Long Cycle Life for Sodium-Ion/Lithium-Ion Batteries. Electrochim. Acta 2020, 361, 137041. [Google Scholar] [CrossRef]
- Chen, K.H.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C fast Charging of Li-ion Batteries with Graphite/Hard Carbon Hybrid Anodes. Adv. Energy Mater. 2021, 11, 2003336. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Wu, H.; Yuan, R.; Zong, M.; Li, Y.; Dravid, V.; Ai, W.; Wu, J. A Hybrid Lithium Storage Mechanism of Hard Carbon Enhances Its Performance as Anodes for Lithium-Ion Batteries. Carbon 2021, 178, 443–450. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.-J.; Xu, L.-Q.; Feng, B.; Hu, J.-B.; Chang, S.-S.; Liu, G.-G.; Liu, Y.; Xu, B.-H. Tannin-Derived Hard Carbon for Stable Lithium-Ion Anode. Molecules 2022, 27, 6994. https://doi.org/10.3390/molecules27206994
He M-J, Xu L-Q, Feng B, Hu J-B, Chang S-S, Liu G-G, Liu Y, Xu B-H. Tannin-Derived Hard Carbon for Stable Lithium-Ion Anode. Molecules. 2022; 27(20):6994. https://doi.org/10.3390/molecules27206994
Chicago/Turabian StyleHe, Ming-Jun, Lai-Qiang Xu, Bing Feng, Jin-Bo Hu, Shan-Shan Chang, Gong-Gang Liu, Yuan Liu, and Bing-Hui Xu. 2022. "Tannin-Derived Hard Carbon for Stable Lithium-Ion Anode" Molecules 27, no. 20: 6994. https://doi.org/10.3390/molecules27206994
APA StyleHe, M.-J., Xu, L.-Q., Feng, B., Hu, J.-B., Chang, S.-S., Liu, G.-G., Liu, Y., & Xu, B.-H. (2022). Tannin-Derived Hard Carbon for Stable Lithium-Ion Anode. Molecules, 27(20), 6994. https://doi.org/10.3390/molecules27206994








