Enhanced Visible-Light-Driven Photocatalytic Activity by Fe(Ш)-Doped Graphitic C3N4
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of g-C3N4
2.2. Characterization
2.3. Photocatalytic Properties Characterization
3. Results and Discussion
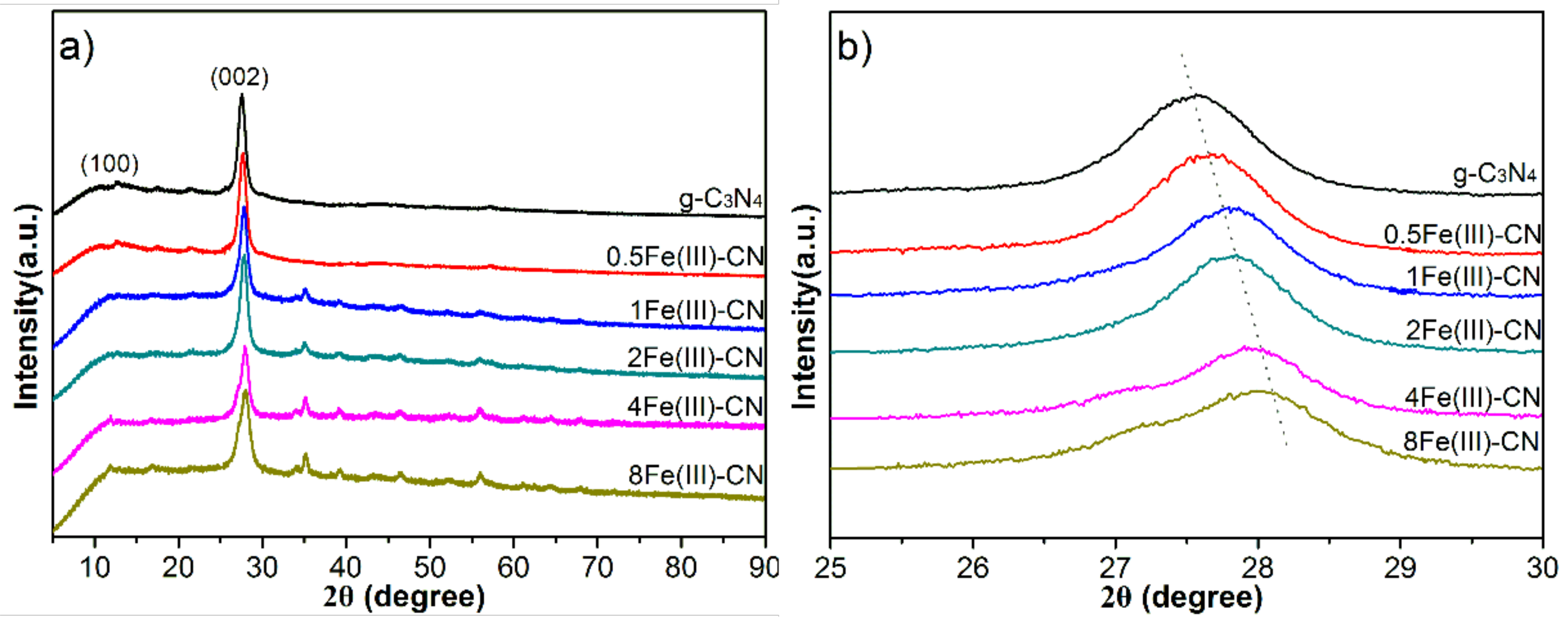
3.1. XRD Characterization
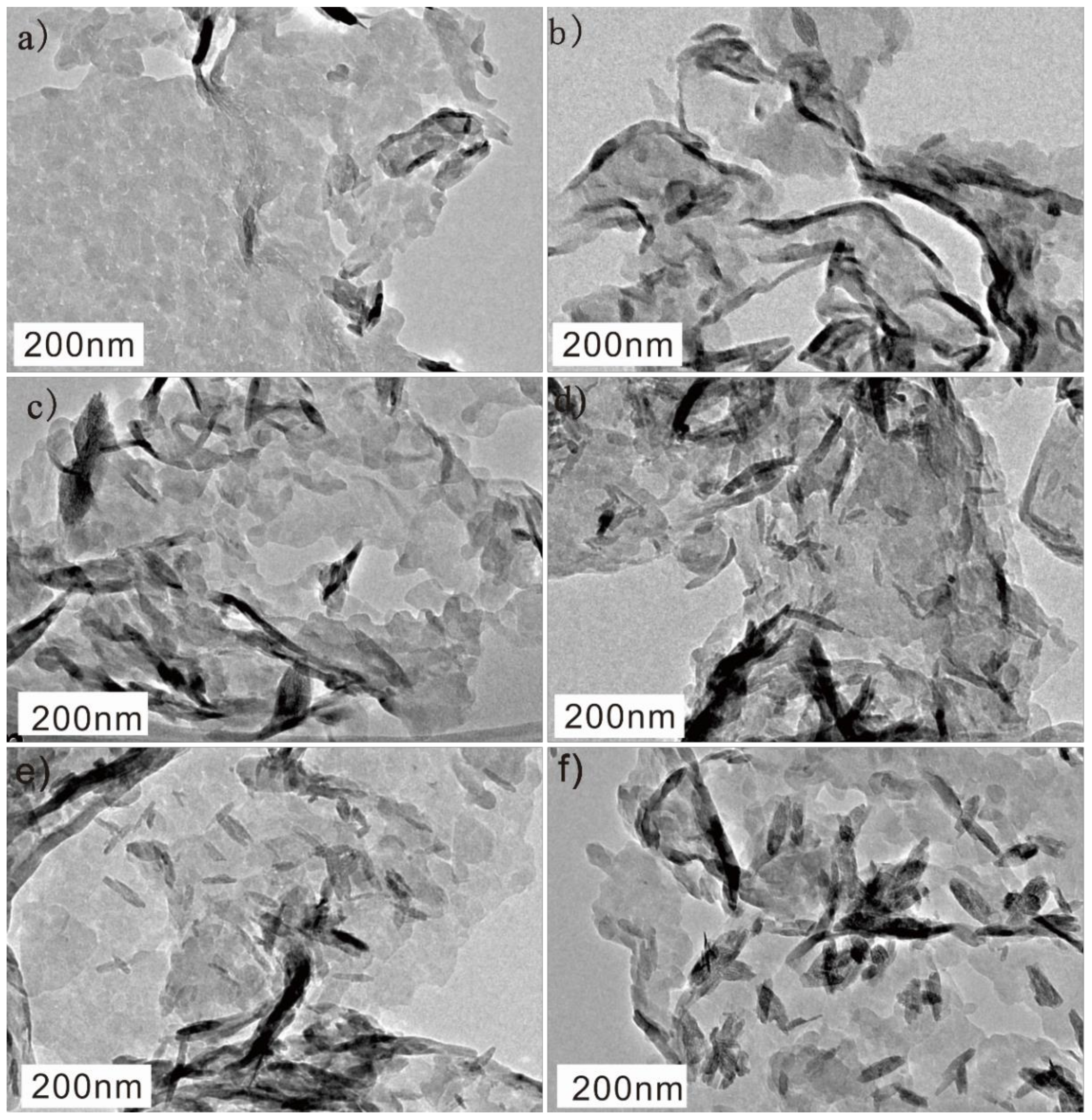
3.2. TEM Characterization
3.3. FT-IR Spectra
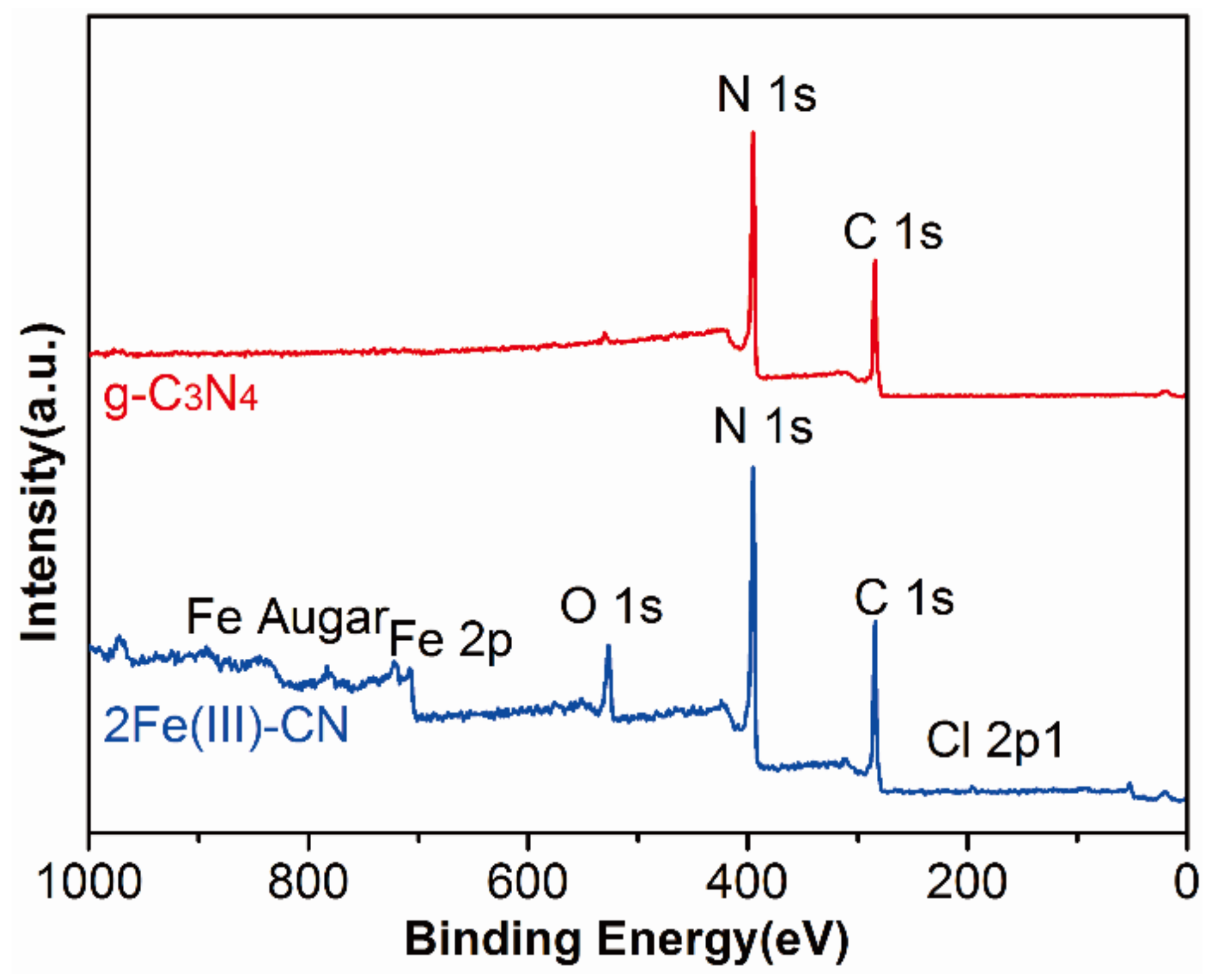
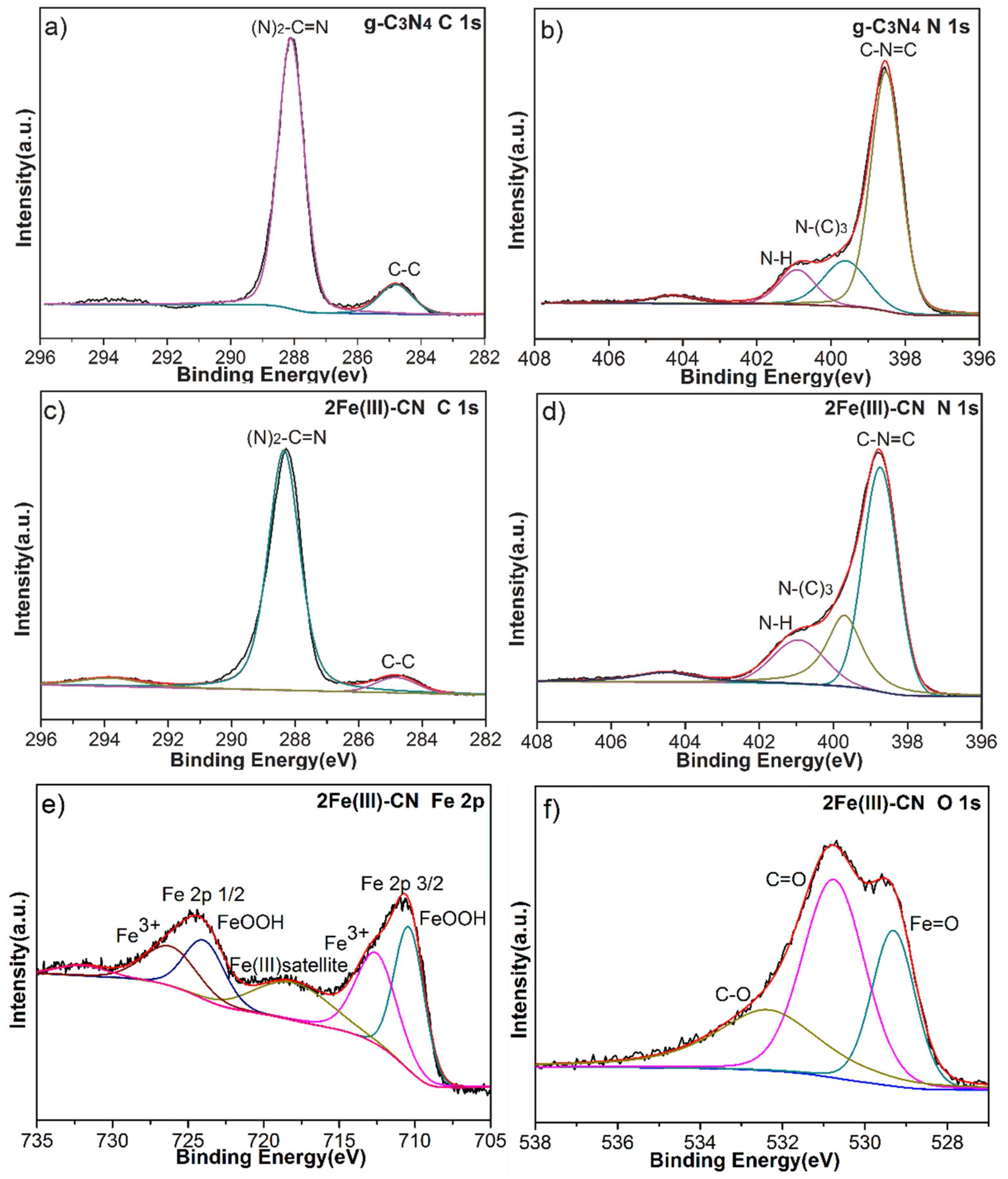
3.4. Chemical Compositions
3.5. UV-Visible Diffuse Reflection Spectra
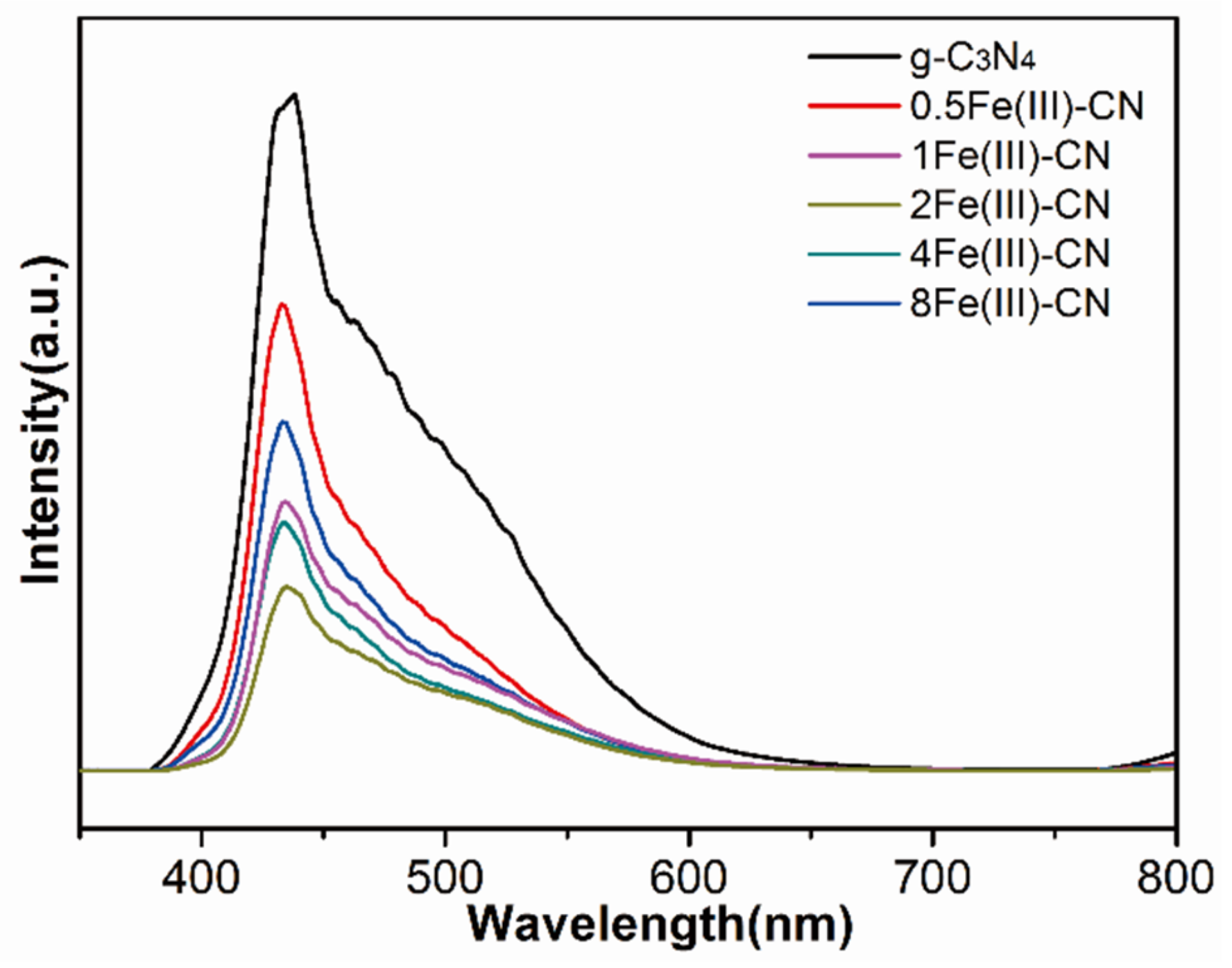
3.6. PL Measurements
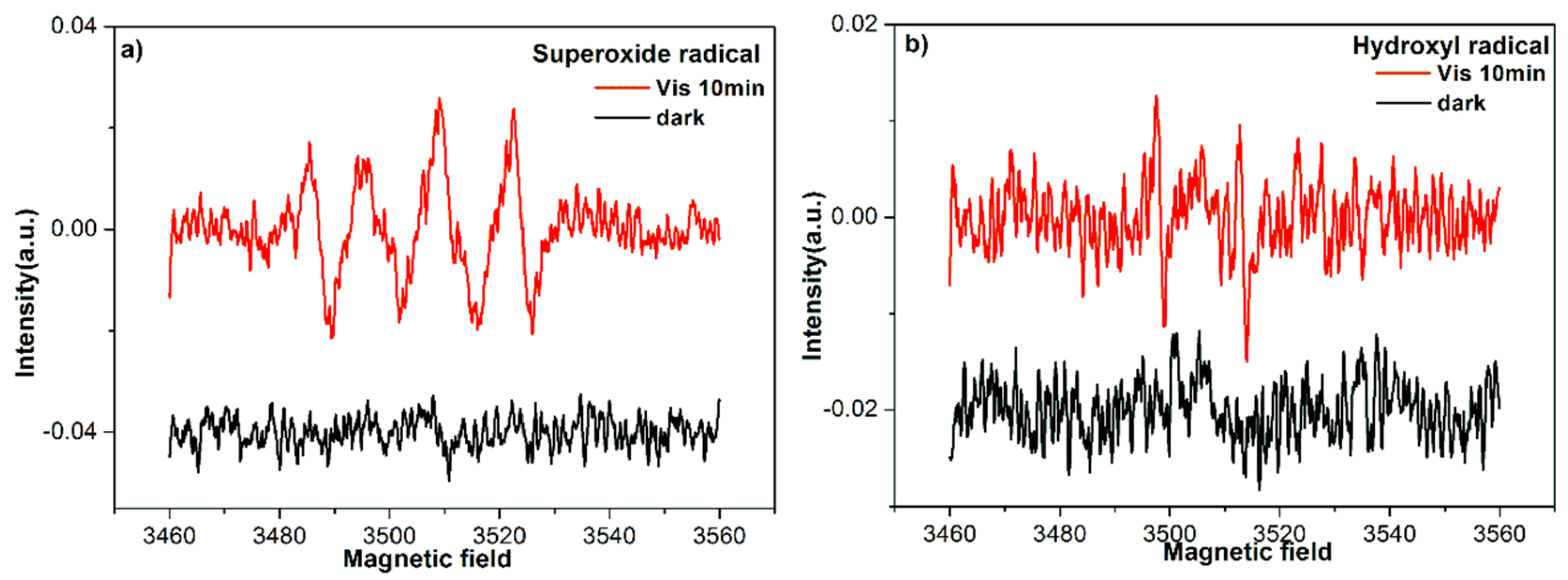
3.7. Electron Spin Resonance Spectra
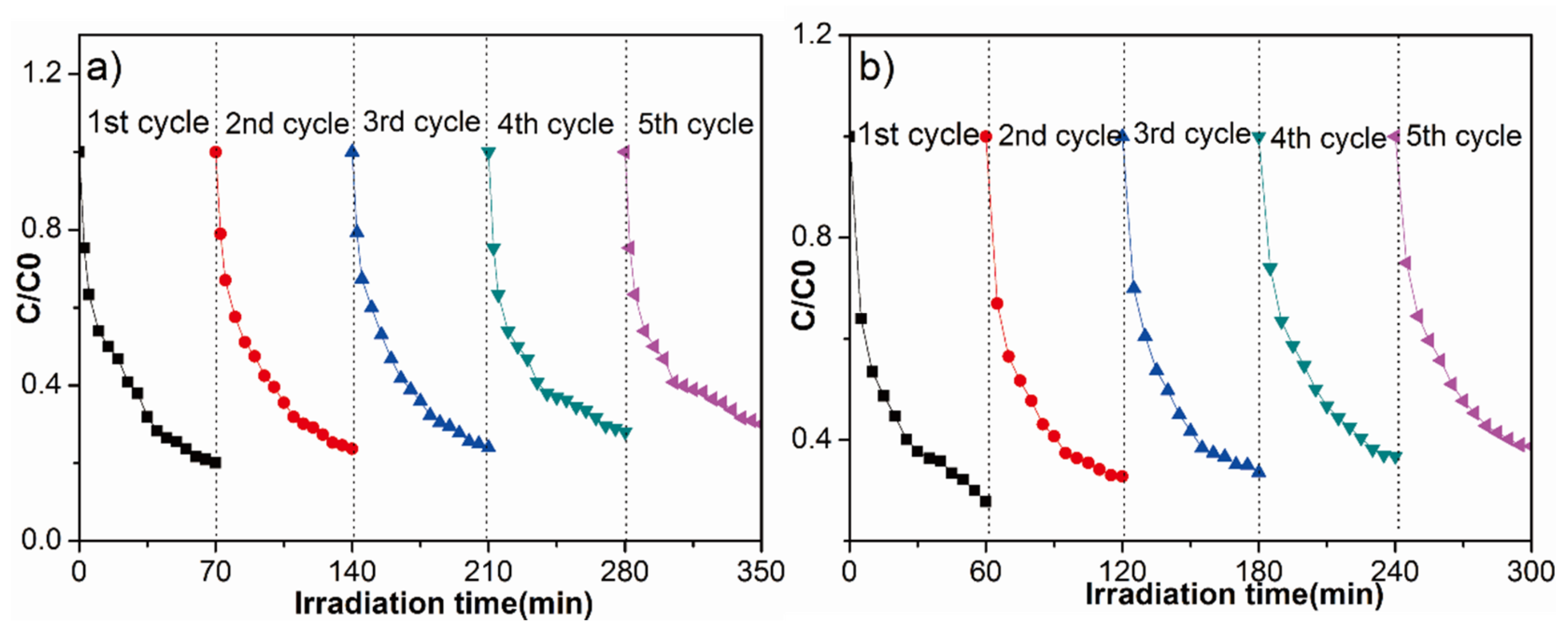
3.8. Photocatalytic Activity
3.9. Mechanism Discussion
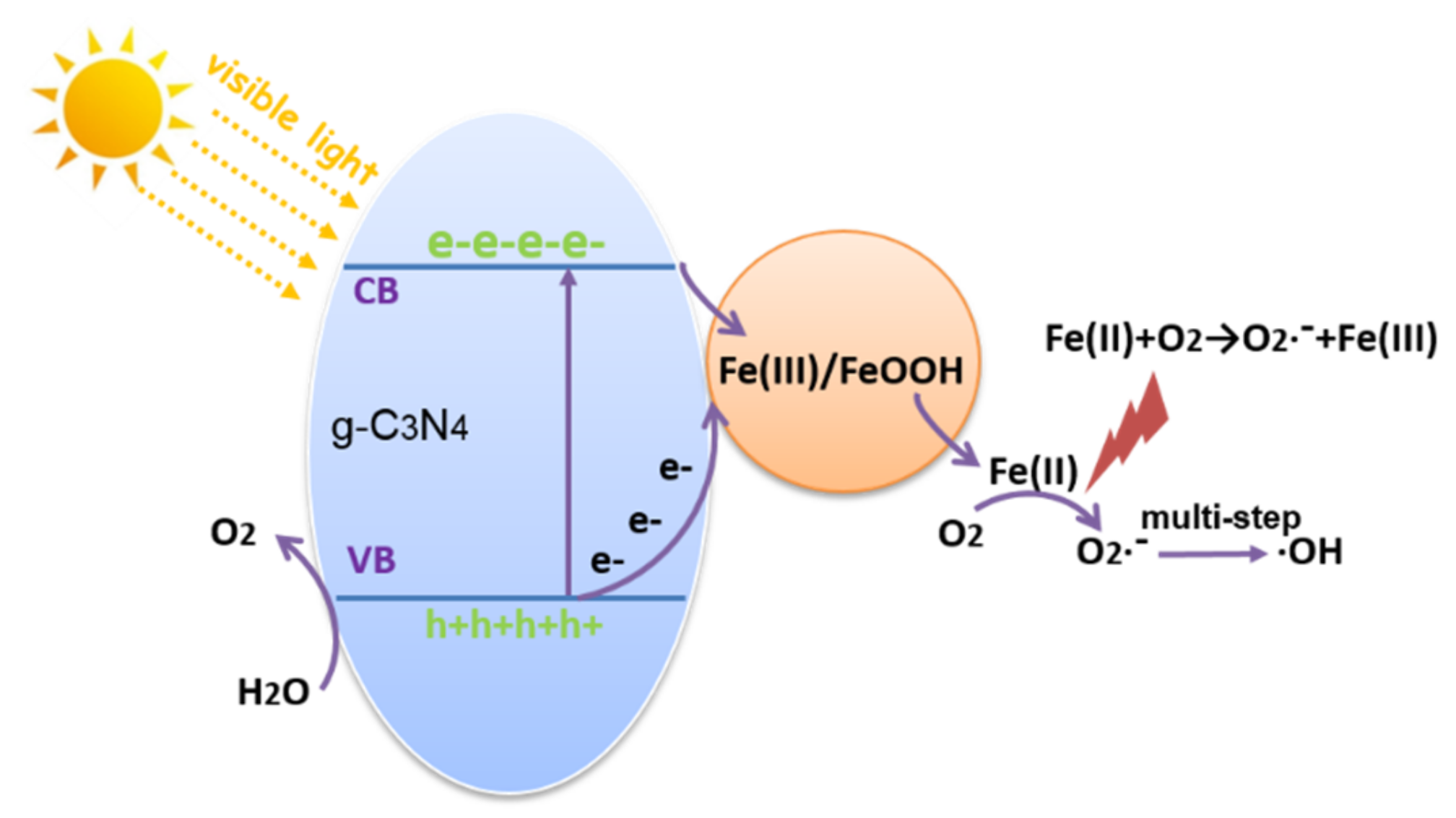
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B -Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Chen, D.; Ye, J. Hierarchical WO3 hollow shells: Dendrite, sphere, dumbbell, and their photocatalytic properties. Adv. Funct. Mater. 2008, 18, 1922–1928. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Yang, L. ZnO–SnO2 hollow spheres and hierarchical nanosheets: Hydrothermal preparation, formation mechanism, and photocatalytic properties. Adv. Funct. Mater. 2007, 17, 59–64. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Chen, B.; Zhang, M.; Guo, Z.; Zhang, P.; Zhang, Z.; Sun, Y.; Shao, C.; Liu, Y. Enhancement of the visible-light photocatalytic activity of In2O3–TiO2 nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 4, 424–430. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Sun, C. Highly efficient Z-Scheme Ag3PO4/Ag/WO3−x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B Environ. 2015, 179, 363–371. [Google Scholar] [CrossRef]
- Chen, J.-B.; Li, K.-N.; Li, X.-F.; Fan, J.-J.; LÜ, K.-L. Preparation and Modification of Crystalline Carbon Nitride. Chinese J. Inorg. Chem. 2021, 37, 1713–1726. [Google Scholar]
- Chen, J.; Kang, N.; Fan, J.; Lu, C.; Lv, K. Carbon nitride for photocatalytic water splitting to produce hydrogen and hydrogen peroxide. Mater. Today Chem. 2022, 26, 101028. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Ou, X.; Li, R.; Li, Q.; Fan, J.; Lv, K. Strategies for the Fabrication of 2D Carbon Nitride Nanosheets. Acta Phys.-Chim. Sin. 2021, 37, 2008010. [Google Scholar] [CrossRef]
- Li, K.; Zhou, W.; Li, X.; Li, Q.; Carabineiro, S.A.C.; Zhang, S.; Fan, J.; Lv, K. Synergistic effect of cyano defects and CaCO3 in graphitic carbon nitride nanosheets for efficient visible-light-driven photocatalytic NO removal. J. Hazard. Mater. 2023, 442, 130040. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.-K.; Lee, S. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.-M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Chen, J.; Fang, S.; Shen, Q.; Fan, J.; Li, Q.; Lv, K. Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques. Catalysts 2022, 12, 962. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Wang, Y.; Li, D.; Zhu, Y. Dramatic Activity of C3N4/BiPO4 Photocatalyst with Core/Shell Structure Formed by Self-Assembly. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2011, 108, 100–107. [Google Scholar] [CrossRef]
- Wang, J.-C.; Cui, C.-X.; Li, Y.; Liu, L.; Zhang, Y.-P.; Shi, W. Porous Mn doped g-C3N4 photocatalysts for enhanced synergetic degradation under visible-light illumination. J. Hazard. Mater. 2017, 339, 43–53. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-Containing Carbon Nitride Compounds: A New Functional Organic-Metal Hybrid Material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.; Guo, Y.; Zhang, Z.; Fang, X. Grafting Fe(III) species on carbon nanodots/Fe-doped g-C3N4 via interfacial charge transfer effect for highly improved photocatalytic performance. Appl. Catal. B Environ. 2017, 205, 173–181. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; Miyauchi, M.; Hashimoto, K. An Efficient Visible-Light-Sensitive Fe(III)-Grafted TiO2 Photocatalyst. J. Phys. Chem. C 2010, 114, 16481–16487. [Google Scholar] [CrossRef]
- Lu, Z.; Song, W.; Ouyang, C.; Wang, H.; Zeng, D.; Xie, C. Enhanced visible-light photocatalytic performance of highly-dispersed Pt/g-C3N4 nanocomposites by one-step solvothermal treatment. RSC Adv. 2017, 7, 33552–33557. [Google Scholar] [CrossRef]
- Xia, P.; Liu, M.; Cheng, B.; Yu, J.; Zhang, L. Dopamine Modified g-C3N4 and Its Enhanced Visible-Light Photocatalytic H2-Production Activity. ACS Sustain. Chem. Eng. 2018, 6, 8945–8953. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Sun, Y.; Zhu, J.; Li, F.; Mu, H.; Zhao, J. Enhanced Schottky effect of 2D-2D CoP/g-C3N4 interface for boosting photocatalytic H2 evolution. Nanoscale 2018, 10, 12315–12321. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Xue, J.; Peng, X.; Li, S.; Bi, Q.; Tang, C.; Zhang, L. Oxalate enhanced synergistic removal of chromium(VI) and arsenic(III) over ZnFe2O4/g-C3N4: Z-scheme charge transfer pathway and photo-Fenton like reaction. Appl. Catal. B -Environ. 2021, 282, 119578. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Zhong, H.; Zhu, Z.; Chen, W. In situ stable growth of β-FeOOH on g-C3N4 for deep oxidation of emerging contaminants by photocatalytic activation of peroxymonosulfate under solar irradiation. Chem. Eng. J. 2020, 400, 125872. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Song, W.; Liu, M. Enhanced Visible-Light-Driven Photocatalytic Activity by Fe(Ш)-Doped Graphitic C3N4. Molecules 2022, 27, 6986. https://doi.org/10.3390/molecules27206986
Lu Z, Song W, Liu M. Enhanced Visible-Light-Driven Photocatalytic Activity by Fe(Ш)-Doped Graphitic C3N4. Molecules. 2022; 27(20):6986. https://doi.org/10.3390/molecules27206986
Chicago/Turabian StyleLu, Zhao, Wulin Song, and Minghao Liu. 2022. "Enhanced Visible-Light-Driven Photocatalytic Activity by Fe(Ш)-Doped Graphitic C3N4" Molecules 27, no. 20: 6986. https://doi.org/10.3390/molecules27206986
APA StyleLu, Z., Song, W., & Liu, M. (2022). Enhanced Visible-Light-Driven Photocatalytic Activity by Fe(Ш)-Doped Graphitic C3N4. Molecules, 27(20), 6986. https://doi.org/10.3390/molecules27206986



