Anti-Adipogenic Effects of Salicortin from the Twigs of Weeping Willow (Salix pseudolasiogyne) in 3T3-L1 Cells
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Compounds
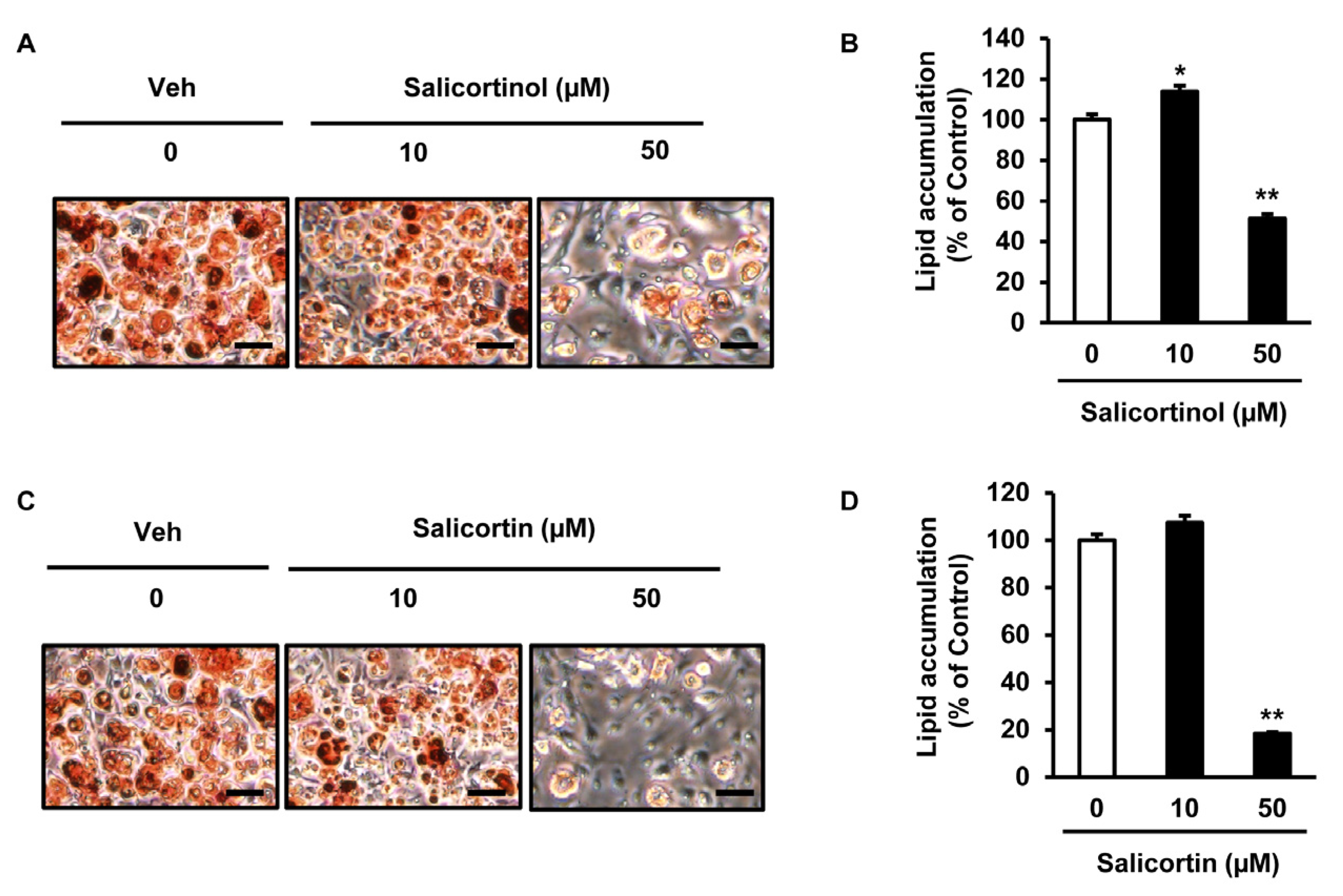
2.2. Effects of the Isolated Compounds on Lipid Accumulation
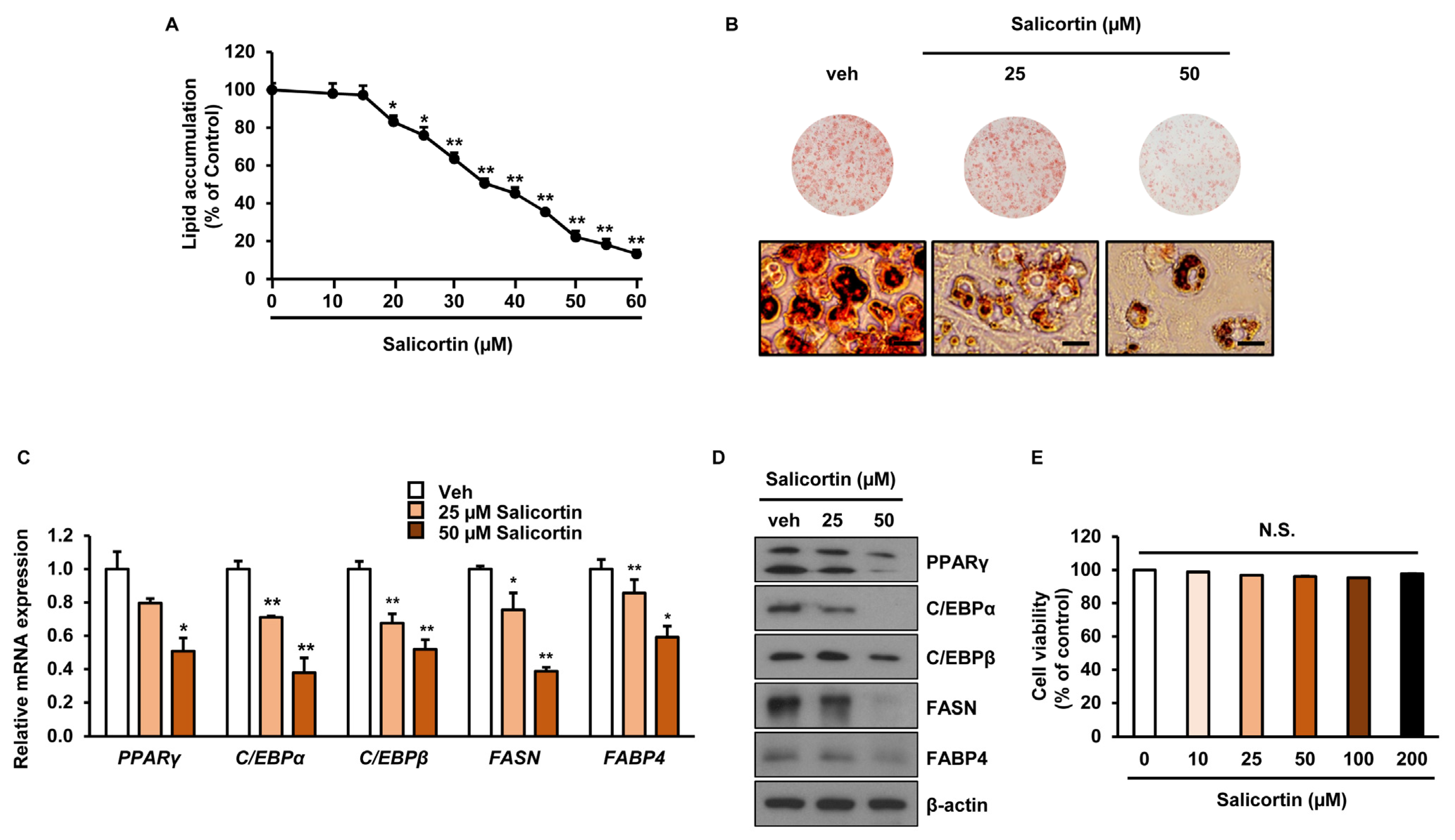
2.3. Salicortin Suppresses Adipogenesis by Attenuating the Expression of Lipogenic and Adipogenic Transcription Factors
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Plant Extraction and Isolation of Compounds
4.4. Compounds Treatment
4.5. Adipocyte Differentiation and Maintenance
4.6. Oil Red O Staining
4.7. Extraction of Proteins and Western Blotting
4.8. Isolation of RNA and Quantitative Polymerase Chain Reaction (qPCR)
4.9. Statistical Analysis
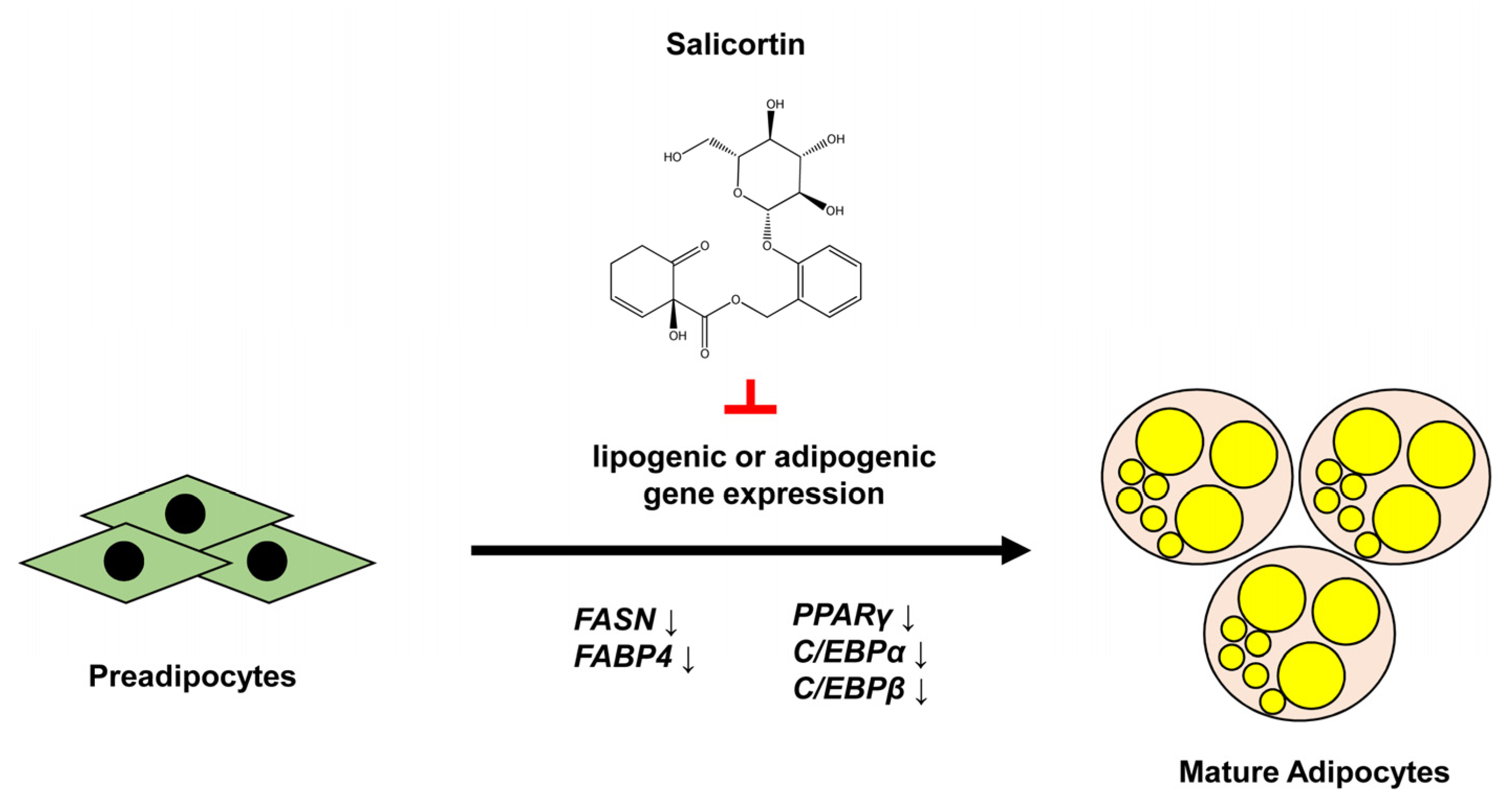
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, S.D. Metabolic complications of obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.P.; Manca di Villahermosa, S.; Martino, G.; Rovella, V.; Noce, A.; De Lorenzo, A.; Di Daniele, N. Obesity-Related Metabolic Syndrome: Mechanisms of Sympathetic Overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Reuss, L.; Wang, Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARgamma Expression and/or Its Transcriptional Activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Colin-Ramirez, E.; Field, C.J.; Madsen, K.L.; Prado, C.M.; Haqq, A.M. Adipose Tissue Development and Expansion from the Womb to Adolescence: An Overview. Nutrients 2020, 12, 2735. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.; Lee, H.W.; Lee, Y.S.; Kim, J.B. Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem. Biophys. Res. Commun. 2004, 321, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.-O.; Choi, I.-Y.; Cho, J.-H.; Shin, H.-M.; Cho, H.-W. Anti-melanogenesis and anti-oxidant of Salix pseudo-lasiogyne water extract in α-MSH-induced B16F10 melanoma cells. Food Agric. Immunol. 2017, 28, 1003–1016. [Google Scholar] [CrossRef]
- Kyeongshik, K.; Euishik, J. Ferns, Fern-Allies and Seed-Bearing Plants of Korea; Iljinsa Seoul: Seoul, Korea, 2003. [Google Scholar]
- Noleto-Dias, C.; Ward, J.L.; Bellisai, A.; Lomax, C.; Beale, M.H. Salicin-7-sulfate: A new salicinoid from willow and implications for herbal medicine. Fitoterapia 2018, 127, 166–172. [Google Scholar] [CrossRef]
- Mahdi, J.G.; Mahdi, A.J.; Mahdi, A.J.; Bowen, I.D. The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential. Cell Prolif. 2006, 39, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.H.; Kang, J.; Yang, H.; Jeong, E.J.; Kim, H.P.; Kim, Y.C.; Sung, S.H. Salicortin-derivatives from Salix pseudo-lasiogyne twigs inhibit adipogenesis in 3T3-L1 cells via modulation of C/EBPalpha and SREBP1c dependent pathway. Molecules 2013, 18, 10484–10496. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic Pregnane Steroid from a Hydractinia-associated Fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharmacal Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An Important Source of Natural Bioactive Compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal Phenols from Woodfordia uniflora Collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.C.; Ko, Y.J.; Kim, S.H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharmacal Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a Styrylpyrone-Fused Steroid with a Hexacyclic 6/5/6/6/6/5 Skeleton from a Mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef]
- Wei, W.; Rena, K.; Yang, X.W. New salicin derivatives from the leaves of Populus euphratica. J. Asian Nat. Prod. Res. 2015, 17, 491–496. [Google Scholar] [CrossRef]
- Feistel, F.; Paetz, C.; Lorenz, S.; Schneider, B. The absolute configuration of salicortin, HCH-salicortin and tremulacin from Populus trichocarpa × deltoids Beaupre. Molecules 2015, 20, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural products in anti-obesity therapy. Nat. Prod. Rep. 2011, 28, 1493–1533. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Martineau, L.C.; Muhammad, A.; Saleem, A.; Herve, J.; Harris, C.S.; Arnason, J.T.; Haddad, P.S. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, part II: Bioassay-guided identification of actives salicortin and oregonin. Planta Med. 2010, 76, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, H.H.; Lee, J.K.; Ye, S.K.; Kim, S.H.; Sung, S.H. Anti-adipogenic activity of compounds isolated from Idesia polycarpa on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013, 23, 3170–3174. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.S.; Kim, Y.K.; Ryu, J.H. Phenolic glycosides as inhibitors of inducible nitric oxide synthase from Populus davidiana in LPS-activated RAW 264.7 murine macrophages. Die Pharm. 2012, 67, 870–873. [Google Scholar]
- Knuth, S.; Schubel, H.; Hellemann, M.; Jurgenliemk, G. Catechol, a bioactive degradation product of salicortin, reduces TNF-alpha induced ICAM-1 expression in human endothelial cells. Planta Med. 2011, 77, 1024–1026. [Google Scholar] [CrossRef]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-kappaB and JNK activation in RAW 264.7 macrophages. BMB Rep. 2014, 47, 318–323. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Im, D.U.; Kim, S.C.; Chau, G.C.; Um, S.H. Carbamazepine Enhances Adipogenesis by Inhibiting Wnt/beta-catenin Expression. Cells 2019, 8, 1460. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, D.U.; Chau, G.C.; Mishra, N.K.; Kim, I.S.; Um, S.H. Novel anti-adipogenic effect of CF3-allylated indole in 3T3-L1 cells. Chem. Biol. Interact. 2022, 352, 109782. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.S.; Chau, G.C.; Baek, K.H.; Um, S.H. A single extra copy of Down syndrome critical region 1–4 results in impaired hepatic glucose homeostasis. Mol. Metab. 2019, 21, 82–89. [Google Scholar] [CrossRef]
- Chau, G.C.; Im, D.U.; Kang, T.M.; Bae, J.M.; Kim, W.; Pyo, S.; Moon, E.Y.; Um, S.H. mTOR controls ChREBP transcriptional activity and pancreatic beta cell survival under diabetic stress. J. Cell Biol. 2017, 216, 2091–2105. [Google Scholar] [CrossRef] [PubMed]


| Name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| PPARγ | GGGTGAAACTCTGGGAGATTCTCC | CAGCAACCATTGGGTCAGCTCT |
| C/EBPα | ACAACATCGCGGTGCGCAAGA | TGCCATGGCCTTGACCAAGGAG |
| C/EBPβ | GTCCAAACCAACCGCACAT | CAGAGGGAGAAGCAGAGAGTT |
| FASN | CGGAAACTGCAGGAGCTGTC | CACGGAGTTGAGCCGCAT |
| FABP4 | TGGAAGCTTGTCTCCAGTGA | AATCCCCATTTACGCTGATG |
| GAPDH | GTCTTCCTGGGCAAGCAGTA | CTGGACAGAAACCCCACTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Lee, D.E.; Park, E.C.; Ra, M.-J.; Jung, S.-M.; Yu, J.-N.; Um, S.H.; Kim, K.H. Anti-Adipogenic Effects of Salicortin from the Twigs of Weeping Willow (Salix pseudolasiogyne) in 3T3-L1 Cells. Molecules 2022, 27, 6954. https://doi.org/10.3390/molecules27206954
Kim HJ, Lee DE, Park EC, Ra M-J, Jung S-M, Yu J-N, Um SH, Kim KH. Anti-Adipogenic Effects of Salicortin from the Twigs of Weeping Willow (Salix pseudolasiogyne) in 3T3-L1 Cells. Molecules. 2022; 27(20):6954. https://doi.org/10.3390/molecules27206954
Chicago/Turabian StyleKim, Hee Jung, Da Eun Lee, Eon Chung Park, Moon-Jin Ra, Sang-Mi Jung, Jeong-Nam Yu, Sung Hee Um, and Ki Hyun Kim. 2022. "Anti-Adipogenic Effects of Salicortin from the Twigs of Weeping Willow (Salix pseudolasiogyne) in 3T3-L1 Cells" Molecules 27, no. 20: 6954. https://doi.org/10.3390/molecules27206954
APA StyleKim, H. J., Lee, D. E., Park, E. C., Ra, M.-J., Jung, S.-M., Yu, J.-N., Um, S. H., & Kim, K. H. (2022). Anti-Adipogenic Effects of Salicortin from the Twigs of Weeping Willow (Salix pseudolasiogyne) in 3T3-L1 Cells. Molecules, 27(20), 6954. https://doi.org/10.3390/molecules27206954







