Fabrication and Characterization of Chitosan–Pea Protein Isolate Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CS–PPI Nanoparticles Preparation
2.3. Particle Size, Polydispersity Index and Zeta Potential Determination
2.4. Zeta Potential of CS–PPI Nanoparticles at Different pH
2.5. Turbidity
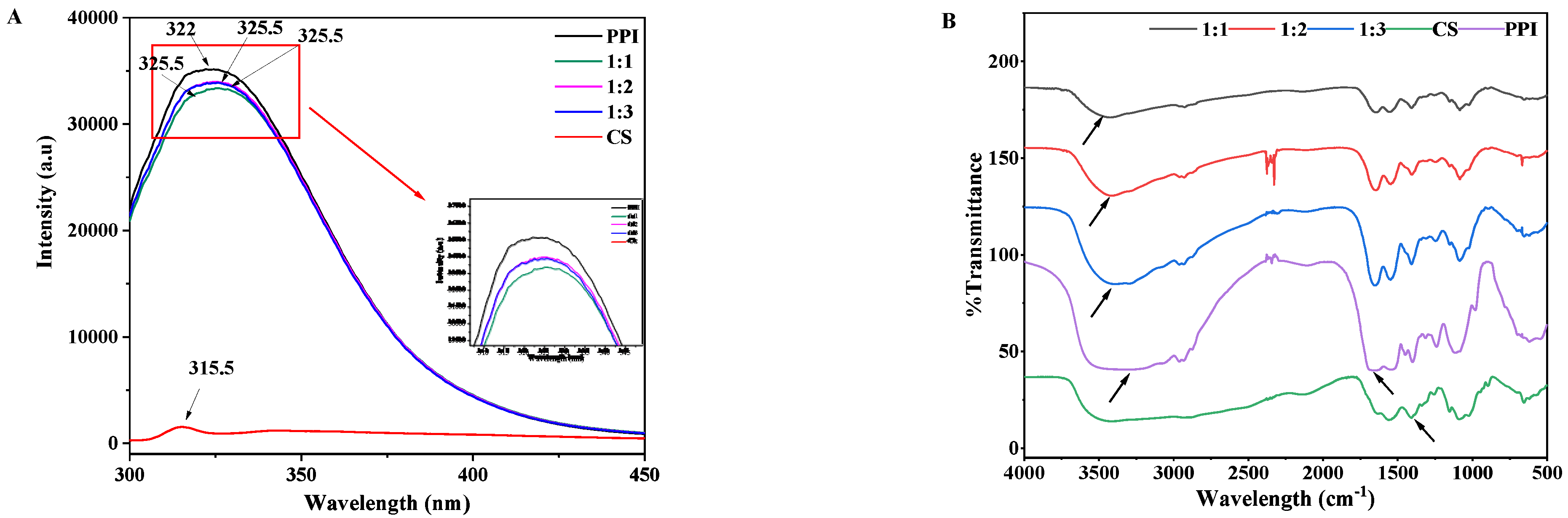
2.6. Endogenous Fluorescence Spectroscopy
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Interface Properties
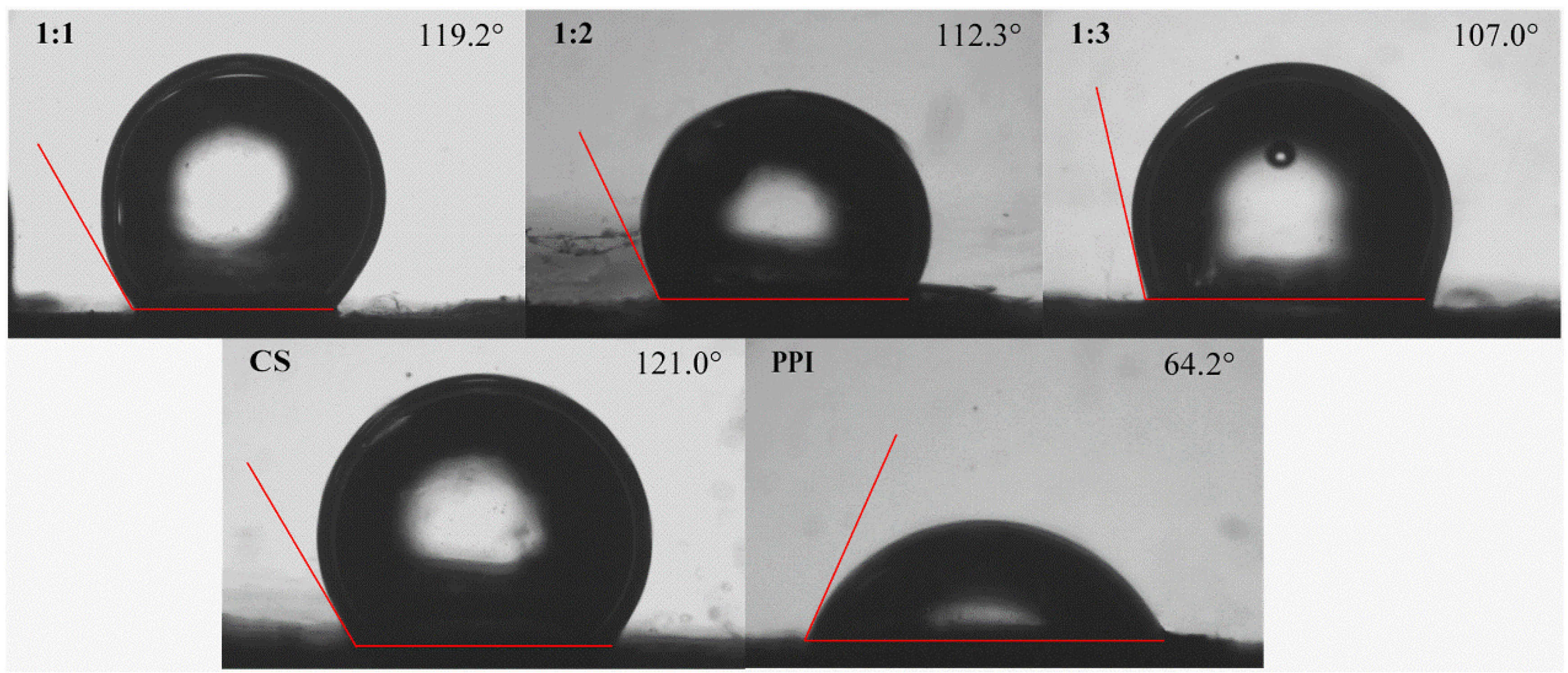
2.8.1. Wettability
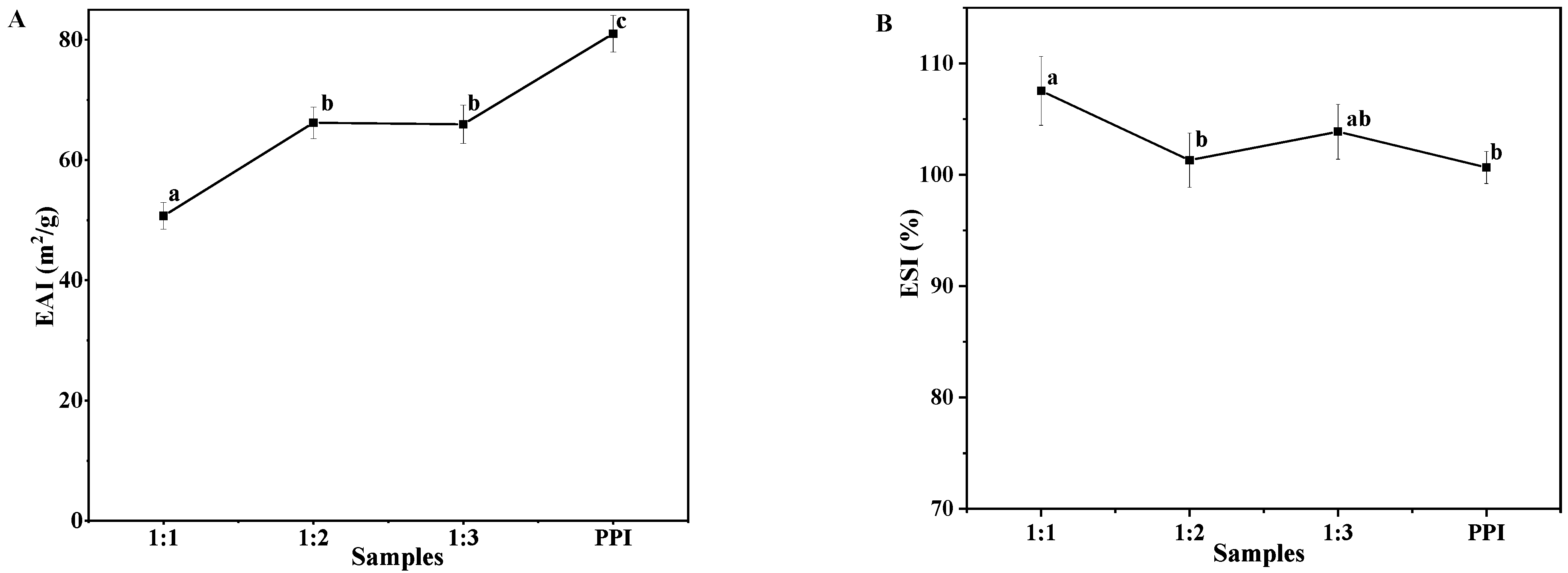
2.8.2. Emulsifying Properties
2.9. Thermal Stability
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Particle Size, PDI and Zeta Potential
3.2. Zeta Potential of CS–PPI Nanoparticles at Different pH
3.3. Turbidity
3.4. Endogenous Fluorescence Spectroscopy
3.5. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
3.6. Interface Properties
3.6.1. Wettability
3.6.2. Emulsifying Properties
3.7. Thermal Stability
3.8. Transmission Electron Microscopy (TEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Dong, D.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Soybean whey protein/chitosan complex behavior and selective recovery of kunitz trypsin inhibitor. J. Agric. Food Chem. 2014, 62, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, H. Characterization of the interactions between chitosan/whey protein at different conditions. Food Sci. Technol. 2019, 39, 163–169. [Google Scholar] [CrossRef]
- Okagu, O.D.; Jin, J.; Udenigwe, C.C. Impact of succinylation on pea protein-curcumin interaction, polyelectrolyte complexation with chitosan, and gastrointestinal release of curcumin in loaded-biopolymer nano-complexes. J. Mol. Liq. 2021, 325, 115248. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.; Yuan, F.; Mao, L.; Gao, Y. Preparation, characterization and stability of pea protein isolate and propylene glycol alginate soluble complexes. LWT Food Sci. Technol. 2019, 101, 476–482. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Cosson, A.; Oliveira Correia, L.; Descamps, N.; Saint-Eve, A.; Souchon, I. Identification and characterization of the main peptides in pea protein isolates using ultra high-performance liquid chromatography coupled with mass spectrometry and bioinformatics tools. Food Chem. 2022, 367, 130747. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Su, J.; Xie, W.; Tu, X.; Yuan, F.; Mao, L.; Gao, Y. Curcumin-loaded pea protein isolate-high methoxyl pectin complexes induced by calcium ions: Characterization, stability and in vitro digestibility. Food Hydrocoll. 2020, 98, 105284. [Google Scholar] [CrossRef]
- Sun, C.; Wu, T.; Liu, R.; Liang, B.; Tian, Z.; Zhang, E.; Zhang, M. Effects of superfine grinding and microparticulation on the surface hydrophobicity of whey protein concentrate and its relation to emulsions stability. Food Hydrocoll. 2015, 51, 512–518. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar] [CrossRef]
- Yuan, Y.; Wan, Z.-L.; Yin, S.-W.; Teng, Z.; Yang, X.-Q.; Qi, J.-R.; Wang, X.-Y. Formation and dynamic interfacial adsorption of glycinin/chitosan soluble complex at acidic pH: Relationship to mixed emulsion stability. Food Hydrocoll. 2013, 31, 85–93. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Jiang, W.; Zhao, H.; Fu, J. Ability of casein hydrolysate-carboxymethyl chitosan conjugates to stabilize a nanoemulsion: Improved freeze-thaw and pH stability. Food Hydrocoll. 2020, 101, 105452. [Google Scholar] [CrossRef]
- Setiowati, A.D.; Saeedi, S.; Wijaya, W.; Van der Meeren, P. Improved heat stability of whey protein isolate stabilized emulsions via dry heat treatment of WPI and low methoxyl pectin: Effect of pectin concentration, pH, and ionic strength. Food Hydrocoll. 2017, 63, 716–726. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Jiang, W.; Du, Q.; Yu, C.; Sun, Q.; Zhang, S. An Enhanced Stability Nanoparticle Preparation by Corn Protein Hydrolysate-Carboxymethyl Chitosan Maillard Conjugates Loaded with Rutin. J. Food Sci. 2019, 84, 1829–1835. [Google Scholar] [CrossRef]
- Xu, W.; Lv, K.; Mu, W.; Zhou, S.; Yang, Y. Encapsulation of α-tocopherol in whey protein isolate/chitosan particles using oil-in-water emulsion with optimal stability and bioaccessibility. LWT Food Sci. Technol. 2021, 148, 111724. [Google Scholar] [CrossRef]
- Ji, Y.; Han, C.; Liu, E.; Li, X.; Meng, X.; Liu, B. Pickering emulsions stabilized by pea protein isolate-chitosan nanoparticles: Fabrication, characterization and delivery EPA for digestion in vitro and in vivo. Food Chem. 2022, 378, 132090. [Google Scholar] [CrossRef]
- Jiang, S.S.; Hussain, M.A.; Cheng, J.J.; Jiang, Z.M.; Geng, H.; Sun, Y.; Sun, C.B.; Hou, J.C. Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate. LWT Food Sci. Technol. 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of hyssop essential oil in chitosan-pea protein isolate nano-complex. LWT Food Sci. Technol. 2021, 144, 111254. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release Off. J. Control. Release Soc. 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, W.N. Micro/nano emulsion delivery systems: Effects of potato protein/chitosan complex on the stability, oxidizability, digestibility and beta-carotene release characteristics of the emulsion. Innov. Food Sci. Emerg. Technol. 2022, 77, 102980. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.Q.; Lin, M.G.; Zhao, R.Y.; Tang, C.H.; Luo, L.; Liu, L. Complex coacervation of chitosan and soy globulins in aqueous solution: A electrophoretic mobility and light scattering study. Int. J. Food Sci. Technol. 2011, 46, 1363–1369. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, H.M.; Gao, J.; Chen, L.Y.; Vasanthan, T. Field pea protein isolate/chitosan complex coacervates: Formation and characterization. Carbohydr. Polym. 2020, 250, 116925. [Google Scholar] [CrossRef]
- Yang, S.N.; Li, X.F.; Hua, Y.F.; Chen, Y.M.; Kong, X.Z.; Zhang, C.M. Selective Complex Coacervation of Pea Whey Proteins with Chitosan to Purify Main 2S Albumins. J. Agric. Food Chem. 2020, 68, 1698–1706. [Google Scholar] [CrossRef]
- Elmer, C.; Karaca, A.C.; Low, N.H.; Nickerson, M.T. Complex coacervation in pea protein isolate-chitosan mixtures. Food Res. Int. 2011, 44, 1441–1446. [Google Scholar] [CrossRef]
- Chao, D.; Jung, S.; Aluko, R.E. Physicochemical and functional properties of high pressure-treated isolated pea protein. Innov. Food Sci. Emerg. Technol. 2018, 45, 179–185. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, X.M.; Liang, Y.R.; He, M.Y.; Geng, M.J.; Huang, Y.Y.; Teng, F.; Li, Y. Effects of electron beam irradiation pretreatment on the structural and functional properties of okara protein. Innov. Food Sci. Emerg. Technol. 2022, 79, 103049. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.Q.; Shu, X.; Yuan, F.; Mao, L.K.; Gao, Y.X. Development of high methoxyl pectin-surfactant-pea protein isolate ternary complexes: Fabrication, characterization and delivery of resveratrol. Food Chem. 2020, 321, 126706. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.T.; Wu, X.L. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for beta-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, M.H.; Chiou, Y.S.; Li, Z.S.; Wei, S.D.; Yin, X.L.; Ding, B.M. Insights from alpha-Lactoalbumin and beta-Lactoglobulin into mechanisms of nanoliposome-whey protein interactions. Food Hydrocoll. 2022, 125, 107436. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.G.; Li, Y.; Yang, Y.J.; Ju, X.R.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Liu, J.; Gao, Y. Fabrication, structural characterization and functional attributes of polysaccharide-surfactant-protein ternary complexes for delivery of curcumin. Food Chem. 2021, 337, 128019. [Google Scholar] [CrossRef]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Luo, Y.; Wang, Q. Carboxymethyl chitosan-soy protein complex nanoparticles for the encapsulation and controlled release of vitamin D(3). Food Chem. 2013, 141, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.S.; Luo, Z.G.; Peng, X.C. Fabrication and Characterization of Quinoa Protein Nanoparticle-Stabilized Food-Grade Pickering Emulsions with Ultrasound Treatment: Interfacial Adsorption/Arrangement Properties. J. Agric. Food Chem. 2018, 66, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Linke, C.; Drusch, S. Pickering emulsions in foods—Opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 1971–1985. [Google Scholar] [CrossRef]
- Zhou, F.-Z.; Huang, X.-N.; Wu, Z.-l.; Yin, S.-W.; Zhu, J.-h.; Tang, C.-H.; Yang, X.-Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, H.N.; Sun, S.; Yu, C.P. The interaction between sodium alginate and myofibrillar proteins: The rheological and emulsifying properties of their mixture. Int. J. Biol. Macromol. 2020, 161, 1545–1551. [Google Scholar] [CrossRef]
- Fan, Y.T.; Peng, G.F.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT Food Sci. Technol. 2021, 149, 111904. [Google Scholar] [CrossRef]
- Mession, J.-L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Turner, E.M.; Sapkota, K.R.; Hatem, C.; Lu, P.; Wang, G.T.; Jones, K.S. Wet-chemical etching of FIB lift-out TEM lamellae for damage-free analysis of 3-D nanostructures. Ultramicroscopy 2020, 216, 113049. [Google Scholar] [CrossRef]



| Samples | Effective Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| PPI | 229.32 ± 4.59 d | 0.340 ± 0.013 ab | −15.86 ± 0.64 f |
| CS | 786.06 ± 10.80 c | 0.376 ± 0.081 a | 38.77 ± 0.53 b |
| 1:1 | 802.95 ± 71.94 bc | 0.337 ± 0.061 ab | 45.74 ± 0.56 a |
| 1:2 | 807.10 ± 86.22 c | 0.308 ± 0.040 ab | 36.82 ± 0.59 c |
| 1:3 | 767.75 ± 110.10 c | 0.311 ± 0.045 ab | 27.58 ± 1.04 d |
| 1:4 | 1108.11 ± 280.50 a | 0.299 ± 0.021 b | 20.58 ± 1.31 e |
| 4:1 | 1042.15 ± 118.00 ab | 0.330 ± 0.036 ab | 46.84 ± 1.11 a |
| 3:1 | 962.76 ± 68.05 ac | 0.348 ± 0.012 ab | 45.75 ± 0.89 a |
| 2:1 | 1119.72 ± 95.84 a | 0.311 ± 0.039 ab | 46.26 ± 0.95 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, Z.; Dai, M.; He, H.; Liang, B.; Sun, C.; Li, X.; Ji, C. Fabrication and Characterization of Chitosan–Pea Protein Isolate Nanoparticles. Molecules 2022, 27, 6913. https://doi.org/10.3390/molecules27206913
Zhang M, Li Z, Dai M, He H, Liang B, Sun C, Li X, Ji C. Fabrication and Characterization of Chitosan–Pea Protein Isolate Nanoparticles. Molecules. 2022; 27(20):6913. https://doi.org/10.3390/molecules27206913
Chicago/Turabian StyleZhang, Man, Zikun Li, Mengqi Dai, Hongjun He, Bin Liang, Chanchan Sun, Xiulian Li, and Changjian Ji. 2022. "Fabrication and Characterization of Chitosan–Pea Protein Isolate Nanoparticles" Molecules 27, no. 20: 6913. https://doi.org/10.3390/molecules27206913
APA StyleZhang, M., Li, Z., Dai, M., He, H., Liang, B., Sun, C., Li, X., & Ji, C. (2022). Fabrication and Characterization of Chitosan–Pea Protein Isolate Nanoparticles. Molecules, 27(20), 6913. https://doi.org/10.3390/molecules27206913








