Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Results
2.1. In Vitro Antibacterial Effect
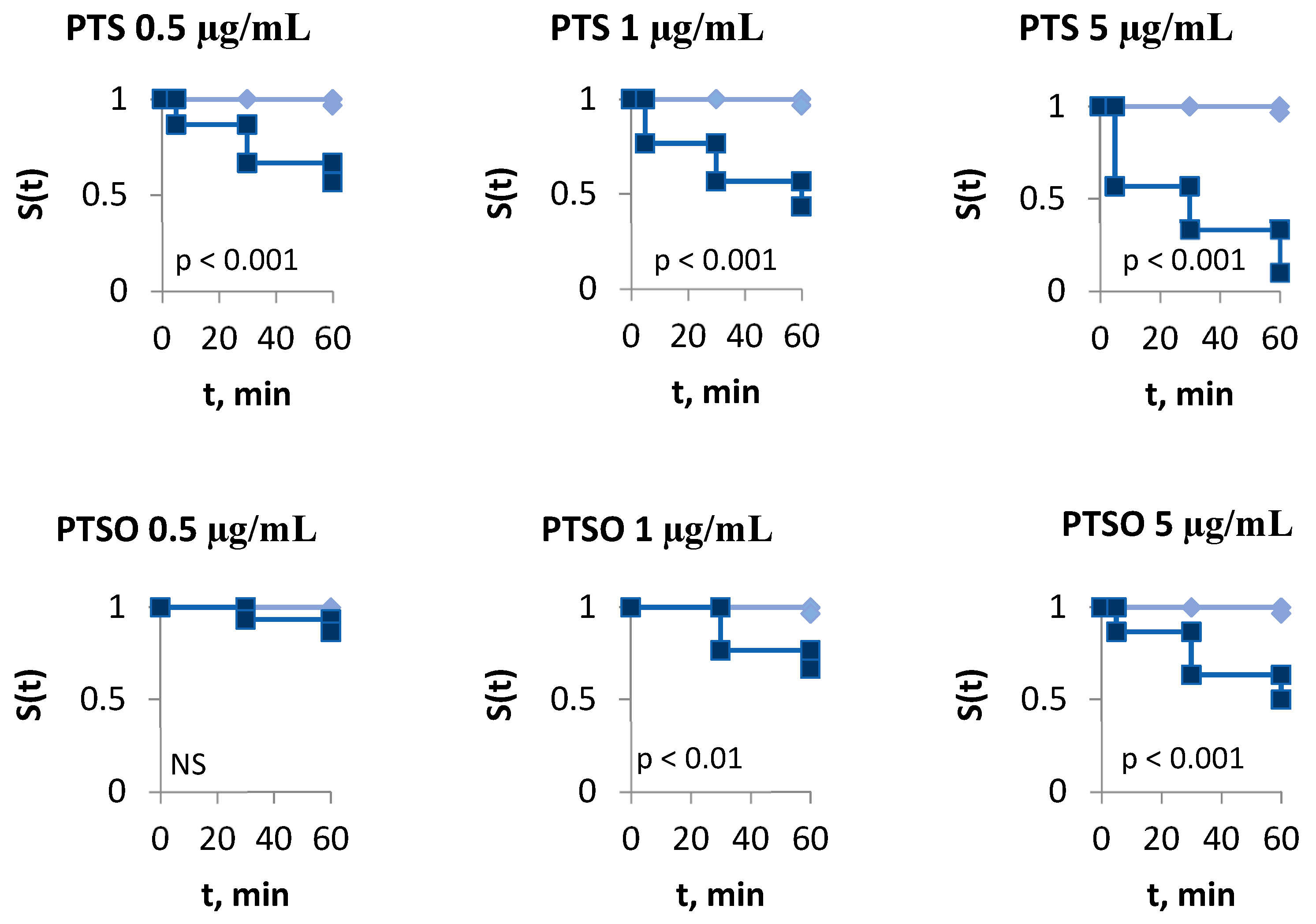
2.2. In Vitro Antiparasitic Effect
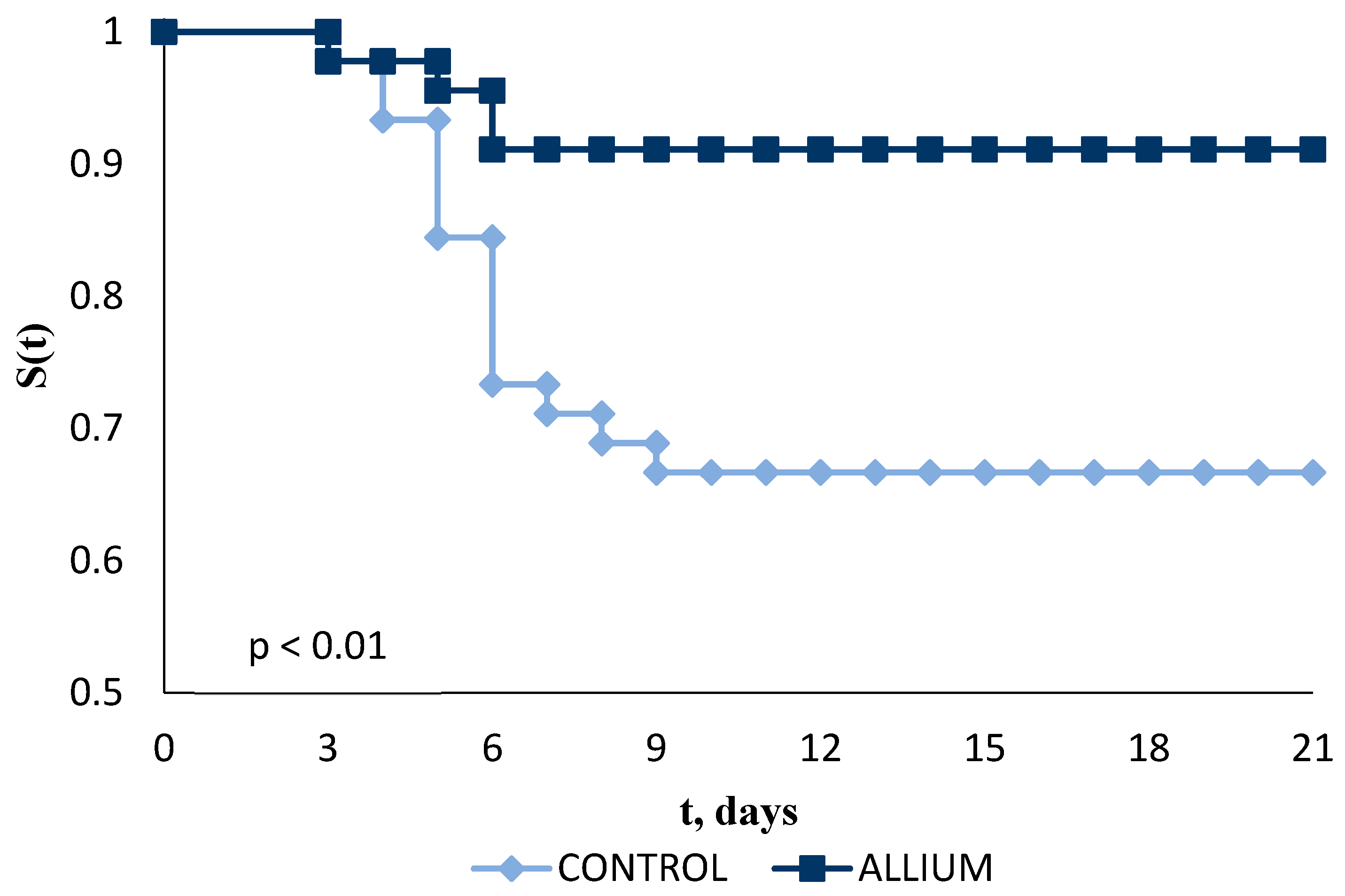
2.3. In Vivo Survival Effect against Photobacterium subsp. Piscicida
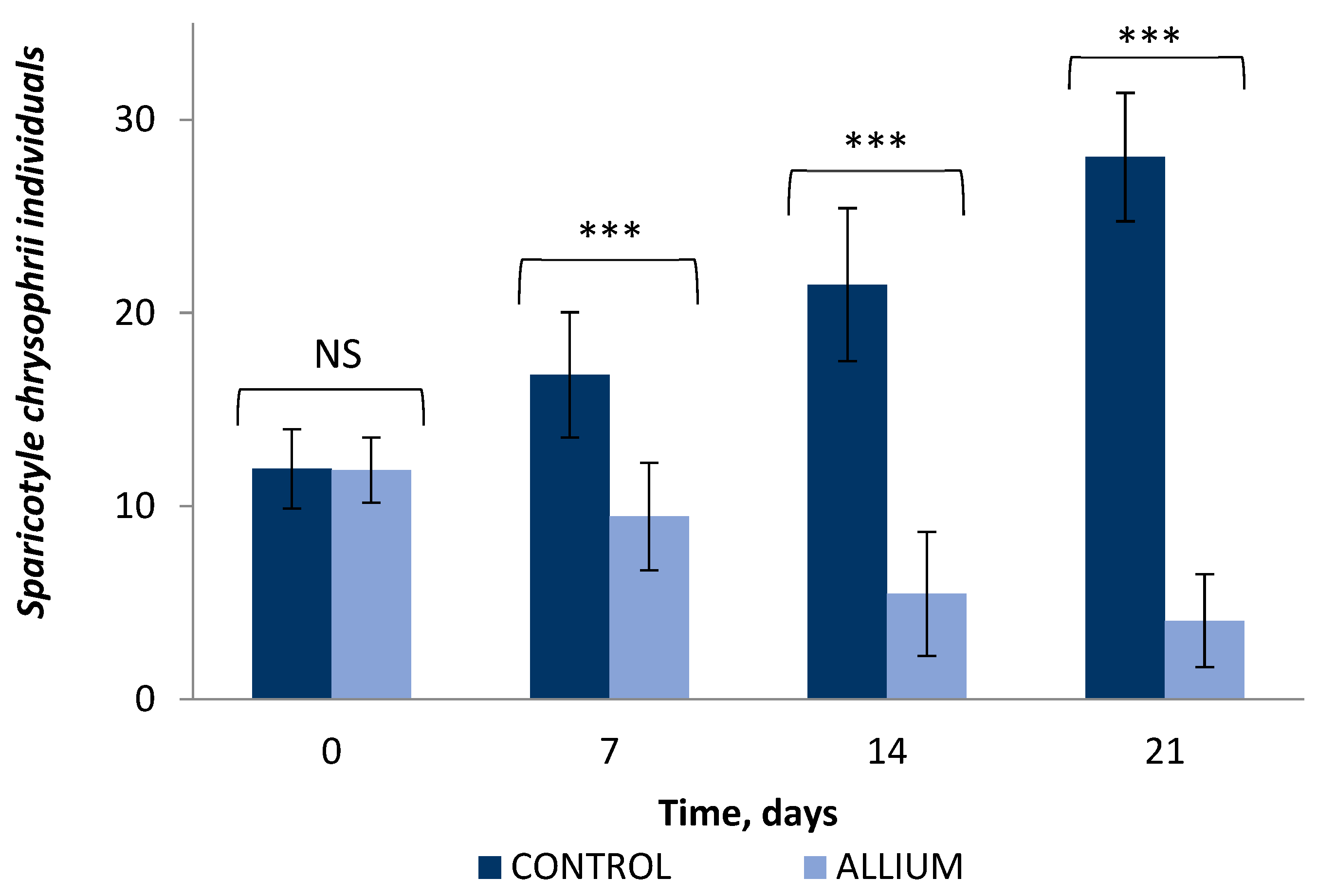
2.4. In Vivo Antiparasitic Effect
3. Discussion
4. Materials and Methods
4.1. Allium Compounds
4.2. Bacterial Strains and Growth Media
4.3. Parasites
4.4. In Vitro Tests
4.4.1. In Vitro Antibacterial Activity
4.4.2. In Vitro Tests for the Evaluation of the Antiparasitic Activity
4.5. In Vivo Challenges
4.5.1. Fish and Experimental Conditions
4.5.2. Diets
4.5.3. Resistance to Experimental Infections against Photobacterium damselae subsp. piscicida
4.5.4. Resistance to Experimental Infections against Sparicotyle chrysophrii
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lucas, J. Aquaculture. Curr. Biol. 2015, 25, R1064–R1065. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thompson, S. The Blue Dimensions of Aquaculture: A Global Synthesis. Sci. Total Environ. 2019, 652, 851–861. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global aquaculture production 1950-2019 (FishstatJ). In FAO Fisheries Division [online]. Rome. Updated 2021. 2021. Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 10 October 2022).
- Pham, T.H.; Cheng, T.C.; Wang, P.C.; Chen, S.C. Genotypic Diversity, and Molecular and Pathogenic Characterization of Photobacterium Damselae Subsp. Piscicida Isolated from Different Fish Species in Taiwan. J. Fish Dis. 2020, 43, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Essam, H.M.; Abdellrazeq, G.S.; Tayel, S.I.; Torky, H.A.; Fadel, A.H. Pathogenesis of Photobacterium Damselae Subspecies Infections in Sea Bass and Sea Bream. Microb. Pathog. 2016, 99, 41–50. [Google Scholar] [CrossRef]
- Antonelli, L.; Quilichini, Y.; Marchand, B. Sparicotyle Chrysophrii (Van Beneden and Hesse 1863) (Monogenea: Polyopisthocotylea) Parasite of Cultured Gilthead Sea Bream Sparus Aurata (Linnaeus 1758) (Pisces: Teleostei) from Corsica: Ecological and Morphological Study. Parasitol. Res. 2010, 107, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Villar-Torres, M.; Montero, F.E.; Raga, J.A.; Repullés-Albelda, A. Come Rain or Come Shine: Environmental Effects on the Infective Stages of Sparicotyle Chrysophrii, a Key Pathogen in Mediterranean Aquaculture. Parasites Vectors 2018, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO/OIE Expert Consultation on Antimicrobial Use in Aquaculture and Antimicrobial Resistance. Available online: https://apps.who.int/iris/handle/10665/133869 (accessed on 17 November 2021).
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to Antibiotics for the Control of Bacterial Disease in Aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of Probiotics in Aquaculture of China—A Review of the Past Decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- Serradell, A.; Torrecillas, S.; Makol, A.; Valdenegro, V.; Fernández-Montero, A.; Acosta, F.; Izquierdo, M.S.; Montero, D. Prebiotics and Phytogenics Functional Additives in Low Fish Meal and Fish Oil Based Diets for European Sea Bass (Dicentrarchus labrax): Effects on Stress and Immune Responses. Fish Shellfish Immunol. 2020, 100, 219–229. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Mikkelsen, L.L.; Sims, I.; Iji, P.A.; Choct, M. Phytobiotics: Alternatives to Antibiotic Growth Promoters in Monogastric Animal Feeds. Recent Adv. Anim. Nutr. Aust. 2005, 15, 131–144. [Google Scholar]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Mnayer, D.; Tabanelli, G.; Stojanovic-Radic, Z.Z.; Sharifi-Rad, M.; Yousaf, Z.; Vallone, L.; Setzer, W.N.; Iriti, M. Plants of the Genus Allium as Antibacterial Agents: From Tradition to Pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar] [PubMed]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic Bioactive Compounds Shape Fish Mucosal Immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; Andreo-Martínez, P.; Mut-Salud, N.; Fonollá, J.; Baños, A. Beneficial Effects of Organosulfur Compounds from Allium Cepa on Gut Health: A Systematic Review. Foods 2021, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Cascajosa-Lira, A.; Prieto Ortega, A.I.; Guzmán-Guillén, R.; Cătunescu, G.M.; de la Torre, J.M.; Guillamón, E.; Jos, Á.; Cameán Fernández, A.M. Simultaneous Determination of Allium Compounds (Propyl Propane Thiosulfonate and Thiosulfinate) in Animal Feed Using UPLC-MS/MS. Food Chem. Toxicol. 2021, 157, 112619. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium Spp. Against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. BioMed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Gálvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to Its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, e1800653. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Arroyo-Manzanares, N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic Derivative Propyl Propane Thiosulfonate Is Effective against Broiler Enteropathogens in Vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Ariza-Romero, J.J.; Zurita-González, M.J.; Martín-Platero, A.M.; Baños, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M.; Peralta-Sánchez, J.M. Allium-Based Phytobiotic Enhances Egg Production in Laying Hens through Microbial Composition Changes in Ileum and Cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; Martín-Platero, A.M.; Casabuena-Rincón, Ó.; Vázquez-Chas, P.; Guillamón, E.; Aguinaga-Casañas, M.A.; Maqueda, M.; et al. Allium Extract Implements Weaned Piglet’s Productive Parameters by Modulating Distal Gut Microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef]
- Sánchez, C.J.; Martínez-Miró, S.; Ariza, J.J.; Madrid, J.; Orengo, J.; Aguinaga, M.A.; Baños, A.; Hernández, F. Effect of Alliaceae Extract Supplementation on Performance and Intestinal Microbiota of Growing-Finishing Pig. Animals 2020, 10, 1557. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved Resistance to Eimeria Acervulina Infection in Chickens Due to Dietary Supplementation with Garlic Metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef]
- Nuñez Lechado, C.; Baños Arjona, A.; Guillamon Ayala, E.; Valero López, A.; Navarro Moll, M.C.; Sanz, A. Use of Propyl Propanethosulfinate and Propyl Propanethosulfonate for the Prevention and Reduction of Parasites in Aquatic Animals. U.S. Patent 9,271,947 B2, 1 March 2016. [Google Scholar]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean Aquaculture in a Changing Climate: Temperature Effects on Pathogens and Diseases of Three Farmed Fish Species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H. Bin Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An Overview of Organosulfur Compounds from Allium Spp.: From Processing and Preservation to Evaluation of Their Bioavailability, Antimicrobial, and Anti-Inflammatory Properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the Garlic (Allium sativum) Properties for Fish Aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef]
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. Phytotherapy as an Alternative for Treating Fish Disease. J. Vet. Pharmacol. Ther. 2015, 38, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Kuo, C.M.; Hong, J.W.; Chou, R.L.; Lee, Y.H.; Chen, T.I. The Effects of Garlic-Supplemented Diets on Antibacterial Activities against Photobacterium Damselae Subsp. Piscicida and Streptococcus Iniae and on Growth in Cobia, Rachycentron Canadum. Aquaculture 2015, 435, 111–115. [Google Scholar] [CrossRef]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The Antioxidant Power of Horseradish, Armoracia Rusticana, Underlies Antimicrobial and Antiradical Effects, Exerted in Vitro. Nat. Prod. Res. 2020, 34, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, J.M.G.; Espinosa, C.; Guardiola, F.A.; Esteban, M.Á. In Vitro Effects of Origanum Vulgare Leaf Extracts on Gilthead Seabream (Sparus aurata L.) Leucocytes, Cytotoxic, Bactericidal and Antioxidant Activities. Fish Shellfish Immunol. 2018, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial Properties of Allicin from Garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The Mode of Action of Allicin: Its Ready Permeability through Phospholipid Membranes May Contribute to Its Biological Activity. Biochim. Biophys. Acta-Biomembr. 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Ruiz, R.; García, M.P.; Lara, A.; Rubio, L.A. Garlic Derivatives (PTS and PTS-O) Differently Affect the Ecology of Swine Faecal Microbiota in Vitro. Vet. Microbiol. 2010, 144, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium Cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2021, 14, 21. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and Chemical Stability of Garlic-Derived Allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Wettenhall, C. Allium: Ecology, Distribution and Cultivation; Plant Science Research and Practices; Nova Science Publishers: Hauppauge, NY, USA, 2020; ISBN 9781536180794. [Google Scholar]
- Yetgin, A.; Canli, K.; Altuner, E.M. Comparison of Antimicrobial Activity of Allium Sativum Cloves from China and Taşköprü, Turkey. Adv. Pharmacol. Sci. 2018, 2018, 9302840. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of Plant Extracts in Fish Aquaculture as an Alternative to Chemotherapy: Current Status and Future Perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Zorriehzahra, M.J.; Adel, M.; Kakoolaki, S.; Seidgar, M.; Akbari, P.; Mehrabi, M.R.; Jadgal, S.; Sakhaie, F.; Fereidouni, M.S. Research Article: Dietary Supplementation of Garlic (Allium sativum L.) Extract Enhances Haematological, Humoral Immune Responses and Disease Resistance of Mugil Cephalus Linnaeus 1758, Larvae against Photobacterium Damselae. Iran. J. Fish. Sci. 2021, 20, 1149–1164. [Google Scholar] [CrossRef]
- Diab, A.S.; Aly, S.M.; John, G.; Abde-Hadi, Y.; Mohammed, M.F. Effect of Garlic, Black Seed and Biogen as Immunostimulants on the Growth and Survival of Nile Tilapia, Oreochromis Niloticus (Teleostei: Cichlidae), and Their Response to Artificial Infection with Pseudomonas Fluorescens. Afr. J. Aquat. Sci. 2008, 33, 63–68. [Google Scholar] [CrossRef]
- Saleh, N.E.; Michael, F.R.; Toutou, M.M. Evaluation of Garlic and Onion Powder as Phyto-Additives in the Diet of Sea Bass (Dicentrarcus labrax). Egypt. J. Aquat. Res. 2015, 41, 211–217. [Google Scholar] [CrossRef]
- Fereidouni, M.S.; Akbary, P.; Soltanian, S.; Fereidouni, M.S.; Akbary, P.; Soltanian, S. Survival Rate and Biochemical Parameters in Mugil Cephalus (Linnaeus, 1758) Larvae Fed Garlic (Allium sativum L.) Extract. Am. J. Mol. Biol. 2015, 5, 7–15. [Google Scholar] [CrossRef]
- Shalaby, A.M.; Khattab, Y.A.; Abdel Rahman, A.M. Effects of Garlic (Allium sativum) and Chloramphenicol on Growth Performance, Physiological Parameters and Survival of Nile Tilapia (Oreochromis niloticus). J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 172–201. [Google Scholar] [CrossRef]
- Wu, N.; Waagbø, R.; Wan, M.; Feijoo, C.G.; Jiang, W.D. Editorial: Gastrointestinal Immunity and Crosstalk with Internal Organs in Fish. Front. Immunol. 2021, 12, 4017. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal Immunity to Pathogenic Intestinal Bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Premkumar, L.S. Transient Receptor Potential Channels as Targets for Phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Myhill, L.J.; Andersen-Civil, A.I.S.; Thamsborg, S.M.; Blanchard, A.; Williams, A.R. Garlic-Derived Organosulfur Compounds Regulate Metabolic and Immune Pathways in Macrophages and Attenuate Intestinal Inflammation in Mice. Mol. Nutr. Food Res. 2022, 66, 2101004. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Newman-Portela, A.M.; Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Agraso, M.D.M.; Bermúdez, L.; Aguinaga, M.A.; Baños, A.; Maqueda, M.; Valdivia, E.; et al. Beneficial Shifts in the Gut Bacterial Community of Gilthead Seabream (Sparus aurata) Juveniles Supplemented with Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO). Animals 2022, 12, 1821. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S.; Sinai, T.; Zilberg, D. Efficacy of Garlic Based Treatments against Monogenean Parasites Infecting the Guppy (Poecilia reticulata (Peters)). Vet. Parasitol. 2014, 203, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Soltani, E.; Ingelbrecht, J.; Soltani, M. Medicinal Herbs and Plants: Potential Treatment of Monogenean Infections in Fish. Rev. Fish. Sci. Aquac. 2020, 28, 260–282. [Google Scholar] [CrossRef]
- Wunderlich, A.C.; de Oliveira Penha Zica, É.; dos Santos Ayres, V.F.; Guimarães, A.C.; Takeara, R. Plant-Derived Compounds as an Alternative Treatment against Parasites in Fish Farming: A Review. In Natural Remedies in the Fight against Parasites; IntechOpen: London, UK, 2017; ISBN 978-953-51-3290-5. [Google Scholar]
- Schelkle, B.; Snellgrove, D.; Cable, J. In Vitro and in Vivo Efficacy of Garlic Compounds against Gyrodactylus Turnbulli Infecting the Guppy (Poecilia reticulata). Vet. Parasitol. 2013, 198, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Millet, C.O.M.; Lloyd, D.; Williams, C.; Williams, D.; Evans, G.; Saunders, R.A.; Cable, J. Effect of Garlic and Allium-Derived Products on the Growth and Metabolism of Spironucleus Vortens. Exp. Parasitol. 2011, 127, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Firmino, J.P.; Vallejos-Vidal, E.; Sarasquete, C.; Ortiz-Delgado, J.B.; Balasch, J.C.; Tort, L.; Estevez, A.; Reyes-López, F.E.; Gisbert, E. Unveiling the Effect of Dietary Essential Oils Supplementation in Sparus Aurata Gills and Its Efficiency against the Infestation by Sparicotyle Chrysophrii. Sci. Rep. 2020, 10, 17764. [Google Scholar] [CrossRef]
- Mladineo, I.; Trumbić, Ž.; Ormad-García, A.; Palenzuela, O.; Sitjà-Bobadilla, A.; Manuguerra, S.; Ruiz, C.E.; Messina, C.M. In Vitro Testing of Alternative Synthetic and Natural Antiparasitic Compounds against the Monogenean Sparicotyle Chrysophrii. Pathogens 2021, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.R.; Liu, Y.M.; Hou, T.L.; Li, C.T.; Zhang, Q.Z. Antiparasitic Efficacy of Crude Plant Extracts and Compounds Purified from Plants against the Fish Monogenean Neobenedenia Girellae. J. Aquat. Anim. Health 2021, 33, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Gaunt, P.S.; Burbick, C.R.; Avendaño-Herrera, R.; Buller, N.; Chuanchuen, R.; Gandhi, M.; Gieseker, C.M.; Marchand, S.; Smith, P.R. Methods for Antimicrobial Broth Dilution and Disk Diffusion Susceptibility Testing of Bacteria Isolated from Aquatic Animals; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Calvo, M.A.; Asensio, J.J. Evaluación de La Eficacia de Productos Antimicrobianos En La Alimentación Animal. Anaporc. 1999, 192, 142–146. [Google Scholar]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus Vulgaris, Thymus Zygis and Thymus Hyemalis Essential Oils. Food Control. 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. In Vitro Antibacterial Activity of Plant Ethanolic Extracts against Fish Pathogens. J. World Aquac. Soc. 2014, 45, 545–557. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; García-Campaña, A.M. A Rapid and Simple UHPLC-ESI-MS/MS Method for the Screening of Propyl Propane Thiosulfonate, a New Additive for Animal Feed. Anal. Methods 2016, 8, 3730–3739. [Google Scholar] [CrossRef]
- Zaiontz, C. Real Statistics Using Excel. Available online: https://www.real-statistics.com/ (accessed on 6 June 2022).

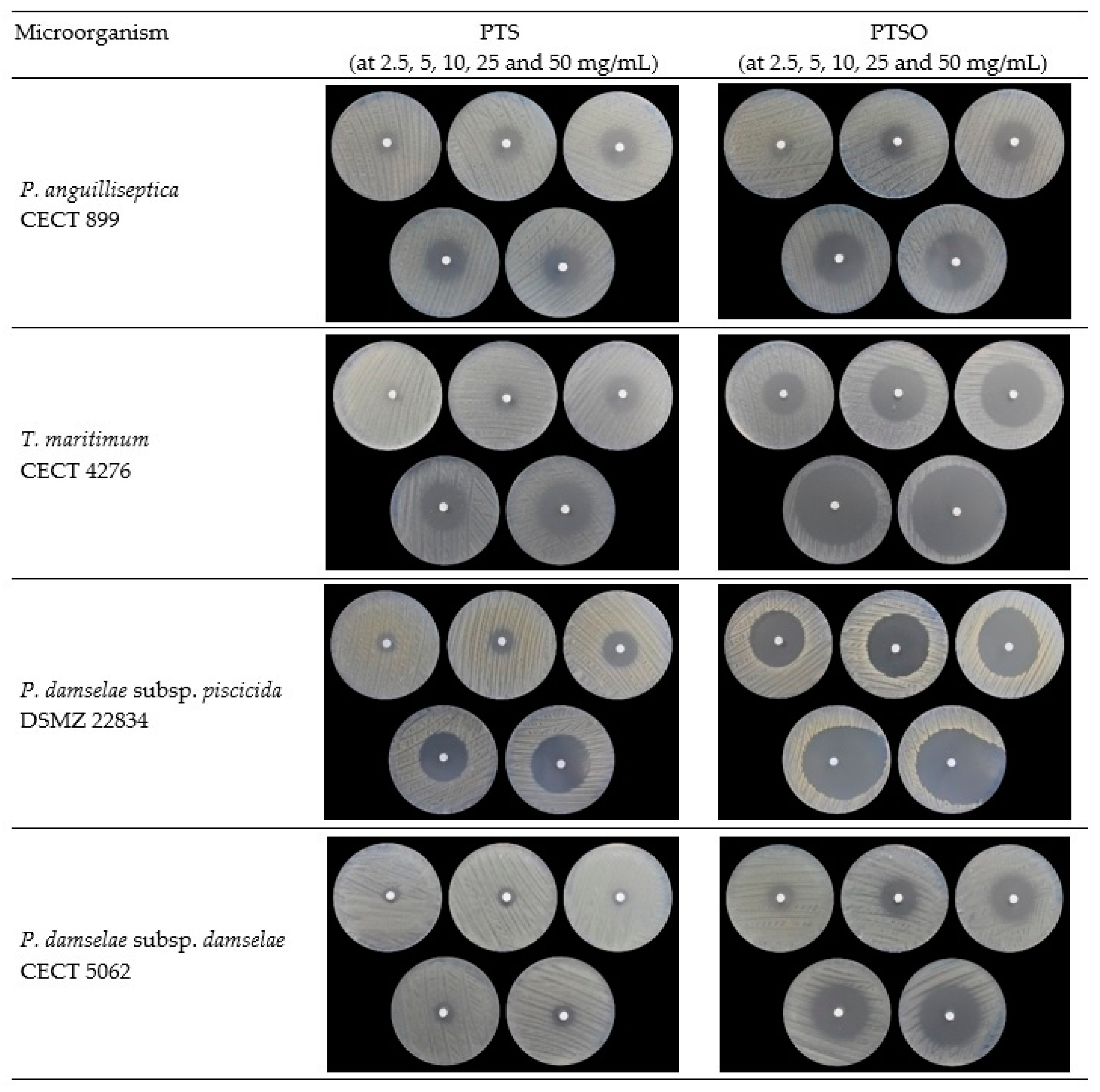
| Compound | Concentration (mg/mL) | P. anguilliseptica | T. maritimum | P. damselae subsp. Piscicida | P. damselae subsp. damselae |
|---|---|---|---|---|---|
| PTS | 2.5 | 12.5 ± 1.12 | 0.0 ± 0.00 | 0.0 ± 0.00 | 11.3 ± 0.83 |
| 5 | 17.5 ± 1.80 | 13.0 ± 1.41 | 11.0 ± 1.41 | 12.8 ± 1.09 | |
| 10 | 19.5 ± 1.80 | 19.0 ± 2.24 | 26.3 ± 2.38 | 13.5 ± 1.12 | |
| 25 | 23.5 ± 1.12 | 30.5 ± 1.80 | 35.0 ± 1.41 | 15.0 ± 0.71 | |
| 50 | 29.8 ± 2.86 | 34.5 ± 1.12 | 43.8 ± 2.38 | 16.5 ± 1.80 | |
| EC50 1(mg/mL) | 12.7563 | 8.1651 | 8.9399 | 11.6285 | |
| PTSO | 2.5 | 14.5 ± 1.12 | 28.5 ± 1.12 | 40.5 ± 1.66 | 18.8 ± 2.86 |
| 5 | 17.5 ± 1.80 | 39.0 ± 1.41 | 47.5 ± 1.80 | 24.5 ± 1.12 | |
| 10 | 23.5 ± 1.80 | 45.5 ± 1.80 | 56.8 ± 2.86 | 30.5 ± 1.12 | |
| 25 | 32.5 ± 1.12 | 59.0 ± 1.41 | 62.5 ± 2.96 | 39.0 ± 1.00 | |
| 50 | 40.5 ± 1.12 | 66.5 ± 1.12 | 68.0 ± 2.24 | 43.5 ± 1.12 | |
| EC50 1(mg/mL) | 14.4796 | 10.5698 | 9.0014 | 10.4522 |
| Strain | PTS (µg/mL) | PTSO (µg/mL) |
|---|---|---|
| Pseudomonas anguilliseptica | 312.5 | 39.06 |
| Tenacibaculum maritimum | 2500 | 1250 |
| Photobacterium damselae subsp. piscicida | 625 | 156.25 |
| Photobacterium damselae subsp. damselae | 625 | 78.125 |
| Strain | Reference | Isolated |
|---|---|---|
| Pseudomonas anguilliseptica | CECT 899 | Anguilla japonica |
| Tenacibaculum maritimum | CECT 4276 | Kidney of diseased black seabream |
| Photobacterium damselae subsp. piscicida | DSM 22834 | Seriola with pseudotuberculosis |
| Photobacterium damselae subsp. damselae | CECT 5062 | Diseased turbot |
| Nutrient Composition | |
|---|---|
| Crude protein% | 45.00 |
| Crude fat% | 20.00 |
| Cellulose% | 2.8 |
| Ash% | 8.00 |
| N.F.E.% | 13.3 |
| Moisture% | 10.00 |
| Calcium% | 1.8 |
| Phosphorus% | 0.9 |
| Gross energy (MJ/kg) | 21.10 |
| Digestible energy (MJ/kg) | 17.5 |
| Protein digestibility% | 90.00 |
| PD/DE (gr/MJ) | 23.14 |
| PTS/PTSO (mg/kg) | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Gómez, J.F.; Aguinaga-Casañas, M.A.; Falcón-Piñeiro, A.; González-Gragera, E.; Márquez-Martín, R.; Agraso, M.d.M.; Bermúdez, L.; Baños, A.; Martínez-Bueno, M. Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies. Molecules 2022, 27, 6900. https://doi.org/10.3390/molecules27206900
Cabello-Gómez JF, Aguinaga-Casañas MA, Falcón-Piñeiro A, González-Gragera E, Márquez-Martín R, Agraso MdM, Bermúdez L, Baños A, Martínez-Bueno M. Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies. Molecules. 2022; 27(20):6900. https://doi.org/10.3390/molecules27206900
Chicago/Turabian StyleCabello-Gómez, Jose F., María Arántzazu Aguinaga-Casañas, Ana Falcón-Piñeiro, Elías González-Gragera, Raquel Márquez-Martín, María del Mar Agraso, Laura Bermúdez, Alberto Baños, and Manuel Martínez-Bueno. 2022. "Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies" Molecules 27, no. 20: 6900. https://doi.org/10.3390/molecules27206900
APA StyleCabello-Gómez, J. F., Aguinaga-Casañas, M. A., Falcón-Piñeiro, A., González-Gragera, E., Márquez-Martín, R., Agraso, M. d. M., Bermúdez, L., Baños, A., & Martínez-Bueno, M. (2022). Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies. Molecules, 27(20), 6900. https://doi.org/10.3390/molecules27206900






