Diastereoselective Synthesis of Highly Functionalized Proline Derivatives
Abstract
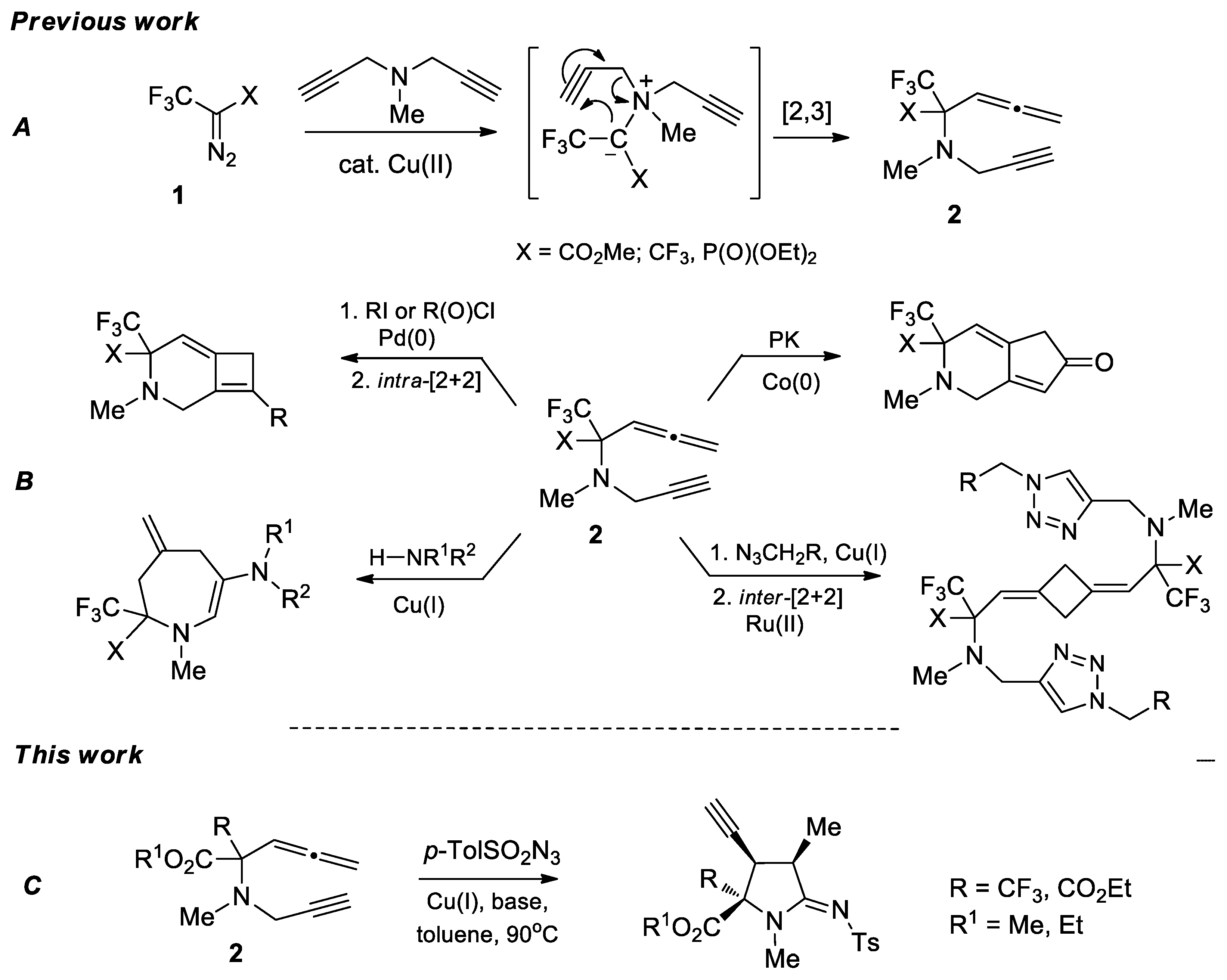
1. Introduction
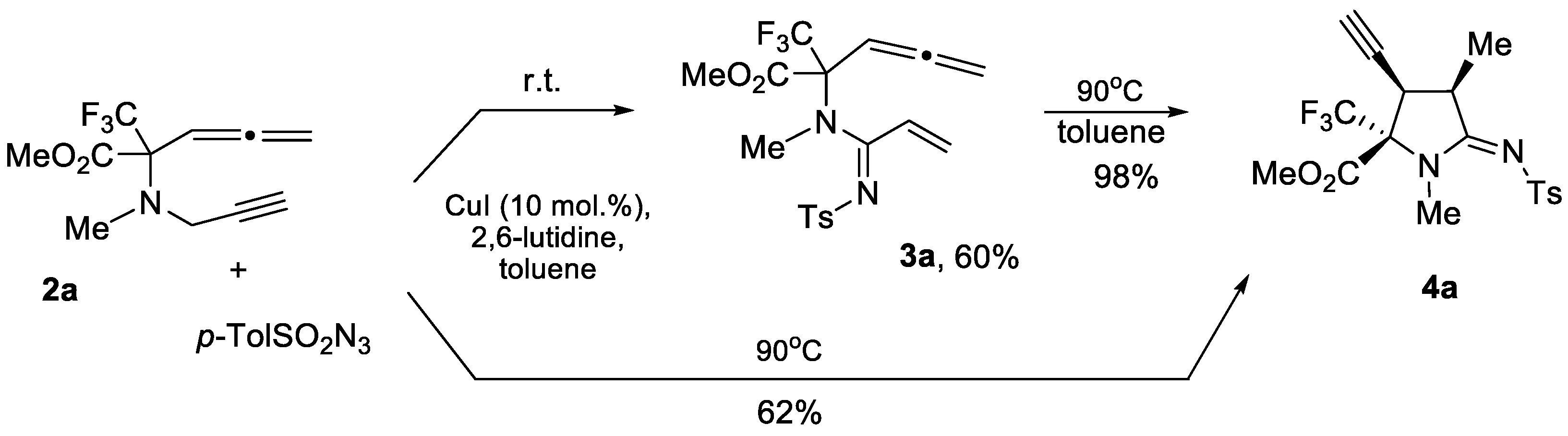
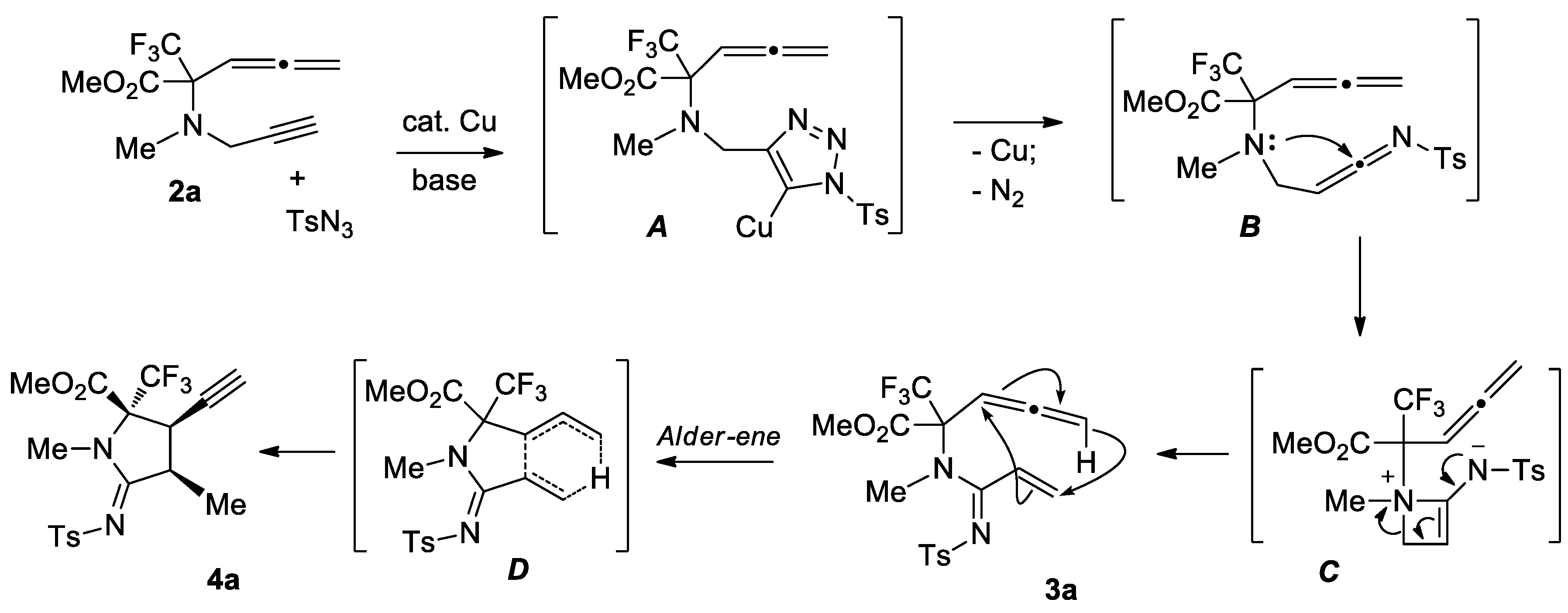
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Preparation of 4a and 4b
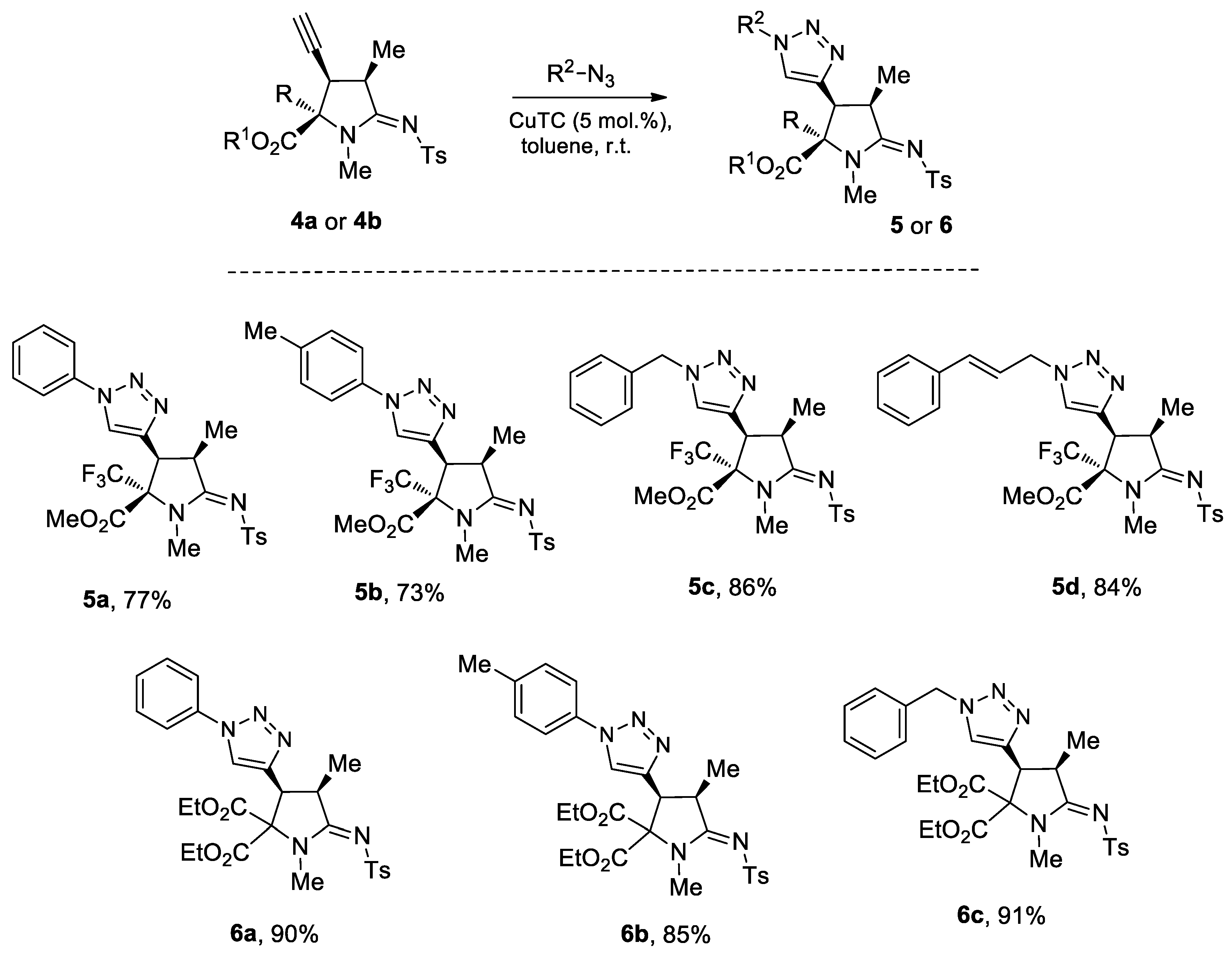
3.3. General Procedure for Preparation of 5a–5d and 6a–6c

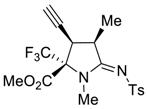
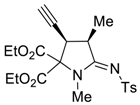
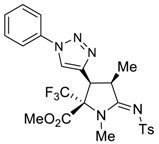
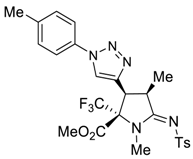
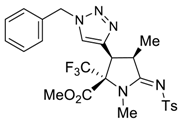
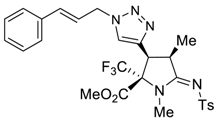

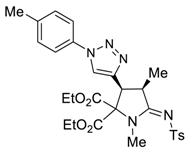

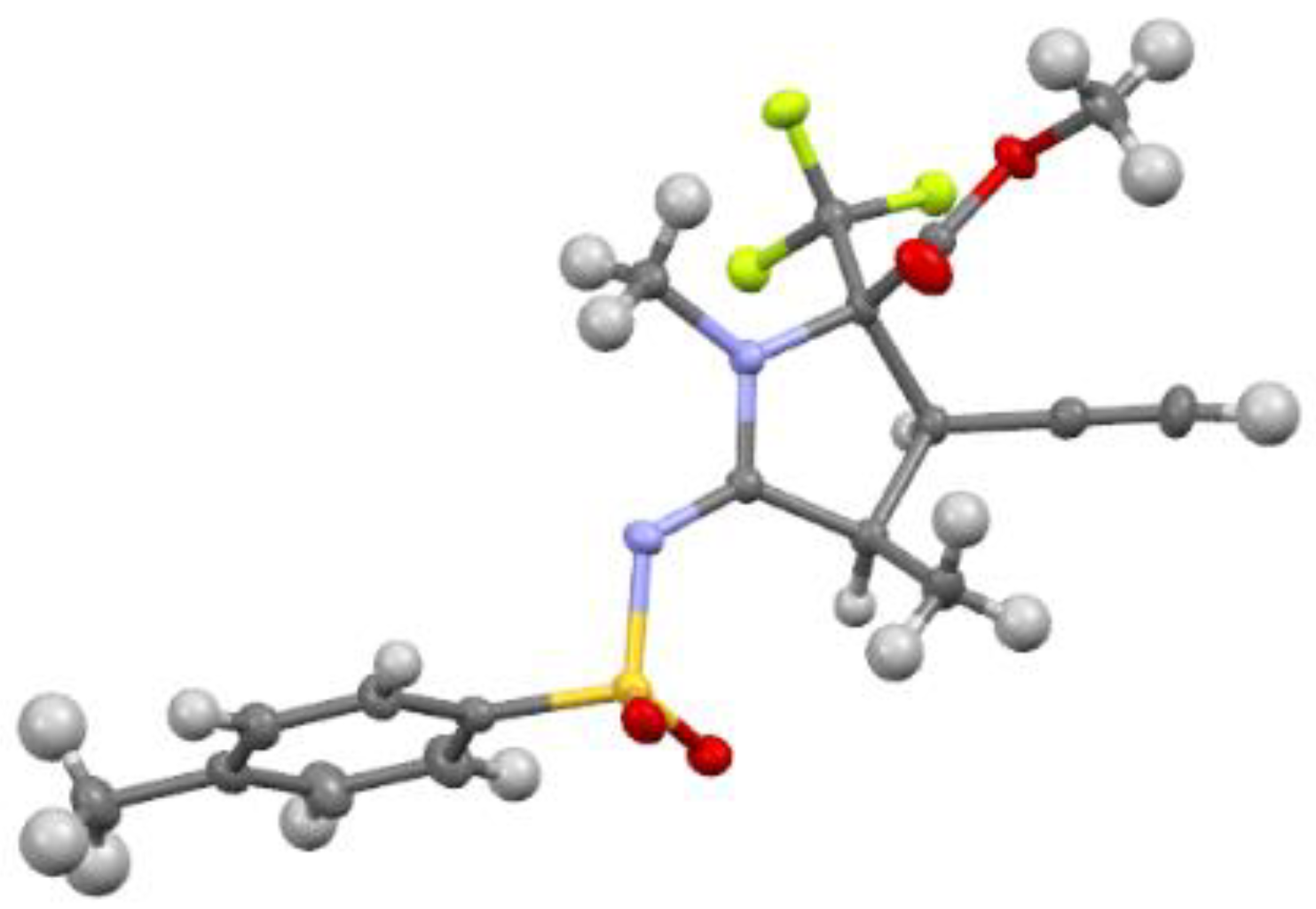
3.4. X-ray Structure Determination of 4a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mauger, A.B. Naturally Occurring Proline Analogues. J. Nat. Prod. 1996, 59, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.P.; Cole, R.J.; Dorner, J.W.; Cox, R.H.; Richard, J.L.; Barnes, C.L.; Van Der Helm, D. Structure and conformation of roseotoxin B. J. Am. Chem. Soc. 1984, 106, 2388–2392. [Google Scholar] [CrossRef]
- Engstrom, G.W.; DeLance, J.V.; Richard, J.L.; Baetz, A.L. Purification and characterization of roseotoxin B, a toxic cyclodepsipeptide from Trichothecium roseum. J. Agric. Food Chem. 1975, 23, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances; Thieme: New York, NY, USA, 2008. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njadarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Flores-Ortega, A.; Jiménez, A.I.; Cativiela, C.; Nussinov, R.; Alemán, C.; Casanovas, J. Conformational Preferences of α-Substituted Proline Analogues. J. Org. Chem. 2008, 73, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R. Pyrrolizidine alkaloids. Nat. Prod. Rep. 2002, 19, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Tolomellia, A.; Ammazzalorsob, A.; Brunob, I.; Amorosob, R. A review of strategies for the development of alkyl prolines in drug discovery. Curr. Bioact. Compd. 2016, 12, 146–160. [Google Scholar] [CrossRef]
- Karoyan, P.; Sagan, S.; Lequin, O.; Quancard, J.; Lavielle, S.; Chassaing, G. Substituted prolines: Syntheses and applications in structure-activity relationship studies of biologically active peptides. Targets Heterocycl. Syst. 2004, 8, 216–273. [Google Scholar] [CrossRef]
- Calaza, M.I.; Cativiela, C. Stereoselective Synthesis of Quaternary Proline Analogues. Eur. J. Org. Chem. 2008, 2008, 3427–3448. [Google Scholar] [CrossRef] [PubMed]
- Naud, J.; Lemke, C.; Goudreau, N.; Beaulieu, E.; White, P.D.; Llinàs-Brunet, M.; Forgione, P. Potent triazolyl-proline-based inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2008, 18, 3400–3404. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Temperini, P.C.; Poulie, B.M.; Staudt, M.; Nielsen, B.; Pickering, D.S.; Bunch, L. A Diversity Oriented Synthesis Approach to New 2,3-trans-Substituted L-Proline Analogs as Potential Ligands for the Ionotropic Glutamate Receptors. ACS Chem. Neurosci. 2020, 11, 702–714. [Google Scholar] [CrossRef]
- Begue, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Purser, S.; Moore, P.R.; Swallowb, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gouverneur, V.; Muller, K. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications; Imperial College Press: London, UK, 2012. [Google Scholar]
- Jlalia, I.; Lensen, N.; Chaume, G.; Dzhambazova, G.; Astasidi, L.; Hadjiolova, R.; Bocheva, A.; Brigau, T. Synthesis of an MIF-1 analogue containing enantiopure (S)-α-trifluoromethyl-proline and biological evaluation on nociception. Eur. J. Med. Chem. 2013, 62, 122–129. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Meng, W.-D.; Qing, F.-L. Synthesis of fluorinated amino acids. Tetrahedron 2004, 60, 6711–6745. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Qing, F.-L. Recent Advances in the Synthesis of Fluorinated Amino Acids. Eur. J. Org. Chem. 2011, 2011, 3261–3278. [Google Scholar] [CrossRef]
- Smits, R.; Cadicamo, C.D.; Burger, K.; Koksch, B. Synthetic strategies to α-trifluoromethyl and α-difluoromethyl substituted α-amino acids. Chem. Soc. Rev. 2008, 37, 1727–1739. [Google Scholar] [CrossRef]
- Moschner, J.; Stulberg, V.; Fernandes, R.; Huhmann, S.; Leppkes, J.; Koksch, B. Approaches to Obtaining Fluorinated α-Amino Acids. Chem. Rev. 2019, 119, 10718–10801. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; White, S.; Graham, D.J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N.A.; Soloshonok, V.A. Tailor-made amino acids and fluorinated motifs as prominent traits in modern pharmaceuticals. Chem. Eur. J. 2020, 26, 11349–11390. [Google Scholar] [CrossRef] [PubMed]
- Bey, P.; Gerhart, F.; Van Dorsselaer, V.; Danzin, C. α-(Fluoromethyl)dehydroornithine and α-(fluoromethyl)dehydroputrescine analogues as irreversible inhibitors of ornithine decarboxylase. J. Med. Chem. 1983, 26, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.T.; Eswarakrishnan, S. Fluorine in Bioorganic Chemistry; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Vorobyeva, D.V.; Mailyan, A.K.; Peregudov, A.S.; Karimova, N.M.; Vasilyeva, T.P.; Bushmarinov, I.S.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Synthesis of functionalized CF3-containing heterocycles via [2,3]-sigmatropic rearrangement and sequential catalytic carbocyclization. Tetrahedron 2011, 67, 3524–3532. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Krylov, I.M.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Thermal [2+2] Cycloaddition of CF3-Substituted Allenynes: Access to Novel Cyclobutene-Containing α-Amino Acids. Synlett 2011, 16, 2321–2324. [Google Scholar] [CrossRef]
- Philippova, A.N.; Vorobyeva, D.V.; Gribanov, P.S.; Godovikov, I.A. Osipov, S.N. Synthesis of functionalized azepines via Cu(I)-catalyzed tandem amination/cyclization reaction of fluorinated allenynes. Molecules 2022, 27, 5195. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Philippova, A.N.; Gribanov, P.S.; Nefedov, S.E.; Novikov, V.V.; Osipov, S.N. Ruthenium-catalyzed dimerization of CF3-containing functional allenes. J. Organomet. Chem. 2021, 951, 121998. [Google Scholar] [CrossRef]
- Eckert, M.; Monnier, F.; Shchetnikov, G.T.; Titanyuk, I.D.; Osipov, S.N.; Dérien, S.; Dixneuf, P.H. Tandem Catalytic Carbene Addition/Bicyclization of Enynes. One-Step Synthesis of Fluorinated Bicyclic Amino Esters by Ruthenium Catalysis. Org. Lett. 2005, 7, 3741–3743. [Google Scholar] [CrossRef]
- Shchetnikov, G.T.; Peregudov, A.S.; Osipov, S.N. Effective Pathway to the α-CF3-Substituted Azahistidine Analogues. Synlett 2007, 2007, 136–140. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Peregudov, A.S.; Röschenthaler, G.-V.; Osipov, S.N. Synthesis of α-CF3-containing triazolyl amino acids as potential neurotransmitters via click-reaction. J. Fluorine Chem. 2015, 175, 60–67. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Petropavlovskikh, D.A.; Godovikov, I.A.; Nefedov, S.E.; Osipov, S.N. Rh(III)-Catalyzed C-H Activation/Annulation of Aryl Hydroxamates with CF3-Containing α-Propargyl α-Amino Acid Derivatives. Eur. J. Org. Chem. 2021, 2021, 1883–1890. [Google Scholar] [CrossRef]
- Petropavlovskikh, D.A.; Vorobyeva, D.V.; Godovikov, I.A.; Nefedov, S.E.; Filippov, O.A.; Osipov, S.N. Lossen rearrange-ment by Rh(III)-catalyzed C-H activation/annulation of aryl hydroxamates with alkynes: Access to quinolone-containing amino acid derivatives. Org. Biomol. Chem. 2021, 19, 9421–9426. [Google Scholar] [CrossRef]
- Horneff, T.; Chuprakov, S.; Chernyak, N.; Gevorgyan, V.; Fokin, V.V. Rhodium-Catalyzed Transannulation of 1,2,3-Triazoles with Nitriles. J. Am. Chem. Soc. 2008, 130, 14972–14974. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Gevorgyan, V. Versatile Reactivity of Rhodium-Iminocarbenes Derived from N-Sulfonyl Triazoles. Angew. Chem. Int. Ed. 2013, 52, 1371–1373. [Google Scholar] [CrossRef]
- Boyer, A. Rhodium(II)-Catalyzed Stereocontrolled Synthesis of Dihydrofuran-3-imines from 1-Tosyl-1,2,3-triazoles. Org. Lett. 2014, 16, 1660–1663. [Google Scholar] [CrossRef]
- Pan, X.-H.; Jiang, P.; Jia, Z.-H.; Xub, K.; Cao, J.; Chen, C.; Shen, M.-H.; Xu, H.-D. Expedient catalytic construction of azabicyclo [4.1.0]/[5.1.0] carbaldehydes via intramolecular cyclopropanation. Tetrahedron 2015, 71, 5124–5129. [Google Scholar] [CrossRef]
- Lv, X.; Liu, S.; Zhou, S.; Dong, G.; Xing, D.; Xu, X.; Hu, W. Asymmetric Three-Component Propargyloxylation for Direct Assembly of Polyfunctionalized Chiral Succinate Derivatives. CCS Chem. 2021, 3, 1903–1912. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ahlquist, M.; Kim, S.H.; Bae, I.; Fokin, V.V.; Sharpless, K.B.; Chang, S. Copper-Catalyzed Synthesis of N-Sulfonyl-1,2,3-triazoles: Controlling Selectivity. Angew. Chem. Int. Ed. 2007, 46, 1730–1733. [Google Scholar] [CrossRef]
- Yoo, E.J.; Bae, I.; Cho, S.H.; Han, H.; Chang, S. A Facile Access to N-Sulfonylimidates and Their Synthetic Utility for the Transformation to Amidines and Amides. Org. Lett. 2006, 8, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Han, H.; Chang, S. Highly Efficient One-Pot Synthesis of N-Sulfonylamidines by Cu-Catalyzed Three-Component Coupling of Sulfonyl Azide, Alkyne, and Amine. J. Am. Chem. Soc. 2005, 127, 2038–2039. [Google Scholar] [CrossRef]
- Cassidy, M.P.; Raushel, J.; Fokin, V.V. Practical Synthesis of Amides from In Situ Generated Copper(i) Acetylides and Sulfonyl Azides. Angew. Chem. Int. Ed. 2006, 45, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Chen, Y.-Y.; Rajagopal, B.; Tu, H.-C.; Chen, K.-L.; Wang, S.-F.; Liang, C.-F.; Tyan, Y.-C.; Lin, P.-C. Thermally Induced Denitrogenative Annulation for the Synthesis of Dihydroquinolinimines and Chroman-4-imines. Chem. Asian J. 2016, 11, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Joyce, L.M.; Drew, M.A.; Tague, A.J.; Thaima, T.; Gouranourimi, A.; Ariafard, A.; Pyne, S.G.; Hyland, C.J.T. A Rare Alder-ene Cycloisomerization of 1,6-Allenynes. Chem. Eur. J. 2022, 28, e202104022. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, G.; Chen, G. Synthesis of α-alkynyl α-amino acids via palladium-catalyzed alkynylation of unactivated C(sp3)-H bonds. Sci. China Chem. 2015, 58, 1345–1348. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
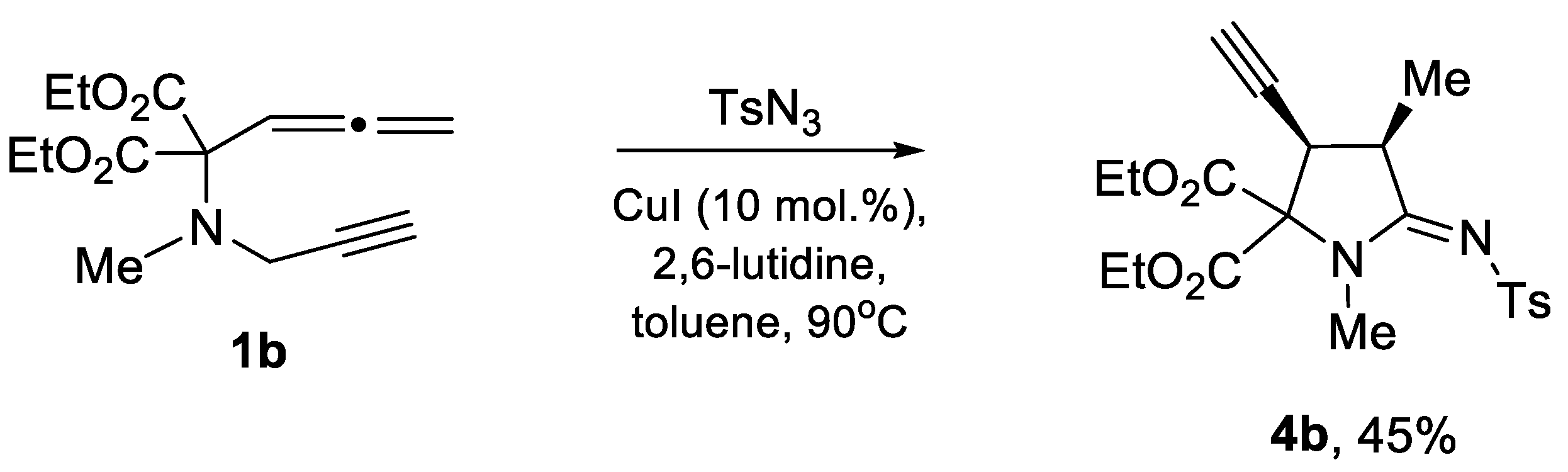
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippova, A.N.; Vorobyeva, D.V.; Gribanov, P.S.; Dolgushin, F.M.; Osipov, S.N. Diastereoselective Synthesis of Highly Functionalized Proline Derivatives. Molecules 2022, 27, 6898. https://doi.org/10.3390/molecules27206898
Philippova AN, Vorobyeva DV, Gribanov PS, Dolgushin FM, Osipov SN. Diastereoselective Synthesis of Highly Functionalized Proline Derivatives. Molecules. 2022; 27(20):6898. https://doi.org/10.3390/molecules27206898
Chicago/Turabian StylePhilippova, Anna N., Daria V. Vorobyeva, Pavel S. Gribanov, Fedor M. Dolgushin, and Sergey N. Osipov. 2022. "Diastereoselective Synthesis of Highly Functionalized Proline Derivatives" Molecules 27, no. 20: 6898. https://doi.org/10.3390/molecules27206898
APA StylePhilippova, A. N., Vorobyeva, D. V., Gribanov, P. S., Dolgushin, F. M., & Osipov, S. N. (2022). Diastereoselective Synthesis of Highly Functionalized Proline Derivatives. Molecules, 27(20), 6898. https://doi.org/10.3390/molecules27206898





