Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation
Abstract
1. Introduction
2. Results and Discussion
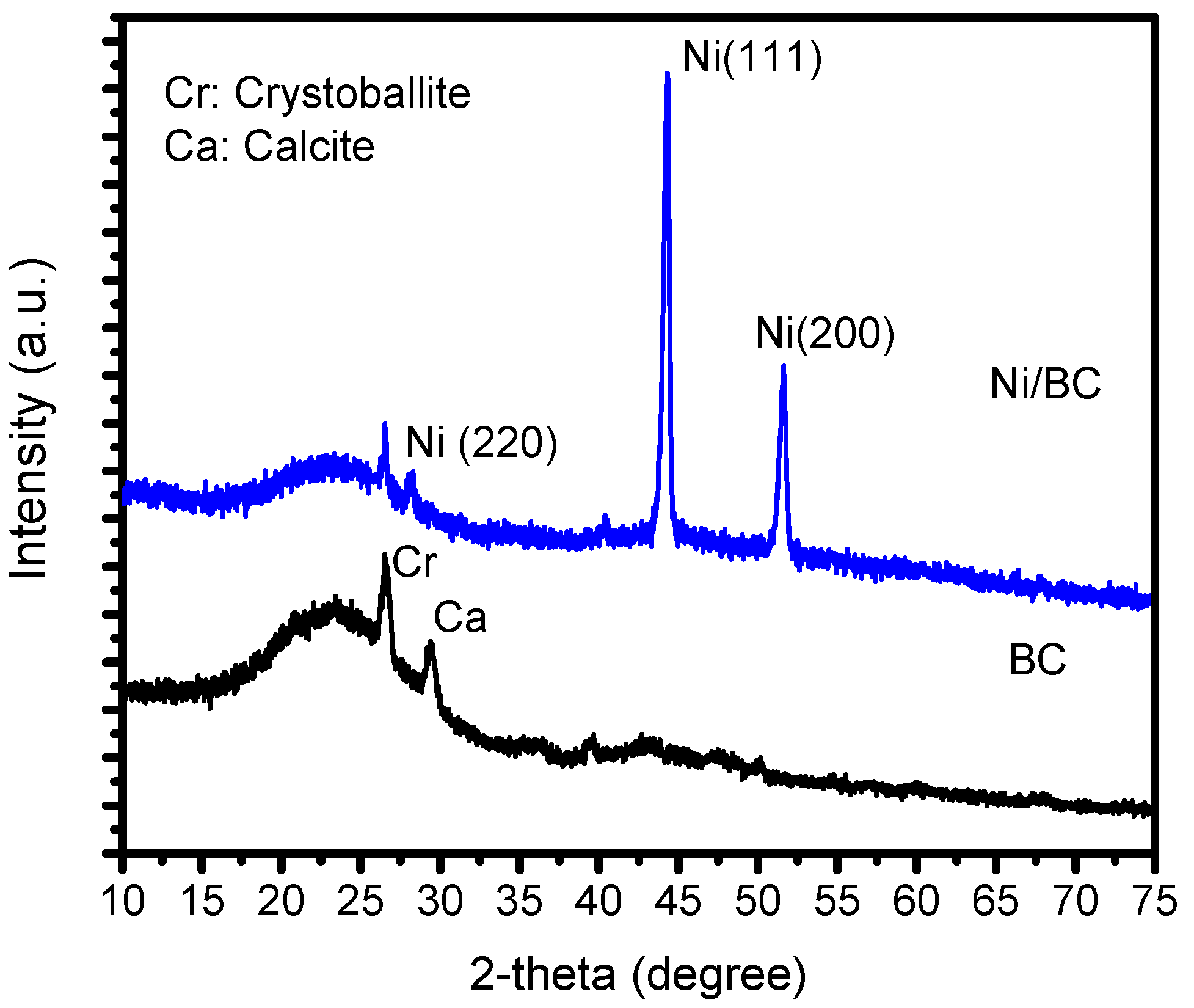
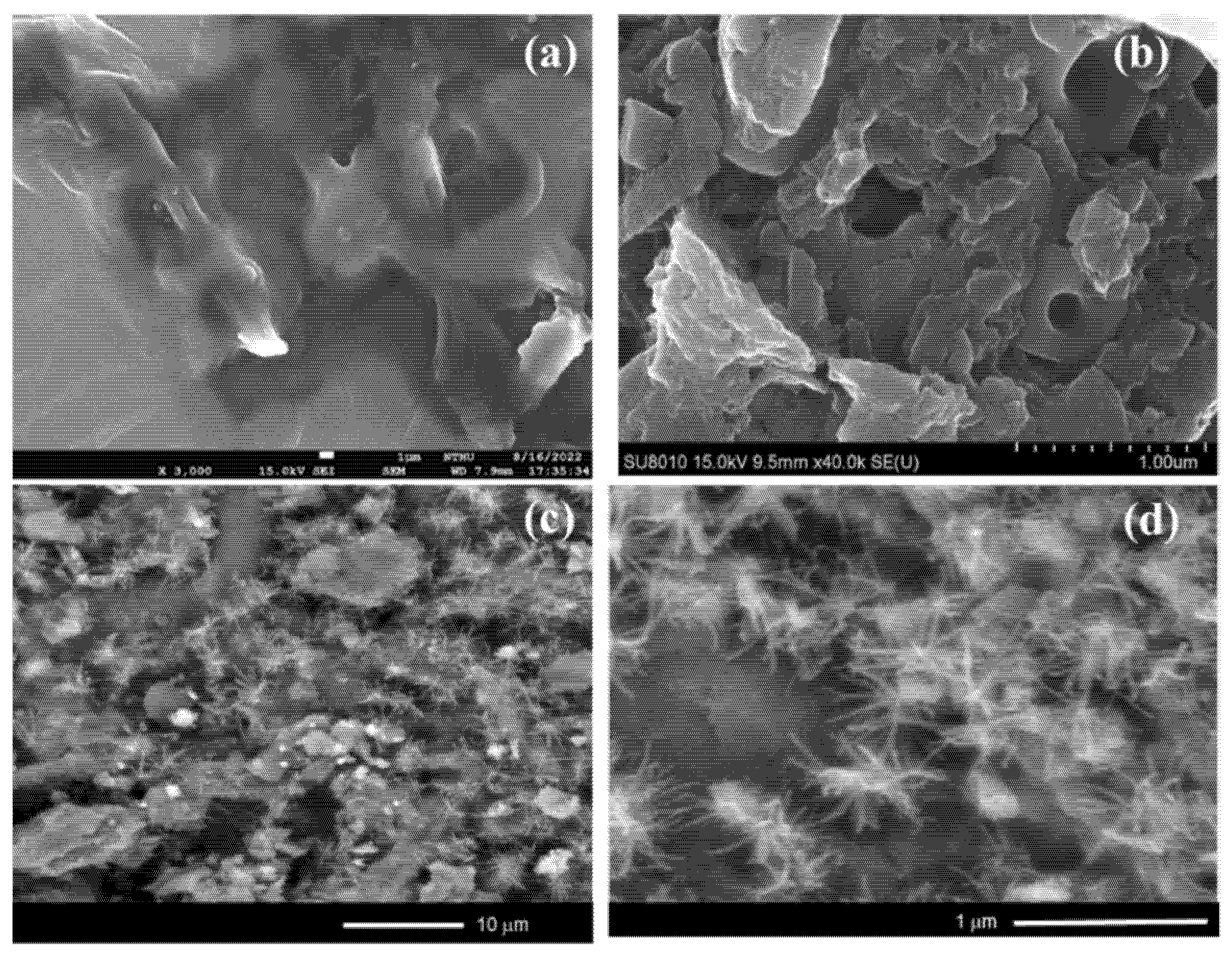
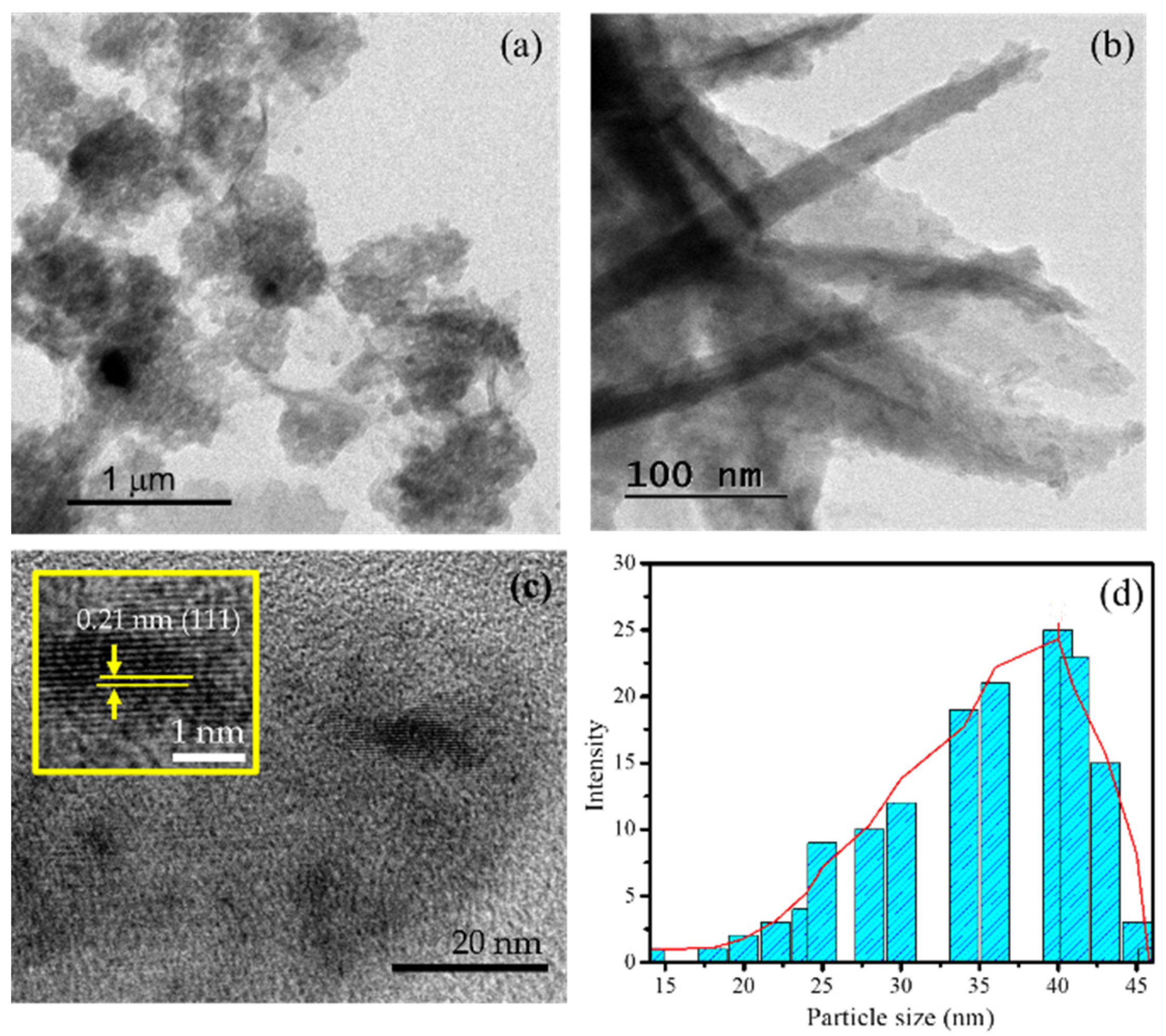
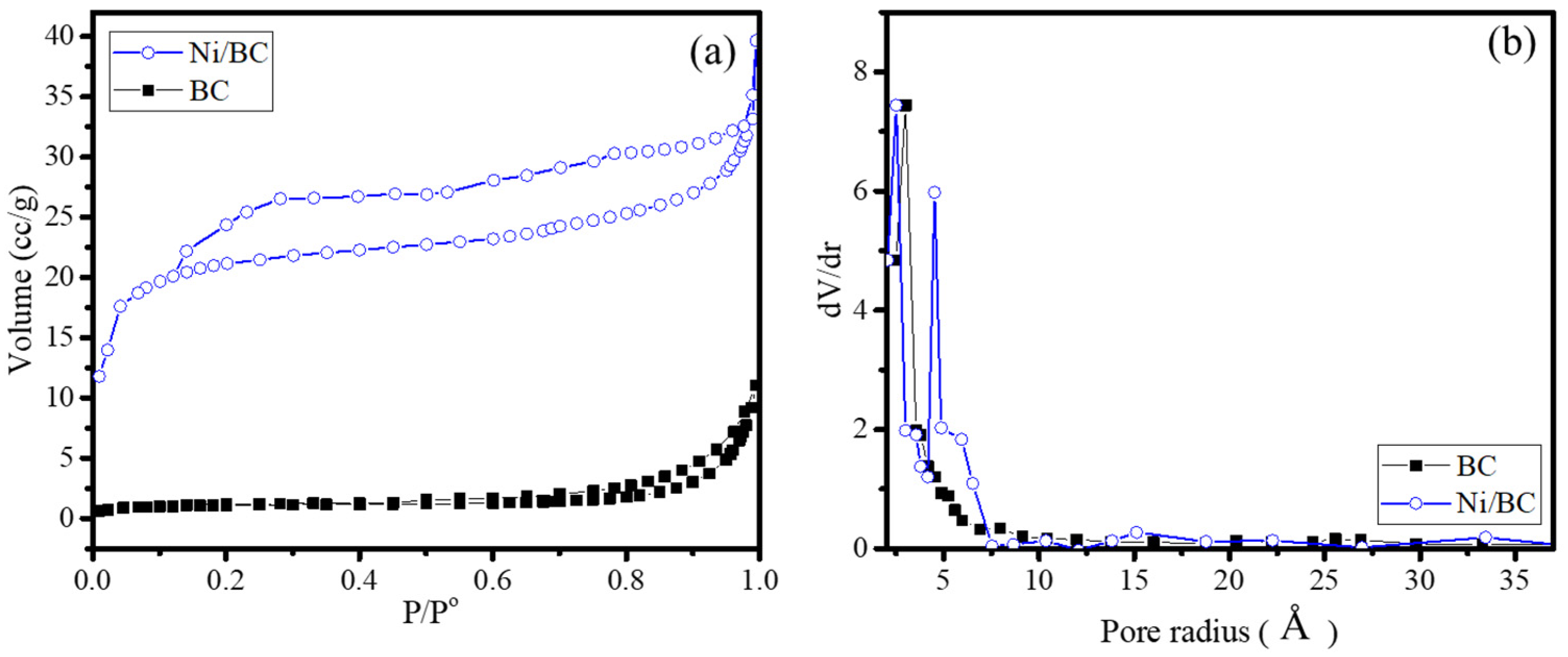
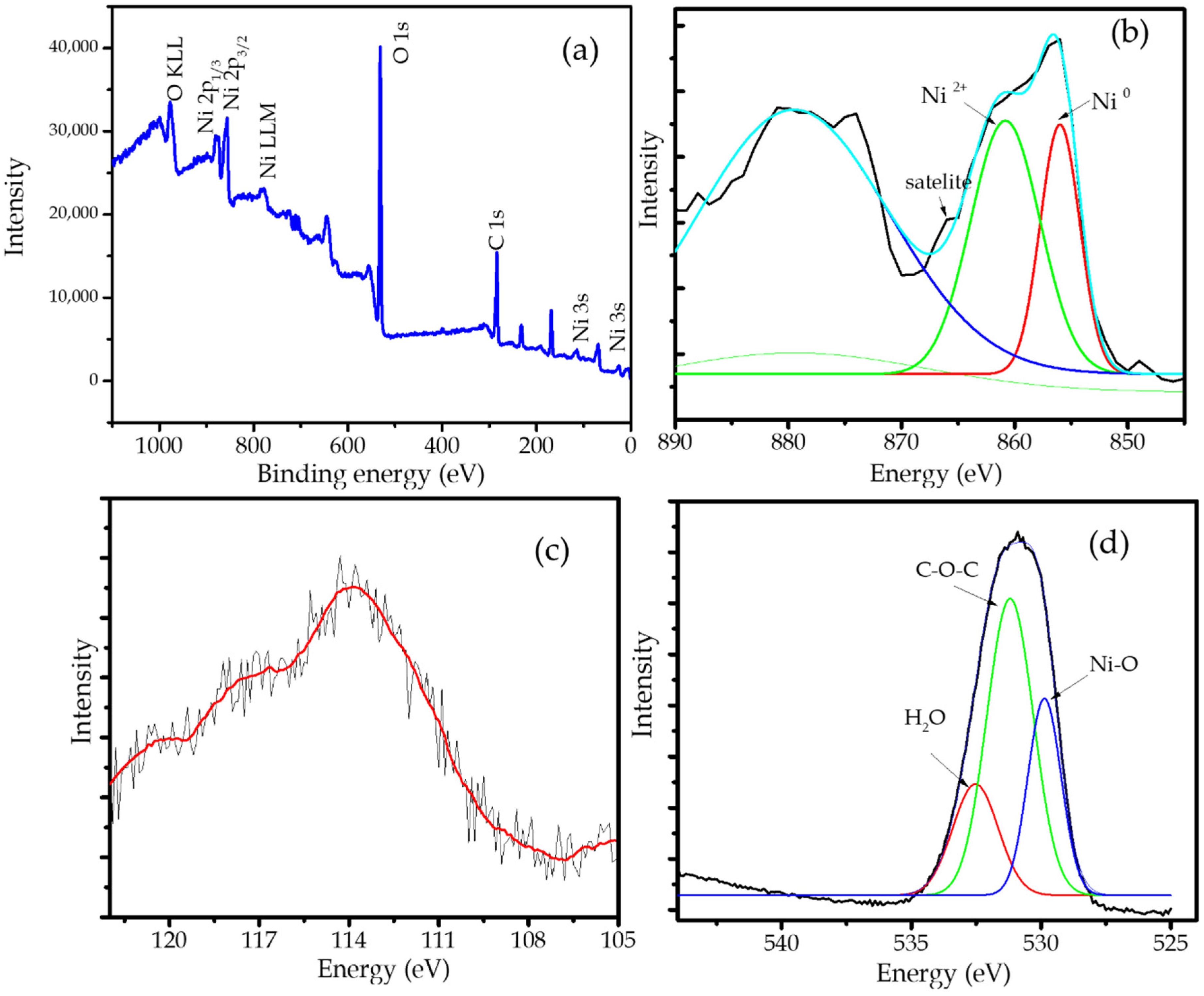
2.1. Physicochemical Characterization
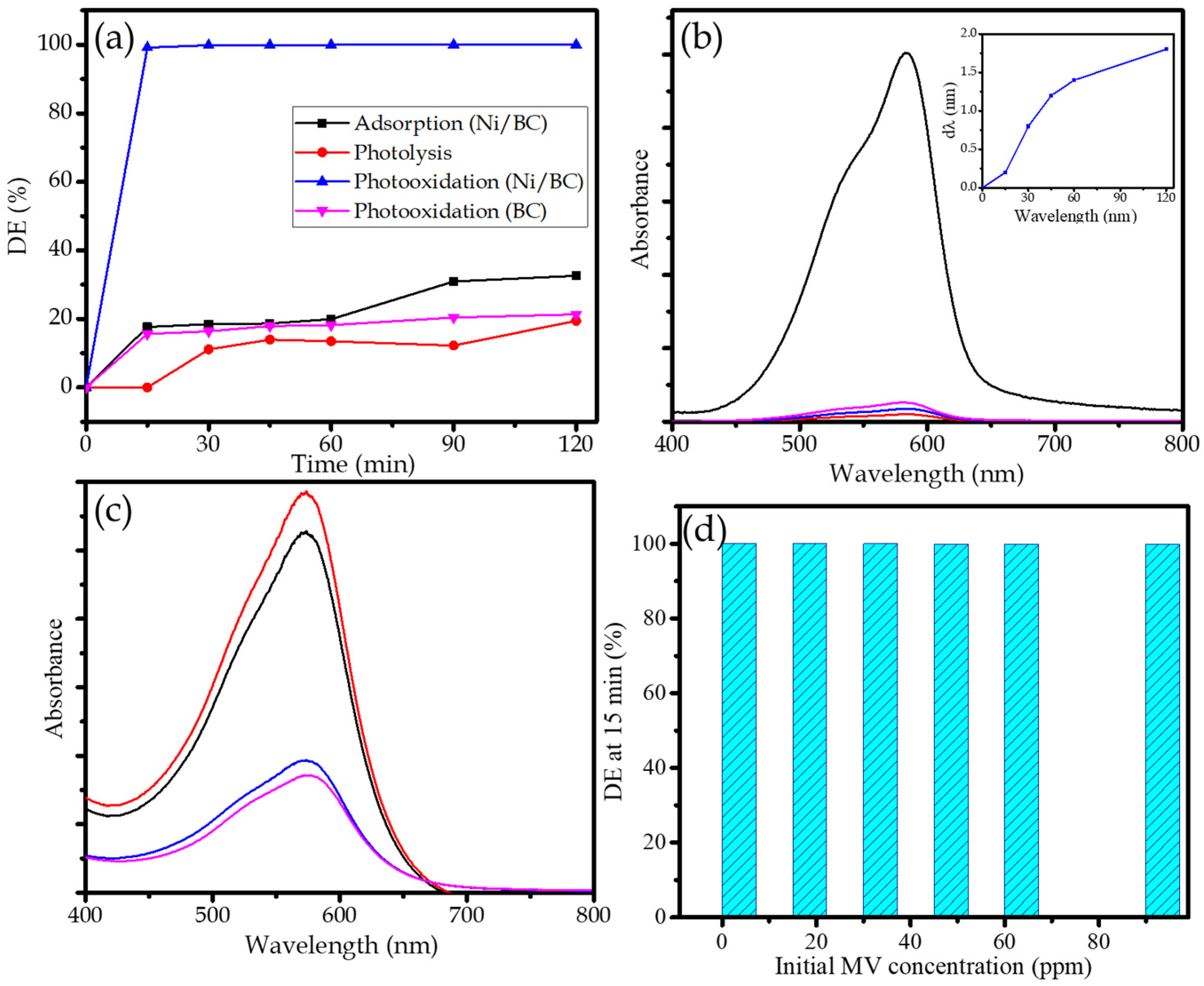
2.2. Photocatalytic Activity
2.3. Effect of pH
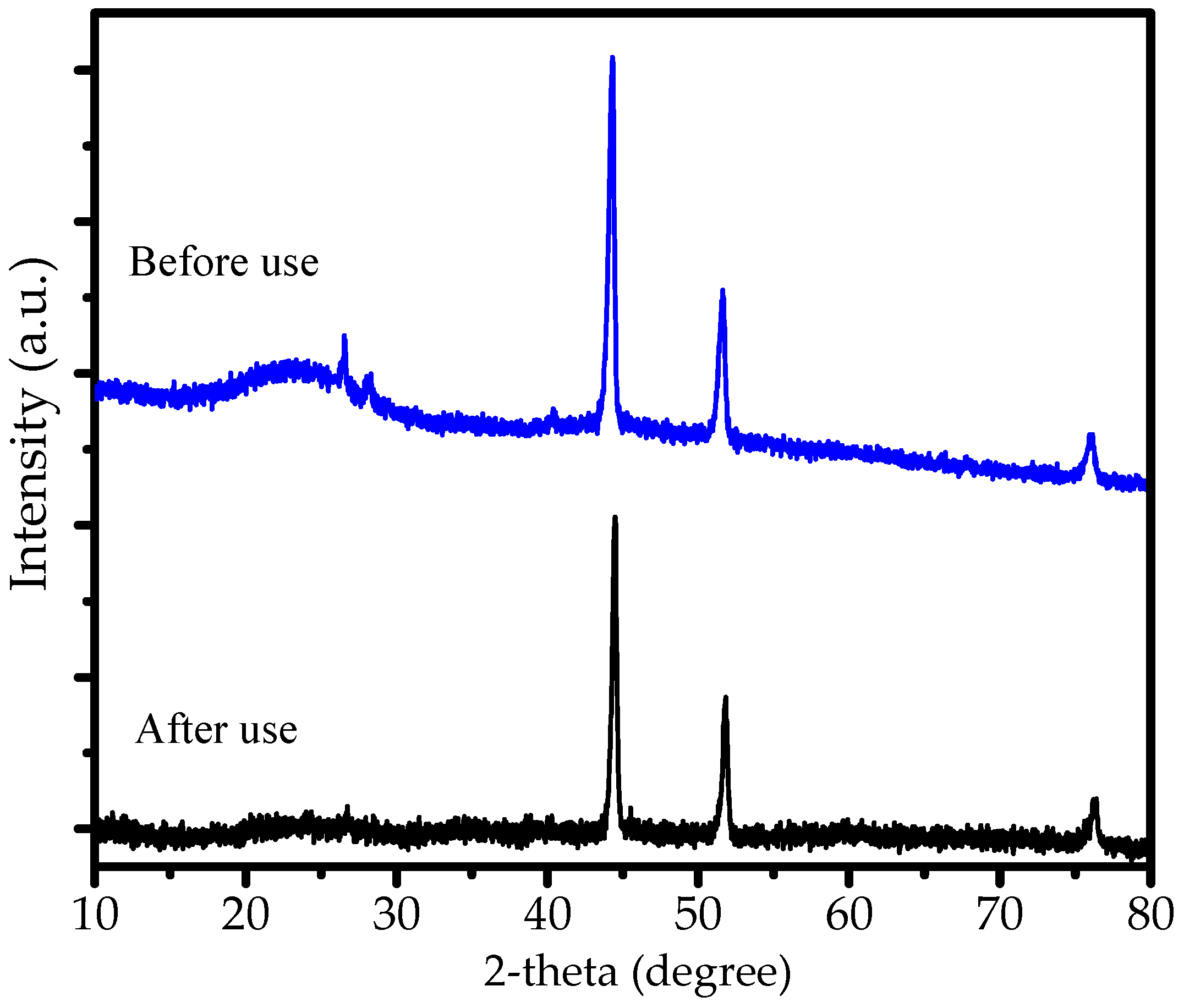
2.4. Reusability and Magnetic Susceptibility
3. Materials and Methods
3.1. Materials
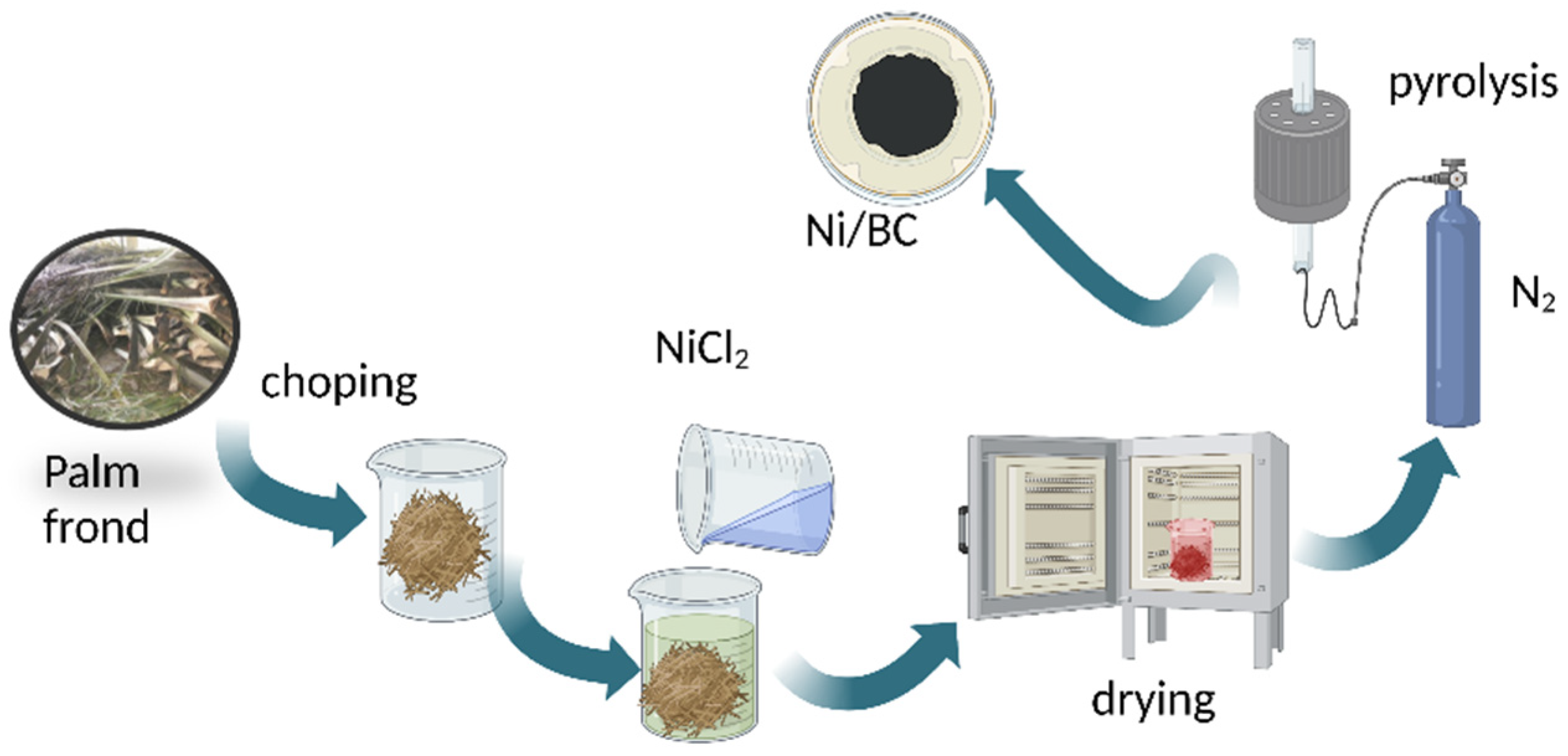
3.2. Preparation Magnetic Ni NPs/Biochar (Ni/BC)
3.3. Physicochemical Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic Degradation of Azo Dye Reactive. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef]
- Ikram, M.; Rashid, M.; Haider, A.; Naz, S.; Haider, J.; Raza, A.; Ansar, M.T.; Uddin, M.K.; Ali, N.M.; Ahmed, S.S.; et al. A review of photocatalytic characterization, and environmental cleaning, of metal oxide nanostructured materials. Sustain. Mater. Technol. 2021, 30, e00343. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Fatimah, I.; Ardianti, S.; Sahroni, I.; Purwiandono, G.; Sagadevan, S.; Doong, R.A. Visible light sensitized porous clay heterostructure photocatalyst of zinc-silica modified montmorillonite by using tris(2,2′-bipyridyl) dichlororuthenium. Appl. Clay Sci. 2021, 204, 106023. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Hidayat, A.; Sagadevan, S.; Kamari, A. Mechanistic insight into the adsorption and photocatalytic activity of a magnetically separable γ-Fe2O3/Montmorillonite nanocomposite for rhodamine B removal. Chem. Phys. Lett. 2022, 792, 139410. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Jeong, T.U.; Ahn, K.H. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. Bioresour. Technol. 2016, 220, 672–676. [Google Scholar] [CrossRef]
- Santosa, D.A.; Tamyiz, M.; Sagadevan, S.; Hidayat, A.; Fatimah, I.; Doong, R. Magnetic Nanocomposite from Bauxite Mining Tailing Waste as Magnetically Separable Catalyst in Catalytic Wet Peroxidation of Tetracycline. Environ. Nanotechnol. Monit. Manag. 2022, 4, 100451. [Google Scholar]
- Lee, T.; Nam, I.H.; Jung, S.; Park, Y.K.; Kwon, E.E. Synthesis of nickel/biochar composite from pyrolysis of Microcystis aeruginosa and its practical use for syngas production. Bioresour. Technol. 2020, 300, 122712. [Google Scholar] [CrossRef]
- Ahghari, M.R.; Soltaninejad, V.; Maleki, A. Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci. Rep. 2020, 10, 12627. [Google Scholar] [CrossRef]
- Jaji, N.D.; Lee, H.L.; Hussin, M.H.; Akil, H.M.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotechnol. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Bashiri, M.; Moeen, S.J. Synthesis of nickel-zinc ferrite magnetic nanoparticle and dye degradation using photocatalytic ozonation. Mater. Res. Bull. 2012, 47, 4403–4408. [Google Scholar] [CrossRef]
- Sadiku, M.; Selimi, T.; Berisha, A.; Maloku, A.; Mehmeti, V.; Thaçi, V.; Hasani, N. Removal of Methyl Violet from Aqueous Solution by Adsorption onto Halloysite Nanoclay: Experiment and Theory. Toxics 2022, 10, 445. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.L. Removal of the methyl violet 2B dye from aqueous solution using sustainable adsorbent Artocarpus odoratissimus stem axis. Appl. Water Sci. 2017, 7, 3573–3581. [Google Scholar] [CrossRef]
- Taghizadeh, F. The Study of Structural and Magnetic Properties of NiO Nanoparticles. Opt. Photonics J. 2016, 06, 164–169. [Google Scholar] [CrossRef]
- Sahoo, Y.; He, Y.; Swihart, M.T.; Wang, S.; Luo, H.; Furlani, E.P.; Prasad, P.N. An aerosol-mediated magnetic colloid: Study of nickel nanoparticles. J. Appl. Phys. 2005, 98, 054308. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Zhang, J.; Li, J. Large scale synthesis and characterization of Ni nanoparticles by solution reduction method. Bull. Mater. Sci. 2008, 31, 97–100. [Google Scholar] [CrossRef]
- Torsello, D.; Ghigo, G.; Giorcelli, M.; Bartoli, M.; Rovere, M.; Tagliaferro, A. Tuning the microwave electromagnetic properties of biochar-based composites by annealing. Carbon Trends 2021, 4, 100062. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Jia, H.; Fan, L.; Liu, X.; Cao, W.; Ai, H.; Wang, Z.; Liu, X. Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water. Processes 2022, 10, 856. [Google Scholar] [CrossRef]
- Arkaan, M.F.; Ekaputri, R.F.; Fatimah, I.; Kamari, A. Physicochemical and photocatalytic activity of hematite/biochar nanocomposite prepared from Salacca skin waste. Sustain. Chem. Pharm. 2020, 16, 100261. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhanasekhar, C.; Venimadhav, A.; Kalpathy, S.K.; Anandhan, S. Pyrolysis-controlled synthesis and magnetic properties of sol–gel electrospun nickel cobaltite nanostructures. J. Sol-Gel Sci. Technol. 2018, 86, 664–674. [Google Scholar] [CrossRef]
- Liu, S.; Mei, J.; Zhang, C.; Zhang, J.; Shi, R. Synthesis and magnetic properties of shuriken-like nickel nanoparticles. J. Mater. Sci. Technol. 2018, 34, 836–841. [Google Scholar] [CrossRef]
- Singh, J.; Patel, T.; Kaurav, N.; Okram, G.S. Synthesis and magnetic properties of nickel nanoparticles. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1731, p. 050036. [Google Scholar] [CrossRef]
- Deepa, E.; Annal Therese, H. Hierarchical Nickel nanowire synthesis using polysorbate 80 as capping agent. Appl. Surf. Sci. 2018, 449, 48–54. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorob’Yov, A.M.; Yukhin, Y.M.; Lyakhov, N.Z. Characterization and Growth Mechanism of Nickel Nanowires Resulting from Reduction of Nickel Formate in Polyol Medium. J. Nanomater. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Hernández-Pérez, I.; Barriga-Arceo, L.D.; Garibay Febles, V.; Suárez-Parra, R.; Paz, R.L.; Santiago, P.; Rendón, L.; Jara, J.A.; Tapia, J.C.E.; González-Reyes, L. Self-organization of nickel nanoparticles dispersed in acetone: From separate nanoparticles to three-dimensional superstructures. J. Saudi Chem. Soc. 2017, 21, 238–244. [Google Scholar] [CrossRef]
- CHEN, K.; ZHANG, T.; CHEN, X.; HE, Y.; LIANG, X. Model construction of micro-pores in shale: A case study of Silurian Longmaxi Formation shale in Dianqianbei area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, Y.; Liu, L.; Hu, C. Effect of nickel salts on the production of biochar derived from alkali lignin: Properties and applications. Bioresour. Technol. 2021, 341, 125876. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Ma, X.; Liu, G.; Yao, Y.; Liu, H.; Chen, H.; Huang, Y.; Chen, C.; Wang, W. Catalytic pyrolysis of tar model compound with various bio-char catalysts to recycle char from biomass pyrolysis. BioResources 2016, 11, 3752–3768. [Google Scholar] [CrossRef]
- Öztürk, S.; Xiao, Y.X.; Dietrich, D.; Giesen, B.; Barthel, J.; Ying, J.; Yang, X.Y.; Janiak, C. Nickel nanoparticles supported on a covalent triazine framework as electrocatalyst for oxygen evolution reaction and oxygen reduction reactions. Beilstein J. Nanotechnol. 2020, 11, 770–781. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q.; Hou, H.; Wu, Y.; Yu, W.; Ji, X.; Shao, L. Nickel nanoparticles supported on nitrogen-doped honeycomb-like carbon frameworks for effective methanol oxidation. RSC Adv. 2017, 7, 14152–14158. [Google Scholar] [CrossRef]
- Cao, X.; Huang, Y.; Tang, C.; Wang, J.; Jonson, D.; Fang, Y. Preliminary study on the electrocatalytic performance of an iron biochar catalyst prepared from iron-enriched plants. J. Environ. Sci. 2020, 88, 81–89. [Google Scholar] [CrossRef]
- Mansour, A.N. Nickel Monochromated Al Kα XPS Spectra from the Physical Electronics Model 5400 Spectrometer. Surf. Sci. Spectra 1994, 3, 221–230. [Google Scholar] [CrossRef]
- Mohammadkhani, F.; Montazer, M.; Latifi, M. Microwave absorption and photocatalytic properties of magnetic nickel nanoparticles/recycled PET nanofibers web. J. Text. Inst. 2019, 110, 1606–1614. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R. Nickel-based cocatalysts for photocatalytic hydrogen production. Appl. Surf. Sci. 2015, 351, 779–793. [Google Scholar] [CrossRef]
- Fatimah, I.; Rubiyanto, D.; Sahroni, I.; Putra, R.S.; Nurillahi, R.; Nugraha, J. Physicochemical characteristics and photocatalytic performance of Tin oxide/montmorillonite nanocomposites at various Sn/montmorillonite molar to mass ratios. Appl. Clay Sci. 2020, 193, 105671. [Google Scholar] [CrossRef]
- Harjati, F.; Citradewi, P.W.; Purwiandono, G.; Fatimah, I. Green synthesis of hematite/TUD-1 nanocomposite as efficient photocatalyst for bromophenol blue and methyl violet degradation. Arab. J. Chem. 2020, 13, 8395–8410. [Google Scholar]
- Devi, H.S.; Singh, T.D.; Singh, H.P.; Singh, N.R. Tailoring of bimetallic NiO-Ag nanoparticles for degradation of methyl violet through a benign approach. J. Mater. Res. 2016, 31, 3459–3471. [Google Scholar] [CrossRef]
- Artagan, Ö.; Vaizoğullar, A.İ.; Uğurlu, M. Activated carbon-supported NiS/CoS photocatalyst for degradation of methyl violet (MV) and selective disinfection process for different bacteria under visible light irradiation. J. Taibah Univ. Sci. 2021, 15, 154–169. [Google Scholar] [CrossRef]
- Kanchana, S.; Vijayalakshmi, R. Photocatalytic degradation of organic dyes by peg and pvp cappcu, ni and ag nanoparticles in the presence of nabh4 in aqueous medium. J. Water Environ. Nanotechnol. 2020, 5, 294–306. [Google Scholar] [CrossRef]
- Ghribi, F.; Boudjemaa, A.; Benmaamar, Z.; Bachari, K. Preparation and Enhanced Visible-Light Photo-Catalytic Dye Degradation Activity of NiZY Composites. Biointerface Res. Appl. Chem. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Comǎnescu, A.F.; Mihaly, M.; Meghea, A. Photocatalytic degradation of organic pollutants using NiO based materials. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2012, 74, 49–60. [Google Scholar]

| Element | Percentage in (wt. %) | |
|---|---|---|
| BC | Ni/BC | |
| O | 40.81 | 15.81 |
| C | 18.17 | 32.86 |
| Al | 12.42 | 1.82 |
| Si | 12.07 | 6.56 |
| K | 8.73 | 0.97 |
| Fe | 6.81 | 1.96 |
| Ca | 0.98 | 1.04 |
| Ni | n.d. | 31.67 |
| Parameter | BC | Ni/BC |
|---|---|---|
| Specific surface area (m2/g) | 3.92 | 74.12 |
| Pore volume (cc/g) | 1.64 × 10−3 | 2.89 × 10−3 |
| Pore radius (Å) | 31.98 | 6.79 |
| MV Concentration (ppm) | First-Order Kinetics | Second-Order Kinetics | ||
|---|---|---|---|---|
| R2 | Kinetics Equation | R2 | Kinetics Equation | |
| 10 | 0.898 | ln C = 0.307 t + 0.305 | 0.999 | 1/C = 66.66 t + 0.167 |
| 20 | 0.901 | ln C = −0.060 t + 2.324 | 0.974 | 1/C = 4.282 t + 49.639 |
| 50 | 0.869 | ln C = −0.053 t + 3.695 | 0.990 | 1/C = 0.619 t − 4.390 |
| 70 | 0.861 | ln C = −0.104 t + 2.635 | 0.955 | 1/C = 0.255 t − 1.534 |
| 80 | 0.867 | ln C = −0.080 t + 3.029 | 0.957 | 1/C = 0.042 t + 0.064 |
| Photocatalyst | DE | Remark | |
|---|---|---|---|
| NiO NPs | 28 | Photocatalysis reaction obeys pseudo-first order kinetics | [39] |
| NiO-Ag bimetal | 32 | Photocatalysis reaction obeys pseudo-first order kinetics at MV concentration of 1 × 10−3 M at neutral pH | [39] |
| Activated carbon-supported NiS/CoS | 60–70 | Degradation efficiency is ranging at 56–78% depending on Ni and Co composition at degradation time of 90 min | [40] |
| Ni NPs | 45 | Ni NPs was synthesized using polyvinyl pyrrolidone (PVP), stabilizer, the reaction was conducted under UV light for 40 min | [41] |
| Ni/Zeolite Y | 94 | Photocatalysis reaction was conducted for 240 min | [42] |
| NiO/SiO2 | 20 | Photooxidation was conducted for 21 min | [43] |
| NiO | 50 | Photooxidation was conducted for 21 min | [43] |
| Ni/BC | >99.5 | Photooxidation efficiency was obtained for MV initial concentration of 10–80 ppm conducted for 30 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, I.; Wijayanti, H.K.; Ramanda, G.D.; Tamyiz, M.; Doong, R.-a.; Sagadevan, S. Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation. Molecules 2022, 27, 6871. https://doi.org/10.3390/molecules27206871
Fatimah I, Wijayanti HK, Ramanda GD, Tamyiz M, Doong R-a, Sagadevan S. Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation. Molecules. 2022; 27(20):6871. https://doi.org/10.3390/molecules27206871
Chicago/Turabian StyleFatimah, Is, Hiroko Kawaii Wijayanti, Galih Dwiki Ramanda, Muchammad Tamyiz, Ruey-an Doong, and Suresh Sagadevan. 2022. "Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation" Molecules 27, no. 20: 6871. https://doi.org/10.3390/molecules27206871
APA StyleFatimah, I., Wijayanti, H. K., Ramanda, G. D., Tamyiz, M., Doong, R.-a., & Sagadevan, S. (2022). Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation. Molecules, 27(20), 6871. https://doi.org/10.3390/molecules27206871










